Flowonix Prometra II, 13827 User Manual

PROMETRA® II PROGRAMMABLE PUMP (REF 13827)
For use with Intrathecal Catheter
MR Conditional
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.

Table of Contents
Introduction ................................................................................................................ 3
Contents ..................................................................................................................... 3
Description ................................................................................................................. 3
Indications .................................................................................................................. 6
Drug Information ........................................................................................................ 6
Contraindications ........................................................................................................ 6
Warnings .................................................................................................................... 7
General .......................................................................................................................... 7
Magnetic Resonance Imaging (MRI) ............................................................................. 8
Precautions ............................................................................................................... 19
General ........................................................................................................................ 19
Implant ........................................................................................................................ 19
Device Compatibility ................................................................................................... 19
Potential Adverse Events .......................................................................................... 20
Clinical Studies .......................................................................................................... 22
Results ......................................................................................................................... 22
Equipment ................................................................................................................ 23
Pump Operation ....................................................................................................... 23
Programmable Features ............................................................................................. 23
Programming Medication Regimens .......................................................................... 24
Pre-Programmed Pump Settings ................................................................................. 26
Pump Alarms ............................................................................................................... 27
Implantation Instructions .......................................................................................... 28
Pre-Implant Pump Programming Set Up .................................................................... 28
Pump Priming Preparation .......................................................................................... 28
Pump Priming .............................................................................................................. 30
Implantation of the Intrathecal Catheter ................................................................... 31
Implantation of the Prometra II Programmable Pump ............................................... 31
Patient Implant Card and Registration ....................................................................... 33
Pump Explantation .................................................................................................... 33
Calculations .............................................................................................................. 34
Patient-Related Variables and Flow Rate Accuracy .................................................... 34
Geographical Elevation ............................................................................................... 34
Temperature Variation ............................................................................................... 35
Flow Rate Accuracy ..................................................................................................... 36
Device Longevity ......................................................................................................... 37
Drug Stability............................................................................................................... 37
PROMETRA® II PROGRAMMABLE PUMP Page 2 of 38

Introduction
The Prometra II Programmable Pump is designed to provide controlled delivery of Infumorph® to the
intrathecal space via the separately supplied Intrathecal Catheter. The Prometra II Pump incorporates
a patented flow activated safety valve (FAV™) that will shut off drug flow to the patient in the event
that a high flow rate is encountered. The Prometra Programmer is a separately supplied handheld,
menu-driven device that enables remote programming of the Prometra II Pump.
Note: The use of the terms “medication” and “drug” throughout this document refer to the use of
Infumorph.
Contents
The following components are sterile and non-pyrogenic:
1 – Prometra II Programmable Pump
1 – Needle, Non-Coring, 0.7 mm (22G) x 38 mm (1.5 in.)
1 – Needle, Catheter Access, 0.9 mm (20G) x 45 mm (1.75 in.)
Non-sterile components:
1 – Patient and Physician Information Packet:
1 – Instructions for Use
1 – Calculations Guide
1 – Patient Guide
2 – Temporary Patient Implant Cards
1 – Sheet of Device ID Stickers
1 – Patient Device Tracking Form
1 – Warranty Card
Description
The Prometra II Pump is a battery-powered, teardrop-shaped pump with a rigid titanium housing and a
triple redundancy flow controller system. To help increase safety, the Prometra II Pump incorporates
a safety valve (flow-activated valve or FAV) that will shut off drug flow to the patient in the event a
high flow rate occurs.
PROMETRA® II PROGRAMMABLE PUMP Page 3 of 38
The triple redundancy flow control system is designed to provide a precise and accurate flow rate. The
flow rate accuracy is independent of normal operating environmental conditions such as altitude,
temperature and reservoir volume.
PROMETRA® II PROGRAMMABLE PUMP Page 4 of 38
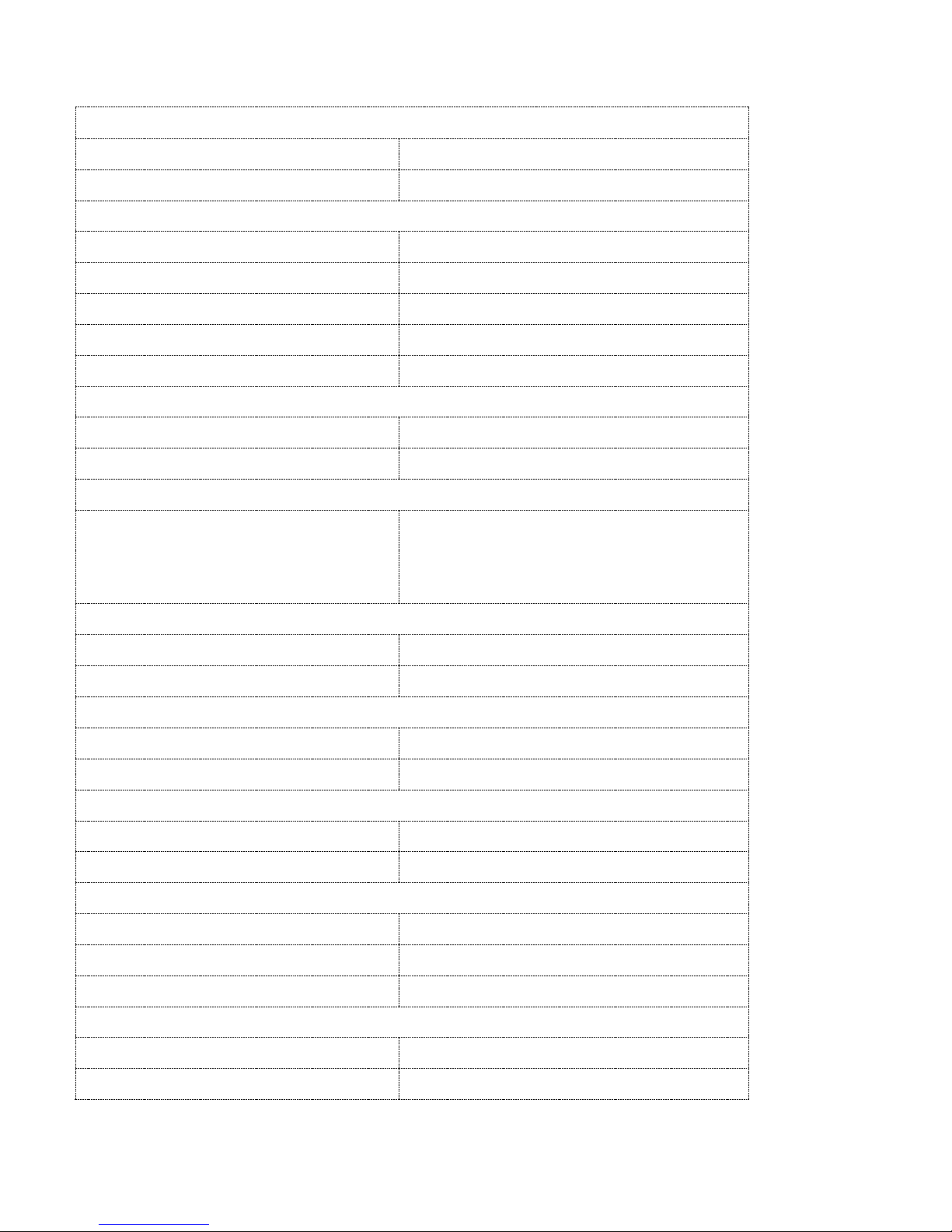
Titanium Polyphenylsulfone access ports
Specifications of the Prometra II Programmable Pump are:
Device Longevity
Pump 10 years at 0.25 mL/day
Septum (Refill and CAP) 1000 punctures maximum
External Properties
Material
Thickness (nominal) 20 mm
Diameter (excluding CAP) 69 mm
Average Volume Displacement 100 mL
Weight, unfilled 150 g
Drug Reservoir
Material Titanium
Usable Capacity 20 mL
Precision Dosing System
Material Titanium
MP35N alloy
Stainless steel
Silicone rubber
Refill Septum
Septum material Silicone rubber
Access needle Huber point, 22G non-coring needle
Catheter Access Septum
Septum material Silicone rubber
Access needle Lancet point with side hole, 20G
Bacterial filter
Material Polyvinylidene fluoride
Pore size 0.22 micron
Flow Rate
Range 0-28.8 mL/day
Accuracy 95.9-97.7% (90% confidence limit)
Refill Interval Not more than 90 days
Flow Activated Valve (FAV)
Material Same as Precision Dosing System
Maximum volume dispersed when closed 10 µl
PROMETRA® II PROGRAMMABLE PUMP Page 5 of 38

The pump is supplied with a Catheter Access needle and a non-coring Refill needle for priming the
pump at implantation. The Patient Information packet contains a patient guide and two patient
implant cards to be completed and given to the patient. Additionally, a federally-mandated patient
device tracking form is included and needs to be completed and returned to Flowonix.
Indications
The Prometra II Programmable Pump is indicated for intrathecal infusion of Infumorph® (preservativefree morphine sulfate sterile solution) or preservative-free sterile 0.9% saline solution (Sodium
Chloride Injection, USP).
Drug Information
Refer to the Infumorph labeling for a complete list of indications, contraindications, warnings,
precautions, dosage administration information and screening procedures.
Contraindications
Implantation of this device is contraindicated when:
• The presence of infection is known or suspected.
• The patient’s body size or anatomy is insufficient to accommodate the size of the implanted pump
or catheter.
• The pump cannot be implanted 2.5 cm (1 in.) or less from the surface of the skin. Deeper implants
could interfere with septum access or telemetry.
• The patient is known or is suspected to be allergic to materials contained in the catheter: silicone
elastomers, barium sulfate, tungsten, polyacetal resin, ink, stainless steel, hydroglide hydro gel
coating, or plastic needle hubs (polypropylene and acrylic based).
• The patient is known or is suspected to be allergic to materials contained in the pump: titanium,
silicone elastomers, polyphenylsulfone, silicone adhesive, polyvinylidene fluoride, MP35N metal
(nickel-cobalt-chromium-molybdenum alloy), or stainless steel (AL29-4, 316L).
• The patient has exhibited a prior intolerance to implanted devices.
• The patient has a spinal column anatomy that would obstruct cerebrospinal fluid flow or that
would prevent intraspinal drug administration.
• The patient has emotional, psychiatric or substance abuse problems that are deemed to prohibit
intrathecal drug administration.
• Contraindications relating to Infumorph must be observed and followed per the approved drug
labeling.
PROMETRA® II PROGRAMMABLE PUMP Page 6 of 38
WARNING: USE OF UNAPPROVED DRUGS (e.g., DRUG COCKTAILS, PHARMACY-COMPOUNDED
IN PUMP FAILURE AND/OR SERIOUS ADVERSE EVENTS INCLUDING DEATH.
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT COULD
RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR DEATH.
Warnings
General
DRUGS, MORPHINE WITH PRESERVATIVES, ETC.) WITH THE PROMETRA II PUMP COULD RESULT
• Prior to infusion of Infumorph into the catheter, medical personnel should be familiar with and
observe all warnings, cautions, contraindications, and instructions as specified by the drug
manufacturer.
• Patients should not undergo hyperbaric therapy since exposure could result in drug underdose.
• Always select and program dosages consistent with the Infumorph® labeling to prevent improper
drug administration.
• In the event of over-medication, refer to the approved Infumorph labeling for appropriate
treatment.
• Clinicians implanting, programming, accessing, or maintaining implanted programmable pumps
must comply with the instructions for use. Technical errors may result in a return of underlying
symptoms, drug withdrawal symptoms, or clinically significant or fatal overdose.
• The Prometra II Programmable Pump components are supplied sterile and non-pyrogenic. The
packages should be examined carefully prior to opening. Do not use the contents if there is any
evidence of damage to the package or package seal that could compromise sterility. Do not
resterilize contents of any damaged or opened packages.
• After use, this device is a biohazard. Handle and dispose of in accordance with accepted hospital
practice and all applicable laws and regulations.
• Do not incinerate or cremate the pump.
• Do not expose the pump to temperatures above 57˚C (134.6˚F) or below 2˚C (35.6˚F).
• The patient has an occupation where he/she would be exposed to high current industrial
equipment, powerful magnets or transmitting towers, such as, electricians, electrical engineers or
MRI technicians.
PROMETRA® II PROGRAMMABLE PUMP Page 7 of 38

DEATH.
Magnetic Resonance Imaging (MRI)
Prometra® and Prometra® II Programmable Pumps Magnetic
Resonance Imaging (MRI) Instruction Guide
GENERAL
MR Conditional
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT
COULD RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR
Warning: Patients should not be exposed to MRI environments until the surgical site following
pump implantation is fully healed.
Warning: EMPTY ALL DRUG SOLUTION FROM BOTH PROMETRA AND PROMETRA II PUMPS PRIOR
TO ENTERING THE MRI ENVIRONMENT. If a patient with a Prometra II Pump requires an
emergent MRI, please see page 13 of these instructions for more details on the potential risks
involved.
Strong magnetic fields, such as those created in MRI scanners, may cause the Inlet and Outlet
Valves to open, resulting in the immediate discharge of the contents of the Drug Reservoir and
Catheter into the patient. This could result in drug overdose that could lead to serious patient
injury or death.
Prior to initiating the MRI procedure, the physician must determine if the patient can safely be deprived of
medication for the length of the MRI procedure. If medication is needed, then alternate means of drug
delivery (such as I.V. administration) should be employed for the duration of the MRI procedure.
Prior to scheduling an MRI scan and upon its completion, pump status should be confirmed by inquiring the
pump to verify pump operation and settings.
Note: Pre-MRI, Post-MRI, and Medical Emergency Use instructions are provided in this document.
SCANNING PARAMETERS
The Prometra® and Prometra® II Programmable Pumps can be safely exposed to an MRI system when ALL of
the following conditions are met:
1. The MRI device has a static magnetic field of 1.5 Tesla.
2. The MRI device has a maximum spatial gradient field of 2,000 Gauss/cm (20 T/m) at 1.5 Tesla.
Warning: Exceeding the 2,000 Gauss/cm (20T/m) at 1.5T limit could result in excessive force or
torque which could lead to patient injury.
PROMETRA® II PROGRAMMABLE PUMP Page 8 of 38

3. A maximum whole body average specific absorption rate (SAR) of 2 W/kg for 20 minutes of safe
scanning in the Normal Operating Mode.
4. All Pre-MRI Instructions must be completed.
NOTE: The MRI conditions for safe scanning detailed in this document only pertain to
the Prometra Pumps implanted in the abdomen. Testing has not been conducted in other
implantation locations or in the presence of other implanted active or passive medical devices. Other
implanted devices (such as pacemakers, abandoned leads, knee implants, etc.) could have conflicting
MR conditions which could lead to patient injury or device malfunction.
Tissue Heating Adjacent to Implant during MR Scans
(1)
®
In non-clinical testing, the Prometra
Pump produced a maximum temperature rise of 1.5°C during 20
minutes of continuous MR scanning in the Normal Operation Mode at a maximum whole-body averaged
specific absorption rate (SAR) of 2 W/kg using a transmit body coil, therefore the Prometra II will experience a
similar maximum temperature rise under the same MR scanning conditions.
The local temperature increase produced by the pump is considered to be below level of concern. In the
unlikely event that the patient experiences uncomfortable warmth near the pump, the MRI scan should be
stopped and the scan parameters adjusted to reduce SAR to comfortable levels.
Warning: Static Magnetic Field
In a 1.5 Tesla MR environment, the pump has a significant magnetically induced deflection force
and very strong torque. The static and gradient magnetic fields produced by an MRI scanner could
potentially interact with the pump and cause vibration. However, when pumps are implanted with
proper techniques, the patient may safely be scanned under the conditions listed above. Not
following the specific conditions may result in serious patient injury. The patient may experience a
tugging and/or vibration sensation at the implant site when placed within the magnetic field. An
elastic garment or wrap will help restrict movement and reduce these sensations while the patient
is in the magnetic field.
Image Artifacts
The programmable pump contains ferromagnetic components that will cause image distortion and localized
voids in regions of the image around the pump. MR image quality will be compromised if the area of interest is
near the pump.
Worst case artifacts measured from the edge of the device in non-clinical tests using a spin echo sequence
were found to extend more than 11 cm from the pump. Image artifacts were reduced up to 36% when
sequences were optimized for imaging (e.g. shorter echo time, decreased water fat shift, etc.). Images of the
head and lower extremities away from the location of the Prometra Pump should be largely unaffected. The
nonclinical testing was performed using the ASTM F2119 GRE and SE sequences in a 1.5T Philips Medical
Systems Intera (software release 12.6.4.3, 2010-12-02) MR system with a body coil in transmit and receive
mode.
1
There are no changes between the Prometra® pump and Prometra® II pump that would significantly affect the
ASTM MRI testing and MRI Scanning Parameters.
PROMETRA® II PROGRAMMABLE PUMP Page 9 of 38
DEATH.
SPECIFIC PRE-MRI INSTRUCTIONS
WARNING: FAILURE TO EMPTY THE PUMP PRIOR TO EXPOSURE TO MRI ENVIRONMENT
COULD RESULT IN DRUG OVERDOSE THAT COULD LEAD TO SERIOUS PATIENT INJURY OR
Prometra® (REF 11827) and Prometra® II (REF 13827) Programmable Pumps
Protocol for Prometra® (REF 11827) and Prometra® II (REF 13827) Programmable Pumps
Pre-MRI Procedure
Warning: EMPTY ALL DRUG SOLUTION FROM BOTH PROMETRA AND PROMETRA II PUMPS PRIOR
TO ENTERING THE MRI ENVIRONMENT. If a patient with a Prometra II Pump requires an
emergent MRI, please see page 13 of these instructions for more details on the potential risks
involved.
Strong magnetic fields, such as those created in MRI scanners, may cause the Inlet and Outlet
valves to open, resulting in the immediate discharge of the contents of the Drug Reservoir and
Catheter into the patient. This could result in drug overdose that could lead to serious patient
injury or death.
The physician must determine if the patient can safely be deprived of medication during the MRI procedure. If
medication is needed then alternative means of drug delivery (such as I.V. administration or analgesic patch)
should be employed.
IF AN MRI PROCEDURE IS NECESSARY, THE PUMP MUST BE EMPTIED of drug solution, not
refilled and the PUMP PROGRAMMED TO 0.0 MG/DAY DRUG FLOW RATE prior to entering the
environment of the MRI.
PERFORM THE FOLLOWING STEPS PRIOR TO ENTERING THE MRI ENVIRONMENT.
1. Pump Inquiry
Inquire the pump with the programmer to verify pump model, the pump is operational and
without errors. Print inquiry page.
WARNING: IF PUMP STATUS CANNOT BE PROPERLY CONFIRMED, DO NOT PROCEED SINCE
THE PUMP MAY NOT BE OPERATING PROPERLY, PLEASE CONTACT FLOWONIX TECHNICAL
SOLUTIONS FOR ASSISTANCE AT: 855-356-9665.
PROMETRA® II PROGRAMMABLE PUMP Page 10 of 38

2. Pump Programming
Set the flow mode to a constant flow rate of 0.0 mg/day. Re-inquire the pump and print inquiry page to
confirm a constant flow rate of 0.0 mg/day.
3. Empty Drug Reservoir
Follow the procedures for emptying the Drug Reservoir in the Refill Kit Instructions for Use.
PROMETRA® II PROGRAMMABLE PUMP Page 11 of 38

SOLUTIONS FOR ASSISTANCE AT: 855-356-9665.
SPECIFIC POST-MRI INSTRUCTIONS
Protocol for Prometra® (REF 11827) and Prometra® II (REF 13827) Programmable Pumps
Post-MRI Procedure
1. Confirm Pump Operational Status –
a. Inquire the pump with the programmer to verify pump operation and settings.
b. Confirm that settings are unchanged from the Pre-MRI settings, e.g., flow rate must be 0.0
mg/day.
c. If the programmer displays any pump errors, proceed to Step 2 “Clear Pump Errors”.
d. If no pump errors are displayed, proceed to Step 3 “Inlet and Outlet Valve Closure
Confirmation”.
WARNING: IF PUMP STATUS CANNOT BE PROPERLY CONFIRMED, DO NOT PROCEED SINCE
THE PUMP MAY NOT BE OPERATING PROPERLY, PLEASE CONTACT FLOWONIX TECHNICAL
2. Clear Pump Errors
a. If pump errors are displayed from the Inquiry performed in Step 1, perform an Emergency Pump
Stop using the programmer, and contact Flowonix Technical Solutions for assistance 855-356-9665.
b. If pump errors are cleared, proceed to Step 3.
3. Confirm Inlet / Outlet Valve Closure
a. Attempt to aspirate the Drug Reservoir through the Refill Port. To aspirate, attach the 22G
non-coring needle (available in Refill Kit) to a sterile syringe.
b. Advance needle through center Refill Port Septum until needle tip resides completely inside
the Drug Reservoir.
c. Pull a vacuum with the syringe for approximately 10 to 30 seconds to confirm Inlet / Outlet Valve
closure.
Warning: If any significant volume (>1ml) is retrieved, it may be indicative that the pump Inlet /
Outlet Valves are open, providing direct access to the catheter/cerebral spinal fluid; If so, DO NOT
proceed with the refill since the pump may not be operating properly. The pump may need to be
explanted and replaced. For questions, Contact Flowonix Technical Solutions for assistance at:
855-356-9665.
4. Refill The Drug Reservoir
a. Proceed to refill the Drug Reservoir in accordance with the refill procedure defined in the
Refill Kit Instructions for Use.
b. Confirm the correct prescription is programmed, or program a new prescription.
Warning: A period of observation should follow the Refill Procedure to closely monitor patients for
clinical symptoms of underdose or overdose based upon Infumorph’s prescribing information.
PROMETRA® II PROGRAMMABLE PUMP Page 12 of 38
 Loading...
Loading...