Page 1

User’s manual
FLIR G300 a
Page 2

Page 3

User’s manual
FLIR G300 a
#T559899; r. AB/35742/35742; en-US
iii
Page 4

Page 5

Table of contents
1 Legal disclaimer ....... ....... ....... .......................... ....... ....... .....................1
1.1 Legal disclaimer ......................................................................... 1
1.2 Usage statistics .......................................................................... 1
1.3 Changes to registry .....................................................................1
1.4 U.S. Government Regulations........................................................1
1.5 Copyright ..................................................................................1
1.6 Quality assurance .......................................................................1
1.7 Patents.....................................................................................1
1.8 EULA Terms ..............................................................................1
2 Safety information .... ....... ....... ....... ................... ....... ....... ....... ..............2
3 Notice to user .. ....... ....... .......................... ....... ....... ................... ....... ... 3
3.1 User-to-user forums ....................................................................3
3.2 Accuracy ..................................................................................3
3.3 Disposal of electronic waste .......................................................... 3
3.4 Training ....................................................................................3
3.5 Documentation updates ............................................................... 3
3.6 Important note about this manual.................................................... 3
3.7 Note about authoritative versions....................................................3
4 Customer help ................................. ....... ....... .......................... ....... ....4
4.1 General ....................................................................................4
4.2 Submitting a question .................................................................. 4
4.3 Downloads ................................................................................5
5 Important note about training and applications ............... ....... ....... ....... ... 6
5.1 General ....................................................................................6
6 Introduction....................... ....... ....... ....... ................... ....... ....... ....... ....7
7 Typical system overview....... ....... ....... ....... ................... ....... ....... ....... ... 8
7.1 Explanation ...............................................................................8
8 Quick start guide ..... ....... ................... ....... ....... ....... ................... ....... ... 9
8.1 Download FLIR Tools...................................................................9
9 Mechanical installation ... ....... ................................. ....... ....... ....... ...... 10
9.1 Mounting interfaces................................................................... 10
9.2 Notes on permanent mounting..................................................... 10
9.3 Vibrations................................................................................ 10
9.4 Further information.................................................................... 10
10 Connectors............... ....... ....... ....... ........................................ ........... 11
10.1 Figure .................................................................................... 11
10.2 Explanation ............................................................................. 11
11 Verifying camera operation..................................... ............................ 12
11.1 Power and analog video ............................................................. 12
11.2 IP Communication..................................................................... 12
12 Network troubleshooting..................... ....... ....... ................................. 13
13 Technical data ... ....... ................... ....... ....... ....... ................................. 14
13.1 Online field-of-view calculator ...................................................... 14
13.2 Note about technical data ........................................................... 14
13.3 Note about authoritative versions.................................................. 14
13.4 FLIR G300 a 14.5° fixed lens ....................................................... 15
13.5 FLIR G300 a 24° fixed lens ......................................................... 19
#T559899; r. AB/35742/35742; en-US
v
Page 6

Table of contents
14 Mechanical drawings ................................................. ....... ....... .......... 23
15 CE Declaration of conformity ................... ........................................ ... 25
16 Detectable gases......................... ....... ............................................... 27
17 Why do some gases absorb infrared energy? . ................... ....... ....... ..... 30
18 Cleaning the camera ........................ ........................................ .......... 33
18.1 Camera housing, cables, and other items....................................... 33
18.1.1 Liquids......................................................................... 33
18.1.2 Equipment.................................................................... 33
18.1.3 Procedure .................................................................... 33
18.2 Infrared lens ............................................................................ 33
18.2.1 Liquids......................................................................... 33
18.2.2 Equipment.................................................................... 33
18.2.3 Procedure .................................................................... 33
19 About FLIR Systems ....... ....... .......................... ....... ....... ....... ............. 34
19.1 More than just an infrared camera ................................................ 35
19.2 Sharing our knowledge .............................................................. 35
19.3 Supporting our customers........................................................... 36
20 Glossary .......... ....... ....... ....... ................... ....... ....... ....... ................... 37
21 Thermographic measurement techniques ......... ................................... 40
21.1 Introduction ............................................................................ 40
21.2 Emissivity................................................................................ 40
21.2.1 Finding the emissivity of a sample...................................... 40
21.3 Reflected apparent temperature ................................................... 44
21.4 Distance ................................................................................. 44
21.5 Relative humidity ...................................................................... 44
21.6 Other parameters...................................................................... 44
22 History of infrared technology... ........................................ .................. 45
23 Theory of thermography................. ....... ....... .......................... ....... ..... 48
23.1 Introduction ............................................................................. 48
23.2 The electromagnetic spectrum..................................................... 48
23.3 Blackbody radiation................................................................... 48
23.3.1 Planck’s law .................................................................. 49
23.3.2 Wien’s displacement law.................................................. 50
23.3.3 Stefan-Boltzmann's law ................................................... 51
23.3.4 Non-blackbody emitters ................................................... 52
23.4 Infrared semi-transparent materials............................................... 54
24 The measurement formula........................... ....................................... 55
#T559899; r. AB/35742/35742; en-US
vi
Page 7

1
Legal disclaimer
1.1 Legal disclaimer
All products manufactured by FLIR Systems are warranted against defective
materials and workmanship for a period of one (1) year from the delivery date
of the original purchase, provided such products have been under normal storage, use and service, and in accordance with FLIR Systems instruction.
Uncooled handheld infrared cameras manufactured by FLIR Systems are warranted against defective materials and workmanship for a period of two (2)
years from the deliverydate of the original purchase, provided such products
have been under normal storage, use and service, and in accordance with
FLIR Systems instruction, and provided that the camera has been registered
within 60 days of original purchase.
Detectors for uncooled handheld infrared cameras manufactured by FLIR Systems are warranted against defective materials and workmanship for a period
of ten (10) years from the delivery date of the original purchase,provided such
products have been under normal storage, use and service, and in accordance
with FLIR Systems instruction, and provided that the camera has been registered within 60 days of original purchase.
Products which are not manufactured by FLIR Systems but included in systems delivered by FLIR Systems to the original purchaser, carry the warranty, if
any, of the particular supplieronly. FLIR Systems has no responsibility whatsoever for such products.
The warranty extends only to the original purchaser and is not transferable. It
is not applicable to any product which has been subjected to misuse, neglect,
accident or abnormal conditions of operation. Expendable parts are excluded
from the warranty.
In the case of a defect in a product covered by this warranty the product must
not be further used in order to prevent additional damage. The purchaser shall
promptly report any defect to FLIR Systems or this warranty will not apply.
FLIR Systems will, atits option, repair or replace any such defective product
free of charge if, upon inspection, it proves to be defective in material or workmanship and provided that it is returned to FLIR Systems within the said oneyear period.
FLIR Systems has no other obligation or liability for defects than those set forth
above.
No other warranty is expressed or implied. FLIR Systems specifically disclaims
the implied warranties of merchantability and fitnessfor a particular purpose.
FLIR Systems shall not be liable for any direct, indirect, special, incidental or
consequential loss or damage, whether based on contract, tort or any other legal theory.
This warranty shall be governed by Swedish law.
Any dispute, controversy or claim arising out of or in connection with this war-
ranty, shall be finally settled by arbitration in accordance with the Rules of the
Arbitration Institute of the Stockholm Chamber of Commerce. The place of arbitration shall be Stockholm. The language to be usedin the arbitral proceedings shall be English.
1.2 Usage statistics
FLIR Systems reserves the right to gather anonymous usage statistics to help
maintain and improve the quality of our software and services.
1.3 Changes to registry
The registry entry HKEY_LOCAL_MACHINE\SYSTEM\CurrentControlSet
\Control\Lsa\LmCompatibilityLevel will be automatically changed to level 2 if
the FLIR Camera Monitor service detects a FLIR camera connected to the
computer with a USB cable. The modification will only be executedif the camera device implements aremote network service that supports network logons.
1.4 U.S. Government Regulations
This product may be subject to U.S. Export Regulations. Please send any inquiries to exportquestions@flir.com.
1.5 Copyright
© 2016, FLIR Systems, Inc. All rights reserved worldwide. No parts of the software including source code may be reproduced, transmitted, transcribed or
translated into any language or computer language in any form or by any
means, electronic, magnetic, optical, manual or otherwise, without the prior
written permission of FLIR Systems.
The documentation must not, in whole or part, be copied, photocopied, reproduced, translated or transmitted to any electronic medium or machine readable form without priorconsent, in writing, from FLIRSystems.
Names and marks appearing on the products herein are either registered
trademarks or trademarks of FLIR Systems and/or its subsidiaries. All other
trademarks, trade names or company names referenced herein are used for
identification only and arethe property of their respective owners.
1.6 Quality assurance
The Quality Management System under which these products are developed
and manufactured has been certified in accordance with the ISO 9001
standard.
FLIR Systems is committed to a policy of continuous development; therefore
we reserve the right to make changes and improvements on any of the products without prior notice.
1.7 Patents
One or several of the following patents and/or design patents may apply to the
products and/or features. Additional pending patents and/or pending design
patents may also apply.
000279476-0001; 000439161; 000499579-0001; 000653423; 000726344;
000859020; 001106306-0001; 001707738; 001707746; 001707787;
001776519; 001954074; 002021543; 002058180; 002249953; 002531178;
0600574-8; 1144833; 1182246; 1182620; 1285345; 1299699; 1325808;
1336775; 1391114; 1402918; 1404291; 1411581; 1415075; 1421497;
1458284; 1678485; 1732314; 2106017; 2107799; 2381417; 3006596;
3006597; 466540; 483782; 484155; 4889913; 5177595; 60122153.2;
602004011681.5-08; 6707044; 68657; 7034300; 7110035; 7154093;
7157705; 7237946; 7312822; 7332716; 7336823; 7544944; 7667198;
7809258 B2; 7826736; 8,153,971; 8,823,803; 8,853,631; 8018649 B2;
8212210 B2; 8289372; 8354639 B2; 8384783; 8520970; 8565547; 8595689;
8599262; 8654239; 8680468; 8803093; D540838; D549758; D579475;
D584755; D599,392; D615,113; D664,580; D664,581; D665,004; D665,440;
D677298; D710,424 S; D718801; DI6702302-9; DI6903617-9; DI7002221-6;
DI7002891-5; DI7002892-3; DI7005799-0; DM/057692; DM/061609; EP
2115696 B1; EP2315433; SE 0700240-5; US 8340414 B2; ZL
201330267619.5; ZL01823221.3; ZL01823226.4; ZL02331553.9;
ZL02331554.7; ZL200480034894.0; ZL200530120994.2; ZL200610088759.5;
ZL200630130114.4; ZL200730151141.4; ZL200730339504.7;
ZL200820105768.8; ZL200830128581.2; ZL200880105236.4;
ZL200880105769.2; ZL200930190061.9; ZL201030176127.1;
ZL201030176130.3; ZL201030176157.2; ZL201030595931.3;
ZL201130442354.9; ZL201230471744.3; ZL201230620731.8.
1.8 EULA Terms
• Youhave acquired a device (“INFRARED CAMERA”) that includes software licensed by FLIR Systems AB from Microsoft Licensing, GP or its affiliates (“MS”). Those installed software products of MS origin, as well as
associated media, printed materials, and “online” or electronic documentation (“SOFTWARE”) are protected by international intellectual property
laws and treaties. The SOFTWARE is licensed, not sold. All rights
reserved.
• IF YOU DO NOTAGREE TOTHIS END USER LICENSE AGREEMENT
(“EULA”), DO NOT USE THEDEVICE OR COPY THE SOFTWARE. INSTEAD, PROMPTLYCONTACT FLIR Systems AB FOR INSTRUCTIONS
ON RETURN OF THE UNUSED DEVICE(S) FOR A REFUND. ANY USE
OF THE SOFTWARE, INCLUDING BUT NOT LIMITED TO USE ON
THE DEVICE, WILL CONSTITUTE YOUR AGREEMENT TOTHIS EULA (OR RATIFICATION OF ANY PREVIOUS CONSENT).
• GRANT OF SOFTWARE LICENSE. This EULA grants you the following
license:
◦ Youmay use the SOFTWAREonly on the DEVICE.
◦ NOT FAULT TOLERANT. THE SOFTWARE IS NOTFAULT TOLER-
ANT.FLIR Systems AB HAS INDEPENDENTLY DETERMINED
HOW TO USE THE SOFTWARE IN THE DEVICE, AND MS HAS
RELIED UPON FLIR Systems AB TO CONDUCT SUFFICIENT
TESTING TO DETERMINE THAT THE SOFTWARE IS SUITABLE
FOR SUCH USE.
◦ NO WARRANTIES FOR THE SOFTWARE. THE SOFTWARE is
provided “AS IS” and with all faults. THE ENTIRE RISK AS TO SATISFACTORY QUALITY, PERFORMANCE, ACCURACY, AND EFFORT (INCLUDING LACK OF NEGLIGENCE) IS WITH YOU.
ALSO, THERE IS NO WARRANTY AGAINST INTERFERENCE
WITH YOUR ENJOYMENT OF THE SOFTWARE OR AGAINSTINFRINGEMENT.IF YOU HAVE RECEIVED ANY WARRANTIES RE-
GARDING THE DEVICE OR THE SOFTWARE, THOSE
WARRANTIES DO NOT ORIGINATE FROM, AND ARE NOT
BINDING ON, MS.
◦ No Liability for Certain Damages. EXCEPTAS PROHIBITED BY
LAW,MS SHALL HAVE NO LIABILITY FOR ANY INDIRECT, SPECIAL, CONSEQUENTIAL OR INCIDENTAL DAMAGES ARISING
FROM OR IN CONNECTION WITH THE USE OR PERFORMANCE OF THE SOFTWARE. THIS LIMITATION SHALL APPLY
EVEN IF ANY REMEDY FAILS OF ITS ESSENTIAL PURPOSE. IN
NO EVENT SHALL MS BE LIABLE FOR ANY AMOUNT IN EXCESS OF U.S. TWO HUNDRED FIFTY DOLLARS (U.S.$250.00).
◦ Limitations on Reverse Engineering, Decompilation, and Dis-
assembly. You may not reverse engineer, decompile, or disassem-
ble the SOFTWARE, except and only to the extent that such activity
is expressly permitted by applicable law notwithstanding this
limitation.
◦ SOFTWARE TRANSFER ALLOWED BUT WITH RESTRICTIONS.
Youmay permanently transfer rights under this EULA only as part of
a permanent sale or transfer of the Device, and only if the recipient
agrees to this EULA. If the SOFTWARE is an upgrade, any transfer
must also include all prior versions of the SOFTWARE.
◦ EXPORT RESTRICTIONS. You acknowledge that SOFTWARE is
subject to U.S. export jurisdiction. You agree to comply with all applicable international and national laws that apply to the SOFTWARE,
including the U.S. Export Administration Regulations, as well as
end-user, end-use and destination restrictions issued by U.S. and
other governments. For additional information see http://www.microsoft.com/exporting/.
#T559899; r. AB/35742/35742; en-US
1
Page 8
2
Safety information
WARNING
Make sure that you read all applicable MSDS (Material Safety Data Sheets) and warning labels on containers before you use a liquid. The liquids can be dangerous. Injury to persons can occur.
WARNING
For equipment with plugs:
Make sure that you install the socket-outlet near the equipment and that it is easy to get access to.
CAUTION
Do not point the infrared camera (with or without the lens cover) at strong energy sources, for example,
devices that cause laser radiation, or the sun. This can have an unwanted effect on the accuracy of the
camera. It can also cause damage to the detector in the camera.
CAUTION
Do not use the camera in temperatures more than +50°C (+122°F), unless other information is specified
in the user documentation or technical data. High temperatures can cause damage to the camera.
CAUTION
Do not apply solvents or equivalent liquids to the camera, the cables, or other items. Damage to the battery and injury to persons can occur.
CAUTION
Be careful when you clean the infrared lens. The lens has an anti-reflective coating which is easily damaged. Damage to the infrared lens can occur.
CAUTION
Do not use too much force to clean the infrared lens. This can cause damage to the anti-reflective
coating.
CAUTION
Applicability: Cameras with an automatic shutter that can be disabled.
Do not disable the automatic shutter in the camera for a long time period (a maximum of 30 minutes is
typical). If you disable the shutter for a longer time period, damage to the detector can occur.
NOTE
The encapsulation rating is only applicable when all the openings on the camera are sealed with their correct covers, hatches, or caps. This includes the compartments for data storage, batteries, and
connectors.
#T559899; r. AB/35742/35742; en-US
2
Page 9
3
Notice to user
3.1 User-to-user forums
Exchange ideas, problems, and infrared solutions with fellow thermographers around the
world in our user-to-user forums. To go to the forums, visit:
http://www.infraredtraining.com/community/boards/
3.2 Accuracy
For very accurate results, we recommend that you wait 5 minutes after you have started
the camera before measuring a temperature.
For cameras where the detector is cooled by a mechanical cooler, this time period excludes the time it takes to cool down the detector.
3.3 Disposal of electronic waste
As with most electronic products, this equipment must be disposed of in an environmentally friendly way, and in accordance with existing regulations for electronic waste.
Please contact your FLIR Systems representative for more details.
3.4 Training
To read about infrared training, visit:
• http://www.infraredtraining.com
• http://www.irtraining.com
• http://www.irtraining.eu
3.5 Documentation updates
Our manuals are updated several times per year, and we also issue product-critical notifications of changes on a regular basis.
To access the latest manuals and notifications, go to the Download tab at:
http://support.flir.com
It only takes a few minutes to register online. In the download area you will also find the lat-
est releases of manuals for our other products, as well as manuals for our historical and
obsolete products.
3.6 Important note about this manual
FLIR Systems issues generic manuals that cover several cameras within a model line.
This means that this manual may contain descriptions and explanations that do not apply
to your particular camera model.
3.7 Note about authoritative versions
The authoritative version of this publication is English. In the event of divergences due to
translation errors, the English text has precedence.
Any late changes are first implemented in English.
#T559899; r. AB/35742/35742; en-US
3
Page 10
4
Customer help
4.1 General
For customer help, visit:
http://support.flir.com
4.2 Submitting a question
To submit a question to the customer help team, you must be a registered user. It only
takes a few minutes to register online. If you only want to search the knowledgebase for
existing questions and answers, you do not need to be a registered user.
When you want to submit a question, make sure that you have the following information to
hand:
• The camera model
• The camera serial number
• The communication protocol, or method, between the camera and your device (for example, HDMI, Ethernet, USB, or FireWire)
#T559899; r. AB/35742/35742; en-US
4
Page 11

4
Customer help
• Device type (PC/Mac/iPhone/iPad/Android device, etc.)
• Version of any programs from FLIR Systems
• Full name, publication number, and revision number of the manual
4.3 Downloads
On the customer help site you can also download the following, when applicable for the
product:
• Firmware updates for your infrared camera.
• Program updates for your PC/Mac software.
• Freeware and evaluation versions of PC/Mac software.
• User documentation for current, obsolete, and historical products.
• Mechanical drawings (in *.dxf and *.pdf format).
• Cad data models (in *.stp format).
• Application stories.
• Technical datasheets.
• Product catalogs.
#T559899; r. AB/35742/35742; en-US
5
Page 12

5
Important note about training and
applications
5.1 General
Infrared inspection of gas leaks, furnaces, and high-temperature applications—including
infrared image and other data acquisition, analysis, diagnosis, prognosis, and reporting—
is a highly advanced skill. It requires professional knowledge of thermography and its applications, and is, in some countries, subject to certification and legislation.
Consequently, we strongly recommend that you seek the necessary training before carrying out inspections. Please visit the following site for more information:
http://www.infraredtraining.com
#T559899; r. AB/35742/35742; en-US
6
Page 13

6
Introduction
The new FLIR G300 a is an optical gas camera unit that can be integrated in housings with
application specific requirements. The FLIR G300 a visualizes greenhouse gas emissions
or volatile organic compounds (VOCs). When integrated in a fixed housing, the system is
perfect for monitoring a pinpointed area over a long period of time, making automatic
around-the-clock monitoring possible.
Key features:
• Can be integrated in application-specific housings.
• Visualizes gas leaks in real time.
• Remote control.
• Inspects without interruption.
• Traces leaks to their source.
The FLIR G300 a detects the following gases:
• 1-pentene
• benzene
• butane
• ethane
• ethanol
• ethylbenzene
• ethylene
• heptane
• hexane
• isoprene
• m-xylene
• methane
• methanol
• methyl ethyl ketone (MEK)
• methyl isobutyl ketone (MIBK)
• octane
• pentane
• propane
• propylene.
• toluene
#T559899; r. AB/35742/35742; en-US
7
Page 14
7
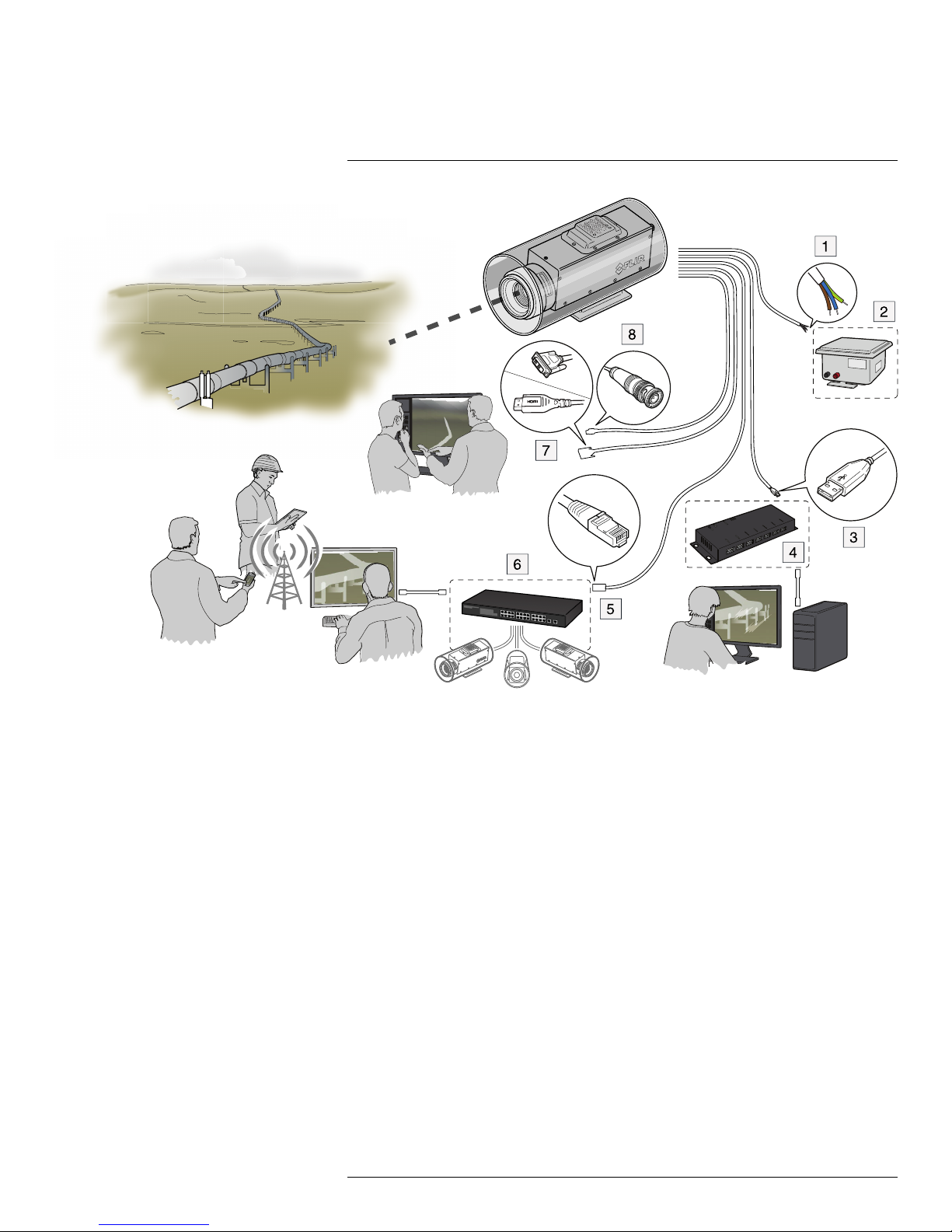
Typical system overview
7.1 Explanation
1. Pigtail cable from the housing:
• Brown: positive (+).
• Blue: negative (–).
• Green/yellow: earth.
2. 10–28 V DC power supply.
3. USB cable.
4. USB hub.
5. Ethernet cable with an RJ45 connector.
6. Ethernet switch.
7. Cable with an HDMI or DVI connector.
8. Video cable with a BNC connector.
#T559899; r. AB/35742/35742; en-US
8
Page 15

8
Quick start guide
Follow this procedure:
1. Connect the power, video, and Ethernet cables to the camera.
2. Connect the video cable from the camera to a display/monitor, and connect the power
cable to a power supply (10–28 V DC). Verify that video output is displayed on the
monitor.
3. Connect the camera to the network using the Ethernet cable.
4. Use FLIR Tools to set up and control the camera. For more information, see section
8.1 Download FLIR Tools, page 9.
8.1 Download FLIR Tools
FLIR Tools lets you quickly create professional inspection reports that clearly show decision makers what you’ve found with your IR camera.
Import, analyze, and fine-tune images easily. Then incorporate them into concise documents to share findings and justify repairs.
Go to the following website to download FLIR Tools:
http://support.flir.com/tools
#T559899; r. AB/35742/35742; en-US
9
Page 16

9
Mechanical installation
9.1 Mounting interfaces
The housing has a mounting interface in the bottom with the following threaded holes.
• 8 × M4 metric threaded holes
• 1 × UNC ¼″-20 standard tripod mount.
There are also holes for positioning, see section 14 Mechanical drawings for more
information.
9.2 Notes on permanent mounting
If the camera unit is to be permanently mounted at the application site, certain steps are
required.
The camera unit needs to be enclosed in a protective housing and, depending on the ambient conditions (e.g., temperature), the housing may need to be cooled or heated by
means of water or air. The distance between the camera unit and the back panel needs to
be large enough to achieve sufficient cooling.
In very dusty conditions the installation might also require a stream of pressurized air directed at the lens, to prevent dust build-up.
9.3 Vibrations
When mounting the camera unit in harsh industrial environments, every precaution should
be taken when securing the unit.
If the environment exposes the unit to severe vibrations, there may be a need to secure
the mounting screws by means of Loctite or another industrial brand of thread-locking
liquid, as well as to dampen the vibrations by mounting the camera unit on a specially designed base.
9.4 Further information
For further information on mounting recommendations and environmental enclosures,
contact FLIR Systems.
#T559899; r. AB/35742/35742; en-US
10
Page 17
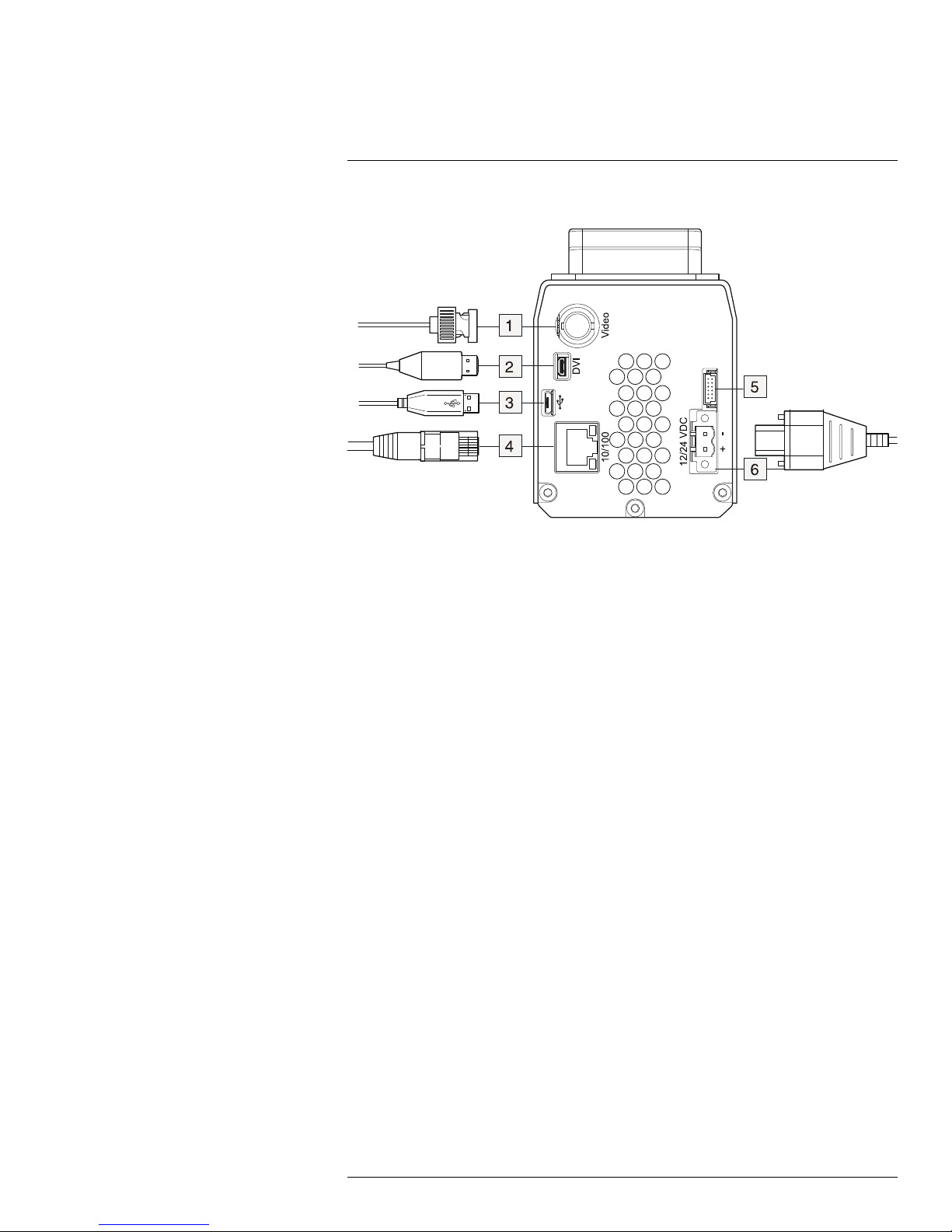
10
Connectors
10.1 Figure
10.2 Explanation
1. Video cable with a BNC connector (for CVBS, composite video output).
2. HDMI cable with a type D connector (for digital video output).
3. USB-A cable (to connect an external USB device to the camera).
4. Ethernet cable with an RJ45 connector (to connect to the network).
Note Only CAT-6 Ethernet cables should be used with this camera.
5. Not used.
6. Power cable for 10–28 V DC power in.
Note The power connector on the camera is polarity protected.
#T559899; r. AB/35742/35742; en-US
11
Page 18

11
Verifying camera operation
Prior to installing the camera, use a bench test to verify camera operation and to configure
the camera for the local network. The camera provides analog video, and can be controlled through IP communications.
11.1 Power and analog video
Follow this procedure:
1. Connect the power, video, Ethernet, and USB.
2. Connect the video cable from the camera to a display/monitor, and connect the power
cable to a power supply.
11.2 IP Communication
It is assumed that a FLIR G300 a system will be set up on an existing network and assigned an IP address from the DHCP server. The MAC address can be found on a label
on the bottom side of the camera.
To detect the camera system on the network, use either FLIR IR Camera Player or FLIR IP
Config. You can download these programs from the following links.
Software Download of software
FLIR Camera Player http://tinyurl.com/ncs5qhd
FLIR IP Config http://tinyurl.com/o5wudd7
The manuals for these programs are included on the User Documentation CD-ROM that
ships with the camera system.
#T559899; r. AB/35742/35742; en-US
12
Page 19

12
Network troubleshooting
Try one of the following if you experience network problems:
• Reset the modem and unplug and replug the Ethernet cable at both ends.
• Reboot the computer with the cables connected.
• Swap your Ethernet cable with another cable that is either brand new or known to be in
working condition.
• Connect your Ethernet cable to a different wall socket. If you are still not able to get online, you are probably experiencing a configuration issue.
• Verify your IP address.
• Disable Network Bridging.
• Disable your Wi-Fi connectivity (if you use it) to ensure that the wired Ethernet port is
open.
• Renew the DHCP license.
• Make sure that the firewall is turned off when you troubleshoot.
• Make sure that your wireless adapter is switched off. If not, the search for the camera
might only look for a wireless connection.
• Normally a modern computer will handle both crossed and uncrossed cable types automatically, but for troubleshooting purposes try both or use a switch.
• Turn off any network adapters that are not connected to the camera.
• For troubleshooting purposes, power both the camera and the computer using a mains
adapter. Some laptops turn off the network card to save power when using the battery.
If none of these steps help you, contact your ISP.
#T559899; r. AB/35742/35742; en-US
13
Page 20

13
Technical data
13.1 Online field-of-view calculator
Please visit http://support.flir.com and click the photo of the camera series for field-of-view
tables for all lens–camera combinations.
13.2 Note about technical data
FLIR Systems reserves the right to change specifications at any time without prior notice.
Please check http://support.flir.com for latest changes.
13.3 Note about authoritative versions
The authoritative version of this publication is English. In the event of divergences due to
translation errors, the English text has precedence.
Any late changes are first implemented in English.
#T559899; r. AB/35742/35742; en-US
14
Page 21

Technical data13
13.4 FLIR G300 a 14.5° fixed lens
P/N: 71502-0101
Rev.: 35207
General description
The FLIR G300 a is a bare infrared camera unit for optical gas imaging (OGI) that visualizes and pinpoints
leaks of volatile organic compounds (VOCs), without the need to shut down the operation. The FLIR
G300 a is used in industrial settings such as oil refineries, natural gas processing plants, offshore platforms, chemical/petrochemical industries, and biogas and power generation plants.
The camera unit is delivered as a bare unit, and is intended for integration in OEM systems.
Benefits
• Improved efficiency: The FLIR G300 a reduces revenue loss by pinpointing even small gas leaks
quickly and efficiently, and from a distance. It also reduces the inspection time by allowing a broad
area to be scanned rapidly and without the need to interrupt the industrial process.
• Increased worker safety: OGI allows gas leaks to be detected in a non-contact mode and from a safe
distance. This reduces the risk of the user being exposed to invisible and potentially harmful or explosive chemicals. With a FLIR G300 a gas imaging camera unit it is easy to scan areas of interest that
are difficult to reach with conventional methods.
• Protecting the environment: Several VOCs are dangerous to human health or cause harm to the environment, and are usually governed by regulations. Even small leaks can be detected and documented
using the FLIR G300 a.
Detects the following gases: benzene, ethanol, ethylbenzene, heptane, hexane, isoprene, methanol,
methyl ethyl ketone, MIBK, octane, pentane, 1-pentene, toluene, m-xylene, ethane, butane, methane,
propane, ethylene, propylene.
Imaging and optical data
IR resolution 320 × 240 pixels
Thermal sensitivity/NETD <15 mK @ +30°C (+86°F)
Field of view (FOV)
Minimum focus distance 0.5 m (1.64 ft.)
Focal length 38 mm (1.49 in.)
F-number 1.5
Focus Automatic using FLIR SDK, or manual
Zoom 1–8× continuous, digital zoom
Digital image enhancement Noise reduction filter, high sensitivity mode (HSM)
Detector data
Detector type Focal plane array (FPA), cooled InSb
Spectral range
Sensor cooling Stirling Microcooler (FLIR MC-3)
MTBF 2 years or 15,000 hours (whichever is greatest), for
Detects following gases Benzene, ethanol, ethylbenzene, heptane, hexane,
14.5° × 10.8°
3.2–3.4 µm
a camera running 24/7 @ +20°C (+68°F)
isoprene, methanol, methyl ethyl ketone, MIBK, octane, pentane, 1-pentene, toluene, m-xylene,
ethane, butane, methane, propane, ethylene,
propylene
Electronics and data rate
Full frame rate 60 Hz
#T559899; r. AB/35742/35742; en-US
15
Page 22
Technical data13
Image presentation
Automatic image adjustment Continuous/manual; linear or histogram based
Manual image adjustment
Image presentation modes
Image modes
Temperature ranges
Temperature range –20°C to +350°C (–4°F to +662°F)
Video streaming
Non-radiometric IR video streaming
Data communication interfaces
Interfaces
Level/span
IR image, high sensitivity mode (HSM)
RTP/MPEG4
• HDMI
• Ethernet
USB
USB Control and image
USB, standard 2.0 High Speed
USB, connector type USB micro
USB, communication TCP/IP socket-based, Microsoft RNDIS or/and
USB, video streaming 640 × 480 pixels at 30 Hz (using USB video class)
USB, image streaming 16-bit 320 × 240 at 30 Hz (using USB video class)
USB, protocols TCP, UDP, RTSP, RTP, HTTP, ICMP, IGMP, ftp,
Ethernet
Ethernet Control, result and image
Ethernet, type 100 Mbps
Ethernet, standard IEEE 802.3
Ethernet, connector type RJ-45
Ethernet, communication TCP/IP socket-based FLIR proprietary
Ethernet, video streaming 640 × 480 pixels at up to 15 Hz
Ethernet, image streaming 16-bit 320 × 240 pixels at up to 10 Hz
Ethernet, protocols TCP, UDP, RTSP, RTP, HTTP, ICMP, IGMP, ftp,
USB video class
DHCP
MPEG-4, ISO/IEC 14496-1 MPEG-4 ASP@L5
DHCP, MDNS (Bonjour), SMB/CIFS
Composite video
Video out Digital video output (image)
Power system
DC operation 10–28 V DC, polarity protected
Power
Start-up time Typically 7 min. @ 25°C (+77°F)
#T559899; r. AB/35742/35742; en-US
• Max. power cooling down @12 V: 13 W
• Steady state @12 V: 9 W
16
Page 23

Technical data13
Environmental data
Operating temperature range –20°C to +50°C (–4°F to +122°F)
Storage temperature range –30°C to +60°C (–22°F to +140°F)
Humidity (operating and storage) IEC 68-2-30/24 h 95% relative humidity +25°C to
Directives
EMC
Shock 25 g (IEC 60068-2-27)
Vibration 2 g (IEC 60068-2-6)
Physical data
Weight 1.4 kg (3.1 lb.), incl. 14.5° lens
Cameras size, incl. lens (L × W × H) 242 × 80 × 105 mm (9.5 × 3.1 × 4.1 in.), incl. 14.5°
Housing material Aluminum
+40°C (+77°F to +104°F) (2 cycles)
• Low voltage directive: 2006/95/EC
• EMC: 2004/108/EC
• RoHS: 2002/95/EC
• WEEE: 2002/96/EC
• EN61000-6-4 (Emission)
• EN61000-6-2 (Immunity)
• FCC 47 CFR Part 15 class A (Emission)
• EN 61 000-4-8, L5
lens
Shipping information
Packaging, type
List of contents
Packaging, weight
Packaging, size
EAN-13 7332558008409
UPC-12
Country of origin Sweden
Cardboard box
• Infrared camera
• Ethernet cable
• FLIR ThermoVision SDK (license only)
• FLIR VideoReport CD-ROM
• Lens cap
• Power supply
• Printed documentation
• USB cable
• Video cable
845188008758
Supplies & accessories:
• T197387; IR lens, 24° with case for GF300, GF309, GF320
• T197388; IR lens, 6° with case for GF300, GF309, GF320, GF346.
• T197385; IR lens, 14.5° with case for GF300, GF309, GF320
• T197692; Battery charger, incl. power supply with multi plugs
• T910814; Power supply, incl. multi plugs
• T198511; Li-Ion Battery pack 7.4V 33Wh
• T911230ACC; Memory card SDHC 4 GB
• 1910423; USB cable Std A <-> Mini-B
• T198509; Cigarette lighter adapter kit, 12 VDC, 1.2 m/3.9 ft.
• T910815ACC; HDMI to HDMI cable 1.5 m
• T910816ACC; HDMI to DVI cable 1.5 m
#T559899; r. AB/35742/35742; en-US
17
Page 24

Technical data13
• T197555; Hard transport case for FLIR GF3xx-Series
• T198585; FLIR VideoReport
• DSW-10000; FLIR IR Camera Player
• T199233; FLIR Atlas SDK for .NET
• T199234; FLIR Atlas SDK for MATLAB
• T198567; ThermoVision™ System Developers Kit Ver. 2.6
• T198566; ThermoVision™ LabVIEW® Digital Toolkit Ver. 3.3
#T559899; r. AB/35742/35742; en-US
18
Page 25

Technical data13
13.5 FLIR G300 a 24° fixed lens
P/N: 71502-0102
Rev.: 35207
General description
The FLIR G300 a is a bare infrared camera unit for optical gas imaging (OGI) that visualizes and pinpoints
leaks of volatile organic compounds (VOCs), without the need to shut down the operation. The FLIR
G300 a is used in industrial settings such as oil refineries, natural gas processing plants, offshore platforms, chemical/petrochemical industries, and biogas and power generation plants.
The camera unit is delivered as a bare unit, and is intended for integration in OEM systems.
Benefits
• Improved efficiency: The FLIR G300 a reduces revenue loss by pinpointing even small gas leaks
quickly and efficiently, and from a distance. It also reduces the inspection time by allowing a broad
area to be scanned rapidly and without the need to interrupt the industrial process.
• Increased worker safety: OGI allows gas leaks to be detected in a non-contact mode and from a safe
distance. This reduces the risk of the user being exposed to invisible and potentially harmful or explosive chemicals. With a FLIR G300 a gas imaging camera unit it is easy to scan areas of interest that
are difficult to reach with conventional methods.
• Protecting the environment: Several VOCs are dangerous to human health or cause harm to the environment, and are usually governed by regulations. Even small leaks can be detected and documented
using the FLIR G300 a.
Detects the following gases: benzene, ethanol, ethylbenzene, heptane, hexane, isoprene, methanol,
methyl ethyl ketone, MIBK, octane, pentane, 1-pentene, toluene, m-xylene, ethane, butane, methane,
propane, ethylene, propylene.
Imaging and optical data
IR resolution 320 × 240 pixels
Thermal sensitivity/NETD <15 mK @ +30°C (+86°F)
Field of view (FOV)
Minimum focus distance 0.3 m (1.0 ft.)
Focal length 23 mm (0.89 in.)
F-number 1.5
Focus Automatic using FLIR SDK, or manual
Zoom 1–8× continuous, digital zoom
Digital image enhancement Noise reduction filter, high sensitivity mode (HSM)
Detector data
Detector type Focal plane array (FPA), cooled InSb
Spectral range
Sensor cooling Stirling Microcooler (FLIR MC-3)
MTBF 2 years or 15,000 hours (whichever is greatest), for
Detects following gases Benzene, ethanol, ethylbenzene, heptane, hexane,
24° × 18°
3.2–3.4 µm
a camera running 24/7 @ +20°C (+68°F)
isoprene, methanol, methyl ethyl ketone, MIBK, octane, pentane, 1-pentene, toluene, m-xylene,
ethane, butane, methane, propane, ethylene,
propylene
Electronics and data rate
Full frame rate 60 Hz
#T559899; r. AB/35742/35742; en-US
19
Page 26

Technical data13
Image presentation
Automatic image adjustment Continuous/manual; linear or histogram based
Manual image adjustment
Image presentation modes
Image modes
Temperature ranges
Temperature range –20°C to +350°C (–4°F to +662°F)
Video streaming
Non-radiometric IR video streaming
Data communication interfaces
Interfaces
Level/span
IR image, high sensitivity mode (HSM)
RTP/MPEG4
• HDMI
• Ethernet
USB
USB Control and image
USB, standard 2.0 High Speed
USB, connector type USB micro
USB, communication TCP/IP socket-based, Microsoft RNDIS or/and
USB, video streaming 640 × 480 pixels at 30 Hz (using USB video class)
USB, image streaming 16-bit 320 × 240 at 30 Hz (using USB video class)
USB, protocols TCP, UDP, RTSP, RTP, HTTP, ICMP, IGMP, ftp,
Ethernet
Ethernet Control, result and image
Ethernet, type 100 Mbps
Ethernet, standard IEEE 802.3
Ethernet, connector type RJ-45
Ethernet, communication TCP/IP socket-based FLIR proprietary
Ethernet, video streaming 640 × 480 pixels at up to 15 Hz
Ethernet, image streaming 16-bit 320 × 240 pixels at up to 10 Hz
Ethernet, protocols TCP, UDP, RTSP, RTP, HTTP, ICMP, IGMP, ftp,
USB video class
DHCP
MPEG-4, ISO/IEC 14496-1 MPEG-4 ASP@L5
DHCP, MDNS (Bonjour), SMB/CIFS
Composite video
Video out Digital video output (image)
Power system
DC operation 10–28 V DC, polarity protected
Power
Start-up time Typically 7 min. @ 25°C (+77°F)
#T559899; r. AB/35742/35742; en-US
• Max. power cooling down @12 V: 13 W
• Steady state @12 V: 9 W
20
Page 27

Technical data13
Environmental data
Operating temperature range –20°C to +50°C (–4°F to +122°F)
Storage temperature range –30°C to +60°C (–22°F to +140°F)
Humidity (operating and storage) IEC 68-2-30/24 h 95% relative humidity +25°C to
Directives
EMC
Shock 25 g (IEC 60068-2-27)
Vibration 2 g (IEC 60068-2-6)
Physical data
Weight 1.4 kg (3.1 lb.), incl. 24° lens
Cameras size, incl. lens (L × W × H) 242 × 80 × 105 mm (9.5 × 3.1 × 4.1 in.), incl. 24°
Housing material Aluminum
+40°C (+77°F to +104°F) (2 cycles)
• Low voltage directive: 2006/95/EC
• EMC: 2004/108/EC
• RoHS: 2002/95/EC
• WEEE: 2002/96/EC
• EN61000-6-4 (Emission)
• EN61000-6-2 (Immunity)
• FCC 47 CFR Part 15 class A (Emission)
• EN 61 000-4-8, L5
lens
Shipping information
Packaging, type
List of contents
Packaging, weight
Packaging, size
EAN-13 7332558008416
UPC-12
Country of origin Sweden
Cardboard box
• Infrared camera
• Ethernet cable
• FLIR ThermoVision SDK (license only)
• FLIR VideoReport CD-ROM
• Lens cap
• Power supply
• Printed documentation
• USB cable
• Video cable
845188008765
Supplies & accessories:
• T197387; IR lens, 24° with case for GF300, GF309, GF320
• T197388; IR lens, 6° with case for GF300, GF309, GF320, GF346.
• T197385; IR lens, 14.5° with case for GF300, GF309, GF320
• T197692; Battery charger, incl. power supply with multi plugs
• T910814; Power supply, incl. multi plugs
• T198511; Li-Ion Battery pack 7.4V 33Wh
• T911230ACC; Memory card SDHC 4 GB
• 1910423; USB cable Std A <-> Mini-B
• T198509; Cigarette lighter adapter kit, 12 VDC, 1.2 m/3.9 ft.
• T910815ACC; HDMI to HDMI cable 1.5 m
• T910816ACC; HDMI to DVI cable 1.5 m
#T559899; r. AB/35742/35742; en-US
21
Page 28

Technical data13
• T197555; Hard transport case for FLIR GF3xx-Series
• T198585; FLIR VideoReport
• DSW-10000; FLIR IR Camera Player
• T199233; FLIR Atlas SDK for .NET
• T199234; FLIR Atlas SDK for MATLAB
• T198567; ThermoVision™ System Developers Kit Ver. 2.6
• T198566; ThermoVision™ LabVIEW® Digital Toolkit Ver. 3.3
#T559899; r. AB/35742/35742; en-US
22
Page 29

14
Mechanical drawings
#T559899; r. AB/35742/35742; en-US
23
Page 30

1,19
30,3
3,77
95,8
0,36
9,2
3,1
78,8
5,2
131,99
6,95
176,47
3,14
79,64
Y
(see table)
2,66
67,5
4,13
105
2,66
67,5
1,54
39
3,23
82,04
6,27
159,37
4 F9
+
+
0,040
0,010
0,157 F9
+
+
0,002
0,000
4.5[0,18]
0,71
17,94
0,94
24
8 x M4
1,81
45,9
3,54
90
0,79
20
Z
(see table)
UNC 1/4-20
All dimensions are valid for FOV 14,5
and 24
Center of gravity
X Y
Z
6 deg
0
N/A N/A
14,5 deg
0
36,5
44,66
24 deg
0
N/A N/A
Där ej annat anges/Unless otherwise stated
Kanter brutna
Edges broken
Hålkälsradier
Ra µm
Fillet radii
Ytjämnhet/Roughness
Blad/Sheet
Rev
Ritn nr/Drawing No
ArtNo.
Skala/Scale
Size
Datum/Date
Kontr/Check
Konstr/Drawn
Material
Ytbehandling/Surface treatment
Gen tol
Benämning/Denomination
Denna handling får ej delges annan, kopieras i
sin helhet eller delar utan vårt medgivande .
Överträdelse härav beivras med stöd av gällande lag.
FLIR SYSTEMS AB
This document must not be communicated or
copied completely or in part, without our permission.
Any infringement will lead to legal proceedings.
FLIR SYSTEMS AB
A3
Utdrag ur/Excerpt from ISO 2768-m
±0,1
±0,2
±0,3
±0,5
±0,8
(400)-1000
(120)-400
(30)-120
(6)-30
0,5-6
ISO 2768-mK
1(1)
1:2
-
Mathijs Mooij
B
T198650
G300a Basic Dimensions
FRGU
2014-05-19
2015-12-07
C. HARJU
Ändrad av/Modified by
Ändrad/Modified
1 2 3 4 5 6 7 8 9 10
A
B
C
D
E
F
G
H
1 32 54
C
F
B
D
G
E
A
-
Page 31

15
CE Declaration of conformity
#T559899; r. AB/35742/35742; en-US
25
Page 32

Page 33
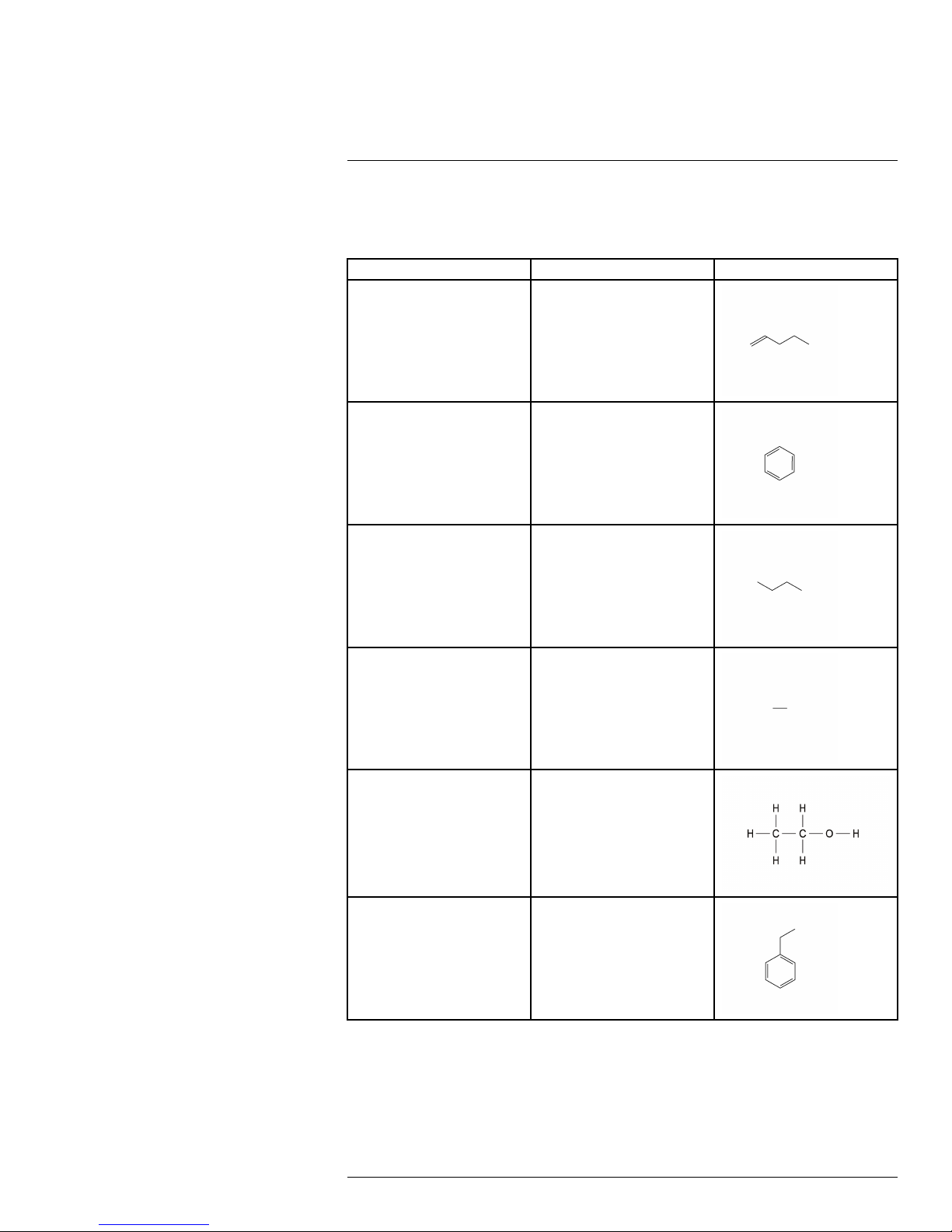
16
Detectable gases
The FLIR G300 a camera has been engineered and designed to detect various gases.
This table lists the gases that FLIR Systems has tested at various concentrations within
the laboratory.
Common name Molecular formula Structural formula
1-Pentene C
5H10
Benzene
Butane
Ethane
Ethanol
C
C
C
C
6H6
4H10
2H6
2H6
O
Ethylbenzene
#T559899; r. AB/35742/35742; en-US
C
8H10
27
Page 34
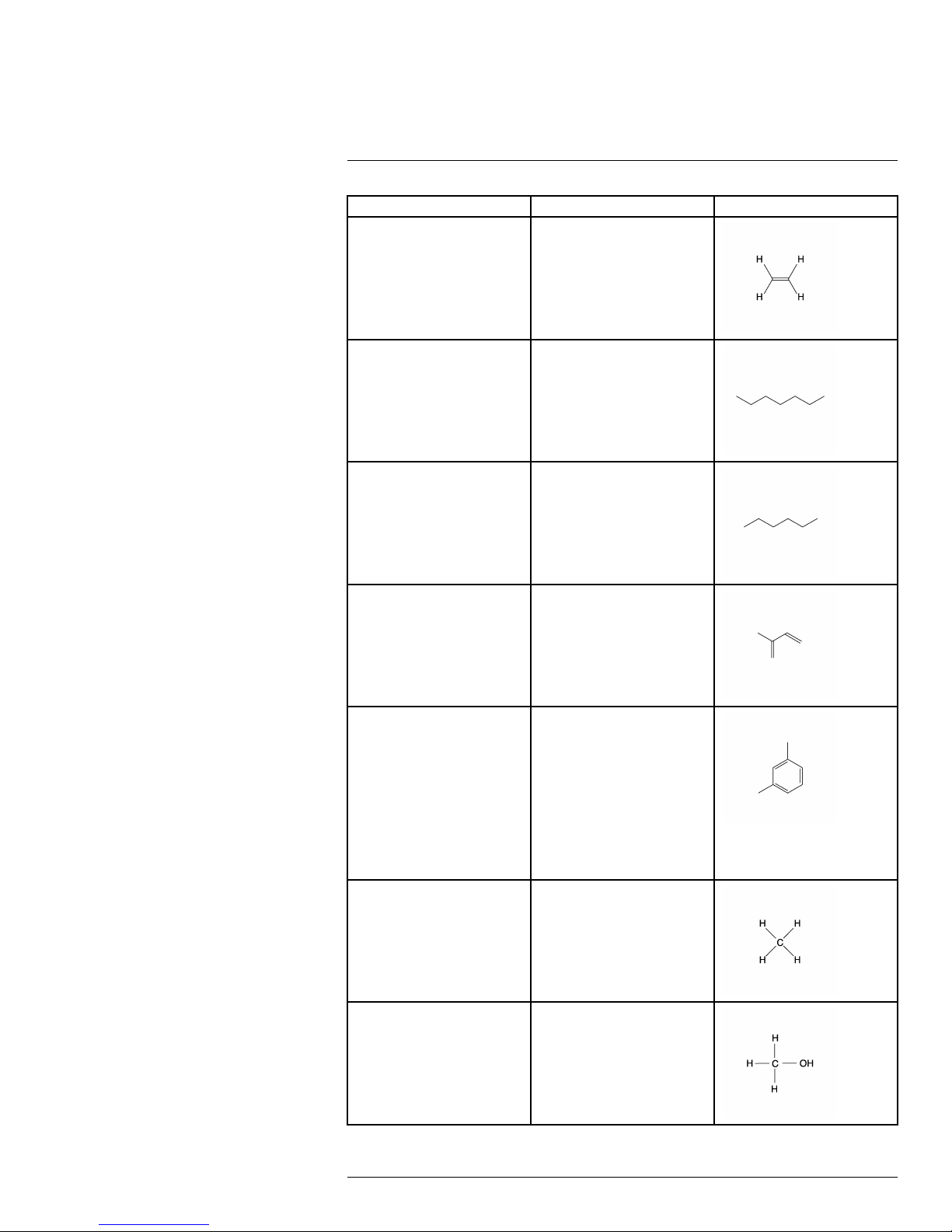
Detectable gases16
Common name Molecular formula Structural formula
Ethylene
C
2H4
Heptane
Hexane C
Isoprene C
m-Xylene C
C
7H16
6H14
5H8
8H10
Methane CH
Methanol
#T559899; r. AB/35742/35742; en-US
CH
4
O
4
28
Page 35
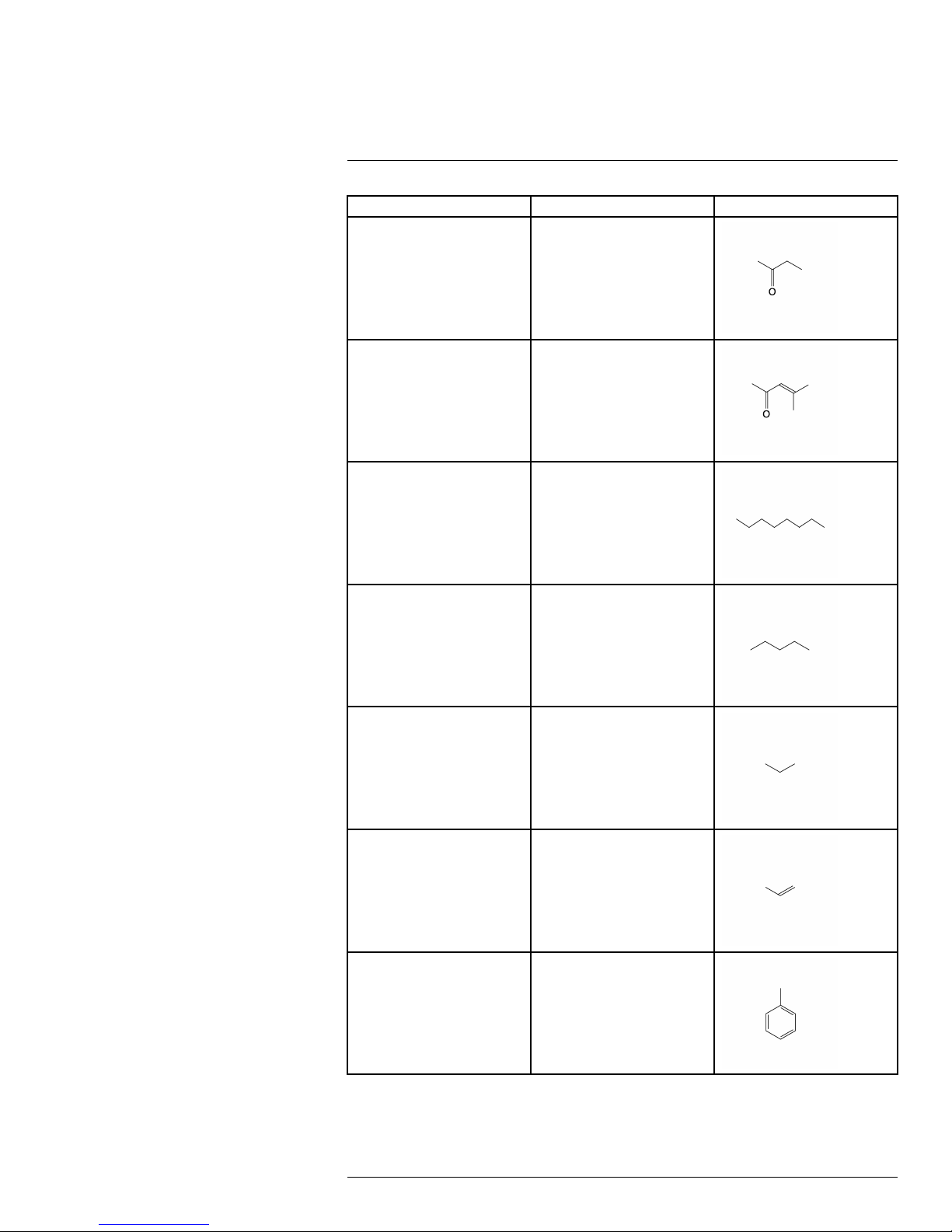
Detectable gases16
Common name Molecular formula Structural formula
Methyl ethyl ketone
C
O
4H8
MIBK
C
6H10
Octane C8H
Pentane C
Propane C
5H12
3H8
O
18
Propylene C
Toluene C
#T559899; r. AB/35742/35742; en-US
3H6
7H8
29
Page 36
17
Why do some gases absorb
infrared energy?
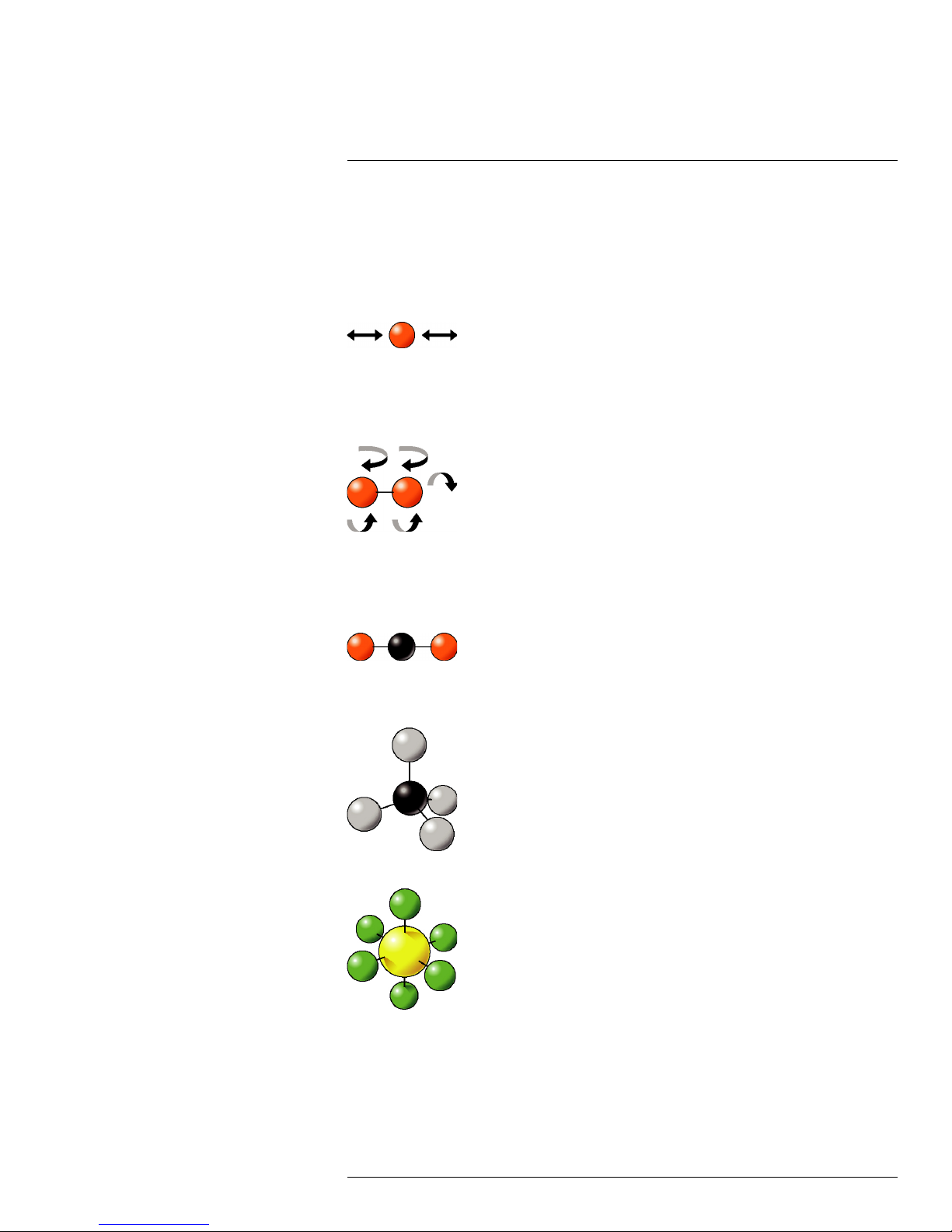
From a mechanical point of view, molecules in a gas could be compared to weights (the
balls in the figures below), connected together via springs. Depending on the number of
atoms, their respective size and mass, the elastic constant of the springs, molecules may
move in given directions, vibrate along an axis, rotate, twist, stretch, rock, wag, etc.
The simplest gas molecules are single atoms, like helium, neon or krypton. They have no
way to vibrate or rotate, so they can only move by translation in one direction at a time.
Figure 17.1 Single atom
The next most complex category of molecules is homonuclear, made of two atoms such
as hydrogen (H
their axes in addition to translational motion.
), nitrogen (N2)and oxygen (O2). They have the ability to tumble around
2
Figure 17.2 Two atoms
Then there are complex diatomic molecules, such as carbon dioxide (CO2), methane
(CH
), sulfur hexafluoride (SF6), and styrene (C6H5CH=CH2) (these are just a few
4
examples).
Figure 17.3 Carbon dioxide (CO2), 3 atoms per molecule
This assumption is valid for multi-atomic molecules.
Figure 17.4 Methane (CH4), 5 atoms per molecule
Figure 17.5 Sulfur hexafluoride (SF6), 7 atoms per molecule
#T559899; r. AB/35742/35742; en-US
30
Page 37
17
Why do some gases absorb infrared energy?
Figure 17.6 Styrene (C6H5CH=CH2), 16 atoms per molecule
Their increased degrees of mechanical freedom allow multiple rotational and vibrational
transitions. Because they are built from multiple atoms, they can absorb and emit heat
more effectively than simple molecules. Depending on the frequency of the transitions,
some of them fall into energy ranges that are located in the infrared region where the infrared camera is sensitive.
Transition type Frequency
9
Rotation of heavy molecules 10
Rotation of light molecules and
vibration of heavy molecules
Vibration of light molecules.
Rotation and vibration of the
structure
Electronic transitions 10
–1011Hz Microwaves, above 3 mm/0.118
11
10
–1013Hz Far infrared, between 30 μm and
13
10
–1014Hz Infrared, between 3 μm and 30
14
–1016Hz UV–visible
Spectral range
in.
3 mm/0.118 in.
μm
In order for a molecule to absorb a photon via a transition from one state to another, the
molecule must have a dipole moment capable of briefly oscillating at the same frequency
as the incident photon. This quantum mechanical interaction allows the electromagnetic
field energy of the photon to be “transferred” or absorbed by the molecule.
FLIR Systems cameras take advantage of the absorbing nature of certain molecules, to
visualize them in their native environments.
FLIR Systems focal plane arrays and optical systems are specifically tuned to very narrow
spectral ranges, in the order of hundreds of nanometers, and are therefore ultra selective.
Only gases absorbent in the infrared region that is delimited by a narrow band pass filter
can be detected.
Since the energy from the gases is very weak, all camera components are optimized to
emit as little energy as possible. This is the only solution to provide a sufficient signal-tonoise ratio. Hence, the filter itself is maintained at a cryogenic temperature: down to 60 K
in the case of the FLIR Systems LW camera that was released in the beginning of 2008.
Below, are the transmittance spectra of two gases:
• Benzene (C
• Sulfur hexafluoride (SF
)—absorbent in the MW region
6H6
)—absorbent in the LW region.
6
#T559899; r. AB/35742/35742; en-US
31
Page 38
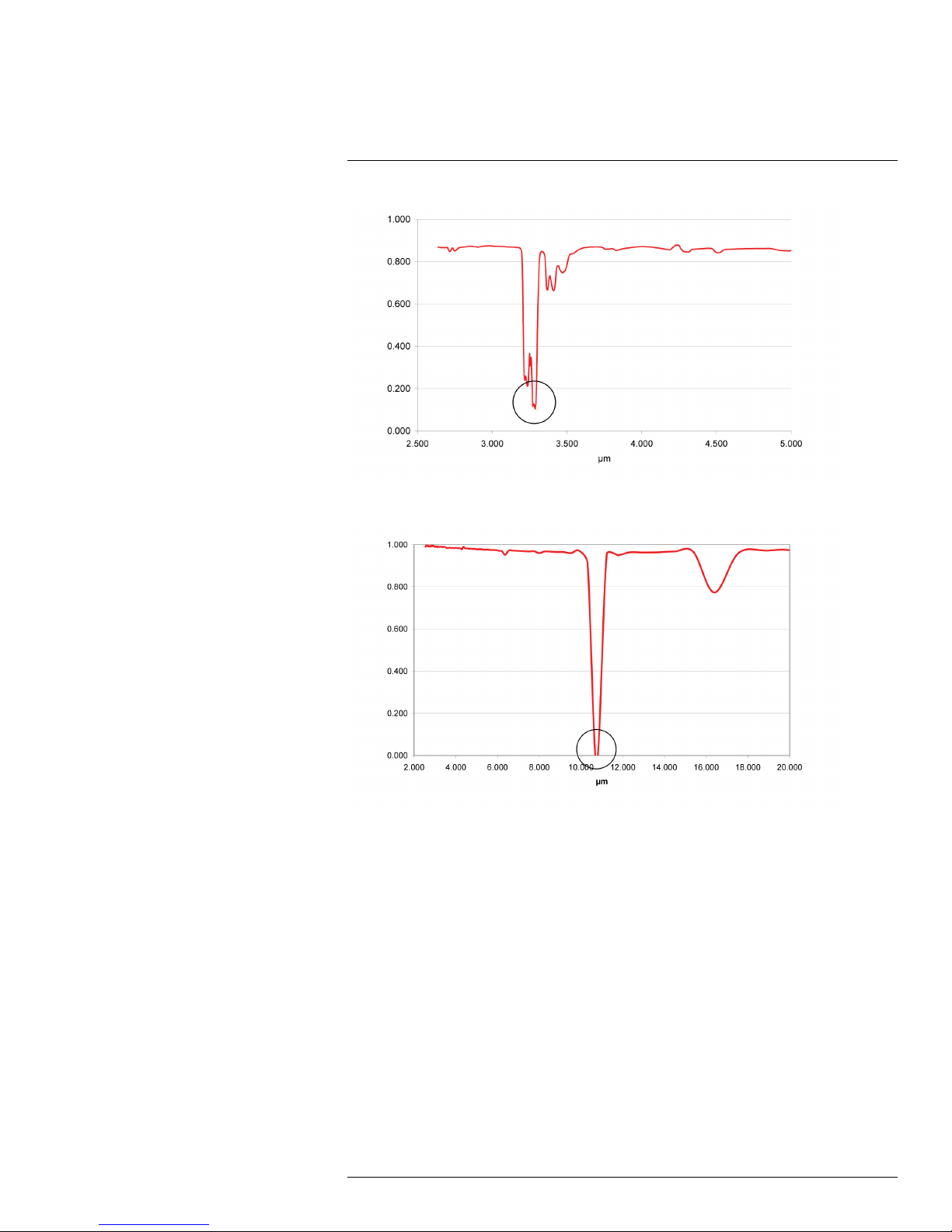
17
Why do some gases absorb infrared energy?
Figure 17.7 Benzene (C6H6). Strong absorption around 3.2/3.3 μm
Figure 17.8 Sulfur hexafluoride (SF6). Strong absorption around 10.6 μm
#T559899; r. AB/35742/35742; en-US
32
Page 39
18
Cleaning the camera
18.1 Camera housing, cables, and other items
18.1.1 Liquids
Use one of these liquids:
• Warm water
• A weak detergent solution
18.1.2 Equipment
A soft cloth
18.1.3 Procedure
Follow this procedure:
1. Soak the cloth in the liquid.
2. Twist the cloth to remove excess liquid.
3. Clean the part with the cloth.
CAUTION
Do not apply solvents or similar liquids to the camera, the cables, or other items. This can cause damage.
18.2 Infrared lens
18.2.1 Liquids
Use one of these liquids:
• A commercial lens cleaning liquid with more than 30% isopropyl alcohol.
• 96% ethyl alcohol (C
18.2.2 Equipment
Cotton wool
18.2.3 Procedure
Follow this procedure:
1. Soak the cotton wool in the liquid.
2. Twist the cotton wool to remove excess liquid.
3. Clean the lens one time only and discard the cotton wool.
WARNING
Make sure that you read all applicable MSDS (Material Safety Data Sheets) and warning labels on containers before you use a liquid: the liquids can be dangerous.
CAUTION
2H5
OH).
• Be careful when you clean the infrared lens. The lens has a delicate anti-reflective coating.
• Do not clean the infrared lens too vigorously. This can damage the anti-reflective coating.
#T559899; r. AB/35742/35742; en-US
33
Page 40

19
About FLIR Systems
FLIR Systems was established in 1978 to pioneer the development of high-performance
infrared imaging systems, and is the world leader in the design, manufacture, and marketing of thermal imaging systems for a wide variety of commercial, industrial, and government applications. Today, FLIR Systems embraces five major companies with outstanding
achievements in infrared technology since 1958—the Swedish AGEMA Infrared Systems
(formerly AGA Infrared Systems), the three United States companies Indigo Systems, FSI,
and Inframetrics, and the French company Cedip.
Since 2007, FLIR Systems has acquired several companies with world-leading expertise
in sensor technologies:
• Extech Instruments (2007)
• Ifara Tecnologías (2008)
• Salvador Imaging (2009)
• OmniTech Partners (2009)
• Directed Perception (2009)
• Raymarine (2010)
• ICx Technologies (2010)
• TackTick Marine Digital Instruments (2011)
• Aerius Photonics (2011)
• Lorex Technology (2012)
• Traficon (2012)
• MARSS (2013)
• DigitalOptics micro-optics business (2013)
• DVTEL (2015)
Figure 19.1 Patent documents from the early 1960s
FLIR Systems has three manufacturing plants in the United States (Portland, OR, Boston,
MA, Santa Barbara, CA) and one in Sweden (Stockholm). Since 2007 there is also a manufacturing plant in Tallinn, Estonia. Direct sales offices in Belgium, Brazil, China, France,
Germany, Great Britain, Hong Kong, Italy, Japan, Korea, Sweden, and the USA—together
#T559899; r. AB/35742/35742; en-US
34
Page 41

19
About FLIR Systems
with a worldwide network of agents and distributors—support our international customer
base.
FLIR Systems is at the forefront of innovation in the infrared camera industry. We anticipate market demand by constantly improving our existing cameras and developing new
ones. The company has set milestones in product design and development such as the introduction of the first battery-operated portable camera for industrial inspections, and the
first uncooled infrared camera, to mention just two innovations.
Figure 19.2 1969: Thermovision Model 661. The
camera weighed approximately 25 kg (55 lb.), the
oscilloscope 20 kg (44 lb.), and the tripod 15 kg
(33 lb.). The operator also needed a 220 VAC generator set, and a 10 L (2.6 US gallon) jar with liquid
nitrogen. To the left of the oscilloscope the Polaroid
attachment (6 kg/13 lb.) can be seen.
Figure 19.3 2015: FLIR One, an accessory to
iPhone and Android mobile phones. Weight: 90 g
(3.2 oz.).
FLIR Systems manufactures all vital mechanical and electronic components of the camera
systems itself. From detector design and manufacturing, to lenses and system electronics,
to final testing and calibration, all production steps are carried out and supervised by our
own engineers. The in-depth expertise of these infrared specialists ensures the accuracy
and reliability of all vital components that are assembled into your infrared camera.
19.1 More than just an infrared camera
At FLIR Systems we recognize that our job is to go beyond just producing the best infrared
camera systems. We are committed to enabling all users of our infrared camera systems
to work more productively by providing them with the most powerful camera–software
combination. Especially tailored software for predictive maintenance, R & D, and process
monitoring is developed in-house. Most software is available in a wide variety of
languages.
We support all our infrared cameras with a wide variety of accessories to adapt your equipment to the most demanding infrared applications.
19.2 Sharing our knowledge
Although our cameras are designed to be very user-friendly, there is a lot more to thermography than just knowing how to handle a camera. Therefore, FLIR Systems has founded
the Infrared Training Center (ITC), a separate business unit, that provides certified training
courses. Attending one of the ITC courses will give you a truly hands-on learning
experience.
#T559899; r. AB/35742/35742; en-US
35
Page 42

19
About FLIR Systems
The staff of the ITC are also there to provide you with any application support you may
need in putting infrared theory into practice.
19.3 Supporting our customers
FLIR Systems operates a worldwide service network to keep your camera running at all
times. If you discover a problem with your camera, local service centers have all the equipment and expertise to solve it within the shortest possible time. Therefore, there is no need
to send your camera to the other side of the world or to talk to someone who does not
speak your language.
#T559899; r. AB/35742/35742; en-US
36
Page 43

20
Glossary
absorption (absorption factor)
atmosphere The gases between the object being measured and the camera, nor-
autoadjust A function making a camera perform an internal image correction.
autopalette The IR image is shown with an uneven spread of colors, displaying
blackbody Totally non-reflective object. All its radiation is due to its own
blackbody
radiator
calculated atmospheric
transmission
cavity radiator A bottle shaped radiator with an absorbing inside, viewed through the
color
temperature
conduction The process that makes heat diffuse into a material.
continuous
adjust
convection
dual isotherm An isotherm with two color bands, instead of one.
emissivity
(emissivity
factor)
emittance Amount of energy emitted from an object per unit of time and area
environment
estimated atmospheric
transmission
external optics Extra lenses, filters, heat shields etc. that can be put between the
filter A material transparent only to some of the infrared wavelengths.
FOV Field of view: The horizontal angle that can be viewed through an IR
FPA Focal plane array: A type of IR detector.
graybody An object that emits a fixed fraction of the amount of energy of a
The amount of radiation absorbed by an object relative to the received radiation. A number between 0 and 1.
mally air.
cold objects as well as hot ones at the same time.
temperature.
An IR radiating equipment with blackbody properties used to calibrate
IR cameras.
A transmission value computed from the temperature, the relative hu-
midity of air and the distance to the object.
bottleneck.
The temperature for which the color of a blackbody matches a specif-
ic color.
A function that adjusts the image. The function works all the time,
continuously adjusting brightness and contrast according to the image content.
Convection is a heat transfer mode where a fluid is brought into motion, either by gravity or another force, thereby transferring heat from
one place to another.
The amount of radiation coming from an object, compared to that of a
blackbody. A number between 0 and 1.
2
(W/m
)
Objects and gases that emit radiation towards the object being
measured.
A transmission value, supplied by a user, replacing a calculated one
camera and the object being measured.
lens.
blackbody for each wavelength.
#T559899; r. AB/35742/35742; en-US
37
Page 44

20
Glossary
IFOV Instantaneous field of view: A measure of the geometrical resolution
of an IR camera.
image correction (internal or
A way of compensating for sensitivity differences in various parts of
live images and also of stabilizing the camera.
external)
infrared Non-visible radiation, having a wavelength from about 2–13 μm.
IR infrared
isotherm A function highlighting those parts of an image that fall above, below
or between one or more temperature intervals.
isothermal
cavity
A bottle-shaped radiator with a uniform temperature viewed through
the bottleneck.
Laser LocatIR An electrically powered light source on the camera that emits laser ra-
diation in a thin, concentrated beam to point at certain parts of the object in front of the camera.
laser pointer An electrically powered light source on the camera that emits laser ra-
diation in a thin, concentrated beam to point at certain parts of the object in front of the camera.
level The center value of the temperature scale, usually expressed as a
signal value.
manual adjust A way to adjust the image by manually changing certain parameters.
NETD Noise equivalent temperature difference. A measure of the image
noise level of an IR camera.
noise Undesired small disturbance in the infrared image
object
parameters
A set of values describing the circumstances under which the meas-
urement of an object was made, and the object itself (such as emis-
sivity, reflected apparent temperature, distance etc.)
object signal A non-calibrated value related to the amount of radiation received by
the camera from the object.
palette The set of colors used to display an IR image.
pixel
Stands for picture element. One single spot in an image.
radiance Amount of energy emitted from an object per unit of time, area and
angle (W/m
2
/sr)
radiant power Amount of energy emitted from an object per unit of time (W)
radiation The process by which electromagnetic energy, is emitted by an object
or a gas.
radiator A piece of IR radiating equipment.
range
The current overall temperature measurement limitation of an IR camera. Cameras can have several ranges. Expressed as two blackbody
temperatures that limit the current calibration.
reference
temperature
A temperature which the ordinary measured values can be compared
with.
reflection The amount of radiation reflected by an object relative to the received
radiation. A number between 0 and 1.
#T559899; r. AB/35742/35742; en-US
38
Page 45

20
Glossary
relative
humidity
Relative humidity represents the ratio between the current water vapour mass in the air and the maximum it may contain in saturation
conditions.
saturation
color
The areas that contain temperatures outside the present level/span
settings are colored with the saturation colors. The saturation colors
contain an ‘overflow’ color and an ‘underflow’ color. There is also a
third red saturation color that marks everything saturated by the detector indicating that the range should probably be changed.
span
The interval of the temperature scale, usually expressed as a signal
value.
spectral (radiant) emittance
temperature
difference, or
Amount of energy emitted from an object per unit of time, area and
wavelength (W/m
2
/μm)
A value which is the result of a subtraction between two temperature
values.
difference of
temperature.
temperature
range
The current overall temperature measurement limitation of an IR cam-
era. Cameras can have several ranges. Expressed as two blackbody
temperatures that limit the current calibration.
temperature
scale
The way in which an IR image currently is displayed. Expressed as
two temperature values limiting the colors.
thermogram infrared image
transmission
(or transmittance) factor
transparent
isotherm
Gases and materials can be more or less transparent. Transmission
is the amount of IR radiation passing through them. A number be-
tween 0 and 1.
An isotherm showing a linear spread of colors, instead of covering the
highlighted parts of the image.
visual Refers to the video mode of a IR camera, as opposed to the normal,
thermographic mode. When a camera is in video mode it captures or-
dinary video images, while thermographic images are captured when
the camera is in IR mode.
#T559899; r. AB/35742/35742; en-US
39
Page 46

21
Thermographic measurement
techniques
21.1 Introduction
An infrared camera measures and images the emitted infrared radiation from an object.
The fact that radiation is a function of object surface temperature makes it possible for the
camera to calculate and display this temperature.
However, the radiation measured by the camera does not only depend on the temperature
of the object but is also a function of the emissivity. Radiation also originates from the surroundings and is reflected in the object. The radiation from the object and the reflected radiation will also be influenced by the absorption of the atmosphere.
To measure temperature accurately, it is therefore necessary to compensate for the effects
of a number of different radiation sources. This is done on-line automatically by the camera. The following object parameters must, however, be supplied for the camera:
• The emissivity of the object
• The reflected apparent temperature
• The distance between the object and the camera
• The relative humidity
• Temperature of the atmosphere
21.2 Emissivity
The most important object parameter to set correctly is the emissivity which, in short, is a
measure of how much radiation is emitted from the object, compared to that from a perfect
blackbody of the same temperature.
Normally, object materials and surface treatments exhibit emissivity ranging from approximately 0.1 to 0.95. A highly polished (mirror) surface falls below 0.1, while an oxidized or
painted surface has a higher emissivity. Oil-based paint, regardless of color in the visible
spectrum, has an emissivity over 0.9 in the infrared. Human skin exhibits an emissivity
0.97 to 0.98.
Non-oxidized metals represent an extreme case of perfect opacity and high reflexivity,
which does not vary greatly with wavelength. Consequently, the emissivity of metals is low
– only increasing with temperature. For non-metals, emissivity tends to be high, and decreases with temperature.
21.2.1 Finding the emissivity of a sample
21.2.1.1 Step 1: Determining reflected apparent temperature
Use one of the following two methods to determine reflected apparent temperature:
#T559899; r. AB/35742/35742; en-US
40
Page 47

Thermographic measurement techniques21
21.2.1.1.1 Method 1: Direct method
Follow this procedure:
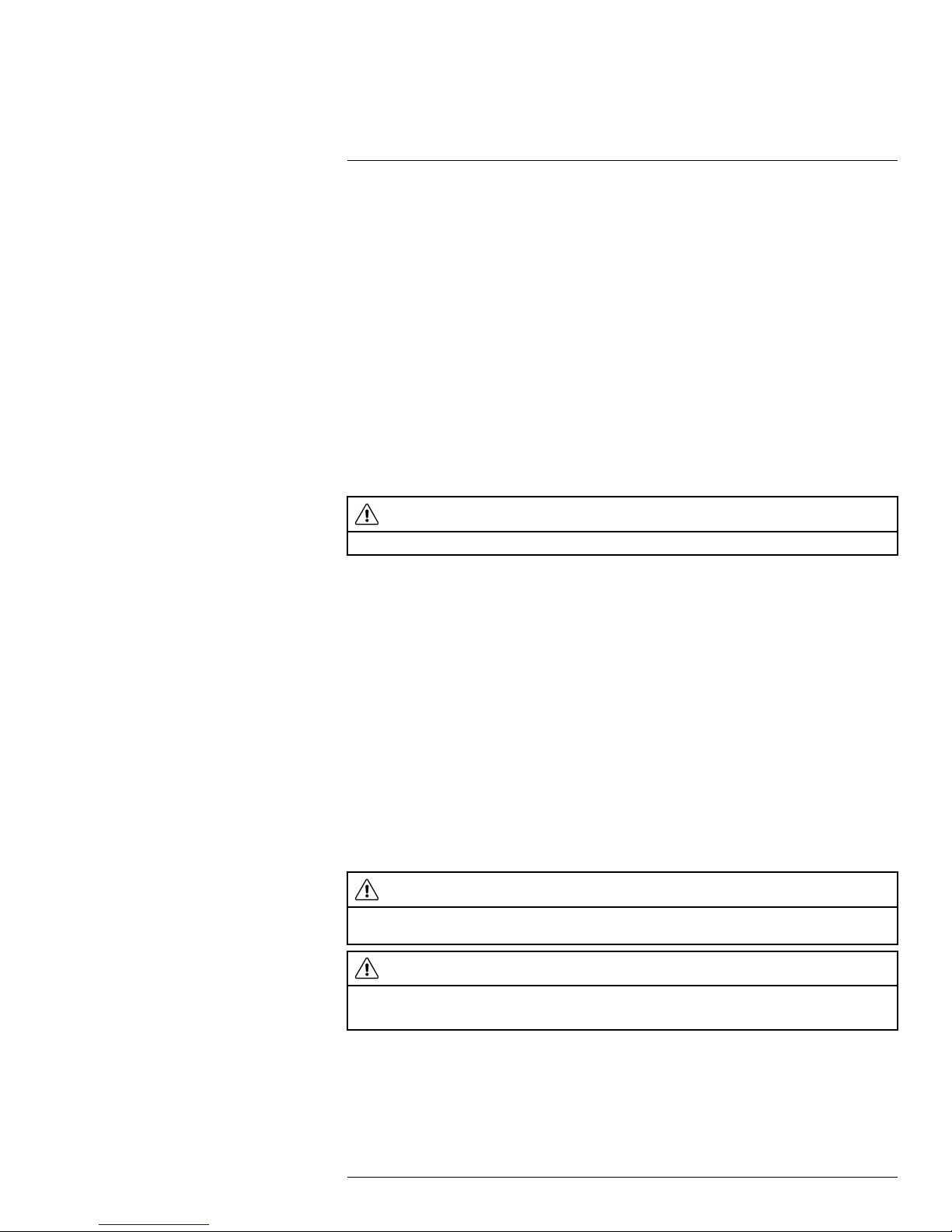
1. Look for possible reflection sources, considering that the incident angle = reflection angle (a = b).
Figure 21.1 1 = Reflection source
2. If the reflection source is a spot source, modify the source by obstructing it using a
piece if cardboard.
Figure 21.2 1 = Reflection source
#T559899; r. AB/35742/35742; en-US
41
Page 48
Thermographic measurement techniques21
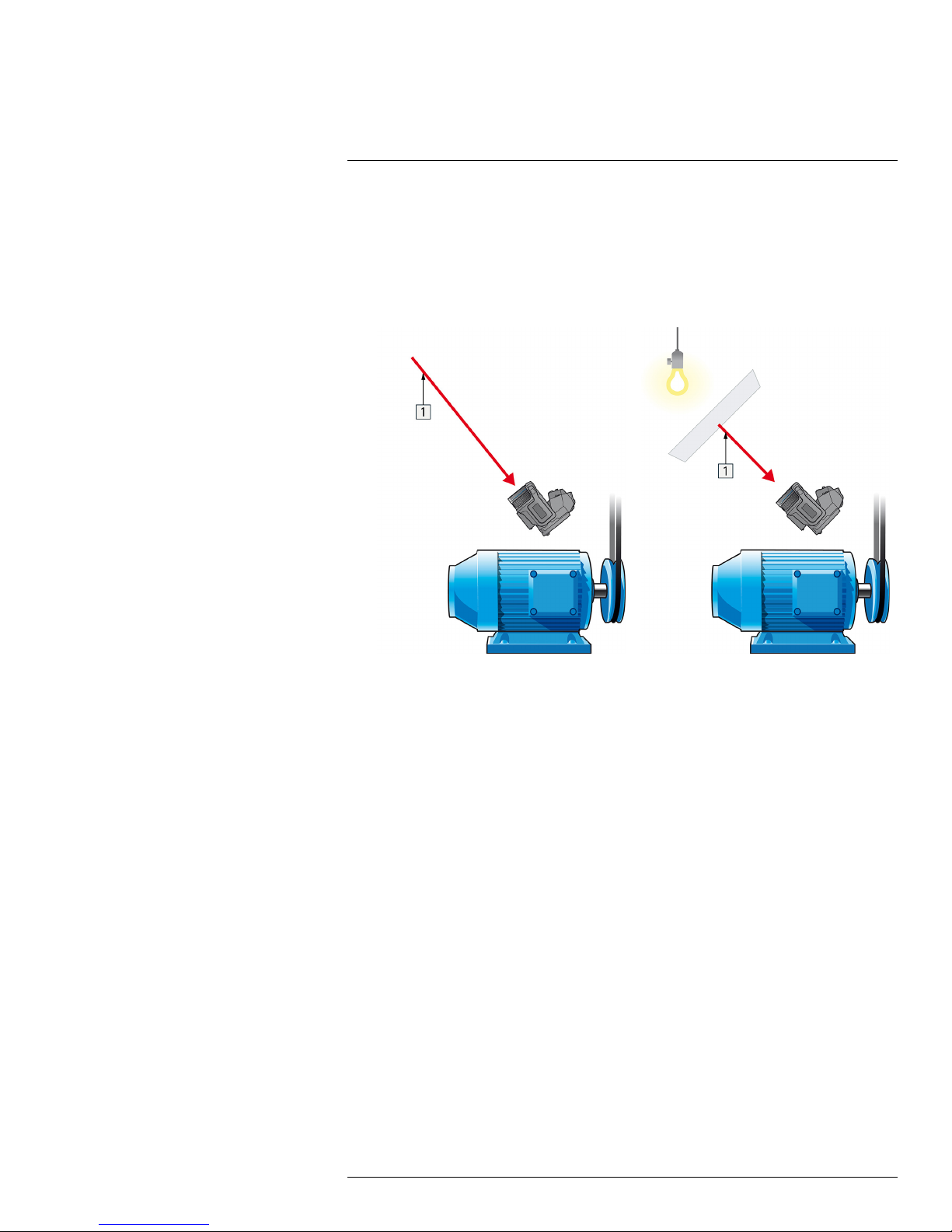
3. Measure the radiation intensity (= apparent temperature) from the reflecting source using the following settings:
• Emissivity: 1.0
• D
: 0
obj
You can measure the radiation intensity using one of the following two methods:
Figure 21.3 1 = Reflection source Figure 21.4 1 = Reflection source
Using a thermocouple to measure reflected apparent temperature is not recommended for
two important reasons:
• A thermocouple does not measure radiation intensity
• A thermocouple requires a very good thermal contact to the surface, usually by gluing
and covering the sensor by a thermal isolator.
21.2.1.1.2 Method 2: Reflector method
Follow this procedure:
1. Crumble up a large piece of aluminum foil.
2. Uncrumble the aluminum foil and attach it to a piece of cardboard of the same size.
3. Put the piece of cardboard in front of the object you want to measure. Make sure that
the side with aluminum foil points to the camera.
4. Set the emissivity to 1.0.
#T559899; r. AB/35742/35742; en-US
42
Page 49
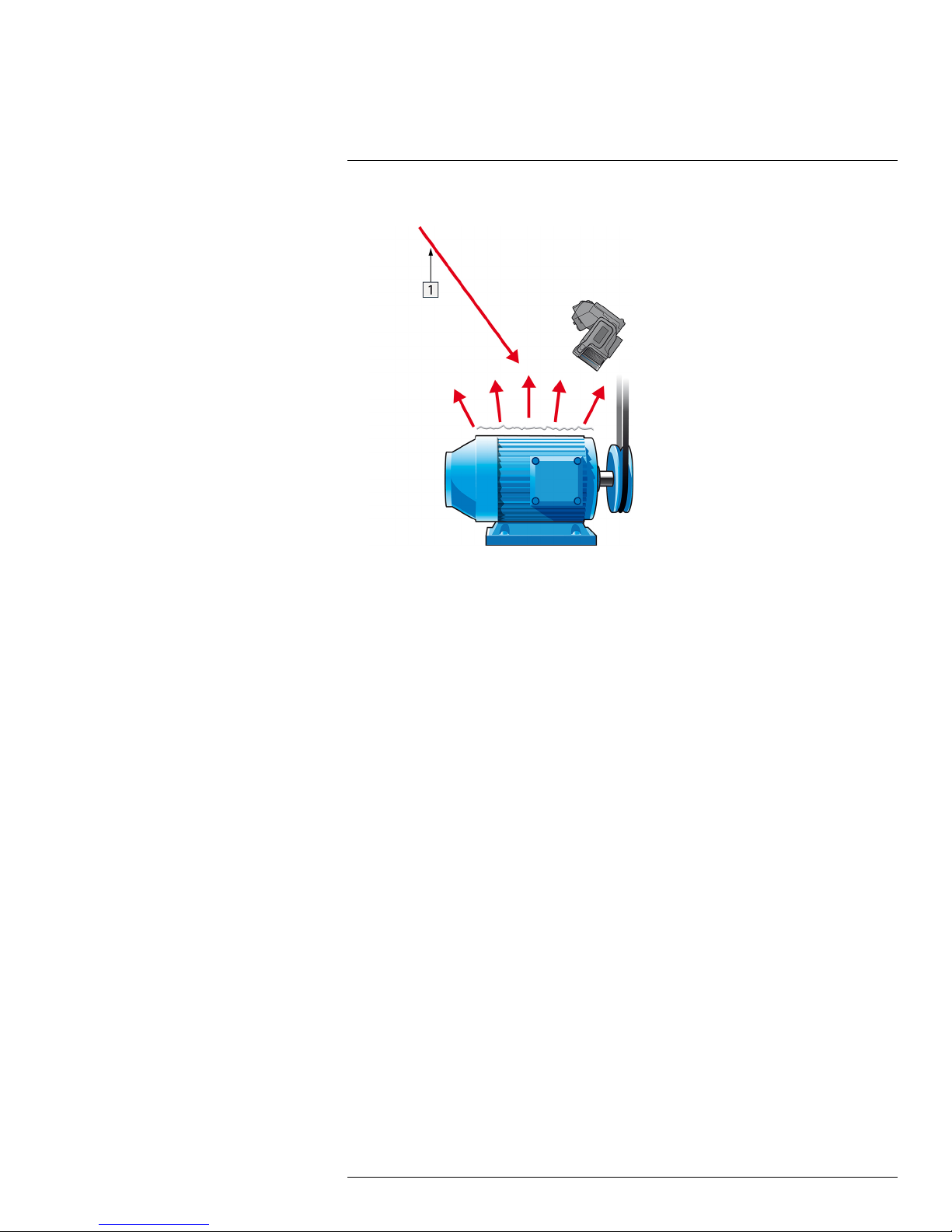
Thermographic measurement techniques21
5. Measure the apparent temperature of the aluminum foil and write it down.
Figure 21.5 Measuring the apparent temperature of the aluminum foil.
21.2.1.2 Step 2: Determining the emissivity
Follow this procedure:
1. Select a place to put the sample.
2. Determine and set reflected apparent temperature according to the previous
procedure.
3. Put a piece of electrical tape with known high emissivity on the sample.
4. Heat the sample at least 20 K above room temperature. Heating must be reasonably
even.
5. Focus and auto-adjust the camera, and freeze the image.
6. Adjust Level and Span for best image brightness and contrast.
7. Set emissivity to that of the tape (usually 0.97).
8. Measure the temperature of the tape using one of the following measurement
functions:
• Isotherm (helps you to determine both the temperature and how evenly you have
heated the sample)
• Spot (simpler)
• Box Avg (good for surfaces with varying emissivity).
9. Write down the temperature.
10. Move your measurement function to the sample surface.
11. Change the emissivity setting until you read the same temperature as your previous
measurement.
12. Write down the emissivity.
Note
• Avoid forced convection
• Look for a thermally stable surrounding that will not generate spot reflections
• Use high quality tape that you know is not transparent, and has a high emissivity you
are certain of
• This method assumes that the temperature of your tape and the sample surface are the
same. If they are not, your emissivity measurement will be wrong.
#T559899; r. AB/35742/35742; en-US
43
Page 50

Thermographic measurement techniques21
21.3 Reflected apparent temperature
This parameter is used to compensate for the radiation reflected in the object. If the emissivity is low and the object temperature relatively far from that of the reflected it will be important to set and compensate for the reflected apparent temperature correctly.
21.4 Distance
The distance is the distance between the object and the front lens of the camera. This parameter is used to compensate for the following two facts:
• That radiation from the target is absorbed by the atmosphere between the object and
the camera.
• That radiation from the atmosphere itself is detected by the camera.
21.5 Relative humidity
The camera can also compensate for the fact that the transmittance is also dependent on
the relative humidity of the atmosphere. To do this set the relative humidity to the correct
value. For short distances and normal humidity the relative humidity can normally be left at
a default value of 50%.
21.6 Other parameters
In addition, some cameras and analysis programs from FLIR Systems allow you to compensate for the following parameters:
• Atmospheric temperature – i.e. the temperature of the atmosphere between the camera
and the target
• External optics temperature – i.e. the temperature of any external lenses or windows
used in front of the camera
• External optics transmittance – i.e. the transmission of any external lenses or windows
used in front of the camera
#T559899; r. AB/35742/35742; en-US
44
Page 51

22
History of infrared technology
Before the year 1800, the existence of the infrared portion of the electromagnetic spectrum
wasn't even suspected. The original significance of the infrared spectrum, or simply ‘the infrared’ as it is often called, as a form of heat radiation is perhaps less obvious today than it
was at the time of its discovery by Herschel in 1800.
Figure 22.1 Sir William Herschel (1738–1822)
The discovery was made accidentally during the search for a new optical material. Sir William Herschel – Royal Astronomer to King George III of England, and already famous for
his discovery of the planet Uranus – was searching for an optical filter material to reduce
the brightness of the sun’s image in telescopes during solar observations. While testing
different samples of colored glass which gave similar reductions in brightness he was intrigued to find that some of the samples passed very little of the sun’s heat, while others
passed so much heat that he risked eye damage after only a few seconds’ observation.
Herschel was soon convinced of the necessity of setting up a systematic experiment, with
the objective of finding a single material that would give the desired reduction in brightness
as well as the maximum reduction in heat. He began the experiment by actually repeating
Newton’s prism experiment, but looking for the heating effect rather than the visual distribution of intensity in the spectrum. He first blackened the bulb of a sensitive mercury-inglass thermometer with ink, and with this as his radiation detector he proceeded to test
the heating effect of the various colors of the spectrum formed on the top of a table by
passing sunlight through a glass prism. Other thermometers, placed outside the sun’s
rays, served as controls.
As the blackened thermometer was moved slowly along the colors of the spectrum, the
temperature readings showed a steady increase from the violet end to the red end. This
was not entirely unexpected, since the Italian researcher, Landriani, in a similar experiment
in 1777 had observed much the same effect. It was Herschel, however, who was the first
to recognize that there must be a point where the heating effect reaches a maximum, and
that measurements confined to the visible portion of the spectrum failed to locate this
point.
Figure 22.2 Marsilio Landriani (1746–1815)
#T559899; r. AB/35742/35742; en-US
45
Page 52

22
History of infrared technology
Moving the thermometer into the dark region beyond the red end of the spectrum, Herschel confirmed that the heating continued to increase. The maximum point, when he
found it, lay well beyond the red end – in what is known today as the ‘infrared wavelengths’.
When Herschel revealed his discovery, he referred to this new portion of the electromagnetic spectrum as the ‘thermometrical spectrum’. The radiation itself he sometimes referred to as ‘dark heat’, or simply ‘the invisible rays’. Ironically, and contrary to popular
opinion, it wasn't Herschel who originated the term ‘infrared’. The word only began to appear in print around 75 years later, and it is still unclear who should receive credit as the
originator.
Herschel’s use of glass in the prism of his original experiment led to some early controversies with his contemporaries about the actual existence of the infrared wavelengths. Different investigators, in attempting to confirm his work, used various types of glass
indiscriminately, having different transparencies in the infrared. Through his later experiments, Herschel was aware of the limited transparency of glass to the newly-discovered
thermal radiation, and he was forced to conclude that optics for the infrared would probably be doomed to the use of reflective elements exclusively (i.e. plane and curved mirrors). Fortunately, this proved to be true only until 1830, when the Italian investigator,
Melloni, made his great discovery that naturally occurring rock salt (NaCl) – which was
available in large enough natural crystals to be made into lenses and prisms – is remarkably transparent to the infrared. The result was that rock salt became the principal infrared
optical material, and remained so for the next hundred years, until the art of synthetic crystal growing was mastered in the 1930’s.
Figure 22.3 Macedonio Melloni (1798–1854)
Thermometers, as radiation detectors, remained unchallenged until 1829, the year Nobili
invented the thermocouple. (Herschel’s own thermometer could be read to 0.2 °C (0.036 °
F), and later models were able to be read to 0.05 °C (0.09 °F)). Then a breakthrough occurred; Melloni connected a number of thermocouples in series to form the first thermopile.
The new device was at least 40 times as sensitive as the best thermometer of the day for
detecting heat radiation – capable of detecting the heat from a person standing three meters away.
The first so-called ‘heat-picture’ became possible in 1840, the result of work by Sir John
Herschel, son of the discoverer of the infrared and a famous astronomer in his own right.
Based upon the differential evaporation of a thin film of oil when exposed to a heat pattern
focused upon it, the thermal image could be seen by reflected light where the interference
effects of the oil film made the image visible to the eye. Sir John also managed to obtain a
primitive record of the thermal image on paper, which he called a ‘thermograph’.
#T559899; r. AB/35742/35742; en-US
46
Page 53

22
History of infrared technology
Figure 22.4 Samuel P. Langley (1834–1906)
The improvement of infrared-detector sensitivity progressed slowly. Another major breakthrough, made by Langley in 1880, was the invention of the bolometer. This consisted of a
thin blackened strip of platinum connected in one arm of a Wheatstone bridge circuit upon
which the infrared radiation was focused and to which a sensitive galvanometer responded. This instrument is said to have been able to detect the heat from a cow at a distance of 400 meters.
An English scientist, Sir James Dewar, first introduced the use of liquefied gases as cooling agents (such as liquid nitrogen with a temperature of -196 °C (-320.8 °F)) in low temperature research. In 1892 he invented a unique vacuum insulating container in which it is
possible to store liquefied gases for entire days. The common ‘thermos bottle’, used for
storing hot and cold drinks, is based upon his invention.
Between the years 1900 and 1920, the inventors of the world ‘discovered’ the infrared.
Many patents were issued for devices to detect personnel, artillery, aircraft, ships – and
even icebergs. The first operating systems, in the modern sense, began to be developed
during the 1914–18 war, when both sides had research programs devoted to the military
exploitation of the infrared. These programs included experimental systems for enemy intrusion/detection, remote temperature sensing, secure communications, and ‘flying torpedo’ guidance. An infrared search system tested during this period was able to detect an
approaching airplane at a distance of 1.5 km (0.94 miles), or a person more than 300 meters (984 ft.) away.
The most sensitive systems up to this time were all based upon variations of the bolometer
idea, but the period between the two wars saw the development of two revolutionary new
infrared detectors: the image converter and the photon detector. At first, the image converter received the greatest attention by the military, because it enabled an observer for
the first time in history to literally ‘see in the dark’. However, the sensitivity of the image
converter was limited to the near infrared wavelengths, and the most interesting military
targets (i.e. enemy soldiers) had to be illuminated by infrared search beams. Since this involved the risk of giving away the observer’s position to a similarly-equipped enemy observer, it is understandable that military interest in the image converter eventually faded.
The tactical military disadvantages of so-called 'active’ (i.e. search beam-equipped) thermal imaging systems provided impetus following the 1939–45 war for extensive secret
military infrared-research programs into the possibilities of developing ‘passive’ (no search
beam) systems around the extremely sensitive photon detector. During this period, military
secrecy regulations completely prevented disclosure of the status of infrared-imaging
technology. This secrecy only began to be lifted in the middle of the 1950’s, and from that
time adequate thermal-imaging devices finally began to be available to civilian science
and industry.
#T559899; r. AB/35742/35742; en-US
47
Page 54
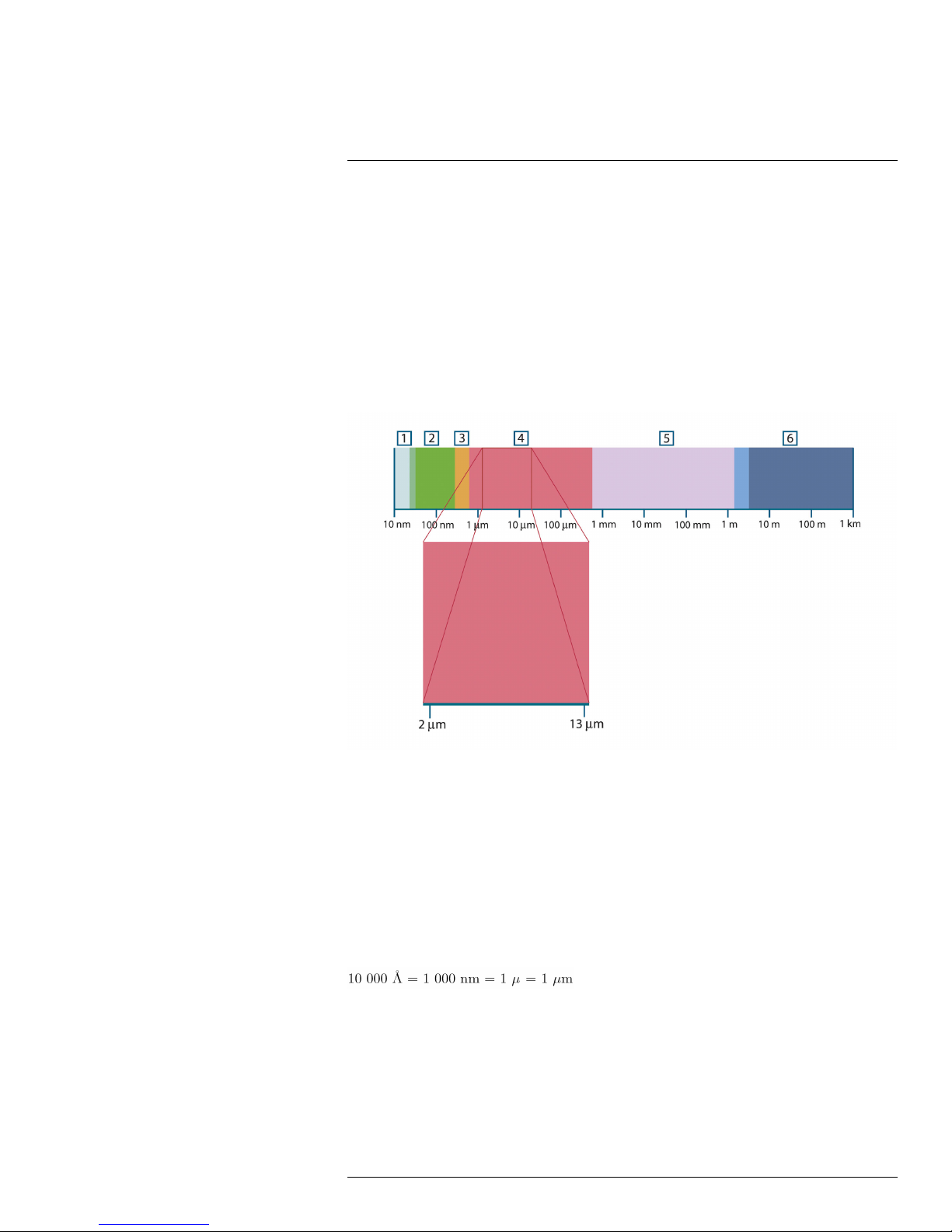
23
Theory of thermography
23.1 Introduction
The subjects of infrared radiation and the related technique of thermography are still new
to many who will use an infrared camera. In this section the theory behind thermography
will be given.
23.2 The electromagnetic spectrum
The electromagnetic spectrum is divided arbitrarily into a number of wavelength regions,
called bands, distinguished by the methods used to produce and detect the radiation.
There is no fundamental difference between radiation in the different bands of the electromagnetic spectrum. They are all governed by the same laws and the only differences are
those due to differences in wavelength.
Figure 23.1 The electromagnetic spectrum. 1: X-ray; 2: UV; 3: Visible; 4: IR; 5: Microwaves; 6: Radiowaves.
Thermography makes use of the infrared spectral band. At the short-wavelength end the
boundary lies at the limit of visual perception, in the deep red. At the long-wavelength end
it merges with the microwave radio wavelengths, in the millimeter range.
The infrared band is often further subdivided into four smaller bands, the boundaries of
which are also arbitrarily chosen. They include: the near infrared (0.75–3 μm), the middle
infrared (3–6 μm), the far infrared (6–15 μm) and the extreme infrared (15–100 μm).
Although the wavelengths are given in μm (micrometers), other units are often still used to
measure wavelength in this spectral region, e.g. nanometer (nm) and Ångström (Å).
The relationships between the different wavelength measurements is:
23.3 Blackbody radiation
A blackbody is defined as an object which absorbs all radiation that impinges on it at any
wavelength. The apparent misnomer black relating to an object emitting radiation is explained by Kirchhoff’s Law (after Gustav Robert Kirchhoff, 1824–1887), which states that a
body capable of absorbing all radiation at any wavelength is equally capable in the emission of radiation.
#T559899; r. AB/35742/35742; en-US
48
Page 55

23
Theory of thermography
Figure 23.2 Gustav Robert Kirchhoff (1824–1887)
The construction of a blackbody source is, in principle, very simple. The radiation characteristics of an aperture in an isotherm cavity made of an opaque absorbing material represents almost exactly the properties of a blackbody. A practical application of the principle
to the construction of a perfect absorber of radiation consists of a box that is light tight except for an aperture in one of the sides. Any radiation which then enters the hole is scattered and absorbed by repeated reflections so only an infinitesimal fraction can possibly
escape. The blackness which is obtained at the aperture is nearly equal to a blackbody
and almost perfect for all wavelengths.
By providing such an isothermal cavity with a suitable heater it becomes what is termed a
cavity radiator. An isothermal cavity heated to a uniform temperature generates blackbody
radiation, the characteristics of which are determined solely by the temperature of the cavity. Such cavity radiators are commonly used as sources of radiation in temperature reference standards in the laboratory for calibrating thermographic instruments, such as a
FLIR Systems camera for example.
If the temperature of blackbody radiation increases to more than 525°C (977°F), the
source begins to be visible so that it appears to the eye no longer black. This is the incipient red heat temperature of the radiator, which then becomes orange or yellow as the temperature increases further. In fact, the definition of the so-called color temperature of an
object is the temperature to which a blackbody would have to be heated to have the same
appearance.
Now consider three expressions that describe the radiation emitted from a blackbody.
23.3.1 Planck’s law
Figure 23.3 Max Planck (1858–1947)
Max Planck (1858–1947) was able to describe the spectral distribution of the radiation
from a blackbody by means of the following formula:
#T559899; r. AB/35742/35742; en-US
49
Page 56
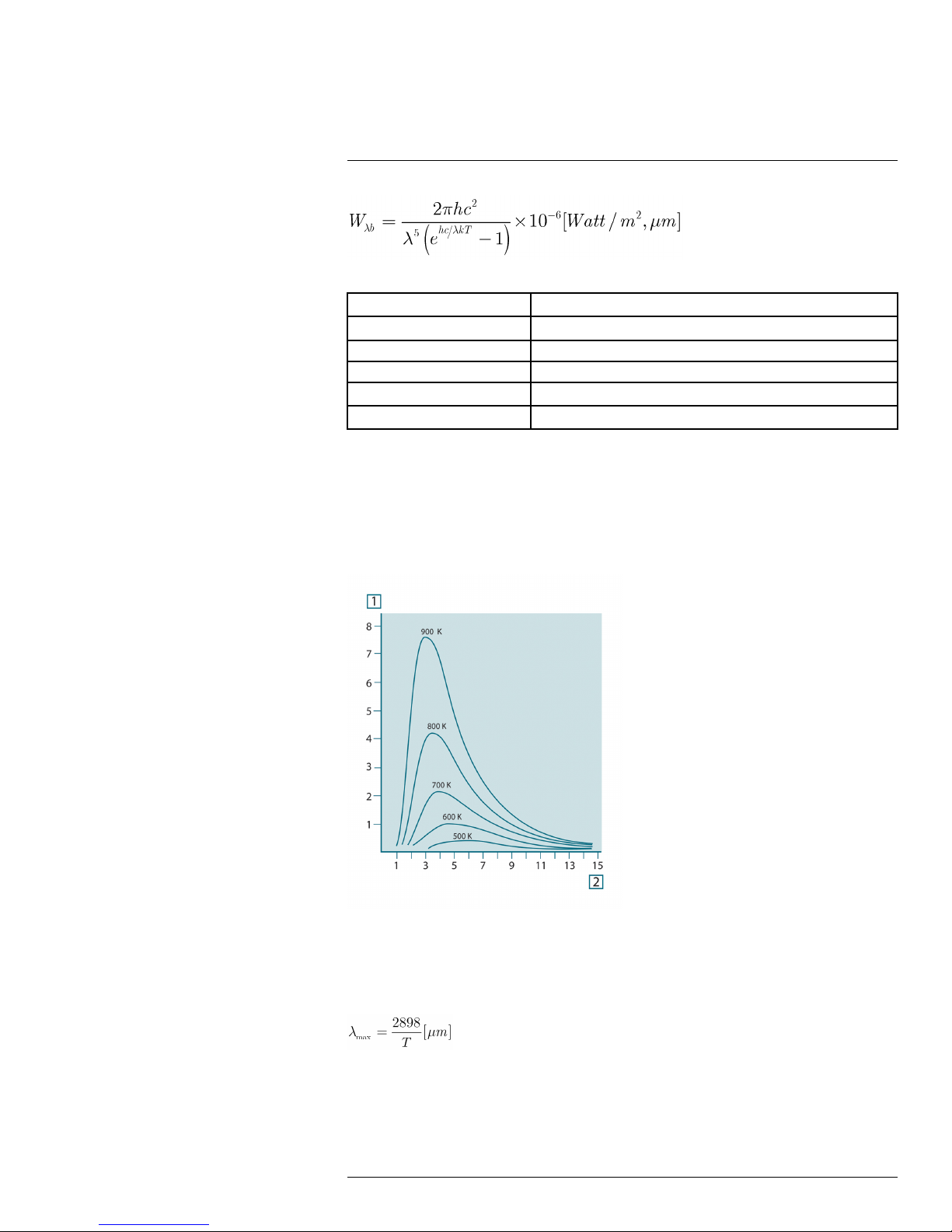
23
Theory of thermography
where:
W
λb
c
h Planck’s constant = 6.6 × 10
k
T Absolute temperature (K) of a blackbody.
λ Wavelength (μm).
Blackbody spectral radiant emittance at wavelength λ.
Velocity of light = 3 × 10
Boltzmann’s constant = 1.4 × 10
8
m/s
-34
Joule sec.
-23
Joule/K.
Note The factor 10-6is used since spectral emittance in the curves is expressed in Watt/
2
m
, μm.
Planck’s formula, when plotted graphically for various temperatures, produces a family of
curves. Following any particular Planck curve, the spectral emittance is zero at λ = 0, then
increases rapidly to a maximum at a wavelength λ
and after passing it approaches zero
max
again at very long wavelengths. The higher the temperature, the shorter the wavelength at
which maximum occurs.
Figure 23.4 Blackbody spectral radiant emittance according to Planck’s law, plotted for various absolute
temperatures. 1: Spectral radiant emittance (W/cm
2
× 103(μm)); 2: Wavelength (μm)
23.3.2 Wien’s displacement law
By differentiating Planck’s formula with respect to λ, and finding the maximum, we have:
This is Wien’s formula (after Wilhelm Wien, 1864–1928), which expresses mathematically
the common observation that colors vary from red to orange or yellow as the temperature
of a thermal radiator increases. The wavelength of the color is the same as the wavelength
calculated for λ
#T559899; r. AB/35742/35742; en-US
. A good approximation of the value of λ
max
for a given blackbody
max
50
Page 57
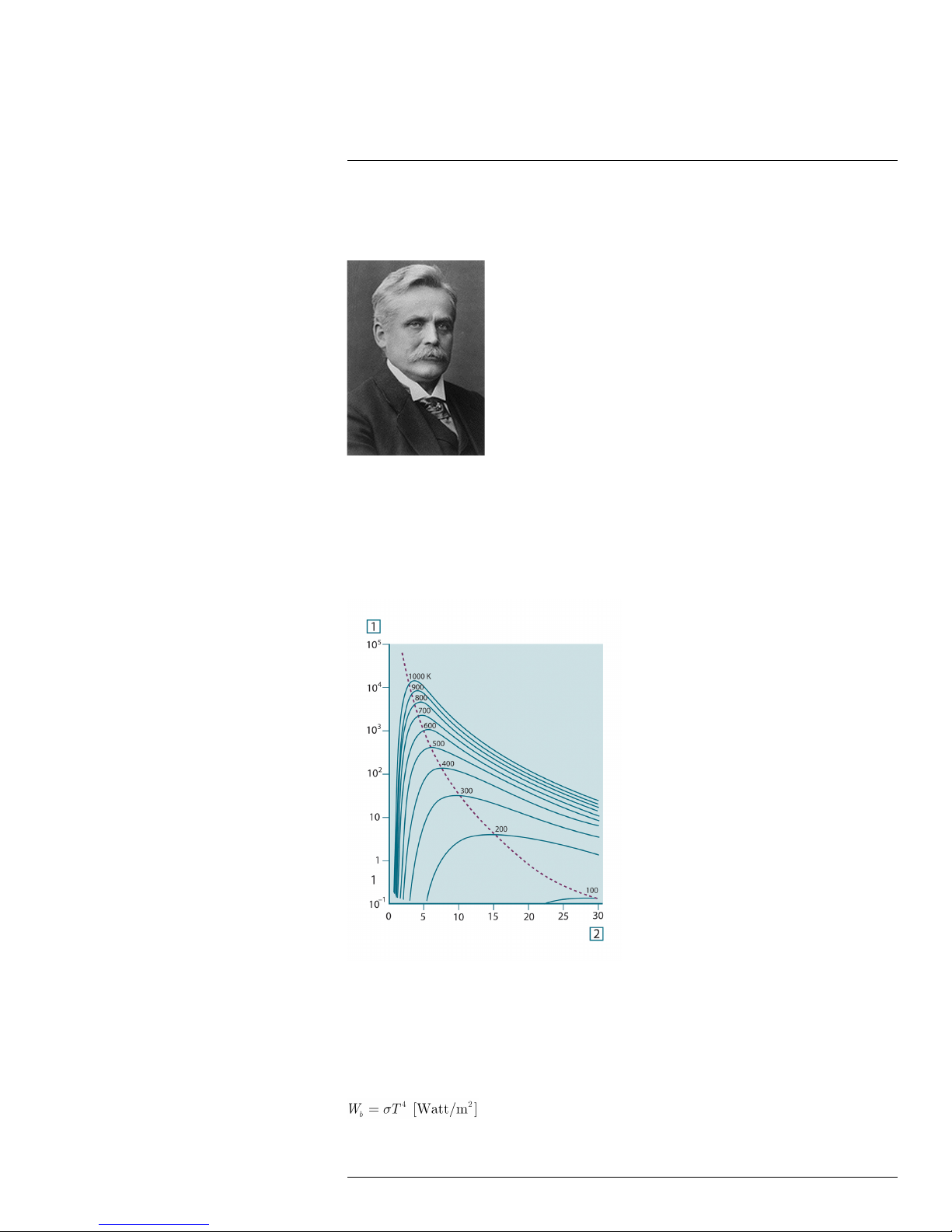
23
Theory of thermography
temperature is obtained by applying the rule-of-thumb 3 000/T μm. Thus, a very hot star
such as Sirius (11 000 K), emitting bluish-white light, radiates with the peak of spectral radiant emittance occurring within the invisible ultraviolet spectrum, at wavelength 0.27 μm.
Figure 23.5 Wilhelm Wien (1864–1928)
The sun (approx. 6 000 K) emits yellow light, peaking at about 0.5 μm in the middle of the
visible light spectrum.
At room temperature (300 K) the peak of radiant emittance lies at 9.7 μm, in the far infrared, while at the temperature of liquid nitrogen (77 K) the maximum of the almost insignificant amount of radiant emittance occurs at 38 μm, in the extreme infrared wavelengths.
Figure 23.6 Planckian curves plotted on semi-log scales from 100 K to 1000 K. The dotted line represents
the locus of maximum radiant emittance at each temperature as described by Wien's displacement law. 1:
Spectral radiant emittance (W/cm
2
(μm)); 2: Wavelength (μm).
23.3.3 Stefan-Boltzmann's law
By integrating Planck’s formula from λ = 0 to λ = ∞, we obtain the total radiant emittance
(W
) of a blackbody:
b
#T559899; r. AB/35742/35742; en-US
51
Page 58

23
Theory of thermography
This is the Stefan-Boltzmann formula (after Josef Stefan, 1835–1893, and Ludwig Boltzmann, 1844–1906), which states that the total emissive power of a blackbody is propor-
tional to the fourth power of its absolute temperature. Graphically, W
represents the area
b
below the Planck curve for a particular temperature. It can be shown that the radiant emittance in the interval λ = 0 to λ
is only 25% of the total, which represents about the
max
amount of the sun’s radiation which lies inside the visible light spectrum.
Figure 23.7 Josef Stefan (1835–1893), and Ludwig Boltzmann (1844–1906)
Using the Stefan-Boltzmann formula to calculate the power radiated by the human body,
2
at a temperature of 300 K and an external surface area of approx. 2 m
, we obtain 1 kW.
This power loss could not be sustained if it were not for the compensating absorption of radiation from surrounding surfaces, at room temperatures which do not vary too drastically
from the temperature of the body – or, of course, the addition of clothing.
23.3.4 Non-blackbody emitters
So far, only blackbody radiators and blackbody radiation have been discussed. However,
real objects almost never comply with these laws over an extended wavelength region –
although they may approach the blackbody behavior in certain spectral intervals. For example, a certain type of white paint may appear perfectly white in the visible light spectrum, but becomes distinctly gray at about 2 μm, and beyond 3 μm it is almost black.
There are three processes which can occur that prevent a real object from acting like a
blackbody: a fraction of the incident radiation α may be absorbed, a fraction ρ may be reflected, and a fraction τ may be transmitted. Since all of these factors are more or less
wavelength dependent, the subscript λ is used to imply the spectral dependence of their
definitions. Thus:
• The spectral absorptance α
= the ratio of the spectral radiant power absorbed by an ob-
λ
ject to that incident upon it.
• The spectral reflectance ρ
= the ratio of the spectral radiant power reflected by an ob-
λ
ject to that incident upon it.
• The spectral transmittance τ
= the ratio of the spectral radiant power transmitted
λ
through an object to that incident upon it.
The sum of these three factors must always add up to the whole at any wavelength, so we
have the relation:
For opaque materials τλ= 0 and the relation simplifies to:
Another factor, called the emissivity, is required to describe the fraction ε of the radiant
emittance of a blackbody produced by an object at a specific temperature. Thus, we have
the definition:
#T559899; r. AB/35742/35742; en-US
52
Page 59
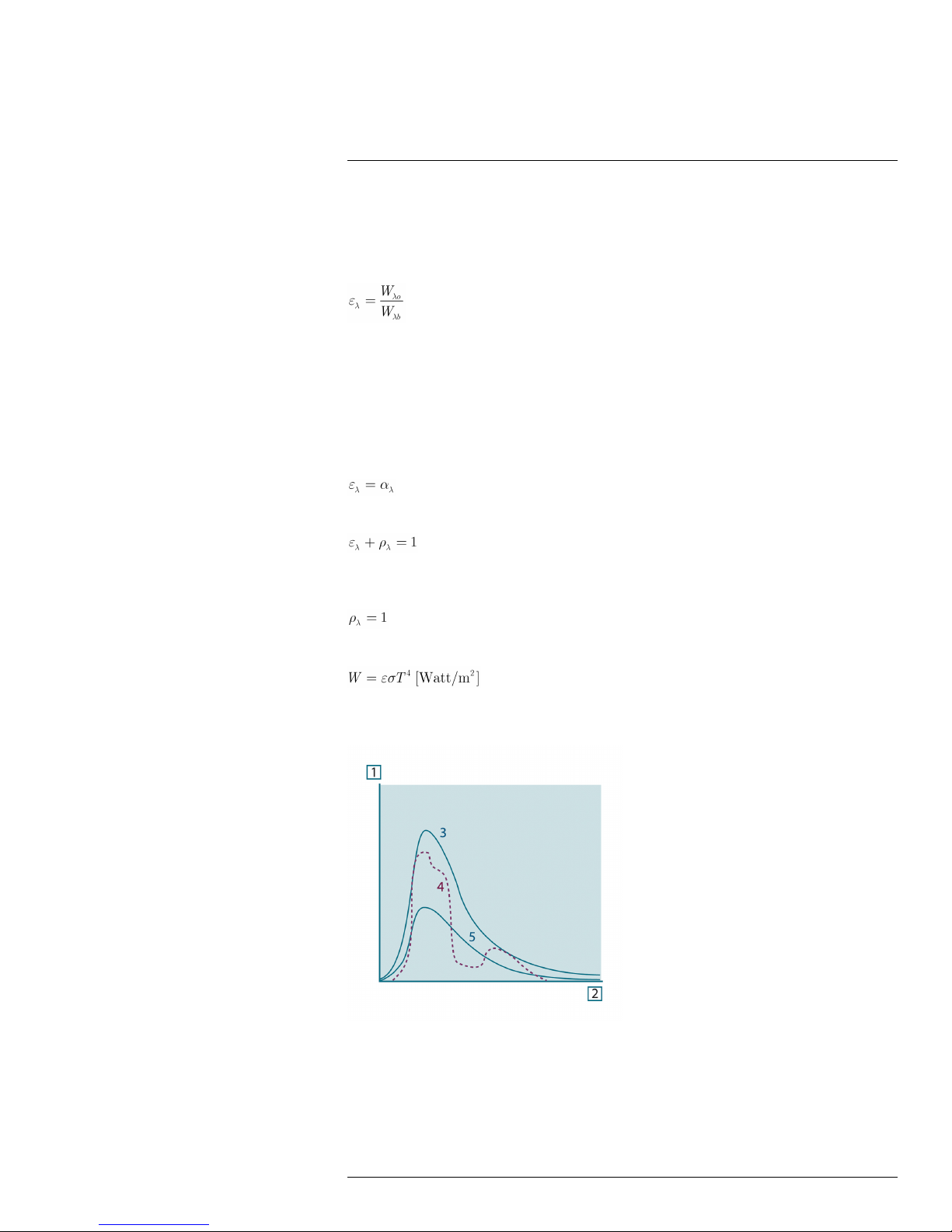
23
Theory of thermography
The spectral emissivity ε
= the ratio of the spectral radiant power from an object to that
λ
from a blackbody at the same temperature and wavelength.
Expressed mathematically, this can be written as the ratio of the spectral emittance of the
object to that of a blackbody as follows:
Generally speaking, there are three types of radiation source, distinguished by the ways in
which the spectral emittance of each varies with wavelength.
• A blackbody, for which ε
• A graybody, for which ε
= ε = 1
λ
= ε = constant less than 1
λ
• A selective radiator, for which ε varies with wavelength
According to Kirchhoff’s law, for any material the spectral emissivity and spectral absorp-
tance of a body are equal at any specified temperature and wavelength. That is:
From this we obtain, for an opaque material (since αλ+ ρλ= 1):
For highly polished materials ελapproaches zero, so that for a perfectly reflecting material
(i.e. a perfect mirror) we have:
For a graybody radiator, the Stefan-Boltzmann formula becomes:
This states that the total emissive power of a graybody is the same as a blackbody at the
same temperature reduced in proportion to the value of ε from the graybody.
Figure 23.8 Spectral radiant emittance of three types of radiators. 1: Spectral radiant emittance; 2: Wavelength; 3: Blackbody; 4: Selective radiator; 5: Graybody.
#T559899; r. AB/35742/35742; en-US
53
Page 60

23
Theory of thermography
Figure 23.9 Spectral emissivity of three types of radiators. 1: Spectral emissivity; 2: Wavelength; 3: Black-
body; 4: Graybody; 5: Selective radiator.
23.4 Infrared semi-transparent materials
Consider now a non-metallic, semi-transparent body – let us say, in the form of a thick flat
plate of plastic material. When the plate is heated, radiation generated within its volume
must work its way toward the surfaces through the material in which it is partially absorbed.
Moreover, when it arrives at the surface, some of it is reflected back into the interior. The
back-reflected radiation is again partially absorbed, but some of it arrives at the other surface, through which most of it escapes; part of it is reflected back again. Although the progressive reflections become weaker and weaker they must all be added up when the total
emittance of the plate is sought. When the resulting geometrical series is summed, the effective emissivity of a semi-transparent plate is obtained as:
When the plate becomes opaque this formula is reduced to the single formula:
This last relation is a particularly convenient one, because it is often easier to measure reflectance than to measure emissivity directly.
#T559899; r. AB/35742/35742; en-US
54
Page 61

24
The measurement formula
As already mentioned, when viewing an object, the camera receives radiation not only
from the object itself. It also collects radiation from the surroundings reflected via the object surface. Both these radiation contributions become attenuated to some extent by the
atmosphere in the measurement path. To this comes a third radiation contribution from the
atmosphere itself.
This description of the measurement situation, as illustrated in the figure below, is so far a
fairly true description of the real conditions. What has been neglected could for instance
be sun light scattering in the atmosphere or stray radiation from intense radiation sources
outside the field of view. Such disturbances are difficult to quantify, however, in most cases
they are fortunately small enough to be neglected. In case they are not negligible, the
measurement configuration is likely to be such that the risk for disturbance is obvious, at
least to a trained operator. It is then his responsibility to modify the measurement situation
to avoid the disturbance e.g. by changing the viewing direction, shielding off intense radiation sources etc.
Accepting the description above, we can use the figure below to derive a formula for the
calculation of the object temperature from the calibrated camera output.
Figure 24.1 A schematic representation of the general thermographic measurement situation.1: Surroundings; 2: Object; 3: Atmosphere; 4: Camera
Assume that the received radiation power W from a blackbody source of temperature
T
on short distance generates a camera output signal U
source
the power input (power linear camera). We can then write (Equation 1):
or, with simplified notation:
where C is a constant.
Should the source be a graybody with emittance ε, the received radiation would conse-
quently be εW
We are now ready to write the three collected radiation power terms:
1. Emission from the object = ετW
transmittance of the atmosphere. The object temperature is T
#T559899; r. AB/35742/35742; en-US
source
.
, where ε is the emittance of the object and τ is the
obj
that is proportional to
source
.
obj
55
Page 62

24
The measurement formula
2. Reflected emission from ambient sources = (1 – ε)τW
tance of the object. The ambient sources have the temperature T
It has here been assumed that the temperature T
, where (1 – ε) is the reflec-
refl
.
refl
is the same for all emitting surfaces
refl
within the halfsphere seen from a point on the object surface. This is of course sometimes a simplification of the true situation. It is, however, a necessary simplification in
order to derive a workable formula, and T
can – at least theoretically – be given a val-
refl
ue that represents an efficient temperature of a complex surrounding.
Note also that we have assumed that the emittance for the surroundings = 1. This is
correct in accordance with Kirchhoff’s law: All radiation impinging on the surrounding
surfaces will eventually be absorbed by the same surfaces. Thus the emittance = 1.
(Note though that the latest discussion requires the complete sphere around the object
to be considered.)
3. Emission from the atmosphere = (1 – τ)τW
mosphere. The temperature of the atmosphere is T
, where (1 – τ) is the emittance of the at-
atm
atm
.
The total received radiation power can now be written (Equation 2):
We multiply each term by the constant C of Equation 1 and replace the CW products by
the corresponding U according to the same equation, and get (Equation 3):
Solve Equation 3 for U
(Equation 4):
obj
This is the general measurement formula used in all the FLIR Systems thermographic
equipment. The voltages of the formula are:
Table 24.1 Voltages
U
obj
U
tot
U
refl
U
atm
Calculated camera output voltage for a blackbody of temperature T
i.e. a voltage that can be directly converted into true requested object
temperature.
Measured camera output voltage for the actual case.
Theoretical camera output voltage for a blackbody of temperature
T
according to the calibration.
refl
Theoretical camera output voltage for a blackbody of temperature
according to the calibration.
T
atm
obj
The operator has to supply a number of parameter values for the calculation:
• the object emittance ε,
• the relative humidity,
• T
atm
• object distance (D
obj
)
• the (effective) temperature of the object surroundings, or the reflected ambient temper-
ature T
• the temperature of the atmosphere T
refl
, and
atm
This task could sometimes be a heavy burden for the operator since there are normally no
easy ways to find accurate values of emittance and atmospheric transmittance for the
#T559899; r. AB/35742/35742; en-US
56
Page 63

24
The measurement formula
actual case. The two temperatures are normally less of a problem provided the surroundings do not contain large and intense radiation sources.
A natural question in this connection is: How important is it to know the right values of
these parameters? It could though be of interest to get a feeling for this problem already
here by looking into some different measurement cases and compare the relative magnitudes of the three radiation terms. This will give indications about when it is important to
use correct values of which parameters.
The figures below illustrates the relative magnitudes of the three radiation contributions for
three different object temperatures, two emittances, and two spectral ranges: SW and LW.
Remaining parameters have the following fixed values:
• τ = 0.88
• T
= +20°C (+68°F)
refl
• T
= +20°C (+68°F)
atm
It is obvious that measurement of low object temperatures are more critical than measuring high temperatures since the ‘disturbing’ radiation sources are relatively much stronger
in the first case. Should also the object emittance be low, the situation would be still more
difficult.
We have finally to answer a question about the importance of being allowed to use the calibration curve above the highest calibration point, what we call extrapolation. Imagine that
we in a certain case measure U
= 4.5 volts. The highest calibration point for the camera
tot
was in the order of 4.1 volts, a value unknown to the operator. Thus, even if the object happened to be a blackbody, i.e. U
obj
= U
, we are actually performing extrapolation of the
tot
calibration curve when converting 4.5 volts into temperature.
Let us now assume that the object is not black, it has an emittance of 0.75, and the trans-
mittance is 0.92. We also assume that the two second terms of Equation 4 amount to 0.5
volts together. Computation of U
by means of Equation 4 then results in U
obj
= 4.5 / 0.75
obj
/ 0.92 – 0.5 = 6.0. This is a rather extreme extrapolation, particularly when considering that
the video amplifier might limit the output to 5 volts! Note, though, that the application of the
calibration curve is a theoretical procedure where no electronic or other limitations exist.
We trust that if there had been no signal limitations in the camera, and if it had been calibrated far beyond 5 volts, the resulting curve would have been very much the same as our
real curve extrapolated beyond 4.1 volts, provided the calibration algorithm is based on radiation physics, like the FLIR Systems algorithm. Of course there must be a limit to such
extrapolations.
#T559899; r. AB/35742/35742; en-US
57
Page 64
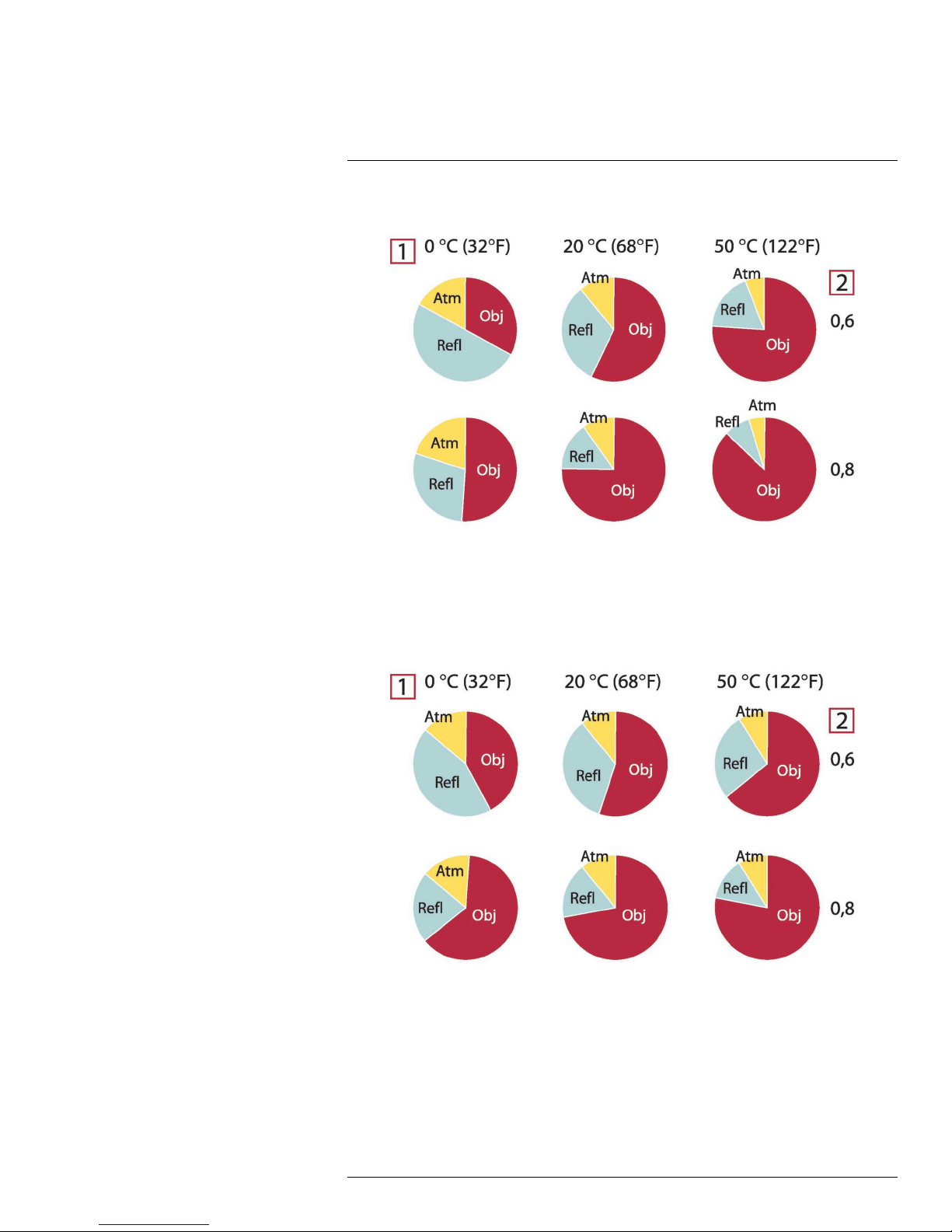
24
The measurement formula
Figure 24.2 Relative magnitudes of radiation sources under varying measurement conditions (SW camera).
1: Object temperature; 2: Emittance; Obj: Object radiation; Refl: Reflected radiation; Atm: atmosphere radiation. Fixed parameters: τ = 0.88; T
= 20°C (+68°F); T
refl
= 20°C (+68°F).
atm
Figure 24.3 Relative magnitudes of radiation sources under varying measurement conditions (LW camera).
1: Object temperature; 2: Emittance; Obj: Object radiation; Refl: Reflected radiation; Atm: atmosphere radiation. Fixed parameters: τ = 0.88; T
= 20°C (+68°F); T
refl
= 20°C (+68°F).
atm
#T559899; r. AB/35742/35742; en-US
58
Page 65

Page 66

A note on the technical production of this publication
This publication was produced using XML — the eXtensible Markup Language. For more information about
XML, please visit http://www.w3.org/XML/
A note on the typeface used in this publication
This publication was typeset using Linotype Helvetica™ World. Helvetica™ was designed by Max Miedinger
(1910–1980)
LOEF (List Of Effective Files)
T501093.xml; en-US; AB; 35742; 2016-05-20
T505471.xml; en-US; 9229; 2013-10-03
T505013.xml; en-US; 35155; 2016-04-21
T505251.xml; en-US; 15388; 2014-06-18
T505771.xml; ; 33311; 2016-02-11
T505432.xml; en-US; 35155; 2016-04-21
T505470.xml; en-US; 12154; 2014-03-06
T505007.xml; en-US; 35155; 2016-04-21
T505004.xml; en-US; 35155; 2016-04-21
T505000.xml; en-US; 35155; 2016-04-21
T505005.xml; en-US; 35155; 2016-04-21
T505001.xml; en-US; 32554; 2016-01-20
T505006.xml; en-US; 32555; 2016-01-20
#T559899; r. AB/35742/35742; en-US
60
Page 67

Page 68

Website
last page
http://www.flir.com
Customer support
http://support.flir.com
Copyright
© 2016, FLIR Systems, Inc. All rights reserved worldwide.
Disclaimer
Specifications subject to change without further notice. Models and accessories subject to regional market considerations. License procedures may apply. Products
described herein may be subject to US Export Regulations. Please refer to exportquestions@flir.com with any questions.
Publ. No.: T559899
Release: AB
Commit:
Head: 35742
Language: en-US
Modified: 2016-05-20
Formatted: 2016-05-20
35742
 Loading...
Loading...