
USER MANUAL
Streamer
STR-4000
®
System
05-12-17
Rev 6.0
Flexcell International Corporation 2730 Tucker Street, Suite 200 Burlington, NC 27215
800-728-3714 (919) 732-1591 FAX: (919) 732-5196 www.flexcellint.com
Culturing Cells in a Mechanically Active Environment™
COPYRIGHT © 2003 FLEXCELL
INTERNATIONAL CORPORATION

FLEXCELL
®
INTERNATIONAL CORPORATION
TABLE OF CONTENTS
1. Getting Started .............................................................................................................. 1
Introduction ........................................................................................................................................ 1
Streamer® Components ...................................................................................................................... 1
Streamer® Setup and Assembly ......................................................................................................... 1
Sterilizing the Streamer® ................................................................................................................... 2
Streamer® Placement in the Incubator .............................................................................................. 3
Using the Pump .................................................................................................................................. 3
Quickstart Instructions ....................................................................................................................... 3
2. StreamSoft™ V4.2 Software ......................................................................................... 5
Installation Instructions ...................................................................................................................... 5
Setting up Parameters in StreamSoft
Main Panel .......................................................................................................................................... 6
General Information Tab ............................................................................................................. 6
System Tab ................................................................................................................................... 7
Pre-Test Configuration ................................................................................................................ 8
Pull-down Menus ......................................................................................................................... 9
Operate Menu ................................................................................................................................... 10
Manual Mode ............................................................................................................................. 10
View Data .................................................................................................................................. 11
Configure Users ......................................................................................................................... 12
Configure Regimes: Setup Parameters ..................................................................................... 13
Configure Testing Apparatus .................................................................................................... 15
How to enter the proper values for your device ....................................................................... 16
Configure System Variables ..................................................................................................... 17
Reinitialize Hardware ................................................................................................................ 18
StreamSoft
™
V4.2 Notes .................................................................................................................. 18
™
V4.2 ..................................................................................... 5
3. Doing an Experiment ................................................................................................. 19
Overview .......................................................................................................................................... 19
Creating a Regime ............................................................................................................................ 19
Setting up an Experiment ................................................................................................................. 20
Filling the System to Eliminate Air Bubbles .................................................................................. 21
Post-Experimental Analysis ............................................................................................................. 21
Application Notes ........................................................................................................... 22
Culturing Cells on Culture Slips® .................................................................................................... 22
Appendix: Parallel Streamer® Shear Stress Numbers ................................................... 23
Warranty Information ..................................................................................................... 24
Contacting Flexcell ......................................................................................................... 25
i
FLEXCELL
®
INTERNATIONAL CORPORATION
1. GETTING STARTED
I
NTRODUCTION
Fluid-induced shear stress occurs in every tissue
in the body as a result of interstitial fluid
movement. Tissue deformation by
compression, tension or shear forces results in
the movement of interstitial fluid around cells.
Fluid movement acts as a transport vehicle for
ions, proteins, carbohydrates and other
molecules capable of movement within the
matrix. As the fluid moves past cell membranes,
a shear stress () is generated. If one assumes
that laminar flow occurs through a parallel-plate
flow chamber, fluid-induced shear stress values
can be determined with the following formula:
where is the shear stress in dyne/cm2, is the
viscosity of the fluid in dynes/cm2, Q is the flow
rate in ml/s, b is the width of the flow channel
in cm, and h is the height of the flow channel in
cm. Shear stress in the vascular system may
vary from less than 1 to more than 35 dyne/cm2.
Fluid shear stress in canaliculi of bone may vary
from 1 to 20 dyne/cm2, while in cartilage it may
be in the range of 1 to 5 dyne/cm2.
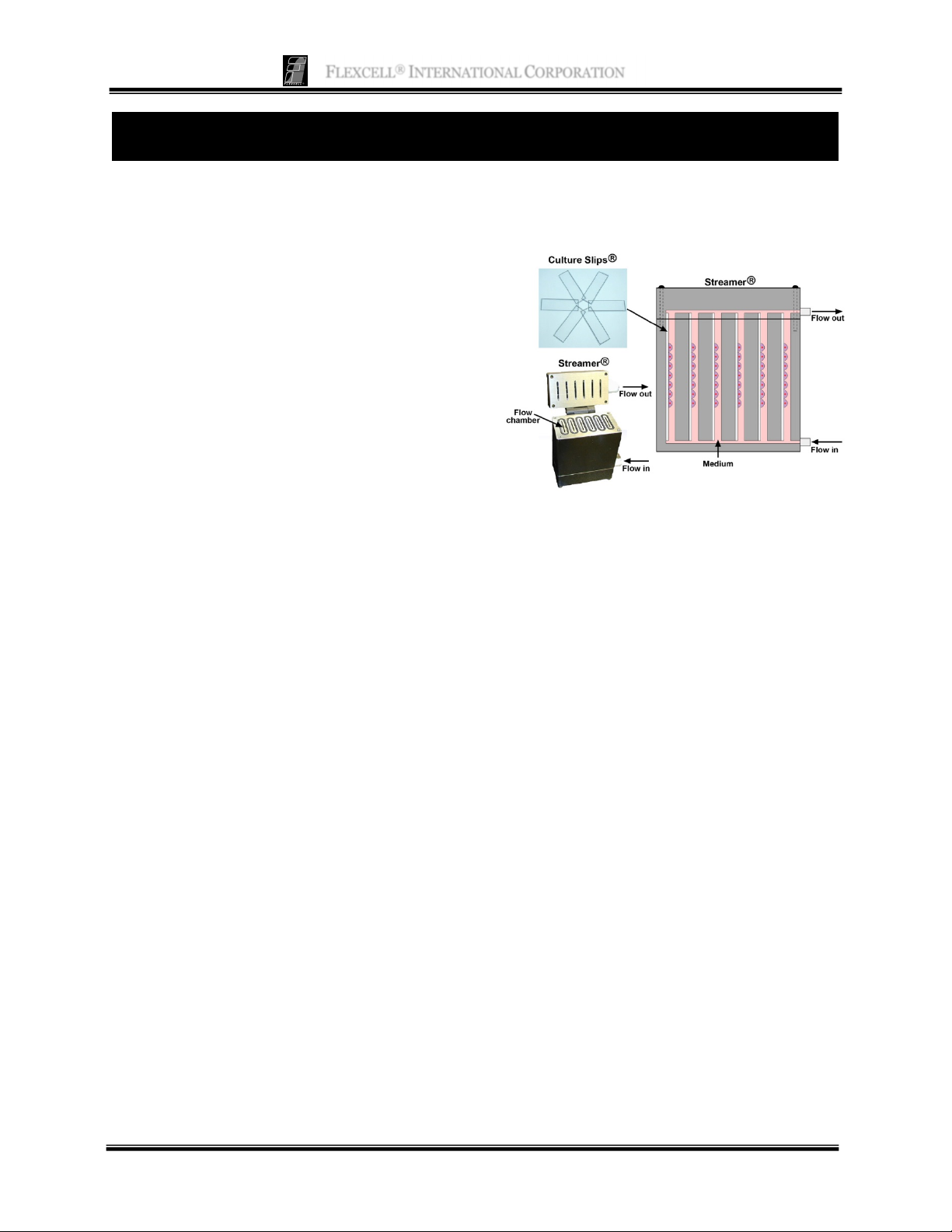
The Streamer
that is used to apply fluid-induced shear stress
to cells grown in a monolayer. The system
includes a six-chamber laminar flow device
designed to hold 75 x 25 x 1 mm Culture Slips®
(Fig. 1). Cells are cultured on these matrixcoated glass slides. StreamSoft software
controls a peristaltic pump, thereby regulating
the flow rate into the chamber and the
magnitude of shear stress applied to the cells.
Shear stress values from 0 to 35 dyne/cm
be achieved depending on the tubing size used.
This six place flow chamber can be used to
assess RNA and protein expression by cells in
response to fluid-induced shear stress, and
= 6Q/bh2
®
is a parallel-plate flow system
2
can
production of secreted molecules into the
perfusate.
Figure 1. Image and schematic of Streamer®
device with cells cultured in monolayer on Culture
Slips
®
.
®
S
TREAMER
C
OMPONENTS
Streamer® Device
Streamer® Tubing (includes quick
disconnect fittings)
Masterflex L/S Peristaltic Pump
RS232 to USB Connector Cable
Pulse Dampeners (2)
®
12 Culture Slips
StreamSoft
Software V4.2
500 ml Culture Medium Collection
Reservoir (includes quick disconnects and
filter)
Dell Inspiron Notebook Computer
(optional)
S
TREAMER® SETUP AND ASSEMBLY
The following instructions are for the full
®
Streamer
system. Once fully assembled in the
incubator, the system should resemble the one
pictured in Figure 2. Always check the tubing
for cracks or leaks prior to use.
1

FLEXCELL
®
INTERNATIONAL CORPORATION
A
B
Figure 2. A) Streamer® system setup on the shelf
in a standard incubator. B) Schematic of Streamer
system setup.
®
1. Connect the medium collection reservoir to
the first pulse dampener with the 3’ (0.9 m)
long piece of beige Phar-Med® tubing. On
the medium reservoir, the quick disconnect
connected to the long tubing extending to
the bottom of the bottle should be used. Do
not use the quick disconnect leading to
the bent tubing in the bottle for this
connection.
2. Move the clamp mounted onto the Phar-
®
tubing to the end closest to the pulse
Med
dampener. Place the middle of this tubing
segment into the pump head. Rotate the
lever to the left to open the pump head for
tubing placement, then rotate the lever to
the right to secure the tubing into the pump
head. When not doing an experiment, the
pump head lever should be rotated to the
left to eliminate pressure on the tubing.
3. Connect the first pulse dampener to the
second pulse dampener with the 3” (7.6 cm)
long segment of silicone tubing.
4. Connect the second pulse dampener to the
inlet port (bottom) of the Streamer
®
with
one of the 2’ (0.6 m) pieces of silicone
tubing.
5. Connect the outlet port (top) of the
Streamer® to the quick disconnect on the
medium bottle that is connected to the short,
bent tubing in the bottle, using the other 2’
(0.6 m ) long piece of silicone tubing.
6. Before the first use, run deionized water
through the entire system to make sure there
are no leaks.
Note: Any of the tubing lengths above can be shorted
or extended according to your setup needs.
S
TERILIZING THE STREAMER
®
All components of the flow system (except the
pump and computer) can be effectively and
safely sterilized in an autoclave at standard
autoclave temperature, pressure and time period
(120 °C, 15 psi, 15-20 minutes). When
autoclaving, leave all system components
connected together. Release the clamp on the
Phar-Med® tubing used between the medium
collection reservoir and the first pulse
dampener. Open the top of the Streamer®
slightly so that steam can reach the inside of the
device. Also, do not place any system
component on top of the pulse dampeners, as
the pulse dampeners may deform under load at
high temperatures. The pulse dampeners should
be placed on top of all system components in
the autoclave.
Note: After autoclaving, check the threaded quick
disconnect fittings on the Streamer® inlet and outlet
and on the pulse dampeners for tightness. If any of
the fittings have become loose, turn them until they
are ¼ turn past finger tight.
If you do not have access to an autoclave you
can use ethylene oxide gas treatment with
subsequent vacuum treatment. 70% ethyl
2

FLEXCELL
®
INTERNATIONAL CORPORATION
alcohol can also be pumped through the system
for cleaning, however, this will not completely
sterilize the system.
S
TREAMER®
I
NCUBATOR
P
LACEMENT IN THE
®
The Streamer
temperature-controlled, CO
system should be placed into a
incubator for
2
experiments. We recommend that the
Streamer® be kept in the incubator for at least
20 minutes before starting an experiment to
assure that the device is at a stable temperature
for cell culture. The pump is placed into the
incubator with the rest of the system. A
containment tray is placed underneath the
system to provide a means to transport the
system to the cell culture hood, and to catch any
fluid should a leak occur. The computer must
not be placed into the incubator.
U
SING THE PUMP
1. Plug the pump into a power outlet (110 V
for North America, 220 V for Europe and
Japan).
2. If using the flow system manually (i.e.
without computer control), ensure that the
correct tubing size is selected on the pump
and the clockwise flow direction. The
standard tubing included with the
®
Streamer
system is MasterFlex L/S 17.
Therefore, you will want to press the “size”
button on the bottom right of the pump face
until the green light is beside the number
“17”.
NOTE: Once you reach the tubing size “25”, the
green light will remain on the same level for two
depressions and the second one will cause the “HP”
LED (near the bottom of the indicator light column)
to light up. The “HP” button specifies the tubing
numbers on the right column of the size listings.
Therefore, when the green light is beside the number
“17” you will want the “HP” light to be off.
Once this is set, the display on the pump
will read the flow rate for that particular
tubing as the pump is running. Consult the
appendix or the data following this manual
to find which flow rate corresponds to the
desired level of shear stress. Use the arrow
keys on the top left of the pump face to
select the required flow rate. Press the blue
start/stop button to initiate and stop flow.
3. If using the system with computer
control, connect the male end of the RS232 cable into the back of the pump and the
USB end into a USB port of the computer.
Turn on the power and start the software.
When the software controller is functional,
“PO1” will appear on the pump display.
Use the software to create a regime with
your desired flowrate(s). See pages 5-18 for
further instructions on using the
StreamSoft™ software.
Q
UICKSTART INSTRUCTIONS
1. Set up the entire system in an incubator. See
page 1 for the system setup.
2. Sterilize the Streamer® and system
components. See page 2 for sterilization
instructions.
3. Connect the cable from the pump to the
serial port of the computer. Turn on the
computer and pump and open up the
™
StreamSoft
software program.
4. Select the Operate menu, then select Users.
Add your name as a user by clicking Add
User, then click the Return button.
5. Select the Operate menu again, then select
Configure Regimes. Type a new name in
the Regime Name field and click on Insert
Step to insert a step into the regime. Create
a regime by entering values in one or more
steps. Once complete, click on Save
Regime. Click Return to exit.
6. On the main screen, click on Configure; this
will open the Pre-Test Configuration
window. Select the appropriate User,
3

FLEXCELL
®
INTERNATIONAL CORPORATION
Regime and Hardware, then click Update.
The regimen is now ready to start.
7. Culture cells on six Culture Slips®. Be sure
that you culture on the side with the Teflon®
rim printed around the borders. Be careful
to plate cells only within this rim. We
recommend allowing cells at least 48 hours
to attach to slides before beginning your
flow experiment.
After cells have attached to slides:
8. Be sure that the Streamer® is closed (the top
lid should be flush with the body of the
device).
9. Place 500 ml of PBS into the medium
container and pump through the system to
flush out impurities. This can be done by
starting your regime or using the manual
mode under the Operate menu in the
software. If you are not using the software,
set the pump to the appropriate tubing size,
set the flowrate at 300 ml/min and press the
start button.
10. After pumping for several minutes, remove
the PBS from the medium container and
replace with 500 ml of sterile tissue culture
medium.
11. Pump the culture medium through the
system to flush out remaining PBS.
Remove the medium and replace with 500
ml of fresh sterile tissue culture medium.
12. Pump the tissue culture medium through
the entire system. Once the system is full,
tilt the pulse dampeners, one at a time, at an
angle of approximately 20 degrees, such
that the direction of the flow is going from
the vertex of the angle to the open end of the
angle. Leave the pulse dampener in this
position until the fluid comes through the
outlet fitting again, then lay the pulse
dampener down horizontally. This process
will allow the pulse dampener to fill to a
level slightly higher than the fittings,
thereby creating a bubble trap for any air
bubbles that may accidentally enter the
system. Do the same with the second pulse
dampener. Once this process is complete,
allow flow to continue and go to the next
step.
13. As the flow continues, check for any air
bubbles visibly trapped within the tubing.
Also check the walls of the medium
container to be sure that no air bubbles have
formed on the sides. If so, swirl the medium
around to release air bubbles from the side
walls.
14. Once the tubing and flow device are filled
with medium and all air bubbles are
eliminated, reverse the flow direction on the
pump to draw the medium level back to the
flow chamber and down past the head of the
chamber, then stop the pump. The fluid
level will have to be estimated once the
fluid can no longer be seen in the tubing
coming from the head of the Streamer®.
15. Tighten the small clamp on the Phar-Med®
tubing just to the right of the pump head so
that the flow path in the tubing is
completely closed off.
16. Turn the lever arm on the MasterFlex
pump all the way to the left to release the
tubing and remove the tubing from the
pump head. Carefully move the tray
containing the Streamer® device, tubing,
pulse dampeners, and fluid collection
reservoir to the tissue culture hood.
®
17. Remove the Streamer
screws and open the
hinged top.
18. Transfer your cells from the incubator to the
tissue culture hood.
19. Using forceps and/or your fingers with
sterile gloves, pick up each Culture Slip®
and place it into each one of the slots in the
flow device. Be sure that the side of the
slide with cells attached is facing the flow
area adjacent to the slot, not the closed
wall of the slot. Gently slide each Culture
Slip® downward until it reaches the bottom
of its chamber. Be careful that the Culture
®
Slip
glass is not chipped against the
stainless steel surface during this process.
4

FLEXCELL
®
INTERNATIONAL CORPORATION
All six slots must be filled to ensure proper
flow rate readings. If you do not wish to
®
use all six Culture Slips
blank Culture Slips
with cells, use
®
for the remaining
slots.
20. Close the top of the device, turn the bolts by
hand, then tighten them with the hex
wrench provided with the system.
21. Move the tray with the system components
back to the incubator. Put the Phar-Med®
tubing back into the MasterFlex® pump
head and clamp the head down.
22. Unscrew the small clamp on the Phar-Med®
tubing to open the flow path to full capacity.
23. Click the Start button in the software (or set
the pump to the desired flowrate and
depress the start button). Your regimen will
start and a green light will go on at the top
right corner of the screen.
24. The expected shear stress and actual value
will be displayed on the graph in real time.
Periodically monitor the flow system for
leaks during the protocol.
25. When the flow regimen is over and the
pump has stopped, remove the Streamer®
system from the incubator as before. Open
the top and remove the slides for
processing.
26. Clean the Streamer® and system with
deionized water. Never leave culture media
in the Streamer® device after an
experiment, as this will corrode the
stainless steel finish over time.
See the instructional video, Streamer®
Assembly, on Flexcell®’s website
(http://www.flexcellint.com/videosinstruct.htm) for a demonstration of how to
assemble the Flexcell® Streamer® and
associated tubing to run with a MasterFlex
Peristaltic Pump.
2. STREAMSOFT™ V4.2 SOFTWARE
I
NSTALLATION INSTRUCTIONS
1. Insert the StreamSoft™ V4.2 DVD into the
DVD-ROM drive on the computer.
2. Double click My Computer (Windows
XP) or Computer (Windows
Vista/Windows 7).
3. Double click the DVD-ROM drive.
4. Double click the Setup installer.
5. The installer will now open and run.
6. On the Product Notification screen, click
Next.
7. On the Destination Directory screen, click
Next.
8. On the License Agreement screen, click I
accept the License Agreement and then
click Next.
9. On the next License Agreement screen,
click I accept the above 2 License
Agreement(s) and then click Next.
10. On the Start Installation screen, click Next.
11. Installation of the required National
Instruments and StreamSoft™ software
will now begin.
12. Once the installation is complete click
Finish and restart the computer.
™
13. Installation of StreamSoft
V4.2 is now
complete.
NOTE: When the Select Pump to Use window
appears when opening the StreamSoft™ V4.2
software, select the pump named MasterFlex
Peristaltic Pump to ensure correct function of the
equipment.
S
ETTING UP PARAMETERS IN
S
TREAMSOFT™
V4.2
Specific parameters will need to be set up in
™
StreamSoft
V4.2 to customize it for your
particular device and system. Setting up
these parameters is extremely important to
5

FLEXCELL
®
INTERNATIONAL CORPORATION
ensure accurate flow results for your
system. For instructions on setting up these
parameters, see Configure Testing Apparatus
MAIN PANEL
G
ENERAL INFORMATION TAB
and Configure System Variables, pages 15-
17. Complete this setup before proceeding
with any experiments.
Function: The default main panel allows the user to verify that the system is running and to stop the
tests at any time.
Buttons and Fields
Status
System 1,2,3,4
General Information
Number is bright green when an experiment is running
These tabs will automatically become highlighted according to the
number of pumps connected to the computer (1 - 4).
Current date and time
6
FLEXCELL
®
INTERNATIONAL CORPORATION
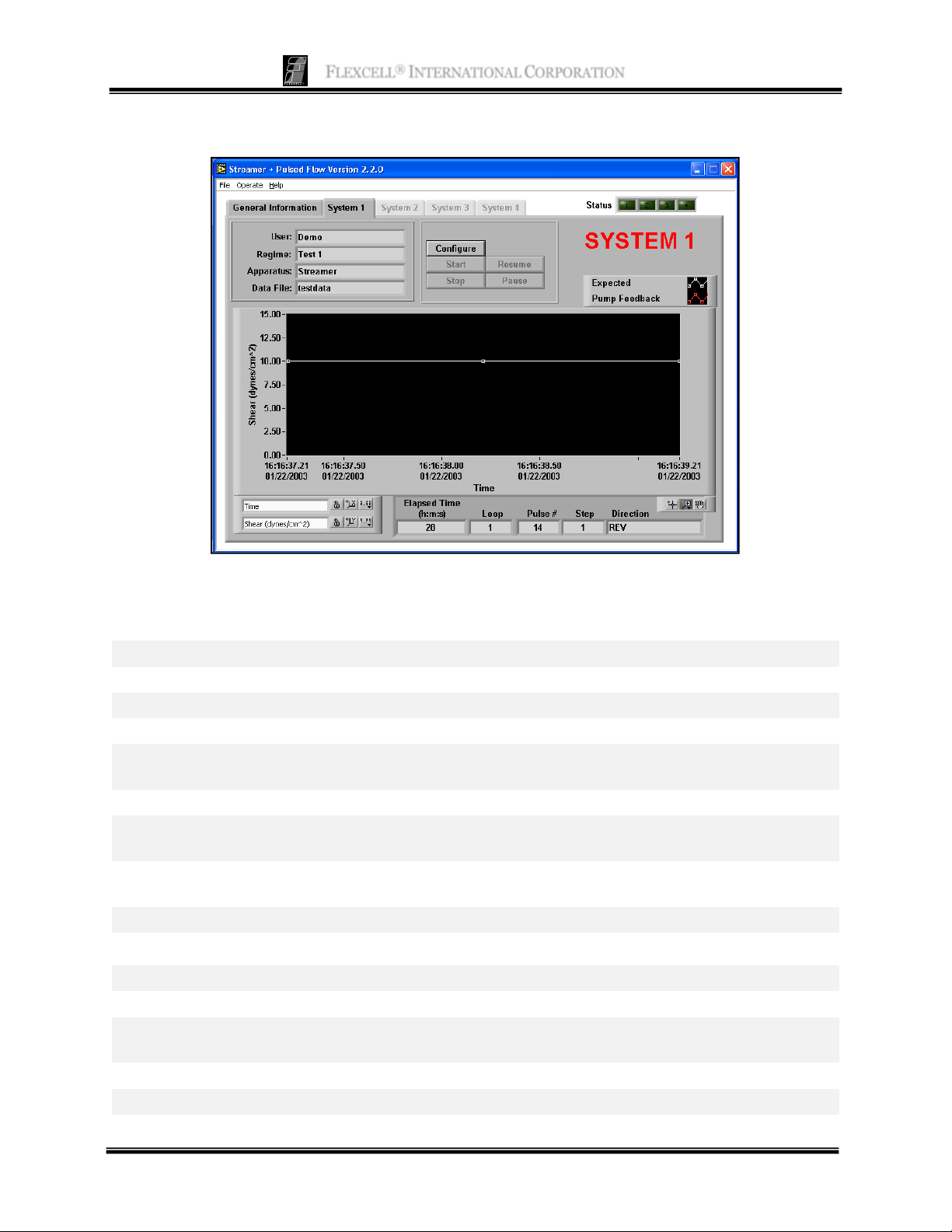
S
YSTEM TAB
Function: This panel is used to run the experiments. Each System tab is identical.
Buttons and Fields
User
Regime
Apparatus
Data File
Configure
Start
Stop
Pause
Resume
Graph
Elapsed Time (h:m:s)
Loop
Pulse #
Step
Direction
Current user in currently configured regime
Regime currently configured
Device being used with the software (Streamer® or FlexFlow™)
Name of file to which data is being saved (if appropriate)
Configure (load) the experiment. The Pre-Test Configuration window will
appear.
Start the experiment.
Terminate the experiment. This button is only active when an experiment is
running.
Suspend the experiment. The pump will stop, but the test regimen is kept in
memory.
Resume a paused experiment.
This graph shows the expected and actual shear stresses during the experiment.
Elapsed time in the current experiment
Current loop in the current step or series of steps
Total number of pulsations (square wave) or oscillations (FWD/REV)
produced by the valves in this regime
Current (active) step in regime
Current flow direction (FWD/REV)
7

FLEXCELL
®
INTERNATIONAL CORPORATION
PRE-T
EST CONFIGURATION
Function: This panel allows the user to configure the parameters of an experiment. It appears when the
user presses the Configure button on the System panel. The information selected here is transferred to
the User, Regime and Apparatus fields on the System panel.
Buttons and Fields
Users
Regimes
List of all users. Select users with the mouse.
List of regimes created by the previously selected user. Select from list by using
the mouse to highlight the desired regime.
List of configured flow devices. Select the device that will be used for the
Apparatus
experiment. Important: Be sure that all parameters have been properly set for
your device in the Configure Testing Apparatus window (see page 15).
Print
Update
Cancel
Help
Print the current panel to a printer or HTML file.
Use the current selections to run the experiment.
Cancel any new selections and use the previously configured setup for the
experiment.
Online help (not currently available)
8

FLEXCELL
®
INTERNATIONAL CORPORATION
P
ULL-DOWN MENUS
This section summarizes the function of each item in the three pull-down menus.
File
Print
Allows user to print a copy of the current panel. This system is configured such that printing
sends a copy of the panel being viewed to a printer or to an html file. If there is no printer
connected to the computer, an error message from the Windows default printer queue will
appear when the user tries to print.
File Operate Help
-Print -Manual Mode -Help
-Exit -View Data -About LabVIEW
-Users
-Configure Regime
-Configure Apparatus
-Configure System
-Reinitialize Hardware
Exit
Allows user to close the program. If the pump is operating at the time of exit, it will continue
running. The keyboard short-cut is Ctrl-Q.
Operate
Manual Mode
View Data
Users
Configure Regime
Configure Apparatus
Configure System
Reinitialize Hardware
Help
Help
Manually control the pump without setting up an experimental regimen.
View shear stress data from a previous experiment.
Add and remove user names.
Create an experimental protocol.
Configure parameters of the flow device so that the software can assign the
flow rates corresponding to the desired shear stress. These parameters
must be set correctly to ensure that the proper shear stress values are
shown. See the manual of your device for the appropriate values.
Configure the system parameters such as data saving, the Com port used and
®
the presence or absence of valves in the system (Osci-Flow
).
This will reinitialize the software to connect the pump and Osci-Flow® (if
present) in the event that a cable is disconnected or the pump is turned off.
Online help (not currently available)
About LabVIEW
Software version information
9

FLEXCELL
®
INTERNATIONAL CORPORATION
OPERATE MENU
M
ANUAL MODE
Function: This panel allows the user to manually control the pump. The actual flow rate and speed of
the pump (RPM) are shown on the graph when the pump is working. Manual mode may be used to
troubleshoot the pump operation. The shear stress value is not shown on this panel since it will depend
on the tubing size and flow chamber used.
Instructions
1. Enter the flow set point (pump speed) either by entering a number in the box or using the mouse to
drag the dial to the desired level.
2. Adjust the seconds between readings to a number between 0 and 5. This is the time between each
update of the pump data on the graph.
3. Click on Press to Start.
4. Click on Press to Stop when ready to stop.
5. Click on Return when done.
10

FLEXCELL
®
INTERNATIONAL CORPORATION
V
IEW DATA
Function: This panel allows the user to view previously collected experimental data in a table format.
Buttons and Fields
File
Table
Export File
Print
Open
Return
Help
The complete file path to the data file being viewed
Contents of the experimental data log file
Export data to a spreadsheet-compatible format
Print a copy of this panel to the Windows default printer or write a copy
to an HTML file.
Open a data file.
Close this panel and return to the Main panel.
Online help (not currently available)
11

FLEXCELL
®
INTERNATIONAL CORPORATION
C
ONFIGURE USERS
Function: This panel allows the user to create or delete users.
Buttons and Fields
Existing Users
User Name
Add User
Delete User
Help
Return
Lists all current users of the system
Field used to enter new users
Add new users to the system.
Delete users from the system.
Online help (not currently available)
Exit this panel and return to the Main panel.
Instructions
To add a user:
1) Type the name into the User Name field.
2) Press the Add User button.
To delete a user:
1) Using the mouse, select the user from the list of Existing Users.
2) Press the Delete User button. If the user has any stored regimes and data sets, the operator will
be prompted to confirm the deletion.
12

FLEXCELL
®
INTERNATIONAL CORPORATION
C
ONFIGURE REGIMES: SETUP PARAMETERS
Function: This panel allows the user to configure (create) a regime.
Buttons and Fields
Existing Users
Regimes for
Selected Users
Regime Name
Time Between
Pump Updates
List of all users; select a user from the list using the mouse.
List of regimens created by the current user. Selecting from this list will
load that regimen and allow the user to view and/or modify that regime.
Name of the current regimen; if creating a new regimen, enter a name in
this field.
Time elapsed between computer updates of the pump parameters; default
is 1 second.
Time interval between each computer sampling of the experimental flow
Time Between
Data Log to File
data. Default value is 10 seconds. For an extremely long test, increase
this interval to reduce the size of the data file.
NOTE: this function only applies when the data saving option is selected in the
Configure System Variables window (see page 17).
This is an estimate of how large the data file would be given the total test
Estimated file
length and the time between data.
size
NOTE: this function only applies when the data saving option is selected in the
Configure System Variables window (see page 17).
Step
Step Name
Current step number selected or being modified
Name of the currently selected step
13

FLEXCELL
®
INTERNATIONAL CORPORATION
Flow Type
ON/HI (s)
OFF/LO (s)
Shear
(dyne/cm^2)
Duration
(h:m:s.ss)
GoTo
Loop
Summary Table
Specifies the direction or type of flow for this step (forward, reverse,
pulsed (square wave), oscillation)
When using pulsed (square wave) or oscillatory flow, specifies how long
the valves remain in a position to allow the fluid flow to continue
unhindered or flow in the forward direction, respectively. For normal
forward or reverse (unidirectional) flow, this value remains at 1.00.
When using pulsed (square wave) or oscillatory flow, specifies how long
the valves remain in a position to stop the fluid flow to the device or
cause it to flow in the reverse direction, respectively. For normal forward
or reverse (unidirectional) flow, this value remains at 1.00.
The value of shear stress to be applied to the cells in this step.
Time to spend in this step (hours:minutes:seconds.milliseconds)
To create a loop, indicate which step to go back to. The GoTo step must
always be a step number before the current step.
Indicates how many times to loop between the GoTo step and the current
step.
This table is a listing of the current steps in the regimen. Selecting a row
from this table will allow the parameters of the step to be viewed and
modified.
Insert Step
Delete Step
New Regime
Delete Regime
Save Regime
Return
Check Shear
Print
Help
Insert a step into the regimen before or after the current step.
Delete the currently selected step.
Clear all parameters and start a new regime. Type in a new name under
Regime Name and select Insert Step.
Delete the currently selected regime.
Save a new or modified regime.
Exit this panel and return to the Main Panel
Check the shear stresses entered in your regime to see if they are
achievable with the apparatus, pump and tubing size that you are using.
Print the current panel to a printer or an HTML file.
Online help (not currently available)
Instructions on how to set all the parameters for an experiment are included in the Doing an Experiment
section of this manual.
14

FLEXCELL
®
INTERNATIONAL CORPORATION
C
ONFIGURE TESTING APPARATUS
Function: This panel allows the user to create, modify or delete a testing apparatus (Streamer® or
FlexFlow flow chamber).
As each Streamer® and FlexFlow device is manufactured to strict dimensional specifications,
the values for the height and width of the chambers must be entered into the software for each
individual device.
These values are measured for your specific device and must be correct for accurate shear stress
measurement. The values can be found in the appendix of the manual for your device.
Buttons and Fields
Testing Apparatus
Name
Flow Factor
List of all flow devices available
When a testing apparatus is selected, this field (and the parameters)
will be updated.
A factor that accounts for any parallel paths in the flow stream. This
number is 6 for the Streamer® and 1 for the FlexFlow.
Hose size determines how fast the pump must move to achieve the
Hose Size
desired flow rate and shear stress level. The sizes listed are standard
for Masterflex
tubing. Select the hose size that you are using with
your system.
Width of the flow area (cm) in a single chamber of the Streamer® or
b
FlexFlow device. This number is found in the back of the manual
for your device listed as Flow Area Width (cm).
15

FLEXCELL
®
INTERNATIONAL CORPORATION
Height of the flow area (cm) in a single chamber of the Streamer® or
h
FlexFlow
device. This number is found in the back of the manual
for your device listed as Flow Area Height (cm).
Viscosity
Print
Save Apparatus
Delete Apparatus
Help
Return
Viscosity of the perfusate/media used in the experiment. The
standard value is 0.01.
Print the current panel to a printer or an HTML file.
Save changes to the apparatus listed in Name.
Deletes apparatus listed in Name.
Online help (not yet available)
Exit this panel and return to the Main panel. Any changes that have
not been saved will be discarded.
H
OW TO ENTER THE PROPER VALUES FOR YOUR DEVICE
Please check the Appendix of the manual for your device for the proper b and h values.
1. Select the Testing Apparatus being used for the experiment or enter a name for a new apparatus in
the Name box.
2. Enter the correct flow factor for your device. This specifies the number of parallel flow chambers
in your device.
3. Select the correct Hose Size for the type of Masterflex tubing being used in the experiment.
4. Enter the proper b and h values for your device.
5. Enter the Viscosity of the perfusate fluid used in the experiment. The default value is 0.01
dynes*s/cm2.
6. Click Save Apparatus button, then click Return to exit this screen.
16
FLEXCELL
®
INTERNATIONAL CORPORATION
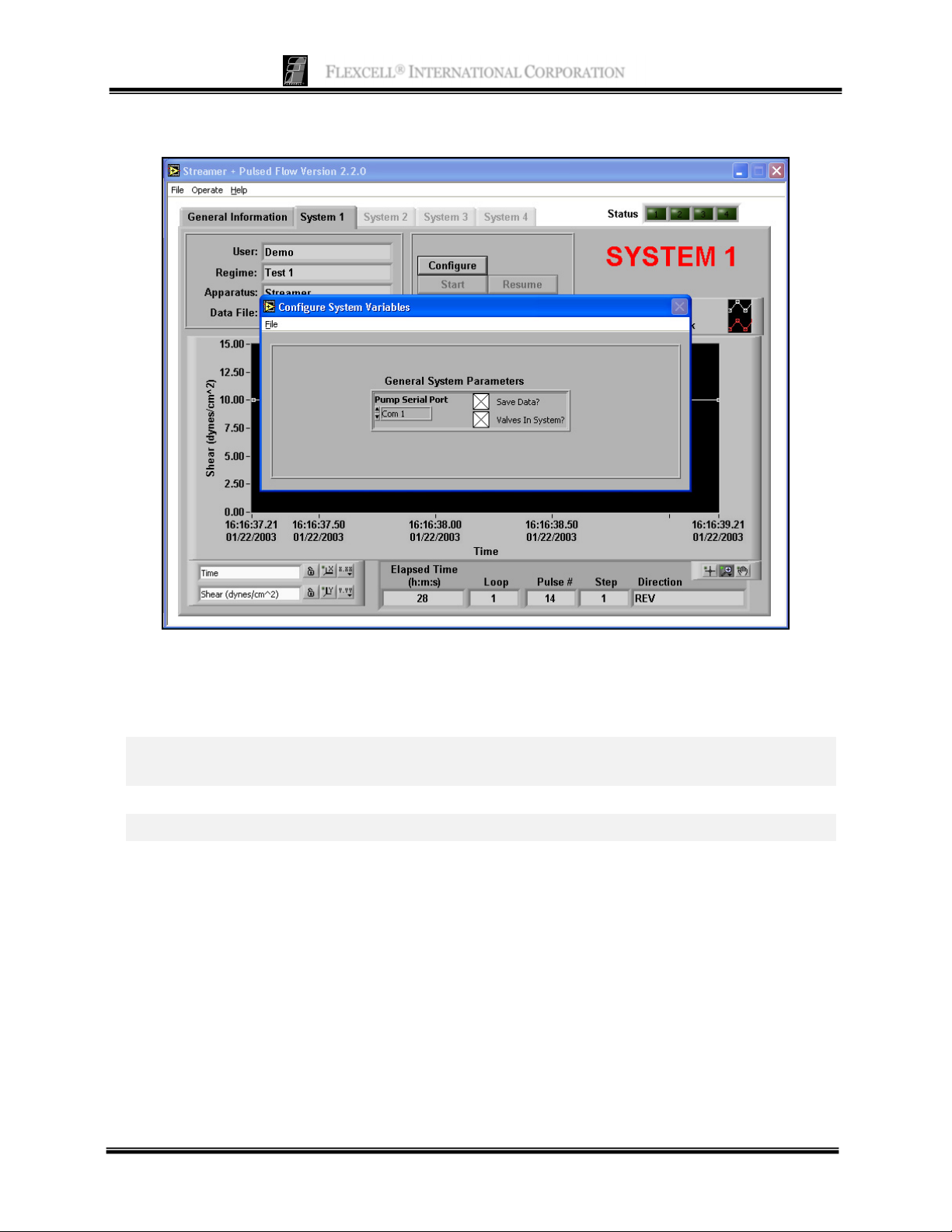
C
ONFIGURE SYSTEM VARIABLES
Function: This panel is used to select three system parameters – Communications port, data saving,
and the presence of valves in the flow system.
Buttons and Fields
Pump Serial Port
Save Data?
Valves in System?
Com 1 is the default port. This should be changed only if there is a
conflict with this port on your computer.
Select this option if you want to save regime data files.
Select this option if you are using the Osci-Flow® Flow Controller.
17
FLEXCELL
®
INTERNATIONAL CORPORATION
R
EINITIALIZE HARDWARE
Function: This panel will appear when the computer program is first started. It will also appear when
the Reinitialize Hardware item is selected in the Operate menu. When the system is properly initiated,
the pump will display PO1. If power to the pump is cycled during experimentation, or communication
is lost, the user should reinitialize the hardware before turning off the program and starting it again.
STREAMSOFT™ V4.2 NOTES
™
When running a regime in StreamSoft
The communication timing to the pump and Osci-Flow® requires full CPU availability. If another
program or operation is running that requires CPU power, it is possible that the pump or valve
timing could be interrupted. This effect may be noticeable when using the Osci-Flow® at a higher
frequency than 2 seconds on, 2 seconds off, or when oscillating the pump speed to create pulsatile
flow.
When the Select Pump to Use window appears when opening the StreamSoft
select the pump named MasterFlex Peristaltic Pump to ensure correct function of the
equipment.
V4.2, do not run other applications on the same computer.
™
V4.2 software,
18

FLEXCELL
®
INTERNATIONAL CORPORATION
3. DOING AN EXPERIMENT
O
VERVIEW
There are two major components to running an
experiment with the Streamer® system:
configuration of the pump software and
preparation of the flow chamber with Culture
®
and cells.
Slips
We suggest that the following steps be done:
1. Culture cells on Culture Slips
the Streamer® system components.
2. Configure a regimen in the StreamSoft
program.
3. Prepare the Streamer® system with media,
then place the Culture Slips® into the
chambers of the Streamer® device in a
sterile environment such as a laminar flow
hood. Move the system to the incubator. Be
sure that you have placed six Culture
Slips® into the device, as any less will
invalidate the shear stress values. Use
blank Culture Slips® if necessary.
4. Assign the user, regime and apparatus for
the experiment.
5. Start the experiment.
6. When the experiment is finished, remove
the Culture Slips® from the Streamer® flow
device and proceed to analyze the cells.
The processes of creating a regimen and placing
the slides into the flow device are described in
further detail in this section.
C
REATING A REGIME
1. From the main panel, select the Configure
Regimes item in the Operate menu.
2. Click on an existing user name.
3. To create a new regimen, click on New
Regime. Enter a name in the Regime Name
field.
4. Click Insert Step; give this step a name in
the Step Name field.
®
and sterilize
5. Click on Save Regime. The regime name
should appear in the Regimes for Selected
Users field at the top.
6. Specify the Flow Type (FWD, REV,
PULSED, OSCILLATION), ON/HI &
OFF/LO times (only when using the pulsed
or oscillation functions; see pages 13-14 for
more details), Shear, and Duration for this
step. Click on Save Regime to save all
information entered up to this point.
NOTE: The Flow Type can only be specified when
using the Osci-Flow® device-- without the ‘Valves in
System’ selection used with the Osci-Flow®, the
Flow Type will remain on FWD. See page 17 for
more details.
If you wish to add additional steps:
1. Click Insert Step. You will be queried as to
whether this step should be inserted before
or after the current step. Click on before or
after according to your preference. Enter
the preferred parameters as in #6. If this step
was inserted after the step entered in #6, you
can also use the GoTo and Loop options to
loop through steps 1 and 2. Under GoTo in
step 2, enter “1”. Under Loop, enter the
number of times that you would like to loop
through steps 1 and 2.
2. Add additional steps as desired. Once the
regime is complete, click on Save Regime.
3. Optional: Check the shear stress(es) in
your regime to be sure that they are
achievable with your apparatus, tubing size
and pump. Click on Check Shear at the
bottom of the Configure Regimes window
while your regime is selected (see page 13).
The Pre-Test Configuration window will
appear (see page 8). Select a user, regime,
and apparatus. Click on Update. The
software will tell you if your shear stresses
are achievable with this apparatus and the
19

FLEXCELL
®
INTERNATIONAL CORPORATION
tubing size and pump assigned to it. Modify
shear stresses if necessary.
4. The regime is now ready to run.
S
ETTING UP AN EXPERIMENT
1. Set up the system in an incubator according
to instructions on page 2.
2. Sterilize the Streamer
®
unit according to
instructions on page 2. Close the lid and
tighten screws until the lid is flush with the
body of the device.
3. Place the Streamer® in the incubator with
the remainder of the system. This will keep
the temperature of the unit at 37°C.
4. Culture cells on 6 Culture Slips®. Be sure
that you culture on the side with the brown
Teflon® rim printed around the borders.
Be careful to plate cells only within this rim.
Allow cells at least 48 hours for full
attachment to slides.
5. Create your regime in the StreamSoft
software.
After cells have attached to slides:
1. Put one bottle of PBS into the system
medium container.
2. Pump the PBS through the system to flush
the tubing and Streamer® device, then
discard the perfusate; this is done to remove
any cytotoxic substances that may have
accumulated during sterilization.
3. Put 500 ml of medium into the medium
container (this may be adjusted later as you
determine your system volume
requirements).
4. Flow the medium through the system to
flush out remaining PBS. Remove medium
and replace with 500 ml of fresh sterile
tissue culture medium.
5. Pump medium through the entire system to
fill the flow device and tubing. Once the
system is full, tilt the pulse dampeners, one
at a time, at an angle of approximately 20
degrees, such that the direction of the flow
is going from the vertex of the angle to the
open end of the angle. Leave the pulse
dampener in this position until the fluid
comes through the outlet fitting again, then
lay the pulse dampener down horizontally.
This process will allow the pulse dampener
to fill to a level slightly higher than the
fittings, thereby creating a bubble trap for
any air bubbles that may accidentally enter
the system. Do the same with the second
pulse dampener. Once this process is
complete, allow flow to continue.
6. As the flow continues, check to be sure that
no air bubbles are visibly trapped within the
tubing. Also check the walls of the medium
container to be sure that no air bubbles have
formed on the sides. If so, swirl the medium
around to release air bubbles from the side
walls.
7. Once the tubing and flow device are filled
with medium and all air bubbles are
eliminated, stop flow, then reverse flow so
that the medium is drawn down to about
80% of the Streamer® body. The fluid level
will have to be estimated once the fluid
flows past the Streamer® outlet fitting.
When the fluid reaches this level, stop the
flow again.
8. Tighten the clamp on the Phar-Med® tubing
just to the right of the pump head so that the
flow path in the tubing is completely closed
off.
9. Turn the lever arm on the MasterFlex
pump all the way to the left to release the
tubing and remove the tubing from the
pump head. Carefully move the tray
containing the Streamer® device, tubing,
pulse dampeners, and fluid collection
reservoir to the tissue culture hood.
10. Remove the Streamer
®
screws and open the
hinged top.
11. Transfer your cells from the incubator to the
tissue culture hood.
12. Using forceps and/or your fingers with
sterile gloves, grasp a Culture Slip® at one
20

FLEXCELL
®
INTERNATIONAL CORPORATION
end. Be careful not to stimulate or crush any
cells on the slide.
13. Place the Culture Slip® into one of the slots
in the Streamer® device. Be sure that the
side with cells attached is facing the flow
area (the shorter slot parallel and adjacent
to the slide slot). Be careful not to chip the
glass against the stainless steel surface.
14. Repeat this for the other Culture Slips®,
making sure the surface with the cells all
face the proper direction in the flow device.
NOTE: All six slots must be filled to ensure proper
flow rate readings. If you do not wish to use all six
Culture Slips® with cells, use blank Culture Slips®
for the remaining slots.
15. Once all Culture Slips® are in the Streamer®
unit, close the lid and tighten the screws
using the hex head tool provided with the
system.
NOTE: As you are moving the Streamer® from this
point on, always position the device vertically such
that the inlet connector is at the bottom and the
outlet connector is at the top.
If you wish to run the Streamer
the remainder of the system with fluid so that all air
is completely out of the Streamer® chamber.
Be aware that any air that accidentally enters the
system may eventually form a dry area at the
topmost slide so that these cells will no longer see
fluid media and shear stress.
Be sure that your system does not regularly see
additional air bubbles (after initial filling and air
bubble elimination) before using the Streamer® on
its side.
®
on its side, first fill
16. Move the tray with the system components
back to the incubator. Put the Phar-Med®
tubing back into the MasterFlex® pump
head and clamp the head down.
17. Unscrew the clamp on the Phar-Med
®
tubing to open the flow path to full capacity.
18. If you are running the experiment manually,
set the pump to the desired flow rate and
press the start button. If running the
experiment under software control, go to
the System tab, click on the Configure
button, assign the User, Regime and
Apparatus. Click Update, then Start.
19. Once the experiment is over, move the
Streamer® back to the tissue culture hood
and remove the slides.
20. Once the slides are removed, place the
Streamer® back into the incubator and run
deionized water through the system to
remove all remaining media. Refresh the
deionized water and run a second or third
time if necessary. Be sure to never leave the
Streamer® with culture media inside as
this will corrode the stainless steel finish
over time.
F
ILLING THE SYSTEM TO ELIMINATE AIR
B
UBBLES
Before using the Streamer® system with cells,
all of the tubing must be filled with media and
all air bubbles removed. To fill the system,
create a regime with two steps, the first in FWD
mode and the second in REV mode. Each step
should be 2 minutes at a shear stress level ½ that
of which your device is capable. This will give
sufficient time for fluid to fill all of the tubing.
As you notice air bubbles in the silicone tubing
at different locations, shake the tubing to release
the air bubbles.
P
OST-EXPERIMENT ANALYSIS
Upon removal of the slides from the flow
device, many post-flow evaluations can be
done.
®
Cells on the Culture Slips
can be fixed
with formalin then permeabilized and
stained with rhodamine Phalloidin and
DAPI to visualize cell alignment.
21

FLEXCELL
®
INTERNATIONAL CORPORATION
Cells can be lysed with appropriate buffer
to collect total RNA or intracellular
proteins.
®
The Culture Slips
can be returned to their
original culture vessel for further incubation
and subsequent collection of cell
supernatant. The medium can then be
assayed for released effector molecules.
The cells can be trypsinized for replating or
counting.
APPLICATION NOTES
C
ULTURING CELLS ON CULTURE SLIPS
®
Culture Slips
are Teflon®-bordered 75 x 25 x 1 mm glass culture surfaces that are either untreated or
bonded with peptides of collagen, elastin, fibronectin (RGD repeat as Pronectin F), laminin (as the
YIGSR peptide). The Teflon
®
border provides a means to culture cells only in the flow area. Bonded
peptides increase cell attachment.
Cells are plated on the growth surface at 10-25,000 cells/cm2 in 3 to 5 ml of medium. Be sure to plate
cells on the side where the Teflon® border is printed. Once the cells are attached, additional medium
is added and the culture vessel placed into a CO2 incubator at 37°C. Once the cells have grown to
confluence (normally 48 hours), the Culture Slips® are removed and inserted into the Streamer® flow
device for the experiment. Once the flow experiment is over, the Culture Slips® can be returned to their
original culture vessel to allow the measurement of secreted molecules post-flow.
If you experience cell detachment problems during flow regimes, try the following protocol for better
cell attachment to the Culture Slips®.
1. Plate ½ of the normal amount of cells on the Culture Slips®.
2. Reduce the media serum concentration (5% preferably) to slow the cell growth rate. This will
give the cells time to make their own protein matrix which will improve attachment.
3. Allow the cells to grow to near confluency (4-5 days).
®
22
FLEXCELL
®
INTERNATIONAL CORPORATION
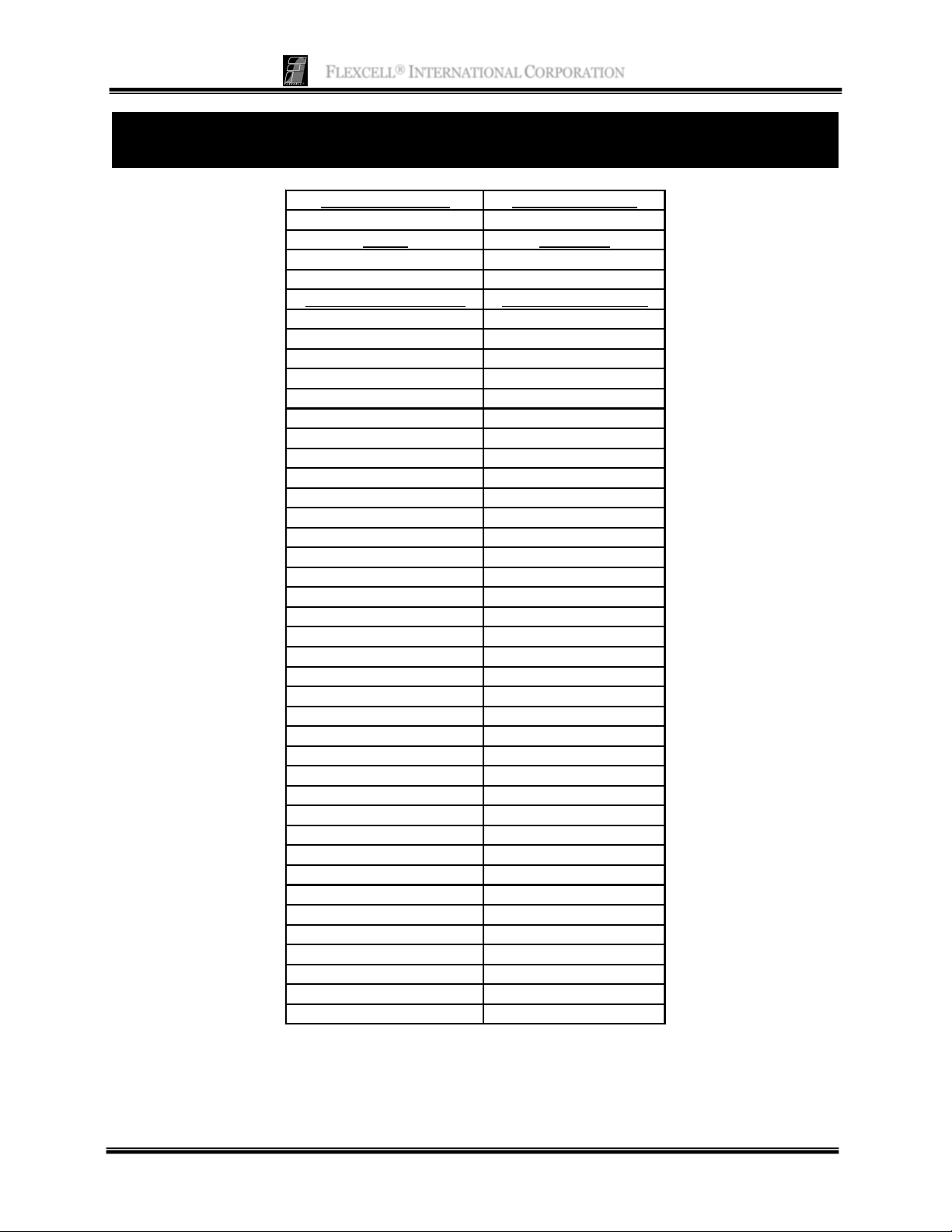
APPENDIX: PARALLEL STREAMER® SHEAR STRESS
NUMBERS
Flow Area Height(cm) Flow Area Width(cm)
0.0513 2.3396
Serial # Flow Factor
SGS-1109 6
System Flow Rate (ml/min) Shear Stress (dyn/cm^2)
00.0
37 1.0
74 2.0
111 3.0
148 4.0
185 5.0
222 6.0
259 7.0
296 8.0
332 9.0
369 10.0
406 11.0
443 12.0
480 13.0
517 14.0
554 15.0
591 16.0
628 17.0
665 18.0
702 19.0
739 20.0
776 21.0
813 22.0
850 23.0
887 24.0
924 25.0
961 26.0
997 27.0
1034 28.0
1071 29.0
1108 30.0
1145 31.0
1182 32.0
1219 33.0
1256 34.0
1293 35.0
23

FLEXCELL
®
INTERNATIONAL CORPORATION
WARRANTY INFORMATION
1. FLEXCELL INTERNATIONAL CORPORATION warrants to the original purchaser/customer all hardware components of the
Streamer
defects in workmanship or materials with the following exceptions, terms and conditions:
a. ITEMS EXCLUDED FROM THE WARRANTY ARE: software, disks, manuals and external peripherals such as printers,
mouse or track ball units, imaging devices, vacuum pumps, air tanks, electric voltage converters, compressors, surge suppressers and all
other accessory equipment.
b. DURING THE WARRANTY PERIOD, the purchaser/customer must notify Flexcell of any warranty claim in writing, by
telephone, fax transmission or email identifying each defective part or specifically describe the exact problem no later than the last day the
warranty is in effect.
c. FLEXCELL AGREES to correct any defect in workmanship or material and supply new or rebuilt parts in exchange for
defective parts upon completion and submission by purchaser/customer of a printed “Parts Return Authorization” form furnished by Flexcell.
Parts must be properly packed in original container and shipped to our factory service center or distributor with all shipping costs prepaid if
the unit is out of warranty coverage. If the original shipping box is not available, Flexcell will send the required protective shipping container.
(Flexcell will recommend the insurance value for parts or equipment to be shipped.) Return carrier shipping costs will be paid by Flexcell
from the service center. The purchaser/customer is solely responsible for payment of custom fees, taxes, holding fees or value added taxes.
d. THIS LIMITED WARRANTY only covers failures due to defects in materials or workmanship which occur during normal
use. It does not cover damage which occurs in shipment or failures of original equipment due to products identified as add-ons not
manufactured by Flexcell International Corporation or its distributors nor does this limited warranty cover damages or failures which result
from accident or disaster such as fire, explosion, flood, wind, lightning, or earthquake or misuse, abuse, neglect, mishandling, misapplication,
alteration, faulty installation, modification or service by anyone other than our factory or distributor. This warranty is extended only to the
original purchaser/customer unless a transfer of ownership is approved by Flexcell in writing.
e. LIMITED LIABILITY. Flexcell or its distributor’s only liability shall be to remedy any defect to comply with its warranty
and return the repaired equipment to function as designed. Under no circumstances shall Flexcell or its distributors be liable for any special
incidental or consequential damages based upon breach of warranty or contract or negligence. Such damages include, but are not limited to:
loss of profits, revenue, loss of data, down time, customer’s material or time.
f. DISCLAIMER OF WARRANTIES: The Limited Warranty expressed in the foregoing language is the only warranty
applicable to this product. Any other warranty, expressed or implied warranty or of merchantability or fitness for a particular purpose are
hereby disclaimed. No oral or written information or advice provided by Flexcell, through its agents or employees, in the use and functioning
of the equipment shall in any way create a warranty or in anyway increase the scope of this limited warranty.
g. DISCLAIMER: LANGUAGE. This warranty document, accompanying instruction manual and supplemental applicable
laws appear in the English language. In the event of any inconsistency in the meaning of the words and terminology and any foreign language
translation, the English language shall prevail.
2. GOVERNING LAW. The performance of the duties and liabilities of the parties under the terms and conditions of this Limited
Warranty shall be governed in all respects by the laws of the Commonwealth of Pennsylvania, the United States of America.
APPLICATION OF STATE LAWS: Some states do not allow the exclusion or limitation of consequential damages nor do some states
allow limitations on how long an implied warranty lasts, so the above limitations may not apply to you. This warranty gives you specific
legal rights and you may also have other rights which vary from state to state.
3. INTERNATIONAL CUSTOMERS. The full text of the foregoing limited warranty and all disclaimers is applicable to international
customers/purchasers except when the purchase was made from an international distributor or reseller, the warranty will be covered through
your distributor or reseller.
If technical advisory support service is not available through your distributor or reseller, for service contact warranty headquarters by phone
or fax.
®
Shear Stress System for a period of one year from the date of delivery to the purchaser/customer to be free from manufacturing
Within the United States only - Toll Free 1-800-728-3714 - Fax: 1-919-732-5196
Email : info@flexcellint.com
Issued April 2011
24

North America
Flexcell International Corporation
2730 Tucker Street, Suite 200
Burlington
NC 27215
USA
Phone: 919-732-1591
800-728-3714 (USA only)
Fax: 919-732-5196
Email: info@flexcellint.com
Web: www.flexcellint.com
Europe
Dunn Labortechnik GmbH
Thelenberg 6
56567 Asbach
Germany
Phone: +49-2683-43094
Fax: +49-2683-42776
Email: info@dunnlab.de
Web: www.dunnlab.de
Japan
LMS CO. LTD.
3-6-7, Hongo,
Bunkyo-ku, Tokyo 113-0033
Japan
Phone: +81-3-5842-4171
Fax: +81-3-5842-4180
Email: intldpt@lms.co.jp
FLEXCELL
®
INTERNATIONAL CORPORATION
CONTACTING FLEXCELL
Taiwan
Nature Opera Biotechnology, Inc.
9F-2, No.70 Sec.4, Cheng Kung Rd.
Nei-Hu Dist.
Taipei
Taiwan
Phone: +886-2-27905097
Fax: +886-2-27931322
Email: nobio@seed.net.tw
Brazil
Sellex, Inc.
5225 Wisconsin Ave, NW
Suite 306
Washington, DC 20015
Phone: 5506-4646
Fax: 5505-7433
Web: www.sellex.com
China, Hong Kong, Malaysia
Bio Excellence International Tech Co., Ltd
Email: slby800@yahoo.com
Web: www.bio-goods.com
South Korea
Lee Baeg Scientific Co., Ltd.
Web: www.lbscience.com
NOTICE
The information in this document is subject to change without notice. Flexcell International
Corporation assumes no responsibility for any errors that may appear in this guide. This manual is
believed to be complete and accurate at the time of publication. In no event shall Flexcell International
Corporation be liable for incidental or consequential damages in connection with or arising from the
use of this manual.
25
 Loading...
Loading...