Flame NebulAIR+ Instructions For Use Manual
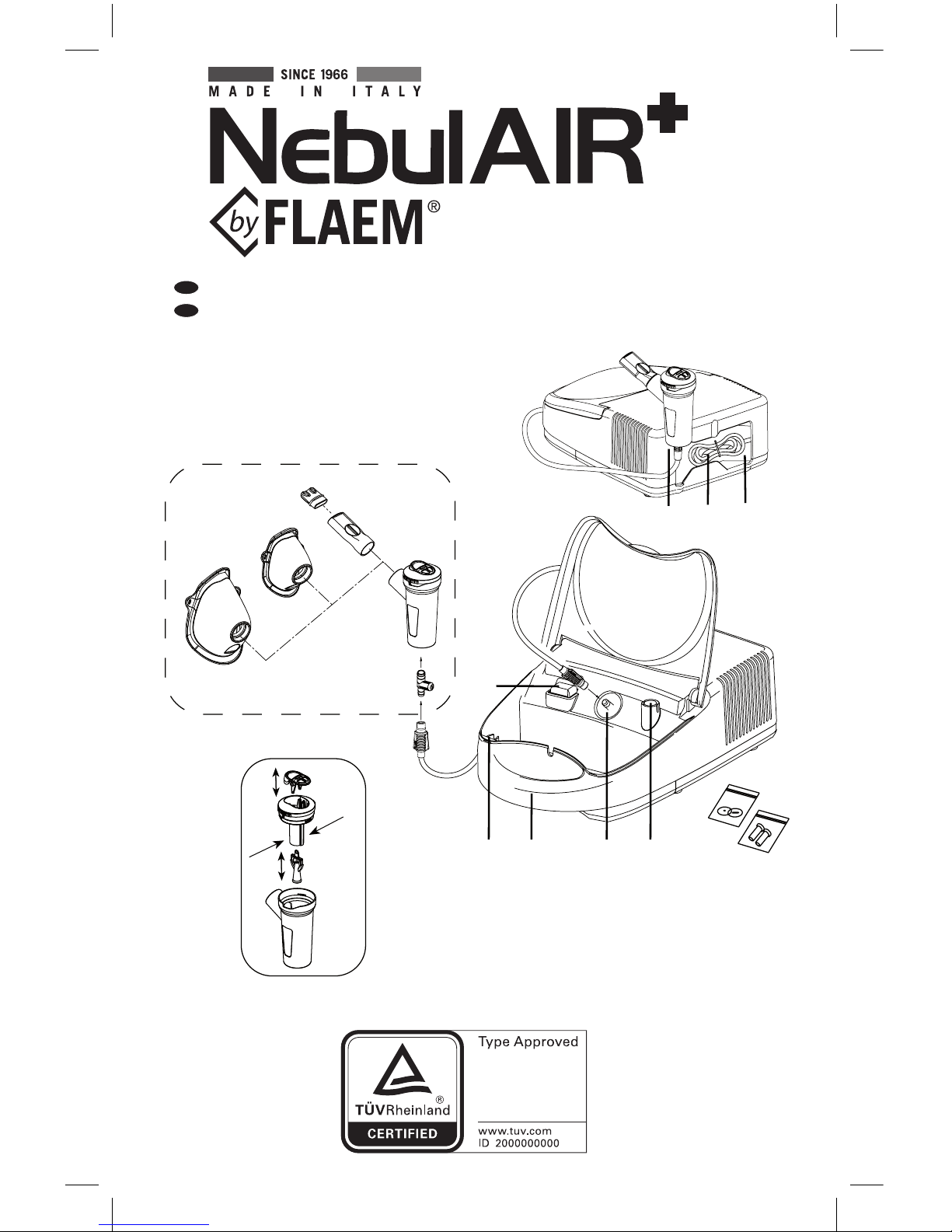
A6A7
A4
A1
A2 A3A5A4
C6
C1
B
A
C
C1.1
C1.2
C1.3
C1.4
C1
C2
C2.1
C3
C4
C5
C7
Assembly diagram - Monteringsskjema
INSTRUCTIONS FOR USE MANUAL
BRUKSANVISNING
NO
GB

1
NebulAIR
+
Mod. NEBULAIR
AEROSOL THERAPY APPARATUS
We are pleased you have purchased our product and we thank you for your trust in us.
We aim atfully satisfying our customers by oering them state-of-the-art products for
the treatment of respiratory diseases.
Read these instructions carefully and retain them for future reference. Only
use the accessory as described in this manual. This is a home medical device to
nebulise and administer medication prescribed or recommended by your doctor
upon assessing the patient’s general conditions. Please note that the full range of
Flaem products is visible on the website www.aem.it
STANDARD EQUIPMENT INCLUDES:
A -
Aerosol apparatus
(main unit)
A1 - On/O switch
A2 - Air intake
A3 - Air lter
A4 - Nebuliser holder
(internal/external)
A5 - Handle for transport
A6 - Cable storage compartment
A7 - Power cable
C -
Accessories
C1 - RF7 Dual Speed Plus Nebuliser
C1.1 - Lower part
C1.2 - Nozzle
C1.3 - Upper part
C1.4 - Speed selector with valve
C2 - Mouthpiece with valve
C2.1 - Exhalation valve
C3 - Non-invasive nosepiece
C4 - Paediatric SoftTouch mask
C5 - Adult SoftTouch mask
C6 - Manual nebulisation control
C7 - Filter replacement kit
B -
Connection tube
(main unit / nebuliser)
IMPORTANT WARNINGS
• This M.D. is also intended for direct use by the patient.
• Before using the product for the rst time, and periodically during its lifetime, check the
integrity of the device structure and of the power cable to make sure there is no damage.
In the event of damage, do not plug in the cable and immediately take the product to an
authorised FLAEM service centre or to your trusted dealer.
• Should your device fail to provide the expected performance, contact the authorised
service centre for clarications.
• The expected medical life of the accessories is 1 year. It is, however, advisable to replace
the nebuliser cup every 6 months in the event of intense use (or earlier if the cup is
obstructed) to always guarantee maximum therapeutic ecacy.
• Children and people who are not self-sucient must use the device under the close
supervision of an adult who has read this manual.
• Some parts of the device are small enough to be swallowed by children; therefore, keep
the device out of the reach of children.
• Do not use the supplied tubing and cables for anything other than their intended use.
These parts could cause a strangling hazard: pay close attention to children and persons
with particular diculties as they are often unable to accurately evaluate danger.
• The apparatus is unsuitable for use in presence of ammable anaesthetic mixture with
air, oxygen or nitrous oxide.
• Always keep the power supply cable away from hot surfaces.
• Keep the power cable away from animals (for example, rodents) which could damage the
insulation.
2
OPERATING INSTRUCTIONS
Before each use, clean hands thoroughly and clean the device as described in the
section on “CLEANING SANITISATION DISINFECTION STERILISATION”. During use,
it is advisable to protect yourself from any dripping. It is recommended that each
person use their own nebulizer cup and accessories to prevent risk of infection due
to contamination.
This device is suitable for the administration of medical substances and not, for
which the administration via aerosol is foreseen; these substances are to be in any
case prescribed by the Doctor. In case of too thick substances, the dilution with a suitable physiological solution could be needed, according to the medical prescription.
1. Plug the power cable (A7) into mains socket that is compatible with the device voltage.
The position of the socket must be such that the device can be easily unplugged from the
mains network.
2. Insert the nozzle (C1.2) in the upper part (C1.3) pressing as shown by the 2 arrows in the
"Connection diagram" in point C1. Insert the Speed selector with valve (C1.4) in the up-
• Do not handle the device with wet hands. Do not use the device in damp environments
(for example, while taking a bath or shower). Do not immerse the device in water; in
the event of immersion immediately disconnect the plug. Do not remove or touch the
immersed device; unplug the power cable rst. Immediately bring the device to an
authorised FLAEM service centre or to your trusted dealer.
• Use the device only in dust-free conditions, otherwise treatment could be compromised.
• Do not wash the device under running water or by immersion and keep it safe from being
sprayed by water or other liquids.
• Do not expose the device to particularly extreme temperatures.
• Do not place the device near sources of heat, in direct sunlight or in excessively hot
rooms.
• Do not obstruct or put objects into the lter or its related housing in the device.
• Never obstruct the air vents located on both sides of the device.
• Always use it on a rigid surface that is clear of obstacles.
• Make sure there is no material obstructing the air vents before each use.
• Do not put any objects in the air vents.
• Repairs, including the replacement of the supply cord, are to be carried out by FLAEM
authorised personnel only, by complying with the information provided by the
manufacturer.
• The average expected duration for the compressor series are: F400: 400 hours, F700: 700
hours, F1000: 1000 hours, F2000: 2000 hours.
• WARNING: Do not modify this device without authorisation from the manufacturer.
• The Manufacturer, the Vendor and the Importer shall be held responsible for safety,
reliability and performance only if: a) the device is used in compliance with the
instructions for use b) the wiring where the device is being used is in compliance with
safety regulations and current laws.
• Interactions: the materials used in contact with medication have been tested with a
vast range of medications. However, in view of the variety and continuous evolution of
pharmaceuticals, interactions cannot be ruled out. We recommend using the medication
as soon as possible once it has been opened and preventing prolonged exposure in the
nebuliser cup.
• The manufacturer must be contacted about any problems and/or unexpected events
concerning operation and for any clarications on use, maintenance/cleaning.
• Interactions: The materials used in the medical device are biocompatible in accordance
with the provisions of Directive 93/42 EC and subsequent amendments. However, the
possibility of occurrence of allergic reactions cannot be entirely excluded.

3
per part (C1.3) as shown in the "Connection diagram" in point C1. Pour the medication
prescribed by the doctor into the lower part (C1.1). Close the nebuliser by turning the
upper part (C1.3) clockwise.
3. Connect accessories as indicated in the “Connection diagram” on the cover.
4. Sit comfortably holding the nebuliser in your hand, place
the mouthpiece onto your mouth or alternatively use the
nose piece or mask. Should you opt for the mask accessory,
place it on your face as shown in the picture (with or without using the elasticated strap).
5. Start the device by means of switch (A1) and breathe deep-
ly in and out. After inhaling, we recommend holding your
breath for an instant to allow the inhaled drops of aerosol
to deposit. Then exhale slowly.
6. Upon completing application, switch o the device and unplug it.
ATTENTION: If after the therapy session an evident deposit of moisture forms within the
pipe (B), detach the pipe from the nebuliser and dry it with the very ventilation from the
compressor; this operation prevents possible blooms of mould inside the pipe.
USE METHODS OF THE RF7 DUAL SPEED PLUS NEBULISER WITH SPEED SELECTION
AND VALVULAR SYSTEM
It is professional, quick, recommended to administer all types of medications, including
the costlier ones, even in patients with chronic diseases. Thanks to the
geometry of the RF7 Dual Speed Plus nebuliser cup internal ducts, we
have obtained a recommended, active particle size to treat even the
lower respiratory tract.
For faster inhalation therapy, position the valve speed selector (C1.4)
pressing on the word MAX with your nger.
For more eective inhalation therapy, position the valve speed selector
(C1.4) pressing on the opposite side of the word Max with your nger.
In this case, you have optimal assumption of the medication, minimising
leaks into the surrounding environment, thanks to the valvular system
with which the nebuliser cup, mouthpiece and mask are equipped.
MAX
SOFTTOUCH FACE MASKS
During inspiration the vent
bends inwards.
During expiration the vent bends
outwards.
The new SoftTouch Masks feature an adaptive edge made
of a soft biocompatible material ensuring an excellent
adherence to the face and a Dispersion Limiting Vent.
These innovations, which make them stand out from the
rest, allow increased sedimentation of medication in the
lungs and reduced dispersion into the air.
Soft
biocompatible
material
Dispersion
Limiting Vent
4
USE OF NEBULIZATION MANUAL CONTROL
To achieve continuous nebulisation action you should not use the manual nebulisation
control (C6), especially in the case of children or persons with reduced physical, sensory, or
mental capabilities. The manual nebulisation control is useful for limiting dispersion of the
medication in the surrounding environment.
To start nebulizing close with
a nger the hole of the nebulizer manual control (C6) and
breathe in gently; we recommend to hold your breath for a
moment so that the inhaled aerosol droplets can be deposited,
meanwhile, to disable nebulizing, remove your nger from
the hole of the nebulizer manual control to avoid the waste
of drug, optimizing its acquisition. Then exhale slowly.
CLEANING, SANITISATION, DISINFECTION, STERILISATION
Switch o the device before any cleaning procedure and unplug the power cable from the
socket.
DEVICE AND TUBING EXTERIOR
Use only a damp cloth with antibacterial soap (non-abrasive and with no solvents of any
sort).
ACCESSORIES
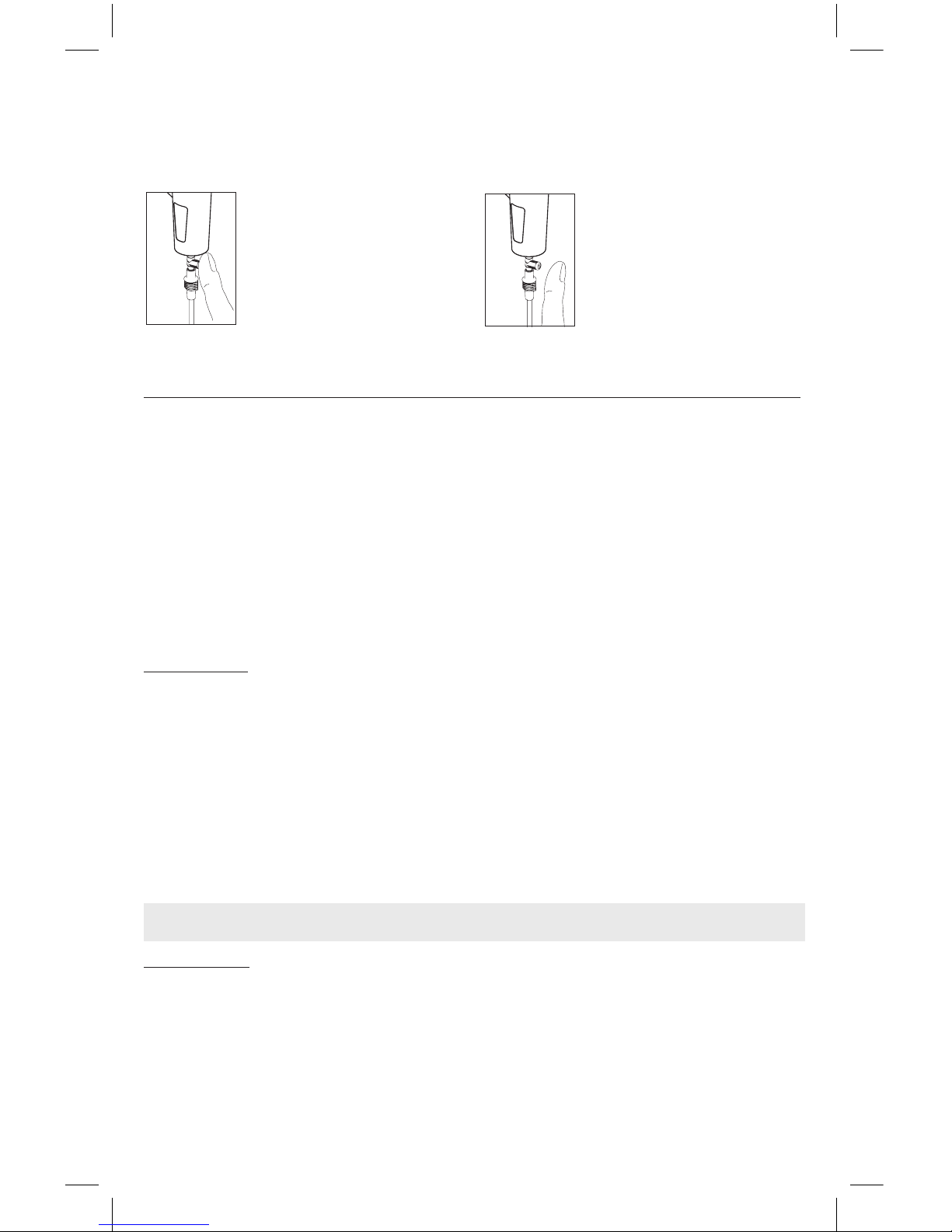
Open the nebuliser by turning the upper part (C1.3) anticlockwise, remove the nozzle
(C1.2) and the speed selector (C1.4) from the upper part (C1.3) as shown in the “Assembly
diagram” in point C1.
Then proceed according to the following instructions.
CLEANING AT HOME - SANITISATION AND DISINFECTION
SANITISATION
Before and after each use, sanitise the nebuliser cup and the accessories, choosing one of
the methods described below.
method A: Sanitise accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 under potable hot
water (approximately 40°C) with a gentle, non abrasive dish detergent.
method B: Sanitise accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 in the dishwasher
with a hot cycle.
method C: Sanitise accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 by immersing
them in a solution of 50% water and 50% white vinegar, then rinse thoroughly under
potable hot water (approximately 40°C).
If you want to also perform the cleaning for DISINFECTION, jump to the DISINFECTION
paragraph.
After having sanitised the accessories, shake them vigorously and place them on a paper
towel. Alternatively, dry them with a jet of hot air (for example, a hair dryer).
DISINFECTION
After sanitising the nebuliser cup and the accessories, disinfect them choosing one of the
methods described below.
method A: Accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 can be disinfected.
The disinfectant must be an electrolytic chloroxidizer (active principle: sodium
hypochlorite) specic for disinfecting, which is available in any pharmacy.
Implementation:
- Fill a container big enough to hold all of the parts to disinfect with a solution of potable
water and disinfectant, according to the proportions indicated on the packaging of the
disinfectant.

5
- Completely immerse each part in the solution, taking care to avoid the formation of air
bubbles on the parts. Leave the parts immersed for the amount of time indicated on the
packaging of the disinfectant associated with the concentration chosen for the solution.
- Remove the disinfected parts and rinse abundantly with warm potable water.
- Dispose of the solution following the instructions provided by the disinfectant
manufacturer.
method B: Sanitise the accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 by boiling
them in water for 10 minutes; use demineralised or distilled water to prevent calcium
deposits.
method C: Sanitise the accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 with a hot
steam steriliser for baby-bottle (not the microwave type). Perform the process faithfully
following the instructions of the steriliser. To ensure that the disinfection is eective,
choose a steriliser with an operating cycle of at least 6 minutes.
After having disinfected the accessories, shake them vigorously and place them on a
paper towel. Alternatively, dry them with a jet of hot air (for example, from a hair dryer).
CLEANING IN A CLINICAL OR HOSPITAL SETTING - DISINFECTION AND STERILISATION
Before disinfection or sterilisation, sanitise the nebuliser cup and the accessories, choosing
one of the methods described below.
method A: Sanitise accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 under potable hot
water (approximately 40°C) with a gentle, non abrasive dish detergent.
method B: Sanitise accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 in the dishwasher
with a hot cycle.
DISINFECTION
Accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5-C6 can be disinfected.
The disinfectant must be an electrolytic chloroxidizer (active principle: sodium
hypochlorite) specic for disinfecting, which is available in any pharmacy.
Implementation:
- Fill a container big enough to hold all of the parts to disinfect with a solution of potable
water and disinfectant, according to the proportions indicated on the packaging of the
disinfectant.
- Completely immerse each part in the solution, taking care to avoid the formation of air
bubbles on the parts. Leave the parts immersed for the amount of time indicated on the
packaging of the disinfectant associated with the concentration chosen for the solution.
- Remove the disinfected parts and rinse abundantly with warm potable water.
- Dispose of the solution following the instructions provided by the disinfectant
manufacturer.
If you want to also perform the STERILISATION, jump to the STERILISATION paragraph.
After having disinfected the accessories, shake them vigorously and place them on a
paper towel. Alternatively, dry them with a jet of hot air (for example, from a hair dryer).
STERILISATION
Accessories C1.1-C1.2-C1.3-C1.4-C2-C3-C4-C5 can be sterilised.
Device: Fractionated vacuum overpressure steam steriliser in accordance with EN 13060.
Implementation: Wrap every single part to be treated with a sterile barrier system or
packaging in accordance with Norm EN 11607. Place the packed components in the steam
steriliser. Run the sterilisation cycle according to the operating instructions of the device by
selecting a temperature of 134°C and a time of 10 minutes rst.
Storage: Store the sterilised parts as per the instructions for use of either the sterile barrier
system or packaging.
The sterilisation procedure is validated in its conformity to ISO 17665-1.
At the end of each use store the device complete with accessories in a dry place away

6
SPARE PARTS
Description Code
- RF7 Dual Speed Plus Nebuliser cup set, comprising:
nebuliser cup, mouthpiece with valve and nosepiece ACO464P
- 2 m tubing connection ACO225P
- Masks set, comprising:
adult mask, paediatric mask and elastic band ACO462P
- Air lter replacement kit (2pcs) ACO164P
from dust.
AIR FILTERING
The device is quipped with an extraction lter (A3) that must
be replaced when it is dirty or changes colour. Do not wash or
reuse the same lter. Regular lter replacement is necessary to
help ensure proper compressor performance. The lter must be
checked regularly.
To replace the lter:
Insert a slotted screwdriver between the edge of the lter and
the body. Lift the lter and extract it by rotating and pulling up. The lter was designed to
always remain secured in its seat. Do not replace the lter during use.
Only use original accessories and spare parts by Flaem, we disclaim any liability in
the event of using non original spare parts or accessories.
 Loading...
Loading...