FitLinxx UWM02 Users Manual
SUBJECT:
A&D MEDICAL DIGITAL BLOOD
PRESSURE MONITOR ASSEMBLY
TEST SPECIFICATION
910-00059-01
DOCUMENT NUMBER:
910-00059-01
UMW02
ASSEMBLY
&
REVISION:
08
PAGE:
1of 18
Copyright © 2003-2004 FitSense Technology
Proprietary Information
Revision History
DATE VERSION DESCRIPTION AUTHOR
05/21/08 01 Initial release of document David W Smith
05/22/08 02 Added details to Functional Verification process David W Smith
06/09/08 03 Added FCC labeling details David W Smith
06/24/08 04 Updated rework instructions to reflect UWM in
location of best performance
7/16/08 05 Added details of test application, setup and test
process.
7/17/08 06 Define all distances in meters David W Smith
7/18/08 07 Clarify the use of System test on starting of test
application.
7/25/08 08 Added FCC statement on section 1.1 David W Smith
David W Smith
David W Smith
David W Smith

SUBJECT:
A&D MEDICAL DIGITAL BLOOD
PRESSURE MONITOR ASSEMBLY
DOCUMENT NUMBER:
910-00059-01
REVISION:
Table of Contents
1 INTRODUCTION.............................................................3
2 INCOMING INSPECTION................................................4
3 INCOMING TEST............................................................4
08
PAGE:
2of 18
4 BP MONITOR REWORK INSTRUCTIONS...................10
5 FUNCTIONAL TEST.....................................................11
6 PACKAGING.................................................................18
SUBJECT:
A&D MEDICAL DIGITAL BLOOD
PRESSURE MONITOR ASSEMBLY
1 INTRODUCTION
1.1 Purpose of this document
The purpose of this document is to specify the assembly and functional test
requirements for the A & D Medical Digital Blood Pressure Monitor with the Universal
Wireless Module (UWM).
This device complies with Part 15 of the FCC Rules. Operation is
subject to the following two conditions: (1) this device may not
cause harmful interference and (2) this device must accept any
interference received, including interference that may cause undesired operation.
DOCUMENT NUMBER:
910-00059-01
REVISION:
08
PAGE:
3of 18
In adherence to FCC regulations any changes or modifications
not expressly approved by FitSense/FitLinxx could void the
user’s authority to operate the device.
1.2 Document Overview
This document in divided into the following section for organizational purposes.
Incoming Inspection; Describes UWM PCB packaging to be received by A&D
Medical.
Incoming Test; Describes the equipment, setup, and process for incoming test of the
UWM PCB.
Rework Instructions; Describes the wiring and mounting of the UWM PCB in the
Digital Blood Pressure Monitor.
Functional Test; Describes the equipment, setup, and process to Functionally Test
the wireless Blood Pressure Monitor containing the UWM PCB.
2 INCOMING INSPECTION
The UWM (P/N 710-00041-01) will be received in an ESD bubble bag with two bar
coded serial number labels and an FCC label as shown below .

SUBJECT:
A&D MEDICAL DIGITAL BLOOD
PRESSURE MONITOR ASSEMBLY
A ID Number label along with the FCC label is to be adhered to the blood pressure
monitor as described in the BP Monitor Rework Instructions. The second ID Number
label can be used on the product carton, packaging or as the manufacturer deems
appropriate.
The bar coded serial number label contains alpha and numerical characters. The
alpha characters represent the Device ID type as a BP monitor. The numerical
characters represents the Device ID Number plus a checksum. The bar code format is
UPC128.
DOCUMENT NUMBER:
910-00059-01
REVISION:
08
PAGE:
4of 18
The UWM PCB can be functionally verified and the corresponding labeling checked
utilizing the Incoming Test process described below. The UWM can be sample tested
per the MIL 105E Level ll Acceptance Plan.
3 INCOMING TEST
3.1 Test Equipment
The following test equipment is required for Incoming Test of the UWM PCB.
● USB based Personal Computer or laptop with Windows XP/2000 O.S. (provided by
A & D Medical)
● Custom Incoming Test Fixture (provided by FitSense)
● 3.30vdc power source for the UWM PCB Test Fixture (provided by A & D Medical)
● Digital Multimeter
● Known good ActiLink (provided by FitSense)
● ActiBPMfgTest test application (provided by FitSense)
3.2 UWM Test application
The System Test portion of the ActiBPMfgTest Manufacturing Test application
should be used for Incoming Test of the UWM PCB. When the application is started,
Select the tab for System Test.
The application (ActiBPMfgTest.exe) is installed in the C:\Program Files\ActiBP
Manufacturing Test. The application can be setup as a shortcut on the desktop.

SUBJECT:
A&D MEDICAL DIGITAL BLOOD
PRESSURE MONITOR ASSEMBLY
A known good ActiLink must be installed in the USB port of the test PC prior to starting
the application. If no ActiLink is present, or the ActiLink is not functioning properly, an
error message “Check Link Connection, Program will close” will be displayed
and the program will exit when the operator clicks on the “OK” tab as shown below.
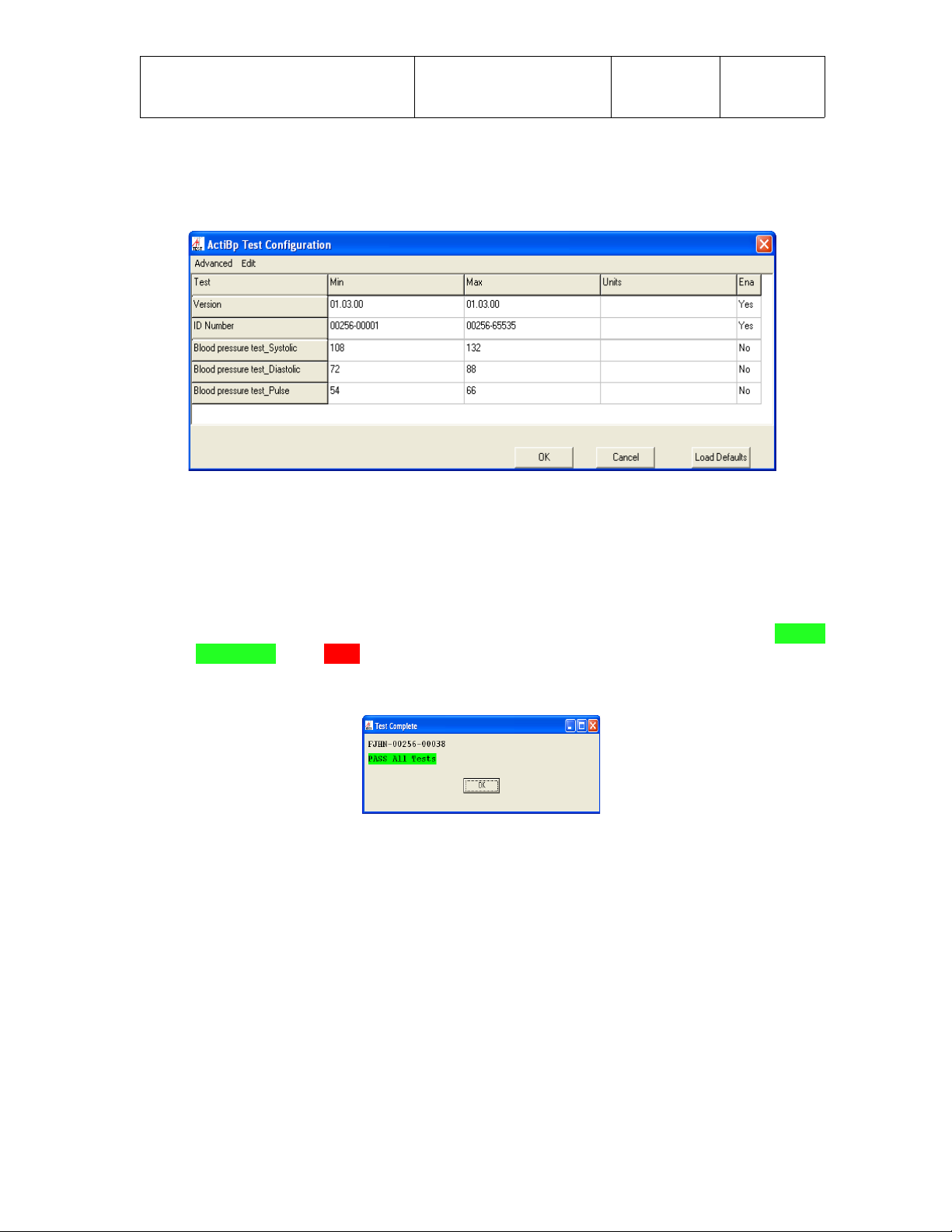
The application has a Test Configuration screen which allows manufacturing engineers
to enable/disable tests and set specific test parameters. Test configuration limits that
can be modified include firmware Version and ID Number. To access the Test
Configuration screen, the engineer would select “Edit”, then “Limits”, then enter the
password.
DOCUMENT NUMBER:
910-00059-01
REVISION:
08
PAGE:
5of 18
No password is set when the application is first installed. Select “OK” to access the
Test Configuration limits screen for the first time. To setup a password, select
“Edit”, then “Limits”. Select “Advanced”, then select “Password”. Enter the new
password and select “OK”. The new password is now required each time to access
the Test Configuration screen.
Once in the Test Configuration screen, the test “limits” should be set as follows for
Incoming Test of the UWM PCB;
● Version - Min and Max values should be set to reflect the latest revision of UWM
PCB firmware. The current firmware Version is 1.3.0
● ID Number - Min and Max values should be set to reflect the range of UWM PCB
ID Numbers for the BP monitor. The current ID Number range is from
00256-00001 through 00256-65535.
In the Test Configuration screen, each sub-test can be enabled or disabled as required
by entering Yes or No in the “Ena” column. For Incoming Test of the UWM PCB, the
tests should be enabled/disabled as follows and as shown in the screen capture below.
● Version - Enabled with a Yes in the Ena column.
● ID Number - Enabled with a Yes in the Ena column.
● Blood pressure test_Systolic - Disabled with a No in the Ena column.
● Blood pressure test_Diastolic - Disabled with a No in the Ena column.
SUBJECT:
A&D MEDICAL DIGITAL BLOOD
PRESSURE MONITOR ASSEMBLY
● Blood pressure test_Pulse - Disabled with a No in the Ena column.
DOCUMENT NUMBER:
910-00059-01
REVISION:
08
PAGE:
6of 18
Once the test limits have been correctly set as described above for Incoming Test of
the UWM PCB, select the “OK” tab. This will save the Test Configuration limit settings,
which will now be utilized each time the test application is started.
When the application has completed testing of a UWM PCB, a message with a PASS
ALL Tests or a FAIL status and the ID Number of the tested PCB will be displayed
across the top of the test screen, as shown below.
All results, pass or fail, are captured in a log file located in C:\Program Files\ActiBP
Manufacturing Test folder. The log file will also contain information regarding the Mfg.
Plant and test Station Number. To set the Plant and test Station Number, select
“Edit”, then “Location”, and enter the specific information. Select the “Ok” tab
when complete.
3.3 UWM Incoming Test Setup
A PC or laptop is required for Incoming Test of the UWM PCB. The PC or laptop
should be placed at one end of a non-metallic table top at a height of 36 inches (0.914
meters) from the floor. The ActiLink should be placed on the opposite side of the the
laptop or PC at a height of 36 inches (0.914 meters) from the floor using a USB
extension cable. Refer to photograph below.
 Loading...
Loading...