Page 1
NOTES TO PRINTER: Proofs accurate for process color only. All spot colors must follow PMS Color
Formula Guide or color swatch specified.
CS DATE: XX/XX/12
TOY YEAR: 2011 Fall
PKG. SIZE:
PKG. SPEC.: Booklet VER.: 1st Run
BLANK SIZE:
5.5” X 8”
13” X 9”
SOFTWARE: InDesign C5
COLOR PROFILE/LPI: Mag CCNB / 150lpi
ITEM NO.: X4003 LANG.: DOM
PART CODE:
ITEM NAME:
HW New Business Rocket Car
X4003-0900
GRAPHIC DESIGNER: Ramen
PI ENGINEER:
PROJECT ENGINEER:
CS VENDOR: IASIK
DATE:
SIGN OFF
(GRAPHIC):
PROOF APPROVAL
X4003_Booklet.indd 1X4003_Booklet.indd 1 16/3/12 5:45 PM16/3/12 5:45 PM
Page 2
In this super science experiment, you will learn how
to make a Rocket Car that travels furiously far! This
set combines physics and fun as an experiment full
of action. This sleek racer will amaze you as it blasts
off at a frenzied pace.
Create a real rocket on wheels using chemistry
and physics. Generate an amazing
REACTION
to 120 feet. Experiment with different amounts
to create your own special fuel mixture. Just
like all science experiments, you must follow
the steps exactly as listed for the experiment
to work.
that may propel your Rocket Car up
CHEMICAL
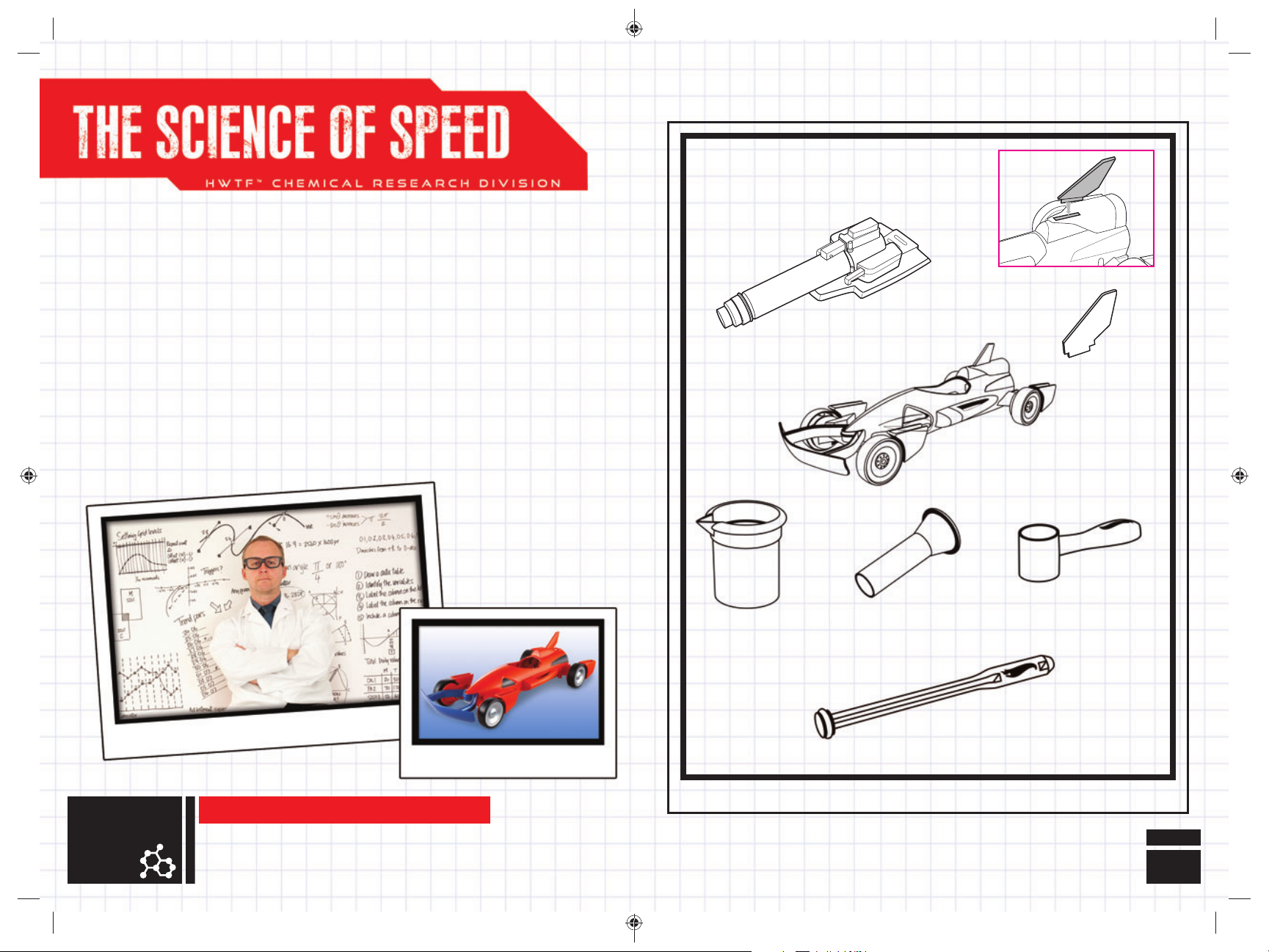
PARTS LIST
N O T E : S P O I L E R R E Q U I R E S O N E - T I M E
ATTACHMENT TO ROCKET CAR.
Launcher
Spoiler
Rocket Car
10
100 ml
(at fi ll line)
Beaker / Cup
CHEMICAL REACTIONS
SCIENCE
FACT
X4003_Booklet.indd 2-3X4003_Booklet.indd 2-3 16/3/12 5:44 PM16/3/12 5:44 PM
DID YOU KNOW?
A chemical reaction is a process that leads to the
transformation of one set of chemical substances
to another.
Funnel
grams
2 Measuring
Cups
Stirrer / Plunger
PAG E
3
Page 3
A rocket is simply a chamber filled with pressurized gas.
A small opening called a nozzle allows the air to esc ape, causing
thrust that propels the rocket. With this project you can make a
Rocket Car that is powered by pressurized gas. The Rocket Car
is one way to observe Newton’s First and Third Laws of Motion.
Because of individual variations in the Rocket Car, yours will
travel different distances and often in unpredictable directions.
Through modifications, you can correct for undesirable results
and improve your car’s efficiency.
ACIDS AND BASES ARE EVERYWHERE
Every liquid you see will probably have either acidic or
basic traits. Scientists use something called the pH scale to
measure how acidic or basic a liquid is.
• What is an Acid?
A n A ci d i s a s ol ut io n t ha t h as an ex ce ss of Hyd ro gen i on s.
It comes from the Latin word “acidus” which means
sharp or sour. Vinegar is one type of acid solution.
• What is a Base?
A Base is a solution that has an excess of Hydroxide
ions. Another word for base is alkali. One example
of this is Sodium Bicarbonate, or Baking Soda.
NEWTON’S LAWS OF MOTION:
• Newton’s First Law
“Objec ts at rest will stay at rest and objects in motion
will s tay in motion in a str aight line unles s acted upon
by an unbalanced force.” In other words, the forces
propelling the rocket car forward must be stronger
than those trying to stop it.
• Newton’s Third Law
“For every action there is always an opposite and
equal reaction.” When an action takes place, like
gases escaping from a rocket, a reaction follows —
in this case, the rocket car runs along the ground.
SIR ISAAC NEWTON
SCIENCE
FACT
DID YOU KNOW?
Sir Isaac Newton is considered by many scholars
to be one of the most infl uential people in human
history!
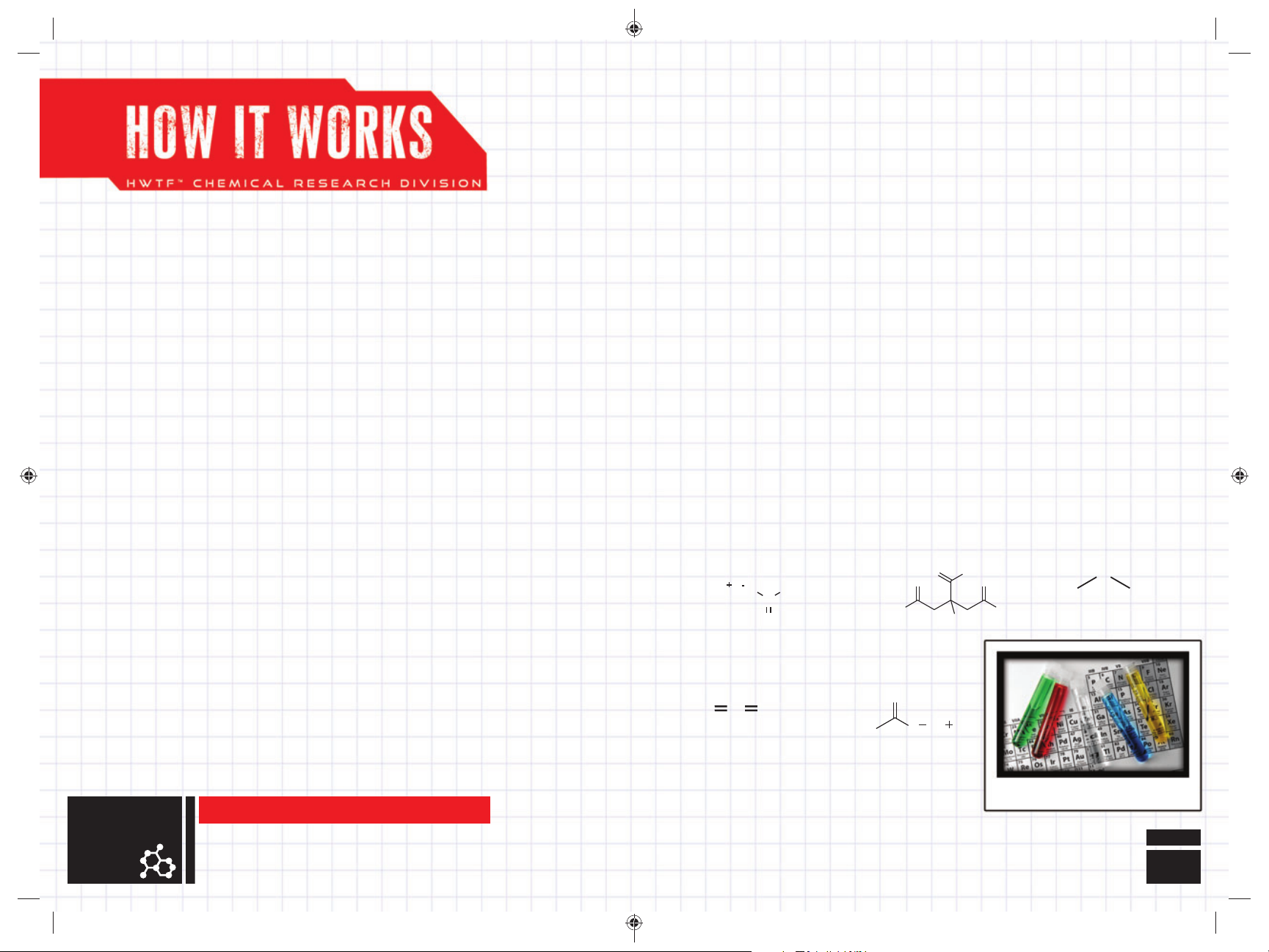
SCIENTIFIC MAKE-UP OF
VARIOUS CHEMICALS
SODIUM BICARBONATE
(Baking Soda)
NaHCO
Na
CARBON DIOXIDE
CO
3
O
O
2
H
C
O
SODIUM ACETATE
OOC
CITRIC ACID + WATER = CITRIC ACID SOLUTION
NOTE: Citric Acid Solution has an equivalent pH value of Vinegar.
CITRIC ACID
HO
CH
COONa
3
O
O
O
C
6H8O7
O
a
N
H
O
O
H
O
H
O
WATER
H
O
2
O
HH
PAG E
5
X4003_Booklet.indd 4-5X4003_Booklet.indd 4-5 19/3/12 12:33 PM19/3/12 12:33 PM
Page 4

EXPERIMENT SAFELY:
- Find a fl at smooth “track” with at least 200 feet of distance.
-
Don’t use the Rocket Car on a street or parking lot where vehicles
are present.
- Always wear safety goggles (not included) when conducting
experiments.
- Always conduct experiments under adult supervision.
- Never point the Rocket Car at anyone once the fuel is loaded.
- Use of the Rocket Car can get messy. Don’t use around areas that
could be damaged.
- Make certain nobody is in front of the Rocket Car during launch.
STEP 1
Fill cup with Vinegar up to fill line.
r
a
g
e
n
i
V
STEP 3
Hold launcher tube vertically
and pour the Vinegar
solution into the tube.
STEP 4
Insert launcher tube into Rocket Car over central tube and
push forward until all 3 latches click together.
Click!
STEP 2
Place funnel over center tube of Rocket Car and pour in 10
grams of Baking Soda then remove funnel.
PAG E
6
X4003_Booklet.indd 6-7X4003_Booklet.indd 6-7 16/3/12 5:44 PM16/3/12 5:44 PM
STEP 5
Shake the assembled Rocket Car vigorously to ensure mixture.
PAG E
7
Page 5

STEP 6
Place assembled Rocket Car on ground.
STEP 7
Place foot on launcher base and wait 20 seconds. Then using other
foot, step on launch button to release Rocket Car.
TRACK THE DISTANCE YOUR ROCKET CAR TRAVELS
After launching your Rocket Car, answer the questions below.
1. Describe how your Rocket Car ran during the first trial run.
2. Did it run on a straight or curved path?
3. How far did it go?
HOW TO CLEAN AND CARE
FOR YOUR ROCKET CAR
IMPORTANT NOTE
If you wait longer or use a mixture of chemicals beyond recommended
amounts, launch may be aborted by loss of pressure and you will need to
restart process using correct amounts. We encourage you to experiment,
but beware material may be lost if recommendations aren’t followed.
Great experiments require properly functioning equipment! Between
each use and after you are done with the experiment, you must clean
out the center cylinder.
STEP 1
Add water.
STEP 2
STEP 8
CLEAN YOUR ROCKET CAR AFTER EVERY LAUNCH.
(INSTRUCTIONS ON PAGE 9)
PAG E
8
X4003_Booklet.indd 8-9X4003_Booklet.indd 8-9 16/3/12 5:45 PM16/3/12 5:45 PM
Shake and pour out to clean.
STEP 3
Ensure the tube for the Sodium Bicarbonate is completely
clean and unobstructed.
PAG E
9
Page 6

EXPERIMENTING WITH FUEL RATIOS
In this experiment, you will adjust the ratios of Vinegar (Acid)
and Baking Soda (Base). Use your distance result from your
first trial as your experiment’s
CONTROL GROUP
.
Acids and Bases are measured by their “pH” level. The pH value
indicates the strength of the Acid or Base on a scale of 0.0 - 14.0.
Acids range from 0.0 - 6.9 and Bases range from 7.1 - 14.0.
A liquid with a pH of 7.0 is neutral, like water, and is neither
an Acid nor a Base. When Acids and Bases mix together, they
react and move towards the neutral 7.0 pH number. If the ratio of
Acid-to-Base is exactly right, they will completely neutralize each
other and the resulting mix will be a 7.0 on the pH scale.
Adjust the levels of Acid and Base in small increments and
notice how your distance changes with each modification
Did your distance improve or decrease with more Acid?
Did your distance improve or decrease with more Base?
The speed and distance your Rocket Car travels is a direct
result of the power of the reaction between your Acid and Base
fuel mixtures, which is a measurement of the strength of the
Acids and Bases you
experiment with! The
stronger the Acids and
Bases, the faster and
farther your Rocket Car
will go!
The farther apart the pH level of the Acid and the Base, the more
vigorous the reaction and more gas is generated in the Rocket
Car fuel tank. This in turn creates more pressure in the closed
system, which then propels the Rocket Car farther and faster
when the gas is released through the nozzle! *
TRACK THE DISTANCE YOUR ROCKET CAR TRAVELS
Use this booklet and test results to answer the following
questions:
1. Which combination of Acid and Base caused the Rocket Car
to travel the greatest distance?
2. How many feet did your Rocket Car travel?
3. What is the chemical reaction that takes place?
CONTROL GROUPS
SCIENCE
FACT
X4003_Booklet.indd 10-11X4003_Booklet.indd 10-11 16/3/12 5:45 PM16/3/12 5:45 PM
DID YOU KNOW?
Control Groups are a vital par t of the
Scientifi c Method, and are used in almost
all experiments!
* A relief valve is installed which will limit the pressure allowed to
build based on pre-determined safety limits.
PAG E
11
Page 7

EXPERIMENTING WITH NON-CARBONATED FUELS
In this experiment, you will use other commonly found noncarbonated liquids instead of Vinegar.
USE DIFFERENT ACIDS
Try orange juice, apple juice, lemon juice, iced tea.
Mix 100 ml of non-carbonated liquid in place of Acid with 10
grams of Base.
which means they are very acidic and have lower pH levels than
other juices (like apple juice, which is not very acidic).
After experimenting with various types of juices, you will be able
to determine which juices are the most acidic with the lowest pH
values, and which ones aren’t very acidic at all. As we discussed,
the lower the pH level of the acidic part of the fuel mixture,
the stronger the reaction will be with the baking soda (sodium
bicarbonate), and the faster and farther the Rocket Car will go!
Did your distance improve or decrease with
different types of Acids?
You may notice that some types of juices work better than
others as fuels in the Rocket Car. Once again, this is due to
the pH levels being
different for all types
of juices, depen ding
on the fruit from
which they are
made. Citric juices,
like orange juice
and especially
lemon juice, have
very low pH levels,
TRACK THE DISTANCE YOUR ROCKET CAR TRAVELS
Use this booklet and test results to answer the following questions:
1. How many feet did your Rocket Car travel?
2. Which liquid produced the best results? Explain why you
think this liquid worked best?
3. Which liquid produced the worst results? Explain why you
think this liquid was not as effective?
PAG E
12
X4003_Booklet.indd 12-13X4003_Booklet.indd 12-13 16/3/12 5:45 PM16/3/12 5:45 PM
PAG E
13
Page 8

X4003_Booklet.indd 14X4003_Booklet.indd 14 16/3/12 5:45 PM16/3/12 5:45 PM
 Loading...
Loading...