
FibroScan
M+
User
Probe
manual
CE
0459
E117M008.7 — Version 7 —
02/2016

M+
PROBE
USER
MANUAL
2
02/2016 - ECHOSENS®
AND
FIBROSCAN®D
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
PROBE
USER
MANUAL
fines
TABLE
1.
PURPOSE
1.1.
Symbols
1.2.
Property
2.
WARNINGS
2.1.
Generality
2.2.
Handling
2.3,Maintenance
3.
MISCELLANEOUS
OF
THE
USER
used
in
the
manual
and
copyright.........................
.ee
the
probe
.9
INFORMATION
CA
3.2.
Registered
4.
INDICATIONS
4.1.
Имепдед
4.3.
Probe
4.4.
2[606841015
4.5.
User
46.
Electricalsafety..........................................
4.7.
Maintenance-relatedsafety................................. e een
trademarks
AND
цзе.............
инете
andexamination
101
456........0..1.0
training
iii
nine
PRECAUTIONS
selectioncriteria...........................................
MANUAL
iii
шинели
의 니 이 의 아 아 아
USE
FOR
마 아 아 마 마 마 마 아 마 아 리 마 러 아 아 라 파
sise
пани
eee
OF
CONTENTS
ereneeeemesenzsenie
нии
нини
ина
아 아
아 아 아 에 어 이 아 리 마 아 아 아 마 나 이 이 이 13
ищении
eee
eee
nana
nene
i
наити
A
5
6
6
7
7
7
7
8
8
8
9
9
10
12
14
14
14
E117M008.7
5.
EXTERNAL
5.1.
Hardware
5.2.
РгоБе
6.
USE
6.1.
User
6.2.
Connecting / disconnecting
6.3.
Handlingtheprobe.......................................
6.4.
End
7.
CLEANING,
7.1.
Cleaning
7.2.
Calibrating
7.3.
Troubleshooting
02/2016 - ECHOSENS®
PRESENTATION
supplied..............................L
дезспрНОп..............
DURING
6.3.1.
6.3.2.
AN
EXAMINATION
recommandations
Proberestingposition.........................................
Grippingtheprobe.................................................
ofexaminatiON..............oocrcconnnsconennronnennenencncnanananancanancnananannnaononannnancannarnonannonennas
MAINTENANCE
чинили
the
AND
ricer
ieri
eee
ee
eee
reni
ernia
шине
нии
нина
probe
.…................................
eee
REPAIRS
нения
нение
eieeienie
nie nie
шин
нии
нение
nice
iii
mene
0...
sere
жении
ezine
an
cenasaniaa
su
anne
nenene
eee
7.1.1.
Cleaning
7.1.2.
Recommended
7.13.
Recommendeddecontamination
AND
FIBROSCANO
the
the
probe
Ge
ARE
REGISTERED
probe
(housing,
cleaning
.ee
TRADEMARKS © COPYRIGHT
cable
products
solutions................................................
and
transducer)...................................
tiene
RR
renen
ECHOSENS
ALL
RIGHT
RESERVED
15
15
15
17
17
17
18
nrrsee
18
18
19
20
20
20
21
22
23
enge
23
3

M+
PROBE
USER
8.
8.1.
MANUAL
TECHNICAL
Ultrasound
CHARACTERISTICS
transducer
ececscessessessssssescessessessessessssetereereesensenseess
4
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
THE
and
required
to
manual.
be
requirements,
PROBE
able
1.
This
User
Echosens
The
present
and
maintenance
the
displayed
Thus,
after
m
connect
m
use
m
carry
Manual
be
carefully
the
the
probe
out
has
held
responsible
user
manual
of
the
data
is
reading
probe
in
accordance
the
maintenance
PURPOSE
no
contractual
details
probe
covered
the
to
the
FibroScan,
on
the
all
designed
in
the
manual,
with
work
of
value
basis
of
FibroScan
technical
the
OF
whatsoever
of
the
the
information
to
be
connected
user
operators
and
probe.
information
shall
clinical
USER
USER
under
contained
for
the
the
FibroScan.
to:
MANUAL
MANUAL
no
circumstances
in
this
manual.
implementation,
Interpretation
may
use
of
Echosens
implicit,
for
Echosens
all
nevertheless
Echosens
loss
or
(imperfection)
physician,
corrections
This
from
Echosens
in
hard
or
software
The
This
problems.
Any
Echosens,
publishes
including,
specific
use
cannot
efforts
have
contain
cannot,
of
business,
consecutive
as
made
manual
is
Echosens
undertakes
copy
or
in
your
product
owner
manual
information
30
been
or
rapidly
updated
contains a chapter
place
this
manual
but
not
limited
in
view
accept
any
made
some
under
data
loss,
damages
error
contained
as
to
this
manual.
on a regular
on
request.
to
send
electronic
format.
possession.
must
keep
or
modification
d'Italie,
"as
is",
without
to
implicit
guarantees
of
providing
simple
responsibility
to
offer a manual
technical
any
of
inaccuracies
circumstances,
business
any
interruption,
type.
In
in
this
User
possible, a hard
basis.
The
Should
any
major
the
physician,
Note
that
this
manual
requests
75013
for
for
troubleshooting
PARIS
France.
guarantees
and
for
the
manual's
that
is
as
and/or
be
held
or
the
event
Manual,
copy
or
most
recent
modifications
as
rapidly
this
does
not
as
long
as
pertaining
of
any
or
merchant
accurate
information.
incorrect
accurate
as
typographical
responsible
for
any
indirect,
of
damages
Echosens
electronic
version
be
made
as
possible, a new
involve
the
product
the
most
to
this
manual
nature,
whether
conditions,
interpretation.
possible,
errors.
for
any
specific,
the
arising
undertakes
document
of
this
manual
to
the
manual,
copy
updating
the
is
used.
commonly
should
explicit
or
or
adaptation
Consequently,
Though
manual may
loss
of
profit,
accidental
from a defect
to
send
the
containing
is
of
the
hardware
encountered
be
all
available
however,
manual
and/
sent
to:
Et17M908.7
02/2016 - ECHOSENSO
AND
FIBROSCANO
ARE
REGISTERED
TRADEMARKS O COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
5

[ETF]
M+
PROBE
USER
MANUAL
1.1.
AN
1.2.
All
manuals
protected
to
legal
whole
Hence,
is
prohibited,
SYMBOLS
This
symbol
Warning:
Instructions
device
This
symbol
Additional
PROPERTY
and
by
copyright,
copyright.
or
in
part,
the
reproduction,
within
USED
means:
see
the
preceded
and
installation
means:
information
AND
documents
all
These
in
any
manner
adaptation
the
limits
IN
THE
ATTENTION
instructions
by
this
if
not
INFORMATION
with
COPYRIGHT
of
all
types
rights
reserved.
manuals
cannot
or
in
or
provided
MANUAL
before
symbol
correctly
no
impact
are
the
Your
be
distributed,
any
form,
translation
by
copyright
using
the
may
cause
followed.
on
instrument
property
right
to
without
of
this
law.
medical
injuries
of
the
copy
translated
prior
written
manual
device.
or
use.
company
this
documentation
or
consent
without
damage
reproduced,
prior
the
Echosens
from
written
medical
and
are
is
limited
either
Echosens.
consent
in
Copyright © —
11/2012 — 02/2016 — Echosens — All
rights
reserved.
6
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
2.1.
GENERALITY
个
2.2.
A
Caution:
HANDLING
The
and
holder
stored
probe
kept
on the
in
Federal
THE
is a fragile
away
from
FibroScan.
its
case.
law
restricts
PROBE
electromechanical
liquids.
In
this
device
Between
the
event
to
two
of
prolonged
PROBE
sale
by
device
examinations,
2.
or
on
that
must
non-use,
USER
MANUAL
WARNINGS
the
order
of a physician.
be
handled
it
should
be
placed
the
probe
FRETE]
with
care
on
should
its
be
2.3.
MAINTENANCE
Maintenance
technician
The
calibration
characteristics
probe
authorized
must
certificate,
operations
by
Echosens.
be
calibrated
the
of
the
probe.
must
not
periodically.
manufacturer
be
performed
no
Beyond
longer
by a third
the
period
guarantees
party
other
indicated
the
performance
than
a
on the
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
u

(U5)
M+
PROBE
USER
3.
3.1.
The
For
shall,
3.2.
MANUAL
MISCELLANEOUS
GUARANTEE
terms
of
guarantee
any
request,
if
applicable,
REGISTERED
are
Echosens
transfer
TRADEMARKS
stated
remains
the
request
in
the
Echosens
available
to a competent
INFORMATION
terms
of
sale
to
the
physician
local
and
representative.
documents.
his/her
appointees
and
Echosens
and
FibroScan
are
registered
trademarks
of
the
company
Echosens.
8
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

4.
INDICATIONS
4.1.
INTENDED
The
FibroScan
ultrasound.
The
measurements
attenuation
body.
The
This
FibroScan
parameter
FibroScan
(VCTETM).
system
device
system
and
estimates
device
USE
with
the
is
designed
with
(CAP:
is
based
AND
M+
probe
to
the
M+
probe
of
tissue
Controlled
on
the
PRECAUTIONS
is
an
active,
non-implantable
be
used
in a doctor's
is
intended
stiffness
Attenuation
Vibration-Controlled
as well
Parameter)
to
as
office.
provide
3.5
MHz
in
Transient
FOR
medical
50
Hz
shear
ultrasound
internal
Elastography
USE
device
wave
coefficient
structures
principle
using
speed
of
of
the
The
FibroScan
shaft
of
the
which
in
turn
subcutaneous
transducer
speed
and
interest,
4
cm
In
addition,
device
E=3xpx
wave
for
the
Concomitantly,
Parameter
propagates
decreases
intensity
attenuation
medium
expressed
Absolute
from
performs a series
of
shear
temporal
which
(which
corresponds
assuming
converts
Vs?
speed
and
purpose
(CAP).
through
exponentially
at
depth
depends
of
propagation.
in
values
different
probe
comprises a single-element
electrodynamic
generates
tissues,
wave
average
can
shear
with p the
of
the
an
and
propagation
speed
be
approximated
to
that
wave
equivalent
comparison
ultrasound
Ultrasound
the
medium.
with
z, a is
principally
medium
the
CAP
dB/m.
for
these
measurements
manufacturers.
transducer.
elastic
shear
then
the
liver.
of
ultrasound
(Vs)
of
propagation
by a cylinder
about 3 cm).
the
liver
is a pure
speed
Vs
density
stiffness
(or
with
other
acquisitions
attenuation
Due
to
depth
(z):
frequency
on
(i)
assesses
ultrasound
This
transducer
wave.
This
wave
During
shear
acquisitions
in
m/s.
This
of
into
equivalent
assumed
Young’s
measurements
are
corresponds
attenuation,
Iz = lo x exp
(f)
dependent
the
ultrasound frequency,
the
value
may
vary
(emission / reception)
measurement
the
shear
with a diameter
elastic,
stiffness E in
to
be
modulus)
used
to
the
(-
of a at
among
transducer
generates a transient
propagates
wave
wave
linear
1000
are
relative
performed
assess
to
the loss
intensity
a(f) x z)
attenuation
the
frequency f =
measurement
through
propagation,
corresponds
through
and
kg/m*.
the
where
the
of 1 cm
isotropic
kPa
using
The
indexes
using
FibroScan
Controlled
of
energy
of
emitted
Iz is
coefficient.
(ii)
the
devices
mounted
vibration,
the
skin,
the
ultrasound
to
measure
to
the
liver
region
and a length
medium,
the
equation
values
for
intended
devices.
Attenuation
as
ultrasound
ultrasound
the
ultrasound
Ultrasound
properties
3.5
MHz
produced
on
the
the
the
spatial
of
of
the
shear
only
(Io)
of
the
and
is
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
9
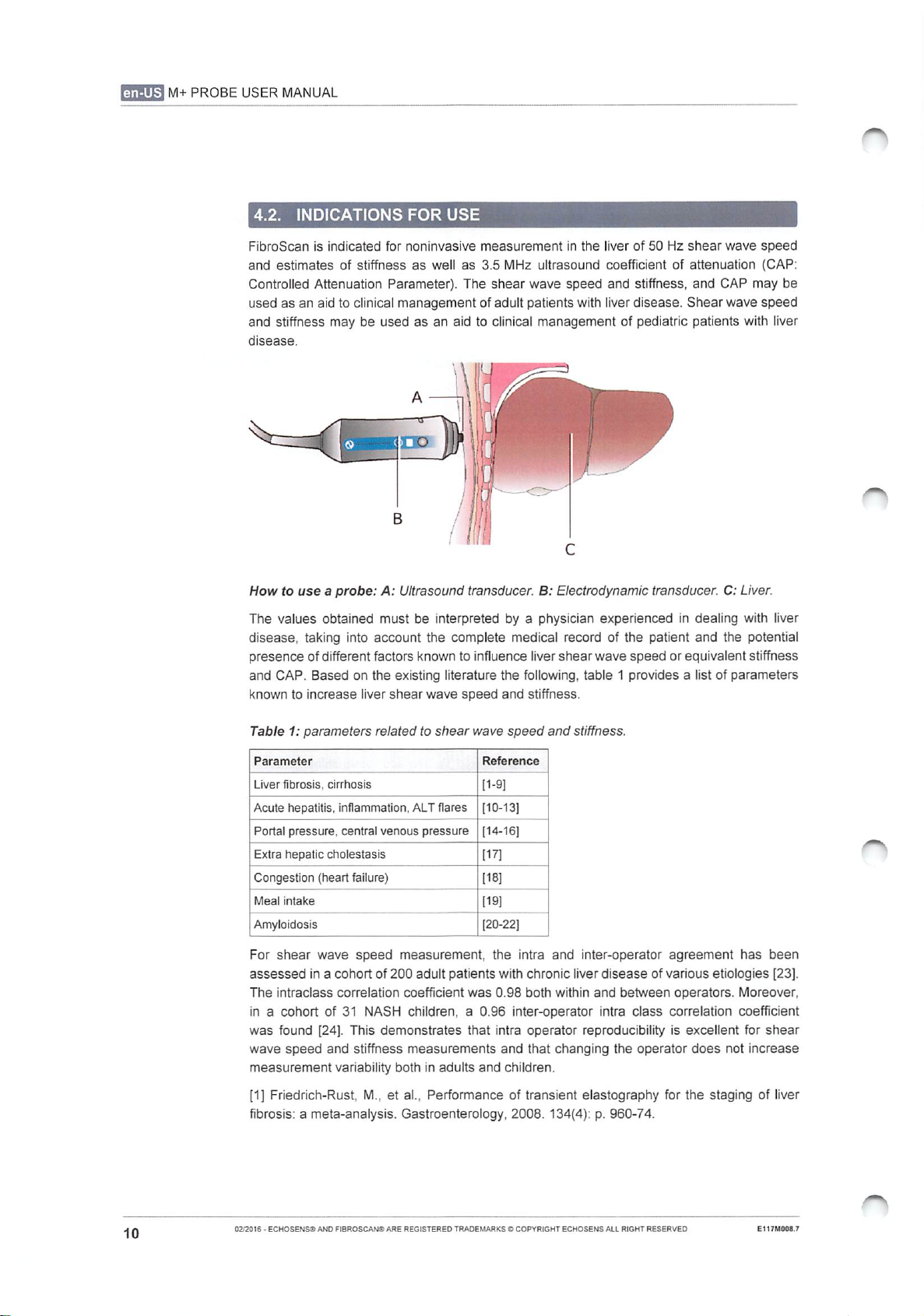
(GUS
M+
PROBE
USER
4.2.
MANUAL
INDICATIONS
FOR
USE
FibroScan
and
estimates
Controlled
used
as
an
and
stiffness
disease.
How
to
use a probe:
The
values
disease,
presence
and
known
CAP.
to
taking
of
Based
increase
is
indicated
of
stiffness
Attenuation
aid
to
clinical
may
be
used
A:
obtained
different
into
on
liver
must
account
factors
the
for
noninvasive
as
well
Parameter).
management
as
an
aid
Ultrasound
be
interpreted
the
complete
known
existing
shear
literature
wave
measurement
as
3.5
MHz
The
shear
of
adult
to
clinical
transducer.
by a physician
medical
to
influence
the
speed
and
in
the
ultrasound
wave
speed
patients
following,
stiffness.
with
management
B:
Electrodynamic
record
liver
shear
table 1 provides a list
liver
of
50
coefficient
and
stiffness,
liver
disease.
of
pediatric
transducer.
experienced
of
the
patient
wave
speed
Hz
shear
of
attenuation
and
Shear
patients
in
dealing
and
or
equivalent
of
wave
speed
(CAP:
CAP
may
wave
speed
with
C:
Liver.
with
the
potential
stiffness
parameters
be
liver
liver
Table
1:
parameters
Parameter
Liver
fibrosis,
Acute
Portal
Extra
Congestion
Meal
Amyloidosis
For
shear
assessed
The
intraclass
in a cohort
was
found
wave
measurement
[1]
Friedrich-Rust,
fibrosis: a meta-analysis.
cirrhosis
hepatitis,
pressure,
hepatic
cholestasis
(heart
intake
wave
in a cohort
of
[24].
speed
and
related
inflammation,
central
venous
failure)
speed
of
correlation
31
NASH
This
demonstrates
stiffness
variability
M.,
to
shear
wave
ALT
flares | [10-13]
pressure
measurement,
200
adult
coefficient
| [14-16]
patients
was
children, a 0.96
that
measurements
both
in
adults
et
al.,
Performance
Gastroenterology,
speed
and
stiffness.
Reference
[1-9]
[17]
[18]
[19]
[20-22]
the
intra
and
inter-operator
with
0.98
chronic
both
liver
within
inter-operator
intra
operator
and
that
changing
and
children.
of
transient
2008.
elastography
134(4):
agreement
disease
and
intra
reproducibility
between
class
the
operator
of
various
correlation
for
p.
960-74.
etiologies
operators.
is
excellent
does
not
the
staging
has
been
[23].
Moreover,
coefficient
for
shear
increase
of
liver
10
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
[2]
Musso,
(NAFLD)
Medicine,
[3]
Shaheen,
Fibrosis: A Systematic
Gastroenterology,
[4]
Shi,
evaluation
[5]
Smith,
analysis
557-76.
Hepatic
[7]
Talwalkar,
fibrosis:
2007.
(8]
Tsochatzis,
liver
G.,
et
al.,
Meta-analysis:
and
diagnostic
2011.
43(8):
et
K.Q.,
et
of
portal
J.O.
and
in
chronic
[6]
Stebbing,
Fibrosis.
systematic
5(10):
disease: A meta-analysis
Journal
J.A.,
p.
1214-20.
E.A.,
accuracy
p.
617-49.
al.,
FibroTest
Review
2007:
p.
al.,
Transient
hypertension
R.K.
hepatitis
J.,
et
al., A Meta-analysis
of
et
al.,
Ultrasound-based
review
et
al.,
1-12.
Sterling,
Clinical
and
Elastography
Natural
of
non-invasive
and
FibroScan
of
Diagnostic
history
tests
for
elastography: a meta-analysis
in
chronic
C.
Alimentary
meta-analysis.
of
diagnostic
liver
disease.
Systematic
Gastroenterology,
review:
Pharmacology
of
Transient
transient
Clinical
for
the
diagnosis
accuracy.
PROBE
of
non-alcoholic
for
liver
the
Prediction
Test
Accuracy.
Liver
Non-invasive
and
Elastography
2010.
elastography
Gastroenterology
of
Journal
USER
disease
of
American
of
diagnostic
Int,
2013.
Therapeutics,
44(3):
p.
214-9.
for
the
severity
of
Hepatology,
MANUAL
fatty
liver
severity.
Hepatitis
accuracy
33(1):
p.
methods
2009.
for
the
Detection
detection
and
of
fibrosis
2011.
disease
Annals
C-Related
Journal
62-71.
of
fibrosis
30(6):
of
hepatic
Hepatology
in
chronic
54(4):
of
of
in
p.
of
p.
650-9.
[9]
Lee,
C.K.,
et
al.,
Advanced
Experience.
Liver
The
Serum
Fibrosis
Journal
Biomarkers
in a United
of
pediatrics,
States
2013.
and
Transient
Cohort:
163(4):
p.
1058-64.
Elastography
The
Boston
as
Predictors
Children's
of
Hospital
[10]
Arena,
transient
[11]
influenced
U.,
elastography.
Coco,
B.,
by
major
et
et
360-9.
{12]
Mueller,
from
[13]
Sagir,
with
acute
[14]
Carrión,
portal
Transplantation,
[15]
Millonig,
Journal
[16]
Vizzutti,
patients
[17]
irrespective
[18]
Lebray,
cardiac
S.,
et
steatohepatitis.
A.,
et
al.,
liver
damage.
J.A.,
hypertension
2006.
G.,
of
Hepatology,
F.,
et
with
HCV-related
Millonig,
G.,
of
fibrosis.
P.,
et
insufficiency.
al.,
Acute
Hepatology,
al.,
Transient
changes
al.,
Increased
World
Transient
Hepatology,
et
al.,
in
patients
12(12):
et
al.,
Liver
2010.
al.,
Liver
et
al.,
Hepatology,
al.,
Liver
Hepatology,
viral
hepatitis
2008.
elastography: a new
of
transaminases.
liver
Journal
of
elastography
Transient
with
p.
1791-8.
stiffness
52(2):
p.
stiffness
cirrhosis.
Extrahepatic
Hepatology,
2008.
stiffness
2008.
increases
47(2):
p.
380-4.
liver
surrogate
Journal
stiffness
Gastroenterology,
2008.
elastography
hepatitis C recurrence
in
is
unreliable
47(2):
is
directly
alcoholic
2010.
p.
592-5.
for
diagnosis
influenced
liver
for
206-10.
measurement
cholestasis
2007.
predicts
45(5):
increases
28(5).
is
an
unreliable
48(6):
p.
marker
2089.
stiffness
of
Viral
disease:
16(8):
detection
after
by
severe
p.
1290-7.
liver
of
liver
values
marker
of
Hepatitis,
differentiating
p.
966-72.
of
cirrhosis
of
advanced
liver
transplantation.
central
venous
portal
stiffness
fibrosis
measured
liver
fibrosis
2007.
14(5):
fibrosis
in
patients
fibrosis
Liver
pressure.
hypertension
(FibroScan)
in
patients
with
by
p.
and
in
E117M008.7
[19]
Mederacke,
resolved
[20]
hepatitis C virus
Janssens,
elastography.
02/2016 - ECHOSENS®
AND
1.,
et
al.,
Food
infection.
E.,
et
al.,
Hepatic
Acta
Gastroenterologica
FIBROSCAN®
ARE
REGISTERED
intake
increases
Liver
International,
amyloidosis
increases
Belgica,
TRADEMARKS © COPYRIGHT
liver
2010.
ECHOSENS
stiffness
2009.
liver
73(1):
ALL
in
29(10):
stiffness
p.
52-4.
RIGHT
RESERVED
patients
p.
1500-6.
measured
with
chronic
by
transient
or
1
1

M+
PROBE
USER
MANUAL
[21]
Lanzi,
A.,
European
[22]
new
[23]
fibrosis
[24]
of
fibrosis
4.3.
The
morphological
m
=
Journal
Loustaud-Ratti,
tool.
Amyloid,
Fraguelli,
in
patients
Nobili,
in
PROBE
recommendations
TP:
Thoracic
SCD:
Skin-to-Capsule
automatic
In
case
of
using
shear
wave
probe.
et
al.,
of
M.,
V.,
et
al.,
pediatric
data:
Perimeter
probe
an
speed
Liver
AL
amyloidosis
Gastroenterology
V.R.,
et
al.,
2011.
18(1):
p.
et
al.,
Reproducibility
with
chronic
liver
Accuracy
nonalcoholic
AND
selection
ultrasound
is
measured
and
EXAMINATION
for
using
measured
Distance
tool.
as a possible
and
Hepatology,
Non-invasive
19-24.
disease.
reproducibility
steatohepatitis.
detection
of
transient
Gut,
SELECTION
the
probes
at
the
xiphoid
assessed
scanner,
with a pressure
with
SCD
elastography
2007.
of
transient
Hepatology,
are
using a tape
an
ultrasound
should
similar
cause
2010.
of
hepatic
56(7):
defined
be
measured
to
the
of
high
liver
22(7):
p.
895-7.
amyloidosis:
in
the
p.
968-73.
elastography
2008.
48(2):
CRITERIA
by the
measure.
scanner
one
at
used
following
or
the
with
stiffness
FibroScan,
evaluation
for
the
diagnosis
p.
442-8.
by
the
point
where
the
FibroScan
values.
a
of
liver
patient's
the
In
case
of
using
the
manual
It
is
not
(chapter
recommended
6.5.11.
automatic
Exam
to
use
probe
type
any
selection
selection
means
SCD.
Four
types
of
examination
measurement
depths
FibroScan®
S1
EXAM
are
that
take
Probe
ΝΟ
‘Thoracic
Perimeter
S2
EXAM
available:
into
account
(TP)
tool,
please
area).
to
compress
S1,
S2, M and
the
liver's
the
depth
Choice
AGE218
YEARS
refer
to
FibroScan
soft
tissues
merely
XL.
They
correspond
beneath
the
skin.
Algorithm
γες
Skin
Capsula
Distance
(SCD)
Probe
XL
EXAM
502
Touch
to
reduce
to
RECOMMENDED
User
the
specific
NOT
12
In
all
02/2016
-
cases,
ECHOSENS®
Echosens
AND
FIBROSCAN®
recommends
ARE
REGISTERED
to
perform
TRADEMARKS
10
©
COPYRIGHT
valid
ECHOSENS
FibroScan
ALL
RIGHT
measurements.
RESERVED
E117M008.7

M+
4.4.
The
following
present
On
On
On
On
On
On
On
PRECAUTIONS
instructions
probe
designed
patients
patients
patients
an
patients
wounds.
pregnant
under
with a thoracic
with
organ
other than
with
women.
skin-to-capsule
active
for
14
FOR
must
the
years
the
implants
USE
be
followed
FibroScan
old.
perimeter
distance
liver.
The
such
in
should
of
less
(SCD)
eyes
and
as
pacemakers,
order
not
than
mucosa
PROBE
to
ensure
be
used
75
cm.
greater
than
must
defibrillators,
USER
patient
in
the
following
25
mm.
absolutely
MANUAL
safety.
be
pumps,
[BBE
Thus,
situations:
avoided.
etc.
the
Moreover,
measurement
The
clinical
EA
presence
with
personnel
The
FibroScan
ALARA
the
(As
of
device.
must
Low
ascites
between
follow
examination
As
Reasonably
normal
should
the
probe
safety
procedures.
be
performed
Achievable).
and
the
liver
may
prevent
prudently
using
from
the
principle
obtaining
of
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS O COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
13

EX
M+
PROBE
USER
MANUAL
4.5.
USER
Only
persons
a
user
certificate
essential
measurements.
This
4.6.
The
with
factory
compliance
the
Safe
for
manual
ELECTRICAL
probe,
IEC
electromagnetic
in
full
indications
use
is
TRAINING
who
have
are
correct
is
not
intended
designed
compliance
and
to
guarantee
and
symbols
no
longer
received
authorized
equipment
to
provide
SAFETY
for
the
FibroScan,
compatibility
with
safety
the
contained
guaranteed
training
to
conduct
use
and
user
(EMC)
and
safe
use
in
in
the
following
in
the
use
of
an
examination
in
order
training.
has
been
manufactured
and
electrical
performance
of
the
medical
this
manual.
main,
the
FibroScan
using
to
obtain
reliable
and
safety
standards.
requirements.
device,
In
the
non-exclusive
unit
and
FibroScan.
and
tested
in
order
to
user
must
cases:
who
possess
Training
reproducible
accordance
It
leaves
the
maintain
this
conform
is
to
the
probe
the
probe
after
prolonged
after
serious
When
safe
Steps
must
technicians
4.7.
MAINTENANCE-RELATED
For
all
maintenance
Echosens,
For
correct
normal
safety
is
visibly
does
damage
use
of
be
taken
for
inspection.
who
will
and
safe
procedures.
damaged,
not
work,
storage
the
probe
to
avoid
operations,
send
use
in
unfavorable
incurred
is
no
its
inadvertent
an
authorized
and
for
during
longer
the
physician
technician.
all
maintenance
conditions,
transport.
possible,
use.
The
SAFETY
operations,
the
probe must
probe
and
should
his/her
the
be
taken
be
handed
appointees
personnel
out
to
should
must
of
service.
authorized
contact
conform
to
14
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
5.
EXTERNAL
5.1.
When
=
Probe
=
User
5.2.
HARDWARE
opening
and
case
Manual
PROBE
the
SUPPLIED
package,
ensure
DESCRIPTION
the
contents
match
PROBE
the
following
USER
MANUAL
PRESENTATION
list:
218]
Housing
The
housing
a
measurement
Probe
housing:
(LED).
D:
Ultrasonic
The
ultrasound
of
the
FibroScan
Measurement
As
soon
as
vibrator
wave)
of
Acquisition
actuates
that
painlessly
acquisitions
lasts
contains
an
trigger
button.
A:
Electrodynamic
transducer.
transducer
unit
in
contact
electrodynamic
of
the
button
this
button
is
pressed
the
electrodynamic
impacts
(emission / reception)
less
than
one
the
tenth
transducer.
probe
with
the
(if
transducer,
patient's
to
of a second.
transducer
is a "Type
patient.
sufficient
skin.
measure
(vibrator),
B:
Measurement
B"
applied
pressure
which
The
ultrasound
the
propagation
an
ultrasound
part,
and
is
exterted
in
turn
generates a shear
transducer
button.
is
on
speed
transducer
C:
Indicator
the
only
component
the
transducer),
wave
performs a series
of
this
shear
and
light
the
(s-
wave.
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
15

ESTE]
M+
PROBE
USER
MANUAL
Indicators
The
indicator
m
On
during
m
Flashing
=
Switched
patients
=
On
during
body.
at
the
Lead
A
lights
FibroScan
lights
off
during
body.
an
exam when
It
is
however
on-screen
(LEDs)
for
the
an
strongly
pressure
start-up
display a status
and
when
probe selected
exam when
the
indicator.
the
operator
recommended
B
as
follows:
standing
when
operator
is
applying
that
an
by
exam
is
applying
the
you
to
launch
starts.
correct
view
an
the
an
exam.
incorrect
pressure
pressure
pressure
to
the
exerted
to
the
patient's
by
looking
Probe
This
1.5 m lead
四
The
probe
before
四
lead:
A:
Connection
connects
The probe
elements
jack
has a red dot
insertion.
The
serial
cable.
the
probe
transducer,
and
must
be
that
number
marked
B:
Connection
to
the
the
probe
handled
should
on
FibroScan
jack,
with
care.
be
aligned
the
connector
jack.
by
and
with
means
of a multi-pin
the
FibroScan connector
the red dot
identifies
the
on
probe
the
FibroScan
uniquely.
jack.
are
fragile
socket
16
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

6
USER
The
following
examination.
=
Hold
the
и
Avoid probe
=
Do
not
immerse
и
Avoid
any
=
Clean
and
maintenance
6.
RECOMMANDATIONS
recommendations
probe
perpendicular
impacts.
the
probe.
liquid
projections
decontaminate
and
repairs).
the
USE
must
to
the
on
the
probe
DURING
be
followed
patient's
medical
with a suitable
skin
device.
M+
AN
during
during
product
PROBE
USER
MANUAL
EXAMINATION
the
different
the
measurements.
(see
paragraph
phases
Cleaning,
218]
of
an
m
и
m
EE
Place
6.2.
Location
To
insert
To
connect
the
probe
after
CONNECTING/
of
the
probe
insert
the
probe
the jack.
Both
the
jack
the
probe
use
in
one
DISCONNECTING
connector:
jack: align
and
socket
lead,
insert
the
the
of
user
probe
are
jack
the
holders
manual.
lead
fragile
elements.
after
or
in
THE
jack's
aligning
its
case.
PROBE
red dot
Handle
the red
with
with
dots.
the
care.
socket's
red
dot
and
E117M008.7
Connecting
m
To
disconnect
pull
the
02/2016 - ECHOSENS®
the
whole
AND
FIBROSCAN®
probe:
the
probe
jack
A:
jack:
back.
ARE
REGISTERED
Red
dot
on
first
pull
TRADEMARKS © COPYRIGHT
probe
the
socket.
jack's
B:
Red
splined
ECHOSENS
dot
sleeve
ALL
RIGHT
on
probe
back
RESERVED
jack.
to
unlock
it,
then
17

ΕΠΕΤΕ]
M+
PROBE
USER
MANUAL
When
unplug
A
probe
examinations.
is
automatically
starting
the
probe
may
an
exam,
until
the
be
disconnected
If
the
probe
closed.
be
sure
end
of
for
is
disconnected
to
follow
the
exam.”
replacement
the
instruction
with
during
in
another
an
examination,
this
probe
message:
between
this
examination
“Do
not
two
Disconnecting
6.3.
HANDLING
个
6.3.1.
When
Probe
the
Refer
probe
the
probe:
to
the
warnings
resting
is
not
in
A:
THE
position
use,
Socket.
B:
Splined
PROBE
in
Chapter 2 concerning
it
must
be
positioned
sleeve.
on
the
the
probe
handling
holder,
of
the
as
probe.
shown.
18
Probe
resting
6.3.2.
Hold
maintained
02/2016 - ECHOSENS®
Gripping
the
probe
position.
the
as
shown.
perpendicular
AND
FIBROSCAN®
ARE
probe
During
to
the skin
REGISTERED
measurements,
surface
TRADEMARKS © COPYRIGHT
of
the
continuously
patient.
ECHOSENS
ALL
make
RIGHT
sure
RESERVED
that
the
probe
E117M008.7
is

M+
PROBE
USER
MANUAL
EXER}
Gripping
6.4.
Once
3.
5.
the
probe:
END
OF
the
examination
Click
on the
Remove
Disinfect
maintenance
Place
Ifthe
any
the
the
probe,
device
=
Press
m
Set
the
=
Disconnect
probe.
=
Store
A:
Patient.
EXAMINATION
is
complete,
button
below
excess
probe
is
the
the
gel
with a suitable
and
repairs.
head
pointing
no
longer
on/off
button
main
switch
the
probe
probe
in
in
its
B:
Operator.
proceed
to
deactivate
holding
the
product
up,
onto
required:
next
to
to
the O position.
as
indicated
case.
as
the
probe,
the
the
monitor
in
C:
Probe.
follows:
probe.
head
indicated
FibroScan
of
paragraph
pointing
the
downwards.
in
paragraph
probe
FibroScan.
Connecting / disconnecting
Cleaning,
holder.
the
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS O COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
19

ETS
M+
PROBE
USER
MANUAL
7.
CLEANING,
In
the
authorized
person
7.1.
Apply
the
Failure
no
longer
event
of
malfunction,
to
service
will
terminate
CLEANING
following
to
observe
be
covered
MAINTENANCE
only
FibroScan
the
guarantee.
recommendations
these
recommendations
by the
guarantee.
the
and
its
accessories. Any
to
clean
staff
may
of
Echosens
or
disinfect
result
in
damage
AND
or
work
the
its
local
performed
probe.
to
the
REPAIRS
representative
by
an
unqualified
probe,
which
will
are
then
Recommendations
=
Always
=
и
=
7.1.1.
ER
Surfaces
e
wear
Observe
Ensure
decontamination
instructions
Carefully
Control
applicable
the
that the
read
and
Cleaning
It
is
must
Gently
remove
eye
protection
expiry
dates
contact
solution
given
on
the
recommendations
Epidemiology
in
the
country.
the
not
necessary
be
cleaned
the
and
of
cleaning
time
are
the
label
(APIC)
probe
to
in
strict
gel
using a soft
gloves
products
and
concentration
appropriate
of
the
cleaning
from
and
(housing,
switch
off
compliance
cloth
to
the
the
prevent
and
of
for
the
product
the
Association
Food
cable
device
with
the
or
wipe.
injury.
decontamination
the
cleaning
equipment
and
the
for
and
Drug
Administration
and
transducer)
before
cleaning
following
solutions.
product
used.
decontamination
Professionals
procedure:
and
Carefully
the
probe.
apply
in
(FDA),
the
solution.
Infection
if
20
02/2016 - ECHOSENSO
AND
FIBROSCANO
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
PROBE
USER
MANUAL
FRETE]
Cleaning
2.
Remove
soaked
If
necessary,
Dry,
if
Wipe
decontamination
Dry,
if
La
Examine
liquid
If
any
damage
representative:
Precautions
Do
not
submerge
Apply
the
cleaning
surface
The
in
damage
Do
probe
order
not
to
be
must
to
ensure
use a surgeon's
the
the
probe:
all
traces
in
the
rinse
necessary,
the
surfaces
A:
of
bodily
recommended
the
cleaned
using a dry
using a soft
solution.
necessary,
the
using a soft
transducer
leakage.
is
observed,
service@echosens.com.
or
soak
the
product
and
cleaned.
be
cleaned
effective
probe.
decontamination.
brush
Wipe.
fluid
by
cleaning
surfaces
cloth.
cloth
dry
cloth.
and
probe
stop
using
probe.
decontamination
after
every
to
clean
cleaning
product.
using a soft
or
wipe
cable
the
use
or
the
probe.
the
soaked
for
any
probe
solution
between
surfaces
cloth
in
the
damage
and contact
to
the
patients.
Even
the
using a soft
soaked
such
use
in
water.
recommended
as
cracks,
Echosens
soft
cloth,
Prior
cleaning
of
flexible
cloth
not
or
wipe
breakage,
or
its
directly
is
necessary
brushes
or
local
on the
could
E117M008.7
Take
care
connector.
7.1.2.
Echosens
m
Pure
=
Detergent
=
Recommended
The
following
=
Abrasive
m
Alkaline
ECHOSENS®
-
02/2016
not
to
introduce
Recommended
recommends
water,
soapy
with
neutral
decontamination
cleaning
products
detergents
FIBROSCAN®
AND
any
use
of
water.
pH
products
(such
as
(pH > 9),
REGISTERED
ARE
cleaning
cleaning
the
following
(5 to
8).
solutions
are
prohibited:
“Cif’
and
bleach,
TRADEMARKS
product
or
products
products:
(see
scouring
etc.
COPYRIGHT
©
decontamination
below).
powders)
ALL
ECHOSENS
RIGHT
solution
RESERVED
into
the
probe
21

EE】
M+
PROBE
USER
m
Sulphuric,
m
Soda,
=
Unleaded
s
Nail
MANUAL
potash,
varnish
acetic,
ammonia,
petrol,
solvent
nitric,
hydrochloric,
etc.
acetone,
and
remover.
MED,
MBK,
and
oxalic
toluene,
acid,
etc.
xylene,
benzene,
trichloroethylene,
etc.
7.1.3.
The
probes.
Ascend
PI-Spray
Thericide
Thericide
Clinell
Tristel
Tuffie
Surfanios
Aniosurf
Wip’Anios
Wip’Anios
Surfa'Safe
Viractean
In
decontamination
decontaminate
Recommended
decontamination
Cleaning
solution
105
Control
Coverage
End-Bac
PI-Spray
and
Spray
III
Spray
Il
Il
Plus
Plus
Wipes
Wipes
Premium
Premium
Premium
SH
addition
solutions
decontamination
System
to
the
list
of
solutions
the
probes.
using
decontamination
recommended
Origin
USA
USA
USA
USA
USA
USA
USA
USA
USA
United
Kingdom
United
Kingdom
United
Kingdom
France
France
France
France
France
France
recommended
quaternary
decontamination
ammonium
below
solutions
are
suitable
Vaporizer
Wipes
Wipes
Wipes
Wipes
Wipes
Vaporizer | Quaternary
Vaporizer | Quaternary
as
for
use
on
the
Type
Vaporizer | Quaternary
Liquid
Liguid
Vaporizer | Quaternary
Liquid
Vaporizer | Quaternary
Vaporizer | Quaternary
Liquid
Liquid
Liquid
an
Active
ingredient
ammonium
Quaternary
ammonium
Ouaternary
ammonium
ammonium
Quaternary
ammonium
ammonium
ammonium
Quaternary
ammonium
Quaternary
ammonium
Quaternary
ammonium
Chlorine
Quaternary
ammonium
Quaternary
ammonium
Quaternary
ammonium
Quaternary
ammonium
Quaternary
ammonium
ammonium
ammonium
solutions,
active
agent
dioxide
any
can
machine
alcohol-free
be
used
and
to
22
02/2016
-
ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS
©
COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
7.2.
The
As
examination.
The
CALIBRATING
probe
contains
The
manufacturer
soon
as
user then has
probe
the
deadline
one
THE
mechanical
must
therefore
no
longer
is
reached,
month
PROBE
parts
guarantees
to
send
that
an
the
be
icon
may
shift
slightly
periodically
the
performance
is
displayed
probe
to
Echosens
PROBE
over
calibrated.
in
the
USER
time.
Beyond
characteristics
information
for
calibration.
MANUAL
this
period,
of
the
window
ΕΠΕΤΕ]
probe.
during
the
the
Despite
strongly
the
recommend,
x
Calibration
7.3.
calibrated.
service
TROUBLESHOOTING
Events
The
probe
"Hardware
"Vibration
In
the
event
Aechosens.com.
presence
icon.
is
no
longer
error"
message.
error"
message.
of a failure
of
the
however,
or
malfunction,
icon,
the
operator
sending
the
probe
Solutions
Contact
Echosens
service@echosens.com.
Check
that
Incorrect
transducer
please
the
or
probe
contact
can
perform
for
calibration
its
local
is
correctly
movement.
Echosens
examinations
as
rapidly
representative:
connected.
or
its
local
as
usual.
as
possible.
representative:
We
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
23
EXE]
M+
PROBE
USER
8.
MANUAL
TECHNICAL
CHARACTERISTICS
Manufacturer
Model
IP
Code
Mechanical
Central
frequency
Measurement — 25
depth
Acoustic
output
Index
Echosens
30
place
d'ltalie
75013
PARIS — France
Type
M+
IPX1:
the
probe,
for
all
and
Elastography
Value
99 % of
(MPa)
(MW)
fe
(MHz)
Zsp
(cm)
Beam
PD
(usec)
PRF
(Hz)
EBD
excluding
operation
are
water.
MI < 1.0
3.5
MHz
mm
to
65
mm
The
acoustical
between M mode
Acoustic
Pre-amendments
Global
(95 % T.L.
values)
|
Associated | pr.a
acoustic | Vo
parameter
|
(Maximum
|
values)
|
|
|
Operating | Control 1 NPL NPL NPL
control
conditions
Maximum
_
Output
for
|
outputs
dimensions
connectors,
mode.
maximal
during
acquisition
measurement
=
X-6
(Cm) 0.37
y-6(cm)
Az.
(cm) 0.68
Ele.
(cm) 0.67
ultrasound | ultrasound | ultrasound
beam beam beam
calibrator | calibrator
is
protected
the
transient
mode.
MI
0.55
0.89
3.38
+
2.57
|
0.47 0.47
500
elastography
(+7
%)
(£3
%)
(£5
%) | 2.57
from
ISPTA.3
(mWicm?)
11.3
|
|1.7(421%)
93.38
0.40
calibrator
vertically
falling
sequence
ee
(4 3 96) | 9.98
(5%) | 2.57
(£6
%)
| 0.37
(+ 6 %) | 0.40
(+ 6 %)
(+ 6 %)
drops
of
which
is a mixed
ISPPA.3,
(Wicm*)
46
|1.7(+21%)
(4: 3 06).
—
(+ 5 %)
(+6
%)
(+ 6 %)
500
24
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
8.2.
ELECTRICAL
CHARACTERISTICS
PROBE
USER
MANUAL
EMI
8.3.
Dimensions
Weight
8.4.
Operating
Operating
Storage
Storage
See
MECHANICAL
158
500
ENVIRONMENTAL
temperature + 10 °C
humidity
temperature
humidity
30 % to
—
20
10 % to
the
FibroScan
user
manual.
CHARACTERISTICS
mm x 52
mm
(L x diameter)
grams
PROPERTIES
to + 40
°C
(+
50
°F
to + 104
75 % relative
°C
to +
85 % relative
70
°C
humidity,
(- 4 °F
humidity,
to +
°F)
non-condensed
158
°F)
non-condensed
E117M008.7
02/2016 - ECHOSENSO
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS O COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
25

EE]
M+
PROBE
USER
MANUAL
INDEX
Application
doesn't
boot,
Calibrating
the
CAP , 10
Cleaning , 0
Copyright , 6
Electrical
characteristics , 25
Environmental
Fuse , 15
Guarantee , 8
indications , 9,
indications
intended
Mains
Maintenance , 0
for
use , 9
lead , 15
23
probe , 23
properties , 25
10,
14
use , 10
Mechanical
characteristics
25
Precautions , 9,
Précautions
precaution
for
Probe , 15
Calibrating
,
23,
cleaning , 20
indicators , 16
Lead , 16
measurement
troubleshooting , 23
Vibrator , 15
Probe
troubleshooting
hardware
problem , 23
incorrect
vibration , 23
13,
14
use , 13
23
button , 15
,
Property , 6
Registered
Repairs , 0
Safety
electrical , 14
maintenance , 14
Selection
Stiffness , 9
Ultrasound
User
criteria
exam
type , 12
probe , 12
training , 14
trademarks , 8
transducer , 24
02/2016 - ECHOSENS®
AND
FIBROSCAND
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
E117M008.7

M+
PROBE
USER
MANUAL
E117M008.7
02/2016 - ECHOSENS®
AND
FIBROSCAN®
ARE
REGISTERED
TRADEMARKS © COPYRIGHT
ECHOSENS
ALL
RIGHT
RESERVED
27

©
ecHosens
Echosens
30
place
75013
FRANCE
Tel:
+33 1 44 82
Fax:
+33 1 44
Web
Site:
www.echosens.com
E-mail:
info@echosens.com
d'Italie
PARIS
82 78
78
~
50
60
 Loading...
Loading...