Page 1

CGM Sensor Insertion and Removal Instructions
IMPORTANT:
• Only physicians who have successfully completed the Eversense CGM Insertion and Removal Training Program and have read and understood the Eversense CGM Sensor Insertion and Removal
Instructions may perform the insertion and removal procedure on patients. Contact Senseonics (in the US toll free at 844-SENSE4U (844-736-7348)) if training has yet to be conducted or if you
experience any diculty or issues with the insertion or removal procedure. Calls received after business hours (8am to 8pm Eastern US time) will be returned within two business days.
To see a list of certified Eversense providers, go to https://www.eversensediabetes.com/find-a-provider/
• All symptoms of infection (e.g., increased temperature, inflammation, redness, pain, tenderness, warmth, swelling or purulence) at the insertion or removal area should be reported. If any of the
above occurs, please advise patients to contact their physician immediately.
• Store the sensor pack refrigerated at the labeled temperature range.
• Review the Eversense CGM System User Guide to help facilitate your patient’s understanding of their new Eversense CGM System and determining their personalized glucose settings.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 1 1/31/19 10:33 AM
Page 2

2
Eversense CGM Sensor Insertion and Removal Instructions
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 2 1/31/19 10:33 AM
Page 3

1
Eversense CGM Sensor Insertion and Removal Instructions
1. Overview of the Eversense
Continuous Glucose Monitoring (CGM) System
Congratulations on having Eversense CGM technology to assist your patients in managing their diabetes. The Eversense CGM System is for people with diabetes to continually measure glucose levels
for up to 90 days from the time of sensor insertion.
Some of the features of the Eversense CGM System:
• Wireless communication with the sensor, smart transmitter and app.
• Long-term sensor wear in the upper arm for up to 90 days.
• Alerts when pre-set Low or High Glucose Alert levels (hypoglycemia or hyperglycemia) are passed.
• Predictive alerts to alert the patient before reaching pre-set Low or High Glucose Alert levels.
• Use of mobile device (e.g., smartphone) to display glucose readings.
• On-body vibe alerts with the smart transmitter even when mobile device is not nearby.
• Provides readings within 40-400 mg/dL (2.2-22.2 mmol/L) range every 5 minutes.
• Trend arrows that show whether glucose values are rising or falling and how fast.
• Graphs and statistics that show glucose results in easy-to-understand formats.
• Removable and rechargeable smart transmitter.
• Event entry capabilities (like meals, exercise and insulin).
• Stores glucose data in the app and on the smart transmitter.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 1 1/31/19 10:33 AM
Page 4

2
Eversense CGM Sensor Insertion and Removal Instructions
Eversense CGM System Components
The System includes:
1) a small sensor inserted subcutaneously by a doctor,
2) a removable smart transmitter worn over the sensor, and
3) a mobile app to display the glucose readings.
Eversense Sensor
The sensor is inserted under the skin (upper arm) and measures glucose in interstitial fluid for up to 90 days. These glucose levels are then calculated
by the smart transmitter and sent to the app.
The Eversense Sensor lasts up to 90 days. The sensor has a silicone ring that contains a small amount of dexamethasone acetate, an anti-inflammatory
steroid drug. The dexamethasone acetate minimizes inflammatory responses, very similar to common medical devices, such as pacemakers.
Specially designed sensor insertion tools are provided for subcutaneous insertion of the sensor. Other equipment necessary for the procedure, but not
included in the Eversense Insertion Tool Pack, is listed in Section 4.
Eversense Smart Transmitter
The removable smart transmitter is worn externally over the sensor and powers the sensor. It wirelessly sends glucose data (via Bluetooth) to the
mobile device app. The smart transmitter also provides on-body vibe alerts based on the pre-set glucose level settings. It has a rechargeable battery
and is reusable for up to one year. Adhesive patches included with the Eversense Insertion Tools Pack are provided for the patient to replace daily.
Eversense App
The Eversense App is a software application that runs on a mobile device (e.g., smartphone) and displays glucose data in a variety of ways. It also
provides alerts based on the pre-set glucose level settings.
Note: Not actual size
Sensor
Eversense App
Smart Transmitter
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 2 1/31/19 10:33 AM
Page 5

3
Eversense CGM Sensor Insertion and Removal Instructions
Continuous glucose monitoring aids in the management of diabetes and glucose control, which can improve your patient’s quality of life. Best results are achieved when the user is fully informed about the
risks and benefits, insertion procedure, follow-up requirements, and self-care responsibilities. Patients should not have the sensor inserted if they cannot properly operate the CGM System.
The CGM System measures glucose in interstitial fluid (ISF) between the body’s cells. Physiologic dierences between ISF and blood from a fingerstick may result in dierences in glucose measurements.
These dierences are especially evident during times of rapid change in blood glucose (e.g., after eating, dosing insulin, or exercising). Glucose levels in ISF lag behind glucose levels in blood by
several minutes.
The sensor has a silicone ring that contains a small amount of an anti-inflammatory drug (dexamethasone acetate). It has not been determined whether the risks associated with injectable dexamethasone
acetate apply to the dexamethasone acetate elution ring inside the sensor. The elution ring releases a small amount of dexamethasone acetate when the sensor comes in contact with body fluids and serves
to minimize the body’s inflammatory response to the inserted sensor. Dexamethasone acetate in the ring may also cause other adverse events not previously seen with the injectable form.
Indications for Use
The Eversense CGM System is indicated for continually measuring glucose levels in adults (18 years or older) with diabetes for up to 90 days.
The system is intended to:
• Provide real-time glucose readings.
• Provide glucose trend information.
• Provide alerts for the detection and prediction of episodes of low blood glucose (hypoglycemia) and high blood glucose (hyperglycemia).
The system is a prescription device. Historical data from the system can be interpreted to aid in providing therapy adjustments. These adjustments should be based on patterns and trends seen over time.
The system is indicated for use as an adjunctive device to complement, not replace, information obtained from standard home blood glucose monitoring devices.
2. Benets and Risks
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 3 1/31/19 10:33 AM
Page 6

4
Eversense CGM Sensor Insertion and Removal Instructions
MRI Safety Information
Non-clinical testing has demonstrated the Eversense Sensor is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
• Static magnetic field of 1.5T or 3.0T
• Maximum spatial field gradient of 2000 gauss/cm (20 T/m)
• Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 W/kg (First Level Controlled Operating Mode)
Under the scan conditions defined above, non-clinical testing results indicate the Eversense Sensor is expected to produce a maximum temperature rise of less than 5.4 °C after 15 minutes of continuous
scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 2.83 inches (72 mm) from the Eversense Sensor when imaged with a gradient echo pulse sequence and a 3T MR system.
The Eversense Smart Transmitter is MR Unsafe and MUST BE REMOVED before undergoing an MRI procedure. Before you undergo an MRI procedure, tell the MRI sta that you have an Eversense Sensor and
Smart Transmitter.
Contraindications
The smart transmitter is incompatible with magnetic resonance imaging (MRI) procedures. The smart transmitter is MR Unsafe and MUST BE REMOVED before undergoing an MRI (magnetic resonance
imaging) procedure. For information on the sensor, please see MRI Safety Information.
The system is contraindicated in people for whom dexamethasone or dexamethasone acetate may be contraindicated.
Mannitol or sorbitol, when administered intravenously, or as a component of an irrigation solution or peritoneal dialysis solution, may increase blood mannitol or sorbitol concentrations and cause falsely
elevated readings of the patient’s sensor glucose results. Sorbitol is used in some artificial sweeteners, and concentration levels from typical dietary intake do not impact sensor glucose results.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 4 1/31/19 10:33 AM
Page 7

5
Eversense CGM Sensor Insertion and Removal Instructions
Warnings
• The Eversense CGM System has not been tested using insertion sites other than the upper arm.
• Patients should always test glucose with a blood glucose meter before making a treatment decision. Using the sensor glucose value to make a treatment decision could result in a high or low
blood glucose.
• If at any time there are symptoms of a low or high glucose level OR if patient symptoms are not consistent with the sensor glucose readings, patients should test glucose levels with a blood
glucose meter.
• Patients should not use a smart transmitter if it is damaged or cracked as this could result in electrical shock.
• Patients should avoid close contact with electromagnetic interference (EMI) while wearing the smart transmitter.
• Antibiotics of the tetracycline class may falsely lower sensor glucose readings. Patients should not rely on sensor glucose readings while taking tetracyclines.
• The bandage should remain covering the incision for 48 hours as this is a standard of care to allow formation of a water-tight seal to help protect against infection. Until it has healed, patients
should always cover the insertion site with a sterile bandage before placing the smart transmitter adhesive over the sensor. Failure to do so could result in infection at the insertion site.
Risks and Side Eects
The glucose alerts and notifications will not audibly notify the user when the sound on their mobile device is turned o. If the system cannot display a glucose value, it also cannot provide glucose alerts.
If the patient is unable to feel the vibration of the smart transmitter he/she may not notice the alerts. The system’s calculated glucose can be slightly dierent from a blood glucose meter. This may cause
an alert to activate at a dierent time than they would have if the system’s values always matched the blood glucose meter values. If the patient does not take frequent blood glucose measurements and
misses an alert, he/she may not be aware of high or low glucose levels. Medical attention may be needed in the event that he/she has high or low glucose and is unaware of it. If the patient does not test
their glucose with a blood glucose meter when symptoms of a low or high blood glucose level appear OR when symptoms are not consistent with the sensor glucose readings, he/she may miss a high or
low glucose event. If a patient does not always test glucose with a blood glucose meter before making a treatment decision, he/she may inadvertently cause a high or low blood glucose value because
actual glucose values can be slightly dierent than the system’s displayed values.
The sensor is inserted by making a small incision and placing it under the skin. This process may cause infection, pain or skin irritation. Additionally, the adhesive may cause a reaction or skin irritation.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 5 1/31/19 10:33 AM
Page 8

6
Eversense CGM Sensor Insertion and Removal Instructions
Precautions
• The sensor and sensor holder are sterile in the unopened, undamaged, sterile package. The sensor should not be used if the sterile package has been opened or damaged.
• A sensor should not be inserted if it has been dropped from a height greater than 30 cm.
• Use only the insertion tools provided in the insertion tool kit to insert the sensor. Other insertion tools may damage the sensor.
• Instruct patients to notify airport security personnel of the presence of the device when going through the security system.
• Patients should NOT exchange smart transmitters with another person. Each smart transmitter can be linked to only one sensor at a time. The system is to be used by a single patient in the home
environment.
• The following medical therapies or procedures have not been tested with the Eversense Sensor and may cause permanent damage to the sensor particularly if used in close proximity to the
device:
– Lithotripsy – The use of lithotripsy is not recommended for people who have an inserted sensor because the eects are unknown.
– Diathermy – DO NOT use diathermy on people who have an inserted sensor. Energy from the diathermy can transfer through the sensor and cause tissue damage in the insertion area.
– Electrocautery – The use of electrocautery near the inserted sensor may damage the device. DO NOT use electrocautery near the sensor.
• Patients should NOT wear the smart transmitter during medical x-rays or computed tomography (CT) scans. To avoid interference with results, the smart transmitter should be removed before
undergoing medical x-ray or CT scans.
• The sensor and smart transmitter should be linked the day of insertion. Failure to link the sensor and smart transmitter could result in a delay in receiving glucose readings.
Warnings (continued)
• The system should only be calibrated using a fingerstick blood sample. Alternative sites (such as forearm or palm) should not be used to calibrate the system.
• Insulin should not be injected and infusion sets for insulin pumps should not be inserted within 4 in (10.16 cm) of the sensor site. If the insulin delivery site is within 4 in (10.16 cm) of the sensor
site, it may interfere with sensor glucose readings and can cause inaccurate glucose readings.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 6 1/31/19 10:33 AM
Page 9

7
Eversense CGM Sensor Insertion and Removal Instructions
Precautions (continued)
• Steroid use – It has not been determined whether the risks usually associated with injectable dexamethasone acetate apply to the use of this dexamethasone acetate elution ring, a highly
localized, controlled-release device. The dexamethasone acetate ring could cause other adverse events not listed or previously seen.
• If the sensor, insertion site or smart transmitter feels warm, the patient should remove the smart transmitter immediately and contact his/her physician for further advice. A warm sensor could
mean there is an infection or a sensor malfunction.
• Patients should NOT attempt to use the Eversense App while operating a motor vehicle.
• Patients should not receive massage therapy near the inserted sensor site. Massage therapy near the sensor site could cause discomfort or skin irritation.
• Patients should use only the AC power adapter and USB cable provided with the smart transmitter when charging the smart transmitter battery. Use of another power supply could damage the
smart transmitter, not allowing glucose readings to be received properly, and could result in voiding the warranty.
• If the patient has any concerns about allergic reaction to adhesive products containing silicone, he/she should contact the physician prior to use. The Eversense adhesive patch should be
discarded after each use of up to 24 hours.
• Patients should not change the unit of measurement unless they have discussed it with their physician. Using the incorrect unit of measure could result in missing a high or low glucose event.
• Entering incorrect blood glucose values for calibration can result in inaccurate sensor glucose readings, which may result in the user missing a high or low glucose event.
• Patients should follow their health care provider’s recommendation for setting their glucose alerts. Incorrectly setting the glucose alerts can result in the user missing a high or low glucose event.
• Patients should pay attention to the glucose alerts the system provides. Failure to appropriately respond to an alert might result in the user missing a high or low glucose event.
• The Eversense NOW Remote Monitoring App does not replace the monitoring regimen as directed by the health care provider.
• The Eversense CGM System has not been tested in the following populations: women who are pregnant or nursing, people under the age of 18, critically ill or hospitalized patients, people
receiving immunosuppressant therapy, chemotherapy or anti-coagulant therapy, those with another active implantable device, e.g., an implantable defibrillator (passive implants are allowed,
e.g., cardiac stents), those with known allergies to or using systemic glucocorticoids (excluding topical, optical or nasal, but including inhaled). The system’s accuracy hasn’t been tested in these
populations, and sensor glucose readings may be inaccurate, resulting in missing a severe low or high glucose event.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 7 1/31/19 10:33 AM
Page 10

8
Eversense CGM Sensor Insertion and Removal Instructions
3. Eversense CGM System Candidates
and Pre-Insertion Activities
Candidate Selection
Per ACE/AACE guidelines*, potential candidates for CGM include those patients:
• Taking insulin to treat their T1 or T2 diabetes, and motivated to optimize their blood glucose management with the addition of new glucose monitoring technology.
• Able to follow device labeling and use their blood glucose meter results to make treatment decisions.
• Have hypoglycemic unawareness/frequent hypoglycemia.
• With their hemoglobin A1c (HbA1c) over target, or with excess glycemic variability – requiring HbA1c lowering without increased hypoglycemia.
Eversense CGM System Candidates
• Must have a compatible Android or IOS device, be familiar with its functionality and have WiFi connectivity. For a list of compatible devices, visit eversensediabetes.com.
• Willing to enter a calibration blood glucose (BG) into the app twice a day.
• Discuss appropriate placement of sensor insertion and smart transmitter wear.
• No known contraindication to dexamethasone acetate.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 8 1/31/19 10:33 AM
Page 11

9
Eversense CGM Sensor Insertion and Removal Instructions
• Is not receiving mannitol or sorbitol, administered intravenously, or as a component of an irrigation solution or peritoneal dialysis solution, as this may increase blood mannitol or sorbitol concentrations
and cause falsely elevated readings of sensor glucose results. Sorbitol is used in some artificial sweeteners, and concentration levels from typical dietary intake do not impact sensor glucose results.
• Is not pregnant or under the age of 18.
* Blevins T, Bode B, Garg S, Grunberger G, Hirsch I, Jovanovic L, et al. Statement by the American Association of Clinical Endocrinologists Consensus Panel on Continuous Glucose Monitoring. Endocrine
Practice, 2010; 16(5): A.
Pre-Insertion Training Activities for Patient
• Download Eversense App to compatible mobile device (requirements are listed in User Guide) and become familiar with functionality.
• Discuss the importance of setting the correct “Unit of Measure” in the Eversense App.
• Go to eversensediabetes.com – view insertion animation video, download Quick Reference Guide (QRG) and/or User Guide for review.
To pair Smart Transmitter with Compatible Mobile Device
• Confirm the patient has downloaded the Eversense CGM App from the App Store or Google Play store.
• Charge smart transmitter for 15 minutes
• Pair smart transmitter to mobile device.
• Set system preferences according to doctor recommendations.
• Instruct patients to bring smart transmitter and mobile device to clinic if it was shipped to patient’s home.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 9 1/31/19 10:33 AM
Page 12

10
Eversense CGM Sensor Insertion and Removal Instructions
The Eversense CGM System Kit comes in three packages: 1) Sensor Pack, 2) Insertion Tools Pack, and the 3) Smart Transmitter Pack.
IMPORTANT: The Sensor Pack and Insertion Tools Pack contain components that are packaged sterile. Both packs are designed for single patient-use only. DO NOT re-use, re-process or re-sterilize
the sensor, blunt dissector, or insertion tool.
Items Not Included: Other procedure instruments, tools and equipment are not included and must be provided by the clinic.
4. Eversense CGM System Kit
1. Eversense Sensor Pack (Sensor in holder)
The Sensor is shipped sterile inside a protective holder for safe handling purposes. You will need to transfer the sensor to the insertion tool before use.
The pouch that holds the sensor is not sterile.
The sensor is approximately 3.5 mm x 18.3 mm and is subcutaneously inserted using the insertion tool. The sensor has a silicone ring that contains an
anti-inflammatory steroid drug (dexamethasone acetate). Upon exposure to body fluids the dexamethasone acetate is eluted from the ring in the area near
the sensor. The dexamethasone acetate minimizes inflammatory responses, very similar to some already available medical devices (e.g., pacemaker leads).
IMPORTANT: Store the sensor pack refrigerated at the labeled temperature range.
Sensor Sensor
holder
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 10 1/31/19 10:33 AM
Page 13

11
Eversense CGM Sensor Insertion and Removal Instructions
2. Eversense Insertion Tools Pack
(Incision Template , Blunt Dissector, Insertion Tool, Tray, Adhesive Patches, and Insertion/Removal Instructions)
The Incision Template is used to guide and mark the incision area on the skin surface by aligning the marking
template to the marked outer edges of the smart transmitter when placed in a comfortable position.
The Blunt Dissector is used to create the subcutaneous pocket for insertion of the sensor. This tool has two depth
guards to help prevent the pocket from being made too deep in the skin. The depth guards have guide marks to
assist in determining the length of the subcutaneous pocket for placing the sensor.
The Insertion Tool is used to insert the sensor inside the subcutaneous pocket created with the blunt dissector. It has
two guide marks on the cannula to assist in proper placement.
The Adhesive Patch (90 patches in pack) has an adhesive side that attaches to the back of the smart transmitter and
a silicone adhesive side that attaches to the skin intended to be changed daily.
3. Eversense Smart Transmitter Pack
(Smart Transmitter, Power Supply, User Guide, Quick Reference Guide)
The Smart Transmitter is the reusable and rechargeable device worn externally over the sensor. The smart transmitter
wirelessly powers the sensor. Use only the Power Supply included in this kit to charge the smart transmitter.
The User Guide and Quick Reference Guide are designed for the patient to learn about their Eversense CGM System.
Incision Point
Marking Template
Blunt Dissector
Guide Marks
Depth Guards
Guide Marks
Insertion Tool
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 11 1/31/19 10:33 AM
Page 14

12
Eversense CGM Sensor Insertion and Removal Instructions
The sensor package, blunt dissector, and insertion tool have been sterilized by the method indicated on the package labels.
Inspect the condition of the sterile package before opening and using the contents.
• DO NOT use the contents if the package is broken or torn, or if contamination is suspected because of a defective sterile package seal.
• DO NOT re-sterilize the sensor or the components by any sterilization method.
• DO NOT use the product if the labeled “Use By” date has passed.
Handling and Storage
• Handle the sensor and all other components with care, using appropriate aseptic technique.
• DO NOT open any of the sterile packages until ready for use.
• Keep sharp instruments away from the kit components.
• DO NOT use the sensor or any kit component if it has been dropped on a hard surface from a height of more than 30 cm.
• Store the sensor package refrigerated at the labeled temperature range.
• Dispose of product packaging in accordance with clinic, administrative and/or local government policy.
5. Product Handling
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 12 1/31/19 10:33 AM
Page 15

13
Eversense CGM Sensor Insertion and Removal Instructions
6. Suggested Equipment
Items Not Included: Other procedure instruments, tools and equipment are not included in insertion tool kit and must be provided by the clinic. Please see list of suggested equipment below.
Materials (or equivalent) suggested for sensor insertion/removal:
• Chlorhexidine OR Betadine solution
• 2-3 Sterile Gauze Pads
• 1 Disposable Sterile Scalpel (e.g., Disposable Sterile Scalpel, #15)
• 1 Sterile Syringe and Needle (for lidocaine injection)
• Steri-Strip Adhesive Skin Closure and/or available sutures (physician preference)
• 1 sterile scissors (e.g., disposable) to cut steri strips
• 1 Sterile Towel Drape
• 1 Sterile Drape with aperture approximately 22 in x 25 in
• 2 Tegaderm™ + Pad Film Dressing
• 1 Lidocaine HCL without epinephrine (1-2 mL)
• 1 Surgical skin marker
• 3 Sterile, non-latex surgical gloves, physician-preferred size
• 1 10 mL sterile saline filled syringe (for insertion only)
• 1 Sterile surgical clamp 10-16cm
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 13 1/31/19 10:33 AM
Page 16
14
Eversense CGM Sensor Insertion and Removal Instructions
Before inserting the sensor, confirm that the patient:
• Does not have allergies to the antiseptic and local anesthetic to be used during insertion.
Note: The procedure below assumes a right handed physician with the patient facing (left arm insertion) or looking away from (right arm insertion) the physician. The dimensions indicated in the
instructions are approximate to give a conceptual context of the insertion.
7. Insertion Procedure
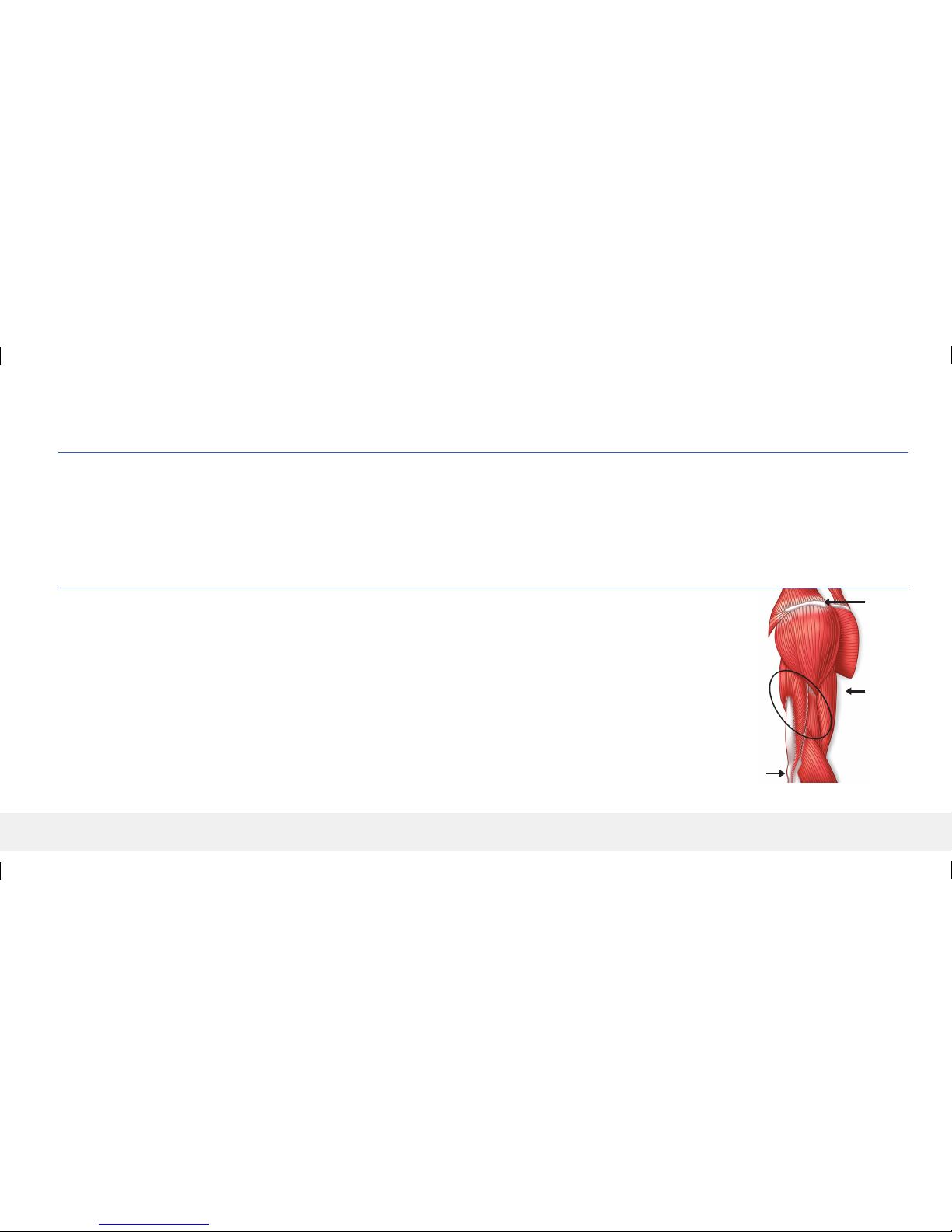
Acromion
Process
Suggested
Insertion
Area
Epicondyle
A. Prep the Insertion Area
1.
With the subject seated on the procedure table, position the smart transmitter on the patient’s arm to select the insertion location for the sensor.
It is recommended to alternate arms for subsequent insertion sites.
Suggested insertion location is approximately at the midway point between the acromion process and the lateral epicondyle.
Things to consider when choosing insertion location:
• It must be comfortable for the user to wear 24/7 for 90 days. Place the smart transmitter on the intended site and confirm that the patient is
comfortable with the placement.
• Not too lateral such that patient cannot easily apply adhesive patch.
• Avoid area with loose skin such as back of arm.
• Avoid areas with scar tissue, tattoo, nevus, or apparent or noticeable blood vessels that could be incised.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 14 1/31/19 10:33 AM
Page 17
15
Eversense CGM Sensor Insertion and Removal Instructions
B. Open the Sensor Pack and Insertion Tools Pack
Over the prepared sterile field, remove the sensor holder from the Sensor pouch and remove the sterile inner tray with tools from the Insertion Tools Pack and place in the sterile procedure field created for
the procedure. Note that the inner tray of the Sensor Insertion Package is sterile and can be placed within the sterile procedure field.
Precautions
• The sensor and sensor holder are sterile in the unopened, undamaged, sterile package. The sensor should not be used if the sterile package has been opened or damaged.
• DO NOT insert a sensor if it has been dropped from a height of 30 cm or more.
• Use only the insertion tools provided in the insertion tool kit to insert the sensor. Other insertion tools may damage the sensor.
2. Once the position for the smart transmitter is selected, mark the corners on the skin.
3. Using the non-sterile incision template, align the template inside the marked lines and mark the skin for the incision using the incision template’s slot.
4. Position the patient in a reclined position preferably on their side, with the elbow flexed up to 90 degrees and the palm resting on the chest or abdomen.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 15 1/31/19 10:33 AM
Page 18

16
Eversense CGM Sensor Insertion and Removal Instructions
2. Align the insertion tool cannula and sensor holder.
Ensure that the blue thumb slide is still in the retracted position.
Align the slot of the sensor holder with the exposed slot of the thumb slide and the triangle on the side of the
sensor holder with the triangle on the insertion tool.
Slots aligned
1. Remove the cap from the end of the sensor holder by pressing the ridged portion and pulling the cap.
Discard the cap.
C. Prepare the Sensor
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 16 1/31/19 10:33 AM
Page 19

17
Eversense CGM Sensor Insertion and Removal Instructions
3. Slide the sensor holder over the insertion tool cannula so that the two triangles are touching at the tip.
4. Depress the blue thumb slide down to unlock and advance it all the way forward until it stops.
This action secures the sensor inside the cannula. The cannula is now visible through the slot in the sensor holder.
DO NOT RETRACT the thumb slide at this step.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 17 1/31/19 10:33 AM
Page 20

18
Eversense CGM Sensor Insertion and Removal Instructions
5. Depress the ridged portion of the sensor holder to remove it from the insertion tool.
Discard the sensor holder. You should see the tip of the sensor at the end of the insertion tool.
6. Place the insertion tool back in its original placement in the tray.
The insertion tool will snap into position in the insertion kit inner tray and the tip of the cannula with the sensor will
be positioned in the preformed well in the tray.
7. Wet the cannula and sensor by filling the preformed well with enough sterile saline (0.95 sterile saline for
injection) to completely cover the cannula and sensor (approximately 10 mL). To ensure proper hydration,
submerge the tip in the well for a few minutes (approximately 5 minutes).
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 18 1/31/19 10:33 AM
Page 21

19
Eversense CGM Sensor Insertion and Removal Instructions
D. Clean and Anesthetize the Insertion Area
1.
If not done previously, position the patient in a reclined position, preferably on their side, with the elbow flexed
up to 90 degrees and the palm resting on the chest or abdomen.
2. Clean and disinfect the insertion area.
Apply disinfectant chlorhexidine to marked area. Cover the arm with sterile drape so opening is around incision site.
3. Anesthetize the insertion area as appropriate.
Local anesthesia (approximately 2 mL of Lidocaine) should be injected approximately 5 mm along the planned
incision (along AB) and approximately 30 mm perpendicular to the planned incision (along CD) which is the planned
track of the blunt dissector tool. (Figure 1).
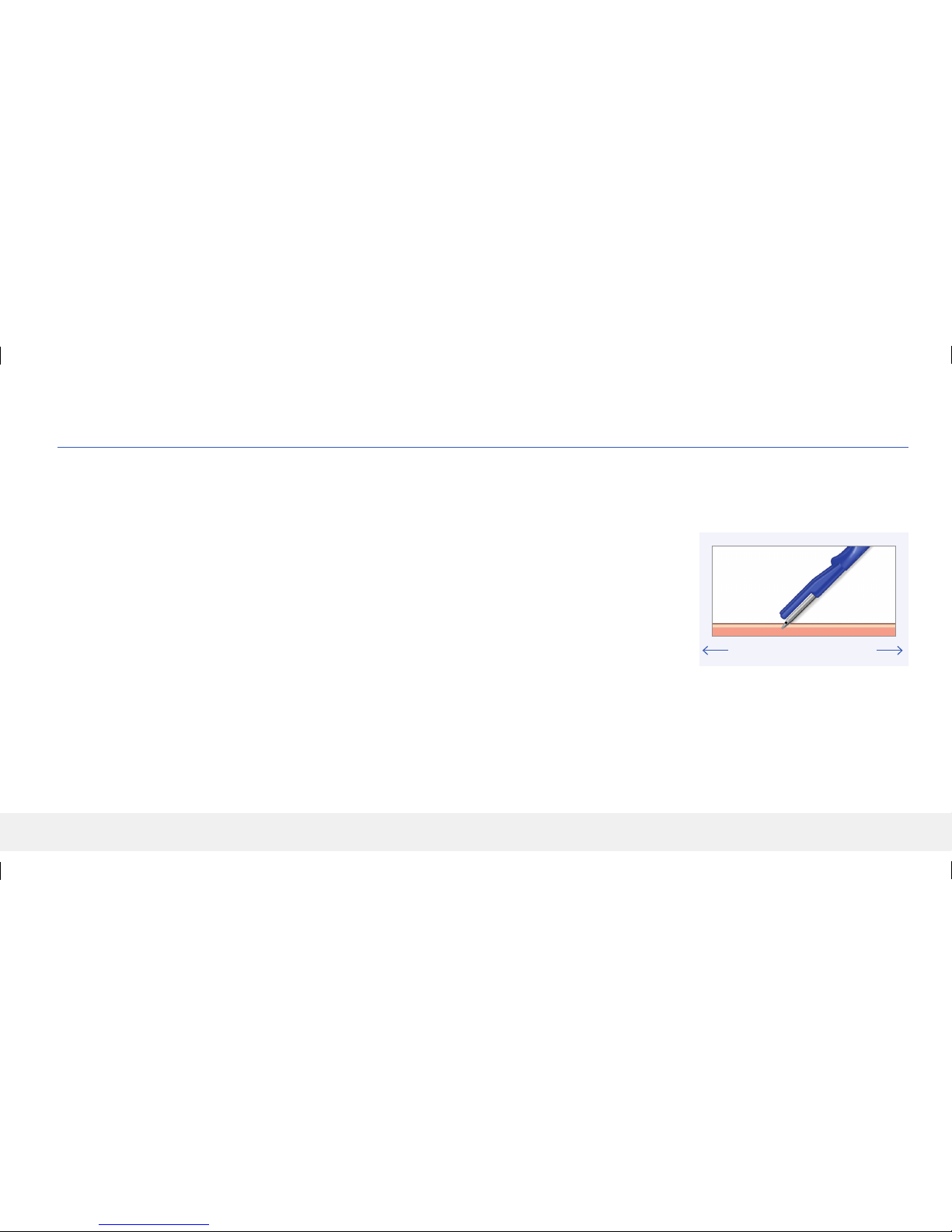
Figure 1
A
D C B
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 19 1/31/19 10:33 AM
Page 22
20
Eversense CGM Sensor Insertion and Removal Instructions
2. Remove the blunt dissector from the tray and introduce the blunt dissector at approximately a 45 degree entry angle at the midline
between A and B (Figures 1 & 2) so that the tip and tapered portion of the blunt dissector are under the skin, and until the depth
guards are touching the skin.
1. Once the insertion area is suciently anesthetized, make an approximately 5 mm incision at the insertion location such that you will be able to create an appropriately sized subcutaneous pocket
approximately 3-5 mm below the skin surface.
Start incision at point B (Figure 1) and go towards point A, until the incision is approximately 5 mm.
E. Make Incision and Subcutaneous Pocket
Figure 2
Elbow
Shoulder
Dissector
AB
Figure 2
Elbow
Shoulder
Dissector
AB
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 20 1/31/19 10:33 AM
Page 23

21
Eversense CGM Sensor Insertion and Removal Instructions
4. Move the blunt dissector toward the shoulder, while maintaining the metallic and plastic parts of the tool in close contact with
the skin to ensure the smallest possible angle of the pocket with respect to the skin (Figure 3).
Continue advancing the tool until the incision between A and B is within the white guide marks on the depth guards
(Approximately 25-30 mm) (Figure 4). Completely retract the blunt dissector and set aside.
Note:
• Pinching and tenting the skin can aid in forming a small space in the skin for insertion.
• Slight rotation of the blunt dissector along the axis of the tool while advancing may be helpful.
• DO NOT create a pocket more than 3-5 mm below the skin. If the sensor is placed too deep, it may be dicult to communicate with
the smart transmitter or to later remove.
• It is important to ensure that the subcutaneous pocket is parallel to and along the same axis as the humerus bone. When you place
the sensor, it should be level in the pocket, which will facilitate communication between the sensor and the smart transmitter.
3. With the tips of the depth guards on the skin and the blunt dissector at the subcutaneous space,
lower the angle of skin entry to approximately 5-10 degrees (Figure 3) taking care to ensure
that the fingers are not under the metal rod or plastic portions of the tool which would cause a
steeper angle.
Figure 3
ElbowShoulder
Dissector
AB
A
D C B
ElbowShoulder
Physician
Figure 4
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 21 1/31/19 10:33 AM
Page 24

22
Eversense CGM Sensor Insertion and Removal Instructions
1. Using approximately a 45 degree entry angle, place the tip of the insertion tool into the incision opening such that the tip of the
cannula is beneath the incision.
2. Similar to steps E3 & E4, lower the entry angle to about 5-10 degrees and advance toward the shoulder following the pocket created
by the blunt dissector.
3. Advance the tool until the incision line is between the first and second marked lines on the cannula.
If necessary, re-use the blunt dissector or widen the incision if excessive force is encountered. DO NOT force the insertion tool into the
incision site.
4. Pushing down on the back of the thumb slide to unlock it, retract the thumb slide to deploy the sensor into the pocket.
The slide locks into place when it has reached the end point. DO NOT re-advance the thumb slide.
5. Lightly palpate the insertion area to confirm that the sensor is in place; remove the insertion tool from the incision.
6. Close and dress the incision in the appropriate manner using adhesive skin closure (e.g., Steri-Strip™) or suture and dressing, making
sure the two sides of the incision are closed together.
F. Sensor Placement and Incision Closure
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 22 1/31/19 10:33 AM
Page 25

23
Eversense CGM Sensor Insertion and Removal Instructions
G. Insertion Tool and Blunt Dissector Disposal
Dispose of used insertion tool and blunt dissector in accordance with clinic, administrative and/or local government policy.
H. Connecting the Eversense CGM System
1.
Confirm the patient’s mobile device has been paired with the Eversense App and has an internet connection.
2. Link the sensor to the smart transmitter.
a. Place the smart transmitter directly over the bandage.
b. On the Eversense App, use the Placement Guide screen to confirm there is a signal.
c. Navigate away from the Placement Guide page once you have confirmed there is a signal.
Note: It may take up to 5 minutes to receive the notification for “New Sensor Detected”. DO NOT remove the smart transmitter from over the insertion site until the linking process is complete.
You may use the Eversense adhesive to place the smart transmitter over the bandage of the insertion site.
Refer to the Eversense CGM System User Guide, Inserting and Linking the Sensor for additional information.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 23 1/31/19 10:33 AM
Page 26

24
Eversense CGM Sensor Insertion and Removal Instructions
8. Post-Insertion Patient CGM Start-Up
Your patients may need assistance in getting started with the Eversense CGM System. Refer to the User Guide and Quick Reference Guide that is included in the smart transmitter pack for information on
getting the smart transmitter and mobile device ready for use.
This includes:
• Charging the smart transmitter.
• Downloading the Eversense App to their mobile device.
• Personalizing the patient’s glucose settings.
• Pairing (connecting) the smart transmitter and app.
• Linking the smart transmitter with the sensor after the sensor is inserted.
Note:
• All but the linking step can be completed before the sensor is inserted.
• Patients do not need to secure the smart transmitter over the sensor during the first 24 hours after insertion. After the sensor is linked to the smart transmitter, the sensor requires 24 hours to stabilize in
the body before glucose values can be calculated by the smart transmitter.
• If the smart transmitter is secured over the sensor within the first 24 hours after insertion, the patient will receive a message indicating a Warm-Up Phase status of the system and will provide the patient
with a 24-hour countdown.
• If the smart transmitter is not secured over the sensor and turned o to avoid vibrations, patient must remember to turn smart transmitter back on at the 24th hour. It will take about 5 minutes after the
smart transmitter is placed over the sensor for the first calibration prompt to be displayed. After calibration is completed, the smart transmitter should not be removed for 15 minutes.
• Glucose readings will appear on screen after successfully completing the 2nd calibration.
Review the Eversense User Guide to help facilitate your patient’s understanding of their new Eversense CGM System and determining their personalized glucose settings.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 24 1/31/19 10:33 AM
Page 27

25
Eversense CGM Sensor Insertion and Removal Instructions
9. Sensor Removal Procedure
1. Using the initial incision point as a guide, palpate and locate the sensor to determine an appropriate incision location. For reference, mark both ends of the sensor if possible to palpate.
Note: If the sensor cannot be located by palpating, the smart transmitter may be used to aid in locating the sensor. To use the smart transmitter to locate the sensor, open the Placement Guide page
in the App. Move the smart transmitter around the sensor insertion area until the screen displays the greatest signal strength. Mark the edges of the smart transmitter at this location and use the incision
template to determine the proper incision location.
2. Mark the incision point on the skin.
If the site of the original incision is within 3-5 mm of the distal tip of the sensor, removal can be accessed through the same location.
A. Locate the Sensor
1.
Position the patient in a reclined position, preferably on their side, with the elbow flexed up to 90 degrees and the palm resting on the chest or abdomen.
2. Clean and disinfect the insertion area.
Prepare the insertion site and surrounding area, using aseptic technique.
3. Anesthetize the insertion area as appropriate for the patient similar to step D3 in section 7.
B. Prep the Removal Area
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 25 1/31/19 10:33 AM
Page 28

26
Eversense CGM Sensor Insertion and Removal Instructions
1. Carefully dissect the subcutaneous tissue until the end of the sensor distal to the incision can be grasped by a small surgical clamp (such as 10 -16cm). Spreading of the tissue through the incision
using the clamp both parallel and perpendicular to the incision may be required to enable visualization and grasping of the sensor with the clamp.
2. Put gentle pressure on the proximal end of the sensor through the skin to help stabilize and facilitate grasping the distal end of the sensor. Use a clamp to grasp the distal end of the sensor and
remove it from the pocket. Rotation of the sensor with the clamp may aide in freeing the sensor from any attached tissue.
3. If the sensor is encapsulated, further dissection may be necessary to grasp and remove the sensor.
D. Remove the Sensor
1.
Close and dress the incision in the appropriate manner using adhesive skin closure (e.g., Steri-Strip™) or suture, making sure the two sides of the incision are closed together.
E. Close and Dress the Incision in the Appropriate Manner
1.
Dispose of sensor according to your area’s local regulations.
F. Sensor Disposal
1.
Push down on the skin over the expected location of the proximal end of the sensor to stabilize it.
2. Create approximately a 5-6 mm incision through the dermis at the location determined in A1.
C. Incision and Pocket Opening
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 26 1/31/19 10:33 AM
Page 29

27
Eversense CGM Sensor Insertion and Removal Instructions
1. Unable to insert blunt dissector through incision
a. Incision may be too small
Increase incision size by 2-3 mm and re-insert the blunt dissector.
b. Refer to tips for proper insertion technique in this document
– Pinching or tenting the skin can aid in forming a small pocket for the insertion.
– Slight rotation of the blunt dissector along the axis of the tool may be helpful.
– DO NOT create a pocket more than 3-5 mm below surface of skin.
2. Unable to advance the insertion tool into the subcutaneous pocket
a. Ensure the insertion tool is below the incision when advancing into subcutaneous pocket
b. Incision size may be too small
Increase incision size by 3-5 mm with scalpel and re-insert the insertion tool.
10. Potential Complications
The insertion and removal of the Eversense Sensor is a minor procedure and requires aseptic technique to minimize the possibility of infection. Please review this document for complete training.
3. Unable to locate the subcutaneous pocket with the insertion tool when inserting the sensor
Re-insert the blunt dissector into incision to ensure subcutaneous pocket is adequate.
4. Subject experiences pain during the procedure
Administer additional local anesthetic as required.
5. Excessive bleeding after incision is made
Apply pressure until bleeding subsides.
A. During Insertion Process
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 27 1/31/19 10:33 AM
Page 30

28
Eversense CGM Sensor Insertion and Removal Instructions
1. Unable to palpate/locate the sensor
Use the Placement Guide on the app and the smart transmitter to find the sensor. Once the
location of the sensor is made with the Placement Guide, mark the position of the smart
transmitter on the skin and use the incision template to mark the point of incision. In some
cases, an ultrasound may be required to locate the proper point of the incision.
2. Excessive bleeding after the sensor is removed
Apply pressure and, if necessary, use sutures to close incision in place of Steri-Strips
TM
.
B. During Removal Process
3. Subject experiences pain during the procedure
Administer additional local anesthetic as required.
4. Tissue encapsulation prevents sensor from moving
Dissect encapsulation by spreading the tissue using the clamp/or other desired instrument
as required. Gently rotate the clamp with the secured sensor to release any small fibrous
tissue encapsulation.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 28 1/31/19 10:33 AM
Page 31

29
Eversense CGM Sensor Insertion and Removal Instructions
11. Device Performance
This section lists Device Performance Characteristics.
Clinical Study Performance
The safety and eectiveness of the Eversense CGM System has been evaluated in several feasibility and pivotal studies. Two studies – PRECISE II (2016) and PRECISION (2018) – were conducted in the U.S.
to evaluate the Eversense CGM System performance in terms of safety and eectiveness. The data collected was analyzed using a new algorithm, SW 602. Accuracy assessments were made at various
points during the studies and subjects were asked to report any adverse events throughout the studies.
PRECISE II Study
PRECISE II was a multi-site, non-randomized single-arm pivotal clinical study. Ninety (90) adults (18 years and older) with type 1 or type 2 diabetes participated in the study across 8 sites in the U.S. Each
had a sensor inserted in the upper arm to collect glucose data but not be displayed to the subject. Some participants had a sensor inserted into each arm for clinical data collection. Participants interacted
with the system to calibrate and address notifications not related to glucose data. Accuracy was measured during day-long clinic visits, with blood taken about every 15 minutes. These visits occurred on
Days 1, 30, 60, and 90. At each visit, sensor accuracy was evaluated relative to a standard laboratory analyzer known as the YSI. Glucose readings were compared at the same moment in time between the
reference analyzer and the continuous device. A safety follow-up visit occurred at Day 100, or ten days after the sensor was removed.
PRECISION Study
PRECISION was a second non-randomized single-arm pivotal clinical study conducted in the U.S. in 3 sites. Thirty-five (35) adults (18 years and older) with type 1 or type 2 diabetes participated in the study
which was similar in study design as PRECISE II, except glucose readings and high/low glucose alerts were not blinded to the participants, and study visits on days 7 and 14 were added. The table below
characterizes accuracy of the system by assessing the sensor glucose values against the YSI reference values.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 29 1/31/19 10:33 AM
Page 32

30
Eversense CGM Sensor Insertion and Removal Instructions
*Data is from primary sensors only; glucose values between 40 and 400 mg/dL.
Since the two studies are similar, the PRECISION study is included in this report. For detailed information on the PRECISE II results, please go to our website www.eversensediabetes.com.
Study
Number of
Participants
Total Number of
Paired CGM and
YSI Values
Percent Within
15/15%
Percent Within
20/20%
Percent Within
30/30%
Percent Within
40/40%
MARD
PRECISE II 90 adults
(18 years and older)
15,753 87% 94% 99% 100% 8.5%
PRECISION 35 adults
(18 years and older)
15,170 85% 93% 98% 99% 9.6%
Table 1 – Accuracy to YSI in PRECISE II* and PRECISION*
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 30 1/31/19 10:33 AM
Page 33

31
Eversense CGM Sensor Insertion and Removal Instructions
Table 2 – Accuracy to YSI in PRECISION Study
Eversense Accuracy to YSI in PRECISION Study
Accuracy was measured by comparing the Eversense sensor glucose values to YSI blood glucose values. For blood glucose values less than or equal to 80 mg/dL, the mean absolute dierence between the
two results was calculated. For values greater than 80 mg/dL, the mean absolute relative dierence was calculated.
YSI Glucose Ranges (mg/dL) Paired CGM-YSI (n) Mean Absolute Relative Dierence (%)
Overall 15,170 9.6
< 40 15 16.2
40 - 60 1,267 8.1
61 - 80 2,212 8.6
81 - 180 5,685 9.7
181 - 300 3,210 7. 7
301 - 350 1,527 6.8
351 - 400 1 ,174 6.5
> 400 80 11.2
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 31 1/31/19 10:33 AM
Page 34

32
Eversense CGM Sensor Insertion and Removal Instructions
Table 3 – Eversense Percentage of Readings in Agreement Overall in the PRECISION study
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 15,170 85.4% 92.8% 98.1% 99.3% 0.7%
40 - 60 1,236 91.9% 96.0% 98.4% 99.3% 0.7%
61 - 80 2,003 87. 3% 94.1% 99.1% 99.6% 0.4%
81 - 180 5,786 80.5% 89.9% 97. 2% 99.0% 1.0%
181 - 300 3,566 84.8% 92.8% 98.1% 99.2% 0.8%
301 - 350 1,628 92.8% 97.5% 99.1% 99.9% 0.1%
351 - 400 951 91.5% 95.8% 98.6% 99.8% 0.2%
Performance in the PRECISION study was also measured by calculating the percentage of sensor glucose readings within 15 mg/dL or 15% of the YSI reference. These tables show the percent agreement at
multiple levels, at dierent glucose ranges, and at dierent days during the sensor wear. Results in the glucose ranges of 80 mg/dL or less reflect the percentage of values within mg/dL, and results in the
glucose ranges over 80 mg/dL reflect the percentage within reference. As an example, glucose values between 40 and 60 mg/dL were within 15 mg/dL of the reference value 91.9% of the time.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 32 1/31/19 10:33 AM
Page 35

33
Eversense CGM Sensor Insertion and Removal Instructions
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 2,665 79.1% 88.9% 95.8% 98.5% 1.5%
40 - 60 274 86.5% 91.2% 96.4% 97.8% 2.2%
61 - 80 378 81.7% 87.8% 97.1 % 98.7% 1.3%
81 - 180 962 76.7% 87.8% 95.6% 98.9% 1.1%
181 - 300 585 80.3% 91.1% 96.1% 97.8% 2.2%
301 - 350 250 77.6% 90.4% 95.2% 99.2% 0.8%
351 - 400 216 74.5% 85.2% 94.0% 99.1% 0.9%
Table 4 – Eversense Percentage of Readings in Agreement on Day 1 in the PRECISION study
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 33 1/31/19 10:33 AM
Page 36
34
Eversense CGM Sensor Insertion and Removal Instructions
Table 5 – Eversense Percentage of Readings in Agreement on Day 7 in the PRECISION study
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 2,926 86.1% 93.3% 98.1% 99.0% 1.0%
40 - 60 214 93.0% 98.1% 99.5% 100.0% 0.0%
61 - 80 404 87. 6% 94.3% 99.3% 99.8% 0.2%
81 - 180 1,053 80.0% 89.3% 96.7% 97.9% 2.1%
181 - 300 630 84.6% 93.5% 97.5% 99.2% 0.8%
301 - 350 385 94.3% 9 7.4% 99.7% 100.0% 0.0%
351 - 400 240 95.0% 97.5% 100.0% 100.0% 0.0%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 34 1/31/19 10:33 AM
Page 37

35
Eversense CGM Sensor Insertion and Removal Instructions
Table 6 – Eversense Percentage of Readings in Agreement on Day 14 in the PRECISION study
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 2,997 88.1% 94.6% 98.8% 99.6% 0.4%
40 - 60 269 87.0% 92.6% 96.7% 98.9% 1.1%
61 - 80 407 84.0% 93.4% 99.5% 100.0% 0.0%
81 - 180 999 82.0% 91.7% 98.0% 99.1% 0.9%
181 - 300 663 91.3% 95.9% 99.4% 99.8% 0.2%
301 - 350 383 97.1% 99.0% 99.5% 100.0% 0.0%
351 - 400 276 96.7% 99.6% 100.0% 100.0% 0.0%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 35 1/31/19 10:33 AM
Page 38

36
Eversense CGM Sensor Insertion and Removal Instructions
Table 7 – Eversense Percentage of Readings in Agreement on Day 30 in the PRECISION study
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 2,284 88.0% 94.3% 98.9% 100.0% 0.0%
40 - 60 209 98.6% 99.5% 100.0% 100.0% 0.0%
61 - 80 416 92.8% 97. 6% 100.0% 100.0% 0.0%
81 - 180 936 81.1% 90.0% 9 7.4% 100.0% 0.0%
181 - 300 492 87.4 % 94.7% 99.8% 100.0% 0.0%
301 - 350 183 99.5% 100.0% 100.0% 100.0% 0.0%
351 - 400 48 100.0% 100.0% 100.0% 100.0% 0.0%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 36 1/31/19 10:33 AM
Page 39

37
Eversense CGM Sensor Insertion and Removal Instructions
Table 8 – Eversense Percentage of Readings in Agreement on Day 60 in the PRECISION study
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 2,133 86.9% 93.7% 98.5% 99.6% 0.4%
40 - 60 165 98.2% 100.0% 100.0% 100.0% 0.0%
61 - 80 176 89.2% 96.6% 100.0% 100.0% 0.0%
81 - 180 880 85.9% 94.2% 98.3% 99.4% 0.6%
181 - 300 592 79.1% 86.8% 97.1 % 99.5% 0.5%
301 - 350 239 96.7% 100.0% 100.0% 100.0% 0.0%
351 - 400 81 98.8% 100.0% 100.0% 100.0% 0.0%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 37 1/31/19 10:33 AM
Page 40

38
Eversense CGM Sensor Insertion and Removal Instructions
Table 9 – Eversense Percentage of Readings in Agreement on Day 90 in the PRECISION study
Percent of CGM System Readings Within
CGM System Glucose
Range (mg/dL)
Paired CGM and
YSI References
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
Overall 2,165 83.9% 92.2% 98.5% 99.3% 0.7%
40 - 60 105 93.3% 100.0% 100.0% 100.0% 0.0%
61 - 80 222 90.5% 96.8% 99.1% 99.1% 0.9%
81 - 180 956 77.7% 86.7% 97.5% 99.2% 0.8%
181 - 300 604 85.9% 94.9% 98.8% 99.2% 0.8%
301 - 350 188 89.9% 98.9% 100.0% 100.0% 0.0%
351 - 400 90 95.6% 98.9% 100.0% 100.0% 0.0%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 38 1/31/19 10:33 AM
Page 41

39
Eversense CGM Sensor Insertion and Removal Instructions
Table 10 – Eversense Agreement when “LO” or “HI” is Displayed in the PRECISION study
Glucose values below 40 mg/dL or above 400 mg/dL are shown on the mobile app as “LO” or “HI”. The table below shows, as a percentage, agreement of sensor glucose values when outside the display
range of 40 mg/dL to 400 mg/dL.
Eversense Alert Performance
The tables in this section show an alert performance assessment. The Confirmed Event Detection Rate shows the percentage of time the Eversense CGM System confirmed the reference value by presenting
an alert within a 15 minute window of a reference value beyond the alert setting threshold. The Missed Detection Rate shows the percentage of time the Eversense CGM System did not present an alert
within a 15 minute window of a reference value beyond the alert setting threshold. The True Alert Rate shows the percentage of time the alert from the CGM system was confirmed by a reference value
within a 15 minute window of the alert being presented. The False Alert Rate shows the percentage of time the alert from the CGM system was not confirmed by a reference value within a 15 minute window
of the alert being presented.
Reference mg/dL
CGM Readings CGM-Ref pairs < 50 < 60 < 70 < 80 > 80 Total
‘LO’
n 6 7 9 9 0 9
% 66.7% 77.8% 100% 100% 0% 100%
Reference mg/dL
CGM Readings CGM-Ref pairs > 340 > 320 > 280 > 240 < 240 Total
‘HI’
n 359 383 399 404 1 405
% 88.6% 94.6% 98.5% 99.8% 0.2% 100%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 39 1/31/19 10:33 AM
Page 42

40
Eversense CGM Sensor Insertion and Removal Instructions
Alert Setting (mg/dL) Confirmed Event Detection Rate Missed Detection Rate True Alert Rate False Alert Rate
Low Alert
60 76% 24% 81% 19%
70 90% 10% 95% 5%
80 94% 6% 96% 4%
90 96% 4% 95% 5%
High Alert
120 99% 1% 96% 4%
140 99% 1% 96% 4%
180 98% 2% 95% 5%
200 98% 2% 95% 5%
220 98% 2% 95% 5%
240 98% 2% 95% 5%
300 93% 7% 94% 6%
Table 11 – Eversense High and Low Glucose Alert Performance (Threshold Only) in the PRECISION study
The table below shows an assessment of the ability of the Eversense CGM System to detect high and low glucose levels, and assess true positive alerts vs false positive alerts. These are shown as a
percentage of alerts provided compared to the YSI reference values at various thresholds.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 40 1/31/19 10:33 AM
Page 43

41
Eversense CGM Sensor Insertion and Removal Instructions
Alert Setting (mg/dL) Confirmed Event Detection Rate Missed Detection Rate True Alert Rate False Alert Rate
Low Alert
60 89% 11% 77% 23%
70 95% 5% 92% 8%
80 97% 3% 93% 7%
90 98% 2% 93% 7%
High Alert
120 99% 1% 96% 4%
140 99% 1% 94% 6%
180 99% 1% 93% 7%
200 98% 2% 94% 6%
220 98% 2% 94% 6%
240 99% 1% 94% 6%
300 95% 5% 92% 8%
Table 12 – Eversense High, Low, and Predictive Alert Performance (Threshold and Predictive) in the PRECISION study
The table below shows an assessment of the ability of the Eversense CGM System to detect high, low, and predictive high and low alerts, and assess true positive alerts vs false positive alerts. These are
shown as a percentage of alerts provided compared to the YSI reference values at various thresholds.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 41 1/31/19 10:34 AM
Page 44

42
Eversense CGM Sensor Insertion and Removal Instructions
Table 13 – Eversense Rate of Change Trend Agreement in the PRECISION Study
Reference Rate of Change (mg/dL/min)
Percent of Matched Pairs in Each Reference
Trend Range for Each CGM ROC Range
CGM Trend (mg/dL/min) < -3 [-3, -2) [-2, -1) [-1, 1] (1, 2] (2, 3] > 3 Total
< -3 14% 20% 30% 36% 0% 0% 0% 135
[-3, -2) 5% 12% 38% 44% 1% 1% 0% 338
[-2, -1) 1% 3% 24% 71% 1% 0% 0% 1,115
[-1, 1] 0% 1% 5% 89% 5% 1% 0% 10,655
(1, 2] 0% 0% 1% 55% 35% 8% 1% 997
(2, 3] 0% 0% 2% 36% 41% 18% 4% 354
> 3 0% 1% 0% 25% 35% 30% 10% 175
Total 57 155 990 11,137 1,085 288 57 13,769
Eversense Rate of Change Trend Agreement
The shaded area of the table below shows agreement between the Eversense glucose trends and the YSI reference trends while glucose is trending at dierent rates (mg/dL per minute). As an example,
when glucose is trending at a rate of between -1 and 1 mg/dL/minute, Eversense glucose trends are in agreement with the reference trends 89% of the time.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 42 1/31/19 10:34 AM
Page 45

43
Eversense CGM Sensor Insertion and Removal Instructions
Table 14 – Concurrence with YSI Range All Days in the PRECISION Study
Eversense Concurrence with YSI Values
The shaded areas of the tables below show what percentage of YSI reference values were in the same range as the sensor glucose values. As an example, when sensor glucose is between 81 and 120 mg/dL,
YSI reference values are in the same range 71% of the time.
Percent of Matched Pairs in Each YSI Glucose Range for Each CGM Glucose Range YSI (mg/dL)
CGM
(mg/dL)
Paired
CGM-YSI (n)
< 40 40 - 60 61 - 80 81 - 120 121 - 160 161 - 200 201 - 250 251 - 300 301 - 350 351 - 400 > 400
40 - 60 1,236 1% 63% 34% 2% 0% 0% 0% 0% 0% 0% 0%
61 - 80 2,003 0% 22% 67% 10% 0% 0% 0% 0% 0% 0% 0%
81 - 120 2,524 0% 2% 17% 71% 10% 0% 0% 0% 0% 0% 0%
121 - 160 2,342 0% 0% 0% 18% 71% 11% 0% 0% 0% 0% 0%
161 - 200 1,727 0% 0% 0% 1% 24% 59% 16% 0% 0% 0% 0%
201 - 250 1,502 0% 0% 0% 0% 1% 19% 65% 14% 1% 0% 0%
251 - 300 1,257 0% 0% 0% 0% 0% 1% 18% 51% 27% 3% 0%
301 - 350 1,628 0% 0% 0% 0% 0% 0% 1% 10% 57% 32% 1%
351 - 400 951 0% 0% 0% 0% 0% 0% 0% 2% 26% 65% 7%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 43 1/31/19 10:34 AM
Page 46

44
Eversense CGM Sensor Insertion and Removal Instructions
Table 15 - Concurrence with YSI Range Day 1 in the PRECISION Study
Percent of Matched Pairs in Each YSI Glucose Range for Each CGM Glucose Range YSI (mg/dL)
CGM
(mg/dL)
Paired
CGM-YSI (n)
< 40 40 - 60 61 - 80 81 - 120 121 - 160 161 - 200 201 - 250 251 - 300 301 - 350 351 - 400 > 400
40 - 60 274 1% 59% 36% 4% 0% 0% 0% 0% 0% 0% 0%
61 - 80 378 0% 14% 65% 19% 1% 0% 0% 0% 0% 0% 0%
81 - 120 516 0% 1% 23% 63% 12% 0% 0% 0% 0% 0% 0%
121 - 160 332 0% 0% 0% 17% 70% 12% 1% 0% 0% 0% 0%
161 - 200 226 0% 0% 0% 1% 23% 58% 17% 0% 0% 0% 0%
201 - 250 268 0% 0% 0% 0% 4% 25% 55% 15% 0% 0% 0%
251 - 300 205 0% 0% 0% 0% 0% 2% 25% 57% 14% 1% 0%
301 - 350 250 0% 0% 0% 0% 0% 0% 4% 27% 42% 26% 1%
351 - 400 216 0% 0% 0% 0% 0% 0% 1% 9% 46% 40% 4%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 44 1/31/19 10:34 AM
Page 47
45
Eversense CGM Sensor Insertion and Removal Instructions
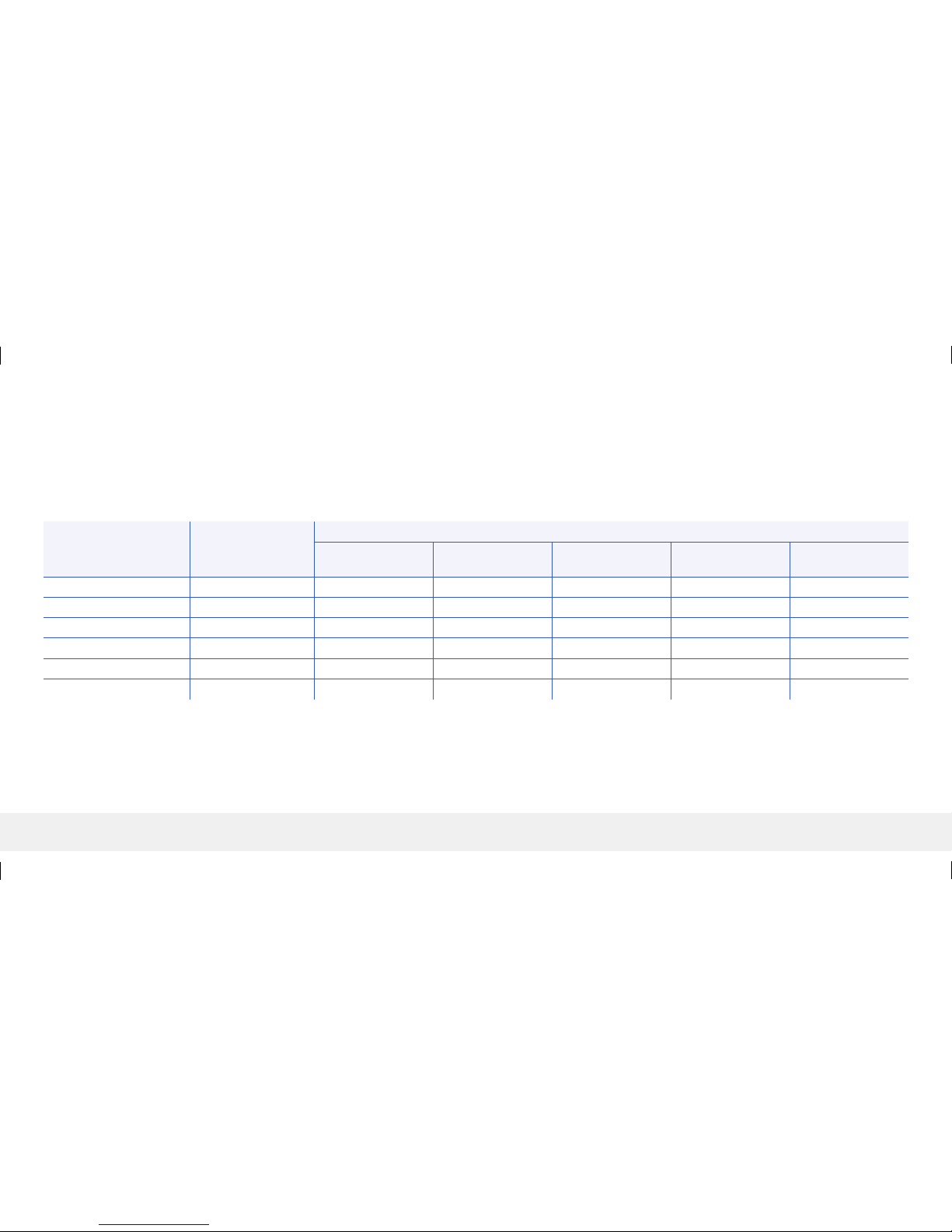
Calibration Stability Agreement
The table below compares the percentage of sensor glucose values to the YSI reference at various time points after a calibration entry. As an example, in the table below, 88% of the Eversense values were
within 15 mg/dL (for reference readings of 80 mg/dL or less), and within 15% (for reference readings over 80 mg/dL) of the reference value 8 to 10 hours after a calibration entry.
Table 16 – Eversense Calibration Stability Agreement in the PRECISION Study
Percent of CGM System Readings Within
Time from Calibration
Number of Paired
CGM-YSI
Percent 15/15% of
Reference
Percent 20/20%
of Reference
Percent 30/30%
of Reference
Percent 40/40%
of Reference
> 40/40%
of Reference
0 - 2 Hours 4,034 86.0% 93.6% 98.0% 99.3% 0.7%
2 - 4 Hours 3,979 85.6% 92.8% 98.4% 99.5% 0.5%
4 - 6 Hours 2,308 84.3% 92.2% 97. 7% 99.0% 1.0%
6 - 8 Hours 1,614 84.3% 92.7% 97.8% 99.4% 0.6%
8 - 10 Hours 1,372 88.0% 94.4% 98.5% 99.6% 0.4%
10 - 12 Hours 1,295 86.1% 92.9% 98.1% 99.2% 0.8%
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 45 1/31/19 10:34 AM
Page 48

46
Eversense CGM Sensor Insertion and Removal Instructions
PARD 9.9%
PCV 7.0%
PARD (Paired Absolute Relative Dierence) and Percent Coecient of Variation
In the PRECISION Study, 27 participants had two sensors inserted. The table below shows the precision of the sensor’s performance by comparing readings between the two sensors worn by each
participant. As an example, the absolute relative dierence between the sensors worn by each person is 9.9%, and the percent coecient of variation is 7.0%, showing the sensor values generally in
agreement throughout the 90 day study period.
Sensor Life
Sensor life measured the percentage of sensors being able to function through the intended 90 day durations of each study. In the PRECISE II study, nine sensors did not function through the 90 day period.
Function stopped at days 10, 30, 53, 67, 70, 80, 81, 83, and 85. The sensor failure on day 10 was due to a software anomaly that has since been resolved. Overall, the analysis estimated that 91% of sensors
remained functioning through 90 days. In the PRECISION study, all sensors functioned through 90 days.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 46 1/31/19 10:34 AM
Page 49

47
Eversense CGM Sensor Insertion and Removal Instructions
Percentage of Sensors that lasted 30, 60, and 90 days
Table 16B PRECISION
Table 16D PRECISION
Table 16A PRECISE II
Table 16C PRECISE II
Days Sensor Life
30 99%
60 97%
90 91%
Days Sensor Life
30 100%
60 100%
90 100%
Mean # of Days Median # of Days
88 91
Mean # of Days Median # of Days
92 91
Days 1 - 30 Days 31 - 60 Days 61 - 90 All Days
Number of Displayed Glucose Values 27 9,674 274,727 266,895 821,296
Expected Number of Glucose Values 285,579 280,478 276,757 842,814
% of Displayed Glucose Values 98% 98% 96% 97%
Data Availability
A total of 97% (821,296 out of expected 842,814) glucose values were available during the 90 day study.
Table 17 – Eversense Data Availability in the PRECISION Study
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 47 1/31/19 10:34 AM
Page 50

48
Eversense CGM Sensor Insertion and Removal Instructions
Safety
Both the PRECISE II and PRECISION studies lasted 90 days, and the number of related adverse events was recorded. The Eversense CGM System was well tolerated in the studies. During the studies’
15,921 sensor wear days, there were no unanticipated adverse events. Twenty-two adverse events were reported in 12 participants, including one serious adverse event related to the removal
procedure. There were no infections. Mild irritation, pain and redness at the sensor insertion site were observed at a low rate of occurrence. None of the adverse events resulted in hospitalization.
Event Type Number of Events Patients (n = 125)
Pain/Discomfort 6 5 (4.0%)
Redness/Erythema 2 1 (0.8%)
Dermatitis at Patch Location 2 1 (0.8%)
Bruising 2 1 (0.8%)
Skin Hyperpigmentation 2 1 (0.8%)
Paresthesia 1 1 (0.8%)
Syncope-vasovagal 1 1 (0.8%)
Pain 1 1 (0.8%)
Device Fragment not Recovered 2 2 (1.6%)
Additional Procedure to Remove Sensor Following First Attempt 3 2 (1.6%)
TOTAL 22 12 (9.6%)
Table 18 – Adverse Events
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 48 1/31/19 10:34 AM
Page 51

49
Eversense CGM Sensor Insertion and Removal Instructions
12. Technical Specications
Sensor Description
Length 18.3 mm
Diameter 3.5 mm
Materials Homopolymer polymethylmethacrylate (PMMA),
Hydroxyethylmethacrylate (HEMA) based Hydrogel, Platinum,
Silicone, Dexamethasone Acetate, epoxy 301-2
Storage Temp Between 36 °F (2 °C) and 46 °F (8 °C)
Sterilization Sterile by Ethylene Oxide
Sensor Holder Description
Materials Acrylonitrile butadiene styrene (ABS) and Polytetrafluoroethylene
(PTFE)
Blunt Dissector Description
Materials Acrylonitrile butadiene styrene (ABS), Stainless Steel
Storage Temp Between 50 °F (10 °C) and 86 °F (30 °C)
Sterilization Sterile by Ethylene Oxide
Insertion Tool Description
Materials Acrylonitrile butadiene styrene (ABS) and Polytetrafluoroethylene
(PTFE); Cyanoacrylate adhesive and Stainless Steel
Storage Temp Between 50 °F (10 °C) and 86 °F (30 °C)
Sterilization Sterile by Ethylene Oxide
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 49 1/31/19 10:34 AM
Page 52

50
Eversense CGM Sensor Insertion and Removal Instructions
Power Supply and Charger Description
Class II
Input AC Input, 100-240Vac, 50/60Hx, 0.3-0.15A
DC Output 5V DC, 1A (5.0 watts)
Moisture Protection IP22
USB Cable* for Charging
and Downloading
Description
Input/Output 5V DC, 1A
Type USB-A to USB micro-B
Length 36 inches (91 cm)
System component Part Number
Eversense Smart Transmitter Kit FG-3300-01-001
Charging Cable FG-6100-00-301
Charging Adapter FG-6201-91-301
Charging Cradle FG-6700-00-301
Eversense Adhesive Patches, White, 30 Pack
Eversense Adhesive Patches, Clear, 30 Pack
FG-6402-01-300
FG-6401-01-300
Eversense Quick Reference Guide LBL-1603-01-001
Eversense CGM User Guide LBL-1602-01-001
Eversense Data Management Software Application FG-5700-01-300
Eversense Mobile Application iOS
Eversense Mobile Application Android
FG-5500-01-300
FG-5600-01-300
Eversense Sensor Kit FG-4200-00-301
Eversense Insertion Tools Kit FG-8211-01-201
*If misused, the USB cable can pose a strangulation risk. The USB cable can be connected to the
power supply/charger and charged using an AC power outlet. To isolate the system, unplug the
charger/power supply from the outlet. If you charge the smart transmitter using a USB port on
your personal computer, ensure the personal computer complies the IEC 60950-1 (or equivalent)
safety standard.
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 50 1/31/19 10:34 AM
Page 53

51
Eversense CGM Sensor Insertion and Removal Instructions
Symbol Explanation
Lot number
Part number
Serial number
Type BF Applied Part
Non-ionizing electromagnetic radiation
Not made with natural rubber latex
Symbol Explanation
Consult accompanying documents
Caution, consult accompanying documents
Use by
Manufacturer
Date of manufacture
Storage temperature limits
Symbols on Packaging and Device
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 51 1/31/19 10:34 AM
Page 54

52
Eversense CGM Sensor Insertion and Removal Instructions
Symbol Explanation
Universal Serial Bus (USB)
FCC ID is assigned to all devices subject to certification
Magnetic Resonance Imaging (MRI) procedures are contraindicated for the
smart transmitter
No known hazards for leaving the sensor inserted in use with MR with a
static magnetic field of 1.5T or 3.0T
European Union WEEE Directive 2012/19/EU
Single use only
Do not re-sterilize
Symbol Explanation
Do not use if package is damaged
Sterilized using Ethylene Oxide
Non-sterile
U.S. (Federal) law restricts the sale of the Eversense CGM System to sale
by or on the order of a physician
Follow instructions for use
Symbols on Packaging and Device (continued)
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 52 1/31/19 10:34 AM
Page 55

3
Eversense CGM Sensor Insertion and Removal Instructions
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 3 1/31/19 10:34 AM
Page 56

© Senseonics, Inc. 2019 PN: LBL-1614-01-201_Rev G 01/2019
Eversense, Eversense Continuous Glucose Monitoring, Eversense CGM, Eversense Sensor,
Eversense Smart Transmitter, Eversense App and the Eversense logo are trademarks of
Senseonics, Incorporated. Other brands and their products are trademarks or registered
trademarks of their respective holders.
Manufactured and Distributed by:
20451 Seneca Meadows Parkway • Germantown, MD 20876-7005 USA
Phone: 844.SENSE4U (844.736.7348) • 301.515.7260
www.eversensediabetes.com
LBL-1614-01-201 Rev G_Insertion Tools Kit PI_EN_R2.indd 4 1/31/19 10:34 AM
 Loading...
Loading...