EUROIMMUN Medizinische Labordiagnostika AG MERGITE! Instructions For Use Manual

Instructions for use
MERGITE!
Wash system for Titerplane slides
MERGITE! 10, MERGITE! 50
EUROIMMUN Medizinische Labordiagnostika AG • 23560 Lübeck (Germany) • Seekamp 31 • Tel. +49 451 5855 -0 • Fax +49 451 5855 -591


The MERGITE! system provides you with efficient support for
processing IFA incubations using the Titerplane technique.
MERGITE! automatically performs the necessary washing processes
on the slides. Up to 50 substrate fields are washed simultaneously
but separately. This ensures a quick washing process without risk of
cross-contamination. A directed liquid flow is gently applied to every
substrate field, providing for a substrate-preserving and efficient
washing. Thus, the MERGITE! washing technology in combination
with standardized washing protocols ensures high reproducibility of
the results.
The different carriers of the MERGITE! system facilitate the handling
and positioning of slides, glass plates and cover glasses.
The device is controlled via an intuitive user software and touch
display. An integrated maintenance assistant reminds the user of all
necessary maintenance and guides the user through the
maintenance steps.
Instructions for use • MERGITE!


This documentation may not be forwarded or duplicated and its
Issued by:
EUROIMMUN Medizinische Labordiagnostika AG
Seekamp
D
Phone:
Fax:
Internet:
contents may not be utilised or communicated unless explicitly
authorised. Non-compliance will entail an obligation to pay
compensation. All rights are reserved in the case of a patent being
granted or the design being registered.
Document number ......................................... YG_0064_A_UK_C04
Version ........................................................................... 01/08/2018
S
oftware version .................................................................... 4.5.0.X
Translation of the original instructions for use
Print format: DIN A4
-23560 Lübeck
+49 (0) 451 5855-0
+49 (0) 451 5855-591
www.euroimmun.de
31
Instructions for use • MERGITE!


Contents
Contents
1 Introduction ................................................... 12
1.1 About these instructions for use ................................................... 12
1.2 Changes to this manual ................................................................. 12
1.3 Meaning of the safety information indicated ................................ 13
1.4 Symbols ........................................................................................... 14
1.5 Guidelines, laws, standards ........................................................... 17
1.6 Warranty .......................................................................................... 17
1.7 Limitation of liability ....................................................................... 17
1.8 Transport and storage requirements ............................................ 18
1.9 Disposal ........................................................................................... 19
1.10 Customer Support .......................................................................... 19
2 Safety ............................................................. 20
2.1 Intended purpose ............................................................................ 20
2.2 Validity ............................................................................................. 20
2.3 Technical safety .............................................................................. 20
2.3.1 Introduction ............................................................................... 20
2.3.2 Prerequisites for the safe use of the device .............................. 21
2.3.3 Device-specific safety instructions ............................................ 21
2.3.4 Instructions for reliable operation of the device ........................ 23
2.4 Note on the required protective equipment .................................. 23
Instructions for use • MERGITE! vii

Contents
2.5 Layout of the safety labels on the product ................................... 24
2.6 In the event of danger .................................................................... 24
2.7 Foreseeable incorrect use ............................................................. 24
2.8 Environmentally hazardous substances/materials ...................... 25
3 Delivery contents and accessories .............. 26
3.1 MERGITE! 10 ................................................................................ 26
3.1.1 Scope of delivery MERGITE! 10 ............................................ 26
3.1.2 Accessories MERGITE! 10 .................................................... 27
3.2 MERGITE! 50 ................................................................................ 27
3.2.1 Scope of delivery MERGITE! 50 ............................................ 27
3.2.2 Accessories MERGITE! 50 .................................................... 28
3.3 Spare parts ...................................................................................... 28
3.4 Supplies ........................................................................................... 29
4 Design and function ...................................... 30
4.1 Description of the functions .......................................................... 30
4.2 Device overview .............................................................................. 31
4.2.1 Connection panel ...................................................................... 32
4.2.2 Container with fill level monitoring ............................................ 33
4.2.3 Carriers ..................................................................................... 34
Carrier plate .............................................................................. 34
Slide carrier ............................................................................... 34
Cover glass carrier .................................................................... 35
Instructions for use • MERGITE!

Contents
5 Installation and start-up ................................ 36
6 Operation ....................................................... 37
6.1 Preparing for washing .................................................................... 37
Preparing the containers ........................................................... 37
Switching the device on ............................................................ 38
6.2 Washing ........................................................................................... 39
Washing Titerplane slides ......................................................... 40
6.3 Supporting the Titerplane incubation technique with
MERGITE! ...................................................................................... 45
Carrying out Titerplane incubation with MERGITE! ................ 45
6.4 Filling or draining the containers .................................................. 49
Draining the waste liquid container ........................................... 49
Filling the container for distilled water ....................................... 50
Filling the wash buffer container ............................................... 50
6.5 Rinsing with the wash buffer ......................................................... 51
Rinsing with wash buffer ........................................................... 51
6.6 Aspirating wash buffer residue ..................................................... 54
Aspirating wash buffer residue ................................................. 54
6.7 Switching the device off ................................................................. 55
Switching the device off ............................................................ 55
7 Administration ............................................... 56
7.1 Information about the device ......................................................... 57
7.2 Assay Manager ............................................................................... 58
Importing an assay ................................................................... 59
Instructions for use • MERGITE! ix

Contents
Exporting an assay ................................................................... 60
Deleting an assay ..................................................................... 60
7.3 Draining MERGITE! ......................................................................... 62
Draining the liquid system of the device ................................... 62
8 Maintenance .................................................. 63
8.1 Maintenance overview .................................................................... 65
8.1.1 Maintenance at the start of work ............................................... 65
8.1.2 Maintenance at the end of work ................................................ 65
8.1.3 Weekly maintenance ................................................................ 65
8.1.4 Monthly maintenance ................................................................ 65
8.1.5 Annual maintenance ................................................................. 65
8.2 Maintenance schedule ................................................................... 66
8.3 Maintenance and cleaning tasks ................................................... 67
Filling the system with distilled water ........................................ 67
8.3.1 Cleaning of the liquid system .................................................... 67
Cleaning the liquid system ........................................................ 67
8.3.2 Cleaning/disinfection of the containers ..................................... 70
Cleaning and/or disinfecting the containers .............................. 70
8.3.3 Cleaning the device and accessories ....................................... 71
Cleaning the device surfaces .................................................... 72
Cleaning the carriers and wash basin lid .................................. 72
8.3.4 Container preparation with SETUP CLEAN .............................. 72
Preparing the container with SETUP CLEAN (1:5) .............. 73
8.4 Measures for storage or transport ................................................ 73
Instructions for use • MERGITE!

Contents
9 Troubleshooting ............................................. 74
9.1 Switching on the device ................................................................. 74
9.2 Liquid system .................................................................................. 74
9.3 Power failure ................................................................................... 76
10 Technical data ............................................... 77
10.1 Mechanical data .............................................................................. 77
10.2 Environmental conditions .............................................................. 77
10.3 Electrical and operating data ......................................................... 78
11 Glossary ......................................................... 79
Instructions for use • MERGITE! xi
1 Introduction
Chapter
New
Changed
Deleted
7
1 Introduction
The present instructions for use are intended for the user and
support safe and efficient operation of MERGITE!.
This document applies for all model versions, is part of the system
and must therefore be stored for the user in its operating
environment.
Read the instructions for use carefully. Familiarity with these
instructions for use is required to operate the device. Pay attention to
the safety information for your safety and that of the environment.
1.1 About these instructions for use
These instructions for use explain
connecting the system components (connection diagram),
operation,
care and maintenance,
disposal.
Installation and commissioning is carried out by the Technical
Customer Support of EUROIMMUN AG.
Abbreviations and special terms are explained in the glossary. If this
document refers to EUROIMMUN AG or EUROIMMUN AG Technical
Customer Support, this can alternatively also be the authorised
representative in the relevant country.
1.2 Changes to this manual
The following changes to the previous version
(YG_0064_A_UK_C03) have been made:
1.2
8 (maintenance menu)
1.4 (type label)
3.1.2, 3.2.2
6. 3 (Introduction)
6.5 step 2)
8.4 instruction steps 1) –
13)
Tab. 1 Table of changes
12/84 Instructions for use • MERGITE!
1 Introduction
TYPE AND/OR SOURCE OF HAZARD
ALTERNATIVE: TYPE OF HAZARD
> Avoidance of hazard.
1.3 Meaning of the safety information indicated
The safety information in this document are clearly highlighted.
WARNING
Possible consequences if the instructions are not observed.
> Avoidance of hazard.
or
WARNING – TYPE OF HAZARD
Possible consequences if the instructions are not observed.
The gravity of the hazard is classified by signal words:
Danger signals the direct risk of fatal or serious injury.
Warning signals the potential risk of serious (irreversible) injury.
Caution signals the potential risk of non-serious (reversible)
injury.
Attention signals the risk of material damage.
Instructions for use • MERGITE! 13/84
1 Introduction
Symbol
Explanation
Symbol
Explanation
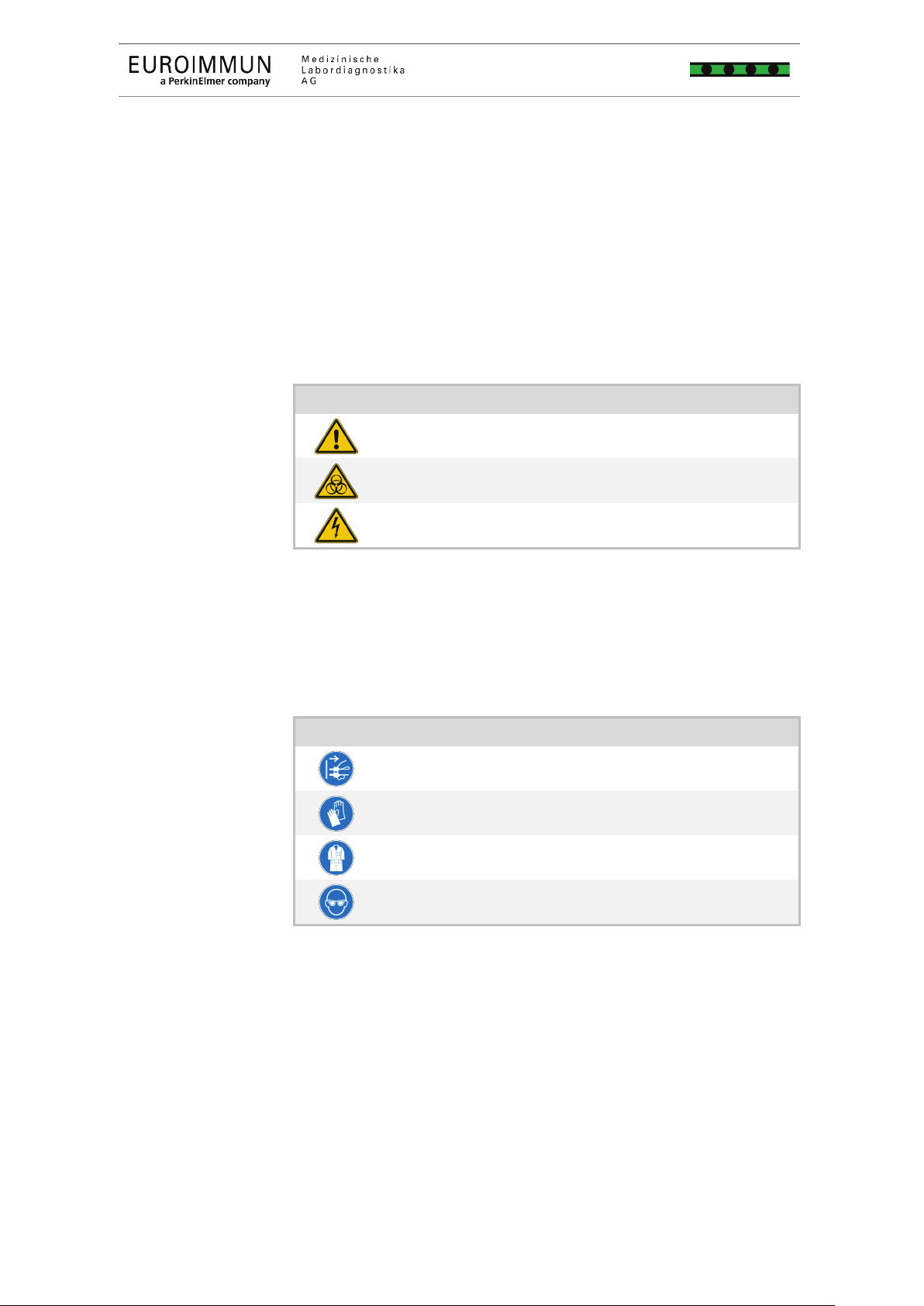
1.4 Symbols
Various symbols can be found on the device, packaging, in the
software and in the instructions for use.
Warning symbols
Warning symbols warn you of hazards. Please observe the
associated safety information.
General hazard sign
Warning of dangerous biological substances
Warning of dangerous electric voltage
Tab. 2 Warning symbols
Mandatory action symbols
Mandatory action symbols point out regulations which must be
observed.
Unplug mains plug
Wear protective gloves
Wear protective gown
Wear eye protection (safety goggles)
Tab. 3 Mandatory action symbols
14/84 Instructions for use • MERGITE!
1 Introduction
Symbol
Explanation
storage
Symbol
Explanation
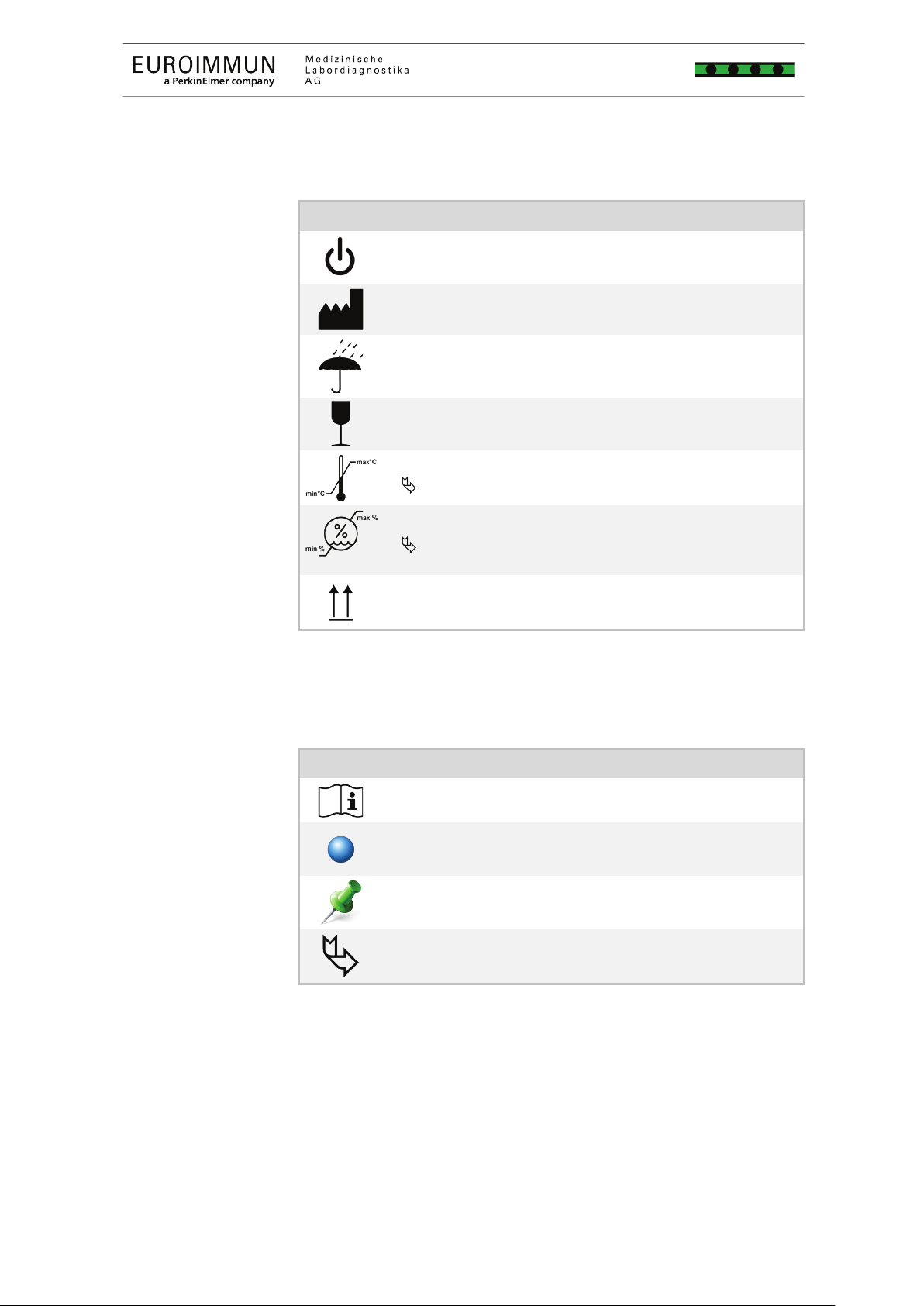
Symbols on the device and the packaging
On/Off switch device
Manufacturer
Protect from moisture
Fragile
Permissible temperature range for storage and transport
Chapter 10.2 Environmental conditions, p. 77
Permissible humidity for storage and transport
Chapter 10.2 Environmental conditions, p. 77
This side up
Tab. 4 Symbols on the device and packaging
Symbols in the instructions for use
Observe the instructions for use
Note
Recommendation
Page or chapter reference
Tab. 5 Symbols in the instructions for use
Instructions for use • MERGITE! 15/84
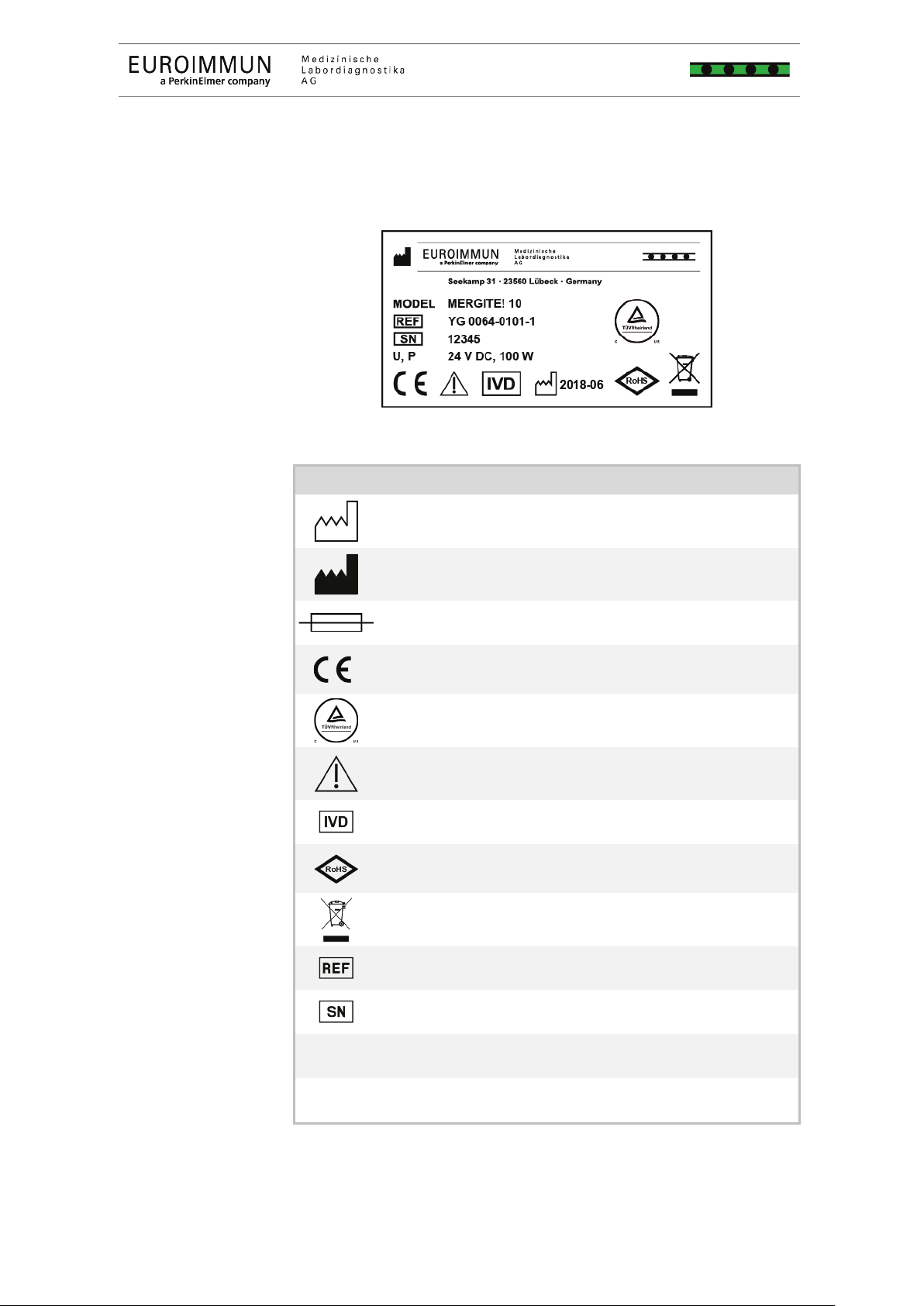
1 Introduction
Symbol
Explanation
the applicable European guidelines)
CSA marking (declaration of conformity that the product complies
Pay attention to the safety information, warnings and precautions in
(Restriction of Hazardous Substances)
U
P
Fig.
Symbols on the type plate
The type plate is located on the back of the device.
1 Type plate (exemple)
Manufacturing date
Manufacturer
Main fuse
CE marking (declaration of conformity that the product complies with
with the applicable guidelines in Canada and the US)
the instructions for use
In vitro diagnostic or IVD medical device
Restriction of use of certain hazardous substances
Marking pursuant to European Directive 2002/96/EC
On Waste Electrical And Electronic Equipment (WEEE)
Item number
Serial number
Supply
Power consumption
Tab. 6 Abbreviations and symbols on the type plate
16/84 Instructions for use • MERGITE!

1 Introduction
1.5 Guidelines, laws, standards
Information on complying with the regulations on device safety
pursuant to the EC declaration of conformity.
MERGITE! was developed and manufactured for in vitro
diagnostics in accordance with the current state of the art in
compliance with the requirements in EC Directive 98/79/EC
The product has been tested for compliance with the relevant
standards for IVD laboratory devices, EN 61010-1 (IEC 61010-1) and
EN 61010-2-101 and corresponds to the specific safety requirements
defined by the regulations on device safety.
Moreover, the product satisfies the requirements of the EN 61326-1
standard (electrical equipment for measurement, control and
laboratory use – EMC requirements) and complies with the limit
values in accordance with DIN EN 55011, Group 1, Class A.
The product also fulfils the ANSI C63.4:2014 (FCC Part 15, Subpart
B) and complies with its limit values.
1.6 Warranty
The statutory warranty periods applicable in the Federal Republic of
Germany, where the product was manufactured, apply.
1.7 Limitation of liability
The validation carried out by the EUROIMMUN Medizinische
Labordiagnostika AG (hereinafter referred to as EUROIMMUN AG
for short) comprises MERGITE! as a system for supporting IFA
protocols in combination with certain EUROIMMUN reagents. As a
result, the acceptance of liability requires that only EUROIMMUN AG
reagents are used together with the system subject to the standard
operating procedures released by EUROIMMUN AG.
By contrast, no liability is accepted if the device is validated in
combination with reagents provided by other manufacturers. An
acceptance of liability also requires that protocols processed using
the MERGITE! are always checked for their correctness by the user.
EUROIMMUN AG is generally liable for breaches of contractual
obligations of its legal representatives or vicarious agents and for tort
only for intentional or grossly negligent actions, and otherwise only in
the event of breach of an essential contractual obligation but limited
in amount to damages foreseeable at the time of conclusion of the
agreement and typical for the agreement.
Instructions for use • MERGITE! 17/84

1 Introduction
This liability limitation does not apply to damages arising from death,
physical injury or damage to health, if a defect is fraudulently
concealed, if a guarantee of quality is not observed, or to liability
according to the Product Liability Act.
EUROIMMUN AG is not liable for consequential damages, lost profit,
interruption of operation or loss of programs or electronic data.
In particular, EUROIMMUN AG also accepts no liability for damages
due to
failure to follow these instructions for use,
use other than the intended use,
natural wear and tear,
assignment of unqualified personnel,
use of information and data from the use of software or
devices without testing for plausibility and completeness,
use of reagents of other manufacturers in devices that have
been purchased by EUROIMMUN AG or provided in another
manner,
use of spare parts or accessories of other manufacturers or
use of hardware or software configurations of other
manufacturers, i.e. lack of compatibility of the EUROIMMUN
software with third party configurations,
independent interventions such as conversions or technical
modifications by the user,
deactivation of safety equipment without permission,
delays in delivery or failed deliveries due to statutory or
official export restrictions or associated conduct by third
parties.
The extent of EUROIMMUN AG’s liability is limited to the relevant
item value.
The general terms and conditions of EUROIMMUN AG also apply
(www.euroimmun.de).
1.8 Transport and storage requirements
The device may only be transported in the transport packaging
provided by EUROIMMUN AG. Contact EUROIMMUN AG for
more information in this respect.
Protect the device against vibrations during storage. For further
transport and storage regulations, please refer to the technical data
( Chapter 10.2 Environmental conditions, p. 77).
18/84 Instructions for use • MERGITE!

1 Introduction
1.9 Disposal
EU guideline on Waste Electrical and Electronic Equipment –
(WEEE) 2012/19/EC
The device may not be disposed of with standard household rubbish.
Please contact EUROIMMUN AG in order to dispose of the device.
RoHS/Reach
The products and processes of EUROIMMUN comply with the
following guidelines with regard to the labelling of substances as well
as the restriction of use of certain hazardous substances:
RoHS 2 (2011/65/EU)
REACH (Regulation (EC) no. 1907/2006), Regulation (EC) no.
765/2008)
1.10 Customer Support
You can reach EUROIMMUN AG Technical Customer Support from
Monday to Friday, between 8:00 am - 5:00 pm.
Telephone: + 49 (0) 451 5855-25550
E-mail: instrumentsupport@euroimmun.de
Instructions for use • MERGITE! 19/84

2 Safety
2 Safety
2.1 Intended purpose
MERGITE! is an in vitro diagnostic device that standardizes and
automates the washing processes of indirect immunofluorescence
tests using the Titerplane
Carriers support the handling of primary samples on glass plates, the
incubation and the mounting of the slides.
MERGITE! is intended exclusively for immunofluorescence tests by
EUROIMMUN AG which use the Titerplane technique.
It does not provide any diagnostics or suggestions for diagnosis.
The system has to be used in laboratory conditions in a defined place
and may not be used in an environment close to the patient. The
system can be used several times per day. It encompasses the
device and the software and may only be used by trained
professional staff.
™
technique.
All results have to be checked by trained medical staff. The diagnosis
or ruling out of an illness as well as an assessment of its likelihood
require a combination of epidemiological, anamnetic, medical and
diagnostic results which have to be taken into account for the
interpretation of the test results.
2.2 Validity
The immunofluorescence tests of EUROIMMUN AG and the
washing processes (assays) on MERGITE! system are subject to
ongoing development. The document YG_0064_UK_WXX (or
YG_0064_CA_WXX for Canada) contains a list of all validated
immunofluorescence tests for the device. It is included in the scope
of delivery.
For further information on using the device with specific test sets,
contact your customer support or visit our customer portal
(www.euroimmun.de).
2.3 Technical safety
2.3.1 Introduction
Dangers can arise for the user if the device is not used correctly, or is
used without due care.
20/84 Instructions for use • MERGITE!
2 Safety
ELECTRIC VOLTAGE
device.
Please observe all the safety instructions, warnings and
precautionary measures included on the device and in these
instructions for use.
Pay attention to all warning symbols on the device and
accessories.
The device must only be used in accordance with its intended
use.
The protective measures for the device may become ineffective if
the device is incorrectly operated.
The device may only be used with the provided accessories.
Familiarise yourself with the device before using it.
Only carry out the maintenance operations listed in these
instructions for use.
2.3.2 Prerequisites for the safe use of the device
The user must be trained to deal with biological material and
have technical knowledge of dealing with laboratory equipment.
This also includes the maintenance work listed in these
instructions for use. Typical qualification levels for this are
biology lab technician or medical lab technician.
The user has been instructed in operating the device by
EUROIMMUN AG.
2.3.3 Device-specific safety instructions
Electrical safety
WARNING
Electric shock
> Only connect the mains adapter to a professionally installed
mains socket with a ground contact.
> Only use the provided mains adapter and mains cable.
> Do not remove any of the housing components, as parts which
carry current could become exposed.
> Immediately disconnect the device from the power supply if
liquid penetrates into the inside of the device or leaks from the
Instructions for use • MERGITE! 21/84
Ensure that the On/Off switch of the device and the mains cable are
always accessible.
2 Safety
BIOLOGICAL SUBSTANCES AND REAGENTS
Avoid skin/mucous membrane contact with the biological
> Observe the instructions for use for the reagents.
DISPLAY DAMAGE
housing on the bottom.
DAMAGE TO THE SLIDE CARRIERS
Note: This combination is stackable.
WARNING
Biological safety
The device uses biological substances and reagents.
Potential risk of infection/skin irritation
>
substances and reagents used.
> Wear suitable protective clothing (protective gown, protective
gloves and if needed, safety goggles).
The use of specific reagents may lead to further hazards. Always pay
attention to the relevant instructions for use when handling these
reagents.
If liquids such as e.g. biological substances or reagents have been
spilled on the device, the contaminated areas must be immediately
cleaned and disinfected using a validated method.
ATTENTION
ATTENTION
Device safety
The display can be damaged through excessive force.
> Do not lift or carry the device by the display, but hold it at the
The supports of the slide carriers are delicate and can easily be
damaged by applying too much force.
> Handle the slide carriers with care.
> Avoid shocks against the slide carriers.
> If you have dropped a slide carrier, check it for damages.
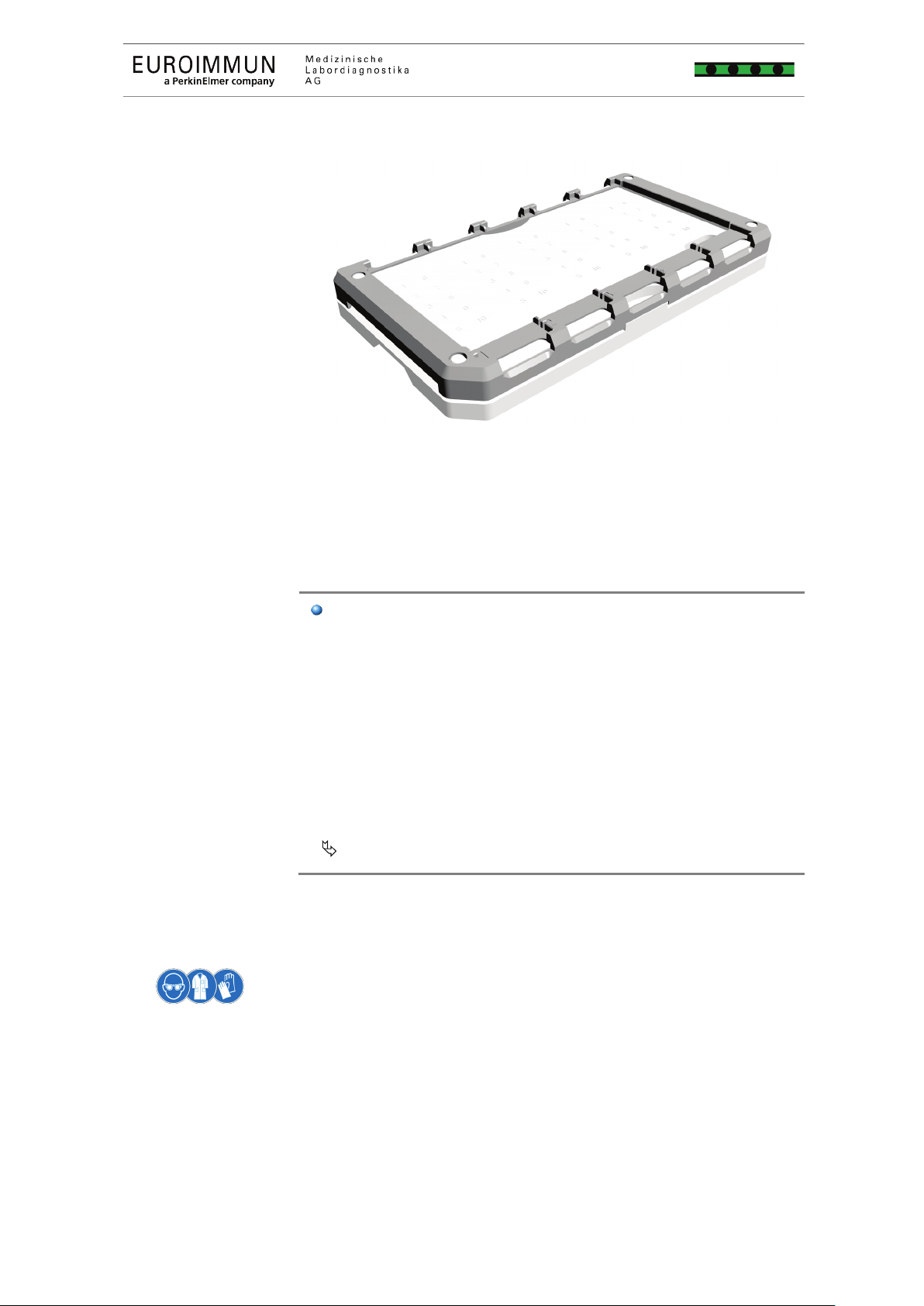
> Store the slide carriers safely on a carrier plate
( Fig. 2, p. 23).
22/84 Instructions for use • MERGITE!
2 Safety
The device must be placed on a stable and level surface.
on the same level as the device, in order to ensure safe
Fig.
2 Slide carrier on carrier plate for storing
2.3.4 Instructions for reliable operation of the device
Note
>
> Always set up the containers for distilled water and wash buffer
conveyance of the liquids.
> Do not expose the device to strong vibrations.
Internal hose connectors could become loose or components
could become damaged.
> Do not expose the device to strong magnetic fields.
Electronic components could become impaired or damaged.
> Comply with the environmental conditions for operation
Chapter 10.2 Environmental conditions, p. 77.
2.4 Note on the required protective equipment
Wear appropriate protective clothing, such as protective gloves and
protective gown and, if necessary, protective goggles when handling
biological material and reagents.
Instructions for use • MERGITE! 23/84
2 Safety
Symbol
Description
Fig.
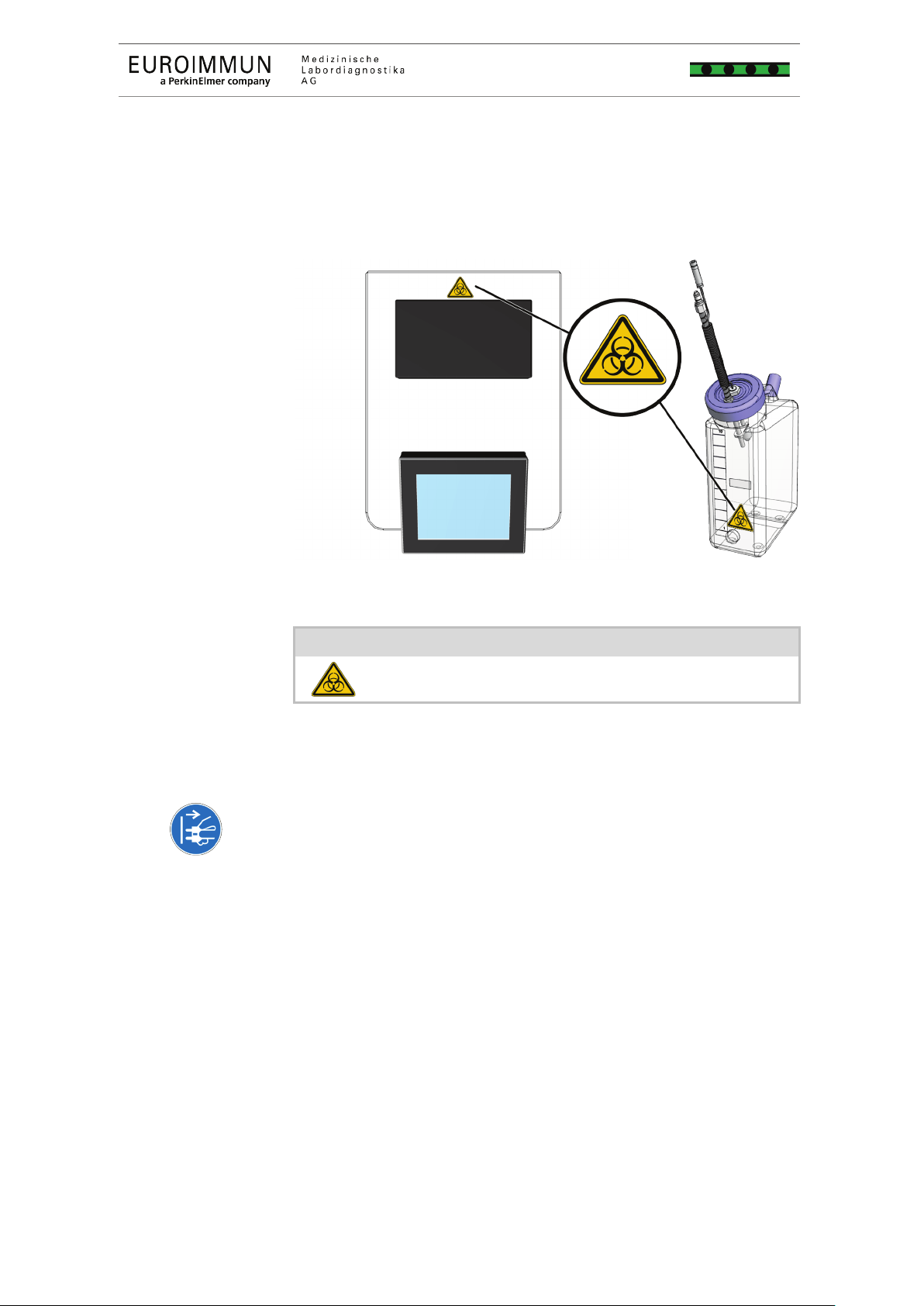
2.5 Layout of the safety labels on the product
The warning symbols on the device refer to a hazard. Replace
damaged or lost warning symbols.
3 Safety indicators
Biohazard
2.6 In the event of danger
In the event of danger, unplug the mains plug in order to disconnect
the device from the power supply.
A dangerous situation is for example the leakage of liquid from the
device.
2.7 Foreseeable incorrect use
MERGITE! must not be used to wash slides from other
manufacturers or other items.
MERGITE! is not intended for cleaning the MERGITE! Titerplane
glass plates.
Only the liquids specified in the EUROIMMUN test sets and in
these instructions for use may be used on MERGITE!.
24/84 Instructions for use • MERGITE!

2 Safety
2.8 Environmentally hazardous
substances/materials
Dispose of the used materials, e.g. slides, glass plates or cover
glasses and the waste liquid in accordance with the statutory
regulations for hazardous biological substances.
The glass plates are suitable for multiple use. For the disinfection,
cleaning and regeneration of the glass plates properties, please refer
to the instructions for use General Instructions for EUROIMMUN
indirect immunofluorescence tests (FI_0001_A_UK_CXX).
Instructions for use • MERGITE! 25/84

3 Delivery contents and accessories
Number
Explanation
1 ×
MERGITE! 10
Order no.: YG 0064-0101-1
5 ×
Titerplane set for the incubation of slides with 10 fields
1 x MERGITE! carrier plate
2 ×
Containers for distilled water and wash buffer
and
MERGITE! 50
1 ×
Container for waste liquid
and MERGITE! 50
5 ×
Coded glass plate
with data matrix coding for slides with 10 fields for MERGITE! 10
1 ×
Device mains adapter MERGITE!
1 ×
Wash basin cover
1 ×
Instructions for use
3 Delivery contents and accessories
The actual delivery contents can deviate from the information
described here in the case of special models, additional ordering
options having been used, or due to the latest technical changes.
3.1 MERGITE! 10
3.1.1 Scope of delivery MERGITE! 10
MERGITE! for 5 slides with 10 fields each
The set includes
1 x MERGITE! slide carrier
1 x MERGITE! cover glass carrier
liquid container with integrated fill level sensor, 5 litres, for MERGITE! 10
waste liquid container with integrated fill level sensor, 10 litres, for MERGITE! 10
Tab. 7 MERGITE! 10 scope of delivery
26/84 Instructions for use • MERGITE!
 Loading...
Loading...