etectRx ET2000150 User Manual

ID-Cap Medication Adherence Feedback System
USER MANUAL
TABLE OF CONTENTS
Glossary ............................................................................................................................................... 4
ID-CAP SYSTEM OVERVIEW .......................................................................................................... 4
User Manual .................................................................................................................................... 4
Device Model Numbers ................................................................................................................ 5
Intended Use .................................................................................................................................. 5
Device Description ........................................................................................................................ 5
The ID-Cap Medication Adherence Feedback System ....................................................................... 5
ID-Capsule (Ingestible Sensor Capsule) ............................................................................................. 6
ID-Cap Reader .................................................................................................................................. 6
ID-Cap Reader Kit ............................................................................................................................. 7
Mobile Phone Application ................................................................................................................ 7
Charging Accessories ....................................................................................................................... 8
System Operation Overview ............................................................................................................ 8
Important Information: Use as An Aid to Measure Medication Adherence ................. 8
Conditions of Use .......................................................................................................................... 9
User Groups..................................................................................................................................... 9
Skills, Training, and Knowledge Required of Patient Users.............................................................. 10
Locations of Use ............................................................................................................................ 10
Operational Contexts of Use .......................................................................................................... 10
Conditions that Could Affect User Interactions with the Device ..................................................... 11
When the Device Should Not Be Used (Contraindications)............................................ 11
Risks and Benefits....................................................................................................................... 12
1

General Warnings and Precautions........................................................................................ 12
Compliant Use .............................................................................................................................. 14
Ordered by Physician ..................................................................................................................... 14
Avoiding Unsafe Use Conditions .................................................................................................... 14
Important Safety Instructions ........................................................................................................ 14
INSTRUCTIONS FOR USE ............................................................................................................. 15
Initial Setup ....................................................................................................................................... 16
Routine Use ....................................................................................................................................... 17
End-of-Therapy Device Return ........................................................................................................... 17
Monitoring the Activity of the Device .................................................................................... 17
Cleaning the ID-Cap Reader .................................................................................................... 18
Maintenance.................................................................................................................................. 18
Storage and Handling ................................................................................................................ 18
Useful Life and Shelf-Life .......................................................................................................... 19
Disposal of Waste Products ...................................................................................................... 20
DEVICE PERFORMANCE................................................................................................................. 20
Nonclinical Performance Test Results ................................................................................... 20
Clinical Study Results – Detection Accuracy and Usability ..................................................... 20
Clinical Study Results - Adverse Events .............................................................................. 21
Device Specifications ................................................................................................................. 21
Quality Assurance ....................................................................................................................... 21
Warranty ........................................................................................................................................... 21
TROUBLESHOOTING ...................................................................................................................... 22
MANUFACTURER CONTACT INFORMATION............................................................................. 24
USER ASSISTANCE ......................................................................................................................... 24
USER MANUAL INFORMATION .................................................................................................... 25
LEGAL NOTICES...................................................................................................................................... 26
Trademark ......................................................................................................................................... 26
Copyright ........................................................................................................................................... 26
APPENDIX A: TECHNICAL INFORMATION ............................................................................... 27
Device Classification................................................................................................................... 27
Symbols Reference Guide ........................................................................................................ 27
2

Environmental Conditions of Use ........................................................................................... 29
Biocompatibility ........................................................................................................................... 30
Protection Against Ingress of Solids and Liquids .............................................................. 30
Regulatory Information ............................................................................................................. 30
Applied Part................................................................................................................................... 30
CISPR Interference Statement ............................................................................................... 31
FCC Interference Statement .................................................................................................... 31
FCC and Canadian Compliance Identifiers .......................................................................... 32
Electromagnetic Compatibility ................................................................................................ 32
Information on the Radio Subsystem ................................................................................... 33
Declaration of Conformity ........................................................................................................ 37
3
Glossary
Caution
etectRx
Hazard
ID-Cap App
ID-Cap Medication Adherence Feedback System
ID-Cap Reader
ID-Capsule
ID-Tag
Medication Adherence
ID-CAP SYSTEM OVERVIEW
CAUTION:
the order of a physician.
User Manual
This User Manual provides important information about the ID-Cap
Medication Adherence Feedback System (ID-Cap System) from etectRx. You
must read and fully understand the contents of this User Manual before
using the System to ensure safe and effective operation of the device.
Federal (United States) law restricts this device to sale by or on
4
To support the safe operation of the device, this User Manual categorizes
damage or lead to minor or moderate injury
or fatal injury
Model Number for the ID
-
Cap Reader
ET70002.10
Model Number for the ID
-
Tag
ET2
0001.5
1
safety instructions into two general types based on potential for harm:
ATTENTION
Hazardous situation which can cause material
WARNING
Hazardous situation which can cause a serious
Device Model Numbers
This User Manual pertains to the following device model numbers:
Intended Use
The ID-Cap Medication Adherence Feedback System consists of a wearable
device for recording of time-stamped ingestion events, including events
signaled by the co-incidence with, or co-ingestion with, the ID-Capsule that
contains an ingestible sensor. When the ID-Capsule is ingested, the ID-Cap
Medication Adherence Feedback System is intended to log, track, and trend
intake times. When co-ingested with medication, the tracking and trending
of intake times may be used as an aid to measure medication adherence.
The ID-Cap Medication Adherence Feedback System enables unattended
data collection for clinical and research applications in the home or clinical
settings.
The patient is an intended operator of the ID-Cap System.
Device Description
The ID-Cap Medication Adherence Feedback System
Your ID-Cap Medication Adherence Feedback System consists of a wearable
ID-Cap Reader, ID-Capsules, and a mobile application. Your ID-Cap Reader
is a pendant that is worn around your neck and collects information from the
ingested ID-Capsules that are taken with your medication. The ingestible
sensor in the ID-Capsule communicates with your ID-Cap Reader after it
5
reaches your stomach. This allows the system to measure the pattern,
WARNING
regularity, and schedule of your medication taking — your medication
adherence. With a simple Bluetooth connection to your mobile device, your
ID-Cap Reader sends your ingestion event data to the ID-Cap mobile
application to help you keep track of how you are taking your medication.
With your permission, this information may be shared with your healthcare
providers to help them improve your health outcomes.
ID-Capsule (Ingestible Sensor Capsule)
The ID-Capsule contains an ingestible sensor called the ID-Tag made of
ingredients that do not easily react with other chemicals and are found in the
food chain. The ingestible sensor communicates with your ID-Cap Reader
after it reaches your stomach. It is powered by your stomach fluid.
Key ingredients of the ingestible ID-Tag that are in human contact include
magnesium, silver chloride, polyimide, and epoxy. The ID-Tag is delivered in
a two-piece gelatin capsule for oral administration. Additional information
about ID-Capsules may be requested from the manufacturer.
DO NOT take an ID-Capsule if you are allergic to
any of its ingredients. Please consult with your
doctor before using.
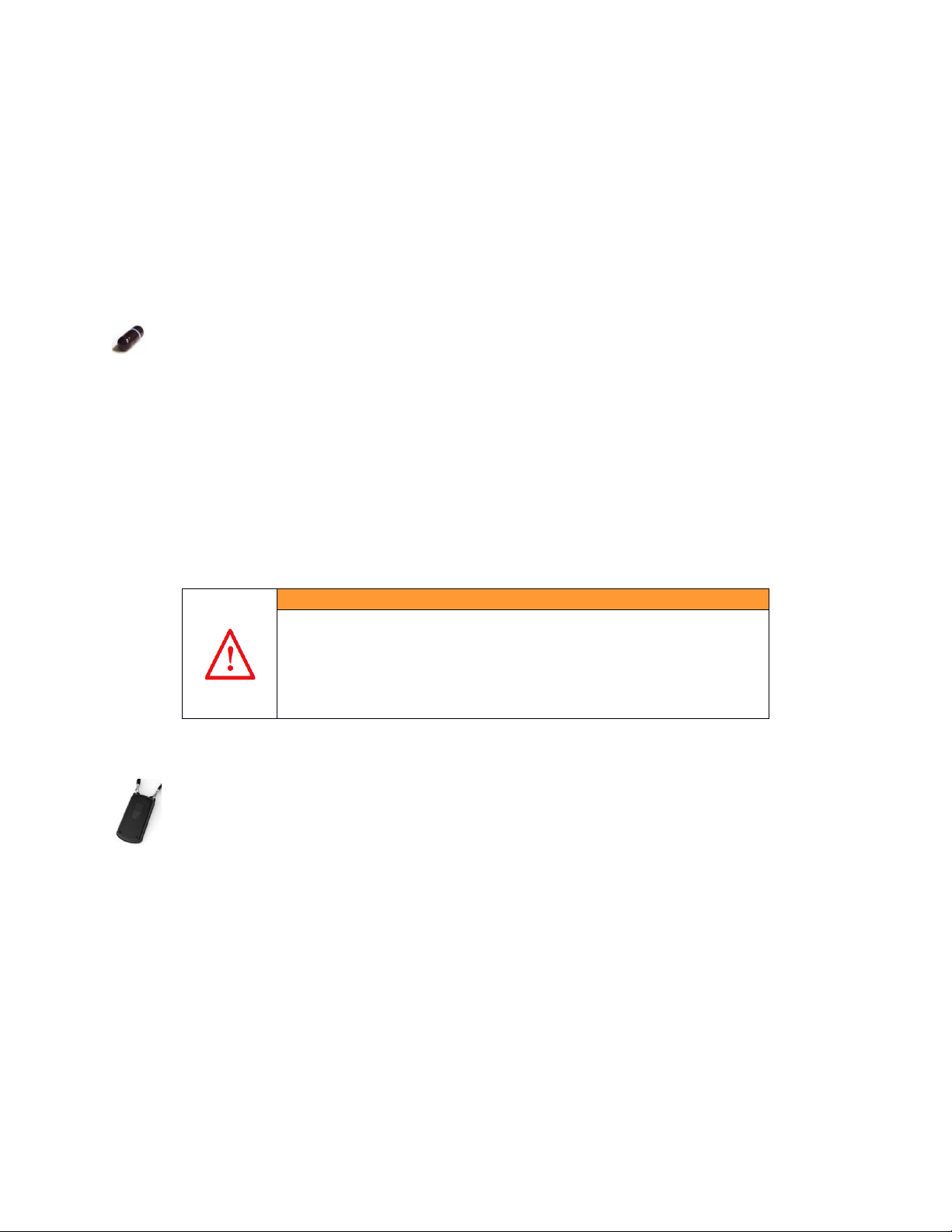
ID-Cap Reader
Your battery-operated ID-Cap Reader records the time the ingestible sensor
is detected. Information from your ID-Cap Reader will transfer to your
mobile phone regularly and automatically as long as the Reader is paired
with the mobile phone as indicated by a flashing green light on the Reader.
6

[ADD PICTURE OF LABELED READER]
work properly when exposed to water.
The ID-Cap Reader is a pendant that is attached to a grey
lanyard provided in the Reader Kit. The lanyard is placed
around the neck so the Reader hangs in the center of the
chest or just below the sternum. The ID-Cap Reader does
not require any adhesives or direct contact with the skin to
receive messages from the ID-Tag.
Your ID-Cap Reader may be worn during most activities, including exercise,
but should be removed prior to any activities that would expose the Reader
to water, including showering, bathing, and swimming.
ATTENTION
DO NOT submerge the ID-Cap Reader in or expose it
to excessive amounts of water. Your ID-Cap Reader
is designed to be weather resistant, but it may not
ID-Cap Reader Kit
Your ID-Cap Reader Kit contains the
device and the following accessories:
• Reader
• Lanyard
• AC Adapter
• USB Charging Cable
• Wireless Charging Pad
• Quick Start Guide
• User Manual
Mobile Phone Application
The ID-Cap Medication Adherence Feedback System may be used with a
mobile application that is loaded on a mobile phone. The mobile application
reports and displays information about ingestion events and ingestion event
7

history, sends notifications and messages to the user, and provides updates
on the status of the ID-Cap Reader and the System. It also allows the user
to manually record an ingestion event that has not been recorded by the IDCap System and to report missed or skipped doses.
Charging Accessories
Your ID-Cap Reader Kit contains the following accessories for charging the
Reader:
• AC Adapter
• USB Charging Cable
• Wireless Charging Pad
This graphic shows the proper set-up of the charging pad in order to
effectively charge the device.
[Insert graphic on setup of charging pad]
System Operation Overview
Important Information: Use as An Aid to Measure Medication
Adherence
When the ID-Capsule is ingested and the Reader records the time-stamped
ingestion event, the ID-Cap System is intended to log, track and trend
intake times. When co-ingested with medication, the tracking and trending
of intake times may be used as an aid to measure medication adherence.
8

The ID-Cap System has been designed to provide reliable information that
will help you and your physician get the most out of your medication. Proper
use by patients and their healthcare providers is essential to the
performance and clinical benefit of the ID-Cap System.
Please keep in mind the following while using the ID-Cap System:
• Always take your medication as prescribed by your physician and
recommended by your pharmacist regardless of the status and use of the
ID-Cap System.
• The ID-Cap System has no effect on your medication.
• With your consent, your doctor or pharmacist may be monitoring your
medication use using the ID-Cap System and may contact you regarding the
information received.
• As with all wireless communications, an ingestion event message will
occasionally not be received by the ID-Cap Reader. Do not be concerned if
this happens, and do not change your prescribed medication regimen or
alter your medication use. If you are experiencing problems with your IDCap System, please refer to the Troubleshooting Section of the User Manual
or contact your pharmacist or doctor. With use of the ID-Cap Mobile App,
you can manually record ingestion events that were not captured by the IDCap System and report missed or skipped doses.
The ID-Cap System should not be used as the sole basis for medication
treatment decisions. Detection accuracy is less than 100%. Patients
instructed by a doctor to take the ID-Capsule with medication may
selectively adhere to one or the other. Therefore, ingestible event data
should be interpreted with caution. Patients should discuss medicationtaking history with their doctor prior to making medication changes.
Conditions of Use
User Groups
There are two distinct user groups based on their potential use of the ID-Cap
System: Patient Users and Clinician Users. Patient Users include individuals
for whom a monitored medication is prescribed and who utilize the ID-Cap
System to capture ingestion events. The intended user population of patients
includes adults 18 years of age and older across a wide range of diverse
9

demographics and medical conditions who are taking an oral medication that
will be monitored.
The user group of Clinician Users include healthcare providers such as
pharmacists, nurses, clinical case managers, and physicians, who will be
involved in the monitoring of patient adherence to medication regimens and
who may facilitate use of the ID-Cap System. Based on their education and
experience, Clinician Users have been trained to care for patients, manage
medication use, and interpret information regarding adherence to prescribed
therapy.
Skills, Training, and Knowledge Required of Patient Users
The patient and/or his or her caregiver must be able to read, understand,
and follow the instructions provided in this User Manual. The patient and/or
his or her caregiver must be able to understand and follow instructions
regarding the taking of oral medications. Use of the ID-Cap System is
reserved for users who have the ability to follow medication ingestion
instructions while also interacting with the ID-Cap System. This System
should not be prescribed to users who might not take their medication as
directed due to confusion with the System.
Locations of Use
The ID-Cap System has been designed for use in the home and clinical
settings. It is not intended for use on aircraft or in other areas where RF
communications are restricted. See Technical Information in Section X of the
User Manual for additional restrictions on locations of use due to
electromagnetic interference.
The patient may use this device while traveling, but the system should not
be used on aircraft or in other locations where RF communications are
restricted. The power cable for the charging pad may not be compatible with
foreign power sources. If this is the case, the user may need an adapter or a
converter to convert to the proper voltage.
Operational Contexts of Use
The ID-Cap System can be utilized with or without use of the optional
etectRx mobile application on the patient’s smartphone.
If the System is operating in a mode without use of the patient’s
smartphone, ingestion event data will be recorded and archived by the IDCap Reader. In this operational mode, ingestion event data will be uploaded
to the monitoring database by the Clinician User after the ID-Cap Reader is
10

returned by the Patient User either at defined intervals during therapy or at
the conclusion of the medication regimen.
If the System is operating in a mode involving use of the etectRx mobile
application on the patient’s smartphone, ingestion event data will be
captured by the ID-Cap Reader, transmitted to the mobile app, and
displayed in the monitoring database.
Conditions that Could Affect User Interactions with the Device
The following conditions could affect user interactions with the ID-Cap
System:
• Technical issues affecting the Reader (e.g., battery)
• Technical issues affecting the ID-Tag or ID-Capsule or messaging between
ID-Tag and Reader
• Technical issues affecting the smartphone and/or mobile app when
operating with the mobile app
• Connectivity issues when operating with the mobile app
• Distractions in the user environment that could interrupt the use process
When the Device Should Not Be Used (Contraindications)
WARNING
DO NOT use the ID-Cap System in patients or
situations in which it is contraindicated.
Contraindications are conditions under which the device should not be used
because the risk of use clearly outweighs any possible benefit. The ID-Cap
System should not be used in the following situations:
• DO NOT use the ID-Cap System if you are pregnant or breastfeeding. Use
in these populations has not been studied.
• The ID-Cap System has not been studied in individuals who are
undergoing magnetic resonance imaging (MRI), cautery, or external
defibrillation procedures. The ID-Cap System has not been tested or
approved for use in the presence of strong magnetic or electrical fields.
DO NOT wear the ID-Cap Reader during magnetic resonance imaging
(MRI), cautery, and external defibrillation procedures. Damage to the
Reader or an unexpected magnetic attraction may result. Please inform
11

your healthcare professional that the ID-Cap Reader must be removed
prior to engaging in one of these procedures.
• DO NOT use the ID-Cap System if you have a cardiac pacemaker or other
implanted electronic medical device. The ID-Cap System has not been
studied in individuals with implanted electronic medical devices.
• DO NOT use the ID-Cap System or ingest the ID-Capsule if you are
unable to take oral medications.
• DO NOT take if you are allergic to any of the ingredients in the ingestible
ID-Capsule.
• DO NOT use the ID-Cap Reader if it has been submerged in liquid or
exposed to excessive amounts of water.
• DO NOT use if you have a significant medical condition which may affect
capsule passage through the gastrointestinal tract.
Risks and Benefits
The ID-Cap System measures and reports the pattern, regularity, and
schedule of your medication taking — your medication adherence. With a
simple Bluetooth connection to your mobile device, your ID-Cap Reader
sends your ingestion event data to the ID-Cap mobile application to help you
keep track of how you are taking your medication. With your permission,
this information may be shared with your healthcare providers to help them
improve your health outcomes.
The ID-Cap System should not be used as the sole basis for medication
adherence determinations or treatment decisions. Detection accuracy is less
than 100%. Patients instructed by a doctor to take the ID-Capsule with
medication may selectively adhere to one or the other. Therefore, ingestible
event data should be interpreted with caution. Patients should discuss
medication-taking history with their doctor prior to making medication
changes.
The risks of the ID-Cap System must be evaluated in light of these benefits
and limitations. The safety and tolerability of the ID-Cap System has been
evaluated in clinical studies of device performance. The reported adverse
effects can be found in Section X of the User Manual.
General Warnings and Precautions
General – ID-Cap System
• Keep components out of reach of children.
12
 Loading...
Loading...