Essilor Instruments Retina 550 User Manual
www.essilor-instruments.com
User manual

CONTENTS
I. INTRODUCTION 5
1. Symbols 6
a. Device symbols 7
2. General warnings 7
3. Normative references 7
a. Community directives 7
b. Technical standards 7
c. Quality management systems standards 8
4. Warranty 8
5. Manufacturer identification 9
II. SAFETY 11
1. Safety warnings 12
2. Device identification plate 12
3. Intended use 13
4. Medical devices classification 14
5. Medical electrical devices classification 15
6. Environmental conditions 15
7. Disposal at the end the useful life 16
8. Manufacturer declarations 17
a. Electromagnetic emissions 17
III. DEVICE DESCRIPTION 19
1. Provision description 20
a. Device Retina550 21
b. Power supply 21
c. Chinrest (optional) 21
d. Electric table (optional) 22
2. Optional accessories 22
3. Technical data 22
IV. DEVICE USE 23
1. How to install the device 24
2. How to connect the device 26
3. How to turn on the device 27
4. How to adjust the chinrest 28
5. How to acquire the image 29
6. How to change the paper for chin cup 31
7. How to turn off the device 31
V. ORDINARY MAINTENANCE 33
1. Safety warnings 34
2. Device cleaning 34
USER MANUAL> CONTENTS

I. INTRODUCTION
The device is the result of a long research period, conducted by experts to give the best union of technical
innovation, quality and design.
The device can be easily used thanks to the guided manual acquiring an the electrical control of the service
functions.
1. SYMBOLS
Within the instructions for use, on the package or on the device, there can be the following symbols:
Symbol Meaning
General danger
Electric shock danger
Obligation. Read the instructions for use
General obligation
Note: Useful information for the user
Prohibition: Forbidden operation
Manufacturer
CE marking (European Regulation on Medical Device)
Symbol of the waste disposal in compliance with the Directive 2012/19/EU (WEEE), and
2011/65/EU (RoHS II)
USER MANUAL> I. INTRODUCTION
The complete user manual is available on a web space. To access, please scan the QR code below
using a dedicated application.
Le manuel utilisateur complet est disponible sur un espace web. Pour y accéder veuillez scanner
le QR code ci-dessous à l'aide d'une application dédiée.
El manual de uso completo está disponible en la web. Para acceder, escanee el código QR que se
encuentra a continuación con la ayuda de una aplicación.
6
Retina550 - Fundus camera > V1 - 06-2017
a. Device symbols
Symbol Meaning
Applied part of type B
Class II device
2. GENERAL WARNINGS
These instructions refer to the model Retina550 ("Device" from now on).
The original manual is in italian.
This device is compliant with marking. Date of first marking: June 2017
Before using the device or if you don't use it since a long time, read these instructions carefully.
Read the instructions given in the instructions manual and reported on the device.
Keep this manual close by for future consultation. If you should decide to sell this appliance to
other people, remember to also include these instructions, complete and readable.
We suggest you keep the original box and packaging, as our free-of-charge service does not
cover any damage resulting from inadequate packaging of the product when this is sent back to
an authorized Service Center.
Verify the presence of damage signs on the device caused by the transport/storage, before using
the device.
It is forbidden to reproduce, totally or partially, texts or images contained in these instructions
without the written authorization of the manufacturer.
The manufacturer reserves himself the right to modify the contents of the instruction for use,
without notice.
3. NORMATIVE REFERENCES
a. Community directives
• Directive 93/42/EEC concerning medical devices.
• Directive 2012/19/EU on waste electrical and electronic equipment (WEEE).
b. Technical standards
• IEC 60601-1 and 3.1: Medical electrical equipment - Part 1: General requirements for basic safety
and essential performance.
• EC 60601-1-2 - edition 4 Collateral Standard: Electromagnetic disturbances - Requirements and tests.
• ISO 15004-1: Ophthalmic Instruments. Fundamental requirements and test methods - Part 1:
General requirements applicable to all ophthalmic instruments.
• ISO 15004-2: Ophthalmic Instruments. Fundamental requirements and test methods - Part 2: Light
hazard protection.
• ISO 14971: Medical devices. Application of risk management to medical devices.
USER MANUAL> I. INTRODUCTION
Retina550 - Fundus camera > V1 - 06-2017
7

c. Quality management systems standards
• ISO 9001:2008 Quality management systems - Requirements.
• ISO 13845:2012 Medical devices. Quality management systems - Requirements for regulatory
purposes.
4. WARRANTY
The manufacturer is responsible for the device conformity to the Community directive 93/42/EEC as
amended by the 2007/47/EC for:
• Features
• Safety and reliability
• CE marking
The manufacturer refuses any responsibility for:
• Installation and activation not activated in conformity to the indications and the precautions reported
in the instructions for use.
• Use not in compliance with the instructions and precautions reported in the instructions for use.
• Use of accessories or spare parts not provided or suggested by the manufacturer.
• Repairs and safety controls not effectuated by expert, qualified, trained and personnel authorized by
the manufacturer.
• Electrical system of the space where the device is installed not in compliance with the technical
standards, the laws and regulations in effect in the country of installation of the device.
• Direct or indirect consequences or damages to objects or persons, originating for the improper use of
the device or erroneous clinical analysis originating from its use.
The manufacturer guarantees the device for 24 months after invoicing. The warranty includes the
substitution, at the manufacturer's or an authorized Service Center, of components and materials and the
labor. The shipping and transport fees are to be paid by the client.
It the warranty are not included:
• Reparations of faults originating from natural disasters, mechanical shocks (fall, hit, etc), electrical
system faults, negligence, improper use, maintenance or reparations carried out with non original
materials.
• Any other improper use or not intended by the manufacturer.
• Damages caused by service lack or inefficiency, originating by causes or circumstances out of the
manufacturers control.
• The parts subject to usage and/or deterioration originating from the normal use and those that might
be broken because of an improper use or maintenance carried out by personnel non-authorized by the
manufacturer.
To ask maintenance interventions or to have technical information about the device, address to a technical
assistance center or directly to the device manufacturer.
The client will not be refunded for damages originating from the device halt.
USER MANUAL> I. INTRODUCTION
8
Retina550 - Fundus camera > V1 - 06-2017

5. MANUFACTURER IDENTIFICATION
ESSILOR INTERNATIONAL
Instruments Division
147 rue de Paris
94220 Charenton le Pont
FRANCE
USER MANUAL> I. INTRODUCTION
Retina550 - Fundus camera > V1 - 06-2017
9

II. SAFETY
1. SAFETY WARNINGS
Electric shock danger. Do not let water fall on the device. Do not immerse the device in water or
other liquids.
Electric shock danger. If the power cables are damaged they must be replaced to prevent any
risk.
Electric shock danger. Unplug the power cable from the mains socket before disinfecting the
device and before any maintenance operation.
Do not use the device if visibly damaged. Periodically inspect the device and the connection
cables to verify if there are damage signs.
Always keep the device out of the reach of children.
Danger of device fall. Do not let the power cord hang in a place where it could be grasped by a
child.
If you notice a wired odor or smoke coming out of the appliance or if it emanates heat, suspend
immediately the use. Keep using a damaged product, or a damaged part, may cause injuries.
It is forbidden to carry out any technical operation on the device that is not recalled or described
in these instructions for use.
It is forbidden to place the device in humid, dusty places or environments subject to sudden
temperature and humidity variations.
It is forbidden to use any extension cable not authorized by the manufacturer.
It is forbidden to use the device outdoors.
The device does not generate and does not receive any electromagnetic interference if it is placed
near other electrical appliances. No preventive or corrective actions are required.
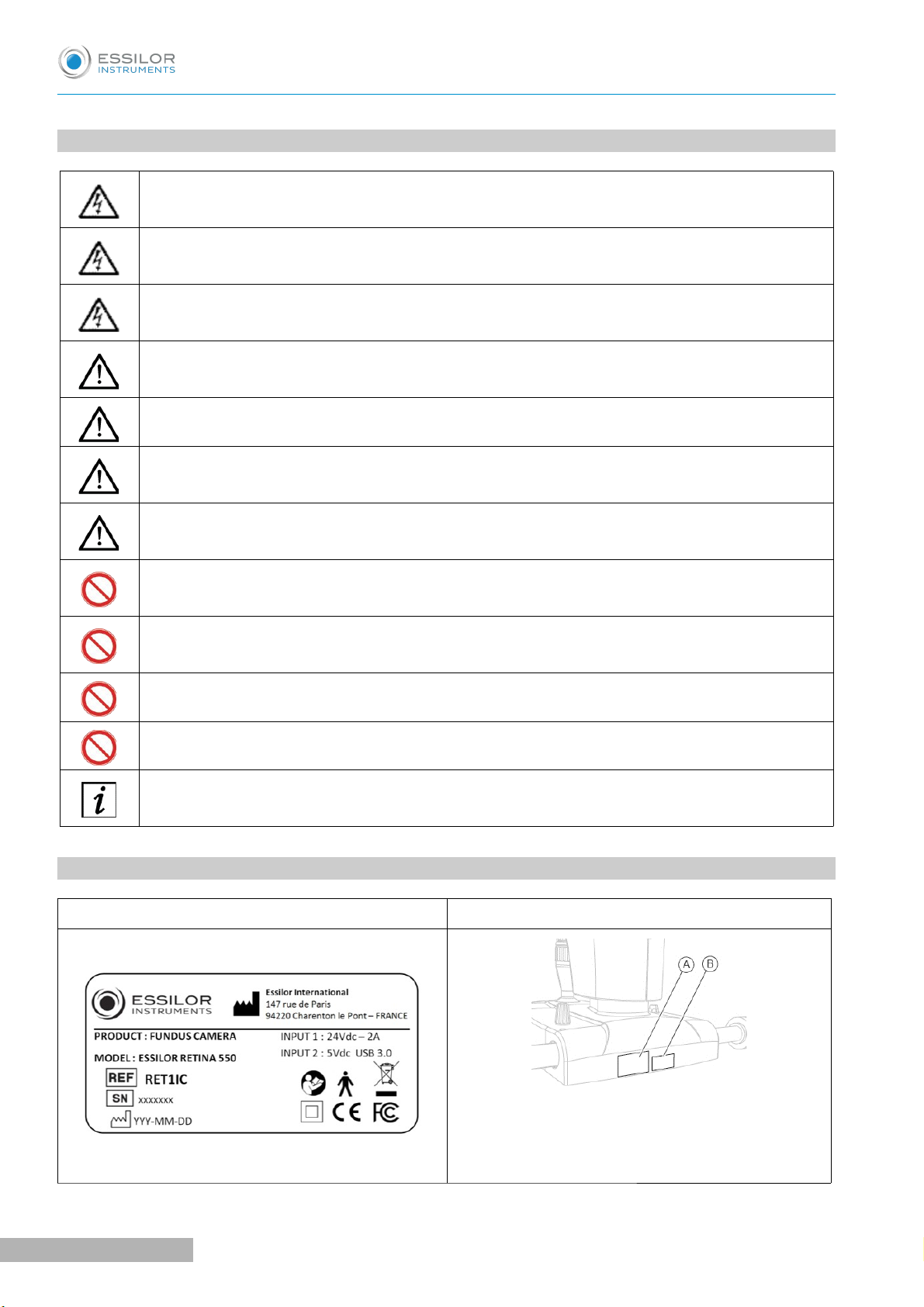
2. DEVICE IDENTIFICATION PLATE
Device data plate Plates position
With:
A: Device data plate
B: Software version plate
USER MANUAL> II. SAFETY
12
Retina550 - Fundus camera > V1 - 06-2017

Power suppliers data plate
3. INTENDED USE
The device Retina550 is a medical electrical device for the non-mydriatic fundus camera.
The device has been designed for the screening, the acquisition and the elaboration of an image of the retina
in the ophthalmic procedure. The device provides a clear and detailed vision of the whole image of the funds
with a real 60°x45° field of view. The device processes retinal images with a minimum flash exposition,
minimizing the discomfort for the patient. The device acquires the image of the retina even when the pupil is
constricted.
The device, with the software allows:
• A guided manual acquisition
• To manage the patients data and to effectuate personalization of researches and statistics
• Acquire simultaneously images in white light mode and IR mode
• Analysis of panoramic images on large field
• Image processing, drawing, measure
• Zoom effects
• Vertical and horizontal cup/disc
• Color control and filters simulation
• Overlay on the image
• Edge enhancement
• Contrast and luminance control and control of each RGB component and range correction
• Image in grayscale, ref fee, and channel separation
• Image with inverted colors
The device must be used only by practitioners, optometrist and opticians, within the limits of the law and the
regulations for the exercise of the profession.
The device must be used in combination with a PC and the software denominated AnaEyes.
Hardware minimum requirements:
• PC Desktop with processor Intel Pentium Dual Core
• 1 GB RAM (2 GB suggested for Windows Vista, Windows 7 and Windows 10)
• USB3.0
• Video board with 512 MB RAM (not shared) and minimum resolution 1280 x 1024 pixels
Software requirements:
Operating systems: Microsoft Windows XP Home, Windows XP Professional, Windows Vista (32 bits) Home
premium, Windows 7 (32 and 64 bits), Windows 10 (32 and 64 bits).
USER MANUAL> II. SAFETY
Retina550 - Fundus camera > V1 - 06-2017
13
 Loading...
Loading...