EPI Mobile Health Solutions W518I User Manual

EPI Mobile Health EPI Mini W518i
Portable ECG Recording User Manual
User Assistance Information
Company Contact Details
EPI Mobile Health Solutions (S) Pte Ltd
8 Ubi View, Serial System Building, #03-01
Singapore 408554
Tel: (+65) 6262 6868
Fax: (+65) 6363 3030
Email: info.enquiries@epi.com.sg
www.epi.com.sg
Inside this box you will find
1. EPI Mini W518i X 1
2. Charger (Model No: AU1050501e) X 1
3. USB cable X 1
4. User Manual X 1
© 2014 EPI Mobile Health Solutions (S) Pte Ltd
LPI-0007-01
EPI document no. LPI-0007-01 Page 1 of 19

Table of Contents
Symbols ............................................................................................................. 3
Indications for Use ............................................................................................. 5
Warnings and Cautions ...................................................................................... 6
Description of Device ........................................................................................ 9
Environmental conditions that affect use ........................................................ 10
Classification of degree of protection/Type BF ................................................ 10
Getting Started ............................................................................................... 10
Charging the device ...................................................................................... 10
Pairing the device with your phone .............................................................. 11
Download Application ................................................................................... 11
Operating Instructions .................................................................................... 12
Recording your ECG ...................................................................................... 12
ECG Manager ................................................................................................ 13
Date/Time ..................................................................................................... 13
Language ...................................................................................................... 15
User Settings ................................................................................................. 15
System Information ...................................................................................... 15
Cleaning and Maintenance .............................................................................. 16
Storage and Transport ..................................................................................... 16
Troubleshooting Guide .................................................................................... 17
Specifications ................................................................................................... 18
Federal Communications Commission (FCC) Notice ......................................... 19
EPI document no. LPI-0007-01 Page 2 of 19
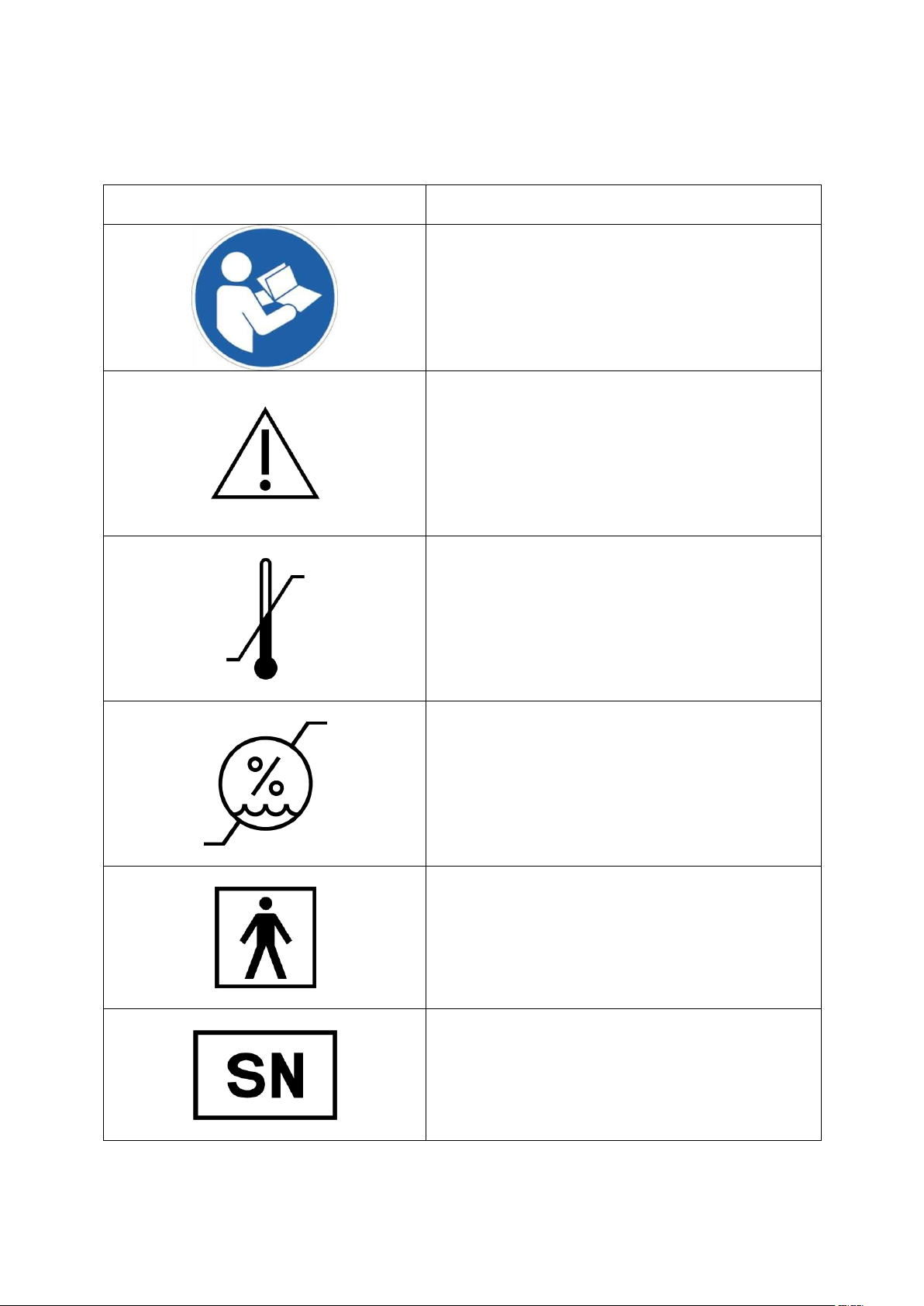
Symbols
Symbols
Meaning
Follow Instructions for Use
Caution. Please refer to safety-related
notes accompanying this instrument
Temperature limitation (Store at)
Humidity limitation (Store at)
Type BF applied part
Serial Number
EPI document no. LPI-0007-01 Page 3 of 19
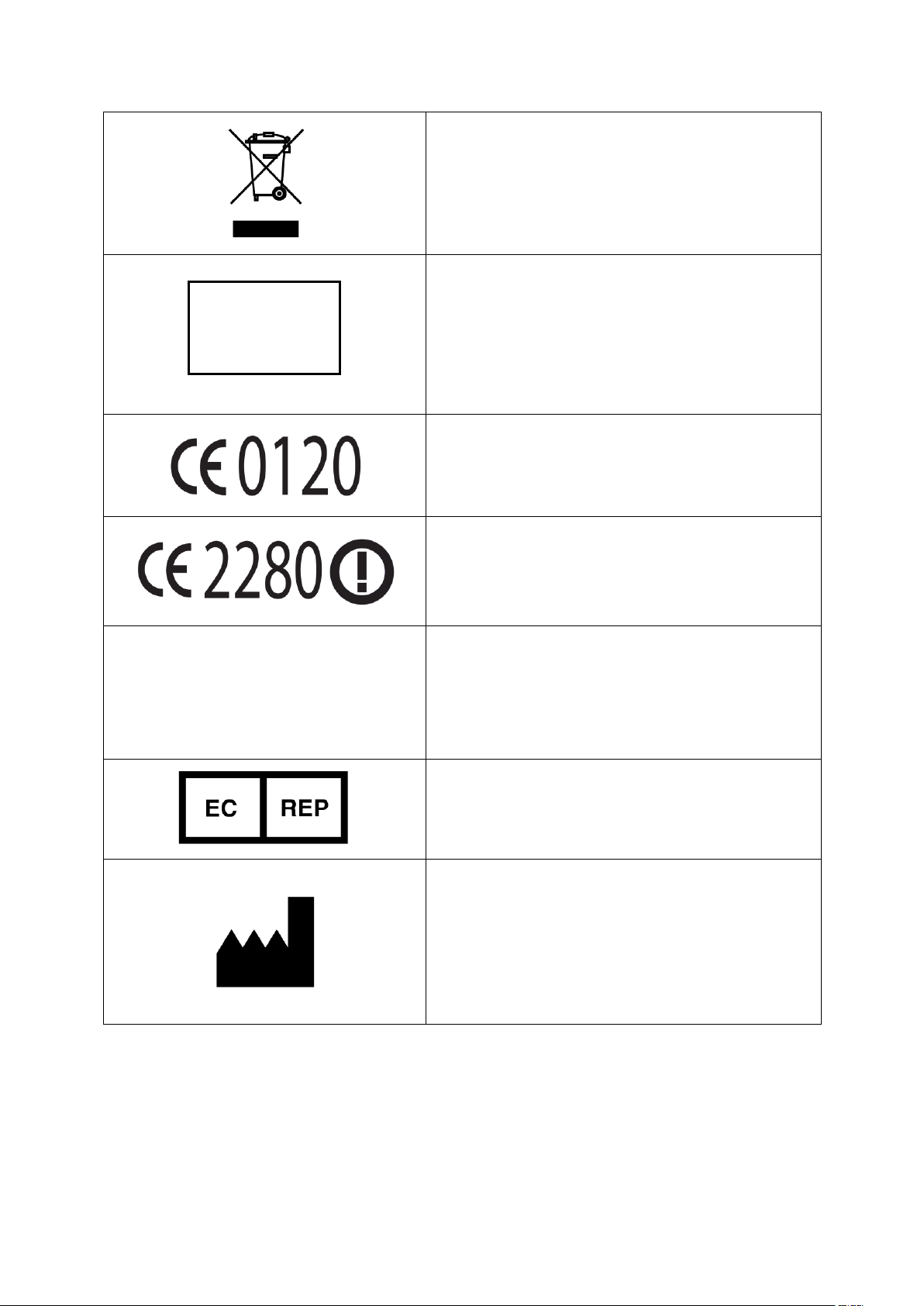
Indicates separate collection for electrical
and electronic equipment (WEEE).
Protected against ingress of solid foreign
objects greater than or equal to 12.5 mm
diameter per IEC 60529. Not protected
against ingress of water
CE Marking indicating conformance to EC
Directive No. 93/42/EEC concerning
medical devices.
CE Marking indicating conformance to
R&TTE directive 1999/5/EC
Rx only
Caution: Federal Law (USA) restricts this
device to sale by or on the order of a
healthcare practitioner
Authorized Representative in the
European Community
Manufacturer name and address
IP20
Note: This device is registered in USA as Rx only for the US market.
EPI document no. LPI-0007-01 Page 4 of 19

For your safety, please follow all operating and safety instructions in
this user guide
Indications for Use
EPI Mini W518 is a portable single lead electrocardiogram (ECG) recording
device. It is capable of recording, storing and sending several leads using
different configurations allowing mobile recording, storing and sending of
ECGs on-the-go. This provides the user with instant access to information
about their heart, within the limitations of a traditional 12 lead ECG. It serves
the purpose of a mobile and highly portable ECG recording device. The device
is used for ad-hoc recording of ECGs when cardiac symptoms are present to
allow the detection of abnormal heart activity. The target patient population
includes but it is not limited to users who are at risk of heart disease, have
existing heart conditions and/or users who show symptoms suggestive of
heart disease. The device is not meant to entirely replace the clinical 12 lead
ECG machine.
ECG data is recorded for 30 seconds each time by the user. Recorded ECG
data is stored on the EPI Mini and can be sent to a Smartphone via Bluetooth.
ECG data sent from the EPI Mini is received and stored for viewing on the
Smartphone using the EPI Mini mobile software application (mobile app).
Users are able to view their ECGs using the mobile app, as well as resend or
delete any of these data via the app to allow the doctor to monitor their
conditions. The ECG data recorded by the device are not designed or
intended for medical diagnosis. Conditions such as arrhythmia can only be
diagnosed by a doctor through a special examination.
EPI document no. LPI-0007-01 Page 5 of 19

Warnings and Cautions
Contraindications
Users who have pacemakers or other stimulators implanted should
not use this device to record any modified pre-cordial leads and
should strictly be limited to using the modified limb leads for ECG
recording (device should be kept at a minimal distance of 9 inches
away from the implanted pacemaker or other stimulators)
For your safety, please take note of the following warnings.
Warnings
Do not use this device in places with flammable substances like
anaesthetic gas or pressurized oxygen.
Do not self-diagnose ECG. Always consult your doctor. Self-
diagnosis or self-treatment may lead to deterioration in your
condition.
Do not use this device around electromagnetic scanning like
MRI scans.
Do not use this device on people with sensitive skin or
allergies.
Do not attempt to use the device over or through clothing.
Use only the original charger and the original USB data cable
for charging. Use of other chargers and USB cables may cause
unwanted electrical risk.
Do not measure ECG when this device is charging.
Do not plug the USB cable to this device during recording of
ECG.
Keep out of reach of infants and small children.
EPI document no. LPI-0007-01 Page 6 of 19
 Loading...
Loading...