eNeura sTMS mini Instructions For Use Manual

sTMS mini
Instructions for Use
sTMS mini Patient Manual
Caution: Federal law (U.S.) restricts this device to
sale by or on the order of a physician.
1

Table of Contents
Before You Begin ......................................................................................................................................................... 3
Intended Use ................................................................................................................................................. 4
Warnings and Precautions ............................................................................................................................ 4
Contraindications .......................................................................................................................................... 6
Clinical Trial and Adverse Reactions .............................................................................................................. 8
Your sTMS mini ............................................................................................................................................................ 8
Getting to Know Your Device ....................................................................................................................................... 9
Using Your Device ...................................................................................................................................................... 11
Setting Up the Device .................................................................................................................................. 11
Recommended Treatment .......................................................................................................................... 11
Preparing for Treatment ............................................................................................................................. 12
Positioning the Device ................................................................................................................................. 14
Delivering the Treatment ............................................................................................................................ 14
Renewing Your Prescription ........................................................................................................................ 15
Replacing the Micro SIM Rx card ................................................................................................................. 15
Additional Information .............................................................................................................................................. 16
Caring For Your sTMS mini......................................................................................................................................... 17
Understanding System Display Messages ................................................................................................................. 18
Troubleshooting ........................................................................................................................................................ 19
Service ......................................................................................................................................................... 20
Return Goods Policy .................................................................................................................................... 20
Technical Specifications ............................................................................................................................................. 20
Operating Environment ............................................................................................................................... 21
Storage Environment ................................................................................................................................... 21
Industry Standard Classification .................................................................................................................. 21
REACH and Warning Statement .................................................................................................................. 22
EMC Compliance and Warning Statement .................................................................................................. 22
EMC Guidance ............................................................................................................................................. 23
Glossary of Abbreviations .......................................................................................................................................... 26
Medical Device Reporting .......................................................................................................................................... 27
Warranty and Limitation of Liability .......................................................................................................................... 27
Customer Care Contact Information ......................................................................................................................... 27
2
Before You Begin
Thank you for choosing the eNeura sTMS mini, the portable single-pulse Transcranial Magnetic Stimulation (sTMS) device. This
manual provides the Information you need to use the sTMS mini. You will see cautions, warnings, and helpful information placed
near the related steps. Call Customer Care if you don’t understand something in this manual.
eNeura and your doctor are committed to helping you manage your migraine pain. The sTMS mini is a new, safe, non-drug therapy
to treat migraine at the first sign of pain.
Please read this entire manual before using the sTMS mini. Learn the contraindications, cautions, warnings and notes about the use
of the device. As the manufacturer, eNeura cannot and does not intend to give medical advice. Contact your doctor for all medical
advice. This manual should be kept near the sTMS mini and be available at all times.
eNeura is committed to the service and support of our customers. If there are any questions about the use of the eNeura sTMS
mini, please contact Customer Care or your local representative at the following:
Manufactured by: eNeura Inc.
715 North Pastoria Avenue
Sunnyvale, CA 94085
eNeura Inc.
715 North Pastoria Avenue
Sunnyvale, CA 94085
Tel: +1 408.245.6400
Toll free (USA only): +1 855.366.8355
Fax: +1 877.874.9584
www.eNeura.com
info@eNeura.com
customercare@eNeura.com
eNeura (UK) Ltd.
6th Floor
One London Wall London EC2Y 5EB
United Kingdom
www.eNeura.co.uk
Tel. +44 (0) 20.3695.4063
Fax: +44 (0) 20.7785.8152
customercare@eneura.co.uk
Authorized Representative
Emergo Europe BV
Prinsessegracht 20
2514 AP The Hague
The Netherlands
Tel: +31 (0) 70.345.8570
Fax: +31 (0) 70.346.7299
www.emergoeurope.eu
Australia Sponsor
Emergo Australia
Level 20, Tower II, Darling Park
201 Sussex Street
Sydney, NSW 2000
Australia
www.emergogroup.com
3
Intended Use
The sTMS mini (The System) is indicated for the acute and prophylactic treatment of migraine headache.
The System is designed for self-treatment and delivers a non-invasive, brief, single pulse of magnetic energy to the back of the head.
This creates a brief electrical current in the brain intended to stop or reduce the effects of migraine headaches. This type of
stimulation is called single-pulse Transcranial Magnetic Stimulation or sTMS.
The System is a drug-free treatment option that can be used in the home or away from home based on your doctor’s instructions.
After treatment, there are no restrictions. You can resume your normal activities.
WARNING: This device should be used under the supervision of a physician.
Keep the sTMS mini out of the reach of children.
Safety and effectiveness have not been established in pregnant women, children under the age of 18
and adults over the age of 65.
The long-term effects of single-pulse transcranial magnetic stimulation are unknown.
Warnings and Precautions
The words WARNING, Precaution and NOTE have special meanings in this manual. Read them throughout the manual to ensure
the safe and effective use of your sTMS mini.
WARNING: A WARNING tells you that the personal safety of the patient may be involved. Ignoring a
WARNING could result in injury to the patient. WARNINGS in the manual are shown in an orange box.
Precaution: A Precaution means that exact steps must be followed to prevent damage to the product. Precautions
in the manual are shown in a purple box.
NOTE: A NOTE gives special information to ease product use or to explain important information.
NOTES in the manual are shown in a dashed box.
4
WARNING: The sTMS mini should be used under the continued supervision of a physician. The
System has been prescribed by your physician only for you.
Inspect the System for any signs of damage before use. Do not use it if it is cracked or wet. If you
suspect damage to the device, call eNeura at: +1 855.366.8355 option 1 for assistance.
Do not operate the System in or near an area where explosive gases are being used or have been
used. Do not operate near gasoline or natural gas.
Do not operate the System in or near the presence of a FLAMMABLE ANESTHETIC MIXTURE WITH AIR
or WITH OXYGEN or NITROUS OXIDE.
Risk of electrical shock. Do not open the System. There are no parts that can be serviced or replaced
by the user. High voltage may be present.
Risk of electrical shock. Do not allow the System or power cords to get wet. Quickly wipe up spills on
or near the sTMS mini. Do not use the System in or near water. For example, do not use while in the
bathtub or shower, in the rain, or while standing in water or on a wet surface.
Do not use the System if the cause of your headache is illness, underlying pathology, trauma or
overuse of medication. See your physician if you are uncertain.
Do not use the System if you have suspected or diagnosed epilepsy or a personal or family history of
seizures. Consult your physician before using the System if a family member has epilepsy or seizures
or if you have had seizures, a head trauma or head injury or take any medication such as tricyclic
antidepressants, neuroleptic agents, or other drugs that lower the seizure threshold.
Do not use the System if you have a history of stroke.
The device is only intended for use when you experience the onset of pain associated with a migraine
headache with aura. The device has not been shown to be effective when treating during the aura
phase before onset of pain.
The System has not been demonstrated as safe or effective when treating cluster headache.
Do not use the System if you use a wearable cardioverter defibrillator (WCD).
The long-term effects of chronic magnetic stimulation are unknown.
Transcranial magnetic stimulation should only be applied to the back of the head as described in the
“Using Your Device” section of this manual.
Do not stimulate over the front of the neck or mouth. Severe spasm of the laryngeal and pharyngeal
muscles may occur and the contractions may be strong enough to close the airway or cause difficulty
in breathing.
Do not stimulate over the upper side of the neck. Stimulation of the carotid sinus nerves, particularly
in patients with a known sensitivity to the carotid sinus reflex, could result in a sudden drop in blood
pressure, slowing of the heart or loss of consciousness.
Do not stimulate the chest or back. The induction of electrical current into the heart may cause
cardiac arrhythmias.
5
Contraindications
WARNING: Failure to follow the restrictions listed below could result in serious injury or death.
The sTMS mini creates a very strong single-pulse magnetic field. The System has been prescribed by your doctor for your use only.
The System may not be used in patients who have metals, conductive materials, or metal-containing implants in their head, neck or
upper body. Metals and conductive materials can be affected by a magnetic field. You should discuss this with your doctor before
use.
Do not use the System if you have a cardiac pacemaker, vagus stimulator (VNS) or other implanted neurostimulator, implanted
cardioverter defibrillator (ICD) or any implanted medical device that stimulates the body or uses any signal from the body .
Talk to your doctor before using the System if you receive an implant. Patients with implants affected by a magnetic field should not
use the System. Examples of such implants include:
Aneurysm clips or coils
Cochlear implants
Cerebral spinal fluid shunts
Bullets or pellets lodged in the head or upper
body
Metal plates, screws, staples or sutures in
skull, neck, shoulders, arms or hands
Dental implants, fillings or other dental appliances are okay and are not affected by the device.
NOTE: If you have an implanted medical device but are not sure if it has metals or conductive
materials, please talk to your doctor before using the sTMS mini.
Radioactive seeds
Magnetically programmable shunt valves
Stents
Filters
Metallic artificial heart valves
Facial tattoos with metallic ink
Electrodes
6
WARNING: The System should not be used while driving, operating machinery or during any activity
in which involuntary muscle contractions may put the user at risk of injury.
Stay at least 2 feet (0.6 meter) from others when using the System. The System could be harmful to
anyone with an electronic implant such as a pacemaker. Anyone with a hearing aid or cochlear
implant may hear an audible click.
The device could be disrupted by RF-emitting equipment including: wireless home network devices,
mobile phones, cordless telephones and their base stations and walkie-talkies. See “EMC Compliance
and Warning Statement” section for additional information on preventing unwanted interference.
Precaution: Keep the System away from other electronic devices that depend-on (receive) or radiate
(transmit) radio frequency energy, when it is powered on.
The operation of the System may be impaired when operated near home devices such as wireless
network routers, mobile phones, cordless telephones and their base stations and walkie-talkies. Keep
the sTMS mini device at least 2 feet (0.6 meter) from these devices when it is powered on and in use.
Keep credit cards, audio/video tapes, computers, computer disks, flash memory sticks, cell phones,
personal digital assistants (PDAs), MP3 players, headphones, digital cameras, portable glucose meters
and other electronic devices or electronic storage media more than 2 feet (0.6 meter) away from the
System when it is in use.
Keep any loose metal objects such as eyeglasses, keys, coins, jewelry, watches and hair clips more than
2 feet (0.6 meter) away from the System when it is in use.
Keep wearable medical devices such as insulin pumps, medicinal pumps, monitors, bone grow
stimulators and Transcutaneous Electrical Nerve Stimulator (TENS) devices more than 2 feet (0.6 meter)
away from the System when it is in use.
Safety and effectiveness have not been established in pregnant women, children under the age of 18
and adults over the age of 65.
Caution should be used for patients with suspected or diagnosed heart problems.
The System is only intended to be serviced or maintained by the manufacturer. Do not attempt to open
the device. The warranty may be invalidated. If the device is opened, contact eNeura at: +1
855.366.8355 option 1.
Keep the System out of the reach of children.
Side effects can include minor dizziness, nausea, vomiting, application site tenderness, muscle spasm,
headache and migraine.
Special precautions regarding Electromagnetic Compatibility (EMC) are required when installing and
using the System. Portable and mobile communications devices can affect proper operation of the
System. See “EMC Compliance and Warning Statement” section of the Instructions for Use for more
information.
7
Clinical Trial and Adverse Reactions
eNeura completed a clinical study in Headache Centers in the United States designed to support safety and effectiveness for use of the
Spring TMS device, an earlier design of the sTMS mini, for the acute treatment and prophylaxis of migraine headache. Baseline
medication and symptoms were recorded for 28 days via patient diary. Subjects were instructed to use the SpringTMS device daily
(morning and evening) and for the acute treatment of attacks for three months with no change in preventive medication. Study results
showed statistically significant reduction in migraine headache days of 2.8 days from a baseline mean of 9.1 days. Forty-six percent of
the patients saw at least a 50% reduction in headache days (responder rate). Reduction in acute medication was 2.9 days. A statistically
significant improvement in quality of life was reported.
For further information about the clinical trial and any adverse reaction, please contact eNeura at 1-855-366-8355, option 1 or consult
your prescribing physician.
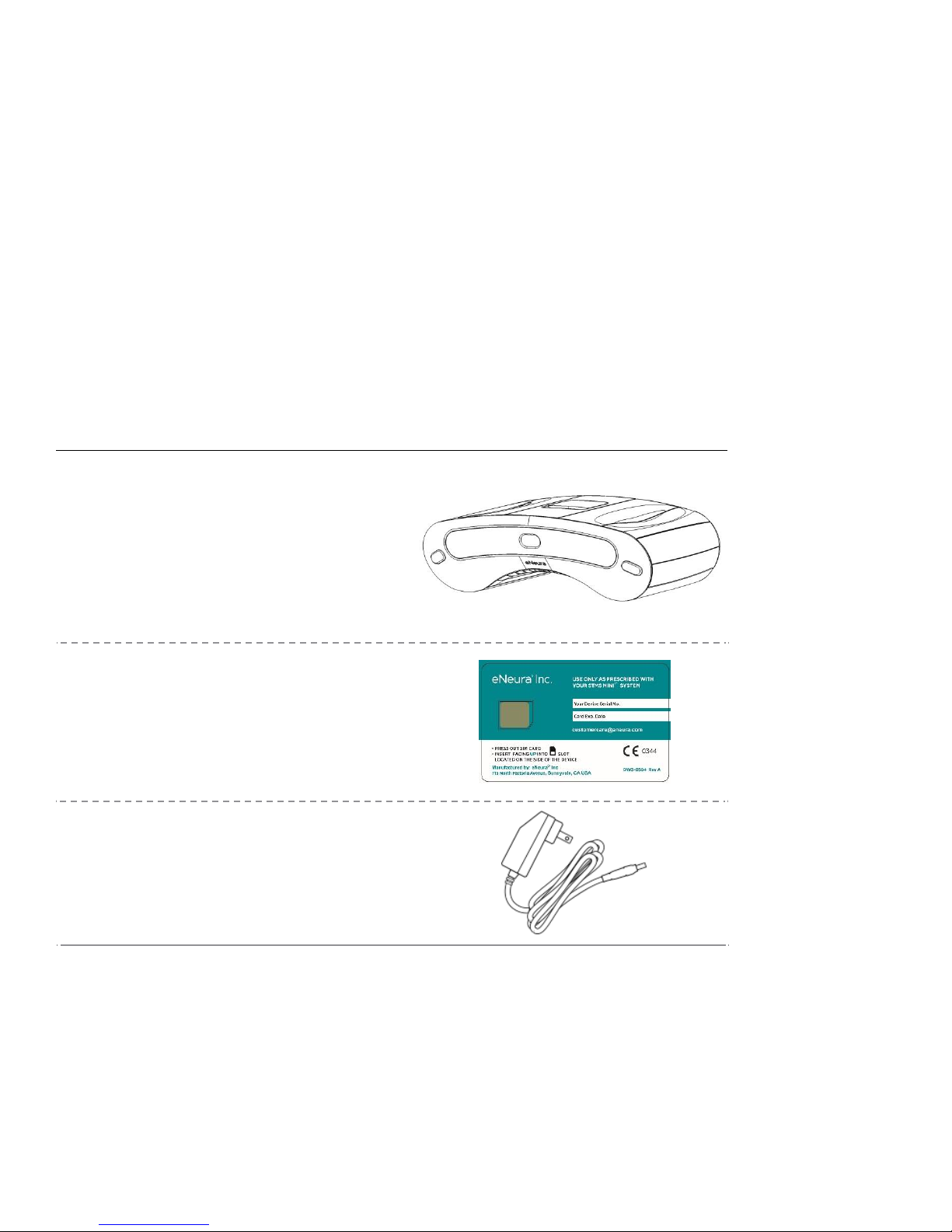
Your sTMS mini
The complete sTMS mini includes this manual and the following items:
The battery-powered, rechargeable device
Prescription card with SIM (Subscriber Identity
Module) Rx card
NOTE: Your first Micro SIM Rx card has been preinstalled
Battery Charger 12V DC 1.5A 18 watts
(reorder no. DWG-0505)
8
–
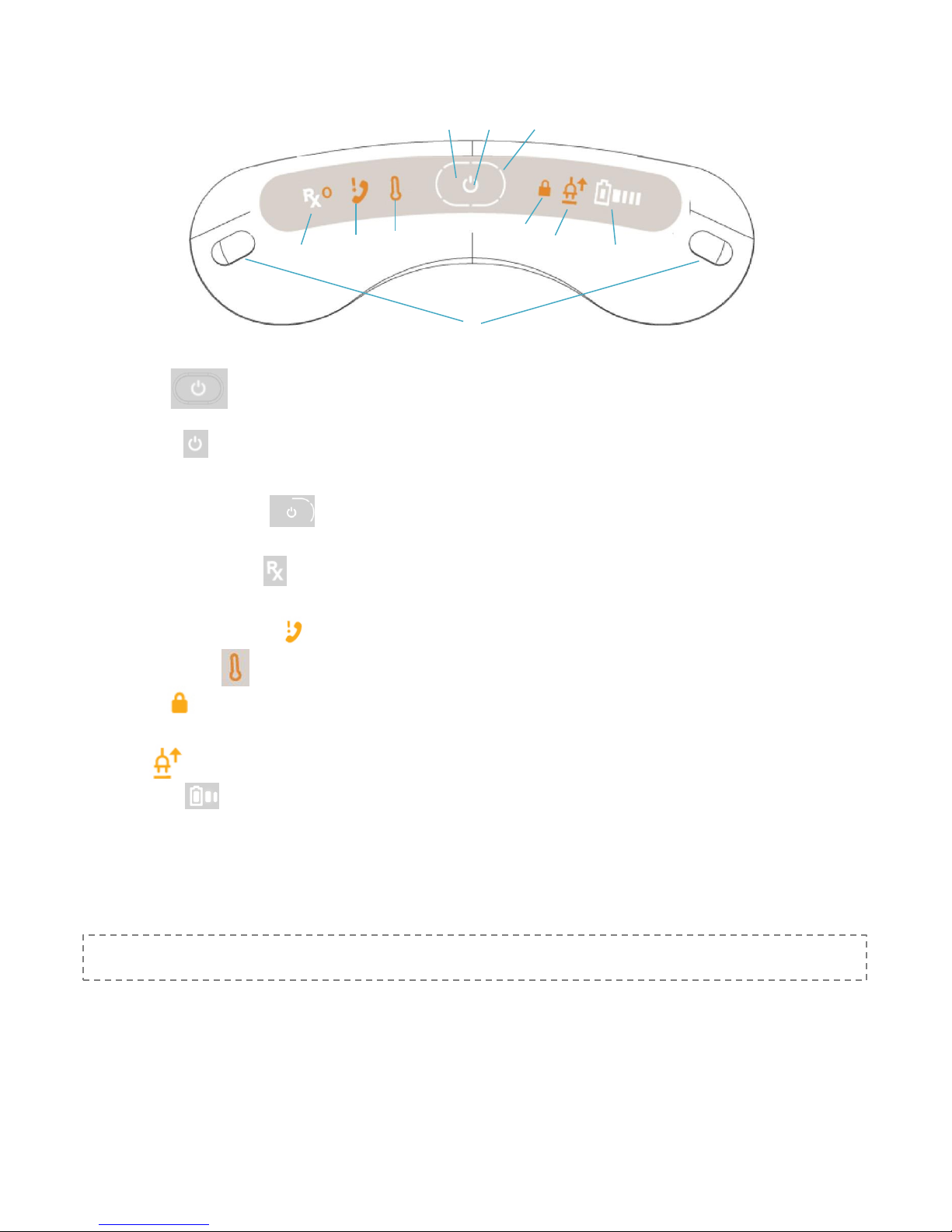
Getting to Know Your Device
A B C
F
G H I
J D E
A. Power Button In the center of the panel on the top of the System. Press the power button to turn the device on and
off.
B. Power Indicator LED light on the top of the System inside the Power button. Static white illumination shows the device is
on and ready.
C. Treatment Progress Indicator LED light around the power button on the top of the System, shows status as it prepares
for treatment.
D. Prescription Status Indicator Located on left side on the top of the System. Confirms a valid Micro SIM Rx card is installed
and shows the status of the prescription programmed on the Micro SIM Rx card.
E. Contact eNeura Customer Care Action required.
F. Temperature warning The device temperature is not in range for safe use.
G. Lock Indicator On the right of the Power button. Indicator is visible when device is turned on if the security lock switch is
enabled.
H. AC Adapter On the right of the Power button. Indicator is lit when the AC adapter is connected to the device.
I. Battery Capacity On right side on the top of the System. Indicates whether or not battery power is enough to allow
treatment.
J. Treatment Buttons On the right and left edge of the panel on the top of the device. Press one or both buttons to deliver a
treatment.
Read “Understanding System Display Messages” for more information.
9
 Loading...
Loading...