EMSI FLEX-MT PLUS Instruction Manual

Instruction Manual
Please read the Instruction
Manual prior to use.
CAUTION: Federal law requires a prescription from your physician before use of this product.

3504 Cragmont Dr. Suite #100 | Tampa, FL 33619
P: 800.588.8383 | 813.931.2369 | F: 800.588.9282 | E: customerservice@wecontrolpain.com

CAUTION: Federal law requires a prescription from your physician before use of this product.

table of contents
1.0 Intended Use .......................................................... 5
2.0 ........................................................ 5
3.0 Contraindications .................................................. 6
4.0 Warnings ................................................................ 6
5.0 Precautions ............................................................. 8
6.0 Adverse Reactions................................................... 9
7.0 Unit Description .................................................... 10
8.0 Specifications .......................................................... 13
9.0 TENS Stimulation Mode Descriptions .................. 14
16.0
Waveform Reference ................................................ 33
10.0 EMS Treatment Functions Descriptions ............... 17
11.0 Instructions For Use ............................................... 18
12.0 Patient Compliance Timer ..................................... 24
13.0 Care and Maintenance ........................................... 25
14.0 Troubleshooting ..................................................... 27
15.0 Declarations-EMC ................................................. 28

5
1.0 | Intended Use
e EMSI Flex-MT® Plus is design for the following:
TENS- Transcutaneous Nerve Stimulation
> Symptomatic relief of chronic intractable pain
> Post traumatic and post surgical pain relief
EMS- Electrical Muscle Stimulation
> Relaxation of muscle spasm
> Increasing local blood circulation
> Muscle re-education
> Prevention or retardation of disuse atrophy
> Prevention of venous thrombosis of the calf muscles immediately after surgery
> Maintaining or increase range of motion
2.0
page will show you how to use and care for your device in the general manner. You should be particularly
familiar with the prescription information and precautions before proceeding.
You should consult with your clinician if you have specific questions or problems regarding the use of your device.
CAUTION: Federal law restricts this device to sale by or on the order of a physician.

3.0 | Contraindications
1. Any electrode placement that applies current to the carotid sinus (front of neck) region.
2. Any electrode placement that causes current to flow transcerebrally (through the head).
3. Any use of this device on patients who have a demand-type cardiac pacemaker.
4.0 | Warnings
1.
2. Stimulation should not be applied over the carotid sinus nerves, particularly in
patients with a known sensitivity to the carotid sinus reex.
3. Stimulation should not be applied over the front of neck or mouth. Severe spasm of the laryngeal
and pharyngeal muscles may occur and the contractions may occur and may be strong
enough to close the airway or cause diculty in breathing.
4. Stimulation should not be applied trans-thoracically in that the introduction of
electrical current into the heart may cause cardiac arrhythmias.
5. Stimulation should not be applied over swollen, infected, or inamed areas or skin eruptions,
e.g., phlebitis, thrombophlebitis, varicose veins, etc.
6. Stimulation should not be applied over, or in proximity to, cancerous lesions.
6

7. For external use only.
8. Do not use device on the eye area.
9.
10. Safety for use during pregnancy or delivery has not been established.
11. Electronic equipment such as ECG monitors and ECG alarms may not operate properly
when TENS is in use.
12. Apply the electrodes to clean, dry and unbroken skin only.
13.
involuntary muscle contractions may put the user at undue risk of injury.
14.
15.
16.
17. TENS is a symptomatic treatment, and as it suppresses the sensation of pain which would otherwise
serve as a protective mechanism.
7

5.0 | Precautions
1. Caution should be used for patients with suspected or diagnosed heart problems.
2. Caution should be used for patients with suspected or diagnosed epilepsy.
3. Caution should be used in the presence of the following:
( a ) When there is a tendency to hemorrhage following acute trauma or fracture;
(b) Following recent surgical procedures when muscle contraction may disrupt the healing process.
( c ) Over the menstruating or pregnant uterus; and
(d ) Over areas of the skin
which lack normal sensation.
4. Some patients may experience skin irritation of hypersensitivity due to the electrical stimulation or
medium, or alternate electrode placement.
5. Electrode placement and stimulation settings should be based on the guidance of the
prescribing practitioner.
6.
the manufacturer.
7. Isolated cases of skin irritation may occur at the site of the electrode placement following
long-term application.
8
9
8. Eectiveness is highly dependent upon patient selection by a person qualified in the management
of pain patients.
9. If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation
amplitude to a comfortable level and contact your physician if problems persist.
6.0 | Adverse Reactions
1. Possible skin irritation or electrode burn under the electrodes may occur.
2. Possible allergic skin reaction to tape or gel may occur.
3.
transmitters, e.g. mobile phones or similar mobile radio equipment, airport security systems, or
metal detection devices (which themselves conform to the EMC regulations), may influence the
proper functioning of the device if such equipment is operated in close proximity and with relatively
high transmitting power.
Flex-MT® Plus meets EMC requirements and is designed in such a way, that under normal conditions,
there is no risk of malfunction caused by electromagnetic interference. However, in the case of signals
from high frequency transmitters, the risk of electromagnetic incompatibility when operated in close
proximity to electronic apparatus cannot be totally ruled out. In unusual circumstances, unintended
functions of the Flex-MT® Plus could be initiated, possibly giving rise to undesirable risks for the patie
nt or
user such as a surge in energy level or ineective treatment parameters.
10
7.0 | Unit Description
ON/OFF Button: Turns the unit ON and OFF.
Amplitude Controls: Controls the “INTENSITY” level of stimulating pulses.
MODE Button: Choose the TENS or EMS stimulation modes.
SET Button: Set the pulse width, pulse rate, ramp time, on time and o time.
TIMER Button: Sets the timer.
INCREASE & DECREASE Button: Increase and decrease pulse width, pulse rate, ramp time, on
time, o time and choose the timer.
LOCK/UNLOCK Button: Locks or unlocks the unit.
Symmetric/Asymmetric Button: Choose symmetric or asymmetric waveform.
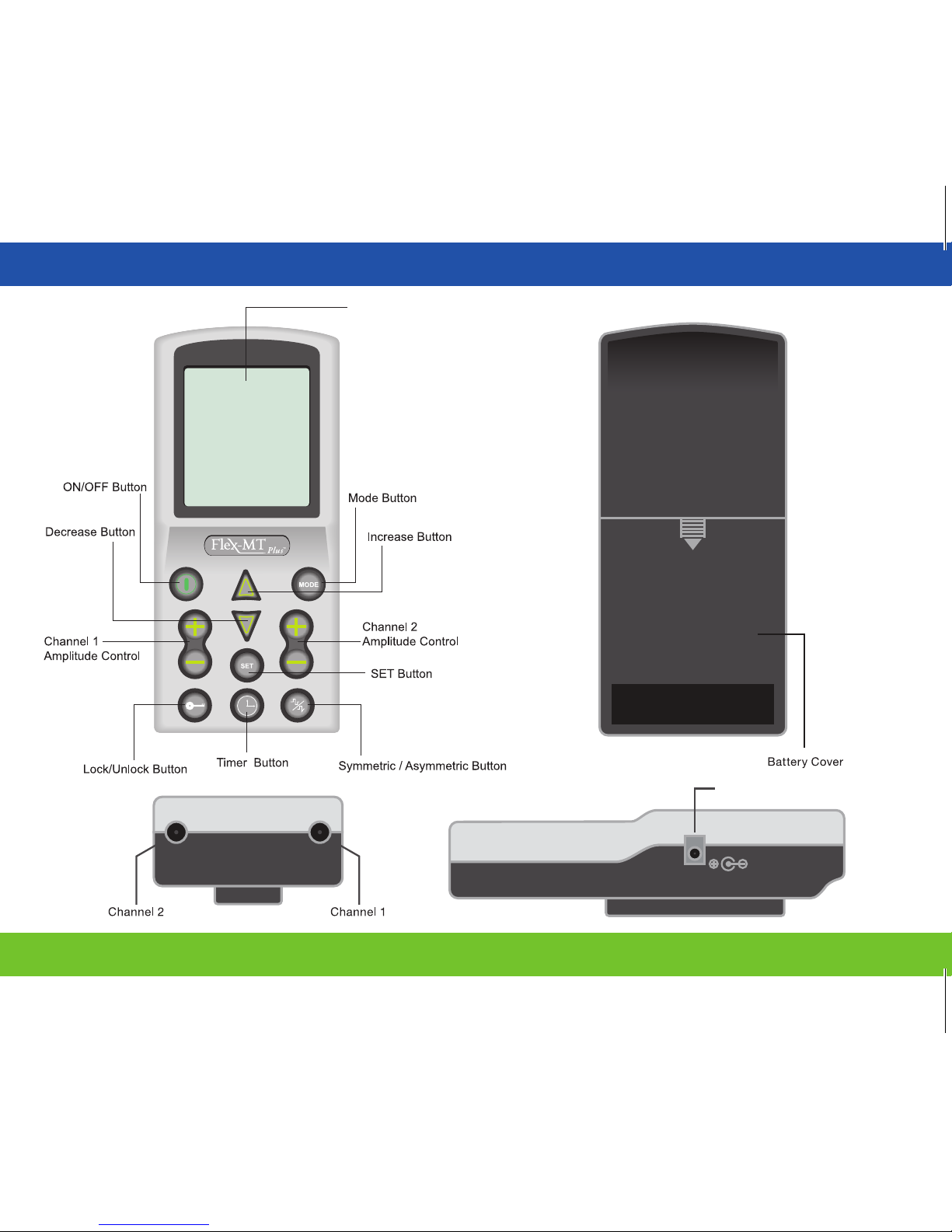
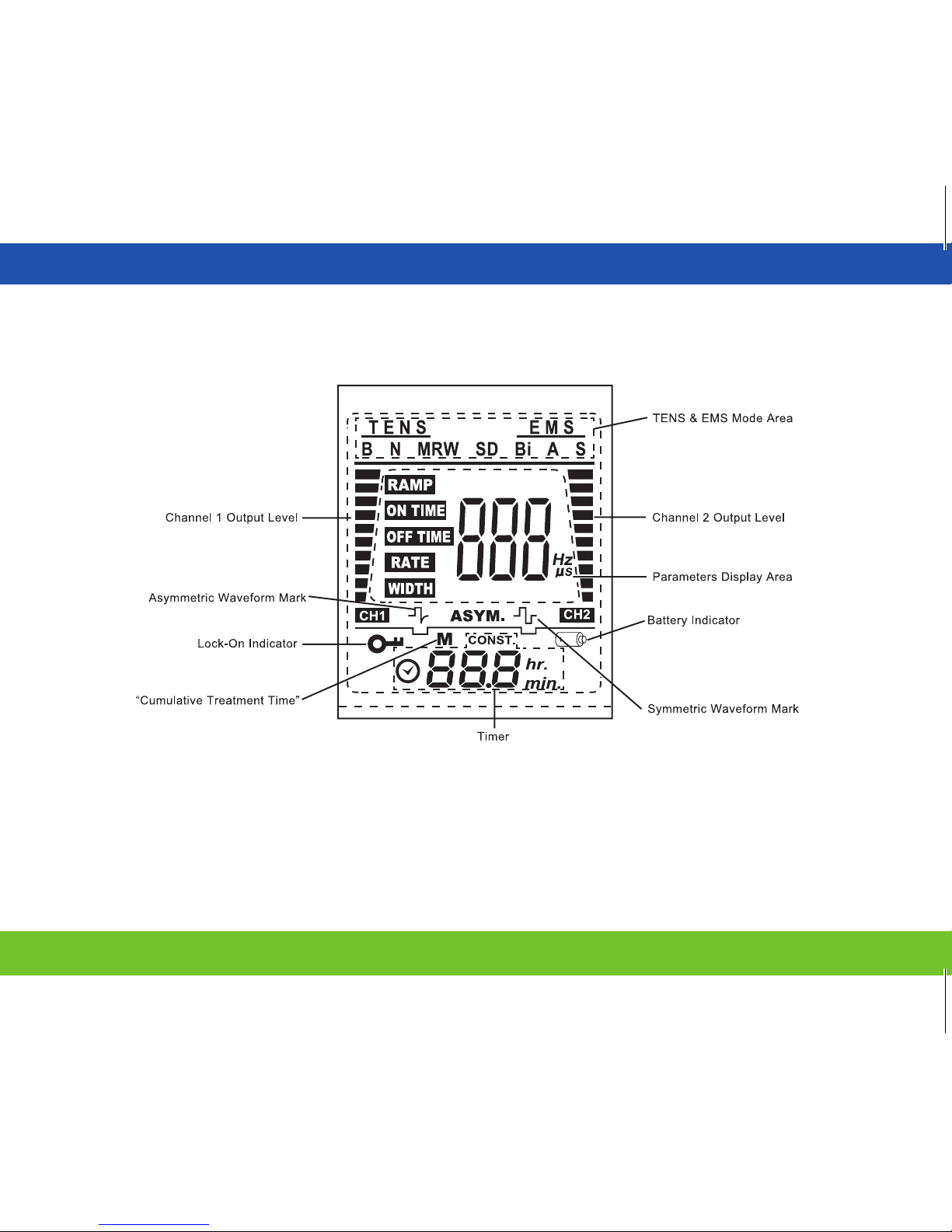
LCD Display
11
Recharger Receptacle
7.0 | Unit Description continued
Function Mark
Electrode Leadwires: Two sets of 1100mm (43 inch)
electrode leadwires which are compatible with commercially
available electrodes (standard 0.08 inch female connection)
are provided with the
Flex-MT® Plus. Each output jack of
the device is designed to accept a lead wire whose connector
complies with FDA 21 CFR Part 898 requirements.
e
Flex-MT® Plus is recommended for use with
the previously cleared Everlife self adhesive
electrodes. (Re-order information on page 26)
Battery Charger:
Input: AC 110V, 50~60Hz, 0.2A
Output: DC 4.8V, 400mA
Accessories
12

13
8.0 | Specifications
EMS/TENS Specifications
Dual, isolated between channels
700 mAh 4.8V Ni-MH rechargeable battery pack
Symmetric or Asymmetric waveform
0~±65V (Loading: 1000Ω)
65mA (Loading: 1000Ω)
Level 1~Level 20: Each level increases ±3.25V
0~65V (Loading: 1000Ω)
65mA (Loading: 1000Ω)
Level 1~20: Each level increases 3.25V
Variable, 50~400 µs
Variable, 2~150 Hz
1~99 seconds
1~99 seconds
Output
Peak Pulse Output
Level
Output
Peak Pulse Output
Level
Channel:
Power Source:
Output waveform:
Symmetric
Asymmetric
Pulse Width:
Pulse Frequency:
* All values + or - 10%
On Time:
O Time:
EMS only
 Loading...
Loading...