Page 1

M-Series Touchcomputer User Guide
M-Series LCD Multi-function Touchcomputer
[19” model shown with optional accessories]
Page 2

Elo Touch Solutions
M-Series Touchcomputer for Healthcare
Applications User Guide
Multi-function Touchcomputer
Revision A
SW200069
1-800-ELOTOUCH (1-800-356-8682)
www.elotouch.com
M-Series Touchcomputer User Guide ii
Page 3

Copyright © 2012 Elo Touch Solutions Inc. All Rights Reserved.
No part of this publication may be reproduced, transmitted, transcribed, stored
in a retrieval system, or translated into any language or computer language, in
any form or by any means, including, but not limited to, electronic, magnetic,
optical, chemical, manual, or otherwise without prior written permission of Elo
Touch Solutions.
Disclaimer
The information in this document is subject to change without notice. Elo
Touch Solutions Inc. Elo Touch Solutions makes no representations or
warranties with respect to the contents herein, and specifically disclaims any
implied warranties of merchantability or fitness for a particular purpose. Elo
Touch Solutions reserves the right to revise this publication and to make
changes from time to time in the content hereof without obligation of Elo Touch
Solutions to notify any person of such revisions or changes.
Trademark Acknowledgments
AccuTouch, Elo TouchSystems, Elo TouchSystems (logo), Elo, IntelliTouch,
iTouch, Elo Touch Solutions, and Elo Touch Solutions (logo) are trademarks.
Windows is a trademark of Microsoft Corporation. Other product names
mentioned herein may be trademarks or registered trademarks of their
respective companies. Elo Touch Solutions claims no interest in trademarks
other than its own.
M-Series Touchcomputer User Guide iii
Page 4
Warnings and Cautions
Warning
Danger - Explosion hazard. Do not use in the presence of flammable
•
anesthetics, and other flammable materials.
• To prevent fire or shock hazards, do not immerse the unit in water or expose
it to rain or moisture.
• Do not use the unit with an extension cord receptacle or other outlets unless the
prongs of the power cord can be fully inserted.
• RISK OF ELECTRICAL SHOCK - DO NOT OPEN. To reduce the risk of
electrical shock, DO NOT remove the back of the equipment or open the
enclosure. No user-serviceable parts are inside. Refer servicing to qualified
field service engineers only.
• Uninsulated voltage within the unit may have sufficient magnitude to cause
electrical shock.
Avoid contact with any part inside the unit.
• This device complies with the electromagnetic emission and immunity
standards and is limited to the standards that are listed on pages 41 and 45.
Other devices which are not designed to withstand emission levels as
specified in the medical device standards may be susceptible to interference
from this device. Subjecting the device to conditions beyond the rated
performance capabilities may result in emissions in excess of the standard. If
it is determined that this device produces electromagnetic or other
interference it must be disconnected from power until the cause of the problem
has been determined and resolved. If it is determined that this device is
functioning improperly due to electromagnetic and other interference it must be
disconnected from power until the cause of the problem has been determined
and resolved.
• Elo Touch Solutions recommends that after its useful life (or after sustaining
unrepairable damage), customers dispose of the touchcomputer and its power
supply in an environmentally sound manner. Acceptable methods include the
reuse of parts or whole products and the recycling of products, components,
and materials. Please consult and obey national state, and local laws and
ordinances governing the safe disposal of electronic equipment.
• Do not modify this equipment without authorization of the manufacturer.
This product consists of devices that may contain mercury, which must be recycled
or disposed of in accordance with local, state, or federal laws.
M-Series Touchcomputer User Guide iv
Page 5
Caution
Power cord is used as a disconnection device. To de-energize
equipment, disconnect the power cord.
This unit must follow the national requirement and local state laws to
dispose unit.
Before connecting the cables to your Elo touchcomputer, make
sure all components are powered OFF.
Only approved components complying with IEC 60601-1 series can be
connected to 15MX/19MX Touchcomputer for Healthcare Applications in
Patient Environment. The use of ACCESSORY equipment not complying
with the equivalent safety requirements of this equipment may lead to a
reduced safety of the resulting system. Consideration relating to the
choices of accessory equipment should include: Use of accessory in the
patient environment.· Evidence that the safety certification of the
accessory has been performed in accordance to the appropriate IEC
60601-1 and/or IEC 60601-1-1 harmonized national standard.
For continued safety
• This unit only complies to the above standards if used with a
medical grade power cord.
• A medical grade power supply, such as the one specified, is
required for use in a medical application.
Please do not touch the patient and the touchcomputer output
connecter at the same time.
The touchmonitor is to be used with following adaptor:
1. XP Power / AHM85PS12
or
2. Elo Touch Solutions / FSP110-RAAM
Disposing of your old product
Within the European Union
EU-wide legislation, as implemented in each Member State, requires that
waste electrical and electronic products carrying the mark (left) must be
disposed of separately from normal household waste. This includes monitors
and electrical accessories, such as signal cables or power cords. When you
need to dispose of your display products, please follow the guidance of your
local authority, or ask the shop where you purchased the product, or if
applicable, follow any agreements made between yourself and the supplier of
this product.
The mark on electrical and electronic products only applies to the current
European Union Member States.
Elo Touch Solutions recommends that after its useful life (or after sustaining
unrepairable damage), customers dispose of the touchcomputer and its power
supply in an environmentally sound manner. Acceptable methods include the
reuse of parts or whole products and the recycling of products, components,
and materials. Please consult and obey national, state, and local laws and
ordinances governing the safe disposal of electronic equipment.
M-Series Touchcomputer User Guide v
Page 6

Explanation of Symbols:
• This symbol alerts the user to important information concerning the operation and
!
maintenance of this unit, which should be read carefully to avoid problems.
• This symbol means DC Current.
• This symbol means ON/OFF stand-by switch.
• Consult instructions for use.
Medical and Healthcare Application Disclaimer:
It is the sole responsibility of any person intending to commercialize, market or
use any of Elo Touch Solutions, Inc. products for medical or healthcare
applications to ensure that such product is adequate and appropriate for
the person's intended use and complies with all applicable laws, regulations,
codes and standards including but not limited to the European Union Medical
Device Directive, United States Federal Food, Drug, and Cosmetic Act,
regulations of the United States Food and Drug Administration (FDA), and for
obtaining and maintaining any required regulatory approvals including but not
limited to any required market clearances. Elo has not sought nor received any
rulings from the FDA or any other federal, state, or local government agency or
notified body as to the safety, effectiveness or appropriateness of its product
for such applications. Persons intending to evaluate or use Elo's product for
medical or healthcare purposes must rely on their own medical and legal
judgment without any representation on the part of Elo.
M-Series Touchcomputer User Guide vi
Page 7

Classification
E354528
07EG
With respect to electrical shock, fire in accordance with ANSI/AAMI ES60601-1 and
CAN/CSA- C22.2 No. 60601-1
This touchcomputer is a Class I (GROUNDED) DEVICE.
This touchcomputer is classified NO APPLIED PARTS EQUIPMENT.
Protection against harmful ingress of water:
INGRESS PROTECTION (IP21)
This touchcomputer shall be classified as ORDINARY EQUIPMENT, not
intended or evaluated for use in the presence of flammable anesthetic mixture
with air, oxygen, or nitrous oxide.
Mode of Operation: CONTINUOUS OPERATION.
Environmental conditions for transport and storage
Temp. Operating
Storage / Transportation
0°C to 35°C
-30°C to 60°C
Humidity (non-condensing)
Storage / Transportation 5% to 95%
Operating 20% to 80%
Altitude
Storage / Transportation 0 to 12,192m
Operating 0 to 2,000m
15MX/19MX Touchcomputer for Healthcare Applications is intended for general use in
hospital environment for data collection and display for reference. It shall not be used with
life-supporting system
For full Product Specifications refer to Appendix C
M-Series Touchcomputer User Guide vii
Page 8

European Standards and Classifications
Standards: EN 60601-1-2: 2007
The EMC limits and test methods are referred to the following standards:
Emission: Immunity
CISPR 11: 2009+A1:2010 IEC 61000-4-2: 2008
EN55011: 2009+A1: 2010
(Group 1, Class A)
IEC 61000-3-2:
2005+A1:2008+A2:2009
IEC 61000-3-3; 2008 IEC 61000-4-5: 2005
IEC 61000-4-6: 2008
IEC 61000-4-8: 2009
IEC 61000-4-11: 2004
IEC 61000-4-3: 2006+A1:2007+A2:2010
IEC 61000-4-4: 2011
M-Series Touchcomputer User Guide viii
Page 9
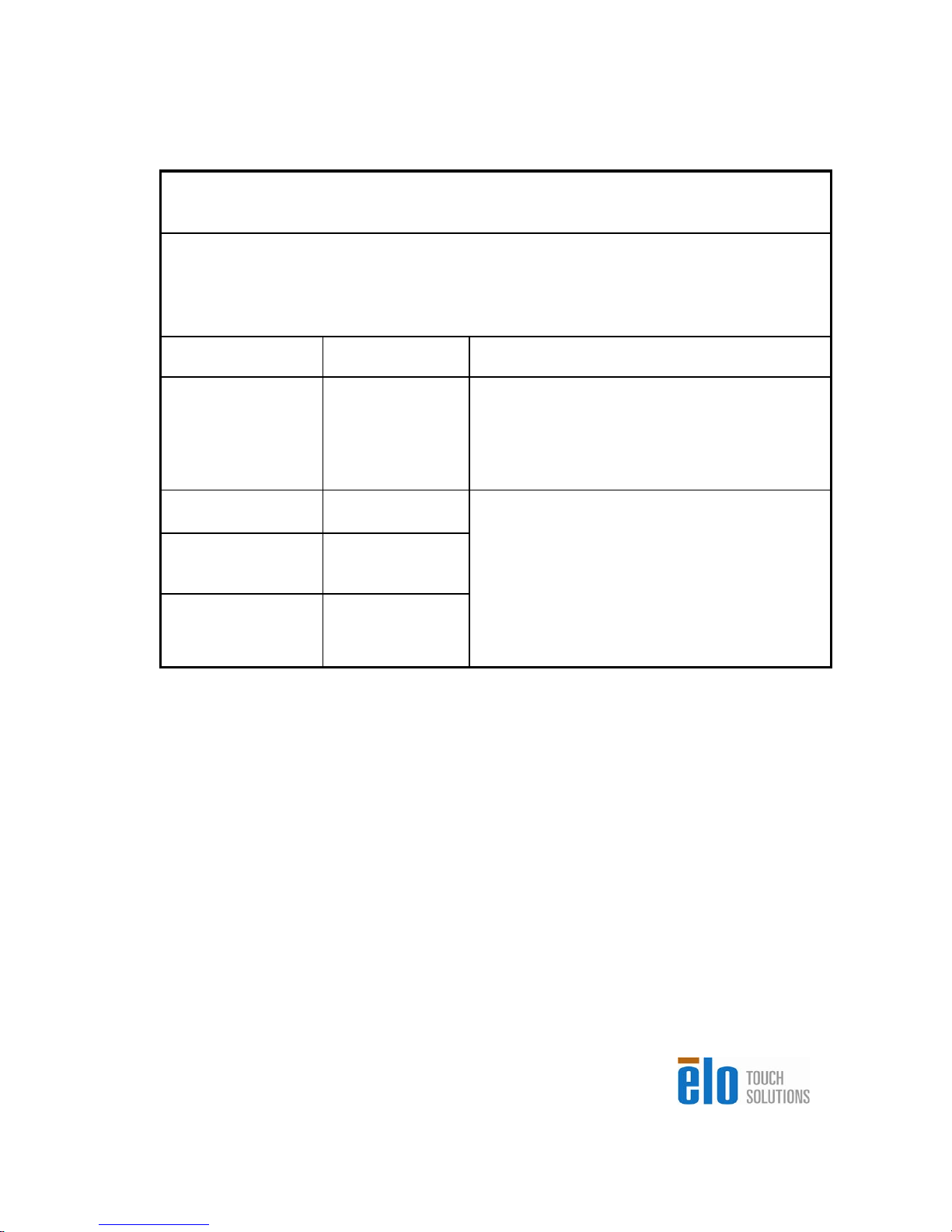
s
Guidance and manufacturer’s declaration-electromagnetic
immunity
for all EQUIPMENT AND SYSTEMS
Guidance and manufacturer’s declaration-electromagnetic
emissions
The 15MX/19MX Touchcomputer for Healthcare Applications is intended for use in the
electromagnetic environment specified below. The customer or the user of the 15MX/19MX
Touchcomputer for Healthcare Applications should assure that it is used in such an
environment.
Emissions test Compliance Electromagnetic environment-guidelines
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonics emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Group 1
Class A
Not applicable
Complies
The 15MX/19MX Touchcomputer for Healthcare
Applications uses RF energy only for its internal
function. Therefore, its RF emissions are very
low and are not likely to cause any interference
in nearby electronic equipment.
The 15MX/19MX Touchcomputer for Healthcare
Applications is suitable for use in all establishment
other than domestic and those directly connected
to a low voltage power supply network which
supplies buildings used for domestic purposes.
M-Series Touchcomputer User Guide ix
Page 10
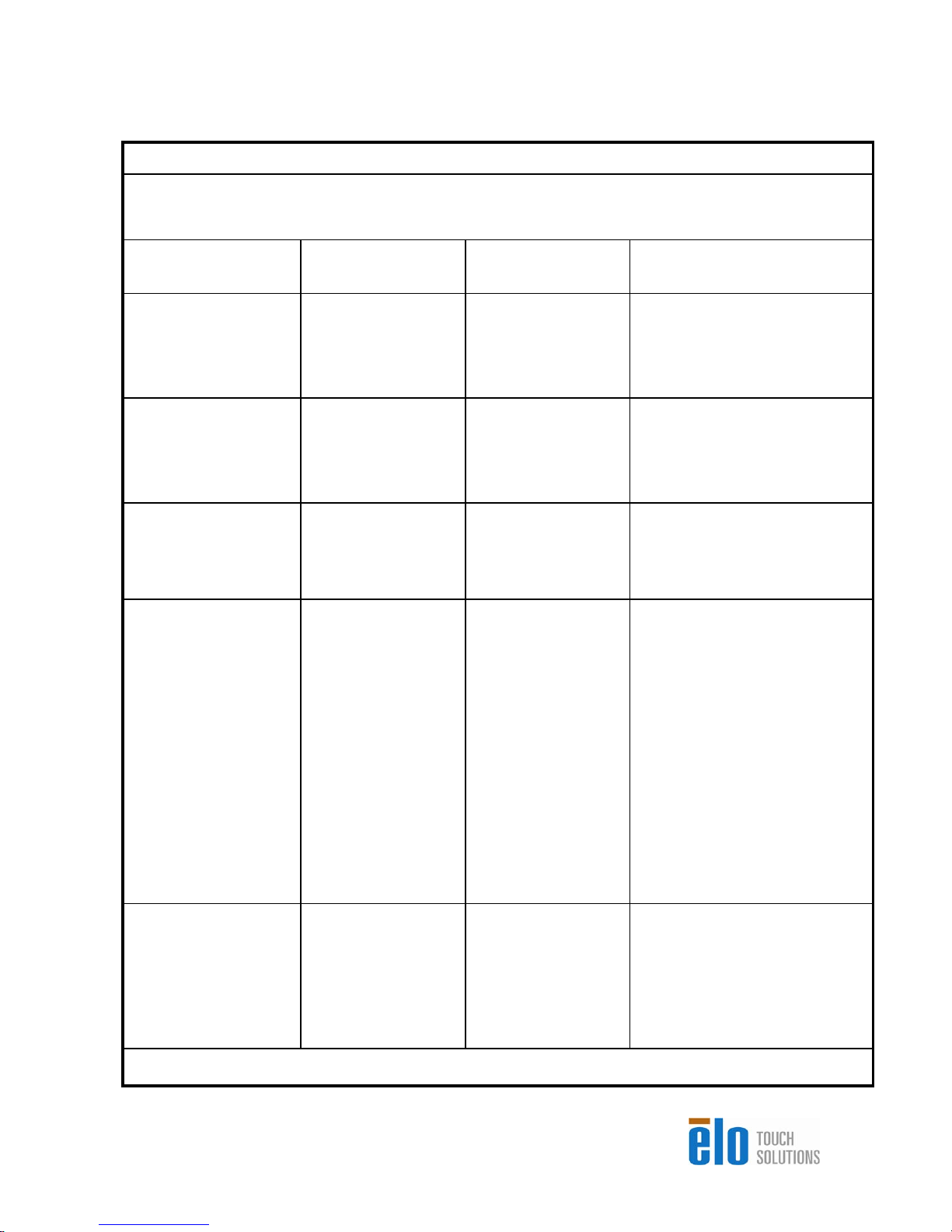
t
t
r
Guidance and manufacturer’s declaration-electromagnetic
immunity
for all EQUIPMENT AND SYSTEMS
Guidance and manufacturer’s declaration-electromagnetic immunity
The 15MX/19MX Touchcomputer for Healthcare Applications is intended for use in the electromagnetic
environment specified below. The customer, or the user of the 15MX/19MX Touchcomputer for Healthcare
Applications, should assure that it is used in such an environment.
Immunity Test IEC 60601 Compliance Level
±
Electrostatic Discharge
(ESD)
IEC 61000-4-2
Electrical Fast
Transient/Burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage Dips, Short
Interruption and Voltage
Variations on Power
Supply Input Lines
IEC 61000-4-11
6 kV contact
±
8 kV air
±
2 kV for power
supply lines
±
1 kV for input/outpu
lines
±
1 kV line(s) to
line(s)
±
2 kV line(s) to earth
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 250 cycles
±
6 kV contact
±
8 kV air
2 kV for power supply
lines
±
1 kV for input/outpu
lines
±
1 kV line(s) to
line(s)
±
2 kV line(s) to earth
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60 % dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 250 cycles
Electromagnetic EnvironmentGuidelines
Floors should be wood,
concrete or ceramic tile. If floors
are covered with synthetic
material, the relative humidity
Mains power quality should be
that of a typical commercial or
hospital environment.
Mains power quality should be
that of a typical commercial or
hospital environment.
Mains power quality should be
that of a typical commercial or
hospital environment. If the user
of the 15MX/19MX Touchcompute
for Healthcare Applications
requires continued operation
during power mains interruptions,
It is recommended that the
15MX/19MX Touchcomputer for
Healthcare Applications be
powered from an uninterruptible
power supply or a battery.
Power Frequency
(50/60 Hz) Magnetic
Field
IEC 61000-4-8
NOTE UT is the A.C. mains voltage prior to application of the test level.
3 A/m 3 A/m
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical commercial
or hospital environment.
M-Series Touchcomputer User Guide x
Page 11
e
e
Guida nce and ma nufa ctur er’s declaration-electromagnetic immunity
for all EQUIPMENT AND SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacturer’s declaration-electromagnetic immunity
The 15MX/19MX Touchcomputer for Healthcare Applications is intended for use in
the electromagnetic environment
Touchcomputer for Healthcare Applications should assure that it is used in such an
environment.
Immunity Test IEC 60601 Compliance
Conducted RF
Radiated RF
IEC 61000-4-3
3 Vrms
3 V/m
80 MHz to 2.5
GHz
specified
Level
3 Vrms
3 Vrms
below. The user of the 15MX/19MX
Electromagnetic
Environment-Guidelines
Portable and mobile RF
communications equipment should be
used no closer to any part of the
15MX/19MX Touchcomputer for
Healthcare Applications and should
assure that it is used in such an
environment, including cables, than th
recommended separation distance
calculated from the equation applicabl
to the frequency of the transmitter.
Recommended separation distance
where P is the maximum output
power rating of the transmitter in
watts (W) according to the transmitter
manufacturer and d is the
recommended separation distance
in metres(m)
Field strengths from
transmitters, as determined by an
electromagnetic site survey
be less than the compliance level in
each frequency range
Interference may occur in the
vicinity of equipment marked with
the following symbol:
80MHz to 800 MHz
800 NHz to 2.5GHz
fixed
2
.
RF
1
, should
M-Series Touchcomputer User Guide xi
Page 12
.
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and
reflection
from structures, objects and people.
3. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the 15MX/19MX
Touchcomputer for Healthcare Applications is used exceeds the applicable RF compliance
level above, the 15MX/19MX Touchcomputer for Healthcare Applications should be
observed to verify normal operation. If abnormal performance is observed, additional
measures may be necessary, such as reorienting or relocating the 15MX/19MX
Touchcomputer for Healthcare Applications.
4. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 Vrms
M-Series Touchcomputer User Guide xii
Page 13
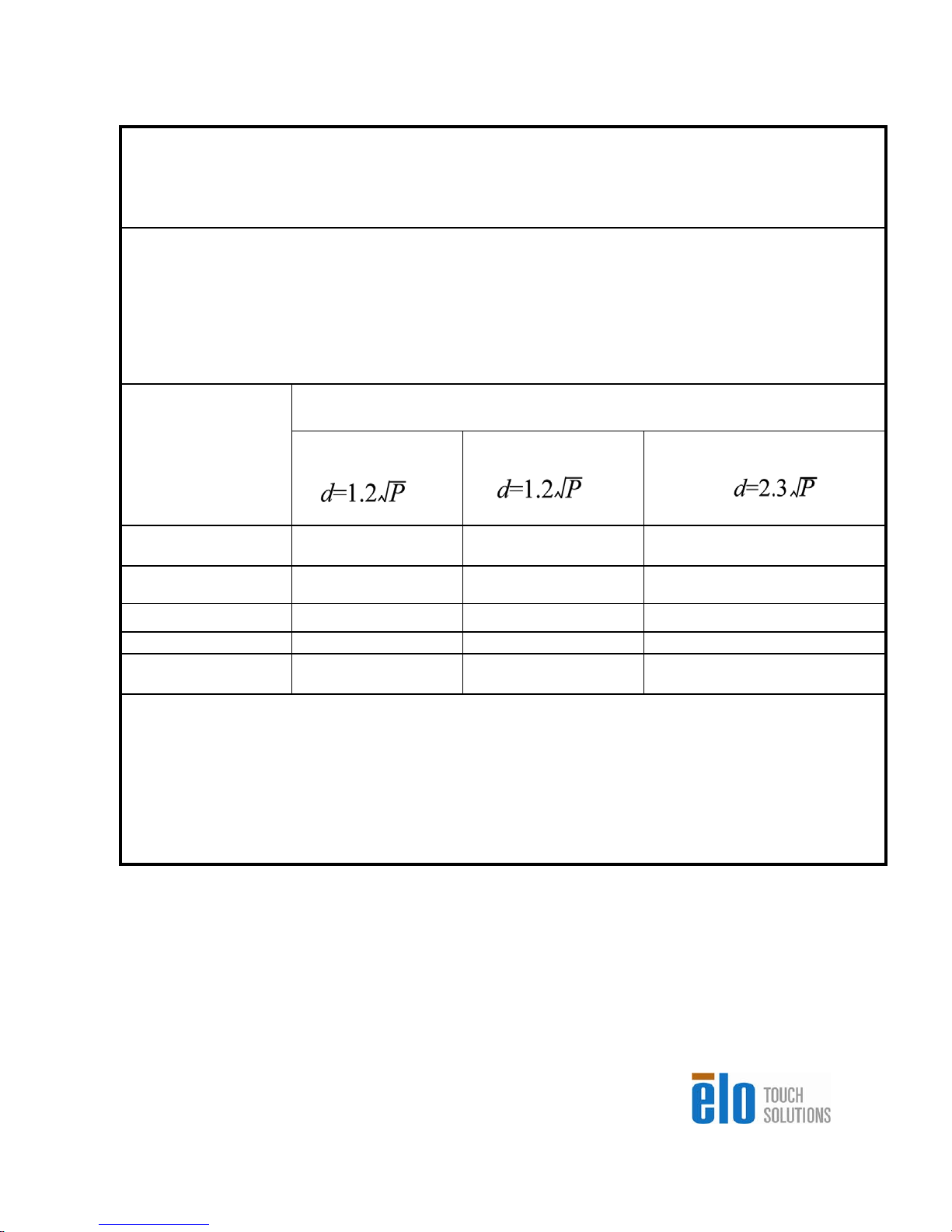
Recommended separation distances between portable
and mobile RF
communications equipment and the 15MX/19MX Touchcomputer for Healthcare
Applications for all EQUIPTMENT AND SYSTEMS that are not
LIFE-SUPPORTING
The 15MX/19MX Touchcomputer for Healthcare Applications is intended for use in an electromagnetic
environment in which radiated RF disturbances are controlled. The customer or the user of the
15MX/19MX Touchcomputer for Healthcare Applications can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications (equipment)
and the 15MX/19MX Touchcomputer for Healthcare Applications as recommended below according to
the maximum output power of the communications equipment.
Rated Maximum
Output Power of
Transmitter
(W)
Separation Distance According to Frequency of Transmitter
150 kHz to 80 MHz
80MHz to 800 MHz
800 MHz to 2.5 GHz
0.0
1
0.
1
1 1.
13. 3. 7.4
10
0
For transmitters rated at a maximum output power not listed above, the recommended separation
distanced in metres (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
0.1
2
0.3
7
1
2
0.1
2
0.3
7
1.
1
2
0.23
0.74
2.3
23
M-Series Touchcomputer User Guide xiii
Page 14
Table of Contents
Chapter 1: Setup......................................................................................1
Unpacking Your Touchcomputer ....................................................................................................1
Touchcomputer Operating System ..................................................................................................2
Accessing the Hard Disk..................................................................................................................2
Calibrating the Touchscreen............................................................................................................3
Securing the M-Series AIO to a Mounting Bracket.........................................................................4
Chapter 2: Operation...............................................................................5
User Buttons.....................................................................................................................................6
Using the Input/Output Panel...........................................................................................................8
Chapter 3: Options and Upgrades.........................................................9
Peripherals and Accessories.............................................................................................................9
Magnetic Stripe Reader (MSR) .....................................................................................................10
Wireless Card.................................................................................................................................11
Solid State Drive............................................................................................................................11
Webcam .........................................................................................................................................11
Barcode Scanner ............................................................................................................................12
Smart Card Reader.........................................................................................................................12
RFID Reader..................................................................................................................................13
USB Handset..................................................................................................................................14
TV Tuner........................................................................................................................................14
3 Meter DC extension cable...........................................................................................................14
AC Cable........................................................................................................................................15
Chapter 4: Safety and Maintenance.....................................................16
Safety .............................................................................................................................................16
Care and Handling .........................................................................................................................17
Chapter 5: Technical Support...............................................................18
Chapter 6: Technical Specifications....................................................18
Touchcomputer Specifications.......................................................................................................18
M-Series Touchcomputer User Guide xiv
Page 15

Technical Assistance......................................................................................................................18
Regulatory Information............................................................................19
Warranty ...................................................................................................27
Index ..........................................................................................................29
M-Series Touchcomputer User Guide xv
Page 16

This chapter discusses how to set up and test your touchcomputer. For information
on peripheral options, refer to Chapter 3, “Options and Upgrades.”
Unpacking Your Touchcomputer
Check that the following items are present and in good condition:
C H A P T E R
1
SETUP
M-Series Touchcomputer User Guide
1
Page 17

Touchcomputer Operating System
The touchcomputer may be delivered without an operating system. An operating
system must be installed before many of the features of the touchcomputer can be
used.
The hardware used on the touchcomputer is compatible with many common
operating systems.
Accessing the Hard Disk
The hard disk is factory installed and located under the hard disk cover on the back
of the unit. To access the hard drive remove the one screw holding the cover in
place and remove the hard drive bracket to access the hard drive if necessary.
Remove this screw to gain access to
the hard disk.
M-Series Touchcomputer User Guide
2
Page 18

Calibrating the Touchscreen
The touchscreen is pre-calibrated for accurate touch response.
If for any reason the touchscreen needs to be recalibrated, right-click the Elo icon
in the Taskbar and then click “Properties.” The following window opens.
NOTE: Calibration is not applicable on projective capacitive touchscreen
models.
NOTE: OS and touchscreen drivers must be installed for Elo icon to appear in
the Taskbar
Click the Align button. This launches the calibration program. The window shown
below opens. Follow the instructions to calibrate the touchscreen.
M-Series Touchcomputer User Guide
3
Page 19

Securing the M-Series AIO to a Mounting Bracket
Use the four pre-tapped holes to secure the touchcomputer to the mounting
bracket. The holes are designed to work with ISO metric M4 screws. Mounting
screws are not included. Select the length screw that will provide adequate thread
engagement to provide a reliable connection to the mounting bracket. The correct
length screw for your application should be commonly available at a hardware
store. Refer to the figure below for the location of the 4 holes.
Mounting Diagram
M-Series Touchcomputer User Guide
4
Page 20

C H A P T E R
2
OPERATION
This chapter describes the user button functions and I/O panel.
All adjustments made to the user buttons are reset upon a power cycle to the
touchcomputer.
M-Series Touchcomputer User Guide
5
Page 21

User Buttons
User buttons are capacitive buttons. Use of finger or appropriate stylus is required for proper
function.
Power Button
The power button function is different depending on the state of the touchcomputer.
• When the touchcomputer is booted into an operating system the power button will turn off
and on the display backlight
• When the touchcomputer is in a sleep state such as hibernate the power button will wake
up the system
• After an operating system shutdown event occurs and power is still applied to the
touchcomputer the power button will boot the computer.
Reading Light Function
Touch the reading light icon and use the +/- function adjustment icons to adjust the intensity.
Current setting is displayed between +/- icons.
Night Light Function
Touch the night light icon and use the +/- function adjustment icons to adjust the intensity. Current
setting is displayed between +/- icons.
M-Series Touchcomputer User Guide
6
Page 22

Screen Brightness Function
Touch the screen brightness icon and use the +/- function adjustment icons to adjust the intensity.
Current setting is displayed in-between +/- icons
.
Audio Volume Function
Touch the audio volume icon and use the +/- function adjustment icons to adjust the intensity.
Current setting is displayed in-between +/- icons.
Function Adjustment
The function adjustment icons are illuminated when enabled. Touching one of the function icons
will enable the function adjustment symbols and display the current setting on the dots between
the +/- symbols.
Use the “-“ symbol to decrease the intensity of the function and use the “+” symbol to increase the
intensity of the function. The current setting will be immediately updated and saved.
After a short time the selected function will become deselected. Other functions can be selected
before the current function is deselected.
+ Computer Shutdown
Press and hold the volume icon and the power button icon for 3 seconds to hard shutdown the
computer. The icons will blink to confirm.
The power button can be used to turn the computer back on.
M-Series Touchcomputer User Guide
7
Page 23

Using the Input/Output Panel
To access the input/output (I/O) ports, remove the cable cover on left side of the
back of the unit.
Install the I/O cables in the respective ports and use plastic cable ties to fasten the
cables to the cable restraints shown below.
Below are the I/O descriptions and location of the cable restraints:
Note: As a safety precaution, always leave the cable cover attached when the system is
powered on.
M-Series Touchcomputer User Guide
8
Page 24

Peripherals and Accessories
The following peripherals and accessories are available for the M-Series
touchcomptuers.
• Magnetic stripe reader (MSR)*
C H A P T E R
3
OPTIONS AND UPGRADES
• Wireless adapter
• Solid State Drive (SSD)*
• Webcam
• Barcode Scanner (2D area array)*
• Smart Card Reader*
• RFID Reader*
• USB Handset*
• TV Tuner*
• 3 Meter DC extension cable*
• AC Cable*
* Optional Accessory
Note: Software drivers and applications for all peripherals are available upon request
M-Series Touchcomputer User Guide
9
Page 25

Magnetic Stripe Reader (MSR)
A magnetic stripe reader (MSR) is an optional M-Series touchcomputer peripheral.
Software application and drivers can be found on the web at:
www.elotouch.com
The MSR is a USB 2.0 device that reads all three data stripes on standard credit
cards or driver’s licenses conforming to ISO/ANSI standards. The MSR has foreign
language capability. The card is read by sliding it through the guide with the
magnetic stripe facing up when placed in the guide in the front of the M-Series
product.
The MSR features are:
• Reads up to 3 tracks of information
• Bi-directional swipe reading
• Superior reading of high jitter, scratched, and worn MagStripe cards
• Reliable for over 1,000,000 card swipes
• Reads ISO7811, AAMVA, and most other card data formats
• PC software makes configuration changes easy
• Swipe speeds from 3 to 60 inches per second
• Interfaces: USB-KB and USB-HID
• Fully supports USB 2.0
M-Series Touchcomputer User Guide
10
Page 26

Wireless Card
A wireless adapter is a standard peripheral in the M-Series touchcomputer.
Typical specifications for the wireless card are:
• Mini PCI-E module
• IEEE 802.11 a/b/g/n compliant
Solid State Drive
A solid state drive can be used to replace the original hard disk drive. This addition
provides additional performance and is more mechanically reliable in harsh
environments.
Access to the original hard disk drive is through the right side cover on the back of
the M-Series touchcomputer.
Webcam
Part number: E520117
An integrated webcam is included in every M-Series touchcomputer. The Webcam
module is a USB-powered device that includes a 2.0 megapixel camera. This
webcam is capable of 2.0MP video at 5fps. This webcam can run on the drivers
that are already preset in many popular operating systems.
Webcam module specifications are shown in the table below:
Feature Description
2.0MP Webcam w/2 x Digital
Microphone 2 Million Pixel Webcam Module
USB Type 1.0, 1.1 or 2.0
1600x1200, 1280x1024, 640x480, 352x388,
Video Resolution
320x240, 176x144, 160x120
M-Series Touchcomputer User Guide
11
Page 27

Auto Frame Rate
Field Of View (FOV) 66°
Focus Range 40cm ~ Infinity
Barcode Scanner
A 2D area array barcode scanner is an optional M-Series touchcomputer
peripheral. The barcode scanner has integrated illumination and is compatible
with many barcode symbologies.
The barcode scanner supports a typical reading range shown in the table below.
5fps max @ 2.0 megapixels (1600x1200);
24fps max @ VGA mode (640x480)
Symbology / X-Dim Typical Range*
100% U.P.C. 46 mm to 419 mm (1.8˝ to 16.5˝)
5 mil Code 39 64 mm - 163 mm (2.5˝ - 6.4˝)
10 mil Code 39 28 mm - 338 mm (1.1˝ - 13.3˝)
6.7 mil PDF417 46 mm - 185 mm (1.8˝ - 7.3˝)
10 mil Data Matrix 53 mm - 203 mm (2.1˝ - 8.0˝)
Resolution, linear bar codes: 0.127 mm (5.0 mil)
Resolution, 2D matrix codes: 0.169 mm (6.7 mil)
* Performance may be impacted by bar code quality and environmental conditions
Smart Card Reader
A contact smart card reader is an optional M-Series touchcomputer peripheral.
The smart card reader supports ISO 7816 microprocessor or memory cards.
M-Series Touchcomputer User Guide
12
Page 28

RFID Reader
An RFID reader is an optional M-Series touchcomputer peripheral.
If installed, the RFID reader is capable of reading tags presented within a few
centimeters of the symbol shown below on the front of M-Series touchcomputer.
Compatible RFID standards are shown below:
• 14443A Mifare Classic
• 14443A Mifare Desfire
• 15693 ICODE
M-Series Touchcomputer User Guide
13
Page 29

USB Handset
A USB Handset is an optional M-Series touchcomputer peripheral. Included in the
kit is a bracket and handset cradle that is required for correct function.
Part number: E709063
TV Tuner
A USB TV Tuner kit is available as an optional accessory. There are three options
available. All options include a 1.2 meter USB extension cable that can be plugged
into the I/O area under the cable cover so that the TV Tuner can be installed
outside of the M-Series device.
Part number: E421980
• Japan TV Tuner Kit that supports the ISDB standard
• Europe TV Tuner Kit that supports the DVB standard
Part number: E378259
• United States TV Tuner Kit that supports the ATSC standard
Part number: E177386
3 Meter DC extension cable
A 3 Meter DC extension cable can be used to replace the original 5 Meter DC
extension cable.
Part number: E175539
M-Series Touchcomputer User Guide
14
Page 30

AC Cable
A country specific AC cable can be used to replace the original AC Cable. There
are two options.
• UK-HK 1.8 Meter AC cable
Part number: E010063
• Japan 1.8 Meter AC cable
Part number: E520117
M-Series Touchcomputer User Guide
15
Page 31

C H A P T E R
4
SAFETY AND MAINTENANCE
Safety
Important information regarding the proper setup and maintenance of your touchcomputer:
• To reduce the risk of electric shock, follow all safety notices and never open the
touchcomputer case.
• Turn off the product before cleaning (refer to “Care and Handling” on page 17 for
proper cleaning methods).
• Your touchcomputer is equipped with a 3-wire, grounding power cord. The power cord
plug only fits into a grounded outlet. Do not attempt to fit the plug into an outlet that has
not been configured for this purpose. Do not use a damaged power cord. Only use the
power cord that comes with your Elo Touch Solutions touchcomputer. Use of an
unauthorized power cord might invalidate your warranty.
• The slots located on the sides and top of the touchcomputer case are for ventilation.
Do not block or insert anything inside the ventilation slots.
• It is important that your touchcomputer remains dry. Do not pour liquid into or onto your
touchcomputer. If your touchcomputer becomes wet, do not attempt to repair it
yourself. Contact Elo Touch Solutions Customer Service for instructions.
• Guidance and manufacturer’s declaration-electromagnetic immunity for all
EQUIPMENT AND SYSTEMS reports are available upon request.
M-Series Touchcomputer User Guide
16
Page 32

Care and Handling
The following tips help keep your touchcomputer functioning at the optimal level.
Power down the unit and disconnect from the electrical mains prior to engaging in
any cleaning activities.
Take care to ensure no leakage of solution into the system. IF ANY FLUID
INGRESS INTO THE UNIT DURING CLEANING IS SUSPECTED, DO NOT
POWER ON THE UNIT. Call the manufacturer at the contact numbers listed
below to have your unit inspected by a qualified technician prior to powering on.
Careful application of dilute aqueous cleaning solutions (non-ammonia based)
according to directions is recommended. Do not spray cleaning solution directly
onto touchscreen; always use a cloth or towel that has been lightly pre-moistened
with cleaning solution.
Do not use organic solvents (e.g., turpentine, varsol, and benzene), abrasive
cleansers or compressed air to clean touchscreen displays or touchcomputer.
For disinfection, careful application of alcohol-based disinfectants or branded
commercially available quaternary ammonium compounds (e.g.,, PRO-SANTM
brand, TB QuatTM brand, etc.) is recommended. Use as little as practicable to
reduce the risk of fluid ingress into the unit.
Warning
This product consists of devices that might contain mercury, which must be
recycled or disposed of in accordance with local, state, or federal laws. (Within this
system, the backlight lamps in the touchcomputer display contain mercury.)
M-Series Touchcomputer User Guide
17
Page 33

Touchcomputer Specifications
For touch computer specifications go to www.elotouch.com
C H A P T E R
5
TECHNICAL SUPPORT
TECHNICAL SPECIFICATIONS
NOTE: Not all operating systems or options are supported in all regions. Please
contact your local Elo TouchSystems representative for details.
Technical Assistance
There are three methods to obtain contact information for technical assistance on
the touchcomputer:
• The web
• The phone
Using the Web
For technical support, go to www.elotouch.com/go/contactsupport.
For current Elo news, product updates, and announcements, or to register to
receive our Touchcomputer newsletter, go to www.elotouch.com/go/news
Using the Phone
.
For technical support, see the table at the end of the user guide for contact
information.
M-Series Touchcomputer User Guide
18
Page 34

REGULATORY INFORMATION
I. Electrical Safety Information
A) Compliance is required with respect to the voltage, frequency, and current
requirements indicated on the manufacturer’s label. Connection to a different
power source than those specified herein may result in improper operation,
damage to the equipment, invalidation of warranty, or a fire hazard if the
requirements are not followed.
B) There are no operator-serviceable parts inside this equipment. There are
hazardous voltages generated by this equipment which constitute a safety hazard.
Service should be provided only by a qualified service technician.
C) This equipment is provided with a detachable power cord which has an integral
safety ground wire intended for connection to a grounded safety outlet.
1) Do not substitute the cord with other than the provided approved type. Under
no circumstances should an adapter plug be used to connect to a 2-wire outlet
as this will defeat the continuity of the grounding wire.
2) The equipment requires the use of the ground wire as a part of the safety
certification. Modification or misuse can provide a shock hazard that can result
in serious injury or death.
3) Contact a qualified electrician or the manufacturer if there are questions
about the installation prior to connecting the equipment to main power.
II. Emissions and Immunity Information
A) Notice to Users in the United States: This equipment has been tested and
found to comply with the limits for a Class A digital device, pursuant to Part
15 of FCC Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This equipment
generates, uses, and can radiate radio frequency energy, and if not installed
and used in accordance with the instructions, may cause harmful
interference to radio communications.
B) Notice to Users in Canada: This equipment complies with the Class A
limits for radio noise emissions from digital apparatus as established by
the Radio Interference Regulations of Industry Canada.
C) Notice to Users in the European Union: Use only the provided power cords
M-Series Touchcomputer User Guide
19
Page 35

and interconnecting cabling provided with the equipment. Substitution of
provided cords and cabling may compromise electrical safety or CE Mark
Certification for emissions or immunity as required by the following
standards:
This Electronic Equipment is required to have a CE Mark on the
manufacturer’s label which means that the equipment has been tested to
the following Directives and Standards: This equipment has been tested
to the requirements for the CE Mark as required by EMC 2004/108/EC
and LVD 2006/95/EC indicated in European Standards EN55022,
EN55024, and EN60950-1, as well as these standards for medical
electrical equipment. EN 60601-1 and EN60601-1-2 (including EN55011
Class A).
D) General Information to all Users: This equipment generates, uses and can
radiate radio frequency energy. If not installed and used according to this
manual the equipment may cause interference with radio and television
communications. There is, however, no guarantee that interference will
not occur in any particular installation due to site-specific factors.
1) In order to meet emission and immunity requirements, the user must
observe the following:
a) Use only the provided I/O cables to connect this digital device
with any computer.
b) To ensure compliance, use only the provided manufacturer’s
approved line cord.
c) The user is cautioned that changes or modifications to the
equipment not expressly approved by the party responsible for
compliance could void the user’s authority to operate the
equipment.
2) If this equipment appears to cause interference with radio or television
reception, or any other device:
a) Verify as an emission source by turning the equipment off and on.
b) If you determine that this equipment is causing the interference, try
to correct the interference by using one or more of the following
measures:
i) Move the digital device away from the affected receiver.
ii) Reposition (turn) the digital device with respect to the affected
receiver. iii) Plug the digital device into a different AC outlet so
the digital device and the receiver are on different branch
circuits.
iv) Disconnect and remove any I/O cables that the digital device
does not use. (Unterminated I/O cables are a potential
M-Series Touchcomputer User Guide
20
Page 36

source of high RF emission levels.)
v) Plug the digital device into only a grounded outlet receptacle.
Do not use AC adapter plugs. (Removing or cutting the line
cord ground may increase RF emission levels and may also
present a lethal shock hazard to the user.)
If you need additional help, consult your dealer, manufacturer, or an experienced
radio or television technician.
Federal Communication Commission Interference Statement
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that
may cause undesired operation.
This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with the instructions, may
cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this
equipment does cause harmful interference to radio or television reception, which
can be determined by turning the equipment off and on, the user is encouraged to
try to correct the interference by one of the following measures:
-Reorient or relocate the receiving antenna.
-Increase the separation between the equipment and receiver.
-Connect the equipment into an outlet on a circuit different from that
to which the receiver is connected.
-Consult the dealer or an experienced radio/TV technician for help.
FCC Caution: Any changes or modifications not expressly approved by the party
responsible for compliance could void the user's authority to
operate this equipment.
This transmitter must not be co-located or operating in conjunction with any other
antenna or transmitter.
M-Series Touchcomputer User Guide
21
Page 37

Exposure to radio frequency radiation
The radiated output power of this device is far below the FCC radio frequency
exposure limits. Nevertheless, the device shall be used in such a manner that the
potential for human contact during normal operation is minimized.
In order to avoid the possibility of exceeding the FCC radio frequency exposure
limits, human proximity to the antenna shall not be less than 20 cm (8 inches)
during normal operation.
Industry Canada statement
This device complies with RSS-210 of the Industry Canada Rules. Operation is
subject to the following two conditions: (1) This device may not cause harmful
interference, and (2) this device must accept any interference received, including
interference that may cause undesired operation.
Radiation Exposure Statement
This equipment complies with IC radiation exposure limits set forth for an
uncontrolled environment. This equipment should be installed and operated with
minimum distance 20cm between the radiator & your body.
This Class A digital apparatus complies with Canadian ICES-003.
Ce dispositif est conforme à la norme CNR-210 d'Industrie Canada applicable aux
appareils radio exempts de licence. Son fonctionnement est sujet aux deux
conditions suivantes: (1) le dispositif ne doit pas produire de brouillage
préjudiciable, et (2) ce dispositif doit accepter tout brouillage reçu, y compris un
brouillage susceptible de provoquer un fonctionnement indésirable.
Déclaration d'exposition aux radiations
Cet équipement est conforme aux limites d'exposition aux rayonnements IC
établies pour un environnement non contrôlé. Cet équipement doit être installé et
utilisé avec un minimum de 20 cm de distance entre la source de rayonnement et
votre corps.
Cet appareil numérique de la classe A est conforme à la norme NMB-003 du
Canada.
M-Series Touchcomputer User Guide
22
Page 38

Europe – EU Declaration of Conformity
This device complies with the essential requirements of the R&TTE Directive
1999/5/EC. The following test methods have been applied in order to prove
presumption of conformity with the essential requirements of the R&TTE Directive
1999/5/EC:
EN 60950-1: 2006+A11: 2009+A1: 2010+A12: 2011
Safety of Information Technology Equipment
EN 50364: 2010
Limitation of human exposure to electromagnetic fields from devices operating in
the frequency range 0 Hz to 300 GHz, used in Electronic Article Surveillance (EAS),
Radio Frequency Identification (RFID) and similar applications
EN 62479: 2010
Assessment of the compliance of low power electronic and electrical equipment
with the basic restrictions related to human exposure to electromagnetic fields (10
MHz to 300 GHz)
EN 300 328 V1.7.1: 2006
Electromagnetic compatibility and Radio spectrum Matters (ERM); Wideband
Transmission systems; Data transmission equipment operating in the 2,4 GHz ISM
band and using spread spectrum modulation techniques; Harmonized EN covering
essential requirements under article 3.2 of the R&TTE Directive
EN 302 291-1 V1.1.1: 2005
Electromagnetic compatibility and Radio spectrum Matters (ERM) - Short Range
Devices (SRD) - Close Range Inductive Data Communication equipment
operating at 13,56 MHz - Part 1: Technical characteristics and test methods
(Endorsement of the English version EN 302291-1 V1.1.1 (2005-07) as German
standard)
EN 302 291-2 V1.1.1: 2005
Electromagnetic compatibility and Radio spectrum Matters (ERM) - Short
Range Devices (SRD) - Close Range Inductive Data Communication equipment
operating at 13,56 MHz - Part 2: Harmonized EN under article 3.2 of the R&TTE
Directive (Endorsement of the English version EN 302291-2 V1.1.1(2005-07) as
German standard)
EN 301 489-1 V1.8.1: 2008
Electromagnetic compatibility and Radio Spectrum Matters (ERM);
ElectroMagnetic Compatibility (EMC) standard for radio equipment and services;
Part 1: Common technical requirements
EN 301 489-3 V1.4.1: 2002
Electromagnetic compatibility and Radio Spectrum Matters (ERM);
ElectroMagnetic Compatibility (EMC) standard for radio equipment and services;
Part 3: Specific conditions for Short-Range Devices (SRD) operating on
frequencies between 9 kHz and 40 GHz
M-Series Touchcomputer User Guide
23
Page 39

EN 301 489-17 V2.1.1 2009
Electromagnetic compatibility and Radio spectrum Matters (ERM);
ElectroMagnetic Compatibility (EMC) standard for radio equipment and
services; Part 17: Specific conditions for 2,4 GHz wideband transmission
systems and 5 GHz high performance RLAN equipment
This device is a wideband transmission system (transceiver), intended for use in all
EU member states and EFTA countries, except in France and Italy where
restrictive use applies.
In Italy the end-user should apply for a license at the national spectrum authorities
in order to obtain authorization to use the device for setting up outdoor radio links
and/or for supplying public access to telecommunications and/or network services.
Česky
[Czech]
Elo Touch Solutions, Inc. tímto prohlašuje, že tento 15MX/19MX
Touchcomputer je ve shodě se základními požadavky a dalšími
příslušnými ustanoveními směrnice 1999/5/ES.
Dansk
[Danish]
Undertegnede Elo Touch Solutions, Inc. erklærer herved, at følgende
udstyr 15MX/19MX Touchcomputer overholder de væsentlige krav og
øvrige relevante krav i direktiv 1999/5/EF.
Deutsch
[German]
Hiermit erklärt Elo Touch Solutions, Inc., dass sich das Gerät
15MX/19MX Touchcomputer in Übereinstimmung mit den
grundlegenden Anforderungen und den übrigen einschlägigen
Bestimmungen der Richtlinie 1999/5/EG befindet.
Eesti
[Estonian]
Käesolevaga kinnitab Elo Touch Solutions, Inc. seadme 15MX/19MX
Touchcomputer vastavust direktiivi 1999/5/EÜ põhinõuetele ja
nimetatud direktiivist tulenevatele teistele asjakohastele sätetele.
English Hereby, Elo Touch Solutions, Inc., declares that this 15MX/19MX
Touchcomputer is in compliance with the essential requirements and
other relevant provisions of Directive 1999/5/EC.
Español
[Spanish]
Por medio de la presente Elo Touch Solutions, Inc. declara que el
15MX/19MX Touchcomputer cumple con los requisitos esenciales y
cualesquiera otras disposiciones aplicables o exigibles de la Directiva
1999/5/CE.
Ελληνική
[Greek]
ΜΕ ΤΗΝ ΠΑΡΟΥΣΑ Elo Touch Solutions, Inc. ΔΗΛΩΝΕΙ ΟΤΙ
15MX/19MX Touchcomputer ΣΥΜΜΟΡΦΩΝΕΤΑΙ ΠΡΟΣ ΤΙΣ
ΟΥΣΙΩΔΕΙΣ ΑΠΑΙΤΗΣΕΙΣ ΚΑΙ ΤΙΣ ΛΟΙΠΕΣ ΣΧΕΤΙΚΕΣ ΔΙΑΤΑΞΕΙΣ
ΤΗΣ ΟΔΗΓΙΑΣ 1999/5/ΕΚ.
Français
[French]
Par la présente Elo Touch Solutions, Inc. déclare que l'appareil
15MX/19MX Touchcomputer est conforme aux exigences essentielles
et aux autres dispositions pertinentes de la directive 1999/5/CE.
Italiano
[Italian]
Con la presente Elo Touch Solutions, Inc. dichiara che questo
15MX/19MX Touchcomputer è conforme ai requisiti essenziali ed alle
altre disposizioni pertinenti stabilite dalla direttiva 1999/5/CE.
Latviski
[Latvian]
Ar šo Elo Touch Solutions, Inc. deklarē, ka 15MX/19MX
Touchcomputer atbilst Direktīvas 1999/5/EK būtiskajām prasībām un
M-Series Touchcomputer User Guide
24
Page 40

citiem ar to saistītajiem noteikumiem.
Lietuvių
[Lithuanian]
Šiuo Elo Touch Solutions, Inc. deklaruoja, kad šis 15MX/19MX
Touchcomputer atitinka esminius reikalavimus ir kitas 1999/5/EB
Direktyvos nuostatas.
Hierbij verklaart Elo Touch Solutions, Inc. dat het toestel 15MX/19MX
Nederlands
[Dutch]
Malti
[Maltese]
Touchcomputer in overeenstemming is met de essentiële eisen en de
andere relevante bepalingen van richtlijn 1999/5/EG.
Hawnhekk, Elo Touch Solutions, Inc., jiddikjara li dan 15MX/19MX
Touchcomputer jikkonforma mal-ħtiġijiet essenzjali u ma provvedimenti
oħrajn relevanti li hemm fid-Dirrettiva 1999/5/EC.
Magyar
[Hungarian]
Alulírott, Elo Touch Solutions, Inc. nyilatkozom, hogy a 15MX/19MX
Touchcomputer megfelel a vonatkozó alapvetõ követelményeknek és
az 1999/5/EC irányelv egyéb elõírásainak.
Polski
[Polish]
Niniejszym Elo Touch Solutions, Inc. oświadcza, że 15MX/19MX
Touchcomputer jest zgodny z zasadniczymi wymogami oraz
pozostałymi stosownymi postanowieniami Dyrektywy 1999/5/EC.
Elo Touch Solutions, Inc. declara que este 15MX/19MX
Português
[Portuguese]
Touchcomputer está conforme com os requisitos essenciais e outras
disposições da Directiva 1999/5/CE.
Elo Touch Solutions, Inc. izjavlja, da je ta 15MX/19MX Touchcomputer
Slovensko
[Slovenian]
v skladu z bistvenimi zahtevami in ostalimi relevantnimi določili
direktive 1999/5/ES.
Elo Touch Solutions, Inc. týmto vyhlasuje, že 15MX/19MX
Slovensky
[Slovak]
Suomi
[Finnish]
Touchcomputer spĺňa základné požiadavky a všetky príslušné
ustanovenia Smernice 1999/5/ES.
Elo Touch Solutions, Inc. vakuuttaa täten että 15MX/19MX
Touchcomputer tyyppinen laite on direktiivin 1999/5/EY oleellisten
vaatimusten ja sitä koskevien direktiivin muiden ehtojen mukainen.
Svenska
[Swedish]
Härmed intygar Elo Touch Solutions, Inc. att denna 15MX/19MX
Touchcomputer står I överensstämmelse med de väsentliga
egenskapskrav och övriga relevanta bestämmelser som framgår av
direktiv 1999/5/EG.
III. Agency Certifications
The following certifications have been issued for the touchcomputer:
Canada cUL
Canada IC
Europe TUV, CE
Japan VCCI
FCC
United States UL
Russia GOST-R
M-Series Touchcomputer User Guide
25
Page 41

IV. Ingress Protection
The 15MX/19MX Touchcomputer is designed to have an ingress protection rating of IP21 over
the range of angles shown in the diagram below.
M-Series Touchcomputer User Guide
26
Page 42

WARRANTY
Except as otherwise stated herein or in an order acknowledgment delivered to
Buyer, Seller warrants to Buyer that the Product shall be free of defects in
materials and workmanship. With the exception of the negotiated warranty
periods; the warranty for the touchcomputer and components of the product is 3
years.
Seller makes no warranty regarding the model life of components. Seller’s
suppliers may at any time and from time to time make changes in the components
delivered as Products or components. Buyer shall notify Seller in writing promptly
(and in no case later than thirty (30) days after discovery) of the failure of any
Product to conform to the warranty set forth above; shall describe in commercially
reasonable detail in such notice the symptoms associated with such failure; and
shall provide to Seller the opportunity to inspect such Products as installed, if
possible. The notice must be received by Seller during the Warranty Period for
such product, unless otherwise directed in writing by the Seller. Within thirty (30)
days after submitting such notice, Buyer shall package the allegedly defective
Product in its original shipping carton(s) or a functional equivalent and shall ship to
Seller at Buyer’s expense and risk.
Within a reasonable time after receipt of the allegedly defective Product and
verification by Seller that the Product fails to meet the warranty set forth above,
Seller shall correct such failure by, at Seller’s options, either (i) modifying or
repairing the Product or (ii) replacing the Product. Such modification, repair, or
replacement and the return shipment of the Product with minimum insurance to
Buyer shall be at Seller’s expense. Buyer shall bear the risk of loss or damage in
transit, and may insure the Product. Buyer shall reimburse Seller for transportation
cost incurred for Product returned but not found by Seller to be defective.
Modification or repair, of Products may, at Seller’s option, take place either at
Seller’s facilities or at Buyer’s premises. If Seller is unable to modify, repair, or
replace a Product to conform to the warranty set forth above, then Seller shall, at
Seller’s option, either refund to Buyer or credit to Buyer’s account the purchase
price of the Product less depreciation calculated on a straight-line basis over
Seller’s stated Warranty Period.
M-Series Touchcomputer User Guide
27
Page 43

THESE REMEDIES SHALL BE THE BUYER’S EXCLUSIVE REMEDIES FOR
BREACH OF WARRANTY. EXCEPT FOR THE EXPRESS WARRANTY SET
FORTH ABOVE, SELLER GRANTS NO OTHER WARRANTIES, EXPRESS OR
IMPLIED BY STATUTE OR OTHERWISE, REGARDING THE PRODUCTS,
THEIR FITNESS FOR ANY PURPOSE, THEIR QUALITY, THEIR
MERCHANTABILITY, THEIR NONINFRINGEMENT, OR OTHERWISE. NO
EMPLOYEE OF SELLER OR ANY OTHER PARTY IS AUTHORIZED TO MAKE
ANY WARRANTY FOR THE GOODS OTHER THAN THE WARRANTY SET
FORTH HEREIN. SELLER’S LIABILITY UNDER THE WARRANTY SHALL BE
LIMITED TO A REFUND OF THE PURCHASE PRICE OF THE PRODUCT. IN NO
EVENT SHALL SELLER BE LIABLE FOR THE COST OF PROCUREMENT OR
INSTALLATION OF SUBSTITUTE GOODS BY BUYER OR FOR ANY SPECIAL,
CONSEQUENTIAL, INDIRECT, OR INCIDENTAL DAMAGES.
Buyer assumes the risk and agrees to indemnify Seller against and hold Seller
harmless from all liability relating to (i) assessing the suitability for Buyer’s intended
use of the Products and of any system design or drawing and (ii) determining the
compliance of Buyer’s use of the Products with applicable laws, regulations, codes,
and standards. Buyer retains and accepts full responsibility for all warranty and
other claims relating to or arising from Buyer’s products, which include or
incorporate Products or components manufactured or supplied by Seller. Buyer is
solely responsible for any and all representations and warranties regarding the
Products made or authorized by Buyer. Buyer will indemnify Seller and hold Seller
harmless from any liability, claims, loss, cost, or expenses (including reasonable
attorney’s fees) attributable to Buyer’s products or representations or warranties
concerning same.
M-Series Touchcomputer User Guide
28
Page 44

INDEX
address, Elo TouchSystems, 36
base, securing, 4
box contents, 1
cables
included, 1
calibration, 2, 3
certifications, 32
contact information, 24
controls, location diagram, 5
customer support, 24
email, Elo TouchSystems, 36
enabling or disabling power, 8
input/output (I/O) panel
accessing, 9
LEDs
location diagram, 5
magnetic stripe reader (MSR)
overview, 11, 13, 14, 15
specifications, 11
maintenance
care and handling, 18
mounting options, 4
on-screen display (OSD)
enabling or disabling menu, 8
operation, 5
options, adding, 10
peripherals
adding, 10
phone number, Elo TouchSystems, 24, 36
ports
accessing, 9
power
enabling or disabling button lock, 8
regulatory information, 25
safety, 18
setup
mounting options, 4
touchscreen calibration, 2, 3
unpacking, 1
specifications
magnetic stripe reader, 11
wireless card, 12
technical support, 20
touchscreen
calibrating, 2, 3
care and handling, 18
upgrades, adding, 10
warranty, 33
website, Elo TouchSystems, 24, 36
wireless card
overview, 12
specifications, 12
M-Series Touchcomputer User Guide
29
Page 45

www.elotouch.com
Get the latest...
•
Product information
•
Specifications
•
News on upcoming events
•
Press release
•
Software drivers
•
Touchcomputer Newsletter
Getting in Touch with Elo
To find out more about the extensive range of Elo touch solutions, visit www.elotouch.com or simply call the
office nearest you:
North America
Elo Touch Solutions Inc.
301 Constitution Drive
Menlo Park, CA 94025
USA
(800) ELO-TOUCH
(800) 356-8682
Tel 650-361-4800
Fax 650-361-4747
customerservice@elotouch.com
Europe
Elo Touch Solutions Inc.
Diestsesteenweg 692
B-3010 Kessel-Lo
Belgium
Tel +32(0)(16)35 21 00
Fax +32(0)(16)35 21 01
elosales@elotouch.com
Asia-Pacific
Sun Hamada Bldg. 2F
1-19-20 ShinYokohama
Kanagawa 222-0033
Japan
Tel +81(45)478-2161
Fax +81(45)478-2180
www.tps.co.jp
© 2012 Elo Touch Solutions Inc. Printed in USA
M-Series Touchcomputer User Guide
30
 Loading...
Loading...