Page 1

Touchmonitor User Guide
1928L 19” LCD Desktop Touchmonitor
5000 Series
Revision B
Page 2

User Guide
1928L 19” LCD Desktop Touchmonitor
Revision B
P/N E116103
Page 3

Copyright © 2007 Tyco Electronics Corporation. All Rights Reserved.
No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval
system, or translated into any language or computer language, in any form or by any means,
including, but not limited to, electronic, magnetic, optical, chemical, manual, or otherwise without prior written permission of Elo TouchSystems.
Disclaimer
The information in this document is subject to change without notice. Elo TouchSystems makes
no representations or warranties with respect to the contents hereof, and specifically disclaims
any implied warranties of merchantability or fitness for a particular purpose. Elo TouchSystems
reserves the right to revise this publication and to make changes from time to time in the content
hereof without obligation of Elo TouchSystems to notify any person of such revisions or changes.
Trademark Acknowledgments
Elo Touchsystems AccuTouch and IntelliTouch are trademarks of Tyco Electronics Corporation.
Other product names mentioned herein may be trademarks or registered trademarks of their respective companies.
iii
Page 4
Warnings and Cautions
!
Warning
• Danger - Explosion hazard. Do not use in the presence of flammable anesthetics, and other
flammable materials.
• To prevent fire or shock hazards, do not immerse the unit in water or expose it to rain or
moisture.
• Do not use the unit with an extension cord receptacle or other outlets unless the prongs of the
power cord can be fully inserted.
• RISK OF ELECTRICAL SHOCK - DO NOT OPEN. To reduce the risk of electrical shock,
DO NOT remove the back of the equipment or open the enclosure. No user-serviceable parts
are inside. Refer servicing to qualified field service engineers only.
• Uninsulated voltage within the unit may have sufficient magnitude to cause electrical shock.
Avoid contact with any part inside the unit.
Caution
!
• Power cord is used as a disconnection device. To de-energize equipment, disconnect the
power cord.
• This unit must follow the national requirement, and local state laws to dispose unit.
• Before connecting the cables to your Elo touchmonitor, make sure all components are
powered OFF.
• The use of ACCESSORY equipment not complying with the equivalent safety requirements
of this equipment may lead to a reduced safety of the resulting system. Consideration
relating to the choices of accessory equipment should include:
• Use of accessory in the patient vicinity.
• Evidence that the safety certification of the accessory has been performed in accordance
to the appropriate IEC 60601-1 and/or IEC 60601-1-1 harmonized national standard.
• For continued safety -
- This unit only complies to the above standards if used with a medical grade power cord.
-A medical grade power supply, such as the one specified, is required for use in a medical
application.
Note:
• This symbol alerts the user to important information concerning the operation and
maintenance of this unit, which should be read carefully to avoid problems.
• This symbol means DC Current.
• This symbol means ON/OFF stand-by switch.
iv
Page 5

!
CAUTION-Life Support
Care must be taken when this touchmonitor is a critical component of a life support system or
device. In case of failure of this touchmonitor, appropriate redundant systems should be incorporated into the system or device to prevent injury to the user or patient.
The following should be an integral part of the safety design of a life support system or device
using this touchmonitor for a critical function.
• An alternate interface or fail-safe must be available should the touchscreen fail to operate.
• The touchscreen interface must not be the only means of control of a critical function.
• An alternate video display should be incorporated into the safety design if used to monitor a
critical function.
• The internal speakers of this touchscreen monitor must not be the sole method of warning of
a critical function.
Critical functions are:
1. Life support devices or systems are devices or systems which, (a) are intended for surgical
implant into the body, or (b) support or sustain life, or (c) whose failure to perform when
properly used in accordance with instructions for use provided in the labeling, can be reason
ably expected to result in significant injury to the user.
2. A critical component is any component of a life support device or system whose failure to
perform can be reasonably expected to cause the failure of the life support device or system,
or to affect its safety or effectiveness.
v
Page 6

Classification
With respect to electrical shock, fire in accordance with UL60601-1 and CAN/CSA C22.2 No.
60601-1
This monitor is a CLASS 1 (GROUNDED) DEVICE.
These displays are classified NO APPLIED PARTS EQUIPMENT.
Protection against harmful ingress of water:
INGRESS PROTECTION(IPX1)
This monitor shall be classified as ORDINARY EQUIPMENT, not intended or evaluated for use
in the presence of flammable anesthetic mixture with air, oxygen, or nitrous oxide.
Mode of Operation: CONTINUOUS OPERATION.
Environmental conditions for transport and storage
Temp. Operating C to 40oC
Storage / Transportation -20oC to +60oC
Humidity (non-condensing)
Operating 30% to 70%
Storage / Transportation 10% to 90%
Altitude Operating 1060hpa.
Storage / Transportation 0 to 40,000ft(12,192m)
o
0
Equivalent to 1013-303 hP.A
(14.7 to 4.4 psia)
For full Product Specifications refer to Appendix C
vi
Page 7

vii
Page 8

Table of Contents
Warnings and Cautions ................................................. iv
Caution-Life Support ...................................................... v
Classification .............................................................. vi
Chapter 1
INTRODUCTION 1
Product Description ................................................ 1
LCD Display Performance Features ....................... 2
19 inch TFT LCD Display Panel ...................... 2
External Medical Grade Power Supply ............ 2
Chapter 2
INSTALLATION AND SETUP 3
Unpacking Your Touchmonitor ............................... 3
Product Overview ........................................... 4
Main Unit ......................................................... 4
Back Unit ......................................................... 4
Side View ......................................................... 4
Base Bottom View ........................................... 5
KensingtonTM Lock ......................................... 5
Touch Interface Connection .................................... 6
Step 1 Connecting the Video Cable ................ 6
Step 2 Connecting the Serial/USB Cable ........ 7
Step 3 Connecting the Speaker Cable ............ 8
Step 4 Connecting the Power Cable ............... 9
Optimizing the LCD Display .................................. 10
Installing the Touch Driver Software ..................... 10
Appendix A
NATIVE RESOLUTION 19
Appendix B
TOUCHMONITOR SAFETY 21
Care and Handling of Your Touchmonitor ................... 22
Appendix C
TECHNICAL SPECIFICATIONS 23
Display Modes ............................................................. 23
Touchmonitor Specifications ........................................ 24
Power Supply Cord Selection ...................................... 25
North America ....................................................... 25
Cord selection for other than North America ........ 26
AccuTouch (resistive) Touchscreen Specifications ..... 28
IntelliTouch (resistive) Touchscreen Specifications ..... 29
Appendix D
CONTACT ELO 31
Contact Elo ............................................................. 31
Replacement Parts ..................................................... 31
REGULATORY INFORMATION 33
WARRANTY 35
INDEX 37
Chapter 3
OPERATION 11
About Touchmonitor Adjustments ................................ 11
Front Panel Controls ..................................... 12
Controls and Adjustment ...................................... 13
OSD Menu Functions .................................... 13
OSD Control Options ..................................... 14
Power LED Display & Power Saving .................... 15
General Power Saving Mode ........................ 15
Display Angle ................................................. 15
Chapter 4
TROUBLESHOOTING 17
Solutions to Common Problems ................................. 17
Page 9

Product Description
C H A P T E R
1
INTRODUCTION
The 1928L is a medical display designed to present information to the operator and the customer.
The 1928L is available in serial and USB(combo) touch interface as well as no touch. The 1928L
functionally consists of a 19” LCD main display with a touchscreen. The main display element
is a 19” diagonal SXGA resolution (1280 x 1024) LCD display. The display consists of an LCD
display and touchscreen. The 1928L is powered by 12 VDC from an external medical grade
power source.
1-1
Page 10

LCD Display Performance Features
19” TFT LCD Display Panel
Display format 1280 x 1024
Display area 376.32 mm (H) x 301.056 mm (V)
Pixel pitch 0.294 mm (H) x 0.294 mm (V)
Contrast Ratio 1300:1( typical)
Brightness
LCD 300 cd/m2 (typical)
AccuTouch 246 cd/m2 (typical)
IntelliTouch 276 cd/m2(typical)
Accutouch transmission 82% (typical)
Intellitouch transmission 92% (typical)
Response time Tr = 15 msec / Tf = 5 msec typical;
Display color 16.7 million colors
Typical vertical viewing angle 89 deg (down) / 89 deg ( up)
Typical horizontal viewing angle 89 deg (left) / 89 deg (right)
12 ms gray to gray response
External Medical Grade Power Supply
The 1928L is powered by an external medical grade universal input AC power source.
Power supply:
• AC power: Input voltage 100 -240 VAC, 1.0A
• Input frequency 50/60 Hz
• DC output Voltage/Current: 12 VDC/4.0A
• Load regulation: ±5% Max.
• Line regulation: ±1% Max.
1-2 Elo Touchmonitor User Guide
Page 11
C H A P T E R
2
INSTALLATION AND SETUP
This chapter discusses how to install your LCD touchmonitor and how to install Elo TouchSystems
driver software.
Unpacking Your Touchmonitor
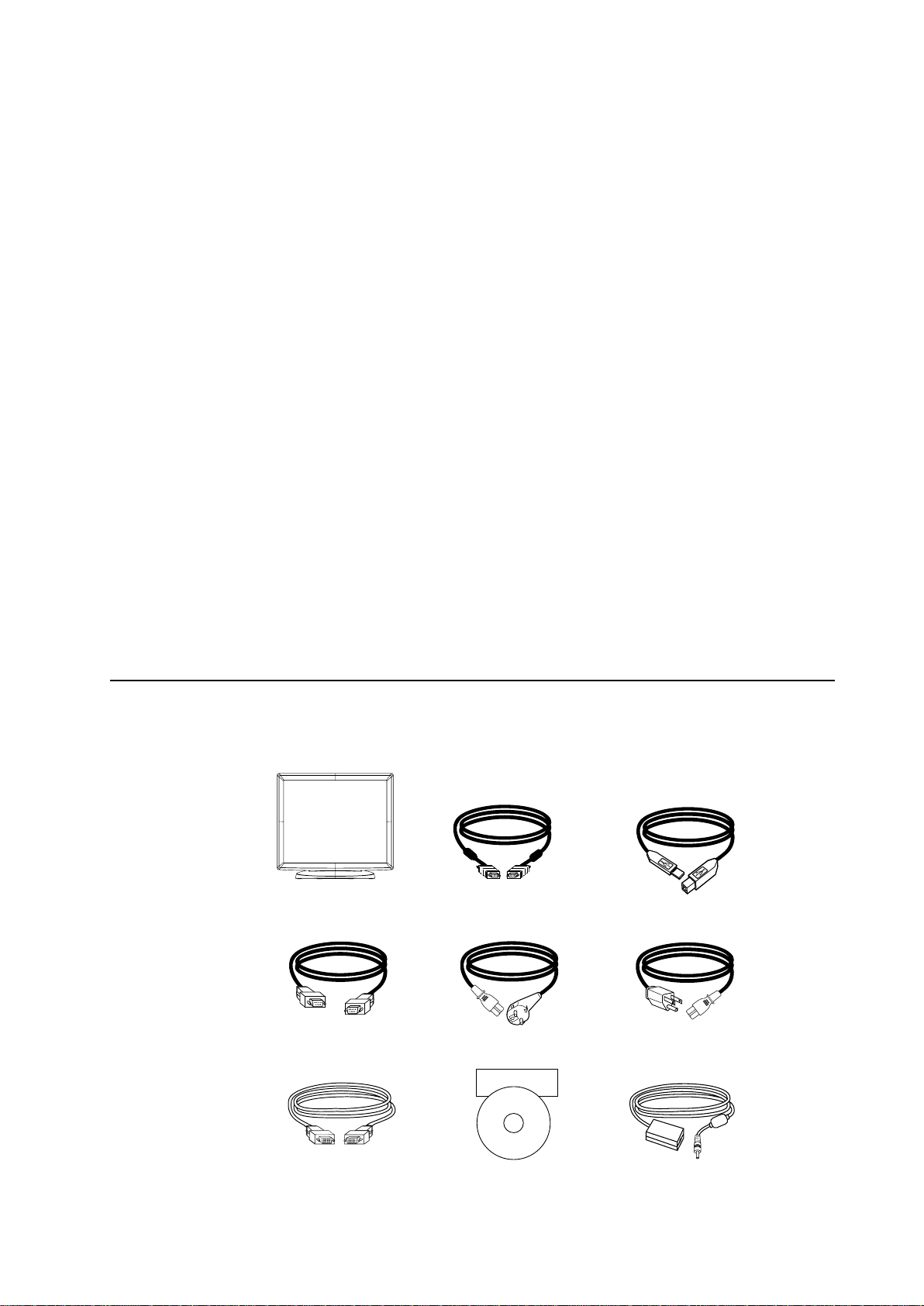
Check that the following items are present and in good condition:
LCD monitor VGA cable USB cable
Serial Cable European power cable Power cable US/Canada
Elo QuickStart
CD
Software
DVI Cable CD and Quick Install Guide Power adaptor
2-3
Page 12
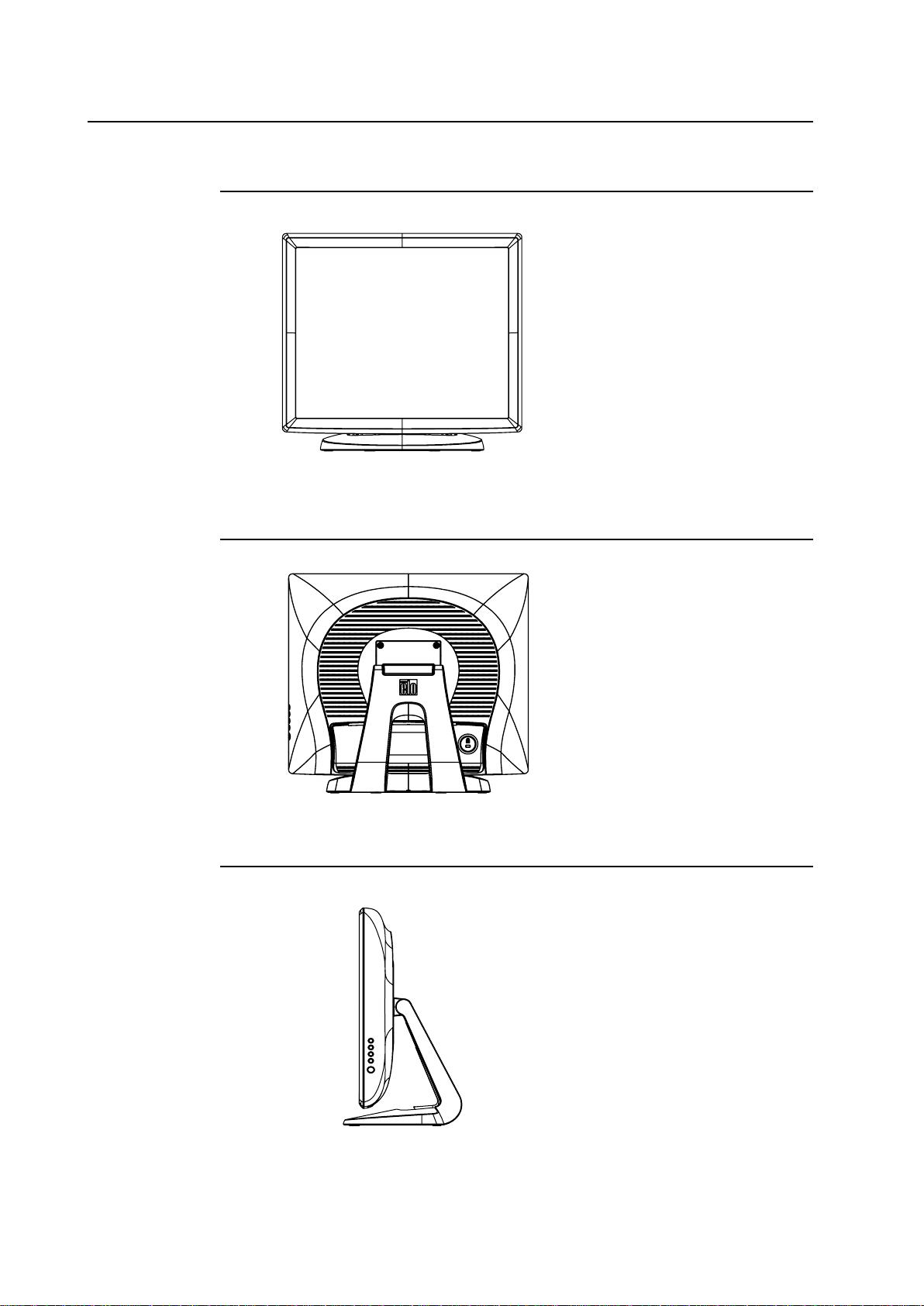
Product Overview
Main Unit
Back Unit
Side View
2-4 Elo Touchmonitor User Guide
Page 13
Base Bottom View
KensingtonTM Lock
The Kensington
TM
lock is a security device that prevents theft. To find out more about this secu-
rity device, go to http://www.kensington.com.
2-5
Page 14
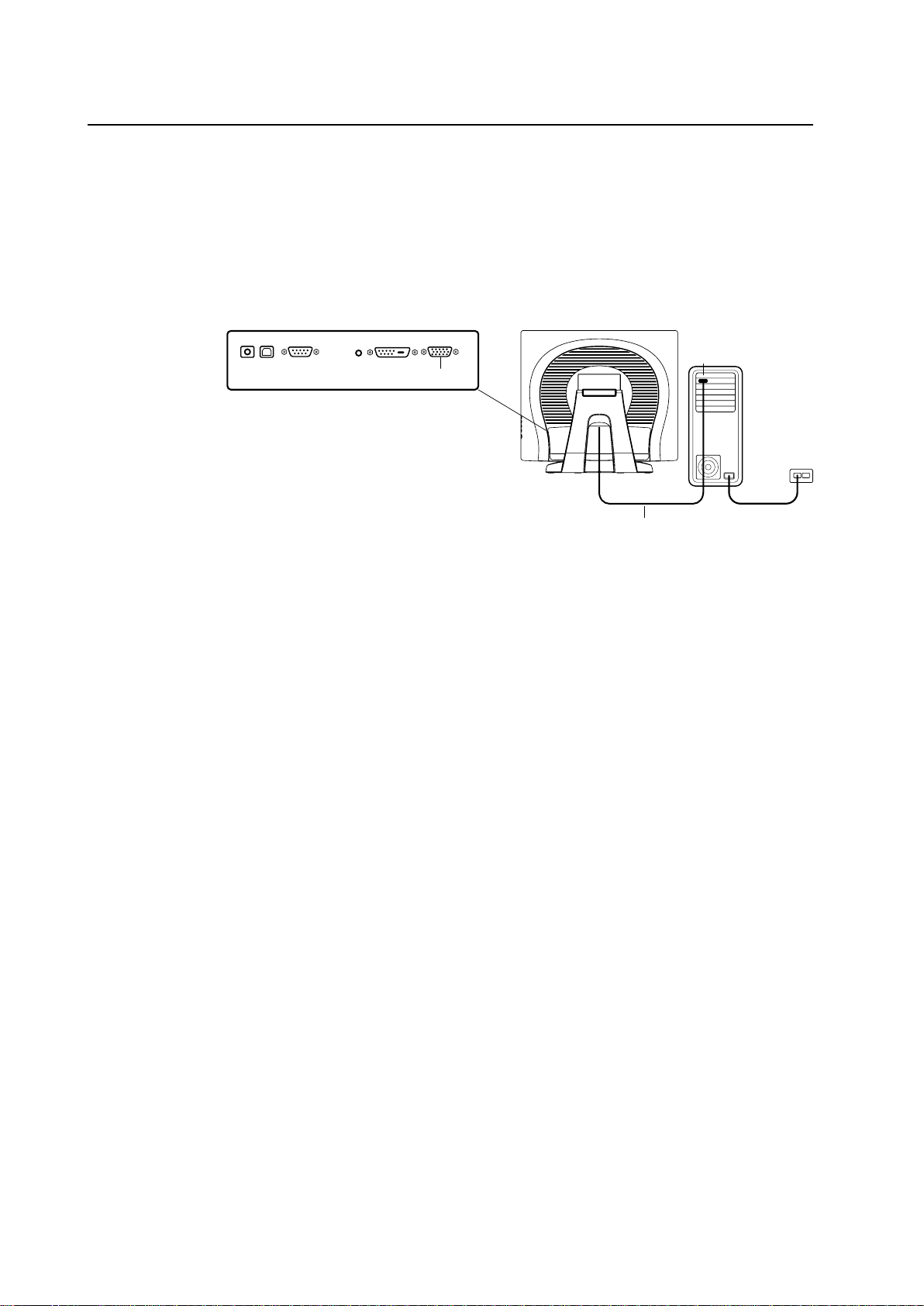
Touch Interface Connection
Note: Before connecting the cables to your touchmonitor and PC, be sure that the computer
and touchmonitor are turned off.
STEP 1-Connecting the Video Cable
CONNECTIONS ON UNDERSIDE
FEMALE 15-PIN
VIDEO CONNECTOR
VIDEO CABLE
VIDEO PORT
• Tilt the screen up and back to access the connection ports.
• Connect the 15-pin video cable (the ferrite bead end) or 24-pin DVI-D cable to the video
port on your PC.
• Connect the other end of the video cable to the video connector on your touchmonitor.
• Secure the cable to your touchmonitor and PC by turning the screws on the connector
clockwise.
2-6 Elo Touchmonitor User Guide
Page 15
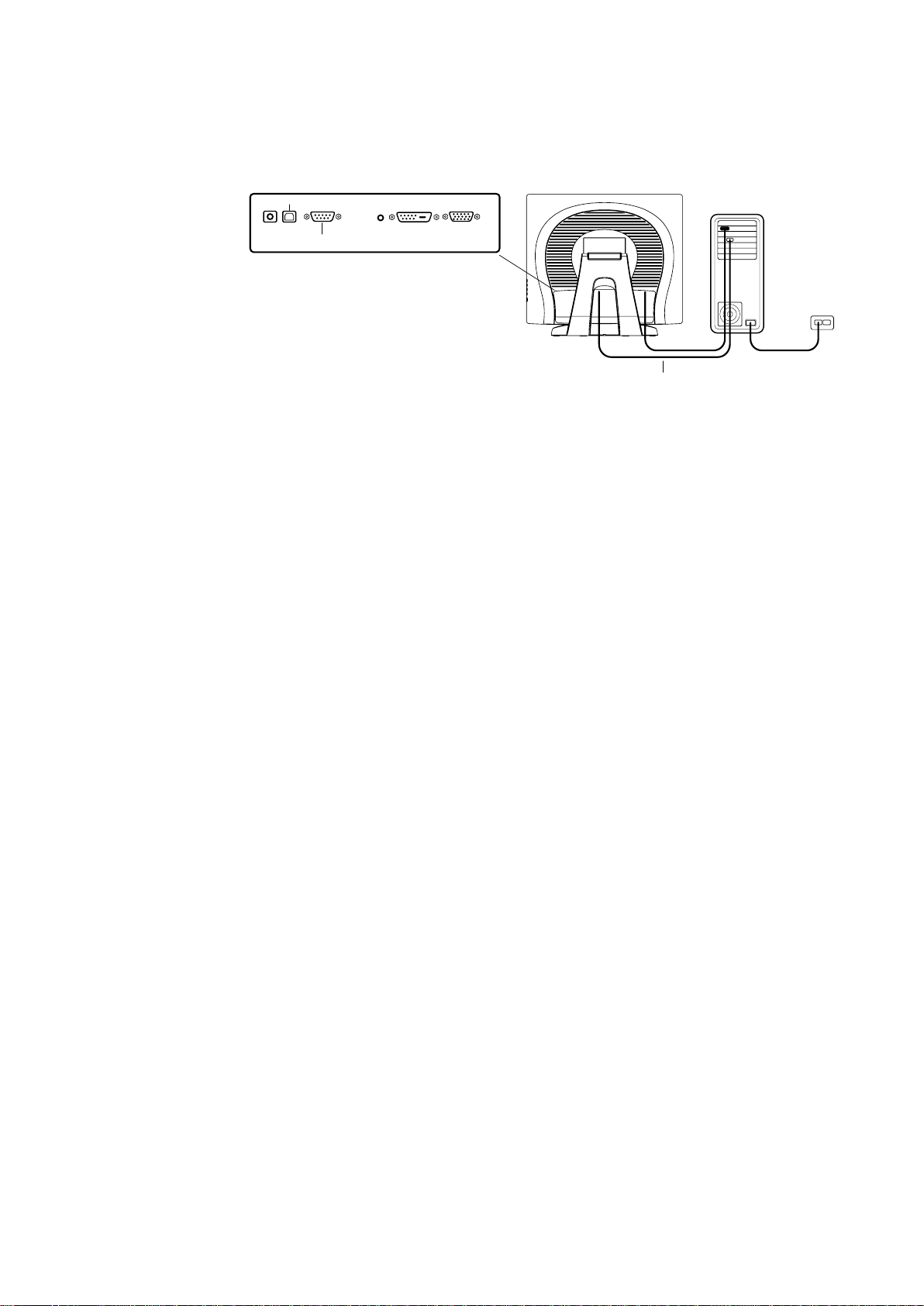
STEP 2-Connecting the Serial and USB Touchscreen Cable
CONNECTORS ON THE UNDERSIDE
USB CONNECTOR
FEMALE 9-PIN SERIAL
TOUCHSCREEN CONNECTOR
SERIAL TOUCHSCREEN CABLE
• Connect the female end of the serial (RS-232) cable to the serial port on your PC, or
connect the USB touchscreen cable to the USB touchscreen connector on the back of your
touchmonitor.
• Connect the male end of the cable to the serial touchscreen connector on your
touchmonitor, or connect the other end of the USB touchscreen cable to your PC.
• Secure the cable to your touchmonitor and PC by turning the screws on the connector.
2-7
Page 16

STEP 3-Connecting the Speaker Cable
CONNECTIONS ON UNDERSIDE
SPEAKER
PORT
SPEAKER CABLE
• Connect the light blue end of the speaker cable to the light blue speaker port to the monitor
(audio in).
• Connect the lime (light green) end of the speaker cable to the lime speaker port on the
computer (audio out).
2-8 Elo Touchmonitor User Guide
Page 17

STEP 4-Connecting the Power Cable
CONNECTIONS ON UNDERSIDE
POWER
POWER CABLE
Depending on where you live, you will use either the European or US/Canadian power cable.
• Connect the female end of the power cable to the medical grade power adaptor.
• Connect the brick power cable into the power port on the touchmonitor.
• Route the cable through the cable management channel.
NOTE: To protect your equipment against risk of damage from electrical surges in the power line,
plug the touchmonitor’s power cord into a surge protector, and then connect the surge
protector to a grounded AC electrical outlet.
2-9
Page 18

Optimizing the LCD Display
To ensure the LCD display works well with your computer, configure the display mode of your
graphic card to make it less than or equal to 1280 x 1024 resolution, and make sure the timing of
the display mode is compatible with the LCD display. Refer to Appendix A for more information
about resolution. Compatible video modes for your touchmonitor are listed in Appendix C.
Installing the Touch Driver Software
Elo TouchSystems provides driver software that allows your touchmonitor to work with your
computer. Drivers are located on the enclosed CD-ROM for the following operating systems:
• Windows XP
• Windows 2000
• Windows Me
• Windows 98
• Windows 95
• Windows NT 4.0
• CE 2.x, 3.0, 4x
• Windows XP Embedded
• Windows 3.x
• MS DOS
• OS/2
Additional drivers and driver information for other operating systems (including Macintosh and
Linux) are available on the Elo TouchSystems web site at www.elotouch.com. Your Elo USB
touchmonitor is plug-and-play compliant. Information on the video capabilities of your
touchmonitor is sent to your video display adapter when Windows starts. If Windows detects
your touchmonitor, follow the instructions on the screen to install a generic plug-and-play monitor.
Refer to the appropriate following section for driver installation instructions.
2-10 Elo Touchmonitor User Guide
Page 19

C H A P T E R
3
OPERATION
About Touchmonitor Adjustments
Your touchmonitor will unlikely require adjustment. Variations in video output and application
may require adjustments to your touchmonitor to optimize the quality of the display.
For best performance, your touchmonitor should be operating in native resolution, that is 1280 x
1024 at 60-75 Hz. Use the display control panel in Windows to choose 1280 x 1024 resolution.
Operating in other resolutions will degrade video performance. For further information, please
refer to Appendix A. All adjustments you make to the controls are automatically memorized.
This feature saves you from having to reset your choices every time you unplug or power your
touchmonitor off and on. If there is a power failure your touchmonitor settings will not default to
the factory specifications. To restore factory set up, choose it from the OSD. See page 14, Recall
Defaults.
3-11
Page 20

Front Panel Controls
1
2
3
4
5
Control Function
1 Menu/Exit Display/Exits the OSD menus.
2 1. Enter contrast of the OSD.
2. Increase value of the adjustment item.
3. Shuffle item clockwise.
3 1. Enter audio volume adjustment.
2. Decrease value of the adjustment item.
3. Shuffle item counter-clockwise.
4 Select Select the adjustment items from the OSD menu.
5 Power Switch Switches the power of the monitor from off to on to off.
(Impotant note: Includes integrated power down delay function,
user must depress power button for five (5) seconds when powering
off)
3-12 Elo Touchmonitor User Guide
Page 21

Controls and Adjustment
OSD Menu Functions
To Display and Select the OSD Functions:
1 Press the Menu key to activate the OSD menu.
2 Use or to move clockwise or counterclockwise through the menu. Press the select key
on the monitor. The parameter will be highlighted when selected.
3 To quit the OSD screen at any time during the operation, press the Menu key. If no keys are
pressed for a short time period, the OSD automatically disappears.
NOTE: The OSD screen will disappear if no input activities are detected from 45 seconds to 255
seconds, depending on the OSD time setting on the OSD of the monitor. The monitor
default is 45 seconds.
3-13
Page 22

OSD Control Options
Control Description
Contrast Increases or decreases contrast.
Brightness Increases or decreases brightness.
V-Position Moves the screen up or down.
H-Position Moves the screen left or right.
Recall Defaults Returns the monitor to its default settings.
Color Balance Press or and “Select” button to select 9300, 6500, 5500, 7500
and USER. Only when selecting USER can you make adjustments
to the R/G/B content. Press Select to restore to factory default setting.
Audio Volume Adjust audio volume of OSD menu.
Sharpness Adjust Sharpness.
Phase Increases or decreases the snow noise of the image after auto
adjustment is made.
Clock The dot clock is fine-adjusted after auto adjust.
OSD H-Position Moves the OSD position horizontally on the screen. When the
“SELECT” button and the button is pressed, the OSD control
menu will move to the right side of the screen. Likewise, when the
“Select” button and the button is pressed, the OSD control
menu will move to the left side.
OSD V-Position Moves the OSD position vertically on the screen. When the
“SELECT” button and the button is pressed, the OSD control
menu will move to the top side of the screen. Likewise, when the
“Select” button and the button is pressed, the OSD control
menu will move to the lower side.
OSD Time Adjsuts the time of OSD icon stays on monitor.
Auto-Adjust Press Auto to enable this function. The Auto-Adjust will
automatically adjust V-Position, H-Position, Clock and Clock-Phase.
OSD Language Select from English, French, German, Spanish, Japanese, Italian,
Chinese and Polish.
Information Description Indicates the current resolution, H-Frequency and V-Frequency.
3-14 Elo Touchmonitor User Guide
Page 23

Power LED Display & Power Saving
General Power Saving Mode
When the power is on and video is present, this LED lights in green. The LED indicates the
different power status with altered LED colors when monitor operates in different modes (see
following table).
Power
Mode Consumption Indicator
On 48w max. Green
Sleep 7w max. Orange
Off 4w NO
Power-Save (No Input)
• The LCD panel background is cut when there is no signal input (AC line power consump
tion of 7w or less).
Note: If the monitor is not to be used for an extended period of time, it is recommended
that the monitor be turned off.
Display Angle
For viewing clarity, you can tilt the LCD forward (up to -5 degrees) or backward (up to 90
degrees).
CAUTION
• In order to protect the LCD, be sure to hold the base when adjusting the LCD.
• For models without a touchscreen take care not to touch the screen.
3-15
Page 24

3-16 Elo Touchmonitor User Guide
Page 25

C H A P T E R
4
TROUBLESHOOTING
If you are experiencing trouble with your touchmonitor, refer to the following table. If the problem persists, please contact your local dealer or our service center. Elo Technical Support numbers are listed on page 31 of this manual.
Solutions to Common Problems
Problem Suggestion(s)
The monitor does not respond Check that the monitor’s Power Switch is on. You turn on the
system.Turn off the power and check the monitor’s power cord
and signal cable for proper connection.
Characters on the screen are dim Refer to the Controls and Adjustments section to adjust the brightness.
The screen is blank During operation, the monitor screen may automatically turn off as
a result of the Power Saving feature. Press any key to see if the
screen reappears.
OSD or power buttons don’t work Refer to the Controls and Adjustments section to adjust the brightness.
“Out of Range” display Check to see that they are not locked out. See page 10.
Check to see of the resolution or vertical frequency of your
computer is higher than that of the LCD display.
Reconfigure the resolution of your computer to make it less than or
equal to 1280 x 1024. 1280 x 1024 is optimal. See Appendix A for
more information on resolution.
Touch doesn’t work Make sure cable is securely attached at both ends.
Power will not shut off immediately Be sure to depree power button for five(5) seconds when powering
off. Unit includes integrated power down delay function.
3-17
Page 26

3-18 Elo Touchmonitor User Guide
Page 27

A P P E N D I X
A
NATIVE RESOLUTION
The native resolution of a monitor is the resolution level at which the LCD panel is designed to
perform best. 1928L native resolution is 1280 x 1024. In almost all cases, screen images look
best when viewed at their native resolution. You can lower the resolution setting of a monitor but
not increase it.
Input Video 19.0” LCD
640 x 480(VGA) Transforms input format to 1280 x 1024
800 x 600 (SVGA) Transforms input format to 1280 x 1024
1024 x 768(XGA) Transforms input format to 1280 x 1024
1280 x 1024(SXGA) Display in Native Resolution
The native resolution of an LCD is the actual number of pixels horizontally in the LCD by the
number of pixels vertically in the LCD. LCD resolution is usually represented by
the following symbols:
VGA 640 x 480
SVGA 800 x 600
XGA 1024 x 768
SVGA 1280 x 1024
A-19
Page 28

As an example, a SVGA resolution LCD panel has 800 pixels horizontally by 600 pixels vertically.
Input video is also represented by the same terms. XGA input video has a format of 1280 pixels
horizontally by 1024 pixels vertically. When the input pixels contained in the video input format
match the native resolution of the panel, there is a one to one correspondence of mapping of input
video pixels to LCD pixels. As an example, the pixel in column 45 and row 26 of the input video
is in column 45 and row 26 of the LCD. For the case when the input video is at a lower or higher
resolution than the native resolution of the LCD, the direct correspondence between the video
pixels and the LCD pixels is lost. The LCD controller can compute the correspondence between
video pixels and LCD pixels using algorithms contained on its controller. The accuracy of the
algorithms determines the fidelity of conversion of video pixels to LCD pixels. Poor fidelity
conversion can result in artifacts in the LCD displayed image such as varying width characters.
A-20 Elo Touchmonitor User Guide
Page 29

A P P E N D I X
B
TOUCHMONITOR SAFETY
This manual contains information that is important for the proper setup and maintenance of your
touchmonitor. Before setting up and powering on your new touchmonitor, read through this
manual, especially Chapter 2 (Installation), and Chapter 3 (Operation).
1. To reduce the risk of electric shock, follow all safety notices and never open the
touchmonitor case.
2. Turn off the product before cleaning
3. Your new touchmonitor is equipped with a 3-wire, Hospital Grade grounding power
cord. The power cord plug will only fit into a grounded outlet. Do not attempt to fit
the plug into an outlet that has not been configured for this purpose. Do not use a
damaged power cord. Use only the power cord that comes with your Elo
TouchSystems Touchmonitor. Use of an unauthorized power cord may invalidate
your warranty.
4. The slots located on the sides and top of the touchmonitor case are for ventilation.
Do not block or insert anything inside the ventilation slots.
5. It is important that your touchmonitor remains dry. Do not pour liquid into or onto
your touchmonitor. If your touchmonitor becomes wet do not attempt to repair it
yourself.
B-21
Page 30

Care and Handling of Your Touchmonitor
The following tips will help keep your Elo touchmonitor functioning at the optimal level.
• To avoid risk of electric shock, do not disassemble the external power adaptor or display
unit cabinet. The unit is not user serviceable. Remember to unplug the display unit from
the power outlet before cleaning.
• Do not use alcohol (methyl, ethyl or isopropyl) or any strong dissolvent. Do not use
thinner or benzene, abrasive cleaners or compressed air.
• To clean the display unit cabinet, use a cloth lightly dampened with a mild detergent.
• Avoid getting liquids inside your touchmonitor. If liquid does get inside, have a qualified
service technician check it before you power it on again.
• Do not wipe the screen with a cloth or sponge that could scratch the surface.
• To clean the touchscreen, use window or glass cleaner. Put the cleaner on the rag and
wipe the touchscreen. Never apply the cleaner directly on the touchscreen .
B-22 Elo Touchmonitor User Guide
Page 31

Display Modes
A P P E N D I X
C
TECHNICAL SPECIFICATIONS
Your Elo touchmonitor is compatible with the following standard video modes:
Item Resolution Type H.Scan(KHz) V. Scan(Hz) Polarity
1 640 x 350 VGA 31.47 70
2 720 x 400 VGA 31.47 70
3 640 x 480 VGA 31.47 60
4 640 x 480 MAC 66 35 6 6 -/ 5 640 x 480 VESA 72 37.86 72 -/ 6 640 x 480 VESA 75 37.5 75 -/7 800 x 600 VESA 56 35.16 56 +/+
8 800 x 600 VESA 60 37.88 60 +/+
9 800 x 600 VESA 75 46.88 75 +/+
10 800 x 600 VESA 72 48.08 72 +/+
11 832 x 624 MAC 75 49.72 75 -/12 1024 x 768 VESA 60 48.36 60 -/13 1024 x 768 SUN 65 52.45 65 -/14 1024 x 768 VESA 70 56.48 70 -/15 1024 x 768 VESA 75 60.02 75 +/+
16 1280 x 1024 SXGA 64 60 +/+
17 1280 x 1024 SXGA 80 75 +/+
18 1152 x 864 SXGA 67.5 75 +/+
19 1280 x 960 SXGA 60 6 0 +/+
C-23
Page 32

Touchmonitor Specifications
Parameter Value
LCD Display 19.0¨ TFT Active Matrix Panel
Pixel Pitch 0.294(H) x 0.294(V) mm
Display Mode VGA 640 x 350 (70 Hz)
VGA 720 x 400 (70 Hz)
VGA 640 x 480 (60 / 72 / 75 Hz)
SVGA 800 x 600 (56 / 60 / 72 / 75 Hz)
SXGA 1280 x 1024(60,70,75 Hz)
Native SXGA 1280 x 1024
Contrast Ratio 1300 : 1 (typical)
Brightness 300 cd/m2 with AT 246 cd/m2, IT 276 cd/m2, IR 276 cd/m
LCD 300 cd/m2 (typical)
AccuTouch 246 cd/m2 (typical)
IntelliTouch 276 cd/m2 (typical)
Response Time Tr = 15 msec, Tf = 5 msec typical ; 12 ms gray to gray
Display Color 16.7 million color, 6 bit with dithering
Viewing Angle (L/R)= -89o/+89o (typical), (U/D) -89o/+89o (typical)
Input Signal VGA Analog Video R.G.B. Analog 0.7V peak to peak
Sync TTL Positive or Negative, Composite Sync, Sync on green
DVI Video Digital TMDS Input
Signal Connector 15 Pin D-Sub, DVI-D
Front Control Power ON / OFF, Menu, Select OSD Contrast, Brightness, H/V-
Position, Recall default, Color Temperature, Sharpness, Phase, Clock
OSD H/V position, OSD Time, Auto Adjust, OSD Language,
Input Select
Plug & Play DDC1 / 2B
Touch Panel AccuTouch , IntelliTouch
Power
External Power Supply* AC 100-240V, 50/60 Hz/1.0A
Monitor DC 12V, 4A
Environmental
Temp. Operating C to 40oC
Storage/Transportation
Humidity (non-condensing)
Operating 30% to 70%
Storage/Transportation
Altitude Operating 1060hpa
Storage/Transportation
Dimensions (H x W x D) 429 x 390 x 212 mm
Weight (Net) 8.4kg.
Certifications UL, C-UL, FCC, CE, VCCI, MPRII, C-TICK
o
0
-20oC to +60oC
10% to 90%
0 to 40,000ft (12,192m)
Equivalent to 1013-303 hP.A
(14.7 to 4.4 psia)
2
* FOR CONTINUED SAFETY - Use only with Hitron Model HES49-12040 adaptor.
C-24 Elo Touchmonitor User Guide
Page 33

Power Supply Cord Selection
North America
Power Supply Cord ¡V Detachable, UL Listed, Type SJT 3 conductor, 18 AWG, configured load
fittings terminating in molded on parallel blade. Grounding type hospital grade attachment plug,
rated at a minimum of 3 amperes.
Basic Cord Type Equivalent Types
SP-2 SPE-2, SPT-2
SP-3 SPE-3, SPT-3
SV SVE, SVO, SVOO, SVT, SVTO, SVTOO
SJ SJE, SJO, SJOO, SJT, SJTO, SJTOO
S SE, SO, SOO, ST, STO, STOO
Grounding reliability can only be achieved when the EQUIPMENT is connected to an equivalent
receptacle marked “Hospital Only” or “Hospital Grade”.
C-25
Page 34

Cord selection for other than North America
For 100 V ac or 220/230/240 V ac operation, the unit is provided with IEC 320 flexible power
cords properly configured for the intended country other than North America. The NOMINAL
cross-sectional area (mm2 Cu) must be 0,75. For assistance in selecting the proper power cord
contact the Elo distributor in your area or contact Elo (see Appendix D, page 23)
Cert. Cert
Country Agency Mark Country Agency Mark
Argentina IRAM Ireland NSAI
Australia SAA Italy IMQ
Austria OVE Japan MITI
Beligum CEBEC Netherlands KEMA
Canada CSA Japan MITI
China CCEE Norway NEMKO
Denmark DEMKO Sweden SEMKO
Finland FEI Switzerland SEV
France UTE United ASTA
Kingdom
Germany VDE BSI
C-26 Elo Touchmonitor User Guide
Page 35

HAR FLEXIBLE CORD
Approval Organization Printed or Embossed
Harmonization Marking
be Located On Jacket or
Insulation of Internal Wiring)
Comite Electrotechnique
Belge (CEBEC)
Verband Deutscher Elektrotechniker (VDE) e.V.
Prufstelle
Union technique de l'Electricite (UTE) USE <HAR> 30 10 30
Instituto Italiano del Marchio di Qualita (IMQ) IEMMEQU <HAR> 10 30 50
British Approvals Service for Electric Cables
(BASEC)
N> V. KEMA KEMA-KEUR <HAR> 10 30 30
CEBEC <HAR> 10 30 10
<VDE> <HAR> 30 10 10
BASEC <HAR> 10 10 30
Alternative Marking
(May Utilizing Black-Red
Yellow Thread (Length
of color Section, mm)
SEMKO AB Svenska Elektriska
Materielkontrollanstalter
Österreichischer Verband fur Elektrotechnik
(ÖVE)
Danmarks Elektriske Materialkontroll <DEMKO> <HAR> 30 10 30
National Standards Authority of Ireland (NSAI) <NSAI> <HAR> 30 30 50
Norges Elektriske Materiellkontroll (NEMKO) NEMKO <HAR> 10 10 70
Asociacion Electrotecnica Y Electronica Espanola
(AEE)
Hellenic Organization for Standardization (ELOT) ELOT <HAR> 30 30 70
Instituto Portages da Qualidade (IPQ) np <HAR> 10 10 90
Schweizerischer Elektro Technischer Verein
(SEV)
SEMKO <HAR> 10 10 50
<ÖVE> <HAR> 30 10 50
<UNED> <HAR> 30 10 70
SEV <HAR> 10 30 90
Elektriska Inspektoratet SETI <HAR> 10 30 90
C-27
Page 36

AccuTouch (resistive) Touchscreen Specifications
Mechanical
Positional Accuracy Standard deviation of error is less than 0.080 in. (2.03 mm).
Equates to less than ±1%.
Touchpoint Density More than 100,000 touchpoints/in2(15,500 touchpoints/cm2)
Touch Activation Force Typical less than 3 ounces(85 grams)
Surface Durability Surface durability is that of glass, Mohs’hardness rating of 7.
Expected Life Performance No known wear-out mechanism, as there are no layers,
coatings, or moving parts. IntelliTouch technology has been
operationally tested to more than 50 million touches in one
location without failure, using a stylus similar to a finger.
Sealing Unit is sealed to protect against splashed liquids, dirt, and
dust.
Optical
Light Transmission (per ASTM 90%
D1003)
Visual Resolution All measurements made using USAF 1951 Resolution Chart,
under 30X magnification, with test unit located approximately
1.5 in (38 mm) from surface of resolution chart. Clear surface:
Excellent, with no noticeable degradation. Antiglare surface:
6:1 minimum.
Gloss (per ASTM D2457 using Antiglare surface: Curved: 60± 20 gloss units or 75 ±15 gloss
a 60-degree gloss meter) units.
Environmental The active area of the touchscreen is resistant to all
Chemical Resistance chemicals that do not affect glass, such as:
Acetone, Toluene, Methyl ethyl ketone, Isopropyl alcohol,
Methyl alcohol, Ethyl acetate, Ammonia-based glass cleaners,
Gasoline, Kerosene, Vinegar
Electrostatic Protection (per Meets Level 4 (15 kV air/8 kV contact discharges).
EN 61 000-4-2, 1995)
C-28 Elo Touchmonitor User Guide
Page 37

IntelliTouch Touchmonitor Specifications
Mechanical
Positional Accuracy Standard deviation of error is less than 0.080 in. (2.03 mm).
Equates to less than ±1%.
Touchpoint Density More than 100,000 touchpoints/in2 (15,500 touchpoints/cm2).
Touch Activation Typically less than 3 ounces (85 grams).
Force
Surface Durability Surface durability is that of glass, Mohs’hardness rating of 7.
Expected Life No known wear-out mechanism, as there are no layers, coatings,
Performance or moving parts. IntelliTouch technology has been operationally
tested to more than 50 million touches in one location without failure,
using a stylus similar to a finger.
Sealing Unit is sealed to protect against splashed liquids, dirt, and dust.
Optical
Light Transmission IntelliTouch 92%, AccuTouch 82%.
(per ASTM D1003)
Visual Resolution All measurements made using USAF 1951 Resolution Chart, under
30X magnification, with test unit located approximately 1.5 in (38
mm) from sur face of resolution chart. Clear surface: Excellent, with
no noticeable degradation. Antiglare surface: 6:1 minimum.
Gloss (per ASTM Antiglare surface: Curved: 60 ±20 gloss units or 75 ± 15 gloss units.
D2457 using a 60degree gloss meter)
C-29
Page 38

C-30 Elo Touchmonitor User Guide
Page 39

Contact Elo
P E N D I X
D
Elo TouchSystems
301 Constifution Drive
Menlo Park, CA 94025
1-800-ELO-TOUCH
(1-800-356-8682)
www.eloinfo.elotouch.com
D-31
Page 40

D-32 Elo Touchmonitor User Guide
Page 41

REGULATORY INFORMATION
I. Electrical Safety Information:
A) Compliance is required with respect to the voltage, frequency, and current requirements
indicated on the manufacturer’s label. Connection to a different power source than those
specified herein will likely result in improper operation, damage to the equipment or pose a
fire hazard if the limitations are not followed.
B) There are no operator serviceable parts inside this equipment. There are hazardous
voltages generated by this equipment which constitute a safety hazard. Service should be
provided only by a qualified service technician.
C) This equipment is provided with a detachable power cord which has an integral safety
ground wire intended for connection to a grounded safety outlet.
1) Do not substitute the cord with other than the provided approved type. Under no
circumstances use an adapter plug to connect to a 2-wire outlet as this will defeat the
continuity of the grounding wire.
2) The equipment requires the use of the ground wire as a part of the safety certification,
modification or misuse can provide a shock hazard that can result in serious injury or
death.
3) Contact a qualified electrician or the manufacturer if there are questions about the
installation prior to connecting the equipment to mains power.
II. Emissions and Immunity Information
A) Notice to Users in the United States: This equipment has been tested and found to comply
with the limits for a Class B digital device, pursuant to Part 15 of FCC Rules. These limits are
designed to provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses, and can radiate radio frequency energy, and if
not installed and used in accordance with the instructions, may cause harmful interference to
radio communications.
B) Notice to Users in Canada: This equipment complies with the Class B limits for radio noise
emissions from digital apparatus as established by the Radio Interference Regulations of
Industrie Canada.
C) Notice to Users in the European Union: Use only the provided power cords and
interconnecting cabling provided with the equipment. Substitution of provided cords and
cabling may compromise electrical safety or CE Mark Certification for emissions or
immunity as required by the following standards:
This Medical Electrical Equipment is required to have a CE Mark on the manufacturer’s
label which means that the equipment has been tested to the following Directives and
Standards: This equipment has been tested to the requirements for the CE Mark as re
quired by medical device Directive (MDD) 93/42/EEC indicated in European Standard
EN60601-1 and EN60601-1-2 (including EN55011 Class B).
33
Page 42

D) General Information to all Users: This equipment generates, uses and can radiate radio frequency energy. If not installed and used according to this manual the equipment may cause
interference with radio and television communications. There is, however, no guarantee that
interference will not occur in any particular installation due to site-specific factors.
1) In order to meet emission and immunity requirements, the user must observe the following:
a) Use only the provided I/O cables to connect this digital device with any computer.
b) To ensure compliance, use only the provided manufacturer’s approved line cord.
c) The user is cautioned that changes or modifications to the equipment not expressly ap
proved by the party responsible for compliance could void the user’s authority to operate
the equipment.
2) If this equipment appears to cause interference with radio or television reception, or any
other device:
a) Verify as an emission source by turning the equipment off and on.
b) If you determine that this equipment is causing the interference, try to correct the interfer
ence by using one or more of the following measures:
i) Move the digital device away from the affected receiver.
ii) Reposition (turn) the digital device with respect to the affected receiver.
iii) Reorient the affected receiver’s antenna.
iv) Plug the digital device into a different AC outlet so the digital device and the
receiver are on different branch circuits.
v) Disconnect and remove any I/O cables that the digital device does not use.
(Unterminated I/O cables are a potential source of high RF emission levels.)
vi)Plug the digital device into only a grounded outlet receptacle. Do not use AC
adapter plugs. (Removing or cutting the line cord ground may increase RF
emission levels and may also present a lethal shock hazard to the user.)
If you need additional help, consult your dealer, manufacturer, or an experienced
radio or television technician.
34 Elo Touchmonitor User Guide
Page 43

WARRANTY
Except as otherwise stated herein or in an order acknowledgment delivered to Buyer, Seller
warrants to Buyer that the Product shall be free of defects in materials and workmanship. The
warranty for the touchmonitors and components of the product is 3 years.
Seller makes no warranty regarding the model life of components. Seller’s suppliers may at any
time and from time to time make changes in the components delivered as Products or components.
Buyer shall notify Seller in writing promptly (and in no case later than thirty (30) days after
discovery) of the failure of any Product to conform to the warranty set forth above; shall describe
in commercially reasonable detail in such notice the symptoms associated with such failure; and
shall provide to Seller the opportunity to inspect such Products as installed, if possible. The
notice must be received by Seller during the Warranty Period for such product, unless otherwise
directed in writing by the Seller. Within thirty (30) days after submitting such notice, Buyer shall
package the allegedly defective Product in its original shipping carton(s) or a functional equivalent and shall ship to Seller at Buyer’s expense and risk.
Within a reasonable time after receipt of the allegedly defective Product and verification by
Seller that the Product fails to meet the warranty set forth above, Seller shall correct such failure
by, at Seller’s options, either (i) modifying or repairing the Product or (ii) replacing the Product.
Such modification, repair, or replacement and the return shipment of the Product with minimum
insurance to Buyer shall be at Seller’s expense. Buyer shall bear the risk of loss or damage in
transit, and may insure the Product. Buyer shall reimburse Seller for transportation cost incurred
for Product returned but not found by Seller to be defective. Modification or repair, of Products
may, at Seller’s option, take place either at Seller’s facilities or at Buyer’s premises. If Seller is
unable to modify, repair, or replace a Product to conform to the warranty set forth above, then
Seller shall, at Seller’s option, either refund to Buyer or credit to Buyer’s account the purchase
price of the Product less depreciation calculated on a straight-line basis over Seller’s stated Warranty Period.
35
Page 44

THESE REMEDIES SHALL BE THE BUYER’S EXCLUSIVE REMEDIES FOR BREACH
OF WARRANTY. EXCEPT FOR THE EXPRESS WARRANTY SET FORTH ABOVE,
SELLER GRANTS NO OTHER WARRANTIES, EXPRESS OR IMPLIED BY STATUTE OR
OTHERWISE, REGARDING THE PRODUCTS, THEIR FITNESS FOR ANY PURPOSE,
THEIR QUALITY, THEIR MERCHANTABILITY, THEIR NONINFRINGEMENT, OR
OTHERWISE. NO EMPLOYEE OF SELLER OR ANY OTHER PARTY IS AUTHORIZED
TO MAKE ANY WARRANTY FOR THE GOODS OTHER THAN THE WARRANTY SET
FORTH HEREIN. SELLER’S LIABILITY UNDER THE WARRANTY SHALL BE LIMITED
TO A REFUND OF THE PURCHASE PRICE OF THE PRODUCT. IN NO EVENT SHALL
SELLER BE LIABLE FOR THE COST OF PROCUREMENT OR INSTALLATION OF SUBSTITUTE GOODS BY BUYER OR FOR ANY SPECIAL, CONSEQUENTIAL, INDIRECT,
OR INCIDENTAL DAMAGES.
Buyer assumes the risk and agrees to indemnify Seller against and hold Seller harmless from all
liability relating to (i) assessing the suitability for Buyer’s intended use of the Products and of any
system design or drawing and (ii) determining the compliance of Buyer’s use of the Products
with applicable laws, regulations, codes, and standards. Buyer retains and accepts full responsibility for all warranty and other claims relating to or arising from Buyer’s products, which include or incorporate Products or components manufactured or supplied by Seller. Buyer is solely
responsible for any and all representations and warranties regarding the Products made or authorized by Buyer. Buyer will indemnify Seller and hold Seller harmless from any liability, claims,
loss, cost, or expenses (including reasonable attorney’s fees) attributable to Buyer’s products or
representations or warranties concerning same.
36 Elo Touchmonitor User Guide
Page 45

INDEX
Numerics
ET1928L-XXWA-1-G
A
About touchmonitor Adjustments, 11
AccuTouch(resistive)Touchscreen Specifications, 28
Audio Volume, 14
Auto-Adjust, 14
B
Back Unit, 4
Base Bottom View, 5
Brightness, 14
C
Care and Handling of your Touchmonitor, 22
Caution-Life Support, v
Classification, vi
Clock, 14
Color Balance, 14
Connecting the Video Cable, 6
Connecting the Serial and USB Touchscreen Cable, 7
Connecting the Speaker Cable, 8
Connecting the Power Cable, 9
Contrast, 14
Control and Adjustment, 13
Cord selection for other than North America, 26
D
Display Angle, 15
Display Modes, 23
E
Electrical Safety Information, 33
Electrostatic Protection, 28
Emissions and Immunity Information, 33
Environmental Chemical Resistance, 28
Expected Life Performance, 28, 29
External Medical Grade Power Supply, 2
F
Front Panel Controls, 12
G
General Power Saving Mode, 15
Gloss, 28, 29
H
H-Position, 14
I
Information Description, 14
Installation and setup, 2
Installing the Touch Driver Software, 10
Intellitouch Touchmonitor Specifications, 29
Introduction, 1
K
KensingtonTM Lock, 2
L
LCD Display Performance Features, 2
Light Transmission, 28
M
Main Unit, 4
N
Native Resolution, 19
O
Operation, 11
Optical Light Transmission, 29
Optimizing the LCD Display, 10
OSD Control Options, 14
OSD Menu Functions, 13
OSD H-Position, 14
OSD Language, 14
OSD or power buttons deosn’t work, 17
OSD V-Position, 14
OSD Time, 14
Out of range display, 14
P
Performance, 29
Phase, 14
Positional Accuracy, 28, 29
Power LED Dispaly & Power Saving, 15
Power-Save, 15
Power will not shut off immediately, 17
Power Supply Cord Selection, 25
Product Overview, 4
I N D E X - 37
Page 46

R
Rear View, 10
Recall Defaults, 14
Regulatory Information, 33
S
Sealing, 28, 29
Sharpness, 14
Side View, 4
Solutions to Common Problems, 17
Surface Durability, 28, 29
SVGA, 19
T
Technical Specifications, 23
Touch Activation Force, 28, 29
Touch Interface Connection, 6
Touch doesn’t work, 17
Touchmonitor Safety, 21
Touchmonitor Specifications, 24
Touchpoint Density, 28, 29
Troubleshooting, 17
U
Unpacking Your Touchmonitor, 3
V
VGA, 19
Visual Resolution, 28, 29
V-Position, 14
W
Warranty, 35
Warnings and Cautions, iv
X
XGA, 19
I N D E X -38
Page 47

Check out Elo’s Web site!
www.elotouch.com
Get the latest...
• Product information
• Specifications
• News on upcoming events
• Press release
• Software drivers
Getting in Touch with Elo
To find out more about Elo’s extensive range of touch solutions, visit our Web site at www.elotouch.com or simply call the office
nearest you:
USA & Headquarters Germany Belgium Japan
Elo TouchSystems Elo TouchSystems GmbH & Co. KG Elo TouchSystems Touch Panel Systems K.K
301 Constitution Drive Haidgraben 6 Diestsesteenweg 692 Sun Homada Bldg. 2F
Menlo Park, CA 94025 D-85521 Ottobrunn B-3010 Kessel-Lo 1-19-20 Shin-Yokohama
USA Germany Belgium Kanagawa 222-0033
Japan
(800) ELO-TOUCH (800-356-8682)
Tel 650-361-4700 Tel +49(89)60822-0 Tel +32(16)35-2100 Tel +81(45)478-2161
Fax 650-361-4747 Fax +49(89)60822-150 Fax +32(16)35-2101 Fax +81(45)478-2180
eloinfo@elotouch.com elosales@elotouch.com elosales@elotouch.com www.tps.co.jp
. Printed in USA
© 2006 Tyco Electronics Corporation
 Loading...
Loading...