Page 1

USER MANUAL
Elo Touch Solutions
2401LM Touchmonitor
SW200127 Rev A
Page 2

Copyright © 2013 Elo Touch Solutions, Inc. All Rights Reserved.
No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated
into any language or computer language, in any form or by any means, including, but not limited to, electronic,
magnetic, optical, chemical, manual, or otherwise without prior written permission of Elo Touch Solutions, Inc.
Disclaimer
The information in this document is subject to change without notice. Elo Touch Solutions, Inc. and its Affiliates
(collectively "Elo") makes no representations or warranties with respect to the contents herein, and specifically
disclaims any implied warranties of merchantability or fitness for a particular purpose. Elo reserves the right to
revise this publication and to make changes from time to time in the content hereof without obligation of Elo to
notify any person of such revisions or changes.
Trademark Acknowledgments
AccuTouch, CarrollTouch, Elo, Elo (logo), Elo Touch, Elo Touch Solutions, Elo TouchSystems, IntelliTouch, iTouch,
SecureTouch, Touch Tools and VuPoint are trademarks of Elo and its Af filiates. Windows is a trademark of Microsoft
Corporation.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 2 of 32
User Manual 2401LM
Page 3

Warnings and Cautions
Warning
• Danger - Explosion hazard. Do not use in the presence of flammable anesthetics, and
other flammable materials.
• To prevent fire or shock hazards, do not immerse the unit in water or expose it to rain
or moisture.
• Do not use the unit with an extension cord receptacle or other outlets unless the
prongs of the power cord can be fully inserted.
• RISK OF ELECTRICAL SHOCK - DO NOT OPEN. To reduce the risk of electrical
shock, DO NOT remove the back of the equipment or open the enclosure. No
user-serviceable parts are inside. Refer servicing to qualified field service engineers
only.
• Uninsulated voltage within the unit may have sufficient magnitude to cause electrical
shock.
• Avoid contact with any part inside the unit.
• This device complies with the electromagnetic emission and immunity standards and
is limited to the standards that are listed on pages 6 and 26. Other devices which are
not designed to withstand emission levels as specified in the medical device
standards may be susceptible to interference from this device. Subjecting the device
to conditions beyond the rated performance capabilities may result in emissions in
excess of the standard. If it is determined that this device produces electromagnetic or
other interference it must be disconnected from power until the cause of the problem
has been determined and resolved. If it is determined that this device is functioning
improperly due to electromagnetic and other interference it must be disconnected
from power until the cause of the problem has been determined and resolved.
• Elo Touch Solutions recommends that after its useful life (or after sustaining
unrepairable damage), customers dispose of the Touchmonitor and its power supply
in an environmentally sound manner. Acceptable methods include the reuse of parts
or whole products and the recycling of products, components, and materials. Please
consult and obey national state, and local laws and ordinances governing the safe
disposal of electronic equipment.
• To avoid risk of electric shock, this equipment must only be connected to supply mains
with protective earth.
This product consists of devices that may contain mercury, which must be recycled or
disposed of in accordance with local, state, or federal laws.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 3 of 32
User Manual 2401LM
Page 4
Caution
Power cord is used as a disconnection device. To de-energize equipment, disconnect
This unit must follow the national requirement and local state laws to dispose unit.
Before connecting the cables to your Elo Touchmonitor, make sure all components
Only approved components complyi ng with IEC60 601-1 ser ies ca n b e connecte d to
For continued safety
the power cord.
are powered OFF.
240 1 L M touch monitor for Healthc ar e Ap p li ca t io ns in Patient Environment. The use of
ACCESSORY equi pment not complying with the equivalent safety requirements of this
equipment may lea d to a reduced saf ety of the resulting system. Consideration relating
to the choices of accessory equipment should include: Use of accessory in the patient
environment.· Evidence that the safety certification of the accessory has been
performed in accordance to the appropriate IEC 60601-1 and/or IEC 60601-1-1
harmonized national standard.
- This unit only complies to the above standards if used with a medical grade power
cord.
- A medical grade power supply, such as the one specified, is required for use in a
medical application.
Please do not touch the patient and the Touchmonitor output connecter at the same
time.
Note:
This symbol alerts the user to important information concerning the operation and
maintenance of this unit, which should be read carefully to avoid problems.
This symbol means DC Current.
This symbol means ON/OFF stand-by switch.
Medical and Healthcare Application Disclaimer:
It is the sole responsibility of any person intending to commercialize, market or use any of Elo
Touch Solutions, Inc. or its family of companies ("Elo") products for medical or healthcare
applications to ensure that such product is adequate and appropriate for the person's
intended use and complies with all applicable laws, regulations, codes and standards
including but not limited to the European Union Medical Device Directive, United States
Federal Food, Drug, and Cosmetic Act, regulations of the United States Food and Drug
Administration (FDA), and for obtaining and maintaining any required regulatory approvals
including but not limited to any required market clearances. Elo has not sought nor received
any rulings from the FDA or any other federal, state, or local government agency or notified
body as to the safety, effectiveness or appropriateness of its product for such applications.
Persons intending to evaluate or use Elo's product for medical or healthcare purposes must
rely on their own medical and legal judgment without any representation on the part of Elo.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 4 of 32
User Manual 2401LM
Page 5

Classification
Wit
h respect to electrical shock, fire in accordance with ANSI/AAMI ES60601-1:2005 and
CAN/CSA C22.2 No. 60601-1-08
This Touchmonitor is a Class I (GROUNDED) DEVICE.
These Touchmonitors are classified NO APPLIED PARTS EQUIPMENT.
Protection against harmful ingress of water:
INGRESS PROTECTION (IPX1)
Touchmonitor shall be classified as ORDINARY EQUIPMENT, not intended or evaluated
This
for
use in the presence of flammable anesthetic mixture with air, oxygen, or nitrous oxide.
Mode of Operation: CONTINUOUS OPERATION.
Environmental conditions for transport and storage
Temp. Operating
0°C to 40°C
Storage / Transportation -20°C to 65°C
Humidity (non-condensing)
Operating
Altitude
2401LM Touchmonitor for Healthcare Applications is intended for general use in hospital environment for data
collection and display for reference. It shall not be used with life-supporting system.
Storage / Transportation 10% to 90%
Operating
Storage / Transportation 0 to 12,192m
0% to 80%
2
0
to 3,000m
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 5 of 32
User Manual 2401LM
Page 6

European Standards and Classifications
Standards: EN 60601-1-2: 2007
The EMC limits and test methods are referred to the following standards:
Emission: Immunity
CISPR11:2009+A1:2010 ED. 5.1(Grp I, Class B) IEC61000-4-2:2008 ED.2.0
AS/NZS CISPR 11: 2011, Grp. 1, Class B IEC61000-4-3:2006+A1:2007 +A2:2010ED.3.2
IEC 61000-4-4: 2012 ED.3.0
EN 61000-3-2: 2006 +A1: 2008+A2: 2009, Class D IEC 61000-4-5: 2005 ED.2.0
IEC 61000-3-3: 2008 IEC 610004-6: 2008 ED.3.0
IEC 61000-4-8: 2009 ED.2.0
IEC 61000-4-11: 2004 ED.2.0
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 6 of 32
User Manual 2401LM
Page 7
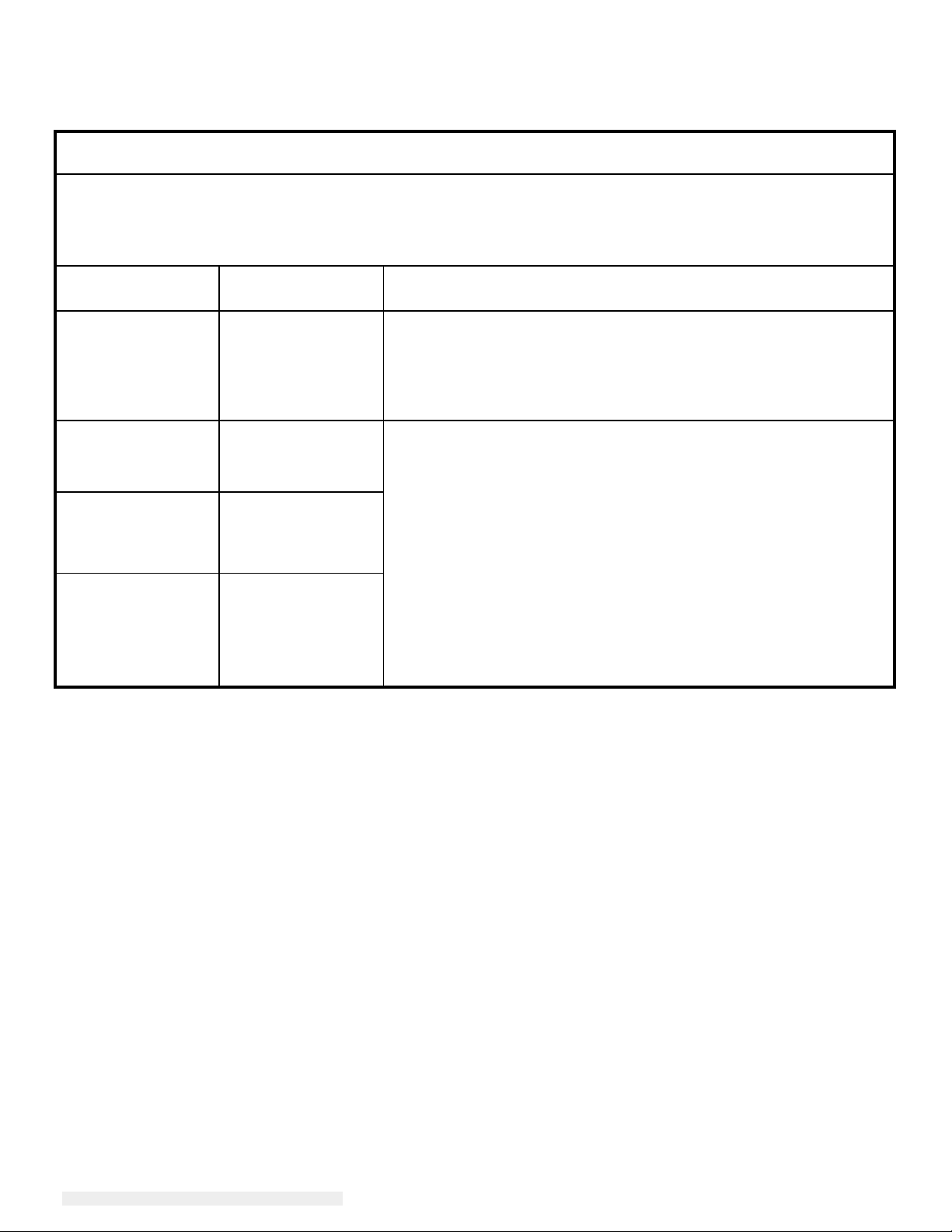
Guidance and manufacturer’s declaration-electromagnetic immunity
for all
EQUIPMENT AND SYSTEMS
Guidance and manufacturer’s declaration-electromagnetic emissions
The 2401LM Touchmonitor for Healthcare Applications is intended for use in the electromagnetic environment
specified below. The customer or the user of the 2401LM Touchmonitor for Healthcare Applications should
assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment-guidelines
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonics
emissions
IEC 61000-3-2
Voltage
fluctuations/flicker
emissions
IEC 61000-3-3
Group 1 The 2401LM Touchmonitor for Healthcare Applications uses RF
energy only for its internal function. Therefore, its RF emissions
are very low and are not likely to cause any interference in nearby
electronic equipment.
Class A The 2401LM Touchmonitor for Healthcare Applications is suitable
for use in all establishments other than domestic and those directly
connected to a low voltage power supply network which supplies
buildings used for domestic purposes.
Class D
Complies
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 7 of 32
User Manual 2401LM
Page 8
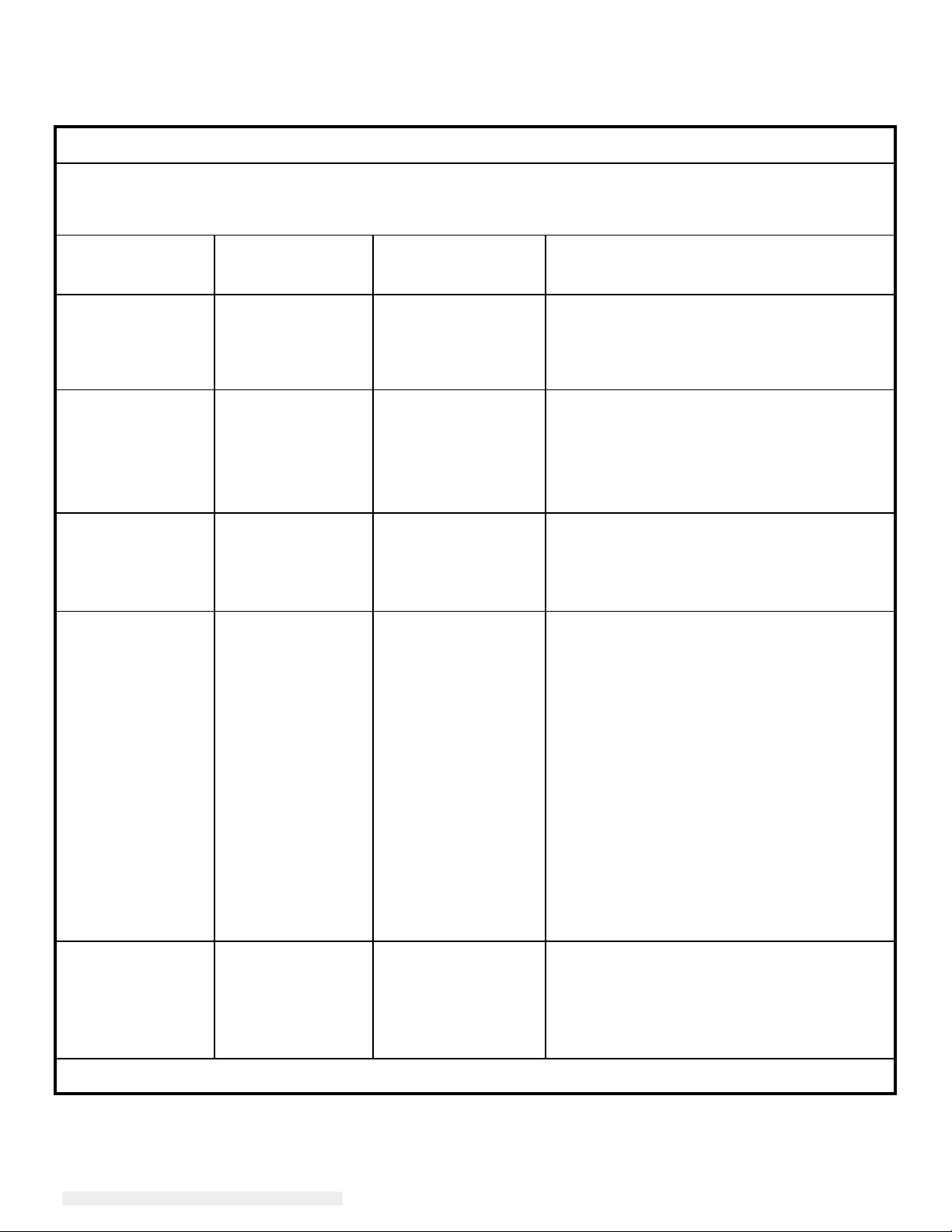
Guidance and manufacturer’s declaration-electromagnetic immunity
for all EQUIPMENT AND
SYSTEMS
Guidance and manufacturer’s declaration-electromagnetic immunity
The 2401LM Touchmonitor for Healthcare Applications is intended for use in the electromagnetic
environment specified below. The customer, or the user of the 2401LM Touchmonitor for Healthcare
Applications,
should assure that it is used in such an environment.
Immunity Test
Level
Electrostatic
Discharge (ESD)
61000-4-2
IEC
Electrical Fast
Tr
ansient/Burst
61000-4-4
IEC
Surge
61000-4-5
IEC
Voltage Dips, Short
Interruption
Voltage
and
Variations
on Power Supply
Input Lines
IEC
61000-4-11
60601
IEC
kV contact
± 6
kV air
± 8
± 2 kV for power
supply lines
kV for
± 1
input/output lines
1 kV line(s) to
±
line(s)
±
2 kV line(s) to
earth
UT
<5%
(>95%
for 0.5 cycle
40%
(60%
dip in UT)
UT
dip in UT)
for 5 cycles
UT
70%
dip in UT)
(30%
for 25 cycles
UT
<5%
(>95%
dip in UT)
for 250 cycles
Compl
supply lines
± 1
± 2
<5%
(>95%
for 0.5 cycle
40%
(60
for 5 cycles
70%
(30%
for 25 cycles
<5%
(>95%
for 250 cycles
iance Level
± 6 kV
2 kV for power
±
± 8
contact
kV air
kV for input/output
Lines
± 1 kV line(s) to
line(s)
kV line(s) to earth
UT
dip in UT)
UT
% dip in UT)
UT
dip in UT)
UT
dip in UT)
Electromagnetic Environment - Guidelines
Floors
tile. If floors are covered with synthetic
mater
least 30%.
should be wood, concrete or ceramic
ial, the relative humidity should be at
Mains power quality should be that of a
typical commercial or hospital environment.
Mains power quality should be that of a
typical commercial or hospital environment.
Mains power quality should be that of a
typical commercial or hospital environment.
If the user of the 2401LM Touchmonitor
for Healthcare Applications requires
continued operation during power mains
interruptions, It is recommended that the
2401LM Touchmonitor for Healthcare
Applications
uninterruptible power supply or a battery.
be powered from an
Power Frequency
(50/60
Magnetic
Hz)
Field
A/m
3
3 A/m
Power frequency magnetic fields should be
at levels char
a typical commercial or
environment.
IEC 61000-4-8
NOTE UT is the A.C. mains voltage prior to application of the test level.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
acteristic of a typical location in
hospital
User Manual 2401LM
SW200127 Rev A - Page 8 of 32
Page 9
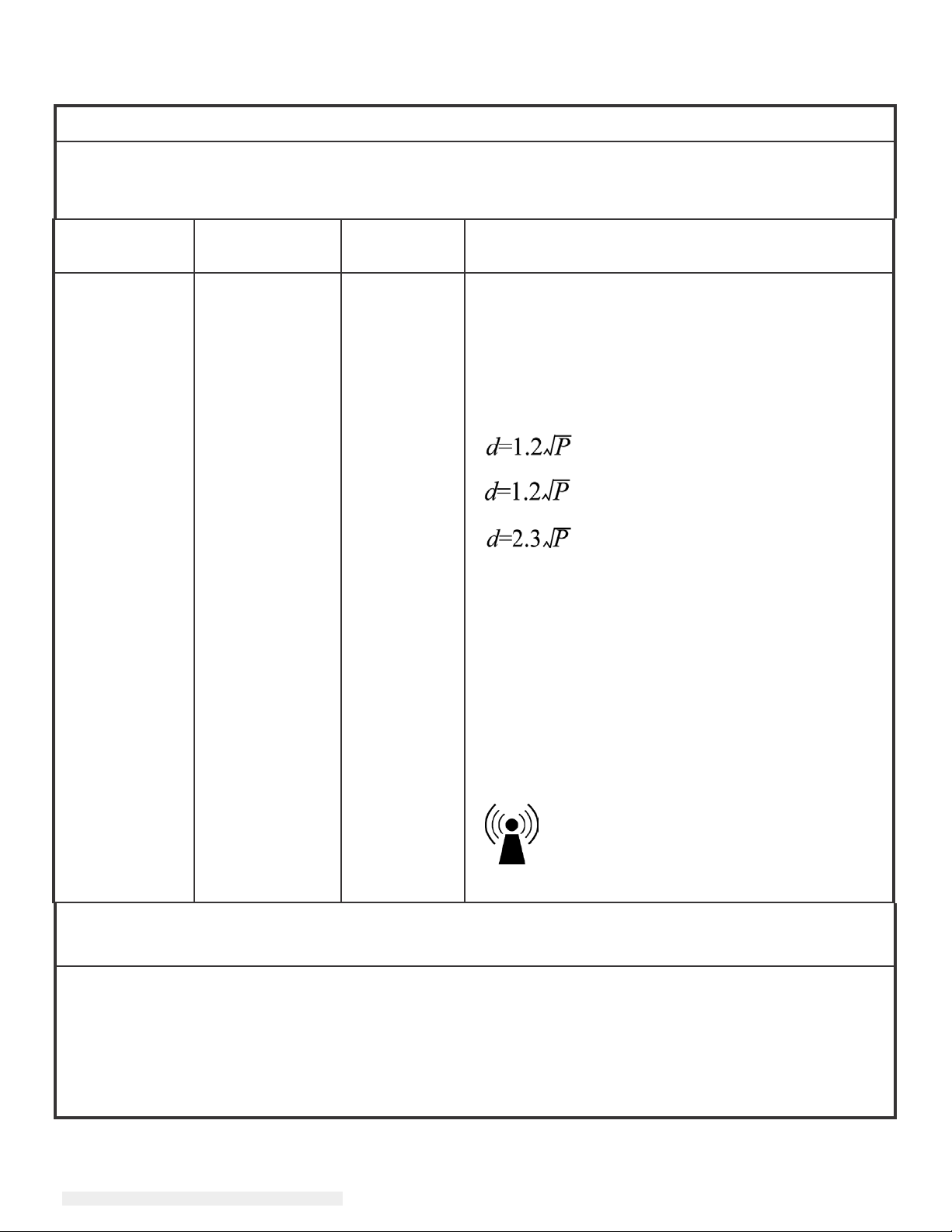
Guidance and manufacturer’s declaration-electromagnetic immunity
SYSTEMS that are not LIFE-SUPPORTING
for all EQUIPMENT AND
Guidance and manufacturer’s declaration-electromagnetic immunity
The 2401LM Touchmonitor for Healthcare Applications is intended for use in the electromagnetic
environment
should assure that it is used in such an environment.
specified below. The user of the 2401LM Touchmonitor for Healthcare Applications
Immunity Test IEC 60601 Test
Level
Conducted RF
Radiated RF
IEC 61000-4-3
3
Vrms
V/m
3
80 MHz to 2.5
GHz
Compliance
Level
3
Vrms
3 Vrms
Electromagnetic Environment-Guidelines
Portable and mobile RF communications equipment
should be used no closer to any part of the 2401LM
Touchmonitor for Healthcare Applications and should
assure that it is used in such an environment,
including cables, than the recommended separation
distance calculated from the equation applicable to the
frequency of the transmitter. Recommended
separation distance
80MHz to 800 MHz
800 MHz to 2.5GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in metres(m)
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey1,
should be less than the compliance level in each
frequency range2.
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
3. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordl ess) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted th eoretically with accuracy. To
assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the 2401LM Touchmonitor for Healthcare Applications
is used exceeds the applicable RF compliance level above, the 2401LM Touchmonitor for Healthcare Applications should
be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary,
such as reorienting or relocating the 2401LM Touchmonitor for Healthcare Applications.
4. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 Vrms.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
User Manual 2401LM
SW200127 Rev A - Page 9 of 32
Page 10
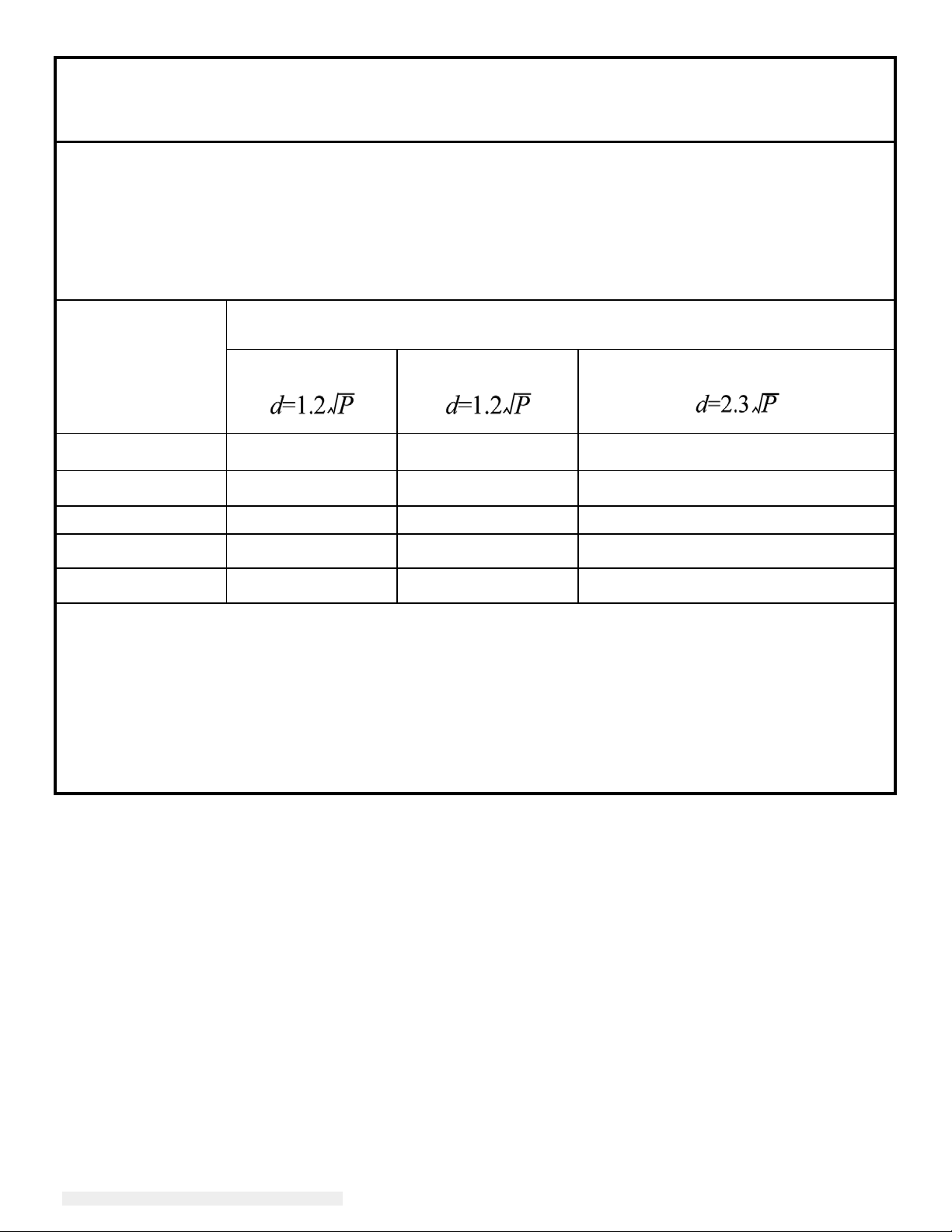
Recommended separation distances between portable
and mobile RF communications equipment
and the 2401LM Touchmonitor for Healthcare Applications for all EQUIPTMENT AND SYSTEMS that are
not LIFE-SUPPORTING
The 2401LM Touchmonitor for Healthcare Applications is intended for use in an electromagnetic
environment in which radiated RF disturbances are controlled. The customer or the user of the 2401LM
Touchmonitor for Healthcare Applications can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications (equipment) and the 2401LM
Touchmonitor for Healthcare Applications as recommended below according to the maximum output
power of the communications equipment.
Rated Maximum
Output Power of
Transmitter
(W)
150 kHz to 80 MHz
Separation Distance According to Frequency of Transmitter
m
80MHz to 800 MHz
800 MHz
to 2.5 GHz
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.2 1.2 2.3
10 3.7 3.7 7.4
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation
distanced in metres (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the
tr
ansmitter manufacturer.
NOTE 1:
NOTE
bsorption and reflection from structures, objects and people.
a
At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
© 2013 Elo Touch Solutions, Inc. All rights reserved.
User Manual 2401LM
SW200127 Rev A - Page 10 of 32
Page 11

Table of Contents
Chapter 1: Introduction ................................................................................ 12
Chapter 2: Installation .................................................................................. 13
Chapter 3: Mounting .................................................................................... 17
Chapter 4: Operation .................................................................................... 19
Chapter 5: Technical Support ..................................................................... 23
Chapter 6: Safety & Maintenance ............................................................... 24
Chapter 7: Regulatory Information ............................................................. 26
Chapter 8: Warranty Information ................................................................ 30
Index .............................................................................................................. 31
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 11 of 32
User Manual 2401LM
Page 12

Chapter 1: Introduction
Product Description
Your new touch monitor combines Elo Touch Solutions’ reliable performance with the latest
developments in touch technology and display design. This combination of features creates a natural
flow of information between a user and the Touchmonitor.
This Touchmonitor incorporates a 8-bit color, active matrix thin-film-transistor LCD panel to provide
high quality display performance. Its Full HD resolution of 1920x1080 is suitable for displaying
graphics and images. Its LED backlight significantly reduces power consumption and eliminates
mercury (compared to CCFL-backlit panels). Other features that enhance this LCD monitor’s
performance are Plug & Play compatibility, built-in speakers and headphone output capability,
on-screen display (OSD) controls, and a family of peripherals including webcam and magnetic stripe
reader.
Precautions
Follow all warnings, precautions and maintenance as recommended in this user manual to maximize
the life of your unit and prevent risks to user safety. See the Safety & Maintenance chapter for more
information.
This manual contains information that is important for the proper setup and maintenance of the unit.
Before setting up and powering on your new touch monitor, read through this manual, especially the
Installation, Mounting, and Operation chapters.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 12 of 32
User Manual 2401LM
Page 13

Chapter 2: Installation
Unpacking the Touch monitor
Open the carton and verify the following items are present:
• Touchmonitor with protective sheet for its face
• Elo TouchTools CD
• Quick Install Guide
• DVI cable
• VGA cable
• USB cable
• AC-DC power adapter
• AC Power Cable
• Audio Cable
• Serial Cable
Connector Panel & Interfaces
Remove the cable cover on the back of the unit to access the Touchmonitor ’s connector panel.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 13 of 32
User Manual 2401LM
Page 14
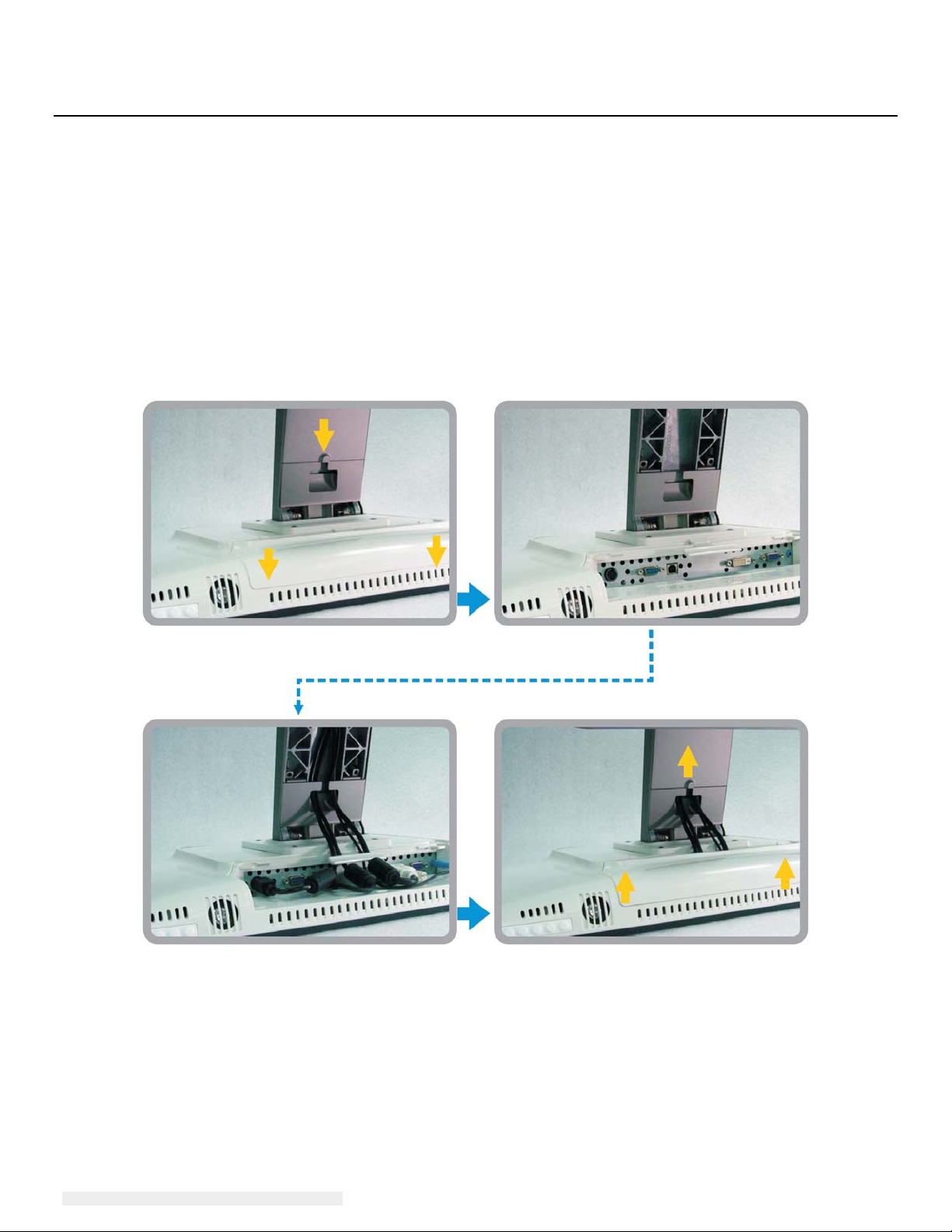
Touchmonitor Connections
1. Connect the DVI or VGA video cables between the monitor’s DVI/VGA input connectors and
your DVI/VGA video source, respectively. Tighten the video cable’s screws for best
performance.
2. Connect the USB touch cable between the monitor’s USB connector and your PC’s USB port.
3. Connect the audio cable between the monitor’s Audio In jack and your audio source.
4. Select the correct power cable for your region. Connect the cable between the AC power
source and the power adapter’s input connector. Connect the power adapter’s DC output
connector to the monitor’s input power jack.
5. Be sure to reinstall the cable cover and secure with appropriate screws. Cables can be
routed inside the stand – remove and replace the stand cable cover for access and routing.
6. The Touchmonitor ships in an OFF state. Press the power button to turn it on.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 14 of 32
User Manual 2401LM
Page 15

Installing the Touch Technology Software Drivers
Some software installation is required for your Touchmonitor to work with your computer.
The drivers for the Windows 7, XP, Vista, WePOS, and 32-bit Server 2003 operating systems are
provided with your Touchmonitor on a CD.
Visit the Elo Touch Solutions website www.elotouch.com for:
• The most up-to-date touch driver versions
• Additional touch driver information
• Detailed touch driver installation guides
• Touch drivers for other operating systems
Download the appropriate driver for your application and follow the onscreen prompts.
For Windows XP, Vista, Server 2003, Server 2008, and WEPOS installations, install the “USB
Touchscreen Drivers” when prompted.
If you do not have the internet available, insert the Elo TouchTools CD into your computer’s
CD-ROM drive. The CD should automatically run the Elo TouchTools application. Select “Install
Driver for This computer”:
© 2013 Elo Touch Solutions, Inc. All rights reserved.
User Manual 2401LM
SW200127 Rev A - Page 15 of 32
Page 16

For Windows 7 installations, check the “Install driver” box under “Elo USB Interfaces – Other
Touchscreens”
After accepting the end-user license agreement, the drivers will finish installing.
Reboot your computer after the install is complete.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 16 of 32
User Manual 2401LM
Page 17

Chapter 3: Mounting
General Mounting Information
The OSD text can be rotated through the OSD menu to better suit your mounting orientation.
The holes located on the top and bottom of the Touchmonitor case are for ventilation. Do not block,
cover, or insert anything inside the ventilation slots.
Rear VESA Mount
A four-hole 100x100mm mounting pattern for M4 screws is provided on the rear of the monitor.
Remove the stand using a Phillips screwdriver to access this mounting interface. The VESA
FDMI-compliant mounting is coded: VESA MIS-D, 100, C
© 2013 Elo Touch Solutions, Inc. All rights reserved.
User Manual 2401LM
SW200127 Rev A - Page 17 of 32
Page 18

Stand Mounting
Threaded through-holes are provided on the bottom of the stand base for mounting or securing.
VESA Mounting Options
The following companies provide VESA mounting devices compatible with your touch monitor :
GCX
800-228-2555
707-773-1100
www.gcx.com
Ergotron
800-888-8458
651-681-7600
www.ergotron.com
Innovative Office Products
800-524-2744
610-253-9554
www.innov-office-prod.com
MRI
800-688-2414
www.mediarecovery.com
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 18 of 32
User Manual 2401LM
Page 19

Chapter 4: Operation
Power
To turn the Touchmonitor on or off, press the Touchmonitor power button once.
The Power Status LED on the bottom of the Touchmonitor functions according to the following table:
Touchmonitor/Computer Module status LED status
OFF OFF
SLEEP ORANGE
ON GREEN
The system consumes low power when in SLEEP and OFF modes. For detailed power consumption
specifications, refer to technical specifications on the Elo website http://www.elotouch.com
Touching the screen will bring the attached host PC out of SLEEP mode (similar to moving the mouse
or pressing a keyboard key).
To improve reliability and reduce wasteful power consumption, disconnect the AC power cable from
the power adapter when long periods of disuse are planned.
Touch
Your IntelliTouch Touchmonitor is factory-calibrated and should not need manual calibration (unless
the input video is not fully scaled to the native resolution, or the touch experience needs to be
calibrated to a specific user).
Video
A display’s native resolution is its width and height measured in number of pixels. Generally, for best
performance, an image displayed on this monitor will look best when your computer’s output resolution
matches this monitor’s native resolution of 1920x1080.
For computer output resolutions at non-native resolutions, the monitor will scale the video to its panel’s
native resolution. This involves stretching or compressing the input image as needed in the X- and
Y-dimensions to fit the display’s native resolution. An unavoidable byproduct of the scaling
algorithms is a loss of fidelity when the computer’s output video image is scaled by the monitor to fit
the display. This loss of fidelity is most apparent when viewing feature-rich images at close distances
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 19 of 32
User Manual 2401LM
Page 20
(for example images containing small-font text).
Your Touchmonitor will likely not require video adjustments. However, for analog VGA video, variations
in video graphic card outputs may require user adjustments through the OSD to optimize the quality of
the Touchmonitor’s displayed image. These adjustments are “remembered” by the Touchmonitor. Also,
to reduce the need for adjustments for different video mode timings, the monitor correctly scales and
displays some of the video industry’s most common video timing modes. Refer to the technical
specifications for this monitor at http://www.elotouch.com for a list of these Preset Video Modes.
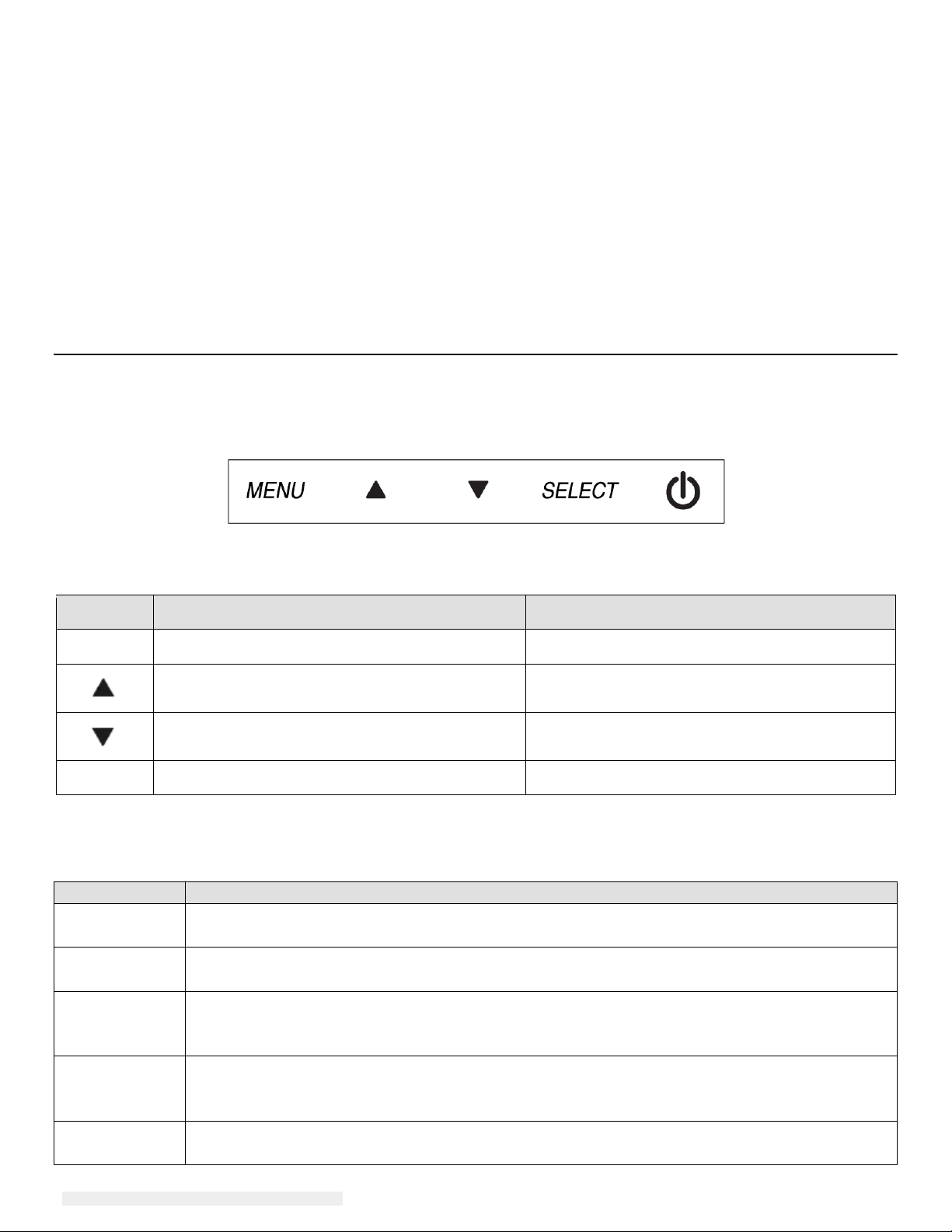
On-Screen Display (OSD)
Four OSD buttons are on the bottom of the monitor. These can be used to adjust various display
parameters.
The buttons and their functionality are:
Button Function when OSD is not displayed: Function when OSD is displayed:
Menu Display OSD main menu Return to previous OSD menu
Display OSD Contrast submenu
Display OSD Brightness submenu
Increase value of selected parameter /
select next menu item
Decrease value of selected parameter /
select previous menu item
Select Enter“Auto Adjust” feature(VGA mode only) Select submenu to enter
Using the OSD buttons controls an on-screen graphical user interface which displays on top of
your input video, allowing intuitive adjustment of the following display parameters:
Parameter Available Adjustment
Contrast
Brightness
Increase/decrease monitor contrast.
Default: best gray-shade performance
Increase/decrease monitor brightness.
Default: maximum
Moves the image vertically on the display in single-pixel increments.
V-position
Default: centered.
Only applicable for VGA input video
Moves the image horizontally on the display in single-pixel increments.
H-position
Default: centered.
Only applicable for VGA input video
Recall
Defaults
Selecting “Recall Defaults” restores all factory default settings for OSD-adjustable
parameters (except OSD Language) and for Preset Video Mode timings.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 20 of 32
User Manual 2401LM
Page 21

Color
Temperature
Volume
Sharpness
Phase
Clock
OSD
H-position
OSD
V-position
OSD Timeout
Auto Adjust
OSD
Language
Input Video
Selects the display’s color temperature. The available color temperatures are 9300K,
6500K, 5500K, 7500K, and User Defined. If the User Defined option is selected, the
user can change the color temperature by changing individual R, G, and B gains on a
scale from 0 to 100.
Default: User Defined with R, G, and B all set to 100.
Adjusts the volume of the internal speakers output.
Adjusts sharpness of the displayed images.
Default: no sharpness adjustment
Allows fine adjustments of the panel’s pixel dot clock phase.
Only applicable for VGA input video
Allows fine adjustments of the panel’s pixel dot clock.
Only applicable for VGA input video
Moves the OSD horizontally on the display
Default: centered.
Moves the OSD vertically on the display
Default: centered.
Adjusts how long a period of OSD button inactivity the Touchmonitor will wait before
closing the OSD. The adjustable range is between 45 and 255 seconds.
Default: 45 seconds
Automatically adjusts the system clock to the input analog VGA video signal, affecting
the H-position, V-position, Clock, and Phase menu items.
Only applicable for VGA input video
Selects which language the OSD information is displayed in. The available
languages are: English, French, German, Spanish, Swedish, Italian, Simplified
Chinese, Polish, and Japanese.
Default: English.
Select input video source : VGA or DVI
Default: VGA
All Touchmonitor adjustments made through the OSD are automatically memorized as soon as they
are entered. This feature saves you from having to reset your choices every time the Touchmonitor is
unplugged or powered off and on. If there is a power failure, the Touchmonitor settings will not default
to the factory specifications.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 21 of 32
User Manual 2401LM
Page 22

OSD and Power Lockouts
Press and hold the “Menu” and “Up” buttons for two seconds to enable/disable the OSD Locking
feature. When the OSD Locking is enabled, pressing any of the Menu, Up, Down, or Select keys will
have no effect on the system.
Press and hold the “Menu” and “Down” buttons for two seconds to enable/disable the Power Locking
feature. When the Power Locking is enabled, pressing the power switch will have no effect on the
system.
Audio
When headphonesare plugged into the headphone output jack, the internal speakers turn off and
audio is played over the headphones.
Volume for the speaker and headphone outputs can be controlled by the OSD.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 22 of 32
User Manual 2401LM
Page 23

Chapter 5: Technical Support
If you are experiencing trouble with your Touchmonitor, refer to the following suggestions. If the
problem persists, please contact your local dealer or contact Elo Touch Solutions Customer Service.
Solutions to Common Problems
Problem Suggested Troubleshooting
The Touchmonitor does not respond when
turning on the system.
Monitor display is dim
Monitor display is blank.
Monitor displays an “Out Of Range”
message
Touch functionality doesn’t work
Check that the AC power cable is properly
connected.
Verify the AC power source is functioning.
Use the OSD to increase the brightness.
Use the OSD to increase the contrast.
If the Power Status LED is blinking, the monitor or
Computer Module may be in SLEEP mode. Press
any key / move the mouse / touch the Touchscreen
to see if the image reappears.
Adjust your computer’s resolution/timing mode to be
within the allowable timing ranges specified for your
Touchmonitor (see website for specifications)
Verify your PC has the latest Elo drivers installed.
Perform the calibration routine provided with the
latest Elo drivers.
Technical Assistance
Visit www.elotouch.com/products for technical specifications for this device
Visit www.elotouch.com/go/websupport for online self-help.
Visit www.elotouch.com/go/contactsupport for technical support.
See this user manual’s last page for worldwide technical support phone numbers.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 23 of 32
User Manual 2401LM
Page 24

Chapter 6: Safety & Maintenance
Safety
To avoid risk of electric shock, follow all safety notices and do not disassemble the T ouchmonitor. They
are not user-serviceable.
The slots located on the sides and top of the Touchmonitor case are for ventilation. Do not block or
insert anything inside the ventilation slots.
The Touchmonitor ships with a 3-wire, grounding power cord. The power cord plug only fits into a
grounded outlet. Do not fit or modify the plug into an outlet that has not been configured for this
purpose. Do not use a damaged power cord. Only use the power cord that came with your Elo Touch
Solutions Touchmonitor. Use of an unauthorized power cord might invalidate your warranty.
Ensure that your installation is equipped to maintain the specified environmental conditions listed in
the Technical Specifications chapter.
Care and Handling
The following tips will help keep your Touchmonitor functioning at an optimal level:
• Disconnect the AC power cable before cleaning.
• To clean the display unit cabinet, use a clean cloth lightly dampened with a mild
detergent.
• It is important that your unit remains dry. Do not get liquids on or inside the unit. If
liquid does get inside, have a qualified service technician check it before you power it on
again.
• Do not wipe the screen with a cloth or sponge that could scratch the surface.
• To clean the Touchscreen, use window or glass cleaner applied to a clean cloth or
sponge. Never apply the cleaner directly to the touchscreen. Do not use alcohol (methyl,
ethyl or isopropyl), thinner, benzene, or other abrasive cleaners.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 24 of 32
User Manual 2401LM
Page 25

Waste Electrical & Electronic Equipment Directive (WEEE)
This product should not be disposed of with household waste. It should be deposited at a
facility that enables recovery and recycling.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 25 of 32
User Manual 2401LM
Page 26

Chapter 7: Regulatory Information
I. Electrical Safety Information:
Compliance is required with respect to the voltage, frequency, and current requirements
indicated on the manufacturer’s label. Connection to a different power source than those specified
herein will likely result in improper operation, damage to the equipment or pose a fire hazard if the
limitations are not followed.
There are no operator serviceable parts inside this equipment. There are hazardous voltages
generated by this equipment which constitute a safety hazard. Service should be provided only by
a qualified service technician.
Contact a qualified electrician or the manufacturer if there are questions about the installation
prior to connecting the equipment to mains power.
II. Emissions and Immunity Information
Notice to Users in the United States: This equipment has been tested and found to comply with
the limits for a Class B digital device, pursuant to Part 15 of FCC Rules. These limits are designed
to provide reasonable protection against harmful interference in a residential installation. This
equipment generates, uses, and can radiate radio frequency energy, and if not installed and used
in accordance with the instructions, may cause harmful interference to radio communications.
Notice to Users in Canada: This equipment complies with the Class A limits for radio noise
emissions from digital apparatus as established by the Radio Interference Regulations of Industrial
Canada.
Notice to Users in the European Union: Use only the provided power cords and interconnecting
cabling provided with the equipment. Substitution of provided cords and cabling may compromise
electrical safety or CE Mark Certification for emissions or immunity as required by the following
standards:
This Information Technology Equipment (ITE) is required to have a CE Mark on the
Manufacturer’s label which means that the equipment has been tested to the following Directives
and Standards: This equipment has been tested to the requirements for the CE Mark as required
by EMC Directive 89/336/EEC as indicated in European Standard EN 55022 Class A and the Low
Voltage Directive 73/23/EEC as indicated in European Standard EN 60950.
General Information to all Users: This equipment generates, uses and can radiate radio
frequency energy. If not installed and used according to this manual the equipment may cause
interference with radio and television communications. There is, however, no guarantee that
interference will not occur in any particular installation due to site-specific factors.
1) In order to meet emission and immunity requirements, the user must observe the following:
a) Use only the provided I/O cables to connect this digital device with any computer.
b) To ensure compliance, use only the provided manufacturer’s approved line cord.
c) The user is cautioned that changes or modifications to the equipment not expressly approved
by the party responsible for compliance could void the user’s authority to operate the equipment.
2) If this equipment appears to cause interference with radio or television reception, or any
other device:
a) Verify as an emission source by turning the equipment off and on.
If you determine that this equipment is causing the interference, try to correct the interference
by using one or more of the following measures:
i) Move the digital device away from the affected receiver.
ii) Reposition (turn) the digital device with respect to the affected receiver.
iii) Reorient the affected receiver’s antenna.
iv) Plug the digital device into a different AC outlet so the digital device and the receiver are on
different branch circuits.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 26 of 32
User Manual 2401LM
Page 27

v) Disconnect and remove any I/O cables that the digital device does not use.(Un-terminated
I/O cables are a potential source of high RF emission levels.)
vi) Plug the digital device into only a grounded outlet receptacle. Do not use AC adapter plugs.
(Removing or cutting the line cord ground may increase RF emission levels and may also present a
lethal shock hazard to the user.)
If you need additional help, consult your dealer, manufacturer, or an experienced radio or
television technician.
III. Agency Certifications
The following certifications and marks have been issued or declared for this monitor:
• CE marking to low voltage directive and EMC directive
• US "NRTL" mark (e.g. UL)
• Canadian “NTRL” mark (e.g. CSA) and ICES EMC labeling
• US FCC, EMC compliance label
• China CCC safety mark and China RoHS marking
• Australia/NZ C-tick EMC mark
• WEEE marking
• RoHS marking
• Korea KC mark EMC
• Russia GOST mark
• Taiwan BSMI mark
• Japan VCCI mark
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 27 of 32
User Manual 2401LM
Page 28
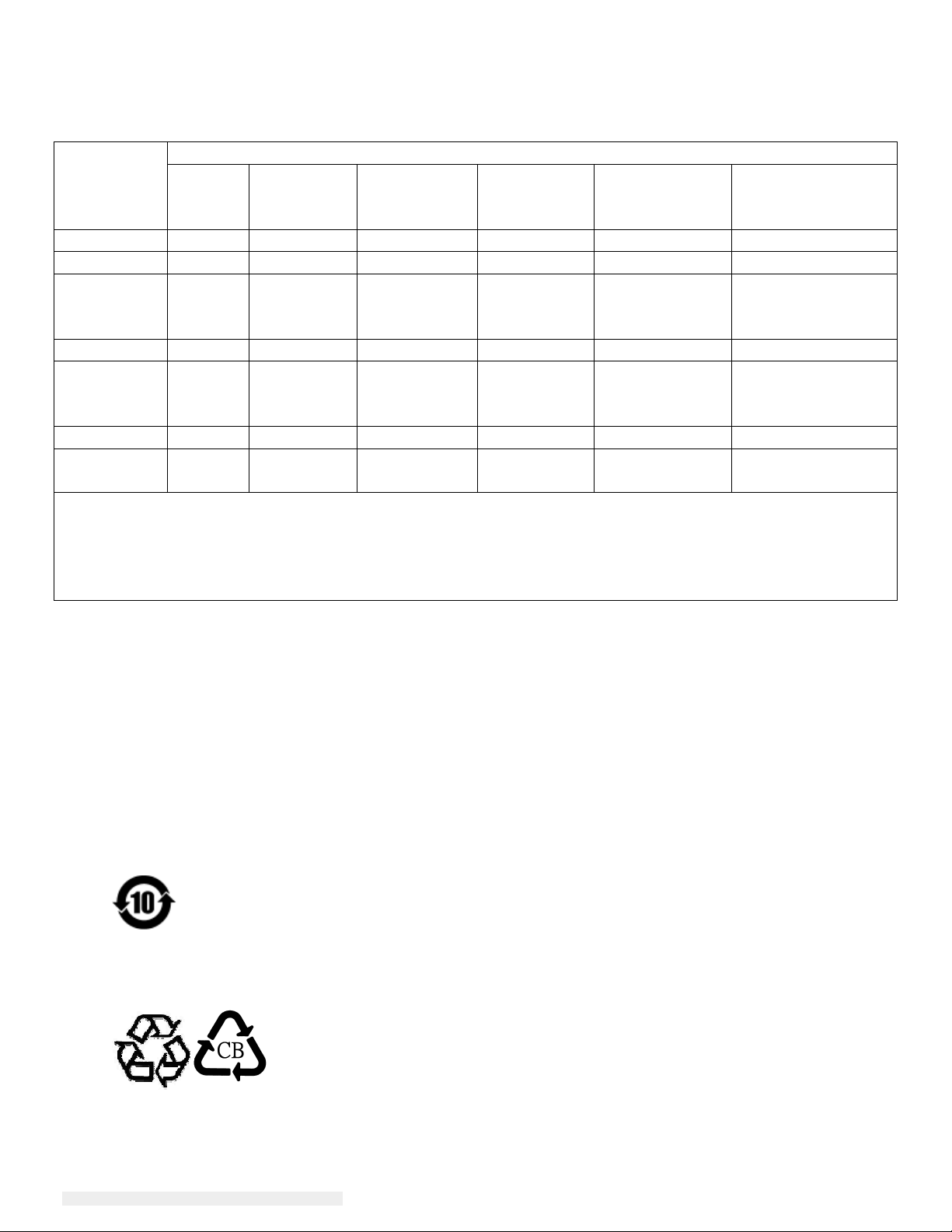
IV. China RoHS
In accordance to Chinese law (Administration on the Control of Pollution Caused by Electronic
Information Products), the section below lists out the name and amount of the toxic and/or
hazardous materials that this product may contain.
Component
Name
Toxic or Hazardous Substances and Elements
Lead(Pb) Mercury(Hg) Cadmium(Cd) Hexavalent
Chromium
(Cr6+)
Polybrominated
Biphenyls
(PBB)
Polybrominated
Diphenyl
Ethers (PBDE)
Plastic Parts O O O O O O
Metal Parts X O O O O O
Wire and
X O O O O O
Cable
Assembly
LCD Panel X O O O O O
Touch
X O O O O O
Screen
Panel
PCBA X O O O O O
Software
O O O O O O
(CD, etc.)
O: Indicates that this toxic or hazardous substance contained in all of the homogeneous materials for
this component is below the limit requirement in SJ/T11363-2006.
X: Indicates that this toxic or hazardous substance contained in at least one of the homogeneous
materials used for this component is above the limit requirement in SJ/T11363-2006. For items
marked with X, exemptions were taken according to EU RoHS.
Explanation of Markings
(1). In accordance with the SJ/T11364-2006 requirement, the electronic information products
are marked with the following pollution control logo. The Environment-Friendly Use Period for
this product is 10 years. The product will not leak or mutate under normal operating conditions
listed below, so that the use of this electronic information product will not result in any severe
environmental pollution, any bodily injury, or damage to any assets.
Operating Temperature:0-35 / Humidity:20%-80% (non-condensing).
Storage Temperature:-20~60 / Humidity:10%~90% (non-condensing).
(2). It is encouraged and recommended that this product be recycled and reused according to
local laws. The product should not be thrown away casually.
V. Power Adapter Specifications
Electrical Ratings:
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 28 of 32
User Manual 2401LM
Page 29

Input: 100-240VAC, 50-60Hz
Output: 12VDC, minimum 3A, LPS
VI. Monitor Specifications
Electrical Ratings:
Input: 12VDC, 3A
Operating Conditions:
Temperature: 0°C - 35°C
Humidity: 20% to 80% (non-condensing)
Altitude: 0 to 3,048m
Storage Conditions:
Temperature: -20°C - 50°C
Humidity: 10% to 90% (non-condensing)
Altitude: 0 to 12,192m
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 29 of 32
User Manual 2401LM
Page 30

Chapter 8: Warranty Information
Except as otherwise stated herein, or in an order acknowledgment delivered to Buyer, Seller warrants to Buyer
that the Product shall be free of defects in materials and workmanship. The warranty for the Touchmonitors and
their components is three years.
Seller makes no warranty regarding the model life of components. Seller’s suppliers may at any time and from
time to time make changes in the components delivered as Products or components.
Buyer shall notify Seller in writing promptly (and in no case later than 30 days after discovery) of the failure of
any Product to conform to the warranty set forth above; shall describe in commercially reasonable detail in such
notice the symptoms associated with such failure; and shall provide to Seller the opportunity to inspect such
Products as installed, if possible. The notice must be received by Seller during the Warranty Period for such
product, unless otherwise directed in writing by the Seller. Within thirty days after submitting such notice, Buyer
shall package the allegedly defective Product in its original shipping carton(s) or a functional equivalent and
shall ship to Seller at Buyer’s expense and risk.
Within a reasonable time after receipt of the allegedly defective Product and verification by Seller that the
Product fails to meet the warranty set forth above, Seller shall correct such failure by, at Seller’s options, either
(i) modifying or repairing the Product or (ii) replacing the Product. Such modification, repair, or replacement and
the return shipment of the Product with minimum insurance to Buyer shall be at Seller’s expense. Buyer shall
bear the risk of loss or damage in transit, and may insure the Product. Buyer shall reimburse Seller for
transportation cost incurred for Product returned but not found by Seller to be defective. Modification or repair,
of Products may, at Seller’s option, take place either at Seller’s facilities or at Buyer’s premises. If Seller is
unable to modify, repair, or replace a Product to conform to the warranty set forth above, then Seller shall, at
Seller’s option, either refund to Buyer or credit to Buyer’s account the purchase price of the Product less
depreciation calculated on a straight-line basis over Seller’s stated Warranty Period.
These remedies shall be the buyer’s exclusive remedies for breach of warranty. Except for the express warranty
set forth above, seller grants no other warranties, express or implied by statute or otherwise, regarding the
products, their fitness for any purpose, their quality, their merchantability, their non-infringement, or otherwise.
No employee of Seller or any other party is authorized to make any warranty for the goods other than the
warranty set forth herein. Seller’s liability under the warranty shall be limited to a refund of the purchase price of
the product. In no event shall Seller be liable for the cost of procurement or installation of substitute goods by
Buyer or for any special, consequential, indirect, or incidental damages.
Buyer assumes the risk and agrees to indemnify Seller against and hold Seller harmless from all liability relating
to (i) assessing the suitability for Buyer’s intended use of the Products and of any system design or drawing and
(ii) determining the compliance of Buyer’s use of the Products with applicable laws, regulations, codes, and
standards. Buyer retains and accepts full responsibility for all warranty and other claims relating to or arising
from Buyer’s products, which include or incorporate Products or components manufactured or supplied by
Seller. Buyer is solely responsible for any and all representations and warranties regarding the Products made
or authorized by Buyer. Buyer will indemnify Seller and hold Seller harmless from any liability, claims, loss, cost,
or expenses (including reasonable attorney’s fees) attributable to Buyer’s products or representations or
warranties concerning same.
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 30 of 32
User Manual 2401LM
Page 31

Index
Agency Certifications, 27
Altitude, 5
Available Adjustment
Auto Adjust, 21
Brightness, 20
Clock, 21
Color Temperature, 21
Contrast, 20
H-position, 20, 21
Input Video, 21
OSD Language, 21
OSD Timeout, 21
Phase, 21
Recall Defaults, 20
Sharpness, 21
Volume, 21
V-position, 20, 21
cable cover, 14
calibration, 19
Care and Handling, 24
China RoHS, 28
Classification, 5
cleaning, 24
Connections, 14
Connector Panel & Interfaces, 13
Disclaimer, 2
electromagnetic immunity, 7
Emissions and Immunity Information, 26
Environmental conditions, 5
European Standards and Classifications, 6
Explanation of Markings, 28
grounding power cord, 24
headphones, 22
INGRESS PROTECTION, 5
Installing Software Drivers, 15
Lockouts, 22
OSD and Power, 22
Mode of Operation, 5
Monitor Specifications, 29
Mounting Information, 17
native resolution, 19
online self-help, 23
On-Screen Display, 20
Operating, 5
OSD, 20
OSD buttons, 20
Out Of Range, 23
Power Adapter Specifications, 28
Power Status LED, 19
Problems, 23
Out of Range, 23
Product Description, 12
Rear VESA Mount, 17
speakers, 22
Stand Mounting, 18
Storage, 5
Technical Assistance, 23
TouchTools, 2, 13, 15
Trademark, 2
Transportation, 5
Unpacking, 13
ventilation, 24
Video, 19
Warnings, 3
Warranty Information, 30
WEEE, 25
internal speakers, 22
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 31 of 32
User Manual 2401LM
Page 32

Check out our website
A
www.elotouch.com
Get the latest...
• Product Information
• Specifications
• Upcoming events
• Press releases
• Software drivers
Getting in Touch with Us
To find out more about the extensive range of Elo touch solutions, visit our website at
www.elotouch.com, or simply call the office nearest you:
North America
Elo Touch Solutions
1033 McCarthy Blvd
Milpitas, CA 95035
Tel 800-ELO-TOUCH
Tel + 1 408 597 8000
Fax +1 408 597 8050
customerservice@elotouch.com
Europe
Tel +32 (0) 16 70 45 00
Fax +32 (0)16 70 45 49
elosales@elotouch.com
sia-Pacific
Tel +86 (21) 3329 1385
Fax +86 (21) 3329 1400
www.elotouch.com.cn
Latin America
Tel 786-923-0251
Fax 305-931-0124
www.elotouch.com
© 2013 Elo Touch Solutions, Inc. All rights reserved.
SW200127 Rev A - Page 32 of 32
User Manual 2401LM
 Loading...
Loading...