Elis DETA AP-20 User Manual
MISSION TO CURE
Electromagnetic Field
Treatment Device
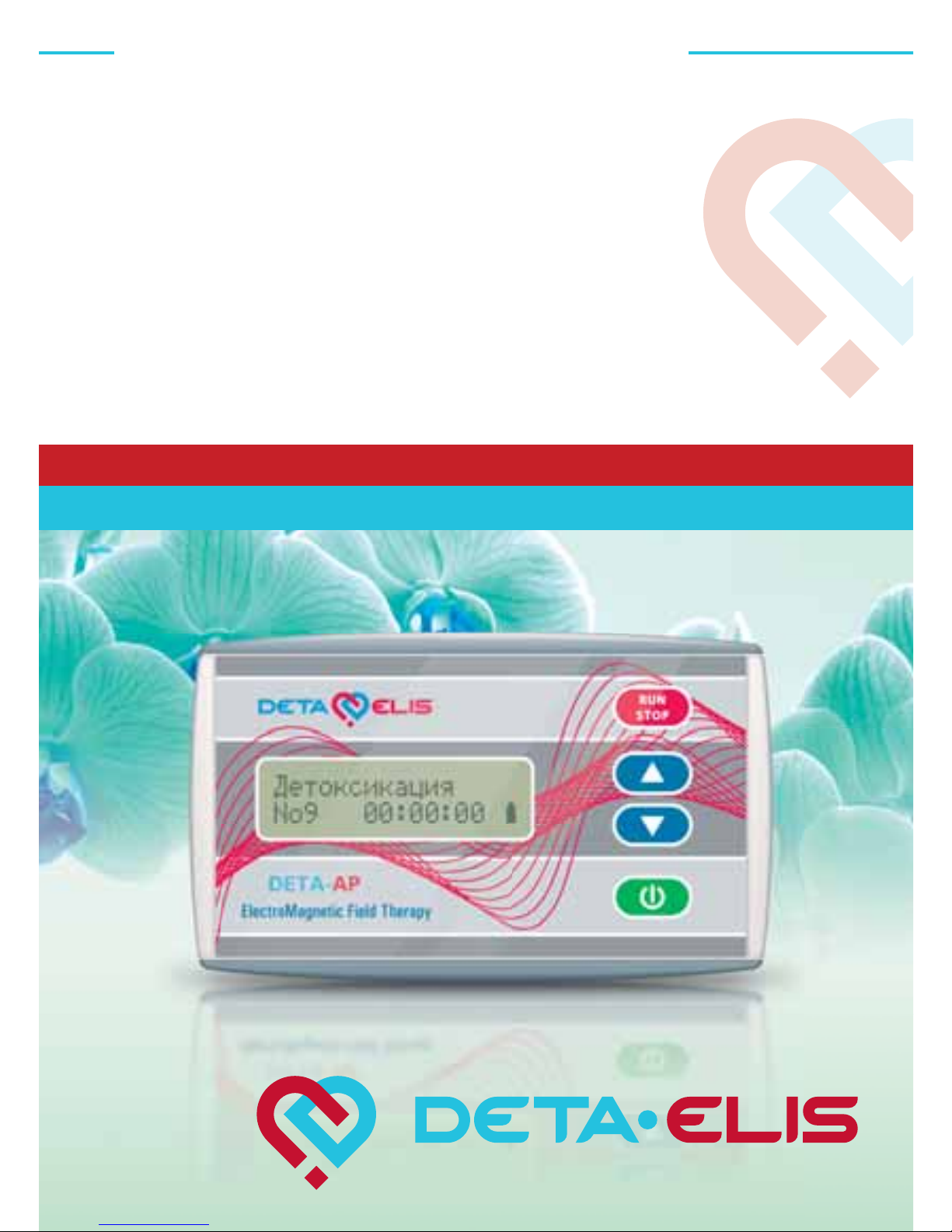
DETA AP-20
Passport
The latest scientifi c opinions on the fi ght against parasites
A unique medical procedure
ELIS Research and Development Enterprise

2
© ELIS Research & Development Enterprise, 2010
All rights reserved. Partial or complete photomechanical
reproduction and recording onto electronic media is prohibited.
Printed in Moscow.

1
1. Introduction 2
2. Purpose 3
3. Main specifi cations of the device 4
4. Package contents 4
5. Information about the device 5
6. Getting started 6
7. O perati on 7
8. Directions for use of the device 8
9. Contraindications to use 9
10. Storage 9
11. Transportation 9
12. Manufacturer’s warranty 9
Contents

2
1. Introduction
Treatment device “DETA-AP-20” TУ 9444-001-27970873-2006
(hereinafter referred to as the device) is designed for electromagnetic therapy.
The device is designed for individual use:
• In clinical practice by doctors of various specializations.
• Outpatient use.
• Home use.
Certifi cate of conformity РОСС RU.ИМ24.В03109
Registration certifi cate No. ФСР 2009/05641
Designation of device in order: device “DETA-AP-20”

3
2. Purpose
Treatment device “DETA-AP-20” is designed for exogenous
bioresonance therapy of a wide range of diseases via the eff ects
of electromagnetic low-energy radiation on the body.
The device operates at frequencies from 0.1 Hz to 10 kHz.
“DETA-AP-20” allows you to conduct therapy for infectious and
diseases and those associated with infection with programs
especially designed for the device. The method of using the
programs is simple, easy to understand, and requires no special
training. The strong therapeutic eff ect is achieved due to the
deep penetrative capability of the electromagnetic fi eld on the
body. The high precision rate of setting the frequency provides
the possibility of aiming at a particular type of infectious pathogen and destroying it.
The therapeutic eff ect when using “DETA-AP-20” is based on
the latest scientifi c opinions set out in guidelines.
The “DETA-AP-20” portable device, programmable with a
computer allows you to conduct therapy using any of 20 programs. Each program is designed to treat a particular disease or
group of diseases.

4
3. Main specifi cations of the device
3.1. Basic parameters and dimensions
3.1.1. The device requires 2 x AA batteries (R6UPR)
3.1.2. Total dimensions of the device: not more than
115 x 70 x 25 mm
3.1.3. Device weight: not more than 0.15 kg
3.2. Frequency range 0.1 Hz ÷ 10 kHz
3.3. Number of programs: 20
3.4. Accuracy of the entire frequency range ± 1%
3.5. Program storage time: minimum 5 years
3.6. Input current during operation: not more than 12.0 mA,
6.0 uA during standby
3.7. Continuous operating time of the device: not less than
20 hours
3.8. Operation mode setup time: not more than 2 seconds
3.9. Average service life of the device: minimum 5 years
4. Package contents
• Medical device “DETA-AP-20”
1 pc.
• Passport
1 pc.
• Guidelines
1 pc.
(as per set of programs supplied)
 Loading...
Loading...