elexxion claros pico User Manual
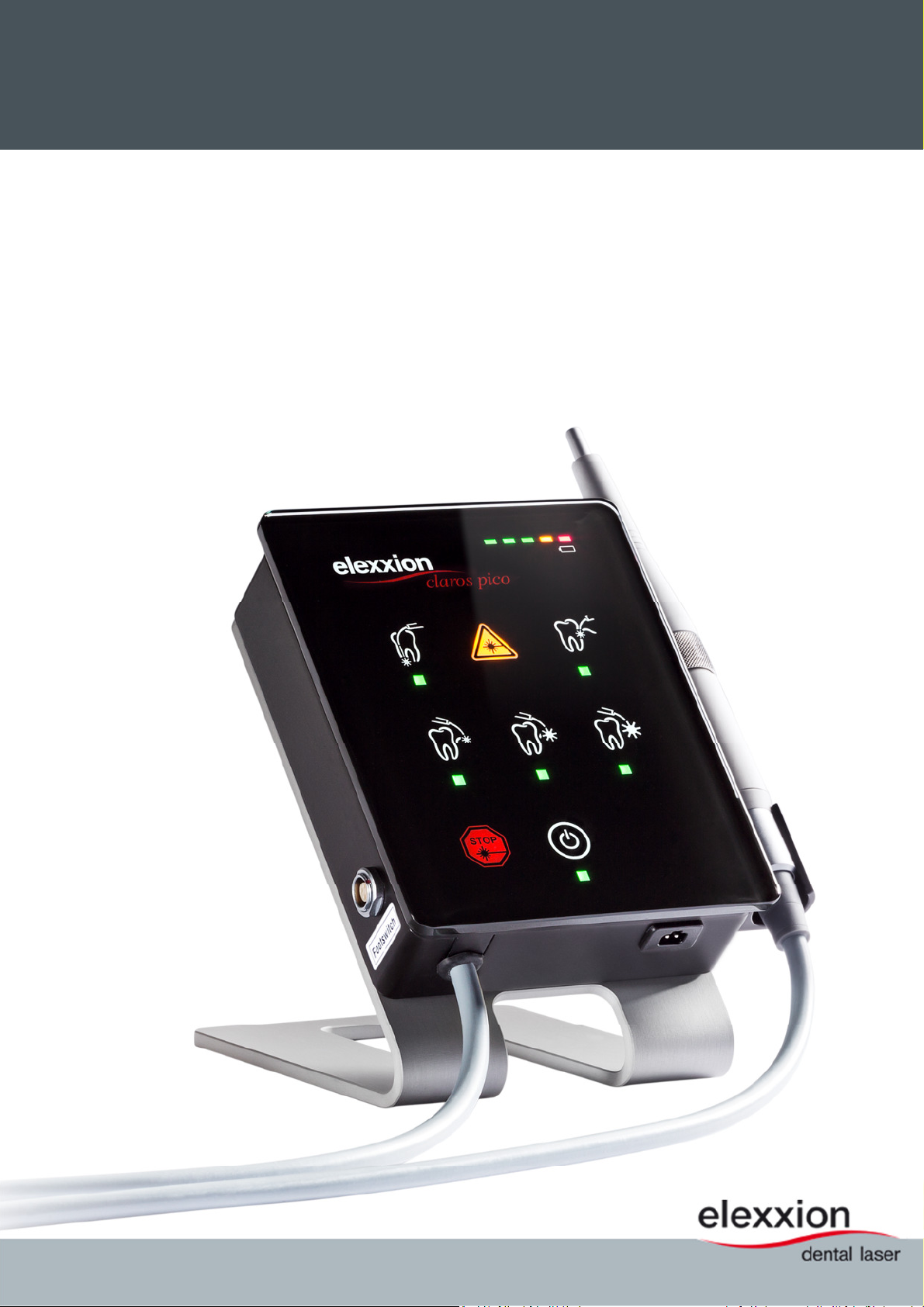
claros pico
user manual
claros pico
ENGLISH / V2.1 / December 2017
image subject to model
claros pico user manual V 2.1 page 1 of 34
Device / type: claros pico
Manufacturer: elexxion AG
Otto-Hahn-Str. 7
78224 Singen
Germany
Tel. 0049-(0)7731-90733-0
Fax 0049-(0)7731-90733-55
E-mail: info@elexxion.com
Serial no.: 35-xxxx
Version: V: 2.0
Release / date: V 2.1/ December 2017
0086
Forwarding and reproduction of this document and communication of its contents are not permitted
unless expressly authorised by the manufacturer. All rights and utility model protection reserved.
© elexxion AG
claros pico user manual V 2.1 page 2 of 34

Content
1
Labelling ........................................................................................................................................................5
1.1 Labels on the device ..............................................................................................................................5
1.2 Labels on accessories ............................................................................................................................7
2 Warning notices ............................................................................................................................................8
2.1 Warning notices – personal hazard ......................................................................................................8
2.2 Warning notices – system hazard .........................................................................................................9
2.3 Warning notices – additional information ............................................................................................9
3 Electromagnetic compatibility (EMC) ........................................................................................................ 10
3.1 General information ........................................................................................................................... 10
3.2 Installation and operation .................................................................................................................. 10
3.3 Guidelines and manufacturer declarations........................................................................................ 10
4 Intended use .............................................................................................................................................. 12
5 Protection and safety regulations .............................................................................................................. 13
5.1 Side-effects......................................................................................................................................... 13
5.2 Mutual interference risks ................................................................................................................... 14
5.3 Contraindications ............................................................................................................................... 15
5.4 Summary assessment of residual risks .............................................................................................. 15
5.5 Handling rechargeable batteries ........................................................................................................ 15
5.5.1 Replacing the battery ................................................................................................................. 15
5.5.2 Charging the battery .................................................................................................................. 15
5.5.3 Operating time ........................................................................................................................... 15
5.5.4 Disposal ...................................................................................................................................... 15
6 Operation ................................................................................................................................................... 16
6.1 Commissioning ................................................................................................................................... 16
6.2 Display and display elements ............................................................................................................. 17
6.3 Program selection and laser operation .............................................................................................. 18
6.3.1 Standby Mode ............................................................................................................................ 18
6.3.2 Program selection mode ............................................................................................................ 18
6.3.3 Laser warning mode ................................................................................................................... 18
6.3.4 Laser ready mode ....................................................................................................................... 18
6.4 Handling handpieces and fibers ......................................................................................................... 19
6.4.1 ergoflex plus ............................................................................................................................... 19
6.4.1.1 Introducing the application fibers .......................................................................................... 19
6.4.1.2 Adjusting the fiber length ...................................................................................................... 21
claros pico user manual V 2.1 page 3 of 34

6.4.2
ergo T ......................................................................................................................................... 21
7 Accessories ................................................................................................................................................. 22
8 Cleaning and sterilisation ........................................................................................................................... 23
8.1 Cleaning .............................................................................................................................................. 23
8.1.1 Packaging ................................................................................................................................... 24
8.2 Sterilisation ........................................................................................................................................ 24
9 Maintenance .............................................................................................................................................. 25
10 Service life .............................................................................................................................................. 25
11 Disposal .................................................................................................................................................. 26
11.1 Packaging ........................................................................................................................................... 26
11.2 Device ................................................................................................................................................. 26
12 Technical data ........................................................................................................................................ 26
12.1 Description of the beam guiding system: .......................................................................................... 26
12.2 Laser aperture: ................................................................................................................................... 26
12.3 Disconnection from the mains (charging mode): .............................................................................. 26
12.4 Technical data power supply: ............................................................................................................ 26
12.5 Technical data base unit: ................................................................................................................... 27
12.6 Accuracy of values displayed: ............................................................................................................ 27
13 Error messages ....................................................................................................................................... 28
14 Calibration .............................................................................................................................................. 28
15 Table of applications /setting ................................................................................................................. 29
16 Notes ...................................................................................................................................................... 34
claros pico user manual V 2.1 page 4 of 34
Symbol
Description
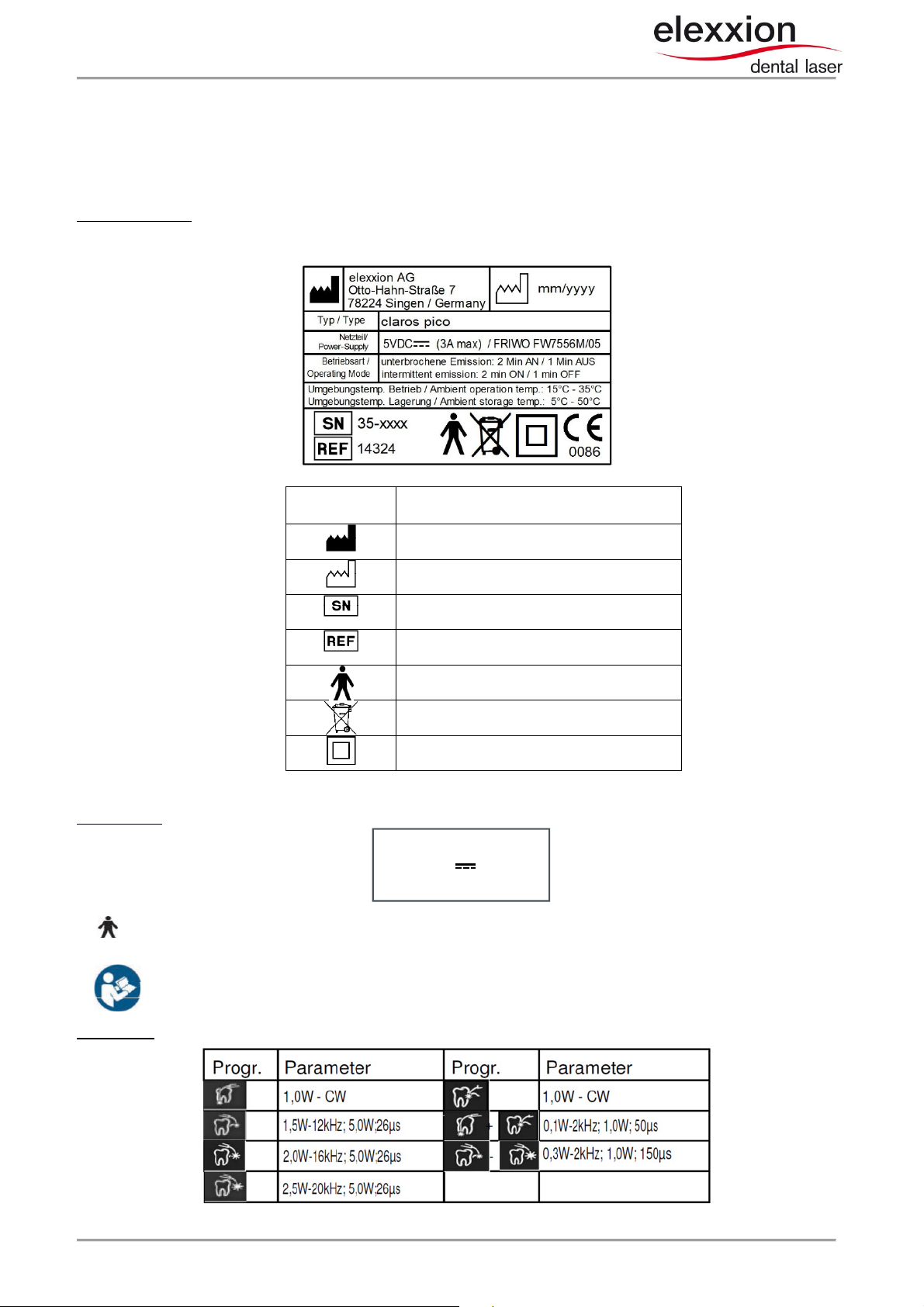
Manufacturer
Date of Manufacture
Serialnumber
Partnumber
Application Part Typ
e B
Do not dispose with household waste
Protection class II
-
Protectionisolation
1 Labelling
1.1 Labels on the device
Information plate: Foil label on bottom of device:
Power supply: Foil label on side of the unit:
On the transmission fiber, below application part type B
On side of casing: Please read operating instructions before use.
Applications: Foil label on the left side of the unit:
5 V DC 3A max
FRIWO W7556M/05
claros pico user manual V 2.1 page 5 of 34
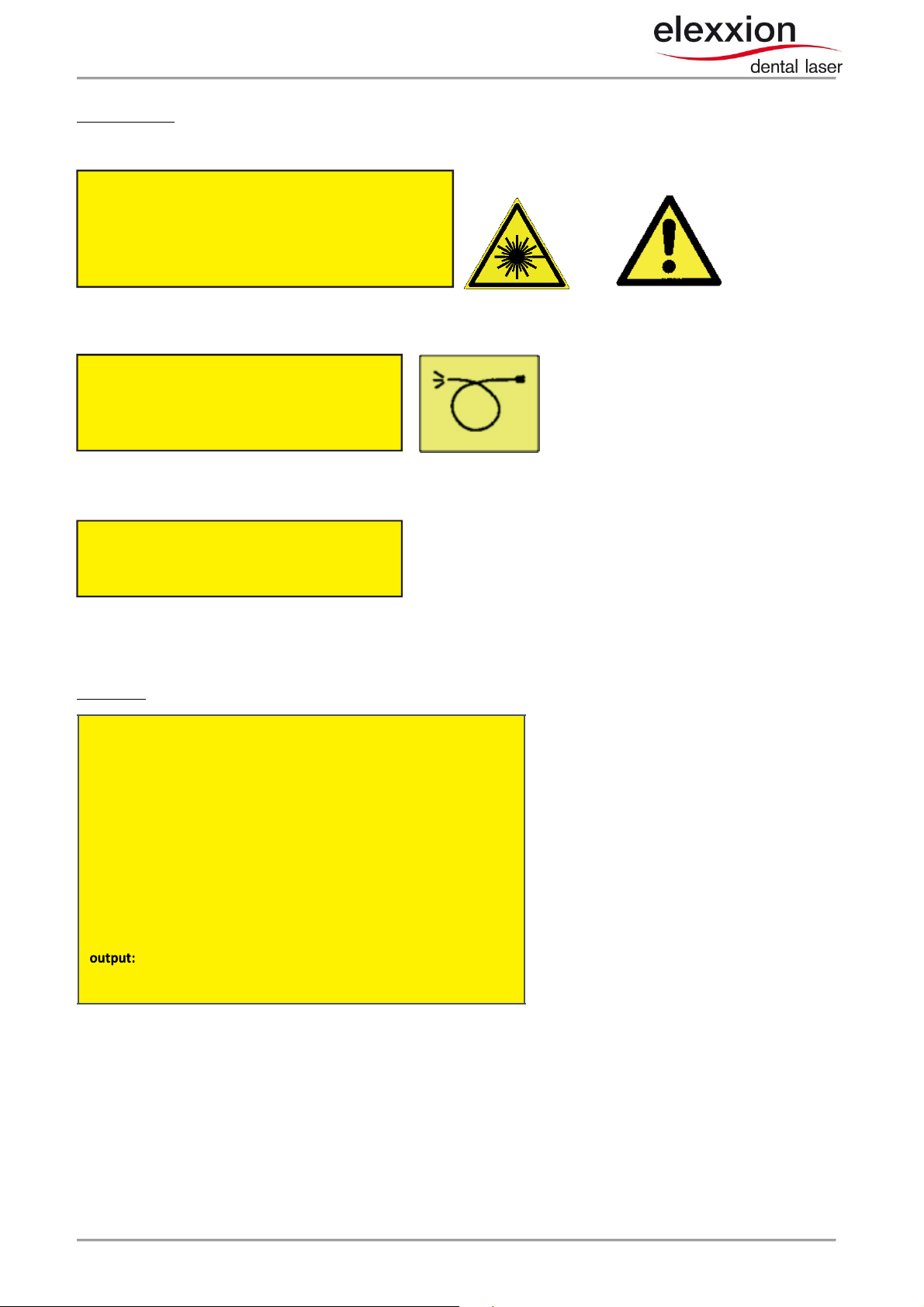
maximum output:
5 W
(peak) 1W (cw)
laser class
4 GaAlAs diode
pulse frequency:
cw –
20.000Hz
pulse duration
: 26µs/50µs/150µs
emitted wavelength:
808 nm
pilot laser:
650 nm
< 1mW
IEC 60825
-
1:2007
Warning labels:
Foil label, yellow/black, on back of device:
Visible and invisible laser
radiation
Avoid irradiating eyes or skin with direct or scattered
radiation
laser class 4
At fiber output on side of the unit:
Laser aperture! Please consult instructions for use!
Visible and invisible
laser radiation
emitted from this aperture
Pilot laser:
Laser radiation do not
stare into bam
laser class 2
(This label is not applied to the device due to lack of space)
Laser type: Foil label, yellow/black, on back of base unit:
claros pico user manual V 2.1 page 6 of 34
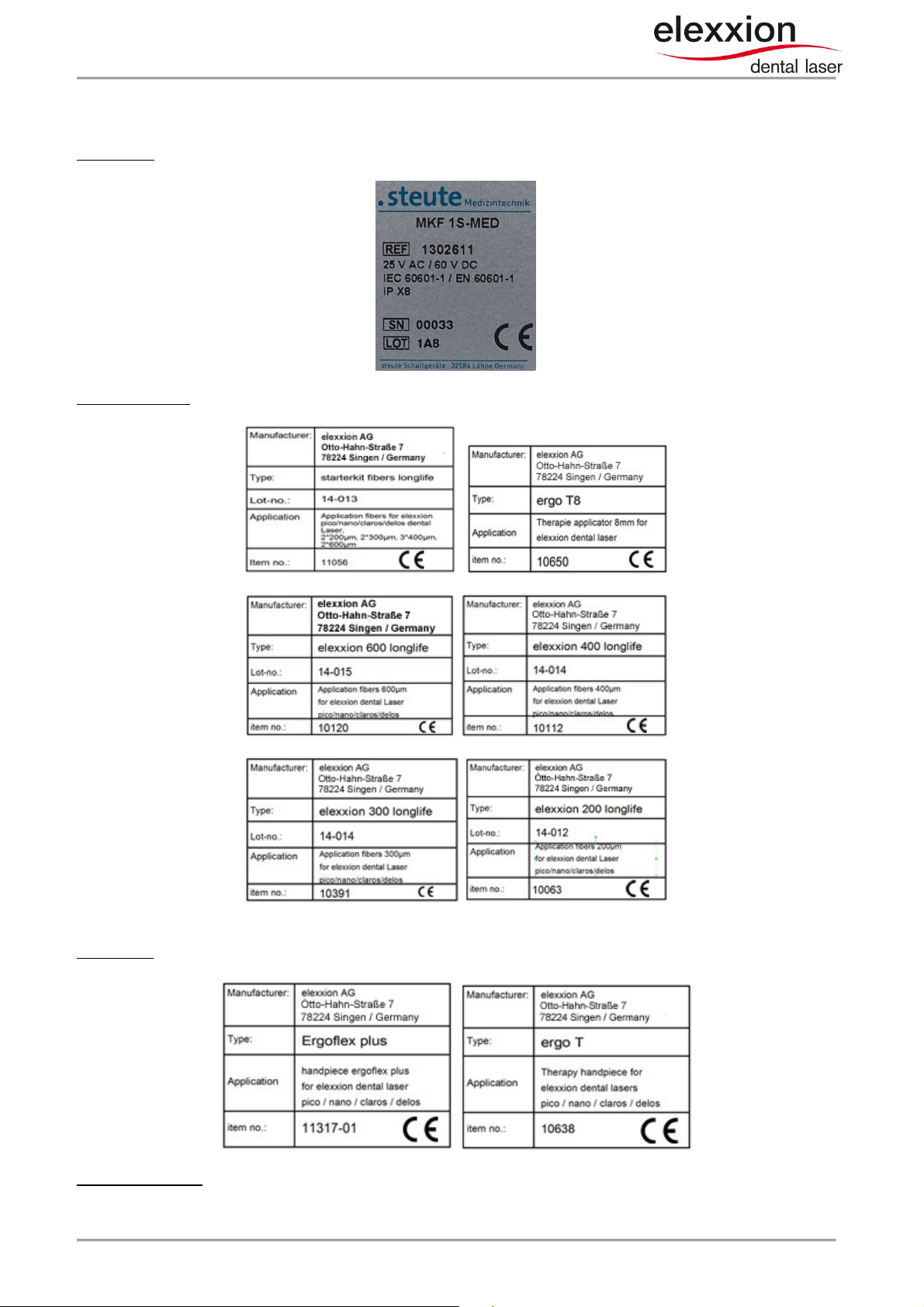
1.2 Labels on accessories
Foot-switch: Foil label on bottom of device
application-fibers: Foil label on bottom of storage-box:
Handpieces: Foil label on bottom of the storage box
Laser safety glasses: Labeling / Instructions from manufacturer
claros pico user manual V 2.1 page 7 of 34

2 Warning notices
2.1 Warning notices – personal hazard
If the following instructions are ignored or not followed correctly, this may result in endangerment of the patient,
operator or support staff.
1. By law, the device may only be sold to or on behalf of a dentist.
2. The energy emitted by the laser light exceeds the tolerance threshold of the eye and can therefore lead to
irreversible damage to the eye. The prescribed safety glasses must be worn by all persons in the treatment room in
order to prevent eye damage. The pilot laser (target beam) is automatically switched on when an application
program is started. Do not look into the beam.
3. Safety glasses with filter level 5 or higher at 808 nm bearing the CE marking in accordance with EN207:1998 must
be worn; these are available from elexxion AG with the name "claros protect". The separate instructions for use for
the laser safety glasses must be observed when using laser safety glasses.
4. The laser danger zone (referred to as the 'treatment room' in the following) is the entire area located within the
range of the laser beam. Warning! As reflections from instruments and equipment introduced into the beam path are
possible, the laser danger zone is only delimited by obstacles around the laser system that are not transparent with
regard to laser radiation (e.g. walls, ceiling, floor, closed doors) . An NOHD value (distance from which the laser can
be observed safely) is not given due to the high mobility of the laser aperture. The entire room in which the laser is
operated must be treated as the laser protection area.
5. The room in which the device is operated must be equipped in accordance with the BGV B2 accident prevention
regulations. The electrical installation must comply with DIN VDE 0100 Part 710. This is the responsibility of the
operator or a laser safety officer appointed by the operator. The BGV B2 accident prevention regulations can be
obtained from elexxion AG upon request.
6. The operator of the laser system must ensure that the treatment room is clearly marked and that nobody enters
the treatment room without safety glasses (see item 2) while the laser is being used.
7. Use is only permitted in rooms designed for medical purposes and which meet the above requirements. Use
anywhere else is not allowed.
8. Only accessories specified by elexxion AG may be used. A list of all accessories can be found in Section 6 of these
instructions for use. In case of doubt, please consult your medical product consultant.
9. Use of the operating equipment in any way other than that described here may result in dangerous irradiation.
10. The device may not be used in explosive atmospheres, irrespective of how these are created.
11. People must not look into the laser beam directly or through optical devices or instruments.
12. Please make sure that the position of the device during charging ensures that the mains adapter can be easily
unplugged from the power supply.
13. Fully disconnect the charger from the power supply when it is not in use for a long period of time.
14. If the emission switch is accidentally activated, an unprotected laser beam may be emitted from the fiber end
depending on the operating status. Please protect the emission switch from accidental activation.
15. Caution: laser smoke may contain viable tissue particles. Please use an extraction system.
16. Please check the condition of the applicator before use. If it is damaged, there is a risk that this may cause cuts –
do not use damaged applicators.
17. The use of flammable anaesthetic gases and oxidising gases such as nitrogen and oxygen must be avoided. Some
materials such as cotton wool that are saturated with oxygen can ignite at high temperatures, as can arise when the
device is used in accordance with its intended use. Solvents, e.g. as contained in adhesives and flammable solutions
that are used for cleaning or disinfection purposes, need time to evaporate before the laser device is operated.
18. Gases produced by the body can also be flammable!
19. Applicators and handpieces must be disinfected/sterilised using an autoclave or by spraying/wiping with
disinfectant before they are first brought into service and before and after each use.
20. It must be ensured that the disinfectant/cleaning agents used have a bactericidal (including TbB), fungicidal and
virucidal (including HBV) effect.
21. The exposure times for disinfectant/cleaning solutions given by the manufacturer must be observed.
claros pico user manual V 2.1 page 8 of 34

22. Servicing and maintenance must be performed by authorised specialist personnel only. A technical safety
inspection must be performed on the laser system at least once a year to maintain safe operation of the system and
to check its performance parameters. (see Section 7.3).
23. The device must be disconnected from the charger before cleaning/disinfecting.
24. If the system is damaged or there are signs that the system is not working properly, operation must be
suspended immediately and the manufacturer notified, as there may be the risk that laser radiation could be emitted
at unforeseeable parts of the device. The therapeutic effect is also no longer ensured.
25. The laser system must not be used to remove tooth hard tissue as this can lead to warming of the tooth tissue
and damage to the dental pulp.
26. When using the surgery programs, the following rule applies: "Start with the lowest power possible and then
increase it later if necessary".
2.2 Warning notices – system hazard
If the following instructions are ignored or not followed correctly, this may result in damage to the system. It may
not be possible to continue with ongoing treatment or this may only be possible with some delay.
27. Please ensure that the device is placed in a secure and stable position on the stand supplied.
28. If the device is brought from a cold environment into a warm environment, please wait a sufficient amount of
time (at least 30 minutes) until the device has reached the ambient temperature before switching it on.
29. Please stop using application fibers if there is less than 1 mm of fiber left (visual check before use).
30. Please handle applicators with care and do not squeeze, twist or apply a heavy load on them.
31. The flexibility of the fibers is limited. Pressing, bending, stretching or compressing the fibers too heavily can cause
them to break.
32. Applicators must not be replaced while the device is ready for use and a program activated, as the emission
switch may be activated by accident.
33. The battery may only be replaced by authorised elexxion AG service personnel. Replacing the battery yourself
may leave the device in a dangerous condition. Caution: risk of fire and explosion!
2.3 Warning notices – additional information
Refers to important and useful additional information. If this information is ignored, this may result in a device
malfunction such as reduced power output or complete loss of function.
34. Do not use pointed objects to operate the membrane keypad.
35. If the intensity of the pilot beam is visibly reduced, a reduction in power output is to be expected, which will
result in the loss of or a reduction in the therapeutic effect. elexxion AG's service centre should be contacted in this
event.
36. The claros pico system is equipped with high-precision optics in the handpiece. Foreign bodies such as dust and
moisture can therefore result in a reduction in power output (loss of or reduction in therapeutic effect). For this
reason, a protective cap or applicator must be placed on the laser aperture when cleaning the handpiece. We also
recommend using a protective cap or applicator when the laser is not in use in order to prevent penetration of dust.
37. The claros pico system complies with applicable electromagnetic compatibility regulations, both with regard to
background radiation interference and the emission of electromagnetic interference. However, it is strongly
recommended that no strong electromagnetic transmitters such as mobile phones, radio remote controls, etc. be
used in the vicinity of the laser system. If an electromagnetic effect / interference is suspected, the system may no
longer be operated until the cause is determined and remedied. Non-compliance may result in risks during use.
claros pico user manual V 2.1 page 9 of 34
3 Electromagnetic compatibility (EMC)
3.1 General information
claros pico is a class A device in accordance with CISPR 11 and is intended for use by medical specialists only. claros
pico is intended for use in settings other than residential settings; the typical electromagnetic environment is that of a
hospital, clinic or doctor's surgery.
3.2 Installation and operation
Electronic devices are sensitive to electrostatic discharge. In order to prevent malfunctions in the claros pico system,
electrostatic charges created by the operator should be prevented by means of ESD protective measures (use of antistatic materials).
In order to prevent disruptions due to electrostatic discharges, the floors should be made of wood or concrete or
covered with ceramic tiles. If the floor is covered with synthetic materials, the relative humidity must be no lower than
40%.
Operators should be familiar with the basic physical processes behind electrostatic charges and how to prevent them.
The claros pico system uses RF energy for its own operation only. The amount of radio frequency interference emitted
is therefore very low and is unlikely to disturb other devices being operated in the vicinity. Nevertheless, it should be
noted that simultaneous operation of the claros pico system together with other devices may result in interference in
the claros pico system or other devices. Care should therefore be taken to ensure that the claros pico system is not
positioned directly next to or above another electronic device.
If it is impossible to avoid positioning the claros pico system in the immediate vicinity of analogue medical
measurement devices, the user of these measurement devices must be made aware that device results should be
observed in order to monitor intended device use in the position selected.
3.3 Guidelines and manufacturer declarations
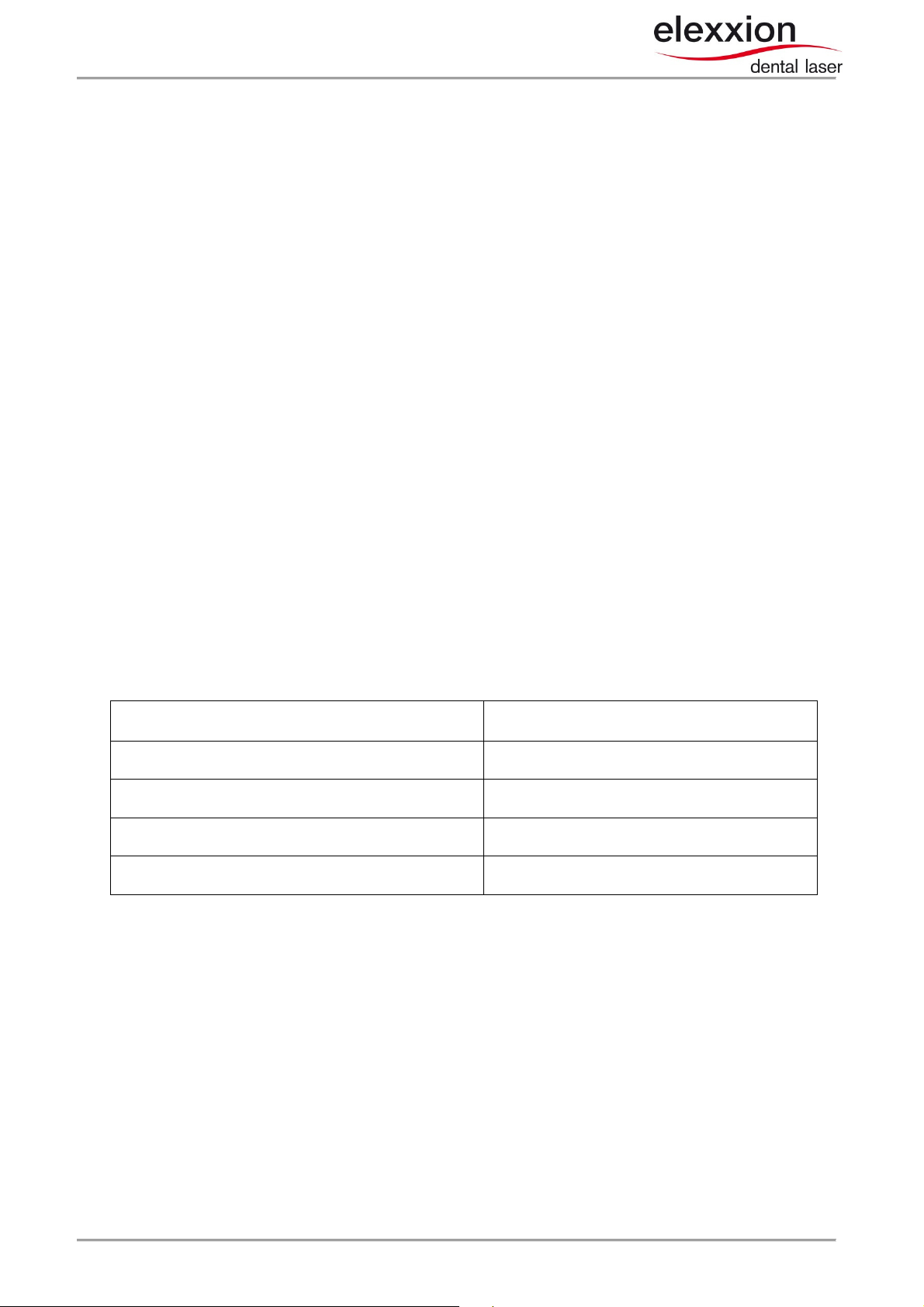
Table 1: Electromagnetic emissions
Emission measurements Compliance
Radiated RF emissions according to CISPR 11
Conducted RF emissions according to CISPR 11
Harmonics according to IEC 61000-3-2
Voltage flucuations / flicker according to IEC 61000-3-3
Group 1 class A
Group 1 class B
Not applicable
claros pico user manual V 2.1 page 10 of 34
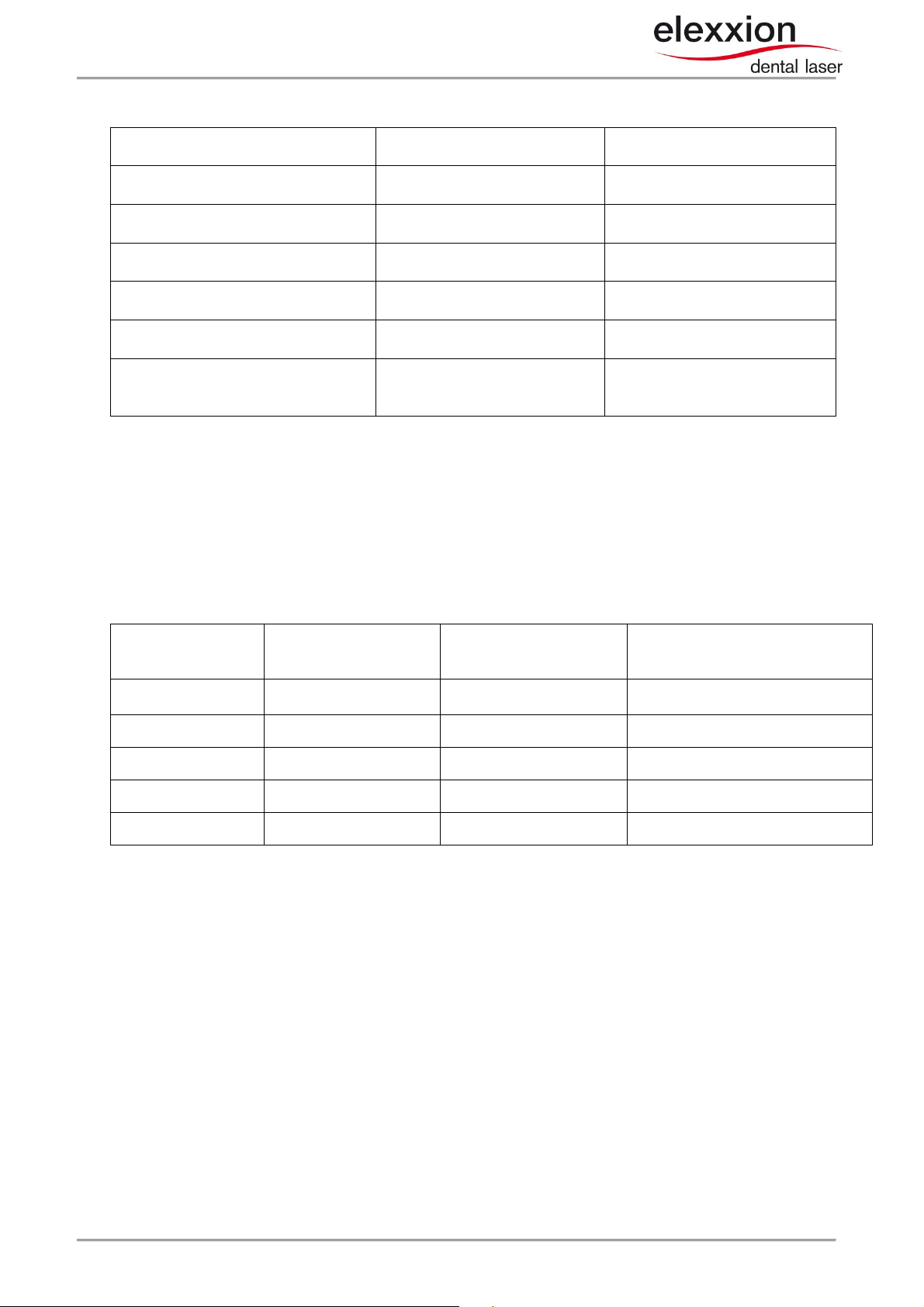
Table 2: electromagnetic immunity
Immunity tests IEC 60601 test level Compliance level
Electrostatic discharge (ESD)
according to IEC 61000-4-2
Radiated RF fields
according to IEC 61000-4-3
Electrical fast transient interference
(burst) according to IEC 61000-4-4
Conducted radio frequency according
to IEC 61000-4-6
Magnetic field at frequency of supply
voltage according to IEC 61000-4-8
Voltage dips an short interruptions
according to EN 61000-4-11
+/- 6 kV contact discharge
+/- 8 kV air discharge
80 MHz-2.5 GHz: 10V/m;
not life-supporting
Not applicable N/A
3V/m 3V/m
3A/m 3A/m
<5 % U / 10 msec
40 % U / 0.1 sec
70 % U / 0.5 sec
+/- 4 kV contact discharge
+/- 8 kV air discharge
80 MHz-2.5 GHz: 10V/m
<5 % U / 10 msec
40 % U / 0.1 sec
70 % U / 0.5 sec
Table 3: Recommended safety distances between portable and mobile telecommunication devices and the
claros pico system
The claros pico system is designed for use in an electromagnetic environment in which RF interference is controlled.
The user of the claros pico system can help to prevent electromagnetic interference by complying with the minimum
distance between portable and mobile RF telecommunication devices and the claros pico system, depending on the
power output. [distances given in meters]
Rated output of
transmitter [W]
0.01 0.01 0.01 0.02
0.1 0.03 0.03 0.06
2 0.14 0.14 0.3
10 0.32 0.32 0.64
100 1 1 2
This results in a safety distance of approximately 14 cm for mobile phones (the transmitted power of which is limited
to around 2 watts) in the D1 and D2 band and 0.3 m in the E band. (assumption: 10V/m compliance level; tested item
not life-supporting)
Safety distance
150 kHz – 80 MHz
Subject to
80 MHz – 800 MHz
Transmitted frequency
800 MHz – 2.5 GHz
claros pico user manual V 2.1 page 11 of 34
 Loading...
Loading...