Page 1

STROBE LIGHT KIT
MODEL AK-520
Assembly and Instruction Manual
Elenco®Electronics, Inc.
ight © 2005, 1994 b
yr
Cop
t of this book shall be reproduced b
No par
y Elenco
®
Electronics
y means;
y an
, Inc.
electronic
ights reser
All r
, photocopying, or otherwise without written permission from the publisher.
ved. Revised 2005 REV-L 753018
Page 2
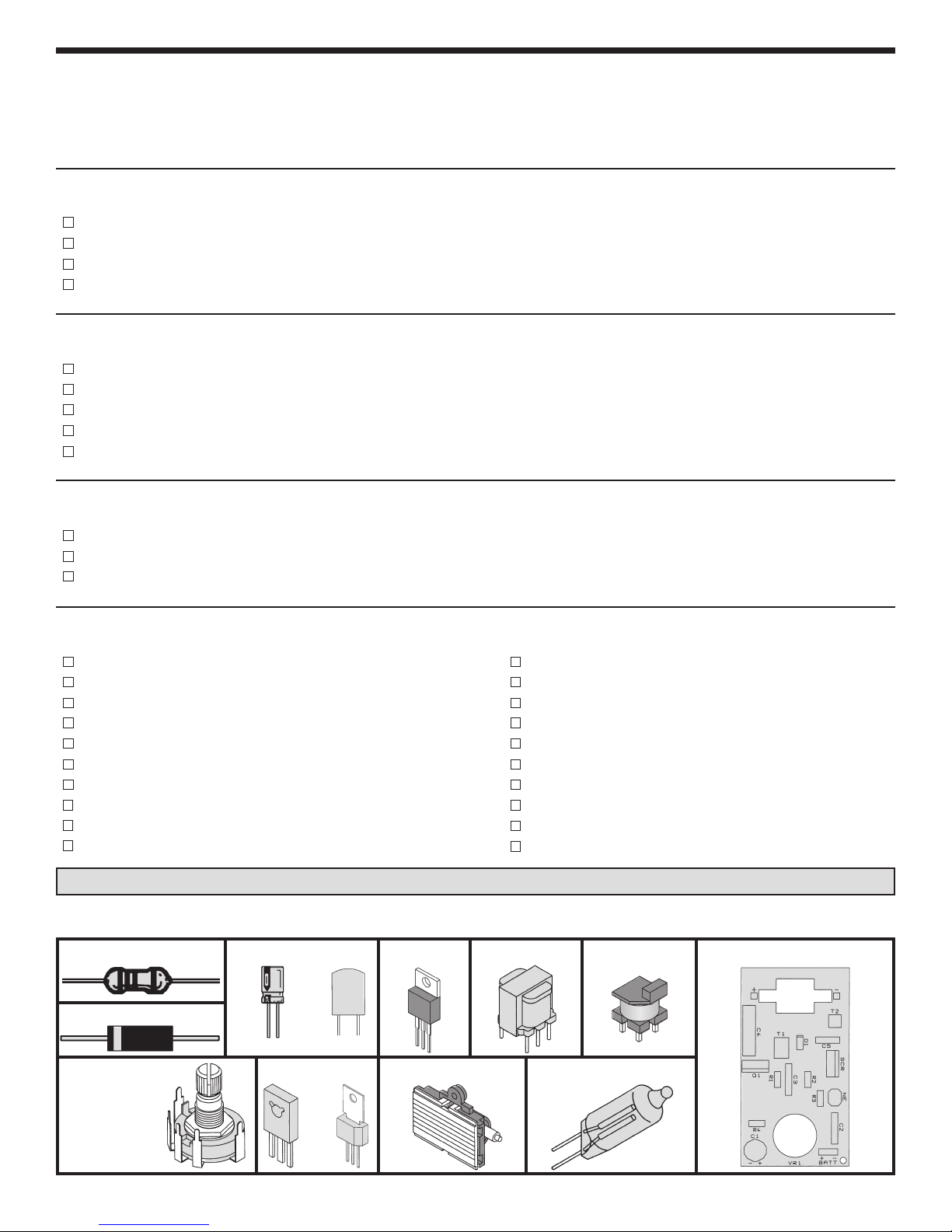
PARTS LIST
If you are a student, and any parts are missing or damaged, please see instructor or bookstore.
If you purchased this kit from a distributor, catalog, etc., please contact Elenco®Electronics (address/phone/email is at the back of this manual) for additional assistance, if needed. DO NOT contact your place of purchase
as they will not be able to help you.
RESISTORS
Qty. Symbol Value Color Code Part #
1 R1 200Ω 5% 1/4W red-black-brown-gold 132000
2 R2, R4 1MΩ 5% 1/4W brown-black-green-gold 171000
1 R3 2MΩ 5% 1/4W red-black-green-gold 172000
1 VR1 2MΩ Potentiometer w/ switch 192732
CAPACITORS
Qty. Symbol Description Part #
1 C5 .033µF 10% 250V Mylar (333) 243319
1 C3 .1µF 10% 100V Mylar (2A104K) 251017
1 C2 .1µF 10% 400V Mylar (2G104K) 25102A
1 C4 .47µF 10% 250V Mylar (474) 254717
1 C1 470µF 10V Electrolytic (Lytic) 284743
SEMICONDUCTORS
Qty. Symbol Description Part #
1 D2
1 Q1 2N6121 Transistor 326121
1 SCR T106D1 / C106D1 SCR 3606D1
1N4004 Diode 314004
MISCELLANEOUS
Qty. Description Part #
1 Trigger coil (T2) 440020
1 Transformer (T1) 440021
1 PC board 517050
1 Neon bulb (NEON) 585020
1 Flash tube 586001
1 Battery holder 590072
1 Bottom case 62PX2GB
1 Knob 622009
1 Top panel 623150
Washer nylon .219 x .1 624031
1
Caution:
Do not mix alkaline
standar
,
d (carbon-zinc), or rechargeable (nickel-cadmium) batteries.
PARTS IDENTIFICATION
Resistor
Diode
Potentiometer
with Switch
Capacitors
Electrolytic
Mylar
SCR Neon
Transistor
Flash Tube
Qty. Description Part #
1 Screw phil pan 2-56 x 7/16”
641260
4 Screw 4 x 1/2” phillips 642465
1 Screw 1.9 x 4mm phillips 643151
1 Nut hex 6mm 644010
1 Nut 2-56 644201
1 Washer flat 7mm 645015
1 Lockwasher #2 646200
1 Tape double-sided 740020
2” Wire 22ga. topcoat red 818120
Solder 9ST4
1
Transformer Trigger Coil
PC Board
or
-1-
Page 3

IDENTIFYING RESISTOR VALUES
Use the following information as a guide in properly identifying the value of resistors.
BAND 1
1st Digit
Color Digit
Black 0
Brown 1
Red 2
Orange 3
Yellow 4
Green 5
Blue 6
Violet 7
Gray 8
White 9
BAND 2
2nd Digit
Color Digit
Black 0
Brown 1
Red 2
Orange 3
Yellow 4
Green 5
Blue 6
Violet 7
Gray 8
White 9
2 Multiplier Tolerance
1
Multiplier
Color Multiplier
Black 1
Brown 10
Red 100
Orange 1,000
Yellow 10,000
Green 100,000
Blue 1,000,000
Silver 0.01
Gold 0.1
BANDS
Resistance
Tolerance
Color Tolerance
Silver +
Gold +5%
Brown +1%
Red +2%
Orange +3%
Green +
Blue +0.25%
Violet +0.1%
10%
0.5%
IDENTIFYING CAPACITOR VALUES
Capacitors will be identified by their capacitance value in pF (picofarads), nF (nanofarads), or µF (microfarads). Most
capacitors will have their actual value printed on them. Some capacitors may have their value printed in the following
manner. The maximum operating voltage may also be printed on the capacitor.
Multiplier
10µF 16V
For the No. 01234589
Multiply By 1 10 100 1k 10k 100k 0.01 0.1
Note: The letter “R” may be used at times
to signify a decimal point; as in 3R3 = 3.3
The letter M indicates a toler
The letter K indicates a tolerance of +
The letter J indicates a toler
103K
100V
First Digit
Second Digit
Multiplier
olerance
T
Maximum Working Voltage
ance of +20%
10%
ance of +5%
The value is 10 x 1,000 = 10,000pF or .01µF 100V
METRIC UNITS AND CONVERSIONS
viation Means Multiply Unit By Or
Abbre
p Pico .000000000001 10
n
µ micro .000001 10
m milli .001 10
– unit 1 10
k kilo 1,000 10
M
nano
mega
.000000001
1,000,000
10
10
-12
-9
-6
-3
0
3
6
1,000 pico units = 1 nano unit
1.
2. 1,000 nano units = 1 micro unit
3. 1,000 micro units= 1 milli unit
1,000 milli units = 1 unit
4.
5. 1,000 units = 1 kilo unit
6. 1,000 kilo units= 1 mega unit
-2-
Page 4
INTRODUCTION
Have you ever seen a lightning flash and wonder
h
ow the light was produced? This strobe light kit not
only explains how a high voltage discharge
produces light, but reproduces those bolts of
lightning in a small glass tube. Even more amazing
is the fact you will be able to control the moment
each flash occurs with a trigger circuit. Strobe lights
THEORY OF OPERATION
WHAT IS A GAS?
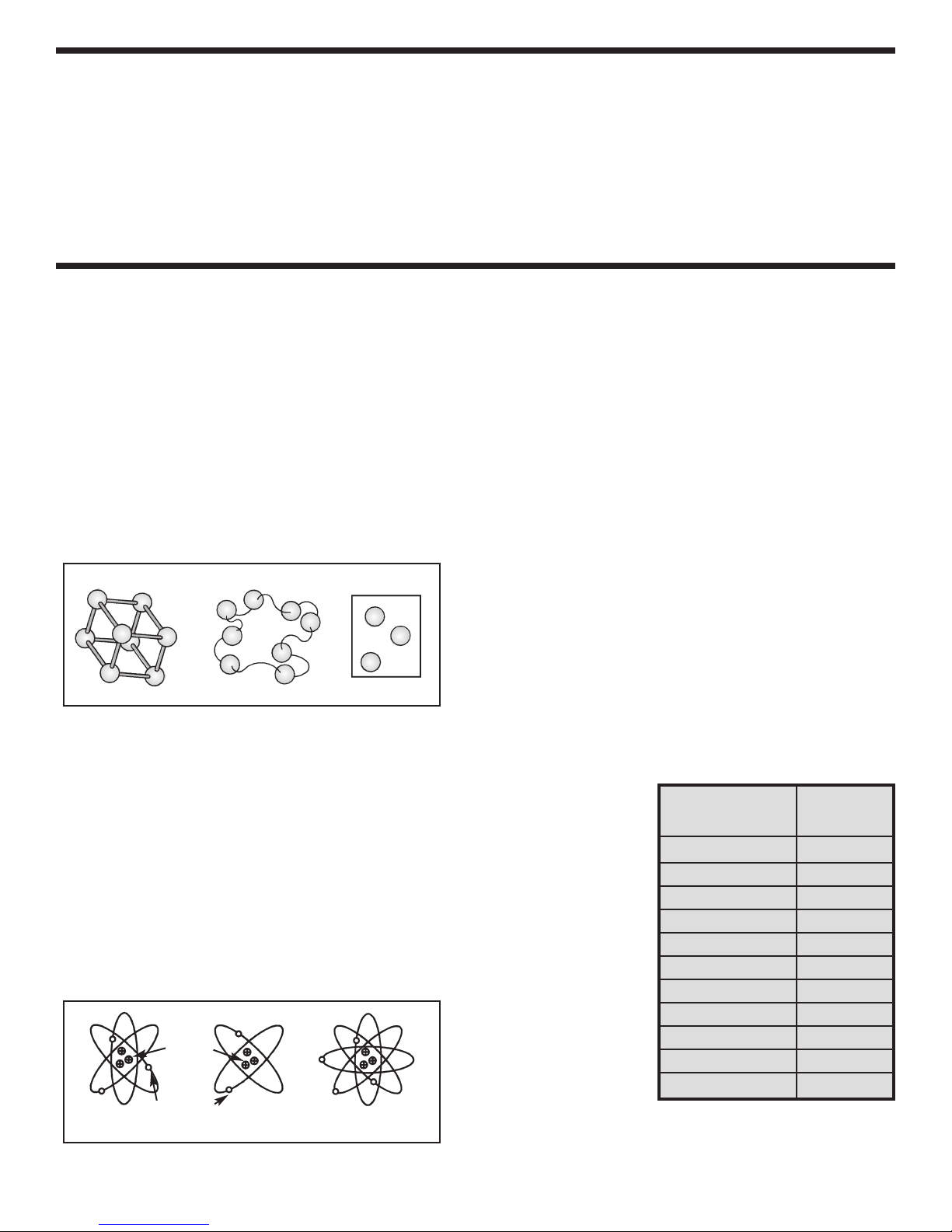
All matter is composed of atoms arranged in
patterns called molecules. In a solid, these
molecules are held in place and cannot move about
easily. In a liquid, the molecules move freely, but are
still loosely bound to each other. In a gas, the
molecules are separated by great distances and
bounce about like ping-pong balls in a large box.
The molecules of a gas are not bound to each other
and will dissipate into the surrounding space if
released from their container. These different states
of matter are shown in Figure 1.
Figure 1
Solid Liquid Gas
The glass tube in your strobe light kit is filled with a
rare gas called Xenon. This gas is used because it
is easy to ionize.
WHAT IS AN ION?
Gas atoms ha
their nor
mal state. There are just as many positively
charged protons as there are negatively charged
electrons. Therefore, the net charge on the atom is
zero. If, however, a negatively charged electron is
ed from one of the atoms
v
remo
a positive charge and it is called a positive ion. This
creation of ions is shown in Figure 2.
Normal Gas
Molecule
ve no electronic charge on them in
, the atom is left with
Figure 2
Protons
Electrons
Positive Ion Negative Ion
are used to stop motion by adjusting the trigger rate
t
o the speed of a moving object. They are also used
to produce light for photography at the moment the
camera shutter is opened. In the text that follows,
mechanical analogies are used to help explain
certain processes that are otherwise difficult to
visualize.
The amount of energy it takes to create an ion is
measured in electron volts. Table 1 shows the
energy needed to produce ions for different gases.
As you can see, Xenon requires much less energy
than Neon to produce ions. If the glass tube in your
kit contained Neon, the amount of energy needed to
ionize the gas would be 1.87 times greater. This
would shorten the life of the batteries by using
almost twice the energy for each flash. It is a law of
nature that opposite charges attract each other and
similar charges repel.
When a gas molecule is
turned into a positive ion, it is attracted to a negative
charge. The positive gas ion is placed in a strong
ic field, it will r
electr
apidly accelerate toward the
negative plate. As it moves, it will strike other gas
molecules, knocking electrons free and creating
more positiv
e ions. These ne
wly created ions will be
attracted by the negative plate, accelerate and
create even more positive ions (see Figure 3). The
avalanche process will contin
ue until all of the gas in
the tube is ionized allowing a large current to flow
through the tube and collapse the electric field. As
the electrons are knoc
ionization process
y release small
the
kets of energy
pac
,
called photons that
radiate from the
tube. The human
eye perceives this
burst of photons as
a brilliant flash of
light.
ked about during the
Gas Ionization
Energy
Helium
Neon
Nitrogen
Hydrogen
Argon
Carbon Mono
xide
Oxygen
ypton 13.3
Kr
ater Vapor 13.2
W
Xenon
Mercur
y 10.4
able 1
T
24.5
21.5
16.7
15.9
15.7
14.2
13.5
11.5
-3-
Page 5

Figure 3
Radiated Energy
Gas Molecule
Positive Ion
Negative Charge on Plate
GENERATING AN ELECTRIC FIELD
In order to ionize the Xenon gas in the glass tube,
the 3 volts DC at the battery must be transformed
into hundreds of volts DC. One of the electronic
devices used to “step up” voltages is called a
transformer. Transformers, however, only work with
AC voltages. You can think of a transformer as a
lever similar to the one shown in Figure 4. A small
movement on the short end of the lever will produce
a large swing on the other end. Since the lever does
not create energy, the power on one end must equal
the power on the other end. Therefore, the force
times the distance on the shor
t end must equal the
force times the distance on the other end (as shown
in Figure 4).
Figure 4
Force of 20 lbs.
2 Inches
2 In. x 20 lbs. = 20 in. x 2 lbs.
Short Side Long Side
Weight = 2 lbs.
Just like the lever, the transformer must have a
moving voltage (AC) to work. If the movement on
the short end of the le
ver equals z
movement on the long end will also be zero.
ewise, if DC is applied to one side of a
Lik
ansformer, the output on the other side will be
tr
zero. Since the transformer cannot create energy,
the power on one side must equal the power on the
other side. Electrical power is measured by
ultiplying the v
m
Figure 5 sho
oltage times the current (V x I).
ws the method used to transform the 3
volts from the battery to 200 volts needed for a
strong electric field.
20 Inches
ero, the
pendulum in a grandfather clock. Once the
pendulum is started in motion, it will use only a
small amount of energy from the main spring to
keep it swinging at the exact same frequency. It is
this stable frequency rate that sets the time
accurately. If the weight is moved up the stick, the
frequency increases. This is called tuning the
frequency of the pendulum. In electronics, an
oscillator circuit also has tunable elements. The
inductor in a tuned electrical circuit is equivalent to
the length of the pendulum (see Figure 6).
Figure 6
Length
Weight
C
Electronic
Tuned Circuit
in Oscillator
By changing the position of the iron core in the
inductor, the inductance can be changed to tune the
oscillator to a desired radio frequency, just like
changing the weight of the pendulum would change
its frequency.
C = Capacitance
L
L = Inductance
MAKING THE FIRST ION
When the electric field is placed across the Xenon
tube nothing happens because there are no ions in
the tube to start the avalache process. A second
ansformer is used to generate a very high voltage
tr
spike on a piece of wire placed along side the tube.
This transformer is called the trigger transformer
since it
“triggers” the a
valanche process by forcing a
few ions to be produced momentarily in the tube.
This process is shown in Figure 7.
Figure 7
Positive Ion
Wire with High
Voltage Spike
Electron F
High Voltage Spike
reed b
y
Tube Filled
with Xenon
Figure 5
3V Battery
3V x .3A = 300V x .003A
DC to AC
erter
v
Con
(Oscillator)
ransformer
T
Diode & Capacitor Converts AC to DC
An oscillator is an electronic circuit similar to the
300 Volts DC
-4-
Page 6

THEORY OF OPERATION
A block diagram is used to break down a system
into sub-systems that are easier to explain. All
strobe lights will have the blocks shown in Figure 8.
The power supply, Block 1, can be either an AC
(Alternating Current) or DC (Direct Current) source of
electrical power. When a low voltage DC source is
used, a battery for instance, the voltage must be
“stepped up” to the proper high voltage required to
Figure 8
1
POWER
SUPPLY
2
GENERATE
HIGH DC
VOLTAGE
BLOCK 1 - Since the power supply in this kit is a
battery, it is a DC source. The low DC voltage must
be con
flash tube.
BLOCK 2 - Figure 5 sho
voltage generator. In this kit, a transistor is used for
an oscillator (Q1 on schematic dr
belo
the secondary also steps up the voltage needed to
flash the xenon tube
verted to a high DC v
w).
Q1 driv
es the pr
.
oltage required b
ws a fundamental high
awing shown
imary of transf
ormer T1 and
y the
produce the avalanche process, as shown in Block 2.
After the high voltage is generated, a trigger pulse is
used to start the avalanche process (Block 3). Once
the gas in the flash tube (Block 4) is ionized, the
resistance of the tube drops and a large current flows
through the tube causing the high voltage to collapse.
The gas in the tube returns to its normal state (not
ionized) and the process starts over.
4
3
TRIGGER
FLASH
TUBE
CIRCUIT
BLOCK 3 - The trigger circuit uses a neon light to
fire an SCR (Silicon Controlled Rectifier). The SCR
acts lik
the primary of transformer T2. A high voltage spike
is produced on the secondary of T2. By using a
piece of wire
the glass tube containing the xenon gas.
BLOCK 4 tube filled with Xenon gas and sealed at each end
with a metal cap. Wires are connected to each of
the metal caps. When a high v
one cap and the other cap is grounded, a strong
electr
e a switch discharging capacitor C4 through
, this trigger v
The flash tube consists of a hollo
ic field will appear across the tube.
oltage is placed close to
w glass
oltage is placed on
SCHEMATIC DIAGRAM
-5-
Page 7

CONSTRUCTION
Introduction
The most important factor in assembling your AK-520 Strobe Light Kit is good soldering techniques. Using the
proper soldering iron is of prime importance. A small pencil type soldering iron of 25 - 40 watts is
recommended. The tip of the iron must be kept clean at all times and well tinned.
Safety Procedures
• Wear eye protection when soldering.
•
Locate soldering iron in an area where you do not have to go around it or reach over it.
• Do not hold solder in your mouth. Solder contains lead and is a toxic substance. Wash your hands
thoroughly after handling solder.
• Be sure that there is adequate ventilation present.
Assemble Components
In all of the following assembly steps, the components must be installed on the top side of the PC board unless
otherwise indicated. The top legend shows where each component goes. The leads pass through the
corresponding holes in the board and are soldered on the foil side.
Use only rosin core solder of 63/37 alloy.
DO NOT USE ACID CORE SOLDER!
What Good Soldering Looks Like
A good solder connection should be bright, shiny,
smooth, and uniformly flowed over all surfaces.
1. Solder all components from
the copper foil side only.
Push the soldering iron tip
against both the lead and
the circuit board foil.
2. Apply a small amount of
solder to the iron tip. This
allows the heat to leave the
iron and onto the foil.
Immediately apply solder to
the opposite side of the
connection, away from the
iron. Allow the heated
component and the circuit
foil to melt the solder.
3. Allow the solder to flow
around the connection.
Then, remove the solder
and the iron and let the
connection cool.
solder should have flowed
smoothly and not lump
around the wire lead.
4.
Here is what a good solder
connection looks like.
The
Component Lead
Foil
Solder
Foil
Solder
Foil
Soldering Iron
Circuit Board
Soldering Iron
Soldering Iron
Types of Poor Soldering Connections
1. Insufficient heat - the
solder will not flow onto the
lead as shown.
2. Insufficient solder - let the
solder flow over the
connection until it is
covered. Use just enough
solder to cover the
connection.
3. Excessive solder - could
make connections that you
did not intend to between
adjacent foil areas or
.
minals
ter
4. Solder bridges - occur
een
ing
ag y
uns betw
our solder
when solder r
circuit paths and creates a
short circuit. This is usually
caused by using too much
solder. To correct this,
simply dr
iron across the solder
bridge as shown.
Rosin
Soldering iron positioned
incorrectly.
Solder
Component Lead
Solder
Soldering Iron
Foil
Dr
Gap
ag
-6-
Page 8

ASSEMBLE COMPONENTS TO THE PC BOARD
Wire 1” - Cut a 1” wire and strip
both ends. Solder one end to
the PC board marked (–).
Wire 1” - Cut a 1” wire and strip
both ends. Solder one end to
the PC board marked (+).
C4 - .47µF (474) mylar cap.
T1 - Transformer
Q1 - Transistor 2N6121 (Fig. A)
R1 - 200Ω 5% 1/4W resistor
(red-black-brown-gold)
C3 - .1µF (2A104K) mylar cap.
R4 - 1MΩ 5% 1/4W resistor
(brown-black-green-gold)
C1 - 470µF lytic capacitor
(see Figure C)
T2 - Trigger transformer
D1 - 1N4004 diode
see Figure B)
(
C5 - .033µF (333) mylar cap.
CR - T106D1/C106D1 SCR
S
(see Figure D)
R2 - 1MΩ 5% 1/4W resistor
(brown-black-green-gold)
R3 - 2MΩ 5% 1/4W resistor
(red-black-green-gold)
NE - Neon lamp
C2 - .1µF (2G104K) mylar cap.
VR1 - 2MΩ potentiometer
(see Figure E)
Figure A
Bend the leads of the transistor in the
direction shown below.
Mount the transistor onto the PC
board in the direction shown below.
3/16”
Bend
leads 90
O
Diodes have polarity. Mount them
with the band in the correct direction,
as shown on the top legend.
Band
Figure B
Epoxy
PC board
marking
Figure E
Cut off the tab on the potentiometer as shown. Install the potentiometer so that the
lack section is even with the PC board as shown.
b
Cut off tab
otentiometer
P
k
Blac
section of
potentio-
meter
Figure C
Electrolytics have a polarity
marking indicating the (–) lead.
The PC board is marked to
show the lead position.
Polarity
marking
(–)
(+)
Figure D
Mount the SCR in the same direction as marked on the PC
board.
Metal
backing
With beveled edge
PC Board
-7-
Page 9

FINAL ASSEMBLY
Flash tube (See Figure 9) -
1
Attach the flash tube to the PC board with the 1.9 x 4mm screw
as shown. Cut and solder two 1” wires from the (+) and (–) points
n the PC board to the flash tube. Then solder the yellow wire
o
from the flash tube to the transformer T2 as shown.
Legend side of
PC board
3
Assemble the PC
board to the front
panel as shown in
Figure 11.
Flash tube
Figure 9
2-56 Nut
#2 Lockwasher
Yellow wire
1” Wires
Case
2
Battery Holder (see Figure 10) - Pass the
attery holder wires through hole in the PC
b
board from the foil side. Solder the red wire
from the battery holder to the (+) point on the
PC board and the black wire to the (–) point.
Black (–)
Red (+)
Figure 10
Double-sided tape
4
Remove the
backing on both
sides of the
double-sided tape
and apply it to the
back of the battery
holder. Now,
place the battery
holder inside of
the case as shown
in Figure 12.
Insert three “AA”
size (alkaline only)
batteries into the
battery holder.
Note: Be sure that
you place the
battery holder at
the bottom of the
back cover as
shown in Figure 12.
Also, make sure
that the three
positive (+) battery terminals are
pushed up against
the battery holder
contacts.
Washer
Front panel
2-56 x 7/16” Screw
Figure 11
Battery holder
7mm Flat washer
ut
6mm He
x n
To p
Figure 12
-8-
Page 10

FINAL ASSEMBLY (continued)
lace the front panel onto the case and secure it with four 4 x
P
1/2” screws (see Figure 13).
4 x 1/2” Screws
nob - Turn the shaft on the pot counter-
K
clockwise all of the way. Install the knob with
the line pointing in the direction as shown in
Figure 14.
Figure 13
Figure 14
CAUTION: High voltage present on the PC board. DO NOT handle it while in operation!
OPERATION
1. Turn the unit on by adjusting the knob clockwise. The unit should flash once per second (1Hz) at the lowest
setting. Increase the flash rate up to four times per second (4Hz) by turning the knob clockwise. Note that
the flash ma
The maximum flash rate can be adjusted to approximately 4 times per second.
y be err
atic when set to the maxim
um position.
-9-
Page 11

TROUBLESHOOTING
Consult your instructor or contact Elenco®Electronics if you have any problems. DO NOT contact your place of
purchase as they will not be able to help you.
1. One of the most frequently occurring problems is poor solder connections. Tug slightly on all of the parts to
make sure that they are indeed soldered.
2. All solder connections should be shiny. Resolder any that are not.
3. Solder should flow into a smooth puddle rather than a round ball. Resolder any connection that has formed
into a ball.
4. Have any solder bridges formed? A solder bridge may occur if you accidentally touch an adjacent foil by
using too much solder or by dragging the soldering iron across adjacent foils. Break the bridge with your
soldering iron.
5. Check the battery voltage with a voltmeter (4.5VDC).
6. Check the voltage across C4 for 250 - 350V. If less, then check the battery, R1, C1, C2, C3, Q1, D1 and/or
T1. If greater, then check R2 - R4, VR1, Neon, SCR, C5, T2 and/or the flash tube.
7. If the Neon flashes but the strobe light doesn’t, then check the SCR, C6, T2, battery voltage and/or the flash
tube.
GLOSSARY
AC Voltage
A voltage that var
Atom The smallest par
Avalanche
An increase in mo
Electric Field The force that exists when a difference in charge occurs.
Electron A tiny negatively charged particle that rotates around the nucleus of an atom.
Electron-volts A unit of energy equal to 1.602 x 10
Energy Effective force. The capacity for doing work.
Force The cause that changes bodies from a state of rest to motion or from motion to rest.
Gas An air-like substance without definite shape or volume, tending to expand indefinitely when
unconfined. One of the three forms in which matter can exist.
Ion An electrically charged particle that enables the flow of electricity.
Liquid One of the three forms in which matter can exist separately and still maintain the character of
that substance.
Neon A gaseous element, inert, colorless, and found in the atomosphere.
Photons A unit of light measurement.
Power The mechanical rate at which energy is exerted or work done.
ies, usually above and below zero volts, thus causing the current to alternate.
t into which matter can be divided and still maintain its identity.
ving particles due to sudden impact.
-19
joules.
Proton The smallest unit of positive charge in an atom.
Solid One of three forms in which matter can exist, having a definite volume and a definite shape.
Transformer A device used for converting an alternating electric current from one voltage to another.
Xenon A gaseous element which belongs to the group of inert gases. It occurs in air in minute traces.
-10-
Page 12

QUIZ
1. All matter is composed of atoms arranged in patterns called _______________.
2. In their normal state, the net charge on a molecule of gas is _______________.
3. When a molecule of gas is positively charged it is called a _______________.
4. Xenon requires less _______________ than Neon to produce ions.
5. A positive ion will accelerate toward a _______________ charged plate.
6. During the ionization and avalanche process, small packets of energy called _______________ radiate from
the glass tube.
7. The _______________ is used to step-up an AC voltage.
8. Electrical power is measured by multiplying _______________ times _______________.
9. The _______________ transformer is used to produce the first ions.
10. A negative ion has on more _______________ than it has protons.
Elenco®Electronics, Inc.
150 Carpenter Avenue
Wheeling, IL 60090
(847) 541-3800
Web site: www.elenco.com
e-mail: elenco@elenco.com
Technical Assistance Hotline: (800) 533-2441
8. Voltage, Current; 9.Trigger; 10. Electron.
Answers: 1. Molecules; 2. Zero; 3. Positive Ion; 4. Energy; 5. Negative; 6. Photons; 7. Transformer;
 Loading...
Loading...