Elamed ALMAG-01 Operation Manual

ALMAG-01
Operating Manual Page 1
CONTENTS
1. PURPOSE OF THE DEVICE ................................................................................................................................... 3
1.1 GENERAL INFORMATION ............................................................................................................................ 3
1.2 INDICATIONS FOR USE ................................................................................................................................ 3
1.3. CONTRAINDICATIONS ................................................................................................................................ 4
2. SPECIFICATIONS ................................................................................................................................................. 4
3. SET OF SUPPLY ................................................................................................................................................... 6
4. OPERATING PRINCIPLE OF THE DEVICE .............................................................................................................. 6
4.1 PHYSIOLOGICAL EFFECT OF PULSED ELECTROMAGNETIC FIELD ON THE HUMAN BODY ............................ 6
4.2 DEVICE DESCRIPTION .................................................................................................................................. 8
4.3 MARKING .................................................................................................................................................. 10
5. SAFETY INSTRUCTIONS .................................................................................................................................... 11
6. PREPARATION FOR USE ................................................................................................................................... 11
7. TREATMENT PROCEDURES............................................................................................................................... 13
7.1. MUSCULOSKELETAL SYSTEM DISEASES .................................................................................................... 13
Osteochondrosis ........................................................................................................................................ 13
Deforming osteoarthritis ........................................................................................................................... 16
Humeroscapular periarthrosis ................................................................................................................... 17
Arthritis ...................................................................................................................................................... 19
Epicondylitis ............................................................................................................................................... 20
Gout ........................................................................................................................................................... 22
Bursitis ....................................................................................................................................................... 23
Myositis ..................................................................................................................................................... 24
Tenosynovitis ............................................................................................................................................. 25
7.2. INJURIES AND THEIR AFTER-EFFECTS ....................................................................................................... 26
Bone fractures ........................................................................................................................................... 26
Internal joint injury .................................................................................................................................... 27
Soft tissue bruises, hematoma, posttraumatic edema .............................................................................. 27
Ligament and muscle injuries .................................................................................................................... 28
Postoperative wounds ............................................................................................................................... 29
Sluggish purulent wounds, phlegmons, burns ........................................................................................... 29
7.3. DISEASES OF PERIPHERAL NERVOUS SYSTEM .......................................................................................... 30
Neuritis ...................................................................................................................................................... 30
Facial nerve neuritis ................................................................................................................................... 30
Radial nerve neuritis .................................................................................................................................. 31
Ulnar nerve neuritis ................................................................................................................................... 32

ALMAG-01
Operating Manual Page 2
Median nerve neuritis ................................................................................................................................ 32
Iciatic nerve (ischias) neuritis ..................................................................................................................... 32
Peroneal nerve neuritis .............................................................................................................................. 33
Plexitis ........................................................................................................................................................ 33
7.4. NEURALGIA .............................................................................................................................................. 34
Trigeminal neuralgia .................................................................................................................................. 34
Occipital neuralgia ..................................................................................................................................... 35
Intercostal neuralgia .................................................................................................................................. 35
7.5. TRAUMAS OF CENTRAL NERVOUS SYSTEM .............................................................................................. 36
Vertebral column and spinal cord traumas ................................................................................................ 36
Disorders of the spinal blood circulation ................................................................................................... 36
7.6. PANCREATIC DIABETES COMPLICATIONS ................................................................................................. 37
Diabetic angiopathy ................................................................................................................................... 37
Diabetic polyneuropathy ........................................................................................................................... 38
7.7. DISEASES OF THE VENOUS SYSTEM OF THE UPPER AND LOWER LIMBS .................................................. 39
Deep vein thrombosis of the lower leg ...................................................................................................... 39
Chronic thrombophlebitis at a stage of trophic disorders.......................................................................... 40
Varicose veins ............................................................................................................................................ 41
8. MAINTENANCE ................................................................................................................................................ 42
9. STORAGE AND TRANSPORTATION ................................................................................................................... 42
10. ACCEPTANCE CERTIFICATE ............................................................................................................................. 43
11. MANUFACTURER'S WARRANTY ..................................................................................................................... 44
Dear Customer!
You have purchased ALMAG-01 Pulsed Electromagnetic Field (PEMF) Therapy Device
(hereinafter referred to as ALMAG) intended for hospital use for treatment and prevention of a
wide range of diseases, as well as for home use by patients upon doctor`s advice. In order to
perform treatment, please follow the guidelines of this Manual.
ALMAG has been included in the List of physiotherapy devices approved for medical
application by the Committee on New Medical Technology under the Ministry of Health of the
Russian Federation (Committee Minutes No. 7 dd. August 9, 1999; Registration Certificate No. FSR
2007/00136 dd. December 14, 2009).
ATTENTION! Prior to first usage of the device, please study this Operating Manual carefully,
and follow its instructions during further use to ensure proper treatment procedure and
effectiveness. When handing the device over to another user, please make sure to hand in the
Manual as well.
This Operating Manual serves as the Manufacturer’s guarantee of the basic parameters and
technical features of the ALMAG device.

ALMAG-01
Operating Manual Page 3
1. PURPOSE OF THE DEVICE
1.1 GENERAL INFORMATION
1.1.1 ALMAG has been designed for therapeutic treatment of the human body by
means of pulsed electromagnetic field, to be performed either by medical staff at
physiotherapy departments of healthcare facilities, or by individual patients at home.
1.1.2 ALMAG operates under normal ambient conditions for products designed for
“moderately cold” climate category: ambient temperatures between +10°C and +35°C,
air pressure from 86.6 kPa to 106.7 kPa (650 - 800 mmHg).
1.1.3 Electrical safety class for the device: Class II Type B according to IEC 60601-1.
1.2 INDICATIONS FOR USE
Musculoskeletal system diseases:
- osteochondrosis
- deforming osteoarthritis
- humeroscapular periarthrosis
- arthritis
- epicondylitis
- gout
- bursitis
- myositis
- tenosynovitis
Injuries and their after-effects:
- bone fractures
- internal joint injuries
- posttraumatic joint contracture
- wounds
- soft tissue bruises
- hematoma
- posttraumatic edema
- ligament and muscle injuries
- postoperative wounds
- keloid scar

ALMAG-01
Operating Manual Page 4
- sluggish purulent wounds, phlegmons, burns
Diseases of peripheral nervous system:
- neuritis
- facial nerve neuritis
- radial nerve neuritis
- ulnar nerve neuritis
- median nerve neuritis
- sciatic nerve (ischias) neuritis
- peroneal nerve neuritis
- plexitis
- neuralgia
- trigeminal neuralgia
- occipital neuralgia
- intercostal neuralgia
Traumas of central nervous system:
- vertebral column and spinal cord traumas
- disorders of the spinal blood circulation
Pancreatic diabetes complications:
- diabetic angiopathy
- diabetic polyneuropathy
Diseases of venous system:
- deep vein thrombosis of the lower leg
- chronic thrombophlebitis
- varicose veins
1.3. CONTRAINDICATIONS
- pyoinflammatory diseases in the acute phase
- pregnancy
- systemic blood diseases
- oncological diseases
- thyrotoxicosis
- alcohol intoxication
- presence of an implanted pacemaker in the treated area

ALMAG-01
Operating Manual Page 5
Inclusions of metal elements in bone tissues are not a contraindication for
therapeutic usage of the device.
2. SPECIFICATIONS
2.1 AC power supply:
~230V (-34,5V; +23V), frequency 50Hz;
~120V (-10V; +6V), frequency 60Hz.
2.2 Power consumption: ≤ 35 VA.
2.3 Weight: max. 0.62 kg.
2.4 Overall dimensions:
Power supply unit - 137x60x45 mm;
Emitter (single piece) - Ø 90mm, 15mm thickness.
Note: max deviation: ±3%.
2.5 The number of emitters - 4.
2.6 Amplitude value of magnetic induction on an emitter’s surface (both flat sides) (20±6) mT.
2.7 Pulse duration: 1.5-2.5 ms.
Magnetic field frequency for each emitter: 6,25 Hz.
2.8 The device has LED indicators that light up when it is connected to the power line
and PEMF is generated.
2.9 The device operates in the following mode within 6 hours: operation period of 22
min for 50Hz versions of the device (or 18 min for 60Hz versions) followed by a 10 min
break.
2.10 The device automatically shuts down:
- after (22±1) min of operation for 230V versions of the device;
- or after (18±1) min of operation for 120V versions of the device.
2.11 The surfaces of the device can be safely disinfected with any solution approved
for disinfection of plastic objects in medical institutions.
2.12 Mean lifetime – 10 (ten) years.
2.13 The device is made of hypoallergenic materials and may be used by hypersensitive patients.
2.14 Maximum temperature after one operation cycle:
- Control unit, max: +45°C;

ALMAG-01
Operating Manual Page 6
- Emitter, max: +41°C
2.15 Class of the device according to MDD 93/42/EEC – Class IIa.
3. SET OF SUPPLY
The complete delivery set includes:
- "ALMAG-01" device;
- Pulsed electromagnetic field (PEMF) indicator;
- Operating Manual.
4. OPERATING PRINCIPLE OF THE DEVICE
4.1 PHYSIOLOGICAL EFFECT OF PULSED ELECTROMAGNETIC FIELD ON THE HUMAN
BODY
According to various scientific data, the therapeutic effect of magnetic fields involves
their ability to control the flow of charged particles and to act on magnetised objects
regardless of their motion state. This results in a positive effect on natural biological
processes as the intracellular and intercellular metabolism intensifies. Magnetic field
therapy thus activates the body`s self- restorative function in the healing process by
direct stimulation - without surgical intervention, drugs, or side effects. Among various
types of magnetic fields, the strongest medical effect is demonstrated by the travelling
pulsed electromagnetic field (that of the ALMAG device), as compared to static or
alternating magnetic fields. This effect is achieved because ALMAG’s PEMF frequency is
within the biological frequencies range of the human organism (4 - 16 Hz).
The unique design of ALMAG’s induction coils (i.e. emitters) ensures PEMF
penetration of up to 8 cm deep into the patient’s tissues, which is successfully applied for
treatment of internal organs diseases. The regular rhythmical travelling PEMF emitted by
the device produces a healing effect on the cells of diseased organs and stimulates
recovery.
The magnetic field also improves the blood flow in the exposed area, thus reducing
blood viscosity and, consequently, the risk of blood clot formation (thrombosis). The
blood vessels are widened, additional capillaries are opened, and their permeability
enhances. All of the above boosts blood circulation in the affected area, supplies the cells
with extra oxygen, and stimulates formation of building and protective proteins in the
cells, whilst removing the inflammation products out of them. This activation of

ALMAG-01
Operating Manual Page 7
metabolism prevents disease progression and accelerates regeneration processes and
recovery of the diseased cells.
Application of low-frequency PEMF as a treatment procedure has a number of
beneficial effects on a patient’s body:
- a sedative effect caused by stimulation of nervous inhibition processes, which
results in emotional stress relief and sleep normalisation;
- cerebral vascular tone release, improvement of cerebral blood circulation, activation
of metabolism processes that increase resistance to cerebral hypoxia;
- lowering of systolic and diastolic pressure down to normal values;
- an analgesic effect created by reduction in the sensitivity of peripheral nervous
receptors;
- improved functionality of the endocrine system due to stabilised production and
release of the respective hormones into blood;
- faster reduction of swelling and dissolution of medications as a result of increased
permeability of blood vessels and epithelial tissue;
- suppression of pathologic processes in the liver, heart and other organs through
enhanced metabolism;
- increased resistance to unfavourable conditions.
Generally, a low-frequency pulsed electromagnetic field produces analgesic, antiinflammatory and anti-edematous effects and stimulates metabolic process. The key
organs of the immune system (thymus gland, spleen, lymph nodes, etc.) are especially
PEMF-responsive, which is confirmed by an increase in leukocyte count (the number of
white blood cells) during the treatment course. In addition to that, exposure to PEMF
affects the biologically active spots all over the human body, causing reflex responses in
the corresponding muscles and inner organs. Continued treatment by ALMAG results in a
smooth build-up of the patient’s adaptation level (the ability of a human organism to
withstand unfavourable environmental load combined with self-healing ability), which is
a valuable support in the treatment of both acute and chronic diseases.

ALMAG-01
Operating Manual Page 8
4.2 DEVICE DESCRIPTION
ALMAG consists of:
- a control unit (pulse generator)
connected to four emitters;
- emitters connection cable;
(2.1±0.1)m
- power cable: (1.2±0.1)m.
All connections of the individual units
are flexible and non-detachable.
Note: the first emitter is the only one
connected to the control unit cable.
The control unit box is made of high
impact polystyrene.
There are two indicator lights (LEDs)
of different colours on the control unit
box. The green light indicates that the
device is connected to the power line.
The yellow LED indicator lights up together with the green one and signals that the
magnetic field is in action. The yellow LED is connected to a timer and turns off 22
minutes after start of operation for 50Hz versions of the device (or 18 min after for 60Hz
versions), thus indicating deactivation of the magnetic field.
Note: For further use, please unplug the device from the power line and then switch it
on again (but at least 10 minutes after shutdown).
Unplug the device after the treatment session is over.
Flashing green indicators in the center of each of the four emitters indicate that the
magnetic field is active, and ALMAG is functioning properly. During operation the
indicators should flash at regular intervals.
ALMAG’s functioning can additionally be checked by applying the PEMF indicator onto
the side marked with “N” of each emitter in turn, while the device is powered. Flashing of
the green light in the middle of the indicator shall confirm the PEMF presence.
For treatment apply ALMAG to the skin on the affected area with the side that has
no LED light and is marked with the “N” sign. The “N” stands for the north pole of the
emitter.
PEMF travels from emitter 1 to emitter 4.
Fig. 1
Control unit
PEMF indicator
2
3
4
Emitter
1

ALMAG-01
Operating Manual Page 9
Due to the high penetrating ability of ALMAG’s magnetic field, the treatment can as
well be performed through clothing, dry or damp gauze bandage, or plaster bandage up
to 1 cm thick.
NOTE: To position the device on the body properly, please follow the instructions
herein. Pay attention to the direction of PEMF travel and the working side of the
emitters.
ALMAG-01
Operating Manual Page 10
4.3 MARKING
The mark defines the device as complying to Class II in terms of electrical safety
according to IEC 60601-1
Warnings on safety and efficacy of operation
Emitters are protected with reinforced insulation
Indicates necessity to refer to the Operational manual
IP 41 The enclosure of this product is protected against access of solid particles and
against vertically falling drops of water.
The mark defines the device as complying to
MDD 93/ 42/EEC,
EN ISO 10993-1,
EN 60601-1,
EN 60601-1-2,
EN 60601-1-11.
!
ALMAG-01
Operating Manual Page 11
5. SAFETY INSTRUCTIONS
5.1 Please study this Operating Manual carefully before use.
5.2 Examine the device before use carefully. Make sure that all parts of the device are
undamaged.
5.3 Place the control unit on a flat surface. Make sure the cable is not twisted or taut.
AC mains voltage:
~230V (-34,5V; +23V), frequency 50Hz
~120V (-10V; +6V), frequency 60Hz
5.4 During chemical disinfection or wiping of the device make sure that the moisture
does not get inside the control unit or the emitters. Protect the device from dampness,
shock and impact.
5.5 CAUTION! Do not use the device if its case, emitters, or cables are damaged.
5.6 Safety instructions for treatment sessions:
a) the first treatment session should last no longer than 20 minutes; if two areas are
under treatment, the total treatment time should not exceed 30 minutes;
b) the first 3 sessions of cervicothoracic area treatment (the area of the neck and the
chest) should last no longer than 10 minutes;
c) using the device on the heart and brain areas is prohibited.
Note: there may be traces of plastic outflow on the working surface of the device
casing, which are not considered as damaging and have no impact on the device
operation.
CAUTION! Do not lift or carry the device by power cable!
Do not place an operational device nearby (less than 0.5 m apart
from) magnetic data carriers, audio, video, and other magnetosensitive devices!
6. PREPARATION FOR USE
- After storage or transportation at temperatures below +10°C or above +35°C, keep
the device in a room at a temperature from +10°C to +35°C for at least 4 hours prior to
plugging it in.
- Before use, wipe the outer surfaces of the control unit and the emitters with a piece
of cloth moistened with 3% solution of hydrogen peroxide mixed with 0.5% solution of a
household detergent, or with 1% solution of chloramine.

ALMAG-01
Operating Manual Page 12
While cleaning, avoid leakage of the disinfectant solution or detergent inside the
control unit or the emitters.
Use the PEMF indicator to check the presence of electromagnetic field on the
emitters of the actuated device by applying the indicator onto the working side of the
emitters (marked with “N” sign) and making sure the green LED turns on.
For treatment, the patient should take a comfortable position in which he/she will
remain until the end of the treatment session.
Carry out the treatment sessions (usually 10-20 for the treatment course) at regular
intervals, preferably before meal. It is not recommended to have meal for at least 1 hour
after the treatment session.
The first few sessions of a treatment course should be carried out daily and should
take no longer than 10 minutes. Increase the duration of the treatment sessions
gradually within 2-3 days until the maximum duration is achieved. The usual time of a
treatment session is 10-20 minutes. A change in the treatment duration is possible upon
doctor’s advice.
Carry out the procedures twice a day. Treat only one disease during one treatment
course. If necessary, repeat the treatment course in 30-40 days, and then in 3-4 months,
that is a total of 3-4 courses a year for one disease.
During these intervals between the treatment courses for one disease, treatment of
another disease is possible, provided there is a 10-day break before starting a new
treatment course.
In case the treatment sessions cause an exacerbation of the disease (increased pain,
dizziness, etc.) or other undesirable symptoms, reduce the frequency of the sessions to
every other day with the same duration. If this does not eliminate the undesirable
symptoms, treatment should be stopped.
PEMF treatment is allowed for patients from 2 years of age and above.
ALMAG has demonstrated a good tolerability among elderly patients and people
suffering from cardiovascular diseases, which makes ALMAG applicable in cases when
other therapeutic methods are not recommended.
The treatment procedure causes a warm sense in the area where the ALMAG emitters
are applied.
In some chronic cases, the patients might experience exacerbation of pain during the
first 3 days of treatment. The symptoms usually disappear after a few sessions.
Due to the deferred effect of exposure to PEMF, improvement can come after 15-20
days of treatment.

ALMAG-01
Operating Manual Page 13
Do not use PEMF therapy after taking alcohol.
7. TREATMENT PROCEDURES
7.1. MUSCULOSKELETAL SYSTEM DISEASES
Osteochondrosis
Osteochondrosis is a degenerative dystrophic disease characterized by deterioration
of intervertebral disks and connective tissues of the backbone and the nervous system.
Intervertebral disks lose their cushioning ability, which results in compression and
deformation of a nerve root, a vessel, or the spinal cord, and causes pain.
The backbone consists of 33-34 vertebrae, which constitute cervical (neck), thoracic
(chest), lumbar (lower back), sacral and coccygeal (tailbone) regions of the spine. The
spaces between the vertebrae are layered with soppy cartilage tissue of the disks whose
function is to absorb the impacts experienced by the spine. Nerve roots are located close
to each disk and connect the spinal cord with other human organs. Spinal cord nerves
influence the functioning of all human organs and systems. If the disks are in a sound
condition, they allow the vertebral segments to move easily and do not pinch the nerves.
If the disks are worn out, they can sag, which will eventually cause the adjacent vertebrae
to get so close to each other that they will touch and irritate the nerve roots during
movement. Without timely intervention, this process can later on develop into spinal disk
herniation, also known as slipped disk.
The mostly affected regions of the spine are the lumbar and cervical ones, while the
thoracic spine degenerates less frequently.
Typical symptoms
The patients with lumbar spinal degeneration suffer from lower back pain caused by
physical exercise, a stiff movement, a long period of tension, or exposure to cold. The
patient may also feel pain in the intestinal tract and genitals, since their nerves are
connected to the nerves of the spinal cord. Slipped disk is often accompanied by shooting
pains and muscle weakness.
Cervical spine disorders are associated with compression of not only nerve roots and
their arteries, but also of the spinal cord and vertebral artery. This comes out in neck pain
shooting up the shoulder or the back of the head. Slipped disk in this vertebral region
can cause pain in the arm, shoulder blade, or the front area of the chest.

ALMAG-01
Operating Manual Page 14
Osteochondrosis of the thoracic spine induces spinal pains, a pain syndrome in
various body organs (heart, stomach, lungs, liver, kidneys, urinary bladder, pancreas) and
dyskinesia, a disorder of the movement function of these organs.
Very often osteochondrosis has neurological complications as a result of nerve ending
compression.
It is recommended to start treatment with a short bed rest for 2-3 days. As the pain
syndrome subsides, strengthening of the back muscle core with appropriate physical
exercises is required.
PEMF therapy with ALMAG is prescribed since the first days of treatment after the
disease was diagnosed.
Therapeutic effect
ALMAG’s anti-inflammatory and anti-edematous action ensures a distinctive painkilling effect. This helps improve the conduction of the nerve endings compressed
between the vertebrae, which, in its turn, benefits the recovery of the functions of the
correlated end organs. PEMF stimulates the blood flow to the diseased area and
increases metabolic activity in the surrounding tissues, leading to gradual recovery of the
disk tissue and normalization of its functions.
A complex therapy combining PEMF treatment courses, physical exercise, and
medication suppresses further disease development and improves the patient’s quality
of life.
Treatment procedure
The best time for the procedure is right before bedtime, since it is not recommended
to put any stress on the backbone afterwards. If the patient is required to stay in the
prone position during an acute stage of the disease, the treatment sessions are carried
out twice a day, in the morning and in the evening, with an interval of at least 6 hours.
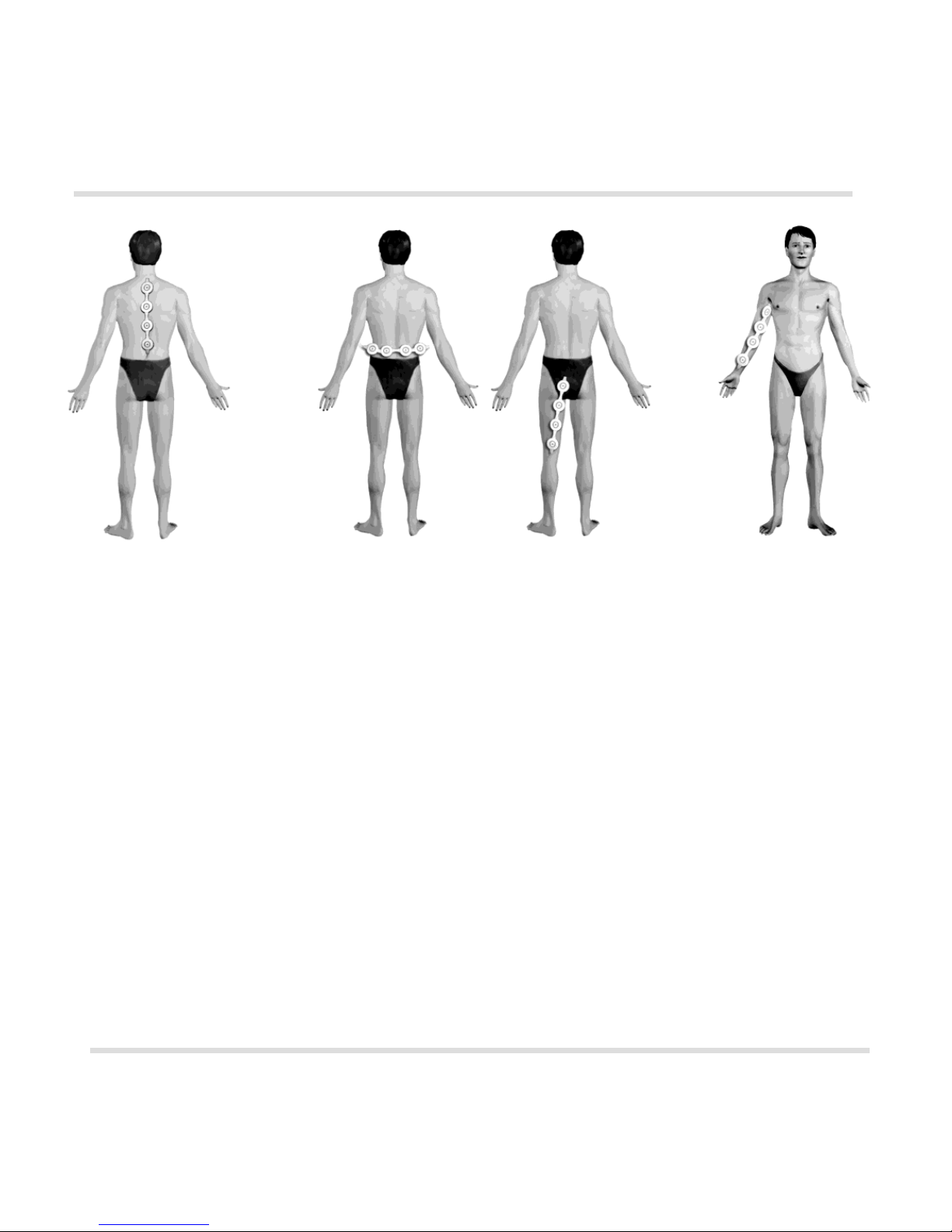
For treatment, ALMAG is placed on the bed along the axis of the patient’s vertebral
column. The patient lies his/her back on the emitters (see Fig. 2).
For the first 3 days of treatment the procedures are to be carried out for 3 minutes 3
times a day. For the next 3 days the procedures is increased up to 5 minutes (3 times a
day). A 1 day break should follow, after which the treatment continues for 6 days
(procedure duration is 10 minutes, 2 times a day). Another 1 day break should be made
before the last 6 days of treatment (procedure duration is 15 minutes, 2 times a day).
Total length of the course is 20 days.
It is undesirable to stand and it is not allowed to sit within 1 hour after the treatment.
ALMAG-01
Operating Manual Page 15
ALMAG is effective for acute osteochondrosis complicated with neuritis, but the
procedures are taken once a day in this case. Treatment begins with the diseased region
of the spine (cervical or lumbar), followed by device application to the areas along the
affected nerves:
- sciatic, tibial, fibular (see Fig. 3);
- radial, ulnar, median (see Fig. 4).
The first 6 days the treatment is to be carried 2 times a day no longer than 5 min. If
required, 2 areas can be treated in this way within one day. Make a pause for 1 day and
continue treatment for the next 6 days (procedure duration is 7-8 minutes, twice a day).
Another 1 day break should be made before the last 6 days of treatment (procedure
duration is 15 minutes, twice a day). Avoid sitting or standing during 30 minutes after the
treatment procedure.
The treatment course can be repeated in 30-40 days after end on the 1st course.
Supportive treatment course should be made in 3-4 months after completion of the
second treatment (procedure duration is 15-20 minutes, total course length is 20 days).
In case for patients with hypertension, blood pressure should be checked before the
treatment procedure and in 30 minutes after it. If blood pressure increases or any other
undesirable symptoms appear, perform treatment every other day with the same or
Fig. 2
Fig. 3
Fig. 4
 Loading...
Loading...