Page 1

For Eko CORE Digital Attachment
and Eko BUNDLE Electronic Stethoscope
Model E4
Electronic Stethoscope System
Page 2

Introduction, Warnings, & Safety
Contact Information
Installation
CORE Use
Cleaning
Warranty
Operating Conditions
CORE Modes and LED States
Eko App Use
Electrical Safety
Contents
4
4
11
11
13
14
15
16
18
19
2
Page 3
The Eko Electronic Stethoscope System (herein referred
to as Eko) is designed to support healthcare professionals
in analyzing cardiac and other internal organ sounds. Eko
includes a device that is attached to a stethoscope (CORE),
a smartphone application (App), and a web application
(Dashboard).
CORE features sound amplification and audio transmission to
a smartphone via Bluetooth that allows the user to open and
playback sounds in a mobile application on compatible iOS
smartphones and tablets. The App provides the ability to save
sounds within select Electronic Health Record (EHR) systems,
share patient recordings with other practitioners, and annotate
notes on recorded audio. Eko is intended for use on pediatric
and adult patients.
CAUTION: Federal (USA) law restricts this device to sale to or
on the order of a clinician.
Please report any injury or adverse event to Eko Devices using
any of the contact methods below. For general and product
related comments, questions, or concerns, please contact Eko
Devices, Inc. directly
Eko Devices, Inc.
2600 10th St. Suite 260
Berkeley, CA 94710
USA
General Assistance and FAQs ekodevices.com/getstarted
Direct Contact support@ekodevices.com
Phone Support 1.844.356.3384
Product Reference and Information www.ekodevices.com
1.1 Introduction, Warnings, and Safety
1.2 For Help and Assistance
3
© 2018 Eko Devices, Inc.
Page 4
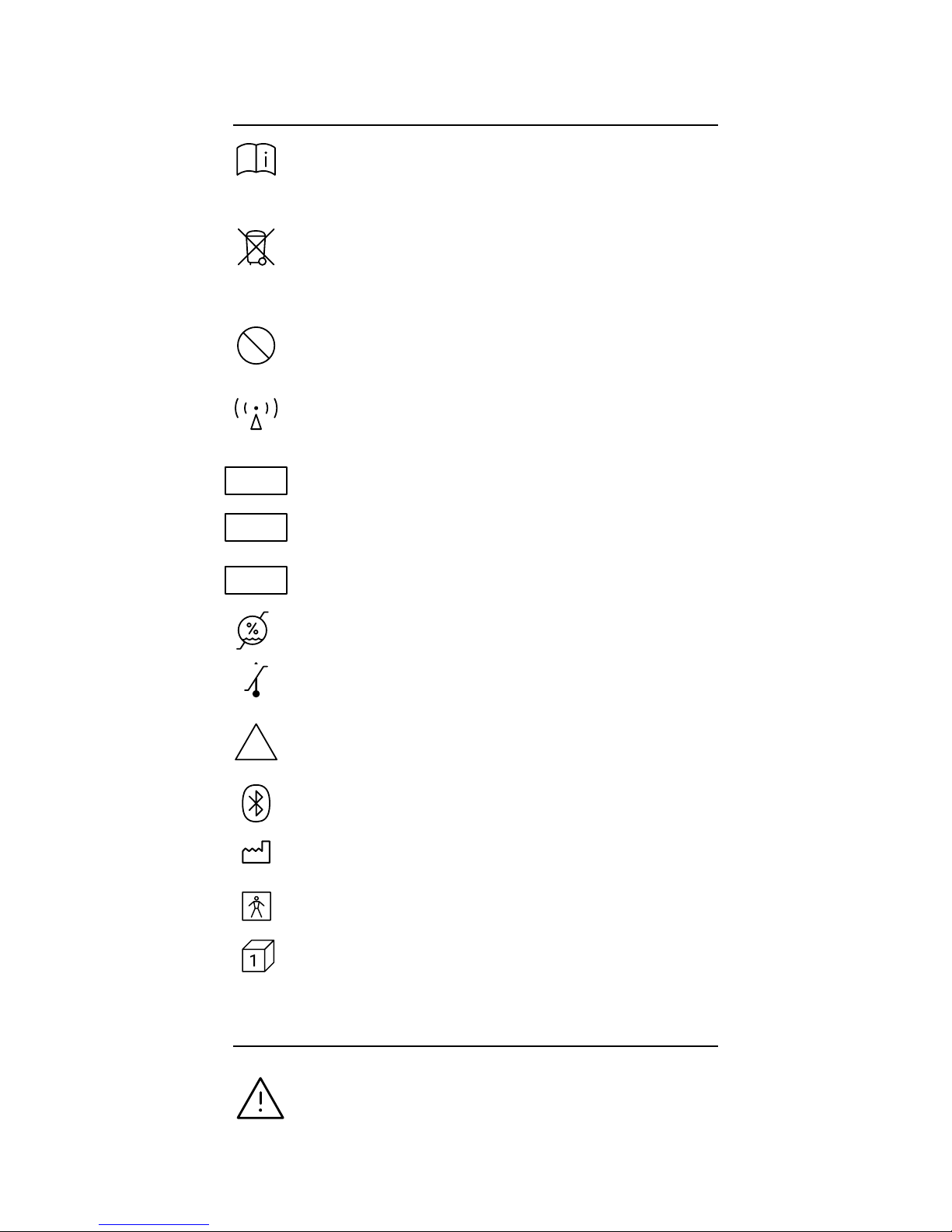
Consult instructions for use.
This product contains electrical and electronic
components and must not be disposed of using
standard refuse collection. Please consult local directives
for disposal of electrical and electronic equipment.
This product and packaging does not contain natural
rubber latex.
This product contains an intentional RF radiator certified
by the FCC.
Catalog Number
Batch Number
Serial Number
Humidity Limit (Operational)
Temperature Limit (Operational)
This product is provided non-sterile. Do not attempt to
re-sterilize the device.
This product uses wireless Bluetooth communication.
Manufacturer (Abbreviation Mfg.)
BF Applied Part
Contents (Quantity)
Indicates a hazardous situation, which if not
avoided, could result in injury and/or property
damage and/or damage to the device.
1.3 Safety Related Labels & Symbols
1.4 Signal Word Consequences
LATEX
15%
93%
40° C
-30° C
NON
STERILE
SN
LOT
REF
4
Page 5
CAUTION:
• To reduce the risk of device interference, keep CORE at
least 1 meter away from all RF emitters including Wifi routers
and radios.
• To reduce the risks associated with infection follow all
cleaning and disinfecting instructions included in this manual.
Establish and follow a cleaning and disinfecting schedule.
• To reduce the risks associated with inaccurate data
acquisition store and operate this stethoscope only as
instructed in this manual. Though there is an acoustic (nonamplified) mode available with this stethoscope, it is highly
recommended that the battery be recharged within thirty
minutes of the LED indicator turning red. Recharge the battery
using only the USB power cord and charger provided with the
device.
• DO NOT immerse the stethoscope in a liquid or subject
it to any sterilization processes other than those described in
this manual.
• To reduce the risks associated with very strong
electromagnetic fields avoid using the stethoscope near
strong radio frequency (RF) signals or portable and/or mobile
RF devices. If sudden or unexpected sounds are heard, move
away from any radio transmitting antennas. Using accessories,
transducers, and cables not produced by Eko Devices may
result in increased RF emissions or decreased immunity of the
Eko Electronic Stethoscope System.
• Please read, understand, and follow all safety information
contained in these instructions prior to using the Eko Electronic
Stethoscope System. It is recommended that these instructions
be retained for future reference.
• To reduce the risk associated with an electrical shock
do not use the stethoscope on patients without the analog
stethoscope’s chest piece in place.
• CORE contains a Bluetooth Class 2 wireless data link.
The maximum radio frequency field strength generated
by the stethoscope is below three volts per meter, a level
that is considered safe to use with other medical devices.
However, audio, video, and other similar equipment may cause
electromagnetic interference. If such devices are encountered
5
© 2018 Eko Devices, Inc.
Page 6

FCC Intentional Radiator Certification
Contains FCC ID: QOQBLE113
Contains IC: 5123A-BGTBLE113
This equipment contains an intentional radiator approved by
the FCC under the FCC ID numbers shown above. This device
complies with Part 15 of the FCC rules. Operation is subject
to the following two conditions: (1) This device may not cause
harmful interference, and (2) this device must accept any
interference received, including interference that may cause
undesirable operation.
NO MODIFICATION: Modifications to this device shall not
be made without the written consent of Eko Devices, Inc.
Unauthorized modifications may void the authority granted
under Federal Communications Commission rules permitting
the operation of this device.
EMC Compliance Europe
This equipment complies with the EMC requirements of the IEC
60601-1-2.
NOTICE:
1.5 EMC Compliance
and cause interference, immediately move CORE away from
that device and/or turn the Bluetooth feature OFF.
• To reduce the risks associated with environmental
contamination follow applicable regulations when disposing
of this stethoscope. CORE contains a lithium-ion polymer
rechargeable battery; please properly dispose of the device as
mandated by local directives.
• No modification of this equipment is allowed. There are no
repairable parts inside CORE.
6
Page 7
Eko is intended to be used as a part of a physical assessment
of a patient by healthcare professionals for diagnostic decision
support in clinical settings. Eko is intended for use on pediatric
and adult patients. It can electronically amplify, filter, and
transfer sounds to the accompanying mobile application for
storage and sharing. It can be used to record heart sounds and
cardiac murmurs, bruits, respiratory sounds and abdominal
sounds during physical examination in normal patients or those
with suspected diseases of the cardiac, vascular, pulmonary or
abdominal organ systems.
There are no known contraindications for Eko, although care
should be taken when considering using the device according to
the warnings and precautions below.
Eko is not life-supporting or life sustaining.
The device is intended to be prescribed by licensed medical
professionals for use on patients during a physical assessment
in a clinical setting. The system provides one source of
data that is significant only when used in conjunction with
clinician oversight and consideration of other relevant patient
information.
Eko should be used only by qualified clinicians. Eko is intended
for use on patients that can be auscultated on normally with an
acoustic stethoscope.
This manual provides instructions for the use of CORE and Eko
web and mobile applications. It is assumed that the user is
familiar with basic website navigation and mobile application
use.
This device is only indicated for use in a hospital, physician’s
office, or other clinical setting. Standard procedures for
auscultation should be followed including background noise
reduction and optimal patient positioning.
1.7 Precautions
1.6 Indications for Use
7
© 2018 Eko Devices, Inc.
Page 8
The privacy of patient health information may be protected
by state, federal, or international/foreign laws that regulate
how such information can be used, stored, transmitted, and
disclosed. The Eko system employs security features that
are compliant with HIPAA policies. Third party access may
be prohibited to such information without obtaining written
authorization from the patient.
The user is fully responsible for understanding and following all
laws that regulate storage, transmission, and disclosure of any
electronic patient data through the use of software. If the user
becomes unable to comply with a law or restriction that applies
to use and disclosure of such data, the user should not proceed
to collect or save such information.
This application may require entry of individually identifiable
health information in order to function. Records are stored and
recalled through the use of patient name, date of birth, and/or
patient ID #. By entering this information, the user assumes any
and all risks of and liabilities incurred with using or transmitting
such information.
1.8 Patient Privacy
In order to transmit sounds to the Eko App, the stethoscope and
device must be connected via Bluetooth, and in order to fully use
certain functions, the mobile device must be connected to the
internet via cellular data connection or Wi-Fi.
CORE uses a Bluetooth Class 2 wireless data link. The Bluetooth
range will be reduced when objects (walls, furniture, people,
etc) are between CORE and a paired mobile device. To improve
Bluetooth connection, reduce the distance and/or allow a line of
sight between CORE and mobile device.
It is highly recommended that users of the Eko Dashboard and
Eko App use device and networking security features to protect
patient data created and stored using this software, in addition
to security features embedded in the system. Please consult
your institution’s technical services to implement appropriate
security measures.
8
Page 9

CORE includes (1) CORE, (2) stethoscope tubing adapters,
(1) micro USB cable, and (1) USB charger. This device is
non-assembled and must be installed by the user. For full
functionality, the system requires an acoustic stethoscope and
smart mobile device with wireless Internet capabilities (not
included). The compatible hardware and software platforms are
listed below.
The Bundle package includes (1) CORE fully assembled with an
acoustic stethoscope, (1) micro USB cable, and (1) USB charger.
The digital electronic stethoscope attachment is referred to
as CORE, while the Eko BUNDLE is an electronic stethoscope
consisting of CORE fully assembled to an acoustic stethoscope.
Compatible Stethoscopes
Eko is designed and tested to work with the 3M Littmann*
Cardiology II/III, WelchAllyn Harvey Elite, and ADC 601
lines of analog stethoscopes. Eko will work with many
other stethoscope brands and models, but no performance
guarantees are claimed using other models or brands.
NOTE: CORE is not compatible with any digital stethoscopes.
System Requirements
The mobile app software can be used on iPhone* 4S, iPhone
5/5C/5S, iPhone 6/6 plus, iPhone 6s/6s plus, iPad* Mini 2/3,
iPad Air/Air 2, iPod Touch 5G, and iPad 3rd and 4th generations
with iOS 7.0 and higher. The mobile app software can also be
used with Android devices with BLE support (Bluetooth 4.0) and
Android 5.0 and above.
CORE uses Bluetooth Smart; mobile devices used must be
compatible with Bluetooth Smart.
*Littmann, 3M , and Cardiology III are registered trademarks of
the 3M Corporation.
*iPhone, iPad, iTunes, and iOS are registered trademarks of
Apple, Inc.
*Bluetooth is a registered trademark of Bluetooth SIG, Inc.
1.9 Contents and Operation
9
© 2018 Eko Devices, Inc.
Page 10
2.1 Installation to Existing Stethoscopes
Not applicable to Eko Bundle
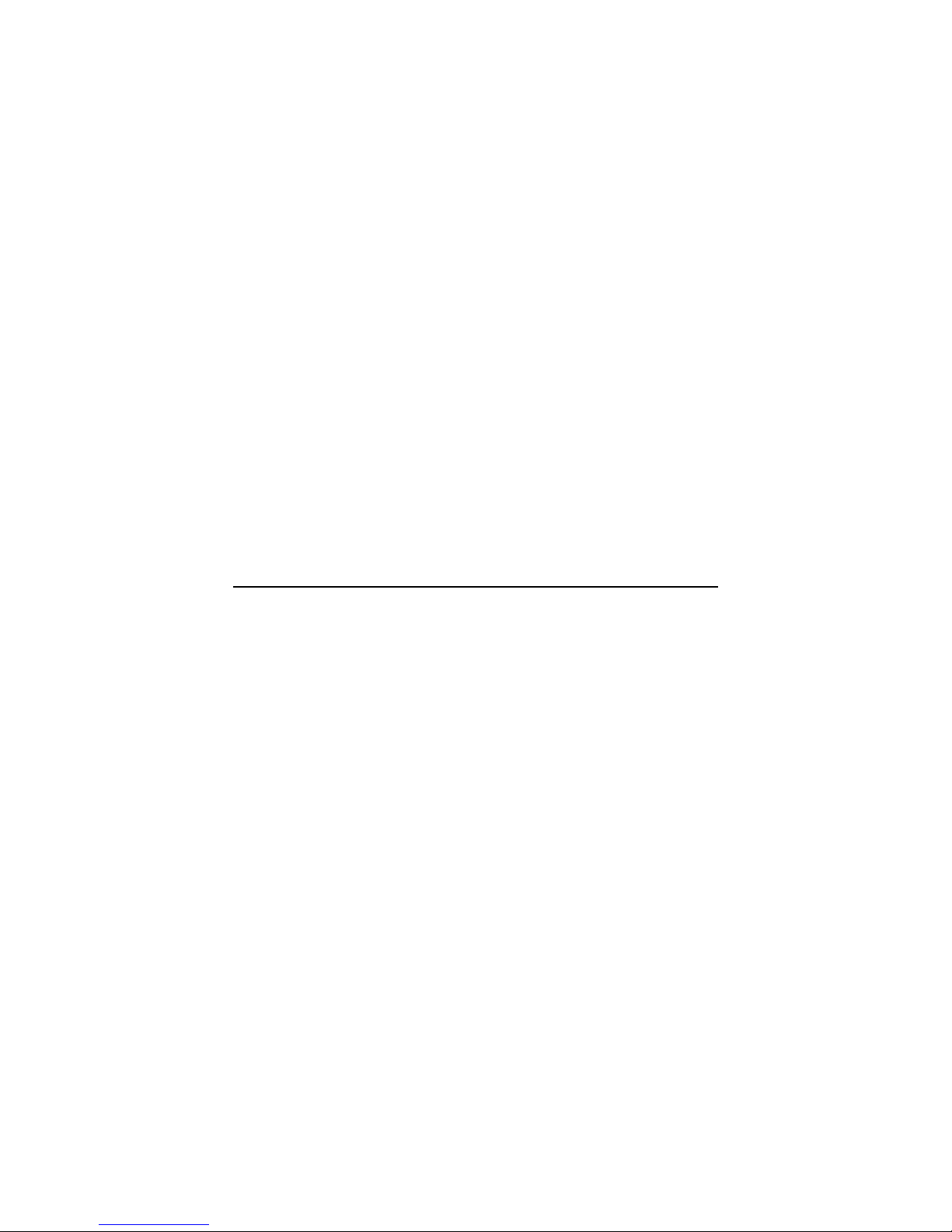
Detach Chest Piece
Remove chest piece of the analog stethoscope manually. Grip
the chest piece with one hand and the tubing in the other, then
twist and pull them apart. This may require some force. See
Fig. 1 & 2
Install CORE
Insert the narrow end of CORE into the tubing of the
stethoscope. The metal stem fits into the hollow opening of
the tube.
NOTE: Ensure the smaller end of CORE is connected to the
stethoscope tubing. See Fig. 3 & 4
Fig. 1 Detach the Chest Piece
Fig. 3 Tubing Adapter
and Chest Piece
Fig. 2 Detached Chest Piece
Fig. 4, Left: CORE on Stethoscope Tubing
Right: Connector on Chest Piece
For more information, click here.
10
Page 11
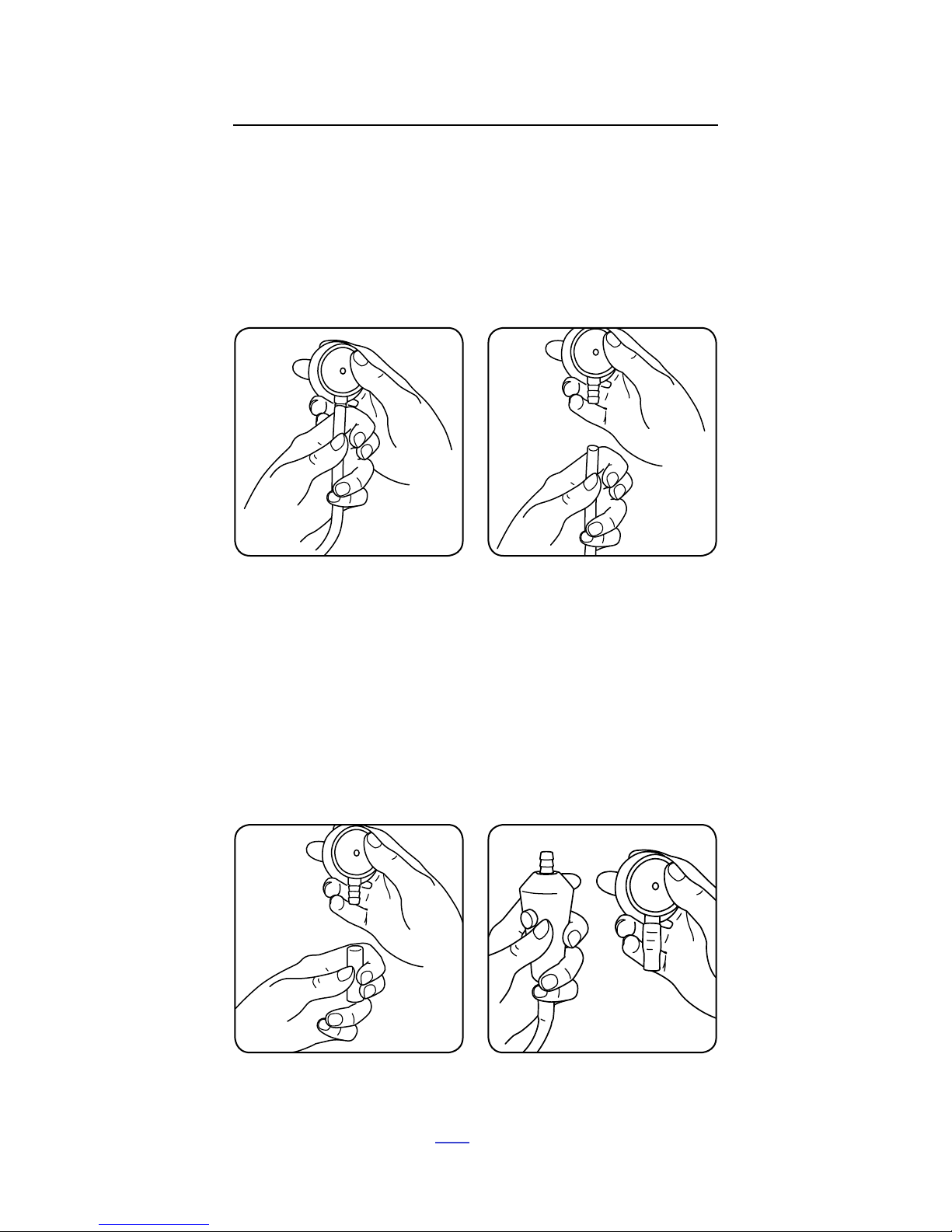
Reattach Tubing and Chest Piece
Attach the additional tubing connector onto the end of CORE
as shown below. Then attach the chest piece into the tubing
connector as it was on the analog stethoscope.
To get the best sound, we recommend you test all Eko-supplied
tubing adapters & select the one that provides the tightest fit
with your analog stethoscope.
Fig. 5 CORE with Attached Tubing Fig. 6 Completed Installation
2.2 CORE Use
Charge Battery
The battery in CORE will need to be charged; insert the included
micro USB cable into the USB port on the device and plug the
other end into the included USB charger. The LED will begin to
flash yellow, signifying that it is charging. The LED will change to
solid yellow when the device is fully charged.
NOTE: CORE will not turn on while it is plugged in and charging.
Power Off
When CORE is turned Off, sounds will be heard as through the
analog stethoscope. “OFF” will be displayed on the toggle when
the device is powered off.
Power On
Depress the power slider to move the switch from the OFF to the
ON position. “ON” will be displayed on toggle when the device
is powered on.
11
© 2018 Eko Devices, Inc.
Page 12

Test the Volume Level
CORE’s sound level can be amplified in 7 increments up to 40X
amplification of an acoustic stethoscope. Change the volume
level by clicking the plus (+) and minus (-) volume buttons on
the side of CORE.
Bluetooth Pairing
First, enable Bluetooth on the selected mobile device. On the
iOS device go to Settings > Bluetooth > and tap the slider to turn
Bluetooth ON.
Then, navigate to the Menu screen by clicking on the top left tab
in the App. Navigate to Hardware > Bluetooth > Select Device
and pair with the device.
The mobile device is now ready to record sounds from CORE. If
Bluetooth pairing is unsuccessful, an error message will appear
in the App and no sounds will be recorded. If the Bluetooth
connection is successful the LED will turn from flashing blue to
green See Section 2.3 for the LED states of the device.
Setting up a PIN
Create a secure 4-digit PIN by logging in to the mobile
application. Navigate to the Menu screen by selecting the icon
on the top left of the Moblie App home screen.
Next, select Account Settings > Create Pin. Follow the
instructions on the screen to create and save a 4 -digit PIN. You
will need to enter your PIN twice for verification purposes.
Adding Notes to Recordings on Moblie App
To create notes on any patient recordings, log into the mobile
application. Access the list of patients by selecting the patients
tab on the top right of the home screen. Select the desired
patient and select a recording to add notes to.
On the bottom of the recording screen, select the Notes icon.
The Notes icon looks like a post-it with writing on it. Select “Add
Note” and begin typing your note. Select the the check mark
to save.
12
Page 13

3.1 Cleaning
Cleaning and Disinfecting Procedure
The stethoscope and CORE should be cleaned between each
patient use. All cleaning instructions pertaining to the original
stethoscope apply.
Under normal conditions it is unnecessary to remove CORE from
the stethoscope tubing for cleaning. All external parts of the
hardware can be cleaned with 70% isopropyl alcohol wipes.
NOTE: DO NOT immerse the device in any liquid or subject it to
any high-pressure/autoclave sterilization processes.
If it becomes necessary to remove CORE, pull the stethoscope
tubing off of the metal stem on both ends of the device. Wipe
all parts of the stethoscope clean with 70% isopropyl alcohol
wipes including CORE’s surface, stethoscope tubing, tubing
connector, and chest piece. Reassemble the stethoscope by
reinserting the metal stems into the stethoscope tubing as
before.
13
© 2018 Eko Devices, Inc.
Page 14

4.1 Warranty
Eko provides a limited warranty for CORE.
Please visit ekodevices.com/warranty for a full description of
the warranty.
14
Page 15

Operating Warnings
Failure to follow care and maintenance recommendations could
result in damage to the internal components of CORE. Internal
damage to the product could cause malfunction of the product,
possibly leading to complete loss of function. If problems are
encountered with CORE, do not attempt to repair it. Please notify
our support team for assistance.
5.1 Operating Conditions
Environmental
The operating range of CORE is -30° to 40°C (-22° to 104°F),
and 15% to 93% relative humidity. The storage and transport
range is -40° to 55°C (-40° to 131° F), and 15% to 93% relative
humidity. Acceptable pressure is 1 atm.
It is recommended to avoid exposure to extreme heat, cold,
solvents and oils. Extreme heats and colds will negatively affect
the lithium ion battery in the device, and may affect battery life.
15
© 2018 Eko Devices, Inc.
Page 16

On
Paired
CORE is ON and paired with
a phone/tablet. The CORE
will stream live audio from the
stethoscope chest piece to the
paired device.
6.1 CORE Modes and Corresponding LED States.
Off CORE is OFF. Sounds from
the stethoscope pass through
unfiltered.
On
Not Paired
CORE is ON but not paired. The
CORE is discoverable and ready
to connect via Bluetooth.
On
Volume Change
CORE is ON and changing
playback volume based on
commands from the volume
buttons or paired phone/tablet.
The LED will blink once for each
volume interval changed.
On (quick blink)
Playback
CORE is ON and playing back
sounds from a paired phone/
tablet.
16
Page 17

On
Battery Expired
CORE is ON and its battery level
is below 10%. The CORE will
no longer stream or playback
audio.
Off
Charging
CORE is OFF and connected to
a power source. The battery is
charging.
Off
Fully Charged
CORE is OFF and connected to
a power source. The battery is
fully charged (100%).
On
Low Battery
CORE is ON and its battery level
is below 25%.
17
© 2018 Eko Devices, Inc.
Page 18
7.1 Eko App
Download the Eko app, available on the App Store® and
Google Play and follow the on-screen instructions to connect
to CORE.
Bluetooth must be enabled in the mobile or desktop’s
Bluetooth settings in order to use CORE with the Eko App.
18
Page 19

8.1 Electrical Safety
Warning: The use of accessories other than those specified, with the exception
of accessories sold by Eko as replacement parts, may result in increased
emissions or decreased immunity of the Eko Electronic Stethoscope System.
Warning: The Eko Electronic Stethoscope System should not be used adjacent
to or stacked with other equipment. If adjacent or stacked use is necessary,
the Eko Electronic Stethoscope System should be observed to verify normal
operation in the configuration in which it will be used.
Guidance and Manufacturer’s Declaration - Electromagnetic
Emission
The Eko Electronic Stethoscope System is intended for use in the
electromagnetic environment specified below. The user of the Eko
Electronic Stethoscope System should assure that it is used in such an
environment.
Applicable
Emissions Test
Compliance Electromagnetic
Environment- Guidance
RF emissions
CISPR 11
Group 1 The Eko Electronic Stethoscope
System uses RF energy only for
its internal function. Therefore,
its RF emissions are very low
and are not likely to cause any
interference in nearby electronic
equipment.
RF emissions
CISPR 11
Class B The Eko Electronic Stethoscope
System is suitable for use in
all establishments, including
domestic establishments and
those directly connected to the
public low-voltage power supply
network that supplies buildings
used for domestic purposes.
Harmonic Emissions
IEC 6100-3-2
Not Applicable
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not Applicable
19
© 2018 Eko Devices, Inc.
Page 20

Guidance and Manufacturer’s Declaration - Electromagnetic
Immunity
The Eko Electronic Stethoscope System is intended for use in the
electromagnetic environment specified below. The user of the Eko
Electronic Stethoscope System should assure that it is used in such an
environment.
Immunity
Test
IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment-
Guidance
Electrostatic
Discharge
(ESD) IEC
61000-4-2
+/- 6 kV
contact
+/- 8 kV
+/- 6 kV
contact
+/- 8 kV air
Floors should be wood,
concrete, or ceramic tile.
If floors are covered with
synthetic material, the
relative humidity should
be at least 30%
Electrical Fast
Transient/
Burst
IEC 610004-4
+/- 2 kV
for supply
lines
+/- 1 kV
for input/
output
lines
Not
Applicable
Surge
IEC 610004-5
+/- 1kV
line(s) to
line(s)
+/- 2 kV
line(s) to
earth
Not
Applicable
Voltage
dips, short
interruptions
and voltage
variations on
power supply
input lines
IEC 610004-11
< 5% U
T
(>95% dip
in UT) for
0.5 cycle
40% U
T
(60% dip
in UT) for 5
cycle
70% U
T
(30% dip in
UT) for 25
cycle
< 5% U
T
(>95% dip
in UT) for
5 sec
Not
Applicable
20
Page 21
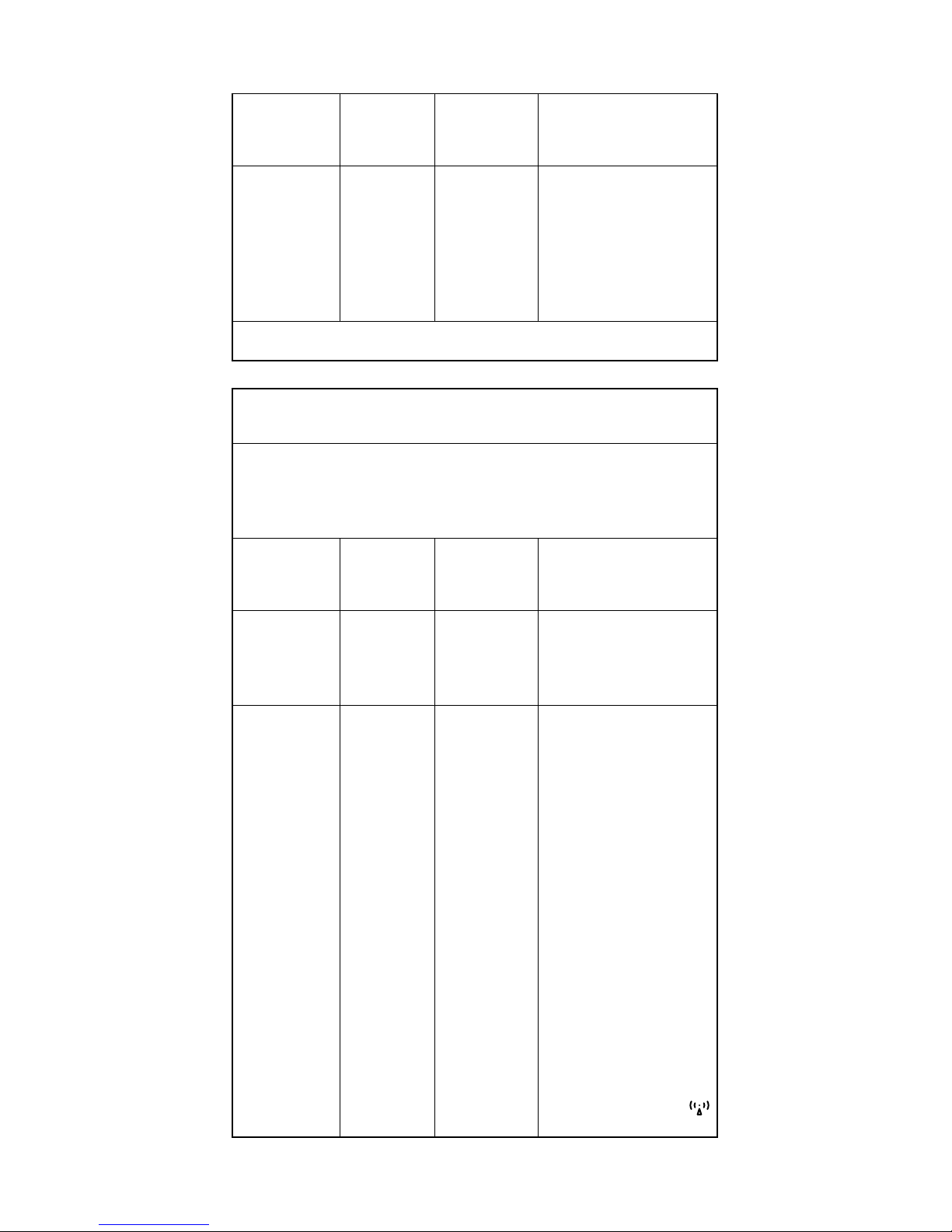
Guidance and Manufacturer’s Declaration - Electromagnetic
Immunity
The Eko Electronic Stethoscope System is intended for use in the
electromagnetic environment specified below. The user of the Eko
Electronic Stethoscope System should assure that it is used in such an
environment.
Immunity
Test
IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment-Guidance
Conducted
RF
IEC 610004-6
3 Vrms
150 kHz to
80 MHz
Not
Applicable
Radiated RF
IEC 610004-3
3V/m
80 MHz to
2.5 GHz
3 V/m
80 MHz to
2.5 GHz
d = 1.2 √P 80 MHz to
800 MHz
d = 2.3 √P 800 MHz to
2.5 GHz
where P is the maximum
output power rating of the
transmitter in watts (W)
according to the transmitter
manufacturer and d is the
recommended separation
distance in meters (m).
Field strengths from fixed RF
transmitters, as determined
by an electromagnetic site
survey,a should be less than
the compliance level in each
frequency range.
b
Interference may occur in the
vicinity of equipment marked
with the following symbol:
21
© 2018 Eko Devices, Inc.
Immunity
Test
IEC 60601
Test Level
Compliance
Level
Electromagnetic
Environment-Guidance
Power
frequency
(50/60 Hz)
magnetic
field
IEC 610004-8
3 A/m 3 A/m Power frequency
magnetic fields should
be at levels characteristic
of a typical location in
a typical commercial
magnetic field or hospital
environment.
NOTE UT is the a.c. mains voltage prior to application of the test level
Page 22

NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and is affected by absorption and
reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To address the electromagnetic environment due to fixed
RF transmitters, an electromagnetic site survey should be considered.
If the measured field strength in the location in which the Eko Electronic
Stethoscope System is used exceeds the applicable RF compliance level
above, the Eko Electronic Stethoscope System should be observed to verify
normal operation. If abnormal performance is observed, additional measures
may be necessary, such as re-orienting or relocating the Eko Electronic
Stethoscope System.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be
less than 3 V/m.
Recommended Separation Distances Between Portable and Mobile
RF Communications Equipment and the Eko Electronic Stethoscope
System
The Eko Electronic Stethoscope System is intended for use in the
electromagnetic environment in which radiated RF disturbances are
controlled. The user of the Eko Electronic Stethoscope System can help
prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment (transmitters)
and the Eko Electronic Stethoscope System as recommended below,
according to the maximum output power of the communications equipment.
Rated Maximum
Output Power of
Transmitter (W)
Separation Distance According to Frequency of
Transmitter (m)
150 kHz to 80 MHz
d = 1.2 √P
80 MHz to 800 MHz
d = 1.2 √P
800 MHz to 2.5 GHz
d = 2.3 √P
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.2 1.2 2.3
10 3.7 3.7 7.4
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d is meters (m) can be estimated using the equation applicable to
the frequency of the transmitter, where P is the maximum power rating of the transmitter
in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency
range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation
is affected by absorption and reflection from structures, objects and people.
22
Page 23

9.1 Manufacturing & Regulatory Information
Manufactured by:
Eko Devices, Inc.
2600 10th Street, Suite #260
Berkeley, CA 94710 USA
www.ekodevices.com
Australian Sponsor:
Emergo Australia
Level 20
Tower II, Darling Park
201 Sussex Street
Sydney, NSW 2000 Australia
Brazilian Registration Holder:
Macrosul
Rua Júlio Bartolomeu Taborda Luiz, 270 – Tingui
Curitiba - PR, 82820-440, Brazil
© 2018 Eko Devices, Inc. All Rights Reserved
LBL 002, Rev K
EC Authorized Representative:
Emergo Europe
Prinsessegracht 20
2514 AP The Hague
The Netherlands
EC REP
Notified Body:
VTT Expert Services Ltd.
Kemistintie 3, Otaniemi, 02150 Espoo, Finland
(Notified Body No. 0537).
0537
 Loading...
Loading...