Eko Devices DUO E5 User Manual

User Manual, Model E5

P.
2
The Eko Model E5 System is designed to support
healthcare professionals in analyzing cardiac and
other internal organ sounds. The Eko Model E5
System includes a device capturing heart sounds
and ECG readings (herein referred to as DUO), a
smartphone application (App), and a web application
(Dashboard).
DUO features audio and ECG data transmission via
Bluetooth that allows the user to open and playback
sounds and visualize phonocardiograms (PCG) and
electrocardiograms (ECG) in a mobile application on
compatible iOS smartphones and tablets. The App
provides the ability to save audio recordings and
waveforms (PCG and ECG) within Electronic Health
Record (EHR) systems, share patient recordings with
other practitioners, and annotate notes on recorded
audio. Eko is intended for use on pediatric and adult
patients. The device is not intended for infants
weighing less than 10 kg.
CAUTION: Federal (USA) law restricts this device to
sale to or on the order of a clinician.
For general and product related comments, questions,
or concerns, please contact Eko Devices, Inc. directly
Please report any injury or adverse event to Eko using
any of the contact methods below.
Eko Devices, Inc.
2600 10th St. Suite 260
Berkeley, CA 94710
USA
General Assistance and FAQs
ekodevices.com/getstarted
Direct Contact
support@ekodevices.com
Phone Support
1.844.356.3384
Product Reference and Information
www.ekodevices.com
1.1
Introduction, Warnings, and Safety
1.2
For Help and Assistance
Consult instructions for use.
This product contains electrical and
electronic components and must not be
disposed of using standard refuse collection.
Please consult local directives for disposal of
electrical and electronic equipment.
This product and packaging does not contain
natural rubber latex.
This product contains an intentional RF
radiator certied by the FCC.
Catalog Number
Lot Number
Serial Number
Humidity Limit (Operational)
Temperature Limit (Operational)
This product is provided non-sterile. Do not
attempt to re-sterilize the device.
This product uses wireless Bluetooth
communication
BF Applied Part
Contents (Quantity)
Protection against dust and water jets when
earpieces are attached.
The earpieces are not IP protected.
MR Unsafe
1.3
Safety Related Labels & Symbols
LATEX
10%
95%
45° C
113° F
10° C
50° F
NON
STERILE
SN
LOT
REF
IP55

P.
3
CAUTION
• To reduce the risk of device interference, keep
DUO at least 2 meters away from all RF emitters
including Wi routers and radios when operating
or charging.
• To reduce the risks associated with infection
follow all cleaning and disinfecting instructions
included in this manual. Establish and follow a
cleaning and disinfecting schedule after each use.
• To reduce the risks associated with inaccurate
data acquisition store and operate this device only
as instructed in this manual. It is recommended
that the battery be recharged within thirty minutes
of the LED indicator turning red. Recharge the
battery using only the wireless charging pad
provided with the device.
• DO NOT immerse the device in a liquid or subject
it to any sterilization processes other than those
described in this manual. The device is non-sterile.
• To reduce the risks associated with very strong
electromagnetic elds avoid using the device
near strong radio frequency (RF) signals or
portable and/or mobile RF devices. If sudden
or unexpected sounds are heard, move away
from any radio transmitting antennas. Using
accessories, transducers, and cables not
produced by Eko may result in increased RF
emissions or decreased immunity of DUO.
• Please read, understand, and follow all safety
information contained in these instructions prior
to using DUO. It is recommended that these
instructions be retained for future reference.
• DUO contains a Bluetooth Class 2 wireless
data link. The maximum radio frequency eld
strength generated by the device is below three
volts per meter, a level that is considered safe to
use with other medical devices. However, audio,
video, and other similar equipment may cause
electromagnetic interference. If such devices are
encountered and cause interference, immediately
move DUO away from that device and/or turn the
Bluetooth feature OFF.
• To reduce the risks associated with
environmental contamination follow applicable
regulations when disposing of this device. DUO
contains a rechargeable battery; please properly
dispose of the device as mandated by local
directives.
• No modication of this equipment is allowed.
There are no repairable parts inside DUO.
• Disperse any static electricity before using the
unit.
• Warning: MR-unsafe! Do not expose the device
to a magnetic resonance (MR) environment. The
device may present a risk of projectile injury due
to the presence of ferromagnetic materials that
can be attracted by the MR magnet core. Thermal
injury and burns may occur due to the metal
components of the device that can heat during
MR scanning. The device may generate artifacts
in the MR image. The device may not function
properly due to the strong magnetic and radio
frequency elds generated by the MR scanner.
• This device does not detect or measure all heart
rate, heart rhythm and heart waveform changes.
• The device has not been tested for use on infants
weighing less than 10kgs.
• Conductive parts of electrodes and associated
connectors for Type BF Applied Parts, including
the neutral electrode, should not contact other
conductive parts including earth.
• Do not use DUO while it is charging.
• Do not use on patients with cardiac pacemakers
or other electronic implanted devices.
• Do not use the DUO on a portion of the body with
too much body fat, body hair or very dry skin, a
successful recording may not be possible.
• Do not use DUO while charging your mobile
device, this will cause electrical interference with
the ECG signal.
• Do not store the DUO in extremely hot, cold,
humid, or wet conditions.
• Do not expose to strong electromagnetic elds.
• Do not use as sole basis for medication or
treatment decisions.
• Do not take a recording if the electrodes are dirty.
Clean them rst.
• Do not use the DUO over broken skin or wound
areas.
1.4
Caution

P.
4
1.5
Skin Preparation
Excessive hair, dirty skin, dry skin, or oily skin can
impact the quality of the ECG tracing. Clean the
patient's skin with water and soap and remove excess
hair as needed to achieve the highest quality tracing.
Do not use the DUO over wound areas or areas of
broken skin. Do not use an alcohol based skin cleaner
as this dries out the patient's skin and increases
resistance. Dry the skin vigorously to increase capillary
blood ow to the tissues.
ECG gels or saline solutions can be used on the
electrodes to improve signal quality.
FCC Intentional Radiator Certication
Contains FCC ID: XXXXXXXXXX
Contains IC: XXXXXXXXXXXXXX
This equipment contains an intentional radiator
approved by the FCC under the FCC ID numbers
shown above. This device complies with Part 15 of
the FCC rules. Operation is subject to the following
two conditions: (1) This device may not cause harmful
interference, and (2) this device must accept any
interference received, including interference that may
cause undesirable operation.
NO MODIFICATION: Modications to this device
shall not be made without the written consent of Eko
Devices, Inc. Unauthorized modications may void
the authority granted under Federal Communications
Commission rules permitting the operation of this
device.
EMC Compliance Europe
This equipment complies with the EMC requirements
of the IEC 60601-1-2.
1.6
EMC Compliance
To ensure a proper ECG and PCG recording, clean the
electrodes with an alcohol-based cleaning solution
prior to use.
Cleaning and Disinfecting Procedure
The device and earpieces should be cleaned between
each patient use. All cleaning instructions pertaining
to stethoscopes and ECG units in general apply.
Under normal conditions it is unnecessary to separate
the device from the earpieces for cleaning. All external
parts of the hardware can be cleaned with 70%
isopropyl alcohol wipes.
NOTE: DO NOT immerse the device in any liquid or
subject it to any high-pressure/autoclave sterilization
processes.
1.7
Cleaning

P.
5
The device is intended to be prescribed by licensed
medical professionals. The device may be used on
patients during a physical assessment in a clinical
setting or by patients with a prescription and under the
supervision of a clinician. The system provides one
source of data that is signicant only when used in
conjunction with clinician oversight and consideration
of other relevant patient information.
DUO should be used only by qualied clinicians or
prescribed to patients with an adequate understanding
of the device. DUO is intended for use on patients
that can be auscultated on normally with an acoustic
stethoscope.
This manual provides instructions for the use of
DUO and the Eko App. It is assumed that the user is
familiar with basic mobile application use on iOS™ and
Android™ devices.
Standard procedures for auscultation should be
followed including background noise reduction and
optimal patient positioning. The quality of the ECG is
dependent on proper preparation practices including,
but not limited to, patient body temperature and clean
contact area.
The device uses a Bluetooth Class 2 wireless data
link. The Bluetooth range will be reduced when
objects (walls, furniture, people, etc) are between DUO
and a paired mobile device. To improve Bluetooth
connection, reduce the distance and/or allow a line of
sight between DUO and the mobile device.
In order to transmit sounds and record electrical
activity to the Eko App, DUO and the mobile device
must be connected via Bluetooth, and in order to
fully use certain functions, the mobile device must be
connected to the internet.
It is highly recommended that users of the mobile
App and web Dashboard use device and networking
security features to protect patient data created and
stored using this software, in addition to security
features embedded in the system.
NOTICE: Some of the features of the Eko App require
a minimum internet connection speed. The minimum
recommended upload speed for the mobile app
is 4000 Kbps. 4G cellular data service or similar is
recommended for the App.
The app can be used to visualize waveforms and
tracings without an internet connection, however an
internet connection is necessary to save the data.
1.8
Precautions

P.
6
The Eko Model E5 System is intended to be used by
healthcare professionals to electronically amplify,
lter, and transfer body sounds and single-channel
electrocardiogram (ECG) waveforms. The Eko
Model E5 System also displays ECG waveforms and
phonocardiogram waveforms on the accompanying
mobile application for storage and sharing (when
prescribed or used under the care of a physician).
It can be used to record heart sounds and cardiac
murmurs, bruits, respiratory sounds, and abdominal
sounds during physical examination in normal patients
or those with suspected diseases of the cardiac,
vascular, pulmonary, or abdominal organ systems. The
device can be used on adults and pediatrics.
The data offered by the device is only signicant when
used in conjunction with physician over read as well
as consideration of other relevant patient data.
The device should not be used on infants weighing
less than 10kg.
1.9
Indications for Use
The privacy of patient health information may be
protected by state, federal, or international/foreign
laws that regulate how such information can be used,
stored, transmitted, and disclosed. The Eko system
employs security features that are compliant with
HIPAA policies. Third party access may be prohibited
to such information without obtaining written
authorization from the patient.
The user is fully responsible for understanding and
following all laws that regulate storage, transmission,
and disclosure of any electronic patient data through
the use of software. If the user becomes unable to
comply with a law or restriction that applies to use and
disclosure of such data, the user should not proceed
to collect or save such information.
This application may require entry of individually
identiable health information in order to function.
Records are stored and recalled through the use of
patient name, date of birth, and/or patient ID number.
By entering this information, the user assumes any
and all risks of and liabilities incurred with using or
transmitting such information.
This package includes
(1) Eko DUO
(1) Wireless Touch Charging Pad
(1) 5W / 2A USB Power Adapter
(1) Micro USB to USB Cable
(1) Eko EARPIECE
(6) Silicone Rubber Eartips
(1) Getting Started Instruction Card
For full functionality, the system requires users to
connect their DUO with an internet-enabled smart
mobile device using the Eko App (not included). The
App supports many Apple® and Android™ mobile
devices.
NOTE: DUO can be used with other audio equipment
or headphones through the 3.5mm audio jack.
However, no performance guarantees are claimed
using other audio products.
System Requirements
The mobile app software can be used with iPhone®
4S, 5, 5C, 5S, 6, 6 Plus, 6s, 6SE, 6S Plus, iPad Mini™ 2,
3, iPad Air®/Air 2, and iPad® 3rd/4th generations with
iOS 7.0+ and at least 25MB of free memory.
DUO uses Bluetooth® Smart; mobile devices used
must be compatible with Bluetooth® Smart.
Apple®, iPhone®, iPad®, iPad Air®, and iPad Mini™ are
registered trademarks of Apple, Inc.
Bluetooth® is a registered trademark of Bluetooth SIG, Inc.
1.10
Patient Privacy
1.11
Contents and Operation
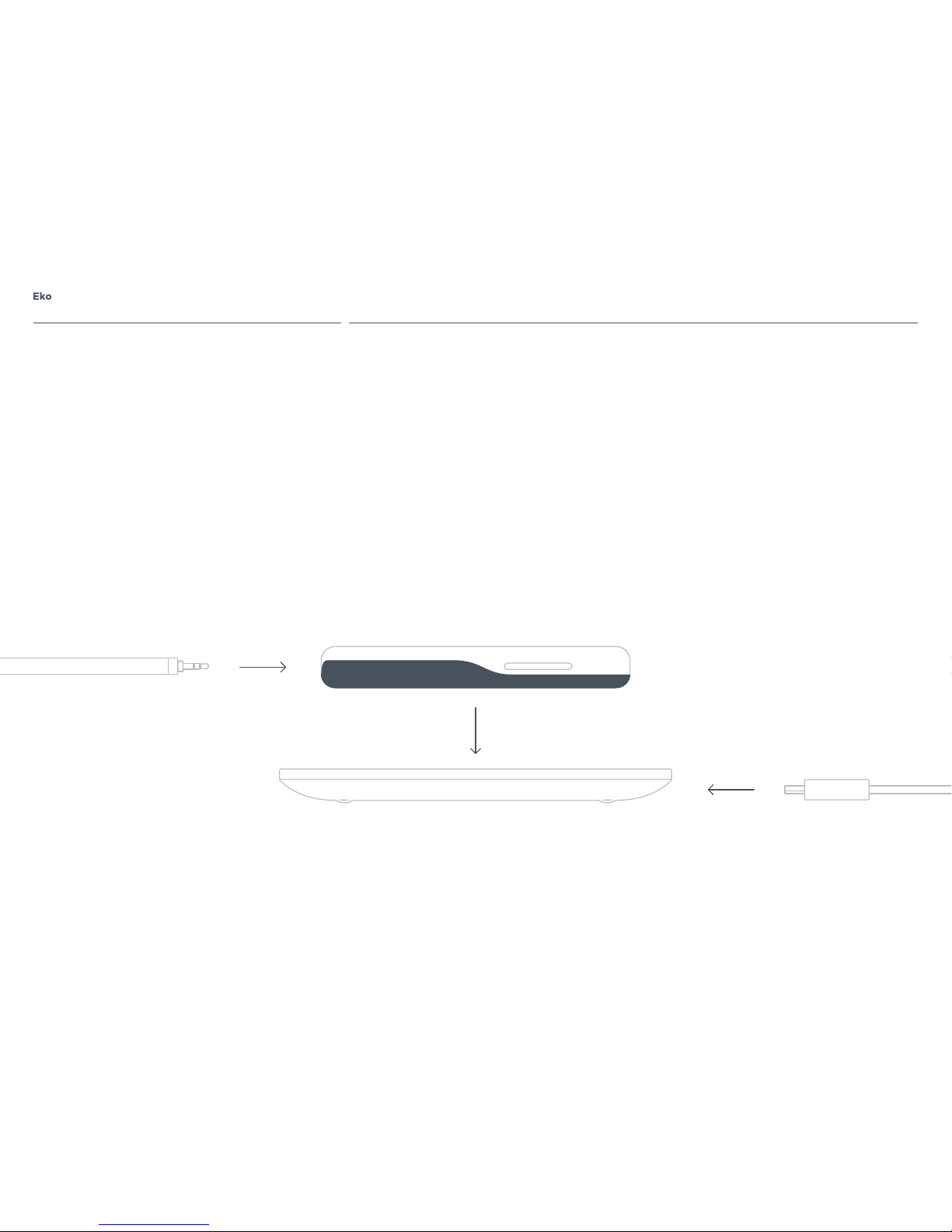
P.
7
Install included EARPIECE by plugging the audio jack
into the 3.5mm audio port at the base of DUO and
tightening the threads.
To charge DUO, place the device on the Wireless
Touch Charging Pad. Connect the charging pad to a
power source using the included USB charging cable
and power adapter. The LED light ring on DUO will
begin to ash to signal the charging status. All of
the LEDs in the ring will light up when the device is
fully charged. DUO should be periodically recharged
even when in storage. Lithium ion batteries slowly
lose charge when in storage and may fall to an
unacceptably low level, damaging the battery.
NOTE: DUO will not operate or connect to the Eko App
while charging.
2.1
Setup
2.2
Charging
Micro USBWireless Touch Charging Pad
EARPIECE
 Loading...
Loading...