eego EE-21 series, EE-22 series, EE-212, EE-211, EE-213 User Manual
...
eego™ amplifier EE-21x EE-22x
User Manual
eego amplifier
EE-211, EE-212, EE-213, EE-214, EE-215 / version 1.2
EE-221, EE-222, EE-223, EE-224, EE-225 / version 1.2
User Manual Revision 5, July 2016
Copyright eemagine Medical Imaging Solutions GmbH.
All rights reserved.

eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 2 of 26 2016-07-11
MANUFACTURER
eemagine Medical Imaging Solutions GmbH
Gubener Str. 47
D-10243 Berlin, Germany
Phone: +49 (0)30 2904 8404
E-Mail: support@eemagine.com
IMPORTANT NOTICES
Following the European Medical Device Directive 93/42/EEC Annex IX, eego amplifier is a CE class IIa
medical device.
This User Manual only covers the EE-21x and EE-22x variants of the eego amplifier. Refer to
dedicated User Manual for other variants.
For US customers only:
Rx only
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.
DISCLAIMER
We have attempted to write this document as accurately as possible. However, mistakes are bound
to occur, and we reserve the right to make changes to the products, which may render parts of this
document invalid.
No part of this document may be copied or reproduced without the explicit permission of the
authors.

eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 3 of 26 2016-07-11
TABLE OF CONTENTS
1 General Information ......................................................................................................................... 4
1.1 About this Document ................................................................................................................. 4
1.2 Intended Use .............................................................................................................................. 4
1.3 Technical Data and Features Overview ...................................................................................... 4
1.4 Safety Warnings ......................................................................................................................... 5
1.4.1 Important information ........................................................................................................ 5
1.4.2 Safety measures .................................................................................................................. 5
1.4.3 Precautionary measures ...................................................................................................... 6
1.4.4 Warnings .............................................................................................................................. 6
1.5 Maintenance and Cleaning ........................................................................................................ 7
1.6 Disposing of Waste Equipment .................................................................................................. 7
1.7 Product Variants ......................................................................................................................... 8
1.8 PC Requirements ........................................................................................................................ 8
1.9 Contact Information ................................................................................................................... 8
2 The eego amplifier, Hardware SETUP description ............................................................................ 9
2.1 The eego amplifier ..................................................................................................................... 9
2.2 Biosignal and Impedance Measurements ................................................................................ 13
2.3 Recording Trigger Input ............................................................................................................ 14
2.4 Mounting Options for the eego amplifier ................................................................................ 14
2.5 Battery and Charger for the eego amplifier ............................................................................. 15
2.6 LED and SOUND Status Indicators for the eego amplifier ....................................................... 16
APPENDIX A: CONNECTORS AND PINNING TABLES .............................................................................. 17
EEG Connectors (Referential) ............................................................................................................ 17
Bipolar Connector .............................................................................................................................. 18
Trigger Connector .............................................................................................................................. 19
Power and USB Connector ................................................................................................................. 20
APPENDIX B: AMPLIFIER TECHNICAL SPECIFICATIONS (EMC) ............................................................... 21
APPENDIX C: EXPLANATION OF SYMBOLS............................................................................................. 23
APPENDIX D: Datasheet......................................................................................................................... 24

eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 4 of 26 2016-07-11
1 GENERAL INFORMATION
1.1 ABOUT THIS DOCUMENT
With this document, we provide you with all information required to set up and start working with
the eego amplifier.
If you encounter problems with the device that cannot be resolved using this document please
contact us directly via support@eemagine.com for further help (please make sure to state the
product ID and serial number of your amplifier in the Subject section of your email).
We will coordinate communication with your dealer in case you purchased the device locally.
1.2 INTENDED USE
The eego amplifier is intended to be used by or under the direction of a physician for acquisition of
EEG signals and to transmit them digitally to a computer.
The device is intended for use on humans.
The device is intended for use in a clinical environment (e.g., hospital, physician’s office, etc).
The device is not intended for use in life support systems.
1.3 TECHNICAL DATA AND FEATURES OVERVIEW
The eego amplifier has been designed as a mobile recording device for high-density EEG and other
electrophysiological signals. It provides access to recorded data over a USB connection to external
software over its signal driver interface. The eego amplifier comes with a built-in rechargeable
battery for mobile applications and recordings of several hours.
This ultimate solution for mobile EEG recording provides maximum 64 channels of referential EEG
and (optionally) synchronously recorded 24 bipolar input signals.
The eego amplifier is used in combination with the eego recording software, a 64-channel or 32-
channel EEG headcap and other optional accessories.
The eego amplifier has one 8-bit trigger input channel accessible through high-density connectors.
Impedance values can be measured for all referential electrodes as well as the reference and patient
ground electrode. An active shielding technique protects the EEG inputs from grid interference noise.
Sampling rate and gain can be set through the user interface of the control software.
Detailed technical specifications, environmental conditions, the available product variants and PC
requirements are given in the APPENDIX D: Datasheet for eego amplifier.

eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 5 of 26 2016-07-11
1.4 SAFETY WARNINGS
The following warnings and cautions apply to the eego amplifier:
Proper use of the eego amplifier depends on careful reading of all instructions and labels that
come with or on the device inaccurate measurements may be caused by incorrect use of the
device.
Non-compliance with warnings and safety regulations may result in severe personal injury!
If you are uncertain or have any questions about operational safety or about any of the warnings and
cautions, then do not hesitate to call or email:
eemagine Medical Imaging Solutions GmbH
Gubener Str. 47
D-10243 Berlin, Germany
Phone: +49 30 29048404
E-Mail: support@eemagine.com
http://www.eemagine.com
For warnings and safety regulations concerning waveguard EEG caps, please refer to the
corresponding manual.
1.4 .1 IM P ORTANT INFORM ATION
There are no known side effects from the use of this equipment.
ONLY APPLICABLE TO THE USA: Under federal law this apparatus may only be sold by or on the
order of a physician or licensed practitioner. The apparatus may only be used under the constant
supervision of or on the instructions of a physician or other authorized medical professional.
1.4 .2 SA FETY MEAS URES
The only external power supply that may be used is the original device supplied
together with the amplifier. DO NOT replace it with anything else. If any third-party
type of power supply is used then patient safety is not guaranteed.
Make sure that the wall socket is well earthed.
Inspect the power cord on a regular basis for damages. Do not operate the device with a
damaged power cord.
The eego amplifier contains LITHIUM-ION BATTERIES. In case of damaged amplifier housing and
to the battery cells inside the housing, metallic Lithium tends to spontaneous formation of
flames when in air contact. Make sure to quarantine any amplifier with damaged battery cells
and inform eemagine support immediately.
Do not connect active sensors or electrodes to any of the inputs of the amplifier.
Do not use the device with adapters, EEG caps or other devices not explicitly listed to be
compatible with the device.
Take care when arranging sensor cables to avoid risks of entanglement or strangulation.
eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 6 of 26 2016-07-11
Reusable electrodes present a potential risk of cross-contamination. Please refer to the
documentation that came with your electrodes for instructions on how to prevent this.
Do not connect the device to a patient undergoing MRI, electro-surgery or defibrillation.
Do not modify the equipment. Never attempt to unscrew any parts of the amplifier.
Do not attempt to open the battery compartment of the amplifier.
ELECTRICAL SHOCK HAZARD: Do not connect electrode inputs to earth ground.
Connecting an earth ground might result in electrocution.
ELECTRICAL SHOCK HAZARD: Servicing of the equipment is to be done by approved
technicians only.
1.4 .3 PRE CAUT IONA RY ME ASURES
When the amplifier is not in use, please make sure to switch it off. The device will switch of
automatically after the batteries are discharged below a given limit. With batteries completely
discharged, the device can only be switched on while the power supply is attached.
The amplifier needs special precautions regarding EMC and needs to be installed and put into
service according to the EMC information provided in APPENDIX B: AMPLIFIER TECHNICAL
SPECIFICATIONS (EMC); the use of accessories other than specified may result in increased
emissions or decreased immunity of the eego amplifier.
The eego amplifier should not be used adjacent to or stacked with other equipment; if this is still
necessary, verify normal operation in the configuration in which it will be used. Do also not stack
multiple eego amplifiers directly on top of each other. Allow for some spacing of several cm in
between.
The use of portable and mobile RF communications equipment can adversely affect the
recording; do not use an operating cellular phone within 30 cm of the amplifier, the cables and
the electrodes to avoid excessive noise on the signals. See also in APPENDIX B: AMPLIFIER
TECHNICAL SPECIFICATIONS (EMC).
Disposable electrodes which are used for electrophysiological measurements may pose a
biohazard. Handle, and when applicable, dispose of these materials in accordance with accepted
medical practice and any applicable local, state and federal laws and regulations.
1.4 .4 WARNING S
Safety and performance of the device are not guaranteed when it is used together
with accessories that were not explicitly listed as compatible.
The device is not defibrillator-proof.
The device is not suitable for use in an inflammable mixture of anesthetics and air, oxygen or
nitrous oxide.
No chemical agents are to be used with the device.
The device is not to be immersed in any liquid and should not be exposed to rain.
If any liquids or moisture penetrate the device or a part thereof, turn the device off, remove the
plug from the wall socket and have the device checked by the manufacturer.
Do not expose the device to direct sunlight, heat from a source of thermal radiation, excessive
amount of dust, moisture, vibration, or mechanical shock.
Do not wind the cables in a loop smaller than 5 cm or bend them sharply, as this may damage the
cables.
eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 7 of 26 2016-07-11
During operation with an active connection via USB to a compatible computer, do not connect
other USB devices, and do not make changes to any connections.
If the eego amplifier is upgraded or replaced for any reason, make sure to have the control
software and interfacing computer checked for compliance by eemagine support before
continuing to work with the device. Similarly have the eego amplifier checked for compliance
when the control software or computer is upgraded or replaced.
During operation place the device in such a way that connectors and cables are easily accessible.
Do not use sharp objects such as pencil-points or pen-tips to manipulate the buttons on the
control panel, as this may cause damage.
Do not use the device whenever parts of it are damaged.
The eego amplifier’s soft-shell must be handled with care and must not be removed from the
device. It may tear otherwise.
1.5 MAINTENANCE AND CLEANING
We would like to give a few general recommendations that may help to keep your equipment in
good shape and ensure high quality of recordings.
The amplifier is mechanically robust – within limits. Try to protect the device as much as possible
from falling and heavy mechanical shocks. Please take special care that the USB cable and the
connectors on either side are not under mechanical stress. A broken USB connection may lead to
expensive and time-consuming repair of the amplifier.
The EEG head-cap and trigger connectors may be attached to the amplifier with the available screws
on the connector in case of expected movement of the patient/subject. The screws do not need to
be extremely tight but just hold the connectors in place, so please tighten the screws gently.
The expected lifetime is 5 years.
If the amplifier requires cleaning, please use a dry cloth to remove dirt (e.g. gel remainders). Do not
use alcohol or cleaning chemicals.
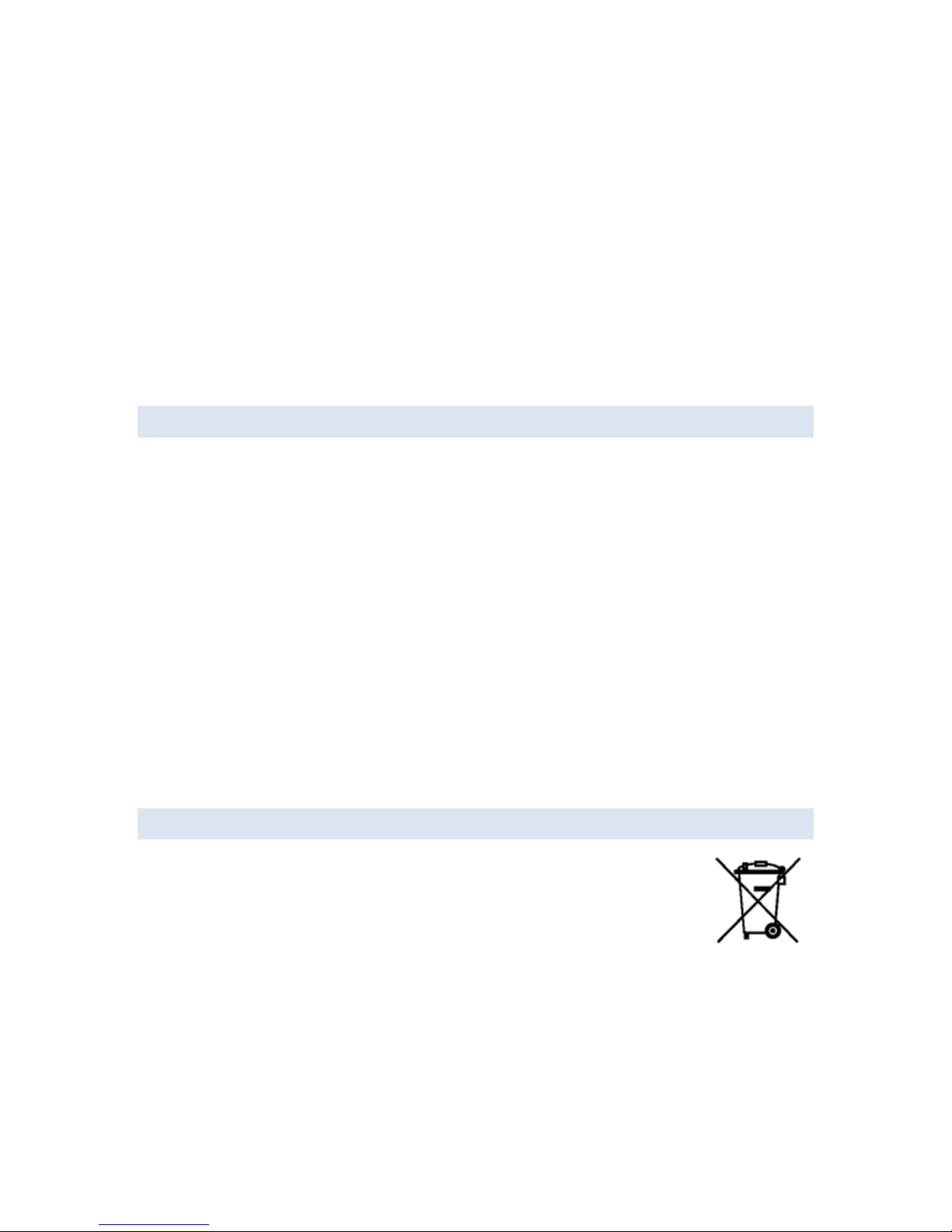
1.6 DISPOSING OF WASTE EQUIPMENT
Various parts of the eego amplifier device bear the symbol for special waste
disposal regulations (EU). The symbol means that the products must not be
disposed of with regular waste. Instead, it is the user’s responsibility to dispose of
waste equipment by handing it over to a designated collection point for the
recycling of waste electrical and electronic equipment. The separate collection and
recycling of your waste equipment at the time of disposal will help to conserve natural resources and
ensure that it is recycled in a manner that protects human health and the environment. For more
information about where you can drop off your waste equipment for recycling, please contact your
local city office, your household waste disposal service, or the manufacturer.

eego amplifier EE-21x EE-22x User Manual
Rev. 5, DRN-PDO-1664 8 of 26 2016-07-11
1.7 PRODUCT VARIANTS
See the APPENDIX D: Datasheet.
1.8 PC REQUIREMENTS
See the APPENDIX D: Datasheet.
1.9 CONTACT INFORMATION
For more information and for reporting problems with eego amplifier, please contact eemagine at:
eemagine Medical Imaging Solutions GmbH
Gubener Str. 47
D-10243 Berlin, Germany
Phone: + 49 30 29048404
E-Mail: support@eemagine.com
http://www.eemagine.com/
 Loading...
Loading...