
EI1121A – Echo PC User manual - Rel. 1.1
E
C
E
USER’S MANUAL
C
H
H
O
O
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
i. INTRODUCTION
i.1. Identification Data
This document represents the User’s Manual of the ECHO, an automatic multiparameter analyzer for clinical chemistry.
The document has to be intended as a functional part of the ECHO analyzer instrument
and the user must read carefully all the sections of this manual, before operate with the
instrument.
The manufacturer doesn’t take any responsibility related to partial or unauthorized copy
of its contents.
i.1.1. Document
• Document Part Number
• Document Revision
• Dated
• Software Version
• Release Date
i.1.2. Instrument
• Instrument Part Number
: EI1121A
: Rel. 1.1
: 03/07/2008
: Rel. 4.23
: 2008
EI0002A
i.1.3. Manufacturer
Edif Instruments s.r.l.
Via Ardeatina, 132
00179 Roma – ITALY
Tel.+39/065127161
Fax +39/065127550
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
email edif@edif.it
i.2. Copyright
This document is property of Edif Instruments S.r.l.
Unauthorized copies or alterations of its content could cause legal actions in order to
preserve manufacturer’s interests.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
i.3. International and European Norms Compliance
This document was written according to UNI EN 591: 1996 standard.
The ECHO equipment meets the following directives:
EN 55001
EN 61326-1
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
i.4. Patents
Currently there are not patents for this instrument.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
i.5. Document Intended Purpose
This document represents the User’s Manual of the ECHO automatic multi-parameter
analyzer.
It is forwarded to laboratory technical personnel that have experience in laboratory
practice and in laboratory automated equipments handling.
NOTE: The Manufacturer declares that the information inside this manual have to
be considered sufficient to perform a proper use of the referred equipment only in
case of readers who have previously followed a manufacturer’s specific training
course.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
i.6. How to Use this Manual
The Manufacturer recommends to read this User’s Manual in any of its parts; in
particular it is necessary to take care of notes, used to specify or to probe concepts
previously treated, and of warnings, used to emphasize potentially risks or hazards.
All the notes and warnings, written in bold print, must be read carefully, paying
particular attention to the following sections:
• Safety (Chapter 1)
• Installation (Chapter 3)
• Maintenance (Chapter 8)
During Laboratory daily operation, it is also suggested by the Manufacturer not to keep
this User’s Manual too far from the ECHO instrument.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
i.7. Index of Contents
Section i - INTRODUCTION
i.1 Identification Data .............................................................................................. pag. 1
i.1.1 Document .................................................................................................... pag. 1
i.1.2 Instrument.................................................................................................... pag. 1
i.1.3 Manufacturer ............................................................................................... pag. 1
i.2 Copyright............................................................................................................ pag. 2
i.3 International and European Norms Compliance ................................................ pag. 3
i.4 Patents................................................................................................................. pag. 4
i.5 Document Intended Purpose............................................................................... pag. 5
i.6 How to Use this Manual ..................................................................................... pag. 6
i.7 Index of Contents ............................................................................................... pag. 7
Section 1 - SAFETY
1.1 General Warnings............................................................................................... pag. 1
1.2 Safety Labels ...................................................................................................... pag. 3
1.3 Safety Warnings ................................................................................................. pag. 4
1.3.1 Installation ................................................................................................... pag. 4
1.3.2 Operations.................................................................................................... pag. 4
1.3.3 Maintenance ................................................................................................ pag. 5
1.3.4 Transport and Storage.................................................................................. pag. 5
1.4 Risks Related to the Use..................................................................................... pag. 6
1.4.1 Human Hazard............................................................................................. pag. 6
1.4.2 Personal Safety Aspects .............................................................................. pag. 7
1.4.3 Information on Infectious Liquids and Parts ............................................... pag. 7
1.4.3.1 Handling............................................................................................. pag. 7
1.4.3.2 Dismantling........................................................................................ pag. 8
1.4.3.3 Contamination.................................................................................... pag. 8
1.5 Recommendations for Optimal Performance ..................................................... pag. 9
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Section 2 - SYMBOLS
2.1 List of Used Symbols and Physical Position Overview ..................................... pag. 1
Section 3 – INSTALLATION
3.1 Equipment Overview.......................................................................................... pag. 1
3.1.1 Supplied Materials....................................................................................... pag. 2
3.1.1.1 Standard Supply List.......................................................................... pag. 2
3.1.1.2 List of Options ................................................................................... pag. 2
3.2 Installation Constrains ........................................................................................ pag. 3
3.2.1 Mechanical .................................................................................................. pag. 3
3.2.2 Environmental .............................................................................................pag. 3
3.2.3 Software....................................................................................................... pag. 3
3.3 Storage................................................................................................................ pag. 4
3.4 Unpacking........................................................................................................... pag. 5
3.4.1 Package Characteristics ............................................................................... pag. 5
3.4.2 Transport Damages Check ..........................................................................pag. 5
3.4.3 Removal From Package............................................................................... pag. 6
3.5 Hardware Installation ......................................................................................... pag. 7
3.5.1 Electrical Connections................................................................................. pag. 7
3.5.2 Instrument Installation Procedure................................................................ pag. 7
3.5.3 Fuses............................................................................................................ pag. 8
3.5.4 Accessories .................................................................................................. pag. 8
3.5.4.1 Tanks.................................................................................................. pag. 8
3.6 Software Installation........................................................................................... pag. 10
3.6.1 Requirements and Recommendations .........................................................pag. 10
Section 4 – THEORY OF OPERATION
4.1 Abstract............................................................................................................... pag. 1
4.2 Principle of Working .......................................................................................... pag. 3
4.3 Bibliography ....................................................................................................... pag. 5
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Section 5 – FUNCTIONS
5.1 Intended Use....................................................................................................... pag. 1
5.2 Instrument Functions Overview ......................................................................... pag. 2
5.3 Functional Subassemblies .................................................................................. pag. 4
5.3.1 Working Area .............................................................................................. pag. 4
5.3.1.1 Reagent Plate ..................................................................................... pag. 5
5.3.1.2 Sample Plate....................................................................................... pag. 6
5.3.1.3 Incubation Plate ................................................................................. pag. 6
5.3.2 Pipetting Subassembly................................................................................. pag. 7
5.3.2.1 Sampling Needle................................................................................ pag. 7
5.3.2.2 Aspiration Needle .............................................................................. pag. 7
5.3.2.3 Syringe ............................................................................................... pag. 7
5.3.2.4 Peristaltic Pump ................................................................................. pag. 7
5.3.3 Flow Cells.................................................................................................... pag. 8
5.3.4 Spectrophotometric Reading ....................................................................... pag. 8
5.4 Management System .......................................................................................... pag. 10
5.4.1 Software Architecture.................................................................................. pag. 10
5.4.1.1 Main Menu......................................................................................... pag. 10
5.4.1.2 Work List ........................................................................................... pag. 10
5.4.1.3 Execution ........................................................................................... pag. 10
5.4.1.4 Results................................................................................................ pag. 10
5.4.1.5 Chemistries ........................................................................................ pag. 11
5.4.1.6 Service................................................................................................ pag. 11
Section 6 – PERFORMANCE CRITERIA AND LIMITATIONS
6.1 General Statement............................................................................................... pag. 1
6.2 Photometer.......................................................................................................... pag. 3
6.2.1 Precision ...................................................................................................... pag. 3
6.2.2 Accuracy...................................................................................................... pag. 4
6.2.3 Linearity ...................................................................................................... pag. 5
6.3 Pipettor ............................................................................................................... pag. 7
6.3.1 Precision ...................................................................................................... pag. 7
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
6.3.2 Accuracy...................................................................................................... pag. 8
6.4 Carry-Over.......................................................................................................... pag. 9
Section 7 – OPERATING PROCEDURES
7.1 Preparation Prior Operations .............................................................................. pag. 1
7.1.1 Safety Recommendations and Remarks ...................................................... pag. 1
7.1.1.1 User Skill Level ................................................................................. pag. 1
7.1.2 Instrument Setup.......................................................................................... pag. 2
7.1.2.1 Instrument Built-in On Line Test....................................................... pag. 2
7.1.3 Sample Handling ......................................................................................... pag. 3
7.1.3.1 Sample Type ...................................................................................... pag. 3
7.1.3.2 Pre-treatment...................................................................................... pag. 3
7.1.3.3 Preservation........................................................................................ pag. 4
7.1.3.4 Sample Minimum Volume................................................................. pag. 4
7.1.3.5 Dead Volume ..................................................................................... pag. 4
7.1.4 Reagent and Disposable Materials ..............................................................pag. 4
7.1.4.1 Use ..................................................................................................... pag. 5
7.1.4.2 Preservation ....................................................................................... pag. 5
7.1.5 Fixture and Accessories............................................................................... pag. 5
7.2 Management System Overview.......................................................................... pag. 6
7.2.1 How To Start ...............................................................................................pag. 6
7.2.2 Main Menu ..................................................................................................pag. 7
7.3 Chemistries Menu .............................................................................................. pag. 9
7.3.1 Edit Methods ............................................................................................... pag. 10
7.3.1.1 Types Selection.................................................................................. pag. 12
7.3.1.2 Filters Selection ................................................................................. pag. 13
7.3.1.3 Incubation Time ................................................................................. pag. 13
7.3.1.4 Stabilization Time.............................................................................. pag. 14
7.3.1.5 Reading Time..................................................................................... pag. 14
7.3.1.6 Reagent 2 – Incubation Time............................................................. pag. 14
7.3.1.7 Reagent Position ................................................................................ pag. 14
7.3.1.8 R1 Reagent Volume .......................................................................... pag. 14
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.9 R2 Reagent Volume........................................................................... pag. 15
7.3.1.10 R3 Reagent Volume ........................................................................... pag. 15
7.3.1.11 Sample Volume.................................................................................. pag. 15
7.3.1.12 Numbers of Calibration Standards..................................................... pag. 15
7.3.1.13 Numbers of Controls.......................................................................... pag. 16
7.3.1.14 Standard Position ...............................................................................pag. 16
7.3.1.15 Control Position .................................................................................pag. 16
7.3.1.16 Standard Value................................................................................... pag. 16
7.3.1.17 Factor ................................................................................................. pag. 17
7.3.1.18 Number of Curves.............................................................................. pag. 18
7.3.1.19 Description of the Curve .................................................................... pag. 19
7.3.1.20 Substrat Depletion.............................................................................. pag. 19
7.3.1.20 bis Sample dilution …………………………………………………. pag. 19
7.3.1.21 Dilution Ratio..................................................................................... pag. 19
7.3.1.22 Linearity ............................................................................................. pag. 20
7.3.1.23 Reagent blank value: min................................................................... pag. 20
7.3.1.24 Reagent blank value: max .................................................................. pag. 20
7.3.1.25 Normal Value: min ............................................................................ pag. 20
7.3.1.26 Normal Value: max............................................................................ pag. 21
7.3.1.27 Measure Unit Selection...................................................................... pag. 21
7.3.1.28 Washings Number.............................................................................. pag. 22
7.3.1.29 Blank Reagent Selection .................................................................... pag. 23
7.4 Patient Handling ................................................................................................. pag. 25
7.4.1 Edit Work List ............................................................................................. pag. 26
7.4.2 Load Work List............................................................................................ pag. 31
7.4.3 Display Work List .......................................................................................pag. 32
7.4.4 Print Work List............................................................................................ pag. 33
7.4.5 Print Profile List .......................................................................................... pag. 33
7.5 Operative Procedure ........................................................................................... pag. 34
7.5.1 Batch............................................................................................................ pag. 36
7.5.1.1 Tests and Control Selection ............................................................... pag. 37
7.5.1.2 Standardisation Procedure.................................................................. pag. 38
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.5.1.3 Execution ........................................................................................... pag. 41
7.5.1.4 Automatic sample dilution …………………………………………. pag. 43
7.5.1.5 Automatic standard dilution ……………………………………….. pag. 43
7.6 Result Handling .................................................................................................. pag. 44
7.6.1 Display Results............................................................................................ pag. 45
7.6.2 Print Results................................................................................................. pag. 46
7.6.3 Repetitions................................................................................................... pag. 47
7.6.3.1 Repetition of One Test ....................................................................... pag. 48
7.6.3.2 Repetition of One Patient................................................................... pag. 49
7.6.4 Controls ....................................................................................................... pag. 50
7.7 Urgent Analysis .................................................................................................. pag. 52
7.8 Dismantling ........................................................................................................ pag. 52
7.8.1 Reagents, Consumable and Toxic ............................................................... pag. 53
7.8.2 Instrument and Components........................................................................ pag. 53
Section 8 - MAINTENANCE
8.1 User Maintenance Basics.................................................................................... pag. 1
8.1.1 Competence ................................................................................................. pag. 1
8.1.2 Cleaning....................................................................................................... pag. 2
8.1.3 Disinfection .................................................................................................pag. 2
8.1.3.1 Instrument Disinfection Procedure .................................................... pag. 2
8.1.3.2 Metallic Needle.................................................................................. pag. 3
8.1.3.3 Waste Tube (External) ....................................................................... pag. 3
8.1.3.4 Waste Tank ........................................................................................ pag. 3
8.2 User Maintenance Warnings .............................................................................. pag. 4
8.3 User Daily Maintenance Procedure.................................................................... pag. 5
8.4 User Weekly Maintenance Procedure ................................................................ pag. 6
Section 9 – TROUBLESHOOTING
9.1 Normal Operation Basics.................................................................................... pag. 1
9.2 Diagnostic System .............................................................................................. pag. 1
9.3 Trouble Sources Classification Remarks............................................................ pag. 1
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
9.4 Correct Operations Messages ............................................................................. pag. 1
9.5 Error Messages List and User Required Actions ............................................... pag. 2
9.6 Competence ........................................................................................................ pag. 8
Section 10 – TECHNICAL SPECIFICATIONS
10.1 Instrument Technical Specification.................................................................... pag. 1
10.1.1 Sample Loading........................................................................................... pag. 1
10.1.2 Sera and Reagent Pipetting Station ............................................................. pag. 2
10.1.3 Incubating Station........................................................................................ pag. 2
10.1.4 Photometric Reading ...................................................................................pag. 2
10.2 Adjustments and Settings ................................................................................... pag. 3
10.3 Electrical Requirements...................................................................................... pag. 4
10.4 Operating Environmental Requirements ............................................................ pag. 5
10.5 Storage Environmental Requirements................................................................ pag. 6
10.6 Emissions............................................................................................................ pag. 7
10.7 Electromagnetic Compatibility........................................................................... pag. 8
10.8 Consumptions ..................................................................................................... pag. 9
Section 11 – SUPPLEMENTARY INFORMATION
11.1 Short Users Instruction ....................................................................................... pag. 1
11.2 Warranty Limitations.......................................................................................... pag. 2
11.3 Spare Parts and Consumables............................................................................. pag. 3
11.4 Ordering Information.......................................................................................... pag. 4
11.5 Technical Assistance .......................................................................................... pag. 5
11.6 Forms.................................................................................................................. pag. 6
Section 12 – GLOSSARY
12.1 List of Acronyms and Abbreviations.................................................................. pag. 1
12.2 List of Terms ...................................................................................................... pag. 2
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
1. SAFETY
The safety precautions and regulations included in this section must be strictly observed
by the User.
The use of the Instrument for purposes other than those specified in Chapter 5 will
automatically invalidate any warranty agreement.
The user must not carry out any kind of alteration on the Instrument, included software
manipulations. Every unauthorized change or modification and not observance of the
maintenance procedures will immediately invalidate warranty agreements.
1.1. General Warnings
All notes and warnings in this Manual which are in bold and/or underlined must be read
carefully, paying particular attention to the following sections:
• Installation (Section 3)
• Disinfections Procedures (Section 8)
All assay methods must be validated on the analyzer before carrying out any laboratory
routine. Such validation must include:
• Make sure that the instrument correctly handling the various reagents, with
reference to:
o Aspiration, transfer and distribution of the liquid
o Liquid mixing (if required) without bubble or froth formation, which may
create problems in later stages of the process.
• Reliability of materials used for the procedure (sample tubes, reagent and/or
control vials, microplates, etc).
• Accurate calculation: ensure that the software calculations give results
comparable to those obtained using the methods specified by the assay
manufacturer.
These procedures are normally validated by the distributor: if the required data are not
available for any particular assay, the user must validate these procedures himself.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Any modification made to pre-validated procedures must be validated again.
The User should always check the proper closure of the Instrument covers, during the
use of the instrument.
The User must always assure himself that all Instrument technical activities related to
installation, performance verification and repair are carried out by qualified personnel.
All precautions and recommended practices normally used in the laboratory (Good
Laboratory Practice) must be followed: this is particularly important because the
diagnostic tests are carried out automatically by the analyzer.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it
EI1121A – Echo PC User manual - Rel. 1.1
1.2. Safety Labels
Infective tank warning
This label is placed on the external tank for the waste and near the peristaltic pumps. It
informs the user about the contamination risk for the part where it is placed.
ATTENZIONE
SERBATOIO POTENZIALMENTE INFETTO
WARNING
POTENTIALLY INFECTIVE TANK
Electrical risk warning
This label is placed on the back of the instrument and it informs the user about electrical
risk.
WARNING
HIGH VOLTAGE INSIDE DO
NOT OPEN CABINET, REFER
TO QUALIFIED PERSONNEL
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
1.3. Safety Warnings
To reduce the risk of electric shock, keep away from live circuits. The instrument must
be connected to a ground point. The instrument is equipped with a three-core cable to be
connected to a 220 Vac or 110 Vac power socket.
1.3.1. Installation
On customer demand, the installation, verification and calibration of the instrument,
will be carried out by qualified technical personnel. They should take reasonable steps
to ensure that the location in which the equipment is to be installed meets the
environmental and electromagnetic specifications for the analyzer. Any significant
movement of the equipment should be carried out by qualified personnel.
If the instrument has the refrigerated plate option, a free air space of at least 10 cm is
necessary to allow air-cooling inlet, placed on the bottom of the instrument, on the left
side.
1.3.2. Operations
If the ECHO is used for automated clinical chemistry analysis; the Manufacturer
reminds the Users that failures following safety precautions and regulations could
produce false negative results reporting.
For these reasons, particular care must be carried out by the operator to the following
points:
• Use only test procedures or assays pre-validated by the Manufacturer or its
distributor;
• Don’t introduce any modification on pre-validated procedures or assays;
• Avoid to use vials other then Manufacturer suggested types;
Particular care must be observed for the instructions provided by the Manufacturer in
order to perform correct switching on and off procedures.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
NOTE: The Manufacturer reminds to the User that safety precautions failure
could produce human hazard, risk or damages both for operator and patient.
1.3.3. Maintenance
The User must carry out the maintenance procedures quoted in Chapter 8, in order to
assure a correct operation of the instrument and the reliability of the assay results.
NOTE: The Manufacturer remarks to the User that continuous Instrument visual
inspection is the first and easier way to guarantee the best equipment
performances.
User’s maintenance tasks must observe the following safety recommendations:
• Read carefully the instruction on Chapter 8 before starting with maintenance;
• Clean every part of the Instrument only using soft cloths;
• Remove immediately spills or sketches from the working planes, if any;
• Use only non corrosive solutions;
All User maintenance tasks must be carried out with the Instrument in OFF state
and with the main power cable disconnected from the power socket.
1.3.4. Transport and Storage
Take care during the transport and the unpacking of the instrument.
To store the instrument read carefully Section 10.6 ‘Storage Environmental
Requirements’.
WARNING
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
1.4. Risks Related to the Use
The Manufacturer reminds that the use of the Instrument doesn’t assure the absence of
exposure to BIOHAZARD so the Instrument must be always handled as potentially
infected device.
The Manufacturer declares that information provided through this manual have to be
consider sufficient to perform a “free of risk” usage of the referred equipment only in
case of readers that previously exceed a manufacturer’s specific training course.
To reach the full throughput condition of the Instrument and to assure at the same time
the user safety, the Manufacturer recommends, considering both the operator and the
patient point of view that the daily operators have to be also previously trained to the
operating system basic usage.
The Manufacturer also assumes that all precautions and recommended practices
normally used in the laboratory (Good Laboratory Practice) must be applied by the
Instrument operators.
NOTE: To avoid running additional risks, do not make any unauthorised
modifications; any kind of alteration should be made with the prior authorisation
of the manufacturer.
1.4.1. Human Hazards
Particular safety items against human hazards are the following:
• Do not eat, drink or smoke in the laboratory;
• Wear the lab coats at all time and particularly near the Instrument placement
point.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
1.4.2. Personal Safety Aspect
The Manufacturer declares that the Instrument and all its internal parts were designed
and manufactured to prevent any possible risk for the operator safety.
It is mandatory, for the operator safety, to install an emergency breaker at not more than
one meter from the Instrument.
1.4.3. Information on Infectious Liquids and Parts
The use of the instrument doesn’t assure the absence of exposure to biohazard.
The Manufacturer remarks to the User that all parts of the Instrument that could come
into direct contact with blood or sera, including positive controls, and/or reagent must
always be treated as potentially infected materials.
The instrument must be always handled as potentially infected device.
1.4.3.1. Handling
When handles potentially contaminated sera, the operator must wear:
• Lab coat
• Disposable gloves
• Safety glasses
in order to avoid splashes and/or spilling of the infectious liquids that could come into
contact with any exposed part of the body.
Take special care when you handle the following parts of the instrument:
WARNING
• Metal needles
• Waste tank
because they could be contaminated: they come in contact with sera and reagents.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Eventual tools and instruments used during technical intervention must be sterilised,
before putting them back into their cases.
1.4.3.2. Dismantling
All waste materials, including both liquids and solid, must be dismantled according with
regional laws and local regulations.
The Manufacturer remarks to the user that all waste materials must always be
treated as potentially infected materials.
In case of replacement of faulty parts, each broken parts, dismounted from the
Instrument just by manufacturer or local distributor qualified personnel, must be
handled as potentially infected materials.
WARNING
The Manufacturer declares that all technical personnel have exceed a specific training
course related to infected materials treatment.
All parts dismounted from the Instrument that could have been entered in direct
contact with possible contamination sources must be subjected to a valid
decontamination process before leaving the User’s medical laboratory.
The dismantling of the product should be carried out by applying the national
regulations, referring to the local environment authority, taking into consideration that
the instrument is manufactured with no noxious materials for the environment.
1.4.3.3. Contamination
Risks of contamination exist only during the operations of loading/unloading; with
regard to this, see section 1.4.3. (Information on Infectious Liquids and Parts). During
the instrument operation there are not contamination risks because the instrument, in
this step, doesn’t need the user intervention.
WARNING
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
1.5. Recommendations for Optimal Performance
The User must observe the following recommendations in order to get the specified
performance from the instrument.
• Fill sample vials out of the instrument, in order to avoid spilling of liquids on
the ECHO working area.
• The user must ensure that, when placing test tubes or vials into the analyzer,
these are properly seated.
• If the laboratory does not have back up power supplies, a standby system
(UPS) providing at least 1.5 kW, should be used to provide power during shortterm cuts.
WARNING
Use of the instrument for purposes other than specified, indicated or agreed upon
with the manufacturer, will automatically invalidate all warranties. The
manufacturer reserves the right to take any legal action to protect all its interests.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
2. SYMBOLS
No symbols are applied on this version of ECHO.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3. INSTALLATION
3.1. Equipment Overview
The ECHO is provided with internal LCD screen, that allows to control all its functions
and operation. Its working area is planned to work in safety and with maximum
productivity; it enclose the reagent plate, where are placed the reagent tubes, the sample
plate, that contains the sample vials, ant the incubation plate.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.1.1. Supplied Materials
In the following sections are listed the supplied materials, subdivided in a standard
supply list and in a list of options.
3.1.1.1. Standard Supply List
EDIF CODE OLD CODE
EI0703CV1 P10 0000 0023 Halogen lamp (MATE-N-LOCK connector)
1
1 EI0801B
1 EI0802A
1 EI0710A
1 EI0711A
1 EI0714A
1 EI0715A
1 EI0716A
1 EI0717A
1 EI0702B
1 EI0721A
1 EI0722A
1 EE0203A
1 EI0702B
1 EI1126A
1 EI0732A
1 EE0202B
P10 0000 0008 Tubes kit
P10 0000 0010 Sampling and Aspiration Needles kit
P10 1000 0001 Reagent bottles 25 ml (27 pos.) pack (30 pcs)
P10 1000 0002 Reagent bottle caps 25 ml (27pos.) pack (30 pcs)
P10 0000 0003 Needle cleaning filters pack
P10 0000 0004 Reaction segments pack (100 pcs)
P10 0000 0005 Serum cups pack (1000 pcs)
P10 0000 0006 Thermal paper pack (3 pcs)
P10 0000 0012 Fuses kit (230V)
P10 0000 0017 Water charge bottle
P10 0000 0018 Waste bottle
S10 2100 0009 Null modem host cable
P10 0000 0012 Fuses kit 230V
Brief user manual
Documentation CD
Power cord
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.2. Installation Constrains
During the installation of the ECHO, the operator has to observe mechanical and
environmental constrains in order to assure a correct instrument operation.
3.2.1. Mechanical
ECHO should be positioned on a working bench, at least 1 meter long, free of vibrations
and far from electromagnetic sources (big electrical motors, elevators, therapeutic
equipment, X rays etc.) and with a good ground connection.
The working bench must be a robust table, to prevent the oscillation of the instrument: the
table should be as flat as possible and capable of bearing the weight of the instrument.
3.2.2. Environmental
The place where the instrument will be installed should be possibly an air-conditioned
room, to have a firm temperature and a correct degree of humidity. Avoid the direct
exposure to the sunbeam.
Allow a working space around the instrument of at least 50cm. However, if space
facilities are restricted and the instrument has the refrigerated plate option, the air intake
located on the bottom of the instrument, on the left side, must have a free air space of at
least 10 cm.
3.2.3. Software
The ECHO software is a firmware multitask application purposely realized for this
instrument; it has characteristics of reliability and use facility. It is recorded on the two
EPROMS mounted on the CPU board.
An internal mini-PC with a DOS Terminal program is used for the video, keyboard,
external printer and host interface.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.3. Storage
To store in the right way the instrumentation, it is recommended to store it in a dry area
and to observe the storage environmental requirements listed in section 10.6.
Remember that the instrument must be stored exclusively in its original wood packing
and that the operations for the storage must be carried out only by authorized personnel.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.4. Unpacking
Before proceeding to unpacking the instrument, check that all constrains and
environmental conditions are met – refer to section 10 Technical Specifications.
3.4.1. Package Characteristics
ECHO is normally despatched in a box made of cardboard studied to give the best
protection to the instrument, during transportation in normal conditions. The original
packing can be re-used if the instrument has to be moved or sent back.
3.4.2. Transport Damages Check
Check the package carefully for damages. The eventual damages caused by the
transport should be contested immediately to the shipping company and noted on the
delivery note.
Once the goods are delivered to the shipping company, the responsibility for its integrity
is his until the delivery of the goods.
If you find some anomalies:
1) Do not refuse the shipment
2) On the receipt of delivery, write a note on the fact
3) Do not remove the package! Leave the instrument in the original package, and request
immediate inspection from carrier within 15 days from delivery – if the delivery is
international, the inspection must be requested within 3 days from delivery.
NOTE: Check if the above procedure is in conformity with local regulations and/or
special agreements with the shipper.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.4.3. Removal from Package
Open the boxes and extract the instrument carefully.
Verify that all the parts described in the delivery note are present, including the
welcome kit as well.
Control the serial number of the instrument, if the same corresponds to the one indicated
in the delivery note.
Remove the lid and then remove the two lateral elements that secure the instrument.
At least two persons are needed to move the instrument from its box to the working table.
Lift the instrument up and place it on the working bench (the one on which the
instrument is destined to be operated upon).
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Hardware Installation
ECHO is a sophisticated high precision instrument. A correct installation will assure the
good functionality of the instrument. We suggest to execute the installation by the
technical staff of the I.S.E. S.r.l. or by staff trained by I.S.E. S.r.l. and make sure to
carry out a complete start up of the instrument before operation.
For detailed safety instructions read carefully Section 2.
3.5.1. Electrical Connection
In compliance with the safety precautions listed at the beginning of this manual, to
reduce the risk of electric shock, the instrument should be connected to a ground point.
The instrument is equipped with a three-core cable for connection to a 220 V or 110 V
power socket. Therefore connect the power cord, situated on the back of the instrument,
to a 115 or 230 VAC 50/60 Hz grounded receptacle.
NOTE: Although ECHO is equipped with an internal stabilised power supply, it
does not manage temporary power losses. The installation of backup equipment
with a power rating of 1.5 kW (to be supplied to the load) is strongly
recommended.
3.5.2. Instrument Installation Procedure
Connect all the cables, included the monitor and the printer ones, if you want use an
external printer. A description of the cabling is outlined in the table shown below.
FUNCTION CABLE
Power line Three-pole Bulgin F
Input for printer
Output for monitor
Power line, monitor Three-pole Bulgin M
Centronics
(25 pole M -37 AMP M)
Input for keyboard Multipole shielded
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.5.3. Fuses
Four external protection fuses are inserted into the switch assembly: 3.15A.
3.5.4. Accessories
The ECHO accessories are indispensable parts for the instrument working; the
instrument needs, for its operation, to be connected with two external tanks; one
contains the liquid for the hydraulics filling and the other is used to contain the waste
liquids.
The connection between tanks and instrument is described on the next section.
3.5.4.1. Tanks
To install the waste tank, insert the waste tube coming from the peristaltic pump
positioned on the right side of the instrument (fig. 3.2), into the 1000 ml waste bottle,
furnished with the instrument.
fig. 3.2
Position the bottle of 500 ml containing the washing solution (our code E/LV01) on its
seat, on the right of the diluter module, introduce the tube coming from the diluter valve
into the bottle.
Make sure that the tube of the peristaltic pump is inserted and the rotor is well tight.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
3.6. Software Installation
The ECHO software is installed and configured by specialized personnel, to assure an
optimal working of the analysis system.
3.6.1. Requirements and Recommendations
The ECHO software is optimized to work with this instrument and it uses in the best
way its potentialities.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
4. THEORY of OPERATION
4.1. Abstract
ECHO is an automatic multi-parameter analyzer for Clinical Chemistry developed for
medium or small size labs. This equipment allows to work in “batch mode” or in
Urgency procedure, reaching its maximum productivity (180 tests/ hour) with the
optimized batch procedure. The ECHO can store 64 methods located in two different
panels and each method can use until 3 different reagents. It is possible to set 16
different profiles from these methods. The instrument performs the test repetitions and
dilutions automatically or according to the operator choices.
The Instrument can perform, with automatic processes, the following basic operations:
• Reagent distribution process;
• Incubation control;
• Photometric reading process;
• Data analysis evaluation process;
• Analysis results printout process.
All the above mentioned tasks can be fully automated and software controlled reducing
time and human resources and assuring a final quality level result free from accidental
errors due to the human routine action tolerance.
Each task is executed using both electromechanical devices (such as stepper motors,
pumps, valves, etc.) and electronics part (such as active and passive components, micro
controller and microprocessors, and so on) including software programs.
The ECHO also includes an electronic photometer working on 340-700nm range that
represents the basic instrument for the automatic reading of the analysis results and for
controls evaluation.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
All working procedures must be validated on the analyser before carrying out
every laboratory routine.
These procedures are normally validated by the distributor: if the required data
are not available for any particular assay, the user must validate these procedures
himself.
Any modification to pre-validated routine must be validated again.
The main topics of the ECHO instrument are the following:
• Up to 27 positions for reagent bottles;
• Up to 64 seats for sample vials;
• 96 positions for incubation tubes.
WARNING
• 1 flow cell for reading is available;
• Urgent Access capability;
• Adaptability to a wide variety of tests available on the market;
• Result data evaluation through proved interpolation methods;
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
4.2. Principle of Working
The ECHO executes programmed tests in the following two manners: with optimised
times, Batch Mode, for all the patients; Urgent Patients, one by one.
For the Batch Mode, the ECHO is working according to the following principles: every
cycle is composed of a complete wheel (96 wells). For every cycle it is possible to insert
up to 96 wells. Including also the first well for the blank reagent of each method, a well
for each standard, a well for each control and a well for each test.
For each test you can programme 27 reagents, the tests with 2 reagents will take 2
places.
Each method is executed completely in one cycle (the maximum per method is of 64
sample / test).
The cycle is programmed for methods that don’t use more than 96 wells. The methods
over this limit will be executed in the successive cycle.
At the end of execution, the ECHO will sign it with 3 bips and will come back to the
execution menu to ask for the next cycle, until the execution of all the programmed test.
During the cycle, the end point methods, including the bi-chromatic ones, are
distributed for first and will be measured at the end of the incubation time.
During this interval, the Fixed Time methods, kinetics and differentials are executed.
The ECHO will begin the sampling of the tests of one method, waiting the incubation
time. When the incubation is terminated, the instrument continues alternatively with a
reading of the incubated sample and doing a new sampling.
If the cycle is composed only by End Point methods, the same operational mode is used
to increase the execution as much as possible. When a method has done all the
samplings, the memorized number of washings will be done.
At the beginning of each cycle, an auto zero procedure is executed.
A washing is executed at the beginning and at the end of each cycle.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
For the Urgent Patients, the ECHO is working according to the following principles:
Only the patients signed as Urgent will be taken in consideration. The ECHO will
execute one patient at a time, for all the methods with a maximum of 27 reagents.
At the end of a patient, the instrument will sing with 3 bips, the operator can verify the
reagents and the sampling and can initiate a new execution.
The principles are the same as the ones described in the Batch mode.
For the successive patients the initial washings and the auto zero procedure are not
effected.
The blank reagent is executed only for the first patient on whom the method was
programmed.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
4.3. Bibliography
- Alon R., Bayer E.A. and Wilcheek M. J. (1990). Streptavidin contains an RYD
sequence which mimies the RGD receptor domain of fibroneetin. Biochem. Biophys.
Res. Commun. 170, 1236-1241.
- Brinkley M. (1992). A brief survey of methods for preparing conjugates with dyes,
haptens and cross-linking reagents. Bioconjugate Chem 3, 2-13.
- Chan D.W. Immunoassay Automation Academic Press.
- Diamandis E.P. and Christopoulos T.K. (1991). The biotin-(strep)avidin system:
Principles and applications in biotechnology. Clin. Chem. 37(5), 625-636.
- Engvall E. and Perlmann P. (1971). Enzyme linked immunosorbent assay (ELISA)
III Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in
antigen coated tubes. J. Immunol. Methods 10, 161-170.
- Gosling J.P. (1990). A decade of development in immunoassay methodology:
Review. Chn. Chem. 36, 1408-1427.
- Harlow E. and Lane D. eds. (1988) Antibodies: A Laboratory Manual Cold Spring
Harbor Laboratory, Cold Spring Harbor, NY.
- Ishikawa E., Hashida S., Kohono T. Ad Taka K. (1988). Method for enzymelabeling of antigens, antibodies and their fragments. In Non-isotopic Immunoassay,
T.T. Ngo. ed. pp. 27-55. Plenum Press. New York.
- Johannsson A., Stanley C.J. and Self C.H. (1985). A fast highly sensitive
coloribetric enzyme immunoassay system demonstrating benefits of enzyme
amplification in clinical chemistry. Clin. Chim. Acta 148, 119-124.
- Kabakoff D. (1980). Chemical aspects of enzyme-immunoassay, in Enzyme-
Immunoassay, E.T. Maggio ed., pp. 97-98 CRC Press, Boca Raton, FL.
- Kricka L.J. (1993). Ultrasensitive immunoassay techniques. Review. Clin. Biochem.
26(5), 325-331.
- Kricka L.J. (1994). Selected strategies for improving sensitivity and reliability of
immunoassays. Clin. Chem. 40(3), 347-57.
- Pesce A.J., Krieger N.J. and Michael J.G. (1983). Theories of immunoassay
employing labelled reagents with emphasis on heterogenous enzyme immunoassay,
In Immunoenzymatic Techniques, pp. 127-138. Elesevier, Amsterdam.
- Pesce A.J. and Michael J.G. (1992). Artifacts and limitation of enzyme
immunoassays. J. Immunol Methods 150, 111-119. [Review].
- Peterman J.H. (1991). A Summary of the principle immunoassay data analysis
packages, In Immunochemistry of Solid Phase Immunoassay, J.E. Butler, ed., pp
293-297. CRC Press, Boca Raton, FL.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
- Porstmann B. and Prstman T. (1988). Chromogenic substrates for enzyme
immunoassay, In Non-isotopic Immunoassay, T.T. Ngo, ed., pp. 59-84. Plenum
Press, New York.
- Roberts I.M., Jones S.L., Premier R.R. and Cox J.C. (1991). A comparison of the
sensivity and specificity of enzyme immunoassays and time-resolved
fluoroimmunoassay. J. Immunol. Methods 143, 49-56.
- Wilchek M. and Bayer E.A. (1988). The avidin-biotin colmplex in bioanalytical
applications. Anal. Biochem. 174, 1-32.
- Van Weemen B.K. and A.H.W.M. Schuurs (1971) “Immunoassay using antigenenzyme conjugates” FEBS Letters, 15,232-236.
- Yalow R.S. and Berson S.A. (1959). Assay of plasma insulin in human subjects by
immunological methods. Nature 184, 1648-1649.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
5. FUNCTIONS
5.1. Intended Use
The ECHO is an analyser designed to automate Clinical Chemistry tests.
The ECHO is designed to automate a great part of operations of the long and borings
laboratory routine analysis. It ensures more accuracy and precision of the output, more
productivity and more safety for the operator. In this way ECHO assures saving of time
and money.
In addition to the traditional in-vitro diagnostic testing, that includes hormones, drugs of
abuse, etc., this instrument can be used for a great number of other different
applications.
The use of the instrument for some of the mentioned application (e.g. blood screening)
may require, in same countries, the approval or registration from Government Agencies.
5.2. Instrument Functions Overview
The ECHO is a fully automated, open system that can process most Clinical Chemistry
tests. All operations are automatic and programmable:
• Manual patient sample entry.
• Reagent distribution.
• Incubation (from room temperature to 42°C in the incubation plate).
• Washing.
• Reading.
• Data analysis and printout.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
The ECHO has the following important features:
HARDWARE
ECHO has the following features:
• It is an open system that will not restrict the customer to anyone reagent
supplier.
• Carry-over between samples is eliminated tanks to an optimized washing
procedure.
• It has a photometric station allowing precise and rapid reading of absorbance
for each test.
SOFTWARE
The software is an easy-to-use management system with an interactive communication
between the operator and the system.
The use of this type of software, simply to use, allows the operator to perform precision
testing with minimal computer training.
Additional features of the software include:
• Easy access to menus.
• Easy, step by step programming.
• Fast and accurate printout of test results.
• Reduces the risk of human error.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
5.3. Functional Subassemblies
The ECHO is based on the following functional subassemblies:
• Pipetting subassembly
• Flow cell
• Spectrophotometric reading subassembly
The subassemblies are described in the following sections.
5.3.1. Working Area
The working area (fig. 5.1) contains:
fig. 5.1
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
• Sample rack: primary tube location.
• Reagent rack for 27 standards tanks.
5.3.1.1. Reagent Plate
This plate is located on the left side of the working area end it has 27 positions for the
reagent bottles, so it is possible to contemporarily execute a maximum of 27 different
methods.
fig. 5.2
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
5.3.1.2. Sample Plate
This plate (fig. 5.3) is placed in the middle of the instrument working area and it has 64
holes for inserting serum cups with blank samples, standards and controls.
5.3.1.3. Reaction Plate
This plate (fig. 5.4) has 4 positions for inserting disposable racks with 24 reaction tubes
for each one, so the total capacity is 96 tubes.
fig. 5.3
fig. 5.4
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
5.3.2. Pipetting Subassembly
5.3.2.1. Sampling Needle
The mechanical arm with the sampling needle moves on the plates and for each test first
picks up the reagents, then takes the serum or standard/control (from the serum cup in
the inner plate). Then the needle sends out all the liquid to an incubation tube.
5.3.2.2. Aspiration Needle
After the incubation time, this needle will aspire the liquid from the tube to the flow cell
for measurement. A needle performs three aspirations for each test. The first and the
second aspiration will not remain in the cuvette and will be transferred to the waste
bottle. This will be useful to wash the liquid residues of the previous sample from the
fluidic system, avoiding carry-over results. The third aspiration volume will be kept
inside the cuvette and measured for the reading time selected by the method. After
reading this liquid will be thrown away, thus clearing the flow cell for a new measure.
5.3.2.3. Syringe
The syringe can aspire in two different ways depending to the position of the electro
valve located on its top: it can aspire from the water bottle or from the sampling needle.
The movements of this component regulate the aspiration and the ejection from the
sampling needle and allow cleansing of the tubes and all the fluidic system with
distilled water.
5.3.2.4. Peristaltic Pump
The peristaltic pump can perform a number of steps in the third aspiration (reading
aspiration) variable from the first and the second one. The number of steps can be
modified by user (user parameters) to allow a greater or a lower aspiration in order to
reach the exact position of the liquid front in the inlet tube.
5.3.3. Flow Cell
This component is located under the little metal door of the instrument. It has inlet
tubes coming from the aspiration needle and outlet tubes that going to the peristaltic
pump at the waste bottle.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
I
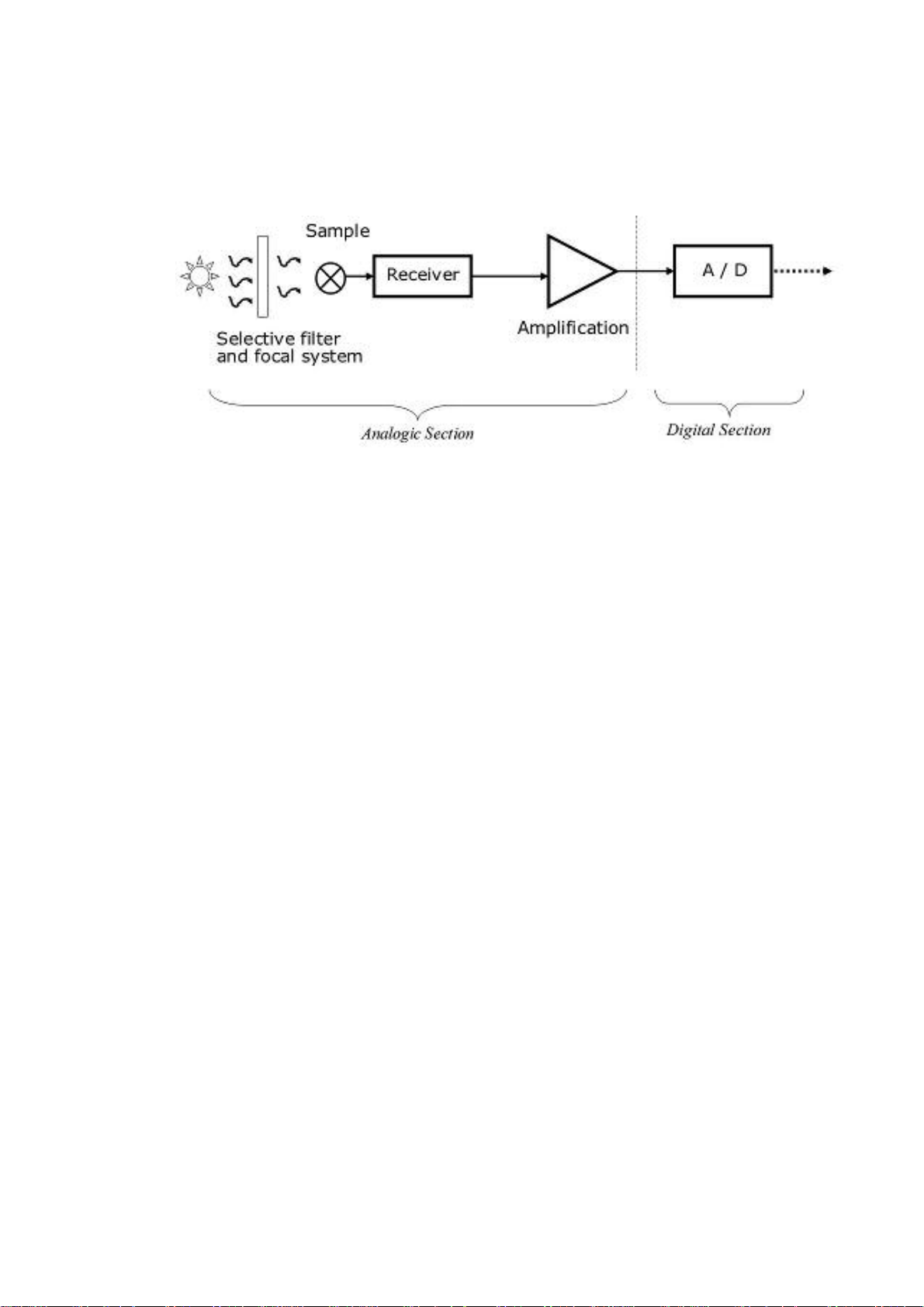
5.3.4. Spettrophotometric Reading
Photometric reading is performed by a spectrophotometer. The liquid is automatically
transported to the reading stations. An internal microprocessor controls the 9selectable
interference filters.
The system measures the optical densities (mono or bichromatic reading) of each test.
The principle on which is based the photometric reading is the Lambert-Beer law.
An incident wave, shooting a solution that holds a substance with homogeneous
concentration, respects this law through its path inside the solution; the law is expressed
by the following formula:
where
KdC
eII
=
0
I Shooting wave amplitude.
0
Transmitted wave amplitude.
)(K Spreading constant of substance (wavelength function).
d Wave optical path inside solution.
C Substance concentration in solution.
Through use of specific reagents in solution for the substance we want to measure, it’s
possible to overvalue the spreading constant for a fixed wavelength, so transmitted
wave amplitude becomes concentration function:
C
I
1
ln
=
KdI
C
I
00
ln
I
This relation allows evaluation of substance concentration by measuring of Absorbance:
I
0
ABS =
=
ln
kdC
I
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it
EI1121A – Echo PC User manual - Rel. 1.1
The measure chain could be represented by the following scheme (fig. 4.1):
fig. 4.1
5.4. Management System
The ECHO software operates under DOS operative system. A series of menus enables
the user to navigate within the programs.
The ECHO software has a list of assay methods pre-programmed by a Technical
Support Representative. This protocol list can be easily modified, deleted or even added
to, using the information provided with a test kit.
The software is password protected with a defined password, that can be modified only
by the authorised user (see the Configuration Section).
5.4.1. Software Architecture
In the following paragraphs are described the main functions of ECHO software, that
allow to set, test and use the instrument.
5.4.1.1. Main Menu
The Main menu enables the user to access the five main software features, described in
the next paragraphs.
5.4.1.2. Work List
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
This section allows the user to program the patients database and to set the tests list for
each patient.
5.4.1.3. Execution
This option gives the access to the section that allows to start the analysis procedure.
5.4.1.4. Results
This option allows to display, print and send the results report of a test session.
5.4.1.5. Chemistries
This software section is used to edit, modify, print or create new assay procedure.
5.4.1.6. Service
The Service function allows to initiate the calibration procedures. Only specialised
personnel have access to this section of the software.
See Service Manual (p/n: ...) for details.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
6. PERFORMANCE CRITERIA AND
LIMITATIONS
6.1. General Statement
This section provides information on the performance of the instrument that need a
peculiar approach to measure them being based on statistic or indirect method
measurements.
This paragraph provides the statement for these items.
Precision: It is the concordance between the repeated measures: it gives the ability of
the instrument to reproduce the same value when handling the same quantity.
Accuracy: It is the ratio between the expected value of the parameter and the true value.
Linearity: Let us assume that the measured values of an ideal-instrument are on a
straight line; the Linearity is the shifting from this ideal straight line of the real-
instrument measures.
The first photometer precision, accuracy and linearity tests are executed in the O.D.
TEST function (F5 SERVICE, F3 DIAGNOSTICS, F2 O.D. TEST) and are necessary
to validate the photometer. The pipettor precision, accuracy and carry-over tests are
executed running a simplified method and use the photometer as the test instrument to
validate the pipettor.
The tests use a kit of four reference solutions of Potassium Bichromate, with the
following nominal O.D. at 340 nm wavelength:
• 2000 mAbs
• 1000 mAbs
• 500 mAbs
• 250 mAbs
The solutions are calibrated by the SYSTEA Srl laboratory using a spectrophotometer
(UV/VIS JENWAY mod. 6405, accuracy of 0,1%). The real certificated O.D. values of
the reference solutions are employed in the tests calculations.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
6.2. Photometer
6.2.1. Precision
This test evaluates the repeatability of the reading. It is performed evaluating the CV%
of a series of readings of the same solution.
The test is performed repeating n=8 times the reading of a concentrated solution of
Potassium Bichromate with an O.D. of 2.000 mAbs at 340 nm wavelength.
After the execution of the eight tests, it is necessary to calculate the CV% :
CV% = Voo / Vm * 100
The acceptance level of the CV% is lower or equal to 2%.
6.2.2. Accuracy
The accuracy test of the photometer is performed together with the precision test,
measuring the average O.D. of a reference concentrated solution of Potassium
Bichromate with an O.D. of 2.000 mAbs at 340 nm wavelength.
So =ODi Soo =ODi²
Vm = So / n
____
Voo = (Soo – n Vm²) / (n-1)
So =ODi
ODm = So / n
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
This value obtained with the instrument in O.D. TEST (F5 SERVICE, F3
DIAGNOSTICS, F2) is compared with the O.D. reference value. The difference
between the two values must be lower than 2%.
6.2.3. Linearity
The linearity test has the purpose to verify the linearity of the optical group (photometer
detector plus preamplifier).
The test is performed using the concentrated solution of Potassium Bichromate with a
nominal O.D. of 0.250, 0.500, 1.000, 2.000 mAbs at 340 nm wavelength.
|ODm – ODr| / ODm * 100 < 2
The four solutions are read with the instrument in the O.D. test menu (F5 SERVICE, F3
DIAGNOSTICS, F2 O.D. TEST) for direct immediate optical density reading.
The solutions are aspirated directly in the cuvette pulling out the aspiration needle.
After the execution of the four tests, it is necessary to calculate the correlation index:
1 2 3 4
Concentrations
(nominal value)
Opt.Densities
Sc = @Ci
@ODi
So =
Ci
ODi
C1
(0,250)
OD1 OD2 OD3 OD4
Scc = @Ci²
Soo =@ODi²
@(Ci .ODi)
Sco =
(0
C2
,500
)
C3
(1,000)
(2
Vcc = n Scc - Sc²
Voo = n Soo - So²
Voc = n Sco – Sc So
C4
,000)
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
_________
Correlation index R = Voc /
To accept the test, the correlation index must be higher or equal to 0,95.
NOTE: The linearity and the correlation index can be calculated automatically
and the graph can be printed using the instrument’s curve calculations.
A special user test must be edited with:
• N. of standards: 4 (or 8 if the solutions are read twice)
• Calculation mode: linear regression
• Curve values (O.D. and std values) taken from the test results
6.3. Pipettor
6.3.1. Precision
D(Vcc .Voo)
This test evaluates the repeatability of the reading of a concentrated solution dispensed
through the ECHO pipettor. It is performed evaluating the CV% of a series of readings
of the same solution and compared with the CV% of the photometer obtained with test
#1.
It is necessary to prepare a special test method:
• type: end-point,
• sample volume: 100
• reagent volume: 400
• reagent position: 1
• incubation time: 0 sec
The test is performed repeating n=8 times the reading of the concentrated solution of
Potassium Bichromate with O.D. 2.000 mAbs at 340 nm wavelength. The concentrated
solution is put in the first 8 sample cups (1 – 8) and distilled water is put in reagent
position 1.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
After the execution of the eight tests, it is necessary to calculate the CV% :
So =
ODi
Vm = So / n
____
CV% = Voo / Vm .100
The acceptance level of the CV% is lower or equal to 5%.
6.3.2. Accuracy
Soo =ODi²
Voo = (Soo – n Vm²) / (n-1)
The test is performed comparing the O.D. obtained diluting the reference solution with
the instrument’s pipettor and the O.D. obtained diluting the reference solution with a
manual pipettor.
It is necessary to prepare four samples using the automatic samples dilution function
and four samples with no dilution programmed, but already diluted with a manual
pipettor:
first program: F1 WORKLIST, F1 INSER.WORK-LIST,
then execute: F2 EXECUTION, F3 AUTOMATIC SAMPLES DILUTION)
The 4 + 4 solutions are read as the previous test #2 (photometer accuracy test).
ODp = optical densities with automatic pipetting
ODr = optical densities with manual reference pipetting
ODpm =ODp / n
ODrm =
ODr / n
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
The two values obtained with the instrument in O.D. TEST (F5 SERVICE, F3
DIAGNOSTICS, F2) are compared. The difference between the two values must be
lower than 4%.
|ODpm – ODrm| / ODrm . 100 < 2
6.4. Carry-Over
This test aims to verify the contamination between a highly concentrated sample and
three following null concentrated samples.
The test is performed using the Potassium Bichromate solution with an O.D. of 2.000
mAbs at 340 nm wavelength in sample position 1 and three tubes of distilled water in
sample positions 2, 3 and 4.
It is necessary to prepare a special test method:
• type: end-point,
• sample volume: 250 µl
• reagent volume: 250 µl
• reagent position: 1
• incubation time: 0 sec
Running the four tests, it is requested that the O.D. of the first blank sample is lower
than 20 mAbs (1% of the concentrated solution).
In case of failure, the O.D. of the two following water samples gives an index of the
carry-over progression over several samples.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7. OPERATING PROCEDURES
7.1. Preparation Prior Operations
In the following paragraph are quoted the necessary information that the user must
know for a correct use of the instrument.
The information about sample handling, reagents and disposable materials are general;
it is recommended to read the respective Manufacturer guides.
7.1.1. Safety Recommendations and Remarks
Read carefully the Safety section for detailed safety instructions.
To reduce risks it is important to follow the safety precautions listed in Section 2.
The Instrument Calibration, the Setting and the Adjustments of the instrument should be
carried out by Technical Support Personnel only.
7.1.1.1. User Skill Level
User appointed for instrument operations must have a good technical skill in medical In
Vitro Diagnostic (IVD) analysis; as highlighted in the Safety section, the use of Good
Laboratory Practice is mandatory to get the proper level of safety when the instrument is
used for medical in-vitro diagnostic use.
It is very important that the user must know the details of the ‘User’s Manual’:
additionally he needs to be trained, according to the manufacturing procedures, to the
use of the instrument for operations.
The user can’t approach to the software’s sections protected from passwords out of his
competence.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.1.2. Instrument Set-up
Switch on the instrument; on the screen will appear the message (fig. 7.1.1):
Press a key and wait until the initialization is performed. It controls the correct
functioning of the principal instrument subassemblies. In case of malfunction will
appear an error message on the screen.
Prior to load the materials required for the instrument run, consisting in reagents, test
supports, samples, etc., carry out the following steps:
• Empty the waste tank: this must be usually performed on instrument shut
down.
• Check that tanks for the distilled water are filled.
7.1.2.1. Instrument Built-in On Line Test
The self-test of the instrument is performed automatically at the instrument start-up.
fig. 7.1.1
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.1.3. Sample Handling
To ensure a correct use of the instrument and to increase the reliability level of the
analysis results it is important to read the following information.
Samples preparation: samples must be adequately brought to room temperature and
checked to be free from clot, fibrin, froth or bubbles. The presence of clot and fibrin
may cause incorrect dispensation of sample.
NOTE: The information about Sample quoted in this is general. We strongly
recommend to read carefully the instruction of the assay Manufacturers.
In case that the ECHO is used for fully automated medical In Vitro Diagnostic (IVD)
analysis, the Manufacturer remind to the Users that failures to follow correct sample
handling procedures could produce false negative results reporting.
WARNING
7.1.3.1. Sample Type
ECHO can be used for fully automated in vitro diagnostic analysis, utilizing samples
like blood, serum, plasma, urine and any liquid solution coming from a sample
preparation.
NOTE: it must be validated that the pipetting system is able to handle the viscosity of
the liquids loaded as samples when they are resulting from a sample
treatment/preparation.
7.1.3.2. Pre-treatment
To avoid ‘in vitro’ alterations that could modify analysis result, the sample must be
preserved according to specific reagent manufacturer instructions.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.1.3.3. Preservation
To avoid ‘in vitro’ alterations that could modify analysis result, the sample must be
preserved according to specific reagent manufacturer instructions.
The general instructions to preserve samples are the following:
Serum or plasma: centrifugate the vial with the blood (after coagulation in case of
serum); put serum or plasma in another vial and refrigerate till the test.
Urine: prepare a sample in a 10 ml vial and refrigerate till the test.
7.1.3.4. Sample Minimum Volume
It is the dead volume plus the volume needed to carry out the analysis.
7.1.3.5. Dead Volume
If the sample volume is less (or equal) than dead volume, the needle can’t aspirate it.
The sample dead volume for standard tubes is <100µl: this may be reduced using vials
with conical shape at the bottom. They need to be properly calibrated by Technical
Service personnel.
7.1.4. Reagent and Disposable Materials
Reagents are the materials that, interacting with sera, generate the reaction at the various
step of the procedure. The reading of the final colour supplies the analysis outcome.
Disposable materials are vials and disposable needle cleaning filters.
Reagent can be loaded in polystyrene tubes 24 ml. It is recommended to not reuse
reagent vials.
NOTE: The information about Reagents and Disposable Materials quoted in this is
general. We strongly recommend to read carefully the instruction of their
Manufacturers.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.1.4.1. Use
Preparing the reagents before or during the test procedure, depending on their stability.
Reagents and samples should be at room temperature (18÷25°C) before beginning the
assay and can remain at room temperature during testing. All containers used for
preparation of reagents must be cleaned thoroughly and rinsed with water before use.
Preparation of reagents and controls: these must be free from froth or bubbles on the
surface of the liquid or inside the test tubes or vials.
Use only disposable needle cleaning filter of the manufacturer’s specified type.
7.1.4.2. Preservation
To preserve reagents and disposable material user must follow carefully manufacturer
instruction.
7.1.5. Fixture and Accessories
For the list and the description of ECHO accessories see Chapter 3.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.2. Management System Overview
The ECHO software runs with DOS operative system, and it is simple to use. The
system is provided with pre-validated test procedures; these procedures can be
modified, added or deleted in easy way.
The software is protected by passwords, that only authorized personnel can modify.
7.2.1. How to Start
After the initialization procedure, the ECHO will ask for an analyst ID number (0-4).
Analyst #0 has full access to the system while analysts #1 to #4 have restricted access
to:
• Edit Methods Read mode only.
• Edit Profiles Read mode only.
• Load / Dump methods No access.
• Copy / Swap methods No access.
• Edit Parameters No access.
• Laboratory Data No access.
After the analyst number, the ECHO will ask for a password. It can be alpha-numeric
and can have up to 6 characters.
If no password is stored, ECHO will ask to insert a new password (Insert new).
The standard password memorized as default is 000000 (6 times 0).
Access to the instrument is denied until a valid password is typed.
The master analyst (#0) can access to the Laboratory Data, where are recorded two lines
for the Laboratory Header printing and the five Analyst names.
Blanking an analyst name will disable that analyst and clear the related password.
Analyst #0 cannot know the password of the other analysts, but can only cancel them.
With the ECHO numeric keyboard, the analyst name can be canceled with the CLEAR /
FEED key.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
If the master analyst has forgotten his password, after typing a wrong password for
analyst #0, the ECHO will display a key number and wait for a master password. The
master analyst must call the assistance, give the key number and get back the master
password. This master password will clear Analyst #0 password and give access to the
instrument only once.
The TEST TOTALS displayed in the CONTROLS menu are, in release 3.xx separated per
analyst. The values displayed are only the tests executed by the selected analyst. Anlyst
#0 can display the totals for all the analysts.
7.2.2. Main Menu
Once user has type his password (fig. 7.1.1), will be displayed the following screen (fig.
7.2.1):
fig. 7.2.1
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
WORK
-
LIST
E
XECUTION
RESULTS
CHEMISTRIES
SERVICE
From this screen is possible to have access to the five main software sections, by
pressing the corresponding key:
F1
Allows the access to the patients manager section.
F2
Allows to run the assay procedures.
F3
This button gives the access to the result section.
F4
The Chemistries section allows to edit, modify, print a chemistry
method or to set up a new method.
F5
The service section access is reserved to authorized personnel
only.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
CHEMISTRIES MENU
Edit
Methods
Print
Methods
7.3. Chemistries Menu
In this section is detailed the procedure to modify the parameters of a test section.
From the Main menu (fig. 7.2.1) press the F4 button; the following window will be
displayed (fig. 7.3.1):
ECHO AUTOMATIC ANALYZER
ISE - EDIF
Firmware version x.xx
F1 F2 F3 F4 F5
EDIT METHODS PRINT METHODS LOAD/DUMP METH. PROFILES PANEL 1/ PANEL 2
fig. 7.3.1.
From this screen is possible to have access to the following six sections, pressing the
corresponding key:
F1
Allows to edits the methodologies.
F2
Allows to print the tests methodologies.
F3
Load/Dump
This button allows to import methods from an external PC.
F4
Profiles
To enter in the Profiles menu.
F5
Panel 1/2
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
To select Panel 1 (Clinic chemistries) or Panel 2
(Turbidimetry), with 32 tests each one.
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1. Edit Methods
To enter in the Edith Methods menu, press the F1 button in fig (7.3.1), the following
screen will be showed (fig. 7.3.2):
fig. 7.3.2
Now select a method typing its code from 01 to 32 (ex.: 02 to select Urea); will be
showed the following window, with all the selected test parameters (fig. 7.3.3):
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Method n. : 2 :
EDIT METHODS
Type: Fixed time UREA
Filters(s): 340 nm Code : BUN :
Sample volume (ul): 5
Reagent 1 Volume (uL): 500
Reagent 2 Volume (uL): 0 Reag. 3 Volume (ul) : 0 :
Number of washes 2
Stabilization time (s) 13 Reading Time (s) : 17 : Cycle : 36
R1 Incubation time (s) 72
Position of reagent 2
Number of standards 1 Number of controls: 1
Position of standard 2 Position of Control : 16 :
Standard value 50.00
Factor 633.1
Susbstrate depletion .7000
Dilution Ratio in Rep .6000 Sample dilution : 0 :
Linearity 200.0 N. of decimals : 4 :
R. Blank value: min .8000 max : 1.800 :
Normal Value min: 15.00 max : 50.00 : mg/dl
> Blanks are NOT used in calculation
> The factor is KEPT in memory Type of test:
F1 F2 F3 F4 F5
SELECT METHODS NEXT METHOD PREV. METHOD SELECT OPTION NEXT OPTION
1= Endpoint
2= Bichromatic
3= Kinetic
4= Fixed time
5= Differential
6= D. sample blank
fig. 7.3.3
The position of the cursor indicates if a parameter is opened for modification or not.
Note: The 5 parameters indicated by are opened by a sub-menu selection in a
window on the right side of the screen, that appears when the cursor is positioned
on one of the parameters.
The parameters are detailed in the following sections.
To exit from the Edit Methods menu, press the PgUp button or the Esc button, then
press F1 to not save the modifications or F2 to save the modifications.
To print the chemistries, press the F2 button from the Chemistries Menu (fig. 7.3.1).
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.1. Types Selection
Use the F4 and F5 buttons to go to the next/previous option. The keys from 1 to 9 of the
keyboard, select directly the correspondent option.
In fig. 7.3.3 in the Type field, is possible to change the name of the method (max. 15
characters) and the code number (max. 3 characters); place the cursor in the field and
press two time the ‘.’ button. The available types are:
• End point.
• Bichromatic.
• Kinetics.
• Fixed time.
• Differential.
• Differential, with blank reagent.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Method n. : 2 :
EDIT METHODS
7.3.1.2. Filter Selection
In the Filters field (fig. 7.3.4) is possible to set the photometer filter. The available
filters will appear in a window on the right of the screen; in case of bi-chromatic
methods, the program will ask for a second filter. The ECHO has 10 filter positions: the
position 0 is
Type:
Filter s(s): 340 n m Code : BU N :
Sam p le volume (ul): 5
Reagent 1 Volume (uL): 500
Reagent 2 Volume (uL): 0 Reag. 3 Volume (ul) : 0 :
Number of washes 2
Sta biliz atio n tim e (s) 13 R ea ding Tim e (s) : 17 : Cy cle : 3 6
R1 Incubation time (s) 72
Position of reagent 2
N umber of standards 1 Num ber of controls: 1
Po sitio n o f standard 2 P osition of C on tro l : 16 :
Standard value 50.00
Factor 633.1
Susbstrate depletio n .7000
Dilution Ratio in R ep .600 0 Sam ple dilution : 0 :
Linearity 200.0 N. of decim als : 4 :
R. Blank value: m in .800 0 max : 1.800 : Filters:
Normal Value min: 15.00 max : 50.00 : mg/dl
> Blanks are NOT used in calcu lation
> The factor is K EPT in memory
F1 F2 F3 F4 F5
SELECT METHODS NEXT METH OD PREV. METHOD SELECT OPTION NEXT OPTION
Fixed time UREA
f
the offset readings.
1= 340 nm
2= 405 nm
3= 492 nm
4= 505 nm
5= 546 nm
6= 578 nm
7= 630 nm
8= 700 nm
or
Note: In case of Bichromatic analysis the instrument will ask you to memorize a second
filter.
7.3.1.3. Incubation Time
Range: 12 – 9999 seconds.
This represent the foreseen time of the reaction. This is the time between the sampling
and reading. The software can modify this time in a small range, adapting it to the cycle
of the instrument.
For the methods with 2 reagents, this time (minimum) is the one between the sampling
of the first and second reagent.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.4. Stabilization Time
Range: 1 – 60 seconds.
It is the time between the pipetting in the measure cuvette and the reading. This time is
necessary to allow the thermo-stabilization and turbulences stabilization.
7.3.1.5. Reading Time
Range: 1 – 254 seconds.
This is the measure time. The sampling A/D converter has a frequency of 200 m/sec.
The sum of the readings during the measure time gives the result.
7.3.1.6. Reagent 2 – Incubation Time
Range: 12 – 9999 seconds.
Set this value different from 0, if method works with two reagents (end point, bichromatic, kinetics or fixed time). It is the time between the pipetting of the reagent 2
and its reading. In case of Incubation Time this is the distribution time between the
reagent 1 and reagent 2.
7.3.1.7. Reagent Position
Range: 1 – 27.
It is the position of each reagent assigned by the user. ECHO has 27 positions for the
reagents, and can work on 27 mono reagents methods for each plate. The methods with
2 reagents are using 2 consecutive positions, reducing in this way the number of
simultaneous methods in use.
The user should assign 32 positions to reagents in two test groups (table 1 / table 2). We
suggest to assign in each group an exclusive positions for the most frequent and usual
tests, and to reserve the other positions for the rare methods or to the other tests which
are rarely simultaneous.
If there are two application with the same position in use, the analyzer will change the
position of the method with the major incubation time.
7.3.1.8. R1 Reagent Volume (µµµµl)
Range: 5 – 800.
Typical: 400 – 500.
It is the reagent volume (in micro liters).
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.9. R2 Reagent Volume (µµµµl)
Range: 5 – 800.
Typical: 5 – 50.
It is the second reagent (in micro liters). This value must be set to 0 for the monoreagent methods. A value different from 0 indicates a method with 2 reagents.
7.3.1.10. R3 Reagent Volume (µµµµl)
Range: 5 – 800.
Typical: 5 – 50.
7.3.1.11. Sample Volume
Range : 5 – 500.
It is the sample volume (in micro liters).
7.3.1.12. Numbers of Calibration Standards
Range: 0 – 8.
This value indicates the number of standards used for the calibration.
0: Value calculated with a factor. A missing factor will cause an error.
1: If the value of the factor is 0, during the analysis cycle the ECHO will
measure the optical density of the standard sample, from the
beginning of the test executions and will calculate the factor. The
factor will be positioned in standard position and will have a defined
value (standard value).
From 2 to 8: These values are used for non linear methods (immunology). In this
case the ECHO will calculate with the number of curves and the
description of the curve. The length of the curve is equal to the
number of the standard; this should be positioned in increasing order,
starting form the position defined by the standard position.
See also the Standardization Procedure (Par. 7.4.1.2).
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.13. Numbers of Controls
Range: 0 – 3.
The ECHO allows to store up to 30 values of control for each method on one level. The
control should be requested by the user during the execution time (see tests and controls
to execute). If this parameter is ‘0’, you can not execute or memorise any control. If this
parameter is ‘1’, a control will be executed at the beginning of the execution of the
corresponding methods, immediately after this a possible standard. The control sample
should be placed in the position defined by the 14 parameter (control of position).
7.3.1.14. Standard Position
Range: 1 – 16
1 – 15 with 1 control
1 – 14 with 2 controls
1 – 13 with 3 control...
These are the positions from 1 to 16 in which the user should place the standards in case
of mono standard methods.
This is the start position from 1 to 16 in which the user should position the standard
samples defined by the 11 parameter (standard number) in case of multi-standard
methods.
The standards should be placed in side by side increasing positions.
If the last standard position is over the position 16, this will cause an error.
7.3.1.15. Control Position
Range: 1 – 16.
This is the position, where the user should place the controls. See parameter 12 (number
of controls).
7.3.1.16. Standard Value
Range: 0.000 – 99999.
This parameter will appear only when the number of standards is ‘1’. This is the theoric
value of the standard used in case of mono standard methods. A null value will cause
an error; when the factor is ‘0’ is necessary to evaluate a new standard.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.17. Factor
Range: 0.000 – 99999.
This parameter will appear only when the number of standard is minor or equal to 1. For
the mono standard or with no standard methods, the factor value is multiply for the
optical density to have a result.
The user should insert the factor, in case of methods without standard.
7.3.1.18. Description of the Curve
This parameter appears only when the number of standards is greater than 1. This
multiple parameter permits the visualization of the values curve: the upper line is the Y
coordinate (O.D.), the inferior line is the X coordinate (Val. Std.).
The curve has as many points as many number of standards has been defined in the
parameter 11. The result is calculated with the Cubic spline interpolation.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Method n. : 2 :
Type of test
EDIT METHODS
Type: Bichromatic IgA
Filters(s): 340 nm 700 nm Code : IGA :
Sample volume (ul): 10
Reagent 1 Volume (uL): 500
Reagent 2 Volume (uL): 0 Reag. 3 Volume (ul) : 0 :
Number of washes 3
Stabilization time (s) 7 Reading Time (s) : 5 : Cycle : 24
R1 Incubation time (s) 312
Position of reagent 2
Number of standards 5 Number of controls: 1
Position of standard 1 Position of Control : 9 :
Interpolation: Cubic spline
Curve O.D. : .0664 .2403 .4283 .7009 .9829
Val. standard: 5.800 16.30 98.00 192.0 389.0
Dilution Ratio in Rep .6000 Sample dilution :1 :
Linearity 200.0 N. of decimals : 4 :
R. Blank value: min .0000 max : 2000. :
Normal Value min: 60.00 max : 360.0 : mg/dl
> Blanks are used in calculation
> The calibration is KEPT in memory 1: Endpoint
2: Bichromatic
3: Kinetic
4: Fixed Time
5: Differential
6: Diff. Sample bl.
F1 F2 F3 F4 F5
SELECT METHODS NEXT METHOD PREV. METHOD SELECT OPTION NEXT OPTION
7.3.1.19. Substrat depletion
Range: 0.000 – 99999.
This parameter is used only kinetic and fixed time type methods.
If the difference between the optical density of the first reading and the optical density
of the blank reagent is greater than the value of the substrat depletion, the following
message will appear: Substrat depletion. .
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
fig. 7.3.5
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.20. Dilution Ratio
Range: Max 1.0 (typical 0.5 – 0.25).
When at the end of a cycle, is requested a repetition of a test with an automatic dilution,
the sample volume is multiplicated by the dilution ratio and the reagent volume is
increased to compensate the reduction of the sample volume.
Sample vol. x dilution ratio
Reagent vol. + Sample vol. x (1 – dilution ratio)
NOTE: it is not possible to use sample volumes after dilution inferior to 3 micro
litres.
7.3.1.20. bis Sample dilution
It is possible to pre-dilute the sample in case of patients with chronicle diseases (for
example diabetes) or for the determination of the Creatinuria.
This dilution is done with the physiological solution or distilled water. The function
has three options:
7.3.1.21. Linearity
Range: 0.000 – 99999.
0 Does not accept pre – dilutions
1 Accepts the pre-dilution and the result is not treated
mathematically (for ex. Pre-diluted at 50 % the final
result will at 50%)
2 Accepts the dilution and the result is treated
mathematically (for exp. Pre-diluted at 50 % the final
result will be calculated multiplying by 2)
If the result is greater than this value, the following message will appear: Not linear
result.
The test should be repeated with the diluted sample (see repetitions).
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.22. Reagent blank value: min
Range: 0.000 – 99999.
If the optical density of the blank reagent is minor to the value determined by this
parameter, the following message will appear: Blank out of range.
7.3.1.23. Reagent blank value: max
Range: 0.000 – 99999.
If the optical density of the blank reagent is greater than the value determined by this
parameter, the following message will appear: Blank out of range.
7.3.1.24. Normal Value: min
Range: 0.000 – 99999.
If the result of a test is minor to the value determined by this parameter, on the screen,
after the value, the L symbol will appear.
7.3.1.25. Normal Value: max
If the result of a test is greater than the value determined by this parameter, on the
screen, after the value, the H symbol will appear.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.26. Measure Unit Selection
Is possible to choose the result measure unit; select the appropriate one from the
windows that appear on the screen (fig. 7.3.6).
Use F4 button to select the option and F5 button to go to next option.
7.3.1.27. Washings Number
Range: 1 – 13 (typical 2).
For the high density reagents use 3 or 4. For creatinine, iron, calcium use 5. This is the
number of the vials and syringe washing, carried out after the sampling, and the number
of washings of the flow cell carried out at the end of the measure. For the last washings
instrument uses 1000 micro liters of distilled water, while for the others it uses 500
micro liters.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
fig. 7.3.6
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.28. Blank Reagent Selection
Is possible to choose between two options (see fig. 7.3.7):
1: NOT USED in calculations
2: be USED in calculations
fig. 7.3.7
In fact during the calculations, ECHO can compare or not compare the optical density
of the blank reagent with the optical density of the sample. In any case a test with the
blank reagent will be executed to check the reagent quality. Typically the end point (bichromatic and differential) uses the blank reagent.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.3.1.29. Factors and Curves Selection
Is possible to choose between two options (see fig. 7.3.8):
1: ERASED daily
2: values are KEPT
fig. 7.3.8
Choosing option 1, the factor is not stored. When a standard is analyzed, the calculated
factor is noted in the parameter 16 (factor) and is used for the successive sample, but it
is discharged after the instrument shut down.
Choosing option 1 the factors are automatically stored. When a standard is analyzed, the
calculated factor is noted in the parameter 16 (factor) and can be used for the future
analysis.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.4 Patients Handling
Pressing the F1 button in the Main Menu (fig. 7.2.1), the following screen will appear
(fig. 7.4.1):
fig. 7.4.1
The available options are the following:
• Keybord Program (by pressing F1): allows to program the patient work list by
the keyboard.
• Load Work List (by pressing F2): with is option is possible to load a work list
from an external PC, using the serial port RS232.
• Display Work List (by pressing F3): displays the work list on the monitor.
• Print Work List (by pressing F4): allows to have a printout of the work list.
• Profiles List (by pressing F5): to display on the monitor the profiles list.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Clear previous patients:
7.4.1 Edit Work List
Pressing the F1 button, the software show three options (fig. 7.4.2):
Press 0 button to not delete the existing database, 1 button to clear the tests but not the
patients in the database and 2 button to clear all database.
After a choice will be displayed the following window (fig. 7.4.3):
0 No
1 Clear only test
2 Clear all
fig. 7.4.2
fig. 7.4.3
In this screen there are some information about the programmed work list, and in
particular:
• Date
• Urgent Edit
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
o 0: corresponds to a normal edition; all the patients will be treated in the
same way.
o
1: first will be treated the urgencies, the patients codifies as Urgent.
• Methods Panel:
methods.
allows the selection of one of the two groups, each one with 32
• Firs Patient: up to 63 samples, the first patient number correspond to the
position number on the sample plate.
• Last Patient: up to 63 samples, the last patient number correspond to the
position number on the sample plate.
• Input test list: this numbers, separated by a dot, identify the tests that will be
executed for all the patients (for fast programming only).
• Profile test list: this numbers, separated by a dot, identify the profiles that will
be executed for all the patients (for fast programming only).
From this screen, pressing the F2 and F5 buttons, is possible to display the tests list and
the profiles list.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
Pressing the F1 button the following screen will be displayed (fig. 7.4.4):
fig. 7.4.4
This screen allows to associate to a specific patient, the test or the profile that we want
execute. On the upper part of the screen there are the patient data, on the lower part
there are the tests.
To recall an existent patient, press F1 button (Select Patient) and type the correspondent
number. An other way is by pressing F2 button (to go to the Next Patient) or F3 button
(to go to the Previous Patient).
A patient is identified by the following data:
• Sample ID code: an ID of 13 alphanumeric characters.
• Urgent (0 / NZ): 0 correspond to not urgent; 1 corresponds to urgent.
• Dil 0%: corresponds to the pre-dilution percentage.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
To create or modify the data of a patient, select it and press the F6 button; the following
screen will appear (fig. 7.4.5):
fig. 7.4.5
From here you can modify all the data that describe a patient.
Once you have selected the patient, you have to set the tests for that patient (refer to fig.
7.7.3). Use the cursor buttons to move though the columns, and in particular:
Move up
Move down
Move left
Enter Move right
Select a test placing on it and pressing the F4 button.
Another way to select a test is typing its numbers (from 1 to 32) on the keyboard; to
cancel the selection type again the test number.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
The Del button allows to delete all the selected tests; the Esc or the Page Up buttons
allow to return to the previous menu.
With the F5 button is possible to set a profile for the selected patients.
On the lower part of the screen will appear the work list program bar (see fig. 7.4.3),
with 63 positions. The symbols on it have the following meanings:
• P = 1 one patient for a test’s list
• R = 1 patient for a test’s list and the previously memorized results.
• C = depending on the selected test, a control can be executed in this position.
• S = depending on the selected test, a standard can be executed in this position.
• . (dot) Indicates a free position.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.4.2. Load Work List
Pressing the F2 button in fig. 7.4.1, the software allows to load a work list from an
external PC, through the serial port RS232.
NOTE: This option is valid only if an external PC is connected.
If the existing work list of the ECHO is not empty, the ECHO will ask if you want to
erase the previous patients. Will be showed three options, type:
• 0 to stop the working list loading and to save the previous work list.
• 1 to load only the tests work list.
• 2 to load the patients and the work list of the test.
At the end of a correct loading the following screen will appear (fig. 7.7.6), to confirm
that the operation is successfully completed:
fig. 7.4.6
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.4.3 Display Work List
Pressing the F3 button in fig. 7.4.1, the software allows to display the work list (see fig.
7.4.7):
fig. 7.4.7
In the Pat column is showed the patient number, in the ID column there is the ID code
of patient, followed by the list of the selected tests (number 1 to 32).
For the urgent patients the work list is visualized in bold. If the work list is longer than
what appears on the screen, press a key to continue and press ESC to terminate.
NOTE: The tests are visualized per code or per number.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.4.4 Print Work List
Pressing the F4 button in fig. 7.4.1, the software allows to print the work list (see fig.
7.4.8):
Press the F1 (fig. 7.4.8) button to print all the patients list, or press the F2 (fig. 7.4.8)
button to print only the urgent patients list.
7.4.5 Print Profile List
Pressing the F5 button in fig. 7.4.1, the software allows to print the profiles list (see fig.
7.4.9):
fig. 7.4.9
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.5 Operative Procedure
To have access to the Execution menu, press F2 button in the main menu screen (fig.
7.2.1); the following screen will appear on the monitor (fig. 7.5.1):
Note: it is possible to enter into the Execution Menu only in case there is already a
Work list in memory.
fig. 7.5.1
Batch mode: ECHO processes all the patients optimizing the times.
Urgent mode: ECHO analyses the sample one by one.
Automatic sample dilution: ECHO dilutes the patients for which the pre-dilution
has been requested on the Work List patient.
Automatic standard dilution: ECHO dilutes the concentrated standard to create the
necessary standards to produce the calibration curve.
For the Optimized Batch mode (F1 button), the ECHO work according the following
principles:
• Every cycle is composed of a complete wheel (96 wells).
• For every cycle is possible to insert up to 96 wells, including the first well for
the blank reagent of each method, a well for each standard, a well for each
control and a well for each test.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
• For each test you can program up to 27 reagents; the tests with 2 reagents will
take 2 places.
• Each method is executed completely in one cycle (the maximum per method is
of 64 sample / test).
• The cycle is programmed for methods that don’t use more than 96 wells. The
methods over this limit will be executed in the successive cycle.
• At the end of execution, the ECHO will sign it with 3 bips and will come back
to the execution menu to ask for the next cycle, until the execution of the entire
programmed test.
• During the cycle, the end point methods, including the bi-chromatic ones, are
distributed for first and will be measured at the end of the incubation time.
During this interval, the Fixed Time methods, kinetics and differentials are
executed. The ECHO will begin the sampling of the tests of one method,
waiting the incubation time. When the incubation is terminated, the instrument
reads the incubated sample and does a new sample alternatively.
If the cycle is composed only by End Point methods, the same operational
mode is used to increase the execution as much as possible. When a method
has done all the samplings, the memorized number of washings will be done.
• At the beginning of each cycle, an auto zero procedure is executed.
• A washing procedure is executed at the beginning and at the end of each cycle.
For the Urgent mode (F2 button), the ECHO work according the following principles:
• Only the patients signed as Urgent will be taken in consideration. The ECHO
will execute one patient for time, for all the methods, with a maximum of 27
reagents.
• At the end of a patient working, the instrument will sing with 3 bips; the
operator can verify the reagents and the sampling and can start a new test
procedure.
• The principles are the same as the ones described in the Batch mode.
• For the successive patients the initial washings and the auto zero procedure are
not effected.
• The blank reagent is executed only for the first patient on whom the method
was programmed.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.5.1 Batch
To run the Batch mode, press F1 button in fig. 7.5.1. Next screen will be showed (fig.
7.5.2):
fig. 7.5.2
There are three steps to execute the assays: Test Selection, Controls Selection and
Standards Selection. Those steps are detailed in the following paragraphs.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.5.1.1 Tests and Control Selection
In this section the operator must confirm the controls and select the tests. To confirm all
the selected tests without the controls, press the F1 button (Continue).
To choose the tests press the F2 button; to choose the controls press F3 button; move
the cursor through the columns, with the following buttons:
Move up
Move down
Move left
Enter Move right
Select or deselect the test/control with the F4 button. To switch all the controls on, press
the F5 button.
In the line with the method, the number of the tests to effect and the position of the
control is indicated (C1 to C9), if a control has been inserted in the parameters.
After this operation press the F1 button to go to the Standardization procedure.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.5.1.2 Standardisation Procedure
In this section, the operator must select the standards to use. The methods are classified
as follows:
fig. 7.5.3
• The standard must be executed.
• The standard can be executed as an option.
• The standard is not necessary.
• The standard must be executed, but it can not be done (error).
Entering into the standardisation procedure, the standards that should be executed are
already signed in bold. The operator can not cancel the selection, but can only select the
standards to execute as optional.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
The standard must be executed when:
• The method has a defined standard, the value of the standard is different from
zero, the factor is zero.
• The method is defined as multistandard and the values of the O.D. curve are
zero.
The standard can be executed as an option:
• The method has a defined standard, the value of the standard and the factor are
different from zero.
• The method is defined as multistandard and the values of the curve are
different from zero.
The standard is not necessary:
• The method does not require a defined standard and the factor is different from
zero.
The standard must be executed, but it can not be done (error)
• The method has no defined standard, the factor is zero. The test can not be
executed.
• The method has a standard with a defined value, the factor is zero and the value
of the standard is zero. The following message will appear on the line 21:
“ERROR” Method .xx. standard value null.
The test can not be executed.
• The method is defined as multistandard and one or more values of the “n”
standard is / are zero.
The following message will appear on the line 21:
“ERROR” Method .xx. N std. Xx, Curve n.x
The test can not be executed.
If you select a standard for a method which does not require it, the ECHO will inform
you with a beep signal.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
In fig. 7.5.3. you can use the following buttons:
F2: Visualizes the programmed tests per reference.
F3: Visualizes the factors.
Under every method name is showed:
For the methods without standard: Factor ? 0
Ex. F 1746
For the methods with one standard: The standard and factor position are? 0
Ex. S1F1000
For the methods multistandard: First standard position and number of
standards.
Ex. S1x8
The number of the curve.
Ex. Curv. 13
F4: Selection and selection cancellation one by one.
F5: Selection of all the standards in option: ON
DEL/CTRL – FEED: Selection of all the standard in option: OFF.
To confirm all the parameters, press F1. The programming of the work execution can
take up to 3 seconds (the opening of a window). After this operation a working cycle
table will appear on the screen (fig. 7.5.4).
fig. 7.5.4
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
In the first line of the screen is showed the number of the tubes to use; in the second line
there is the number of the rack to use, then in the third line there is the number of the
test to execute.
In the seven columns are detailed the method name, the reagent position (R. Pos), the
reagent volume + 2 ml (Val. ml), the execution with factor (F) or mono/multi standards
(S), with the standard/s position, the standard or factor value (Val.), the position of the
control (Ctl. Pos.), the relation between standard and reagent volume (S:R).
The operator should control if everything is placed in the right order, with the correct volumes,
and if the reagent segments (rack) are correct. The operator can change the initial bottle with the
following procedure: press the F2 button and digit number 1, then Enter. The working cycle
will start automatically from the segment 1 (the first well of the segment is always reserved for
the washings).
After all the controls have been carried out, press F1 button to start the execution.
7.5.1.3 Execution
When the execution starts, on the screen will be showed a series of tables that allow to
monitor the instrument operation.
The first table is the ‘Initial Washing’ one; then will be showed the ‘Auto Zero
Procedure’ table, a control is executed on the filters used for the programmed methods.
Furthermore, other tables will indicate, by analysis (in bold), which operation is in
execution, in particular:
• Sampling, or control or test
• Standard incubation or control or test
• Standard reading or curve or test
• Washings
The following image (fig. 7.5.5) shows an example table:
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it
EI1121A – Echo PC User manual - Rel. 1.1
fig. 7.5.5
In this screen are detailed the following information:
Execution: Number of tests to execute.
Wells used: Number of wells used for the cycle execution. The first one is
the well used for the washings:
Total time: Time to execute the cycle (min and sec) + time to programming
the work execution (in sec).
Final time: Remaining time to the end of the work cycle (min and sec).
Well: The last well used for the sampling.
Sample: Standard sample.
Cycle t.: Time to effect a simple cycle in 100 of sec.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.5.1.4 Automatic sample dilution
To run the automatic sample dilution, in case there is at least one patient with the predilution activated in the work list, press the Key F2 – Execution from the Main Menun
and then press F3- Automatic sample dilution.
For ex. In case the patient n. 1 Daniele has in the work list activated the dilution in
percentage, follow the below procedure:
Put an empty serum cup in the position n. 32 of the sample plate (the available positions
for the dilutions are starting from the position 32 of the sample plate).
Put a reagent bottle in the position 27 with distilled water or physiological solution and
press a key.
ECHO will proceed with the dilution.
7.5.1.5 Automatic standard dilution
From the Main Menu press F2 – Execution and F4 – Automatic standard dilution, in
case you want to read for the first time a curve or you want to re-read it to reconfirm the
calibration factors.
For ex. In case of a calibration curve with 5 standards put in standard position 5 on the
sample plate a serum cups full of concentrated standard. Put in reagent position 27 a
reagent bottle with distilled water and press a key.
Note: The above two procedures are suggested on the video.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
DISPLAY RESULTS
PRINT RESULTS
SEND RESULTS
REPETITIONS
CONTROLS
7.6 Results Handling
At the end of the normal cycle execution, the ECHO will inform you by 3 signals and if
there are other memorised tests to execute it will return to the Execution Menu, on the
contrary will be showed the Main menu (fig. 7.2.1).
To read the tests results press the F3 button in the Main menu; the following screen will
appear (fig. 7.6.1):
fig. 7.6.1
The five buttons showed on the bottom of the screen, have the following functions:
F1
Press this button to visualize the results per patient or
per test.
F2
To print the results by group of patients.
F3
Allows to load the results (and the working list) on a
PC.
F4
To organize the repetition by test or by patient.
F5
This function allows visualizes, prints, or erases the
memorized values of the tests and controls.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.6.1 Display Results
Pressing the F1 button in the Results menu (fig. 7.6.1), will be showed the Result Report
menu (fig 7.6.2):
fig. 7.6.2
The F4 and F5 buttons allows to choose the visualization mode, by patient (F4) or by
test (F5).
The F2 and F3 buttons allows to display the next or previous patients/tests.
With the F1 button is possible to choose a patient (or a test) simply typing the patient
(test) number in the Patient (Test) field, on the top of the screen.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.6.2 Print Results
Pressing the F2 button in the Results menu (fig. 7.6.1), will be showed the Print
Patients Report menu (fig 7.6.3):
First sample : 1: Last sample : 63:
PRINT RESULTS
F1
PRINT
fig. 7.6.3
Now insert in the fields the First and the Last patient which should be printed.
Press the key F1 – Print.
PRINT RESULTS
First sample : 1: Last sample : 63:
Form- feed after every sample (0= No, 1= Yes)
fig. 7.6.4
Choosing 0 the patients will be printed in sequence without heading, choosing 1 we will
have a patient report complete of heading printed on the external printer.
NOTE: The patients which have no results will not be printed.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it

EI1121A – Echo PC User manual - Rel. 1.1
7.6.3 Repetitions
After the execution of a tests cycle, the operator can ask some repetitions to be done.
There are 2 functions to use.
Pressing the F4 button in the Results menu (fig. 7.6.1), will be showed the following
screen (fig 7.6.5):
fig. 7.6.5
With this menu, the operator can carry out some repetitions after the execution of a tests
cycle. Two functions are available; pressing the F1 button will repeated one test for all
the programmed patients. With the F2 button will be repeated one test for only one
patient, with the possibility of an auto-dilution.
Edif Instruments s.r.l. – Via Ardeatina, 132 – 00179 Roma – ITALY
Tel.+39/065127161 – Fax +39/065127550
email edif@edif.it
 Loading...
Loading...