EDI VERIS EMS IV, VERIS FMS IV Installation & Service Manual
VERIS™ Service Manual EMS & FMS Stimulators
VERIS™
FMS IV / EMS IV Stimulators
Installation &
Service Manual
Release D
0413
Electro-Diagnostic Imaging, Inc.
http://www.veris-edi.com
Tel 650-631-0120
Fax 650-631-0122
Email: support@veris-edi.com
VG-119-D / 21-Jan-2017 Page 1 of 16

VERIS™ Service Manual EMS & FMS Stimulators
TableofContents
1. IntendedUse,RegulatoryInformation,WarningsandPrecautions..........................................3
1.1. IntendedUse 3
1.2. Contraindications 3
1.3. RegulatoryInformation 3
1.4. WarningsandPrecautions 3
2. Labels....................................................................................................................................................................5
2.1. ComponentLabels 5
2.2. ShippingLabel 5
3. UserProfile.........................................................................................................................................................6
4. ProductLifetime...............................................................................................................................................6
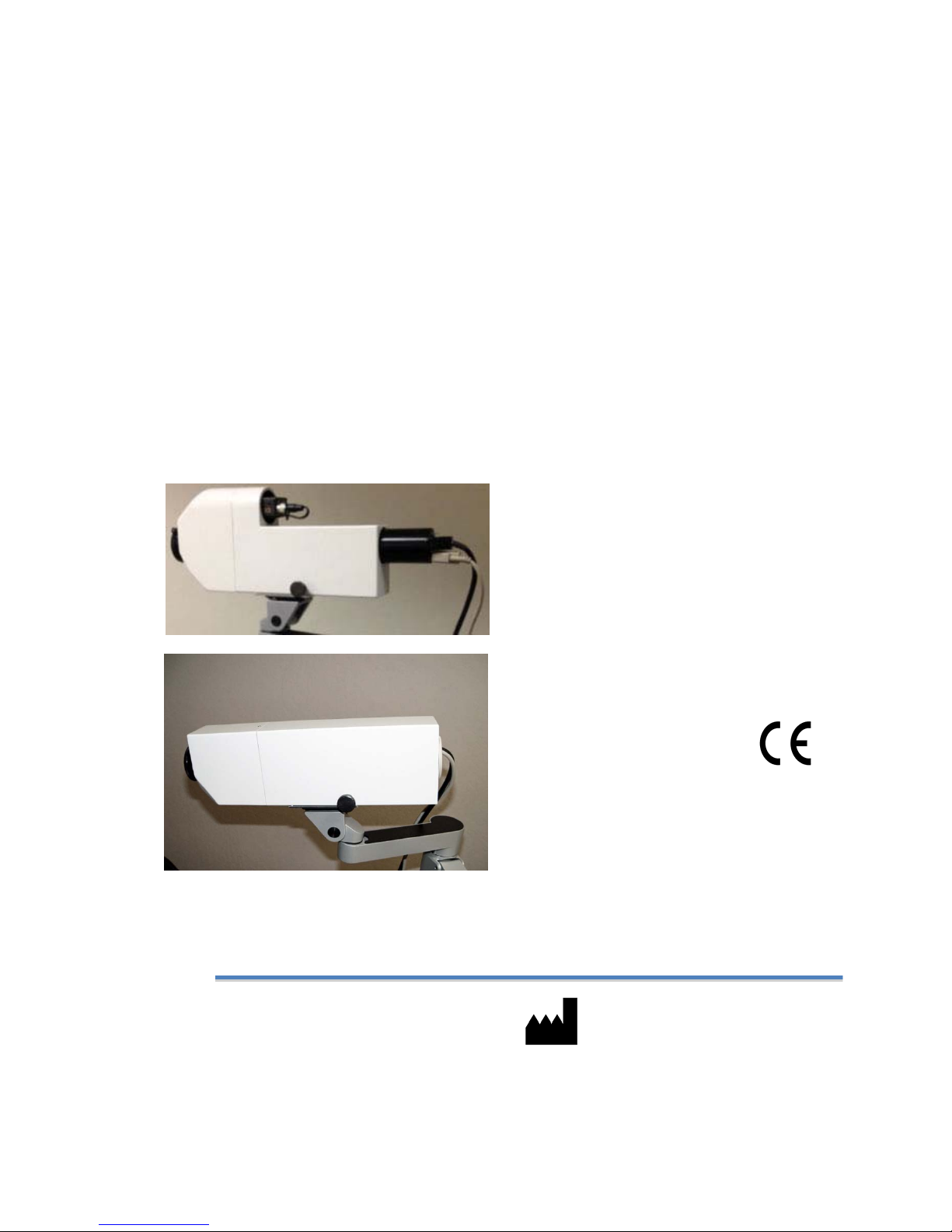
5. ComponentsoftheFMSIV/EMSIVStimulators...............................................................................6
6. TheoryofOperation.......................................................................................................................................6
7. Specifications.....................................................................................................................................................6
8. Installation..........................................................................................................................................................8
9. LuminanceCalibration...............................................................................................................................10
10. GridCalibration.............................................................................................................................................11
11. PreventiveMaintenance............................................................................................................................12
12. Troubleshooting&Repair.........................................................................................................................13
13. Parts...................................................................................................................................................................14
14. Cables,Schematics&Diagrams..............................................................................................................15
15. Software............................................................................................................................................................16
VG-119-D / 21-Jan-2017 Page 2 of 16
VERIS™ Service Manual EMS & FMS Stimulators
1. Intended Use, Regulatory Information, Warnings and Precautions
1.1.
Intended Use
The VERIS system is an electrodiagnostic device used to generate photic signals and to
measure and display the electrical signals generated by the retina and the visual nervous
system. It displays digitized electroretinogram (ERG) and visual evoked potential (VEP)
signals, power spectra and topographic maps. These functions are controlled and interpreted
by trained medical professionals. The device is intended for use in the diagnosis and
management of diseases affecting the function of the retina and the visual pathway.
1.2.
1.3.
1.4.
Contraindications
VERIS should not be used unless clinically indicated or in research with an IRB approved
protocol.
Regulatory Information
VERIS Systems are in compliance with:
FDA - 21 CFR Part 820 - Quality Sy stem Regulations
ISO 13485 - Quality Management Systems - Requirements for regulatory purposes
ISO 14971 - Medical Devices - Application of Risk Management to Medical Devices
IEC 60601-1 - Medical Electrical Equipment - Part 1: General requirements for basic
safety and essential performance
IEC 60601-1-2 - Medical Electrical Equipment - Part 2: General requirements for basic
safety and essential performance - collateral standard: Electromagnetic
VERIS has been tested for radiative emissions and shown to meet the requirements for a
Class A medical device.
Warnings and Precautions
Read this manually carefully before using the VERIS System.
WARNING: To avoid risk of electric shock, this equipment must only be connected to
electrical supply mains with protective earth.
WARNING: To avoid risk of explosion, do not operate the isolation power supply in the
presence of flammable anesthetics or other flammable gases or liquids.
WARNING: Do not operate the isolation power supply on the floor.
WARNING: Do not connect additional multiple outlet devices or extension cords to the
system.
WARNING: Do not connect any device(s) not approved by EDI to the multiple socket
outlets of the isolation power supply.
WARNING: Unauthorized modifications to the VERIS hardware and / or software void the
warranty and may increase the risk of injury to patient and/or operator.
WARNING: All servicing to be undertaken only by qualified personnel. There are no user
serviceable parts inside the unit.
CAUTION: If corneal electrodes are used for data acquisition, follow all instructions
provided by the manufacturer and appropriate regulatory agencies for the use, cleaning,
disinfection, and maintenance of these electrodes.
VG-119-D / 21-Jan-2017 Page 3 of 16
VERIS™ Service Manual EMS & FMS Stimulators
CAUTION: Carefully adhere to best medical practices whenever administering medication
for mydriasis and/or corneal anesthesia. These include proper instructions to the patient,
administration of eye medication, additional care in the event of adverse reactions, and
provision of and/or use of dark glasses after testing.
WARNING: Perform system calibrations as described in this manual at intervals of 2-3
months.
WARNING - Some normative sample data as well as some patient data are provided for some
of the protocols. They are meant for illustration only. Users are encouraged to collect their
own normative data and use VERIS protocols for the statistical analysis.
WARNING - Backup the computer records on a regular basis to prevent the loss of patient
data.
WARNING - The VERIS System in its complete configuration has been tested to meet the
requirements for hospital use in the United States, Canada and Europe. If the VERIS System
is used with customer provided components, testing for safety is the responsibility of the
customer.
Do not sit on the cart or table
.
CAUTION: Federal law restricts the medical use of this device on the order of a physician or
a properly licensed practitioner.
CAUTION: To reduce risk of exposure to airborne pathogens, clean Fresnel lenses, acrylic
screens, and painted surfaces of stimulators with a soft cotton cloth and anti-bacterial window
cleaning solution or alcohol swabs prior to each use.
WARNING: Clean the outside of the objective lens of the FMS and EMS stimulators ONLY
with a high quality lens cleaner and a clean, soft cloth. Antibacterial soft, absorbent
micropore cloth may be used with the lens cleaner. Do not, under any circumstances, remove
this lens from the stimulator.
Recording electrodes can transfer pathogens between patients. Disinfect reusable electrodes
between patients according to manufacturers instructions.
Single use electrodes must be disposed of as biohazardous medical waste.
Recycle electronic equipment after end of life according to local laws and regulations
VG-119-D / 21-Jan-2017 Page 4 of 16
VERIS™ Service Manual EMS & FMS Stimulators
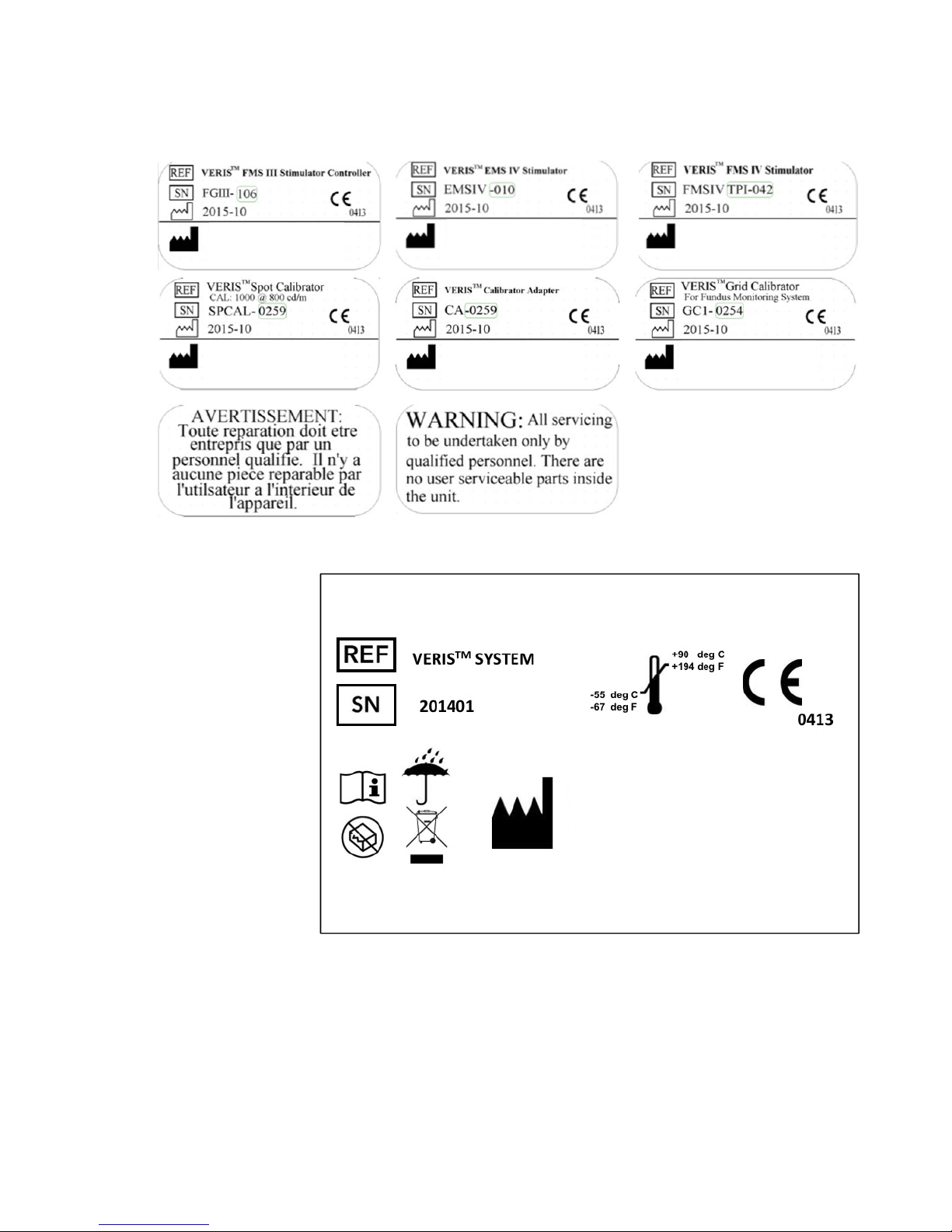
2. Labels
2.1.
Component Labels
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd.,
Milpitas, CA 95035
Made in USA
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd.,
Milpitas, CA 95035
Made in USA
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd.,
Milpitas, CA 95035
Made in USA
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd.,
Milpitas, CA 95035
Made in USA
2.2.
Shipping Label
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd.,
Milpitas, CA 95035
Made in USA
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd.,
Milpitas, CA 95035
Made in USA
VG-119-D / 21-Jan-2017 Page 5 of 16
Electro-Diagnostic Imaging, Inc.
1551 McCarthy Blvd., Suite 114,
Milpitas, CA 95035, USA
T: +1 (650) 631 – 0120
F: +1 (650) 631 – 0122
 Loading...
Loading...