Page 1

Page 2

About this Manual
P/N: 01.54.456718
MPN: 01.54.456718011
Release Date: July, 2015
© Copyright EDAN INSTRUMENTS, INC. 2015. All rights reserved.
Statement
This manual will help you understand the operation and maintenance of the product better. It is
reminded that the product shall be used strictly complying with this manual. User’s operation
failing to comply with this manual may result in malfunction or accident for which EDAN
INSTRUMENTS, INC. (hereinafter called EDAN) can not be held liable.
EDAN owns the copyrights of this manual. Without prior written consent of EDAN, any
materials contained in this manual shall not be photocopied, reproduced or translated into other
languages.
Materials protected by the copyright law, including but not limited to confidential information
such as technical information and patent information are contained in this manual, the user shall
not disclose such information to any irrelevant third party.
The user shall understand that nothing in this manual grants him, expressly or implicitly, any
right or license to use any of the intellectual properties of EDAN.
EDAN holds the rights to modify, update, and ultimately explain this manual.
Product Information
Product Name: Electrocardiograph
Model: SE-301, iSE-301
Responsibility of the Manufacturer
EDAN only considers itself responsible for any effect on safety, reliability and performance of
the equipment if:
Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by EDAN, and
I
Page 3

The electrical installation of the relevant room complies with national standards, and
The instrument is used in accordance with the instructions for use.
Upon request, EDAN may provide, with compensation, necessary circuit diagrams, and other
information to help qualified technician to maintain and repair some parts, which EDAN may
define as user serviceable.
Terms Used in this Manual
This guide is designed to give key concepts on safety precautions.
WARNING
A WARNING label advises against certain actions or situations that could result in personal
injury or death.
CAUTION
A CAUTION label advises against actions or situations that could damage equipment, produce
inaccurate data, or invalidate a procedure.
NOTE
A NOTE provides useful information regarding a function or a procedure.
II
Page 4

Table of Contents
Chapter 1 Safety Guidance ....................................................................................................... 1
1.1 Indications for Use/Intended Use ..................................................................................... 1
1.2 Warnings and Cautions ..................................................................................................... 1
1.2.1 Safety Warnings ........................................................................................................ 2
1.2.2 Lithium Battery Care Warnings ................................................................................ 5
1.2.3 General Cautions ...................................................................................................... 6
1.3 List of Symbols ................................................................................................................. 7
Chapter 2 Introduction ............................................................................................................ 10
2.1 Top Panel ........................................................................................................................ 10
2.2 Bottom Panel ................................................................................................................... 11
2.3 Right Panel ...................................................................................................................... 11
2.4 Back Panel ...................................................................................................................... 11
Chapter 3 Operation Preparations ......................................................................................... 12
3.1 Loading/Replacing Recorder Paper ................................................................................ 12
3.2 Preparing the Patient ....................................................................................................... 13
3.2.1 Instructing the Patient ............................................................................................. 13
3.2.2 Cleaning the Skin .................................................................................................... 13
3.3 Connecting the Patient Cable to the Electrocardiograph and Electrodes ....................... 13
3.4 Attaching Electrodes to the Patient ................................................................................. 14
3.4.1 Reusable Electrodes ................................................................................................ 14
3.4.2 Disposable Electrodes ............................................................................................. 17
3.5 Inspection Before Power On ........................................................................................... 18
Chapter 4 Sampling and Printing ECG ................................................................................. 20
4.1 Entering Patient Information .......................................................................................... 20
4.1.1 Entering Patient Information Manually .................................................................. 20
4.1.2 Entering Patient Information by Acquiring Orders ................................................ 21
4.2 Printing ECG Reports ..................................................................................................... 21
4.3 Sample ECG Reports ...................................................................................................... 22
4.3.1 ECG Reports in the AUTO Mode ........................................................................... 22
4.3.2 PDF Report ............................................................................................................. 24
Chapter 5 Managing ECG Records ........................................................................................ 25
5.1 Transmitting ECG Records to the PC ............................................................................. 25
5.1.1 Transmitting ECG Records through the Network .................................................. 25
5.1.2 Transmitting ECG Records through WIFI Network (Optional) ............................. 26
5.2 Copying ECG Records between the ECG Machine and External Memory ................... 26
5.3 Deleting Patient Records ................................................................................................ 28
5.4 Printing a Patient Record in the File Manager screen..................................................... 28
III
Page 5

Chapter 6 Settings .................................................................................................................... 29
6.1 Work Mode ..................................................................................................................... 29
6.2 Filter ................................................................................................................................ 29
6.3 Record Info Setup ........................................................................................................... 30
6.3.1 Setup 1 .................................................................................................................... 30
6.3.2 Setup 2 .................................................................................................................... 31
CAUTION ................................................................................................................................. 32
6.4 Patient Information Setup ............................................................................................... 33
6.5 Transmission Setup ......................................................................................................... 34
6.6 Lead Setup ...................................................................................................................... 34
6.7 File Setup ........................................................................................................................ 35
6.8 Date&Time Setup ........................................................................................................... 36
6.9 System Maintenance ....................................................................................................... 36
6.10 Other Setup ................................................................................................................... 36
6.11 Advanced Setup ............................................................................................................. 37
Chapter 7 Hint Information .................................................................................................... 38
Chapter 8 Troubleshooting ...................................................................................................... 39
Chapter 9 Cleaning, Care and Maintenance ......................................................................... 41
9.1 General Points ................................................................................................................. 41
9.2 Cleaning .......................................................................................................................... 41
9.2.1 Cleaning the Main Unit .......................................................................................... 42
9.2.2 Cleaning the Patient Cable ..................................................................................... 42
9.2.3 Cleaning the Reusable Electrodes .......................................................................... 43
9.3 Disinfection ..................................................................................................................... 43
9.3.1 Disinfecting the Main Unit ..................................................................................... 43
9.3.2 Disinfecting the Patient Cable ................................................................................ 44
9.3.3 Disinfecting the Reusable Electrodes ..................................................................... 44
9.4 Care and Maintenance .................................................................................................... 44
9.4.1 Recharge and Replacement of Battery ................................................................... 44
9.4.2 Recorder Paper ....................................................................................................... 45
9.4.3 Maintenance of the Main Unit, the Patient Cable and Electrodes .......................... 46
Chapter 10 Accessories ............................................................................................................ 48
Chapter 11 Warranty and Service .......................................................................................... 50
11.1 Warranty ........................................................................................................................ 50
11.2 Contact information ...................................................................................................... 50
Appendix 1 Technical Specifications ...................................................................................... 51
A1.1 Safety Specifications .................................................................................................... 51
A1.2 Environment Specifications ......................................................................................... 52
IV
Page 6

A1.3 Physical Specifications ................................................................................................ 52
A1.4 Power Supply Specifications ....................................................................................... 52
A1.5 Performance Specifications ......................................................................................... 53
Appendix 2 EMC Information ................................................................................................ 55
Appendix 3 Abbreviation ......................................................................................................... 59
V
Page 7
SE-301 Series Electrocardiograph User Manual Safety Guidance
Chapter 1 Safety Guidance
This chapter provides important safety information related to the use of the 3-Channel
Electrocardiograph.
1.1 Indications for Use/Intended Use
The intended use of the 3-Channel Electrocardiograph is to acquire ECG signals from adult and
pediatric patients through body surface ECG electrodes. The electrocardiograph is intended to be
used only in hospitals or healthcare facilities by doctors and trained healthcare professionals. The
cardiogram recorded by the 3-Channel Electrocardiograph can help users to analyze and diagnose
heart disease. However the ECG with measurements and interpretive statements is offered to
clinicians on an advisory basis only.
WARNING
1. This equipment is not designed for intracardiac use or direct cardiac application.
2. This equipment is not intended for home use.
3. This equipment is not intended for treatment or monitoring.
4. This equipment is intended for use on adult and pediatric patients only.
5. The results given by the equipment should be examined based on the overall clinical
condition of the patient, and they can not substitute for regular checking.
1.2 Warnings and Cautions
In order to use the electrocardiograph safely and effectively, and avoid possible dangers caused
by improper operations, please read through the user manual and be sure to be familiar with all
functions of the equipment and proper operation procedures before use.
Please pay more attention to the following warning and caution information.
- 1 -
Page 8

SE-301 Series Electrocardiograph User Manual Safety Guidance
1.2.1 Safety Warnings
WARNING
1. The electrocardiograph is provided for the use of qualified physicians or personnel
professionally trained. They should be familiar with the contents of this user manual
before operation.
2. Only qualified service engineers can install this equipment, and only service
engineers authorized by the manufacturer can open the shell. Otherwise, safety
hazards may happen.
3. Only qualified installation or service engineers can shift the mains supply shift switch
(100V-240V~) according to local mains supply specifications.
4. The EQUIPMENT is protected against malfunction caused by electrosurgery.
5. EXPLOSION HAZARD - Do not use the electrocardiograph in the presence of
flammable anesthetic mixture with oxygen or other flammable agents.
6. SHOCK HAZARD - The power receptacle must be a hospital grade grounded outlet.
Never try to adapt the three-prong plug to fit a two-slot outlet.
7. If the integrity of the external protective conductor is in doubt, the equipment should
be operated by using the built-in rechargeable battery.
8. Do not use this equipment in the presence of high static electricity or high voltage
equipment which may generate sparks.
9. Only the patient cable and other accessories supplied by the manufacturer can be
used. Or else, the performance and electric shock protection can not be guaranteed.
10. The use of patient cable and other accessories not supplied by the manufacturer may
result in increased emissions or decreased immunity of the equipment.
11. The electrocardiograph has been safety tested with the recommended accessories,
peripherals, and leads, and no hazard is found when the electrocardiograph is
operated with cardiac pacemakers or other stimulators.
12. Make sure that all electrodes are connected to the patient correctly before operation.
13. Ensure that the conductive parts of electrodes and associated connectors, including
neutral electrode, do not come into contact with earth or any other conducting
objects.
- 2 -
Page 9

SE-301 Series Electrocardiograph User Manual Safety Guidance
WARNING
14. To avoid a polarization or DC offset voltage, use non-polarizing electrodes(which will
not form a DC offset voltage when subjected to a DC current) such as
silver/silver-chloride types if there is a situation where there is a likelihood that a
defibrillation procedure will be necessary.
15. There is no danger for patients with pacemakers. However, if a pacemaker is used,
the results given by the equipment may be invalid, or lose the clinical significance.
16. If reusable electrodes with electrode gel are used during defibrillation, ECG recovery
will take more than 10 seconds. The manufacturer recommends the use of
disposable electrodes at all times.
17. Electrodes of dissimilar metals should not be used; it may cause a high polarization
voltage.
18. The disposable electrodes can only be used for one time.
19. Do not touch the patient, bed, table or the equipment while using the ECG together
with a defibrillator.
20. Do not touch accessible parts of electrical equipment and the patient simultaneously.
21. The use of equipment that applies high frequency voltages to the patient (including
electrosurgical equipment and some respiration transducers) is not supported and
may produce undesired results. Disconnect the patient data cable from the
electrocardiograph, or detach the leads from the patient prior to performing any
procedure that uses high frequency surgical equipment.
22. If WIFI technology is used, in order to maintain compliance with the FCC RF
exposure guidelines, the wireless should be installed and operated with a minimum
distance of 20cm between the radiator and the human body. Use the supplied
antenna only. There should be no shield in or around the room where WIFI is used.
23. Fix attention on the examination to avoid missing important ECG waves.
24. SHOCK HAZARD - Don't connect non-medical electrical equipment, which has been
supplied as a part of the system, directly to the wall outlet when the non-medical
equipment is intended to be supplied by a multiple portable socket-outlet with an
isolation transformer.
25. SHOCK HAZARD - Don't connect electrical equipment, which has not been supplied
as a part of the system, to the multiple portable socket-outlet supplying the system.
- 3 -
Page 10

SE-301 Series Electrocardiograph User Manual Safety Guidance
WARNING
26. Do not connect any equipment or accessories that are not approved by the
manufacturer or that are not IEC/EN 60601-1 approved to the electrocardiograph.
The operation or use of non-approved equipment or accessories with the
electrocardiograph is not tested or supported, and electrocardiograph operation and
safety are not guaranteed.
27. Any non-medical equipment (such as the external printer) is not allowed to be used
within the patient vicinity (1.5m/6ft.).
28. Multiple portable socket-outlets shall not be placed on the floor.
29. Do not use the additional multiple portable socket-outlet or extension cord in the
medical electrical system, unless it's specified as part of the system by manufacturer.
And the multiple portable socket-outlets provided with the system shall only be used
for supplying power to equipment which is intended to form part of the system.
30. Accessory equipment connected to the analog and digital interfaces must be certified
according to the respective IEC/EN standards (e.g. IEC/EN 60950 for data
processing equipment and IEC/EN 60601-1 for medical equipment). Furthermore all
configurations shall comply with the valid version of the standard IEC/EN 60601-1-1.
Therefore anybody, who connects additional equipment to the signal input or output
connector to configure a medical system, must make sure that it complies with the
requirements of the valid version of the system standard IEC/EN 60601-1-1. If in
doubt, consult our technical service department or your local distributor.
31. Connecting any accessory (such as external printer) or other device (such as the
computer) to this electrocardiograph makes a medical system. In that case,
additional safety measures should be taken during installation of the system, and the
system shall provide:
a) Within the patient environment, a level of safety comparable to that provided by
medical electrical equipment complying with IEC/EN 60601-1, and
b) Outside the patient environment, the level of safety appropriate for non-medical
electrical equipment complying with other IEC or ISO safety standards.
32. All the accessories connected to system must be installed outside the patient vicinity,
if they do not meet the requirement of IEC/EN 60601-1.
- 4 -
Page 11
SE-301 Series Electrocardiograph User Manual Safety Guidance
WARNING
33. If multiple instruments are connected to a patient, the sum of the leakage currents
may exceed the limits given in the IEC/EN 60601-1 and may pose a safety hazard.
Consult your service personnel.
34. The potential equalization conductor can be connected to that of other equipment
when necessary, to make sure that all these devices are connected to the potential
equalization bus bar of the electrical installation.
35. The electrocardiograph shall not be serviced or maintained while in use with a
patient.
36. The appliance coupler or mains plug is used as isolation means from supply mains.
Position the electrocardiograph in a location where the operator can easily access
the disconnection device.
37. The medical electrical equipment needs to be installed and put into service according
to Appendix 2 EMC Information.
38. The equipment should not be used adjacent to or stacked with other equipment, refer
to the recommended separation distances provided in Appendix 2 EMC Information.
39. Portable and mobile RF communications equipment can affect medical electrical
equipment, refer to the recommended separation distances provided in Appendix 2
EMC Information.
40. Assembly of the electrocardiograph and modifications during actual service life shall
be evaluated based on the requirements of IEC60601-1.
1.2.2 Lithium Battery Care Warnings
WARNING
1. Improper operation may cause the lithium battery (hereinafter called battery) to be
hot, ignited or exploded, and it may lead to the declination of the battery capacity. It is
necessary to read the user manual carefully and pay more attention to warning
messages.
2. Only qualified service engineer authorized by the manufacturer can open the battery
compartment and replace the battery, and batteries of the same model and
specification as manufacturer configuration should be used.
- 5 -
Page 12
SE-301 Series Electrocardiograph User Manual Safety Guidance
WARNING
3. DANGER OF EXPLOSION -- Do not reverse the anode and the cathode when
installing the battery.
4. Do not heat or splash the battery or throw it into fire or water.
5. Do not destroy the battery; Do not pierce battery with a sharp object such as a needle;
Do not hit with a hammer, step on or throw or drop to cause strong shock; Do not
disassemble or modify the battery.
6. When leakage or foul smell is found, stop using the battery immediately. If your skin
or cloth comes into contact with the leakage liquid, cleanse it with clean water at once.
If the leakage liquid splashes into your eyes, do not wipe them. Irrigate them with
clean water first and go to see a doctor immediately.
7. Properly dispose of or recycle the depleted battery according to local regulations.
8. Only when the device is off can the battery be installed or removed.
9. Remove the battery from the electrocardiograph when the electrocardiograph is not
used for a long time.
10. If the battery is stored alone and not used for a long time, we recommend that the
battery should be charged at least once every 6 months to prevent over-discharge.
1.2.3 General Cautions
CAUTION
1. Federal (U.S.) law restricts this device to sale by or on the order of a physician.
2. Avoid liquid splash and excessive temperature. The temperature must be kept
between 5 ºC and 40 ºC during operation, and it should be kept between -20 ºC and
55 ºC during transportation and storage.
3. Do not use the equipment in a dusty environment with bad ventilation or in the
presence of corrosive.
4. Make sure that there is no intense electromagnetic interference source around the
equipment, such as radio transmitters, mobile phones etc. Attention: large medical
electrical equipment such as electrosurgical equipment, radiological equipment and
magnetic resonance imaging equipment is likely to bring electromagnetic
interference.
- 6 -
Page 13
SE-301 Series Electrocardiograph User Manual Safety Guidance
CAUTION
5. Ruptured fuse must only be replaced with that of the same type and rating as the
original.
6. The device and accessories are to be disposed of according to local regulations after
their useful lives. Alternatively, they can be returned to the dealer or the manufacturer
for recycling or proper disposal. Batteries are hazardous waste. Do NOT dispose of
them together with house-hold garbage. At the end of their lives hand the batteries
over to the applicable collection points for the recycling of waste batteries. For more
detailed information about recycling of this product or battery, please contact your
local Civic Office, or the shop where you purchased the product.
7. Before use, the equipment, the patient cable and electrodes should be checked.
Replace them if there is any evident defectiveness or aging which may impair the
safety or the performance. Make sure that the equipment is in proper working
condition.
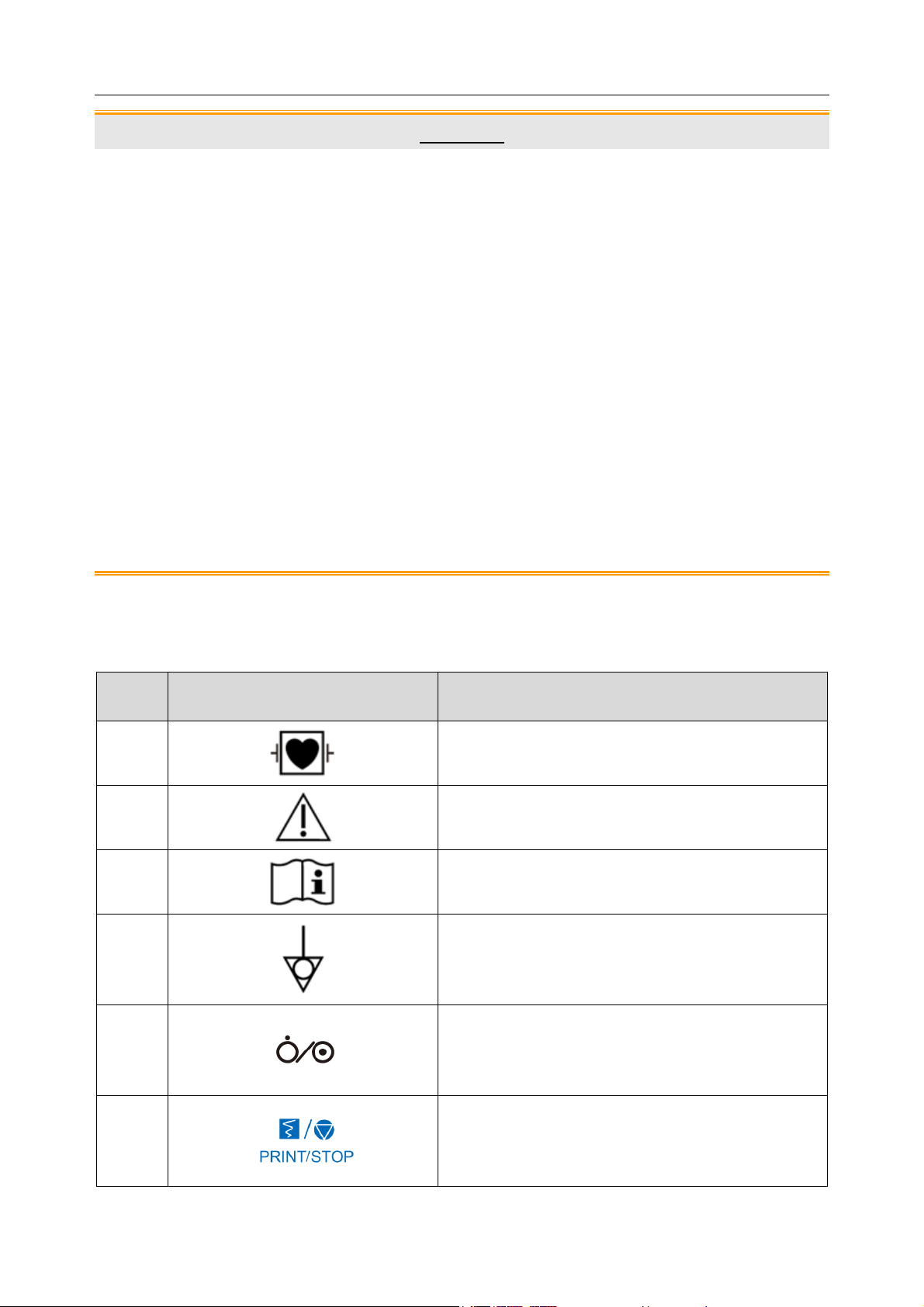
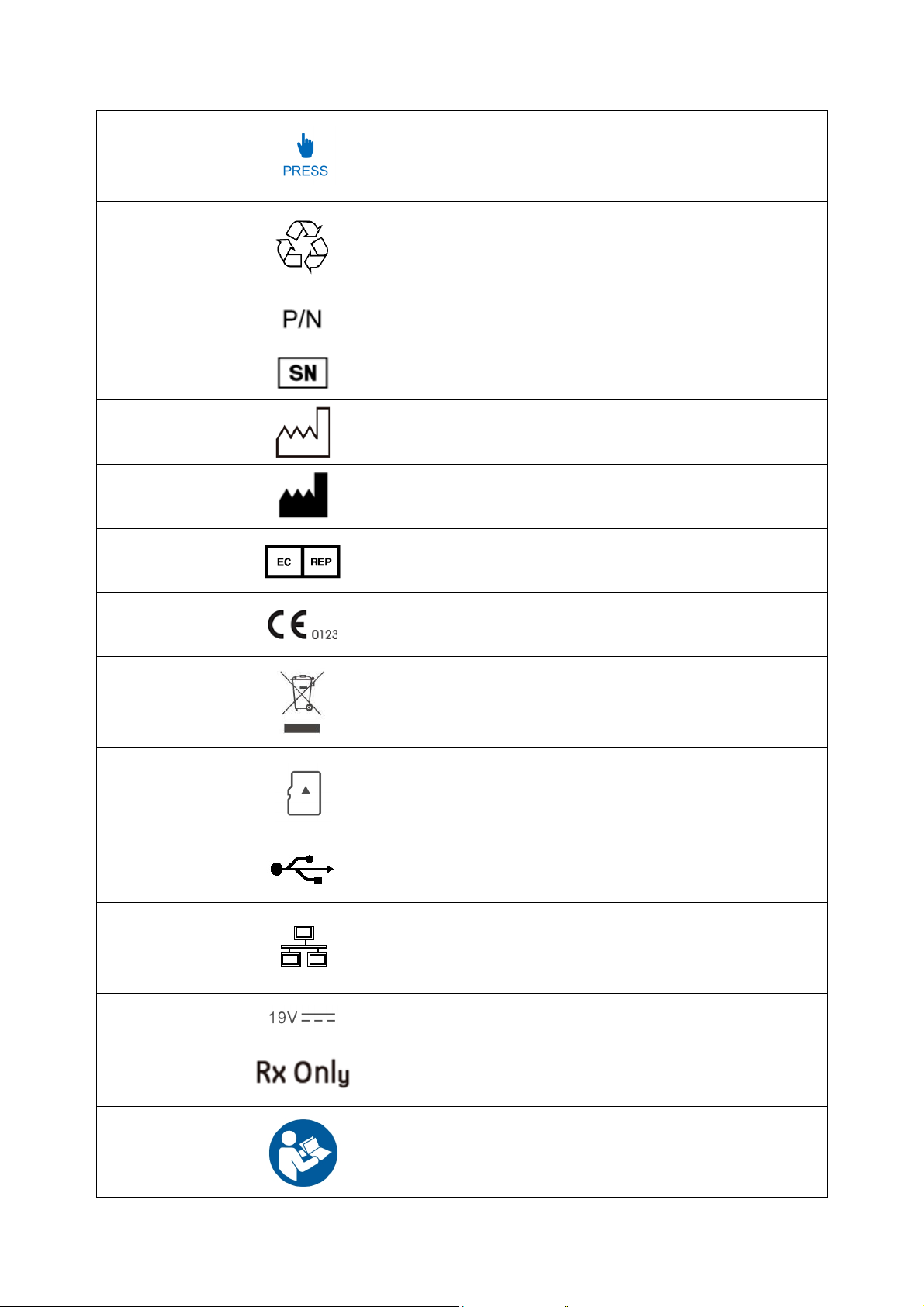
1.3 List of Symbols
No. Symbol Description
1
2
3
4
DEFIBRILLATION-PROOF TYPE CF
APPLIED PART
Caution
Operating instructions
Equipotential grounding
5
6
Power key
Print/Stop key
- 7 -
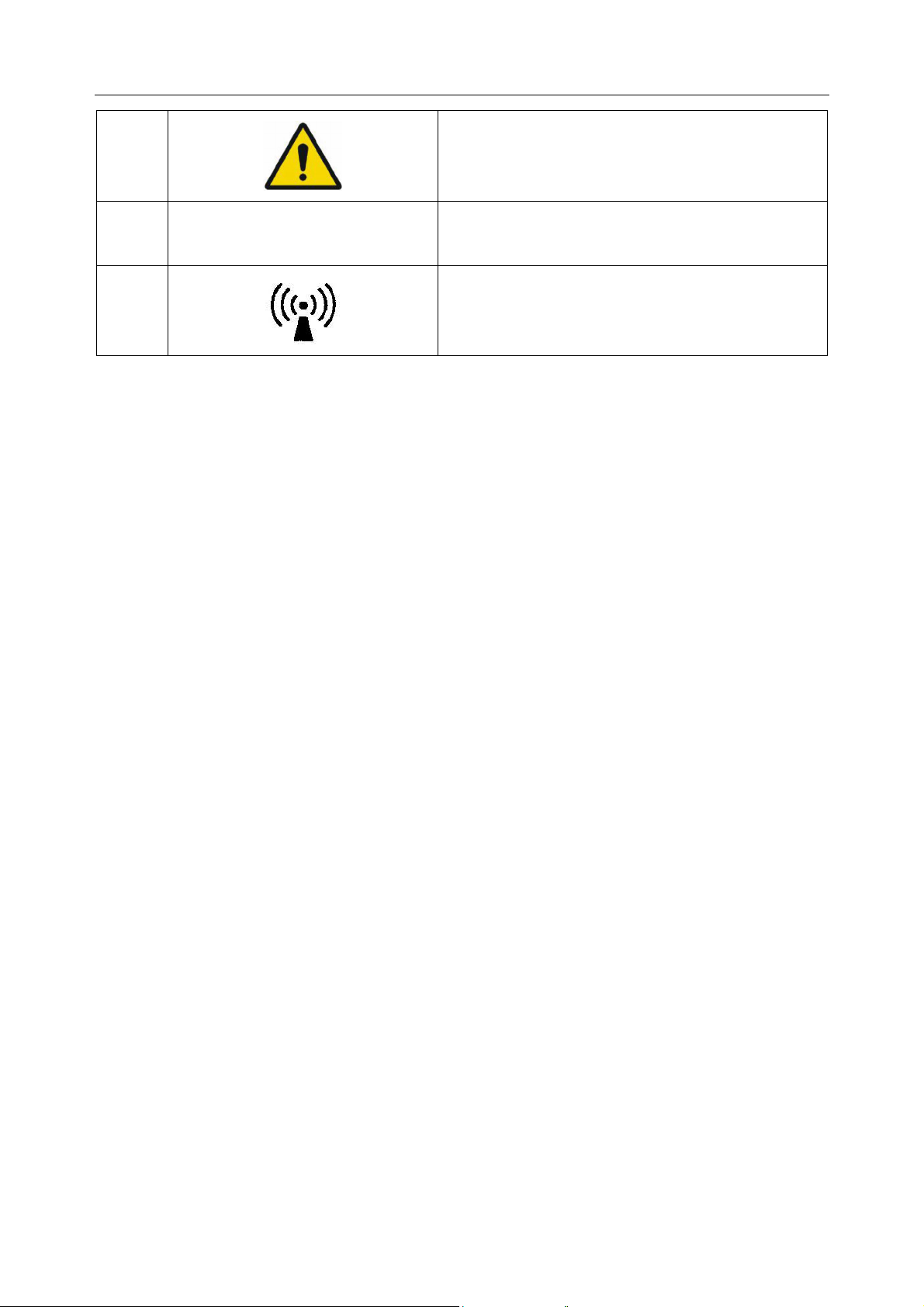
Page 14
SE-301 Series Electrocardiograph User Manual Safety Guidance
7
8
9
10
11
12
13
Casing Button
General symbol for recovery/recyclable
Part Number
SERIAL NUMBER
Date of manufacture
MANUFACTURER
AUTHORISED REPRESENTATIVE IN THE
EUROPEAN COMMUNITY
14
15
16
17
18
19
20
CE marking
Disposal method
SD card slot
USB socket
Net port
Power adapter port
Caution: Federal (U.S.) law restricts this device
to sale by or on the order of a physician
21
Refer to User Manual
(Background: Blue; Symbol: White)
- 8 -
Page 15
SE-301 Series Electrocardiograph User Manual Safety Guidance
Warning
22
(Background: Yellow; Symbol&Outline: Black)
23*
FCC ID: SMQSE301EDAN
SMQSE301EDAN
Federal Communications Commission: FCC ID:
24*
Non- ionizing electromagnetic radiation
NOTE:
1. *Applicable to the Electrocardiograph configured with WIFI module.
2. For details about buttons of the keyboard, refer to Chapter 2.
3. The user manual is printed in black and white.
- 9 -
Page 16

SE-301 Series Electrocardiograph User Manual Introduction
Chapter 2 Introduction
SE-301 3-channel electrocardiograph gathers ECG signals of 12 leads simultaneously. It displays
the operation menu, ECG parameters as well as electrocardiograms.
3-channel ECG waves can be viewed on the LCD screen and printed out by using a high-quality
thermal recorder.
The AUTO, MANU, RHYT, and R-R modes can be chosen freely.
SE-301 series can be powered by the mains supply or a built-in rechargeable lithium battery. Two
models are available: SE-301 with WIFI as an optional configuration, and iSE301 with WIFI as a
standard configuration.
With a 32-bit processor and a large-capacity memorizer, SE-301 has advanced performance and
high reliability. The compact size makes it suitable for clinic, hospital and ambulance use.
Configuration: main unit, power cord, earth wire, patient cable, electrodes, and lithium battery
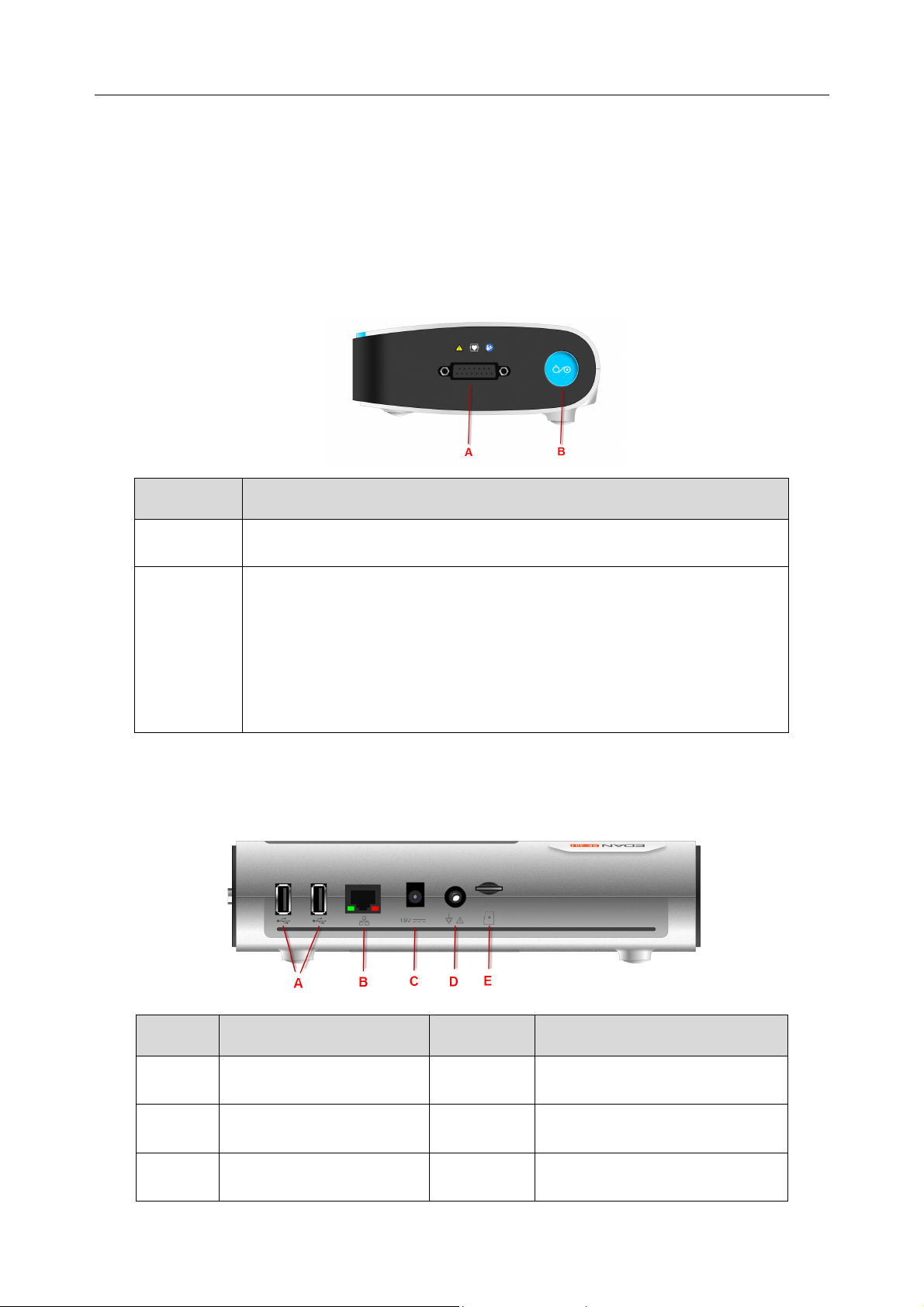
2.1 Top Panel
Figure 2-1 SE-301
No. Description
A
B
Press to release the recorder casing
Press to start/stop ECG sampling
- 10 -
Page 17
SE-301 Series Electrocardiograph User Manual Introduction
2.2 Bottom Panel
The silk screen on the battery compartment indicates the rated voltage.
2.3 Right Panel
.
No. Description
A
B
Patient cable socket
Power key (Long press: switch on/off; short press: sleep mode)
Color when using the mains supply: Green
Color when using built-in battery: Blue
Color when recharging: Orange
2.4 Back Panel
No. Description No. Description
A
B
C
USB socket D Equipotential grounding
- 11 -
E
SD card slot
Net port
Power adapter port - -
Page 18

SE-301 Series Electrocardiograph User Manual Operation Preparations
Chapter 3 Operation Preparations
3.1 Loading/Replacing Recorder Paper
NOTE:
1. When the folded thermal paper is used, the paper roller is unnecessary and must be
taken out.
2. The grid side of the paper should face the thermal print head, and the black marker
on the paper should face the black marker detecting area.
Loading/Replacing Process of Rolled Thermal Paper
Loading/Replacing Process of Folded Thermal Paper
- 12 -
Page 19

SE-301 Series Electrocardiograph User Manual Operation Preparations
3.2 Preparing the Patient
3.2.1 Instructing the Patient
Before attaching the electrodes, greet the patient and explain the procedure. Explaining the
procedure decreases the patient’s anxiety. Reassure the patient that the procedure is painless.
Privacy is important for relaxation. When possible, prepare the patient in a quiet room or area
where others can’t see the patient. Make sure that the patient is comfortable. The more relaxed
the patient is, the less the ECG will be affected by noise.
3.2.2 Cleaning the Skin
Thorough skin preparation is very important. The skin is a poor conductor of electricity and
frequently creates artifact that distorts the ECG signal. By performing methodical skin
preparation, you can greatly reduce the possibility of the noise caused by muscle tremor and
baseline drift, ensuring high-quality ECG waves. There is natural resistance on the skin surface
due to dry, dead epidermal cells, oils and dirt.
To clean the skin
1. Shave hair from electrode sites, if necessary. Excessive hair prevents a good connection.
2. Wash the area thoroughly with soap and water.
3. Dry the skin with a gauze pad to increase capillary blood flow to the tissues and to remove the
dead, dry skin cells and oils.
3.3 Connecting the Patient Cable to the
Electrocardiograph and Electrodes
WARNING
The performance and electric shock protection can be guaranteed only if original patient
cable and electrodes of the manufacturer are used.
- 13 -
Page 20
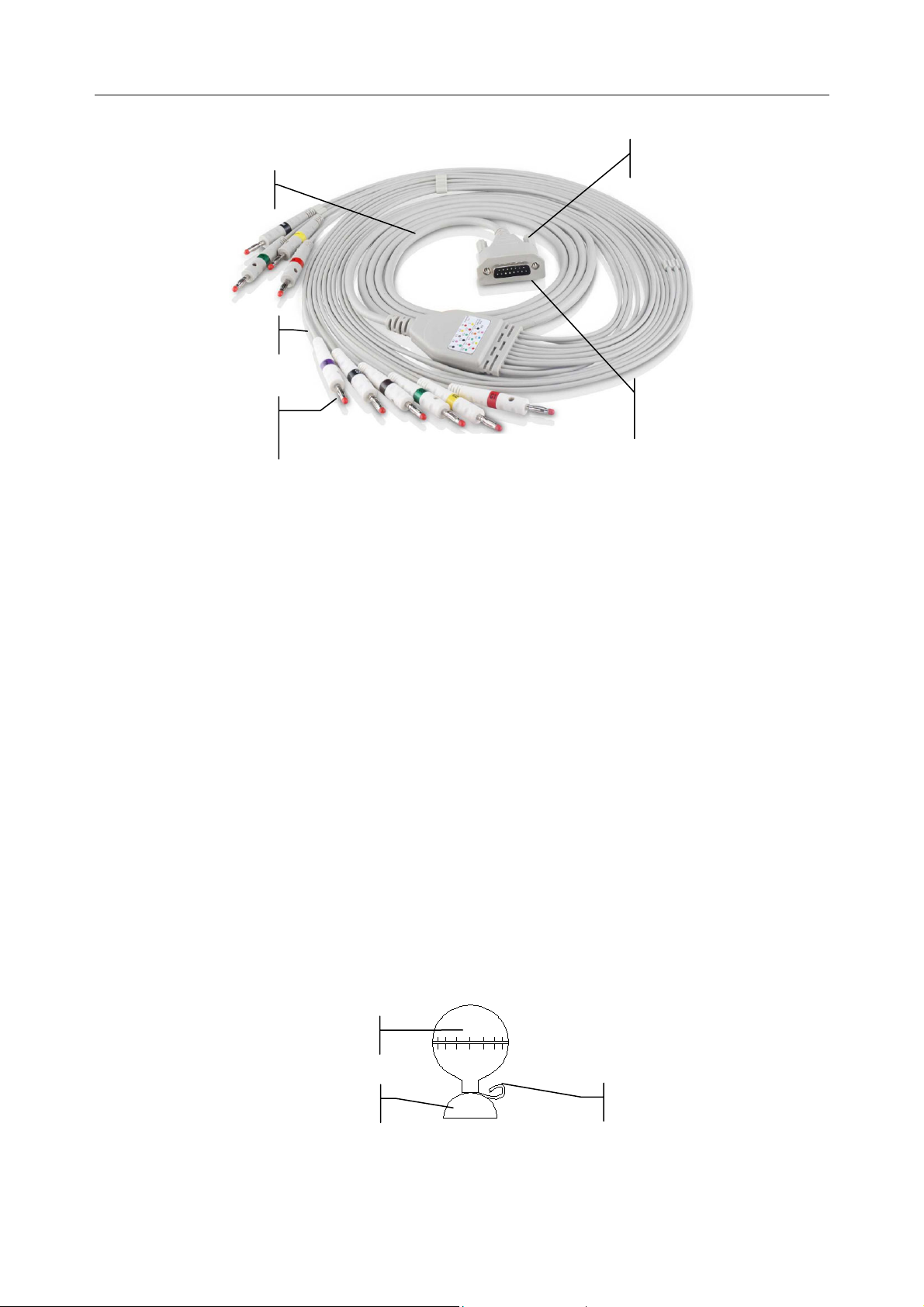
SE-301 Series Electrocardiograph User Manual Operation Preparations
grap
Screw
Main Cable
Lead Wires
Connecting to
Connecting to
Electrodes
Electrocardio
h
1. Connecting the Patient Cable to the Electrocardiograph
Connect the patient cable to the patient cable socket on the right side of the main unit, and then
secure them with two screws.
2. Connecting the Patient Cable to Electrodes
Align all lead wires of the patient cable to avoid twisting, and connect the lead wires to the
corresponding electrodes according to the colors and identifiers. Firmly attach them.
3.4 Attaching Electrodes to the Patient
There are two types of electrode for you to choose, one is the reusable electrodes, and the other is
the disposable electrodes. The uses of the two types of electrode are as shown below:
3.4.1 Reusable Electrodes
Reusable Electrodes is divided into Limb electrode and Chest Electrode, as the following figure
shows:
Suction Bulb
Metal Cup
Electrode
Chest Electrode
- 14 -
Page 21

SE-301 Series Electrocardiograph User Manual Operation Preparations
Electrode
Reed
Clamp
Limb Electrode
The identifiers and color codes of electrodes used comply with IEC/EN requirements. In order
to avoid incorrect connections, the electrode identifiers and color codes are specified in Table
4-1. Moreover the equivalent codes according to American requirements are given in Table 4-1
too.
Table 3–1 Electrodes and Their identifiers and color codes
European American
Electrodes Identifier Color code Identifier Color code
Right arm R Red RA White
Left arm L Yellow LA Black
Right leg N or RF Black RL Green
Left leg F Green LL Red
Chest 1 C1 White/red V1 Brown/red
Chest 2 C2 White/yellow V2 Brown/yellow
Chest 3 C3 White/green V3 Brown/green
Chest 4 C4 White/brown V4 Brown/blue
Chest 5 C5 White/black V5 Brown/orange
Chest 6 C6 White/violet V6 Brown/violet
As the following figure shows, the positions of chest electrodes on the body surface are
C1: Fourth intercostal space at the right border of the sternum
C2: Fourth intercostal space at the left border of the sternum
C3: Fifth rib between C2 and C4
C4: Fifth intercostal space on the left midclavicular line
C5: Left anterior axillary line at the horizontal level of C4
C6: Left midaxillary line at the horizontal level of C4
- 15 -
Page 22

SE-301 Series Electrocardiograph User Manual Operation Preparations
C1
C4
C2
C6
C3
C5
Chest Electrode Connection:
1) Ensure that the electrodes are clean;
2) Align all lead wires of the patient cable to avoid twisting, and connect the lead wires to
the corresponding electrodes according to the colors and identifiers;
3) Clean the electrode area on the chest surface with 75% alcohol;
4) Daub the round area of 25mm in diameter on each electrode site with gel evenly;
5) Place a small amount of gel on the brim of chest electrode’s metal cup;
6) Place the electrode on the chest electrode site and squeeze the suction bulb. Unclench it
and the electrode is adsorbed on the chest;
7) Attach all chest electrodes in the same way.
NOTE: Long-time measurement with a strong negative pressure on the suction bulb
may cause reddening of the skin. When using the electrode on small children or
patients with delicate skin, squeeze the suction ball lightly.
Limb Electrode Connection:
2) Ensure that the electrodes are clean;
3) Align all lead wires of the patient cable to avoid twisting,
and connect the lead wires to the corresponding
electrodes according to the colors and identifiers;
4) Clean the electrode area which is a short distance above
the ankle or the wrist with alcohol;
5) Daub the electrode area on the limb with gel evenly;
6) Place a small amount of gel on the metal part of the limb
- 16 -
Page 23

SE-301 Series Electrocardiograph User Manual Operation Preparations
electrode clamp;
7) Connect the electrode to the limb, and make sure that the metal part is placed on the
electrode area above the ankle or the wrist;
8) Attach all limb electrodes in the same way.
3.4.2 Disposable Electrodes
Disposable Electrode Alligator Clip
Disposable electrode must be used together with the alligator clip.
The electrodes’ positions on body surface are as the following table and figures:
American
European
Electrode placement
label
label
RA R Right deltoid
LA L Left deltoid
Above right ankle (Alternate placement, upper leg as close to torso
RL N or RF
as possible)
Above left ankle (Alternate placement, upper leg as close to torso
LL F
as possible)
V1 C1 Fourth intercostals space at right border of sternum
V2 C2 Fourth intercostals space at left border of sternum
V3 C3 Fifth rib between V2 and V4
V4 C4 Fifth intercostals space on left midclavicular line
V5 C5 Left anterior axillary line at the horizontal level of V4
V6 C6 Left midaxillary line at the horizontal level of V4
- 17 -
Page 24

SE-301 Series Electrocardiograph User Manual Operation Preparations
V1
V4
V2
V3
V6
V5
Disposable Electrode connection
1) Align all lead wires of the patient cable to avoid twisting, and connect the alligator clips to
the lead wires.
2) Clean the electrode areas on the body surface with 75% alcohol.
3) Attach the disposable electrodes to the electrode positions on body surface.
4) Clip the disposable electrodes with the alligator clips.
The quality of ECG waveform will be affected by the contacting resistance between the patient
and the electrode. In order to get a high-quality ECG, the skin-electrode resistance must be
minimized when you attach electrodes to patients.
CAUTION
The disposable electrodes can only be used for one time.
WARNING
1. Make sure that all electrodes are connected to the patient correctly before operation.
2. Make sure that the conductive parts of electrodes and associated connectors,
including neutral electrode, do not come in contact with earth or any other conducting
objects.
3.5 Inspection Before Power On
In order to avoid safety hazards and get good ECG records, the following inspection procedure is
recommended before power-on and operation.
- 18 -
Page 25

SE-301 Series Electrocardiograph User Manual Operation Preparations
1) Environment:
♦ Make sure that there is no electromagnetic interference source around the equipment,
especially large medical electrical equipment such as electrosurgical equipment,
radiological equipment, magnetic resonance imaging equipment etc. Switch off these
devices when necessary.
♦ Keep the examination room warm to avoid muscle action voltages in ECG signals
caused by cold.
2) Power Supply:
♦ If the mains supply is used, please check whether the power cord is connected to the
unit well. The grounded three-phase outlet should be used.
♦ When the battery capacity is low, recharge the battery before use.
3) Patient Cable:
♦ Check whether the patient cable is connected to the unit firmly, and keep it far away
from the power cord.
4) Electrodes:
♦ Check whether all electrodes are connected to lead wires of the patient cable
correctly.
♦ Ensure that the chest electrodes do not contact.
5) Recorder Paper:
♦ Ensure that there is enough recorder paper loaded correctly.
6) Patient:
♦ The patient should not come into contact with conducting objects such as earth, metal
parts etc.
♦ Ensure the patient is warm and relaxed, and breathe calmly.
WARNING
The electrocardiograph is provided for the use of qualified physicians or personnel
professionally trained, and they should be familiar with the contents of this user manual
before operation.
- 19 -
Page 26

SE-301 Series Electrocardiograph User Manual Sampling and Printing ECG
Chapter 4 Sampling and Printing ECG
4.1 Entering Patient Information
4.1.1 Entering Patient Information Manually
On the main screen, the following information is displayed: patient information, system hints,
heart rate, waveforms, current time, battery capacity, WIFI signal (optional), and functional keys.
Click the patient symbol to enter the patient information window, enter the patient information, or
you can configure the patient information items in system setup first.
NOTE: The patient information cannot be set or changed during the printing course.
Item Description
Pacemaker
Select Yes to detect very small pacemaker pulses. However, when
Pacemaker is set to Ye s , the system is very sensitive, and should not be
close to equipment emitting high frequency radiation. High frequency
radiation can interfere with pacemaker pulse detection and normal ECG
acquisition.
NOTE: Pacemaker is recommended to be set to No unless it is
known that the majority of the electrocardiograph usage
will be on patients with pacemakers.
- 20 -
Page 27

SE-301 Series Electrocardiograph User Manual Sampling and Printing ECG
4.1.2 Entering Patient Information by Acquiring Orders
NOTE: To use the order function, the data management software (DMS) of the
manufacturer must be installed in the PC.
Operation procedures are as follows:
1. Connect the electrocardiograph to the PC through the network.
2. Log into the DMS.
3. Set Remote IP, Local IP, Gateway and Subnet Mask in the Transmission Setup window.
4. Click the patient symbol on the main screen to open the patient information window, and then
click Order to open the Order screen.
5. Click Load to download order records from the server.
6. Select an order and click Examine to enter the presampling screen.
4.2 Printing ECG Reports
The operation procedure is as follows:
Select a work mode
NOTE:
Configure printing and system
parameters if necessary
Press PRINT/STOP to start
sampling
MANU
mode
Press PRINT/STOP to stop
sampling
Manually switch lead group
1. The printing mode cannot be changed during the printing course. Stop printing
reports before changing the printing mode.
2. In the MANU mode, press the 1mV/COPY key to print out 1mV mark in the ECG
report.S
- 21 -
Page 28

SE-301 Series Electrocardiograph User Manual Sampling and Printing ECG
4.3 Sample ECG Reports
4.3.1 ECG Reports in the AUTO Mode
Figure 4-1 ECG Reports in the AUTO Mode (a)
Figure 4-2 ECG Reports in the AUTO Mode (b)
Figure 4-1 and Figure 4-2 show an ECG report in the AUTO mode. Template is selected, and
Record Style is set to 3×4.
The ECG report includes:
3×4 ECG waves
ID, Current Date and time
Patient Information:
Measure Information:
HR Heart Rate
P P wave duration: the average P-wave duration from several selected
ID, Name, Age, Gender, Height, Weight, BP, Race, Department,
Exam Room, Medication
dominant beats;
- 22 -
Page 29

SE-301 Series Electrocardiograph User Manual Sampling and Printing ECG
PR P-R interval: the average P-R interval from several selected
dominant beats;
QRS QRS complex duration: the average QRS complex duration from
several selected dominant beats;
QT/QTc Q-T interval: the average Q-T interval from several selected
dominant beats / Normalized QT interval;
P/QRS/T Dominant direction of the average integrated ECG vectors;
RV5/SV1 The maximum of the amplitude of R or R’ wave of one selected
dominant beat from lead V5 / The maximum absolute value of the
amplitude of S or S’ wave of one selected dominant beat from lead
V1;
RV5+S V1
(optional)
RV6/S V2
(optional)
QTcFd
(Optional)
QTcFm
(Optional)
Average Template:
Sum of RV5 and SV1;
The maximum of the amplitude of R or R’ wave of one selected
dominant beat from lead V6 / The maximum absolute value of the
amplitude of S or S’ wave of one selected dominant beat from lead
V2;
Use the Fridericia formula to calculate the QTc interval
Use the Framingham formula to calculate the QTc interval
Average template shows the average value of 10s sampled ECG
signals of every lead.
The broken lines on the template are position markers. They
Diagnosis Information:
Report Confirmed by
Bottom Information:
respectively mark the start and end points of the P and QRS waves,
and the end point of the T wave.
Diagnosis information shows the auto diagnosis result.
Confirmed by the physician
0.67~100Hz (0.67Hz DFT Filter, 100Hz Lowpass Filter),
AC50 (50Hz AC Filter)
- 23 -
Page 30

SE-301 Series Electrocardiograph User Manual Sampling and Printing ECG
25mm/s (Paper Speed)
10mm/mV (Gain)
60 (Heart Rate)
Electrocardiograph Model
V1.0 (Software Version)
SEMIP V1.8 (Algorithm Version)
Institution Name
4.3.2 PDF Report
Figure 4-3 PDF Report
- 24 -
Page 31

SE-301 Series Electrocardiograph User Manual Managing ECG Records
Chapter 5 Managing ECG Records
If you want to save the ECG records, you should set the Auto Save to To E CG or Ext. Memory.
The default value is To EC G . Then the ECG records will be saved in the File Manager or in the
external memory automatically.
Click File on the main screen to enter the file manager screen.
The File Manager allows records to be stored, deleted, printed and transmitted. When there is no
space for more records to be stored in the File Manager, the message MemFull will be displayed.
5.1 Transmitting ECG Records to the PC
NOTE: To transmit ECG records to the PC, data management software (DMS) of EDAN
must be installed in the PC. You should log into the Smart ECG Viewer software
before the transmission.
5.1.1 Transmitting ECG Records through the Network
Connect the PC to the electrocardiograph with an Ethernet cable recommended by the
manufacturer.
Auto Transmission:
1. Choose Setup > Transmission to enter the Transmission Setup window.
2. Set Auto Transmission to On and set Transmission mode to Ethernet.
3. Set the Server IP to the IP of the DMS.
4. Set the first three numbers of the Local IP to the first three numbers of the IP of Smart
ECG Viewer. The last number of the Local IP item can be set at random, but it can’t be the
- 25 -
Page 32

SE-301 Series Electrocardiograph User Manual Managing ECG Records
same as the last number of the IP of the DMS.
5. In the AUTO or RHYT mode, ECG data can be transmitted through the net automatically
after an ECG report is printed out.
Manual Transmission:
1. Choose Setup > Transmission to enter the Transmission Setup window.
2. Set Auto Transmission to Off and set Transmission mode to Ethernet.
3. For IP address setting, refer to step 3 and 4 for auto transmission.
4. To transmit all the data files to the PC, choose More > Trans All in the file management
window.
To transmit a single file, select it and click Trans.
NOTE: The transmission process is long, and please be patient to wait.
5.1.2 Transmitting ECG Records through WIFI Network (Optional)
If the WIFI module is configured, ECG records can also be transmitted through WIFI network.
WARNING
This device complies with Part 15 of the FCC Rules. Operation is subject to the following
two conditions:
1) this device may not cause harmful interference, and
2) this device must accept any interference received, including interference that may
cause undesired operation.
NOTE:
1. This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential installation.
This equipment generates, uses and can radiate radio frequency energy and, if not
installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the equipment and receiver.
- 26 -
Page 33

SE-301 Series Electrocardiograph User Manual Managing ECG Records
- Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
- Consult the dealer or an experienced radio/TV technician for help.
2. Any changes or modifications to this unit not expressly approved by the party
responsible for compliance could void the user's authority to operate the equipment.
5.2 Copying ECG Records between the ECG Machine
and External Memory
1. Connect the external memory to the electrocardiograph.
2. Click File to open the File Manager screen.
3. Choose More > Trans All, and click OK, all the records will be transmitted to the external
memory automatically.
During the transmission, if something wrong happens, the electrocardiograph will give the
error information. Then you should check whether the external memory is connected to the
electrocardiograph well.
4. If you want to import records from the ECGDATA folder of the external memory to the
electrocardiograph, click the Import button, the extended-name of imported records should
be “.dat”.
NOTE: To import records from the external memory to the electrocardiograph, there
should be some records in the folder named ECGDATA in the external memory.
The folder name ECGDATA must be capital letters. You should not change the
name of records in the ECGDATA folder.
5. If you want to export only one record, choose the patient record in the table and click Export.
NOTE:
1. The transmission process is long, and please be patient to wait.
2. During the transmission, the external memory should not be pulled out.
3. Only FAT or FAT32 format can be used when formatting the external memory.
- 27 -
Page 34

SE-301 Series Electrocardiograph User Manual Managing ECG Records
5.3 Deleting Patient Records
1. Open the File Manager screen.
2. If you want to delete all the records, click More and select the Del All button, and then click
OK.
3. If you want to delete a record, choose the patient record in the table, and then click the delete
symbol on the top.
5.4 Printing a Patient Record in the File Manager
screen
1. Open the File Manager screen.
2. If you want to print the patient record, choose the patient record in the list, and then press
PRINT/STOP.
NOTE: If you use USB printer to print the patient record, when the PRINT/STOP key is
pressed, the electrocardiograph begins to analyze data. Then the USB printer
begins to print the ECG record after 8 seconds.
- 28 -
Page 35

SE-301 Series Electrocardiograph User Manual Settings
Chapter 6 Settings
Click Setup on the main screen to display the System Setup screen.
NOTE: The underlined values are system default values.
6.1 Work Mode
Item Description
Sampling Mode
(Only available in
the AUTO mode)
Auto Arrhythmia
Detection
Choose from: Pre-Sample and Real-time
Select Pre-Sample, 10s ECG data sampled before pressing the
PRINT/STOP key will be printed out.
NOTE: When Sampling Mode is set to Pre-Sample, if you press
the PRINT/STOP key before the electrocardiograph
samples for 10s, the recorder will not respond.
When enabled, if arrhythmia is detected in the AUTO mode, a hint will
pop up to ask you whether to print an extra rhythm report after the 12-lead
ECG report.
6.2 Filter
Item Description
AC Filter
Choose from: On
or Off
DFT Filter
EMG Filter
AC filter is used to suppress interference of AC power supply.
NOTE: AC frequency can be set to 50Hz or 60Hz on the Advanced
Setup screen according to local mains supply specifications.
Choose from: 0.01Hz, 0.05Hz, 0.32Hz, or 0.67Hz
DFT Filter greatly reduces the baseline fluctuations without affecting the
ECG signals. The purpose of this filter is to keep the ECG signals on the
baseline of the printout.
The set value is the low limit of the frequency range.
The cutoff frequency can be set to 25Hz, 35Hz, 45Hz or Off
EMG Filter suppresses disturbance caused by strong muscle tremor.
- 29 -
Page 36

SE-301 Series Electrocardiograph User Manual Settings
Lowpass Filter
NOTE: To pass the distortion test, the electrocardiograph has to be configured with the
highest bandwidth in filter settings. Otherwise, ECG signal may be distorted.
The cutoff frequency can be set to 75Hz, 100Hz, 150Hz, 270Hz or 300Hz
Lowpass Filter restricts the bandwidth of input signals.
All the input signals whose frequency is higher than the set cutoff frequency
will be attenuated.
NOTE: Only when EMG Filter is set to Off, can the setting of
Lowpass Filter be effective.
6.3 Record Info Setup
6.3.1 Setup 1
Item Description
Print Out
Speed
Gain
Auto Record Style Choose from: 3×4, 3×4+1R, 1×12, 1×12+1R and 3×2+2×3
Auto Record
Sequence
Choose from: On, Off
Select Off to disable the print function in the AUTO or RHYT mode.
Choose from: 5mm/s, 6.25mm/s, 10mm/s, 12.5mm/s, 25mm/s and
50mm/s
NOTE: The speed is corresponding with the work mode.
Choose from: 1.25mm/mV, 2.5mm/mV, 5mm/mV, 10mm/mV,
20mm/mV, 10/5mm/mV and 20/10mm/mV
10/5mm/mV means that the gain of limb leads is set to 10mm/mV, while
the gain of chest leads is set to 5mm/mV.
Choose from: Sequential or Synchronous
Select Sequential, the lead group is printed one by one in a certain
sequence. The start time of a lead group is just the end time of the
previous lead group.
Select Synchronous, the lead group is printed one by one in a certain
sequence. All leads are printed with the same start time.
AGC
AGC means auto gain control.
Choose from: On or Off
Select On, the gain can be automatically adjusted according to actual
signals.
- 30 -
Page 37

SE-301 Series Electrocardiograph User Manual Settings
Auto Record
Length
Manual Style
Rhythm Record
Mode
Paper Marker
Choose from: Short (2.5s), Medium (5s) and Long (10s)
NOTE: Auto Record Length is corresponding with Record
Choose from 3 channels and 1 channels
Select a style to print the ECG waves in the manual mode.
Choose from: Save Paper or Quickly
Select Save Paper, 10s after pressing the PRINT/STOP key on the main
screen, an ECG report is printed in the RHYT mode.
Select Quickly, pressing the PRINT/STOP key on the main screen to
begin printing an ECG report immediately in the RHYT mode.
Paper Marker is used to identify the start point of each page of the
recorder paper.
Choose from: Ye s or No
Select Ye s if the paper with black markers on the bottom is used, and the
device can identify the start point of each page of the recorder paper while
Device, Sampling Mode and Record Sequence.
printing ECG reports.
6.3.2 Setup 2
Item Description
Measure
Analysis
Template
Choose from: On or Off
When it is set to On, the Measure information will be printed in the ECG
report.
Choose from: On or Off
When it is set to On, the Analysis information will be printed in the ECG
report.
Choose from: 2×6+1R, 3×4 or Off
When it is set to Off, the template will not be printed in the ECG report
Position Marker
Minnesota Code
Choose from: On or Off
When it is set to Off, the template printed in the ECG report will not have
position marker.
Choose from: On or Off
- 31 -
Page 38

SE-301 Series Electrocardiograph User Manual Settings
When it is set to On, the Minnesota Code will be printed in the ECG
report.
Device No.
Baseline
Adjustment
Record Device
Choose from: On or Off
When it is set to On, the Device No. will be printed in the ECG report.
Choose from: Horizontal, Auto or Off
Select Horizontal, the baselines of the lead groups are adjusted
simultaneously, and the baselines of the leads in the same row are on the
same line.
Select Auto, the baselines of the lead groups are adjusted respectively.
Select Off, the baselines of the lead groups are adjusted equally in the
ECG reports.
Choose from: Thermal, HP 1010/1510/2010/1050/2000, HP
M401/2015/2035/1525, HP1020/1020PLUS/1106, or HP 1505
You should connect the corresponding USB printer to the
electrocardiograph before printing with the selected record device.
WARNING
If the printer used is not the type listed above, additional safety
measures (such as applying an isolation transformer to supply the
medical system) should be taken when the safety of the medical
system has not been evaluated. If in doubt, consult our technical
service department or your local distributor.
CAUTION
It is forbidden to connect or disconnect an external memory or a
USB printer during the transmission course.
NOTE:
1. During the USB printing course, pressing the PRINT/STOP key
again cannot stop printing ECG reports.
2. For details of the ECG report printed by the USB printer, please
refer to section 4.3.2 "PDF Report".
3. USB printing is ineffective in the AUTO mode and RHYT mode.
4. Make sure that paper is installed in the USB printer before
printing. Error may occur if no paper is loaded in the USB Printer .
- 32 -
Page 39

SE-301 Series Electrocardiograph User Manual Settings
5. Make sure the type of USB printer connected is matched with
the type you choose in the Record Device. Error may occur if the
USB printer type is not matched.
USB Record Style Choose from: 3×4, 3×4+1R, 3×4+3R, 6×2, 6×2+1R or 12×1
It defines the style of USB report.
Grid of Report
Choose from: On or Off
When it is set to On, the grid will be printed while printing ECG reports
with the thermal recorder or USB printer.
6.4 Patient Information Setup
Item Description
First/Last Name
ID
ID Hint
Choose from: On or Off
When it is set to On, patient name will be divided into first name and last
name.
Choose from: Auto, Time or Manual
Choose from: On or Off
In the AUTO or RHYT mode, when ID is set to Manual and ID Hint is
set to On, if you do not input the patient ID before pressing the
Age
PatInfo Refreshed
H/W Unit
BP Unit
Order Acquired
PRINT/STOP key, a hint will pop up to remind you to input the patient
ID.
Choose from: Age, D.O.B or Age Group
Choose from: On
Select On, the patient information will be refreshed after the ECG report
is printed out and all the leads are off.
Choose from: cm/kg or inch/lb
Choose from: mmHg or kPa
Choose from: On or Off
Select On, the Order item will be displayed in the Patient Information
window and you can acquire orders by clicking it.
or Off
- 33 -
Page 40

SE-301 Series Electrocardiograph User Manual Settings
6.5 Transmission Setup
NOTE:
1. To transmit ECG data to the PC, the Smart ECG Viewer software produced of EDAN
must be installed in the PC. You should log into the Smart ECG Viewer software
before transmission.
2. If the power supply suddenly breakdown during data storage or transmission, file
system error may occur. In this case, the file system should be formatted.
Item Description
Device No. Enter Device No., it should be within 7 ASCII characters.
Auto Transmission
Choose from: On or Off
Select On, ECG data will be transmitted automatically after an ECG
report is printed out in the AUTO or RHYT mode.
Transmission Mode
FTP Information
IP Addresses
Choose from: Ethernet or Wireless
Enter data in the FTP Path, FTP User Name textboxes.
Set Server IP, Local IP, Set Gateway, Set Subnet Mask
For details, please refer to Section 5.1: "Transmitting ECG Records to
the PC".
6.6 Lead Setup
Item Description
Rhythm
Lead1/2/3
Choose from: І, П, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, or V6, the default
NOTE: Rhythm Lead 1/2/3 must be different from each other.
Lead
Sequence
Lead off hint
Choose from: Standard or Cabrera
Lead
Sequence
Lead group 1 Lead group 2 Lead group 3 Lead group
4
aVR, aVL,
Standard
І, II, III
V1, V2, V3 V4, V5, V6
aVF
Cabrera
aVL, І, -aVR II, aVF, III V1, V2, V3 V4, V5, V6
When it is set to On and lead off waves are detected in the presampled waves, a
lead off hint message will be displayed.
- 34 -
Page 41

SE-301 Series Electrocardiograph User Manual Settings
6.7 File Setup
Item Description
Auto Save
Choose from: Off, To ECG or Ext. Memory
Select Off, ECG data will not be saved.
Select To ECG, ECG data in the AUTO or RHYT mode will be saved in
the ECG automatically.
Select Ext. Memory, ECG data in the AUTO or RHYT mode will be
automatically saved to the directory of
ECGDATA\ECG-X\Store\Examination Date of the external memory
after an ECG report is printed out.
NOTE:
1. Please insert the external memory recommended by the
manufacturer. Please set the format to FA T or FAT32 when
formatting the external memory.
2. X in the directory of ECGDATA\ECG-X\Store\Examination
Date can be set in the Device No. textbox in the Transmission
File Format
Del. After Trans.
Or Export
Replace When
Memory Full
SCP File
Setup window.
Choose from: DAT, PDF, SCP, FDA-XML and DICOM
To select SCP/FDA-XML/DICOM, you should first activate the
SCP/FDA-XML/DICOM function on the Advanced Setup screen. For
details, please contact the manufacturer or the local distributor.
Choose from: On or Off
Select On, the files will be automatically deleted from the File Manager
screen after they are transmitted to the PC or exported to the external
memory.
Choose from: On or Off
Select On, if the stored files reaches 200, the files will replace the earliest
one automatically.
Choose from: On or Off
Compression
Select On, the SCP file will be compressed.
- 35 -
Page 42

SE-301 Series Electrocardiograph User Manual Settings
6.8 Date&Time Setup
NOTE: Please set DATE&TIME correctly when it’s the first time you use the
electrocardiograph.
Item Description
Date Mode
Time Mode
Date&Time Enter the current date and time displayed on the main screen and in the
Power off time Set to 0-120
LCD off time Set to 0-120
Choose from: DD-MM-YYYY, MM-DD-YYYY or YYYY-MM-DD
Choose from: 24 Hours or 12 Hours
ECG reports.
This function is only available when the electrocardiograph is powered by
using the mains supply.
6.9 System Maintenance
Import/export the system settings, backup the settings, or load the backup settings
Load factory settings
Set the password to access system settings
6.10 Other Setup
Item Description
Institution Input the institution name manually within 40 ASCII characters.
NOTE: The total number of supported characters may be fewer if
special Latin characters are entered.
Demo Setup
Choose from: Normal, abnormal or Off
When it is set to Normal, the main screen will display demo of normal
ECG signal.
- 36 -
Page 43

SE-301 Series Electrocardiograph User Manual Settings
Grid
Language Select the language displayed on the main screen and in the ECG reports.
Display Colors Set the interface display color
Key Volume
Hint Volume When enabled, the electrocardiograph gives a sound when a hint such as
QRS Volume When enabled, the electrocardiograph gives a sound when an R wave is
Notify Volume When enabled, the electrocardiograph gives a sound after ECG report is
When enabled, the waveforms on the main screen will be displayed with a
background grid.
When enabled, the electrocardiograph gives a short sound when you press
keys.
Lead Off, Overload, Battery Weak etc. is displayed.
detected.
printed.
6.11 Advanced Setup
View the device information, perform system test, etc.
Activate purchased advanced functions.
- 37 -
Page 44

SE-301 Series Electrocardiograph User Manual Hint Information
Chapter 7 Hint Information
Hint information provided by SE-301 and the corresponding causes are listed in Table 10-1.
Table 7–1 Hint Information and Causes
Hint Information Causes
Lead off Electrodes fall off the patient or the patient cable falls off the unit.
Paper? Recorder paper runs out or is not loaded.
PaperErr The system doesn't detect any black signs while the paper style is
set as "Folded" on the System Setup Screen.
BAT WEAK The built-in battery is weak.
Demo The system is in the demonstration mode.
Sampling/Analyzing/
Recording
Transmitting
Transmitting fails! Transmitting ECG data fails.
MemoryFull There is no space for saving more records.
USB Printer / USB
Scanner
ECG signals are being sampled / analyzed / recorded.
ECG data is being transmitted from the electrocardiograph to the
PC through the net or serial cable in the AUTO or RHYT mode.
An external USB printer or a bar code reader is connected to the
USB interface.
- 38 -
Page 45

SE-301 Series Electrocardiograph User Manual Troubleshooting
Chapter 8 Troubleshooting
1) Operating Problems
Q1: I want to save the ECG data without any printing, could it be possible?
A1:
Yes, in the Record Info setup, set Print Out to Off. In the same way, if the
transmission settings have been configured, the ECG data could be transmitted to the
PC without printing.
2) Printing Problems
Q1: There was double impression in printing when I printed ECG reports by using an
ink-jet printer. What’s wrong with it?
A1: It may be the result of the coexisting black and color ink cartridges. Taking out the
color ink cartridge may solve the problem.
Q2: I was encountered with paper-jam, what was I supposed to do?
A2: If it happened for the first time, it might be the result of an inappropriate placement of
the paper. In this case, please open the paper casing, pull the paper out of the paper
tray, tear the pages with rumples, and then put the paper in the paper tray again, adjust
the position of the paper carefully and close the casing.
Q3: The hint PaperErr is displayed on the screen, what should I do?
A3: Check if the paper maker setting is right or might be the result of unsuccessful
detection of the black markers, first open the paper casing so as to clear the error
information, and then check whether the black marker is on the top of the paper.
Reload the paper in the paper tray. If it doesn’t work, change the paper.
If the problem still exists, please contact the manufacturer or the local distributor for
further disposal.
Q4: The hint Paper? is displayed on the screen, what should I do?
A4: Check whether the paper runs out, or the black marker is just facing the black maker
detection window on the thermal printing head.
Reload the paper in the paper tray, close the paper casing firmly. If the problem still
exists, please contact the manufacturer or the local distributor for further disposal.
Q5:
I pressed the PRINT/STOP key, but the ECG didn’t start printing, what’s wrong with
it?
- 39 -
Page 46

SE-301 Series Electrocardiograph User Manual Troubleshooting
A5: Please check whether there is any error information displayed on the screen.
If the hint Paper? or PaperErr is shown on the screen, please deal with it according to
the above-mentioned measures.
If the hint Transfer is shown on the screen, which means that the ECG is transmitting
the data to the PC, please wait a few seconds. You can start the printing after the data
has been transmitted.
If the problem still exists, please contact the manufacturer or the local distributor for
further disposal.
3) Transmitting Problems
Q1: The ECG doesn’t respond to any keys after a long time of transmitting. It transmits
nothing for there is no new data appearing on the interface of the PC software. What
should I do?
A1: Some error may occur during the transmission course, for example, the connection
between the ECG and the net cable may loosen. In this case, please restart the ECG. If
it doesn’t work, please restart the PC.
If the problem still exists, please contact the manufacturer or the local distributor for
further disposal.
4) Main Unit Problems
Q1:
I was doing the examination when the machine suddenly gave out a sound and
displayed the hint Lead Off. What should I do?
A1:
The leads are not connected well. Please check whether the electrodes are connected
to the patient skin well, and then make sure that the patient cable socket is connected
to the patient cable firmly.
If none of the above-mentioned measures take effect, please contact the manufacturer
or the local distributor for further disposal.
Q2: The touch screen is not sensitive after restoring to factory defaults. What should I do?
A2:
Hold down the PRINT/STOP key while switching on the electrocardiograph, the
system will enter the touch screen calibration screen. Operate as indicated on the
screen.
- 40 -
Page 47

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
Chapter 9 Cleaning, Care and Maintenance
Use only the EDAN-approved substances and methods listed in this chapter to clean or disinfect
your equipment. Warranty does not cover damage caused by using unapproved substances or
methods.
Edan Instruments has validated the cleaning and disinfection instructions provided in this User
Manual. It is the responsibility of the healthcare professional to ensure that the instructions are
followed so as to ensure adequate cleaning and disinfection.
9.1 General Points
Keep your electrocardiograph and accessories free of dust and dirt. To prevent the device from
damage, please follow the instructions:
Use only the recommended cleaning agents and disinfectants listed in this manual. Others
may cause damage (not covered by warranty), reduce product lifetime or cause safety
hazards.
Always dilute according to the manufacturer's instructions.
Unless otherwise specified, do not immerse any part of the equipment or any accessories in
liquid.
Do not pour liquid onto the equipment.
Do not allow liquid to enter the case.
Never use abrasive material (such as steel wool or silver polish).
Inspect the electrocardiograph and reusable accessories after they are cleaned and
disinfected.
CAUTION
If you spill liquid on the equipment or accessories, or they are accidentally immersed in
liquid, contact your service personnel or EDAN service engineer.
9.2 Cleaning
If the equipment or accessory has been in contact with the patient, then cleaning and disinfection
is required after each use.
The validated cleaning agents for cleaning the electrocardiograph and patient cable are:
- 41 -
Page 48

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
Mild near neutral detergent
Ethanol (75%)
Isopropanol (70%)
The validated cleaning agent for cleaning the reusable electrodes is:
Mild near neutral detergent
Cleaning agents should be applied or removed using a clean, soft, non-abrasive cloth or paper
towel.
9.2.1 Cleaning the Main Unit
WARNING
Turn off the power before cleaning. The mains supply must be switched off if it is used.
1. Switch off the main unit and disconnect it from the power cord.
2. Wipe the exterior surface of the equipment using a soft cloth dampened with the cleaning
solution until no visible contaminants remain.
3. Wipe off the cleaning solution with a fresh cloth or towel dampened with tap water after
cleaning until no visible cleaning agent remains.
4. Dry the main unit in a ventilated and cool place.
9.2.2 Cleaning the Patient Cable
1. Wipe the patient cable with a soft cloth dampened with the cleaning solution until no visible
contaminants remain.
2. Wipe off the cleaning solution with a fresh cloth or towel dampened with tap water after
cleaning until no visible cleaning agent remains.
3. Wipe off with a dry cloth to remove residual moisture.
4. Leave the patient cable to air dry.
CAUTION
Any remainder of cleaning solution should be removed from the main unit and the patient
cable after cleaning.
- 42 -
Page 49

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
9.2.3 Cleaning the Reusable Electrodes
1. Wipe off with a soft cloth to remove residual gel.
2. Wipe the suction bulbs of chest electrodes and the clamps of limb electrodes with a soft cloth
dampened with the cleaning solution until no visible contaminants remain.
3. Wipe off the cleaning solution with a fresh cloth or towel dampened with tap water after
cleaning until no visible cleaning agent remains.
4. Wipe off with a dry cloth to remove residual moisture.
5. Leave the suction bulbs and clamps to air dry.
9.3 Disinfection
To avoid permanent damage to the equipment, it is recommended that disinfection is performed
only when it is considered as necessary according to your hospital's regulations.
Clean the equipment and reusable accessories before they are disinfected. The validated
disinfectants for disinfecting the electrocardiograph and patient cable are:
Ethanol (75%)
Isopropanol (70%)
The validated disinfectant for disinfecting the reusable electrodes is:
Isopropanol (70%)
If Ethanol or Isopropanol is used for both cleaning and disinfecting, then a new cloth is required
to be used for the disinfection step.
CAUTION
1. Do not use high-temperature, high-pressure vapour or ionizing radiation as
disinfection methods.
2. Do not use chloric disinfectant such as chloride, sodium hypochlorite etc.
3. Clean and disinfect reusable electrodes after each use.
9.3.1 Disinfecting the Main Unit
WARNING
Turn off the power before disinfection. The mains supply must be switched off if it is used.
- 43 -
Page 50

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
1. Switch off the main unit and disconnect it from the power cord.
2. Wipe the exterior surface of the equipment using a soft cloth dampened with the disinfectant
solution.
3. Wipe off the disinfectant solution with a dry cloth after disinfection if necessary.
4. Dry the main unit for at least 30 minutes in a ventilated and cool place.
9.3.2 Disinfecting the Patient Cable
1. Wipe the patient cable with a soft cloth dampened with the disinfectant solution.
2. Wipe off the disinfectant solution with a dry cloth after disinfection.
3. Leave the patient cable to air dry for at least 30 minutes.
9.3.3 Disinfecting the Reusable Electrodes
1. Wipe the suction bulbs of chest electrodes and the clamps of limb electrodes with a soft cloth
dampened with the disinfectant solution.
2. Wipe off the disinfectant solution with a dry cloth after disinfection.
3. Leave the suction bulbs and clamps to air dry for at least 30 minutes.
9.4 Care and Maintenance
9.4.1 Recharge and Replacement of Battery
1) Capacity Identification
The battery capacity can be identified according to the battery symbol in the top right corner
of the LCD screen.
-> -> ->
Capacity is from full to empty.
2) Recharge
SE-301 is equipped with the recharge control circuit together with the built-in rechargeable
lithium battery. When the unit is connected to the mains supply, the battery will be recharged
automatically. During the recharging course, the battery symbol flashes in the top right
corner of the LCD screen. When the battery capacity is full, the symbol stops flashing.
Because of the capacity consumption during the storage and transport course, the battery
- 44 -
Page 51

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
capacity is not full when it is used for the first time. Battery recharge should be considered
before the first use.
NOTE: If the battery has not been used for more than two months, it should be
recharged before use.
3) Replacement
When the useful life of the battery is over, or foul smell and leakage are found, please
contact the manufacturer or the local distributor for replacement.
WARNING
1. Only qualified service engineer authorized by the manufacturer can open the battery
compartment and replace the battery, and the battery of the same model and
specification provided by the manufacturer must be used.
2. Danger of explosion -- Do not reverse the anode and the cathode when installing the
battery.
3. Remove the battery from the electrocardiograph when the electrocardiograph is not
used for a long time.
4. If the battery is stored alone and not used for a long time, we recommend that the
battery should be charged at least once every 6 months to prevent overdischarge.
5. When the battery’s useful life is over, cont act the manufacturer or the local distributor
for disposal or dispose of the battery according to local regulations.
9.4.2 Recorder Paper
NOTE: Recorder paper provided by the manufacturer should be used. Other paper
may shorten the life of the thermal print head. And the deteriorated print head
may lead to illegible ECG reports and block the advance of paper.
Storage Requirements:
♦ Recorder paper should be stored in a dry, dark and cool area, avoiding excessive
temperature, humidity and sunshine.
♦ Do not put the recorder paper under fluorescence for a long time.
♦ Make sure that there is no polyvinyl chloride or other chemicals in the storage
environment, which will lead to color change of the paper.
- 45 -
Page 52

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
♦ Do not overlap the recorded paper for a long time, or else the ECG reports may
trans-print each other.
9.4.3 Maintenance of the Main Unit, the Patient Cable and
Electrodes
The following safety checks should be performed at least every 24 months by a qualified person
who has adequate training, knowledge, and practical experience to perform these tests.
a) Inspect the equipment and accessories for mechanical and functional damage.
b) Inspect the safety related labels for legibility.
c) Inspect the fuse to verify compliance with rated current and breaking characteristics.
d) Verify the device functions properly as described in the instructions for use.
e) Test the protection earth resistance according to IEC/EN 60601-1: Limit: 0.1ohm.
f) Test the earth leakage current according to IEC/EN 60601-1: Limit: NC 500 μA, SFC
1000μA.
g) Test the enclosure leakage current according to IEC/EN 60601-1: Limit: NC 100μA, SFC
500μA.
h) Test the patient leakage current according to IEC/EN 60601-1: Limit: NC a.c. 10μA, d.c.
10μA; SFC a.c. 50μA, d.c. 50μA.
i) Test the patient auxiliary current according to IEC/EN 60601-1: Limit: NC a.c. 10μA, d.c.
10μA; SFC a.c. 50μA, d.c. 50μA.
j) Test the patient leakage current under single fault condition with mains voltage on the
applied part according to IEC/EN 60601-1: Limit: 50μA (CF).
k) Test the essential performance according to IEC/EN 60601-2-25, or methods recommended
by the hospital or local distributor.
The leakage current should never exceed the limit. The data should be recorded in an equipment
log. If the device is not functioning properly or fails any of the above tests, the device has to be
repaired.
WARNING
Failure on the part of the responsible individual hospital or institution employing this
equipment to implement a satisfactory maintenance schedule may cause undue
equipment failures and possible health hazards.
- 46 -
Page 53

SE-301 Series Electrocardiograph User Manual Cleaning, Care and Maintenance
1) Main Unit
♦ Avoid excessive temperature, sunshine, humidity or dirt.
♦ Put the dustproof coat on the main unit after use and prevent shaking it violently when
moving it to another place.
♦ Prevent any liquid from seeping into the equipment, otherwise the safety and
performance of the electrocardiograph can not be guaranteed.
2) Patient Cable
♦ Integrity of the patient cable, including the main cable and lead wires, should be
checked regularly. Make sure that it is conductible.
♦ Do not drag or twist the patient cable with excessive stress while using it. Hold the
connector plug instead of the cable when connecting or disconnecting the patient cable.
♦ Align the patient cable to avoid twisting, knotting or crooking in a closed angle while
using it.
♦ Store the lead wires in a big wheel to prevent any people from stumbling.
♦ Once damage or aging of the patient cable is found, replace it with a new one
immediately.
3) Electrodes
♦ Electrodes must be cleansed after use and make sure there is no remainder gel on them.
♦ Keep suction bulbs of chest electrodes away from sunshine and excessive temperature.
♦ After long-term use, the surfaces of electrodes will be oxidized because of erosion and
other causes. By this time, electrodes should be replaced to achieve high-quality ECG
records.
CAUTION
The device and accessories are to be disposed of according to local regulations after
their useful lives. Alternatively, they can be returned to the dealer or the manufacturer for
recycling or proper disposal.
- 47 -
Page 54

SE-301 Series Electrocardiograph User Manual Accessories
Chapter 10 Accessories
WARNING
Only the patient cable and other accessories supplied by the manufacturer can be used.
Or else, the performance and electric shock protection cannot be guaranteed.
Table 10-1 Accessories List
Accessory Part Number
Power cord (IEC) 01.13.036638
Power cord(AHA) 21.13.036384
01.57.107402
Patient Cable (IEC)
01.57.471500
01.57.110375
Patient Cable (AHA)
01.57.471499
Adult Chest electrodes 01.57.040163
Adult Limb electrodes 01.57.040162
Thermal Recorder Paper 01.57.78076
Paper roller 01.51.19993
Rechargeable Li-ion Battery 21.21.064149
01.57.106902
Patient Cable (IEC)
Patient Cable (AHA)
01.57.107581
01.57.107583
01.57.107048
01.57.107582
01.57.107584
Pediatric Chest Electrodes 01.57.040168
Pediatric Limb Electrodes 01.57.040169
Snap/Banana Socket Adapters 01.13.107449
- 48 -
Page 55

SE-301 Series Electrocardiograph User Manual Accessories
Clip/Snap/Banana Socket Adapter 01.57.040172
Alligator Clip/Banana Socket Adapters 01.57.040173
Adult Disposable Adhesive Electrodes 01.57.471056
Pediatric Disposable Adhesive Electrodes 01.57.471057
Disposable Resting electrodes 01.57.471031
Net Cable 01.13.20096
Thermal Recorder Paper 01.57.78079
Grounding Wire 01.13.114114
U Disk 01.18.052275
SE-301 and accessories are available by contacting the manufacturer or your local distributor.
NOTE:
1. The adult chest electrodes, adult limb electrodes, pediatric chest electrodes and
pediatric limb electrodes are not available in the U.S.
2. The part name may differ in documents, but the part number shall prevail for all
purposes.
- 49 -
Page 56

SE-301 Series Electrocardiograph User Manual Warranty and Service
Chapter 11 Warranty and Service
11.1 Warranty
EDAN warrants that EDAN’s products meet the labeled specifications of the products and will be
free from defects in materials and workmanship that occur within warranty period.
The warranty is void in cases of:
a) damage caused by mishandling during shipping.
b) subsequent damage caused by improper use or maintenance.
c) damage caused by alteration or repair by anyone not authorized by EDAN.
d) damage caused by accidents.
e) replacement or removal of serial number label and manufacture label.
If a product covered by this warranty is determined to be defective because of defective materials,
components, or workmanship, and the warranty claim is made within the warranty period, EDAN
will, at its discretion, repair or replace the defective part(s) free of charge. EDAN will not provide
a substitute product for use when the defective product is being repaired.
11.2 Contact information
If you have any question about maintenance, technical specifications or malfunctions of devices,
contact your local distributor.
Alternatively, you can send an email to EDAN service department at: support@edan.com.cn.
- 50 -
Page 57

SE-301 Series Electrocardiograph User Manual Technical Specifications
Appendix 1 Technical Specifications
A1.1 Safety Specifications
IEC 60601-1:2005/A1:2012
EN 60601-1:2006/A1:2013
Comply with:
Anti-electric-shock type: Class І with internal power supply
Anti-electric-shock degree: Type CF
Degree of protection against
harmful ingress of water:
Disinfection/sterilization
method:
Degree of safety of application
in the presence of flammable
gas:
Working mode: Continuous operation
EMC: CISPR 11 Group 1, Class A
IEC 60601-1-2:2007
EN 60601-1-2:2007/AC:2010
IEC 60601-2-25:2011
Ordinary equipment (Sealed equipment without liquid
proof)
Refer to the user manual for details
Equipment not suitable for use in the presence of
flammable gas
Patient
Leakage
Current:
Patient
Auxiliary
Current:
NC <10μA (AC) / <10μA (DC)
SFC <50μA (AC) / <50μA (DC)
NC <10μA (AC) / <10μA (DC)
SFC <50μA (AC) / <50μA (DC)
- 51 -
Page 58

SE-301 Series Electrocardiograph User Manual Technical Specifications
A1.2 Environment Specifications
Transport & Storage Working
Temperature: -20ºC (-4ºF) ~ +55ºC (+131ºF) +5ºC (+41ºF) ~ +40ºC (+104ºF)
15%RH–95%RH
Relative Humidity:
Non-Condensing
Atmospheric Pressure: 70kPa–106kPa 70kPa–106kPa
15%RH–95%RH
Non-Condensing
A1.3 Physical Specifications
Dimensions
Weight < 1kg (Excluding recorder paper and battery)
Display
224 mm×143 mm×54 mm, ±2 mm
800×480 LCD Screen
A1.4 Power Supply Specifications
Operating voltage =100V--240V~
Mains Supply:
Built-in Lithium Battery Pack:
Operating frequency = 50Hz / 60Hz
Power adapter output voltage: 19V, 2A
Rated voltage = 14.8V
Rated capacity = 2500mAh
When the battery is fully charged, the 3-channel
electrocardiograph can work normally about 8.5 hours. It
can continuously record about 5 hours in Manual mode, and
record at least 500 reports at most in the AUTO mode.
Necessary Charge time: ≤ 3.5 hours
Cycle life ≥ 300 times
- 52 -
Page 59

SE-301 Series Electrocardiograph User Manual Technical Specifications
A1.5 Performance Specifications
Recording
Recorder:
Printing Density
Recorder Paper:
Effective Width:
Paper Speed:
Accuracy of data:
HR Recognition
Technique:
HR Range:
Accuracy:
Thermal dot-matrix recorder
8 dots per mm / 200 dots per inch (amplitude axes)
40 dots per mm / 1000 dots per inch (time axes, @ 25 mm/s)
Folded thermal paper, 80mm×70mm×200pages
Rolled thermal paper, 80mm×20m
72mm
5mm/s, 6.25mm/s, 10mm/s, 12.5mm/s, 25mm/s, 50mm/s
(±3%)
±5% (x-axis), ±5%(y-axis)
Peak-Peak Detection
30 BPM ~ 300 BPM
±1BPM
ECG Unit
Leads:
Acquisition Mode:
A/D:
Resolution:
Time Constant:
Frequency Response:
Sensitivity:
Input Impedance: ≥50MΩ(10Hz)
Input Circuit Current: ≤0.01μA
Input Voltage Range ≤±5 mVpp
Standard 12 leads
simultaneously 12 leads
24bits
0.1575 μV/LSB
≥3.2s
0.01Hz ~ 300Hz (-3dB)
2.5mm/mV, 5mm/mV, 10mm/mV, 20mm/mV, 10/5mm/mV,
20/10mm/mV, AGC
Calibration Voltage: 1mV±3%
- 53 -
Page 60

SE-301 Series Electrocardiograph User Manual Technical Specifications
DC Offset Voltage: ±600mV
Minimum Amplitude: 20 μVp-p
Noise: ≤12.5μVp-p
Multi-channel Crosstalk ≤0.5mm
AC Filter: On / Off
DFT Filter: 0.01Hz, 0.05Hz, 0.32Hz, or 0.67Hz
Filter
EMG Filter: 25Hz / 35Hz / 45Hz / OFF
LOWPASS Filter:300Hz / 270Hz / 150Hz / 100Hz / 75Hz
≥140dB (AC: ON)
CMRR
≥110dB (AC: Off)
Sampling Frequency 16000Hz
Pacemaker Detection
Amplitude -2mV to -700mV & +2mV to +700mV
Width 0.1ms ~ 2.0ms
Sampling Frequency 16,000/sec/channel
WIFI (Optional)
Transmitting Frequency
Frequency Band
2.4GHz
2.400-2.500GHz (2.4 GHz ISM band)
OFDM with BPSK, QPSK, 16-QAM, and 64-QAM
Modulation Type
802.11b with CCK and DSSS
17 dBm for 802.11b DSSS
Transmitting Power
17 dBm for 802.11b CCK
15 dBm for 802.11g/n OFDM
NOTE:
1 Operation of the equipment below the minimum amplitude may cause inaccurate
results.
2 The DE12 ECG board is not available in the U.S.
- 54 -
Page 61

SE-301 Series Electrocardiograph User Manual EMC Information
Appendix 2 EMC Information
Electromagnetic emissions
Guidance and manufacture’s declaration – electromagnetic emission
The Electrocardiograph is intended for use in the electromagnetic environment specified
below. The user of the Electrocardiograph should assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
RF emission
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Guidance and manufacture’s declaration – electromagnetic immunity
The Electrocardiograph is intended for use in the electromagnetic environment specified
below. The user of Electrocardiograph should assure that it is used in such an environment.
Class A
Class A
Complies
Electromagnetic immunity
The Electrocardiograph uses RF energy only
for its internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
equipment.
The Electrocardiograph is suitable for use in
all establishments other than domestic and
those directly connected to the public
low-voltage power supply network that
supplies building used for domestic purposes.
Immunity test IEC 60601 test level Compliance level
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±6 kV contact
±8 kV air
±2 kV for power
supply lines
- 55 -
Electromagnetic
environment - guidance
It is recommended the use
of antistatic materials. If
floor are covered with
synthetic material, the
relative humidity should
be at least 50%.
It is recommended the use
of filters on power input
lines and enough
separation between signal
lines and power lines.
Page 62

SE-301 Series Electrocardiograph User Manual EMC Information
Surge
IEC 61000-4-5
Voltage dips,
short
interruptions and
voltage
variations on
power supply
input lines
IEC 61000-4-11
±1 kV line to line
±2 kV line to ground
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
±1 kV line to line
±2 kV line to ground
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
Mains power quality
should be that of a typical
commercial or hospital
environment.
Mains power quality
should be that of a typical
commercial or hospital
environment.
Power frequency
(50Hz/60Hz)
magnetic field
(>95% dip in UT)
for 5 sec
3A/m 3A/m Power frequency magnetic
(>95% dip in UT)
for 5 sec
fields should be at levels
characteristic of a typical
location in a typical
IEC 61000-4-8
commercial or hospital
environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
- 56 -
Page 63

SE-301 Series Electrocardiograph User Manual EMC Information
Electromagnetic immunity
Guidance and manufacture’s declaration – electromagnetic immunity
The Electrocardiograph is intended for use in the electromagnetic environment specified below.
The customer or the user of Electrocardiograph should assure that it is used in such an
environment.
Immunity
Complianc
Electromagnetic environment -
IEC 60601 test level
test
e level
guidance
Portable and mobile RF
communications equipment should be
used no closer to any part of the
electrocardiograph, including cables,
than the recommended separation
distance calculated from the equation
applicable to the frequency of the
transmitter.
Recommended separation distance
Conducted RF
IEC/
3 V
rms
150 kHz to 80 MHz
3 V
rms
Pd 2.1=
61000-4-6
Radiated RF
IEC
61000-4-3
3 V/m
80 MHz to 2.5 GHz
3 V/m
80 MHz to 800 MHz
Pd 2.1=
800 MHz to 2.5 GHz
Pd 3.2=
Where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter
manufacturer and d is the
recommended separation distance in
metres (m).
Field strengths from fixed RF
transmitters, as determined by an
electromagnetic site survey,a should be
less than the compliance level in each
frequency range.b
Interference may occur in the vicinity
of equipment marked with the
following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
- 57 -
Page 64

SE-301 Series Electrocardiograph User Manual EMC Information
environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the Electrocardiograph
is used exceeds the applicable RF compliance level above, the Electrocardiograph should
be observed to verify normal operation. If abnormal performance is observed, additional
measures may be necessary, such as reorienting or relocating the Electrocardiograph.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communications
equipment and the EQUIPMENT or SYSTEM
Recommended separation distances between
portable and mobile RF communications equipment and electrocardiograph
The electrocardiograph is intended for use in an electromagnetic environment in which radiated
RF disturbances are controlled. The customer or the user of the electrocardiograph can help
prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the electrocardiograph as
recommended below, according to the maximum output power of the communications
equipment.
Rated maximum
output power of
transmitter
(W)
0.01
0.1
1
10
100
Separation distance according to frequency of transmitter
(m)
150 kHz to 80 MHz
Pd 2.1=
80 MHz to 800 MHz
Pd 2.1=
800 MHz to 2.5
GHz
0.12 0.12 0.23
0.38 0.38 0.73
1.2 1.2 2.3
3.8 3.8 7.3
12 12 23
Pd 3.2=
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
- 58 -
Page 65

SE-301 Series Electrocardiograph User Manual Abbreviation
Appendix 3 Abbreviation
Abbr English
BP Blood Pressure
ECG Electrocardiogram/Electrocardiograph
HR Heart Rate
aVF Left Foot Augmented Lead
aVL Left Arm Augmented Lead
aVR Right Arm Augmented Lead
LA Left Arm
LL Left Leg
RA Right Arm
RL Right Leg
ID Identification
AC Alternating Current
USB Universal Serial Bus
AGC Auto Gain Control
NC Normal Condition
SFC Single Fault Condition
- 59 -
Page 66

 Loading...
Loading...