Page 1

Page 2

About this Manual
P/N: 01.54.000805
MPN: 01.54.000805015
Release Date: February 2017
© Copyright EDAN INSTRUMENTS, INC. 2014-2017. All rights reserved.
Statement
This manual will help you understand the operation and maintenance of the product better. It is
reminded that the product shall be used strictly complying with this manual. User’s operation
failing to comply with this manual may result in malfunction or accident for which EDAN
INSTRUMENTS, INC. (hereinafter called EDAN) cannot be held liable.
EDAN owns the copyrights of this manual. Without prior written consent of EDAN, any
materials contained in this manual shall not be photocopied, reproduced or translated into other
languages.
Materials protected by the copyright law, including but not limited to confidential information
such as technical information and patent information are contained in this manual, the user shall
not disclose such information to any irrelevant third party.
The user shall understand that nothing in this manual grants him, expressly or implicitly, any
right or license to use any of the intellectual properties of EDAN.
EDAN holds the rights to modify, update, and ultimately explain this manual.
Responsibility of the Manufacturer
EDAN only considers itself responsible for any effect on safety, reliability and performance of
the equipment if:
Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by EDAN, and
The electrical installation of the relevant room complies with national standards, and
The instrument is used in accordance with the instructions for use.
I
Page 3

Terms Used in this Manual
This guide is designed to give key concepts on safety precautions.
WARNING
A WARNING label advises against certain actions or situations that could result in personal
injury or death.
CAUTION
A CAUTION label advises against actions or situations that could damage equipment, produce
inaccurate data, or invalidate a procedure.
NOTE
A NOTE provides useful information regarding a function or a procedure.
II
Page 4

Table of Contents
Chapter 1 Safety Guidance ........................................................................................................... 1
1.1 Intended Use/Indications for Use ........................................................................................ 1
1.2 Warnings and Cautions ........................................................................................................ 1
1.2.1 General Warnings ...................................................................................................... 1
1.2.2 Battery Care Warnings .............................................................................................. 5
1.2.3 General Cautions ....................................................................................................... 5
1.3 List of Symbols ................................................................................................................... 6
Chapter 2 Introduction .................................................................................................................. 8
2.1 Assembling the System ....................................................................................................... 8
2.1.1 PADECG System ...................................................................................................... 8
2.1.2 DX12(iOS) Transmitter ........................................................................................... 10
2.1.2.1 Keys and Icons .............................................................................................. 11
2.1.2.2 Setting the Menu ........................................................................................... 12
2.2 Installing the Software ...................................................................................................... 12
2.2.1 System Running Environment ................................................................................ 12
2.2.2 About Installation .................................................................................................... 13
2.2.3 Matching DX12(iOS) Transmitter with iOS Tablet ................................................ 13
2.3 Features ............................................................................................................................. 14
Chapter 3 Preparations Before Operation ................................................................................. 15
3.1 Preparing the Patient ......................................................................................................... 15
3.1.1 Instructing the Patient ............................................................................................. 15
3.1.2 Cleaning the Skin .................................................................................................... 15
3.2 Connecting the Patient Cable ............................................................................................ 15
3.3 Attaching Electrodes ......................................................................................................... 16
3.3.1 Electrode Placement ................................................................................................ 17
3.3.2 Attaching the Reusable Electrodes .......................................................................... 18
3.3.2.1 Attaching the Limb Electrodes ...................................................................... 18
3.3.2.2 Attaching the Chest Electrodes ..................................................................... 18
3.3.3 Attaching the Disposable Electrodes ....................................................................... 19
Chapter 4 Operation Instructions .............................................................................................. 20
4.1 Entering Patient Information ............................................................................................. 20
4.2 Sampling ECG Data .......................................................................................................... 21
4.3 Analyzing ECG Data ......................................................................................................... 22
4.4 Printing Reports ................................................................................................................ 24
4.5 Processing Patient Records ............................................................................................... 25
4.6 Configuring the System..................................................................................................... 26
4.6.1 Patient Information Setting ..................................................................................... 26
4.6.2 Sampling Storage Setting ........................................................................................ 27
III
Page 5

4.6.3 Filter Setting ............................................................................................................ 28
4.6.4 Transmission Setting ............................................................................................... 28
4.6.5 Other Setting ........................................................................................................... 29
Chapter 5 Hint Information ........................................................................................................ 30
Chapter 6 Cleaning, Care and Maintenance ............................................................................. 31
6.1 General Points ................................................................................................................... 31
6.2 Cleaning ............................................................................................................................ 31
6.2.1 Cleaning the DX12(iOS) Transmitter ..................................................................... 32
6.2.2 Cleaning the Patient Cable ...................................................................................... 32
6.2.3 Cleaning the Reusable Electrodes ........................................................................... 32
6.3 Disinfection ....................................................................................................................... 33
6.3.1 Disinfecting the DX12(iOS) Transmitter ................................................................ 33
6.3.2 Disinfecting the Patient Cable ................................................................................. 33
6.3.3 Disinfecting the Reusable Electrodes ..................................................................... 34
6.4 Care and Maintenance ....................................................................................................... 34
Chapter 7 Accessories .................................................................................................................. 36
Chapter 8 Warranty and Service ................................................................................................ 37
8.1 Warranty ............................................................................................................................ 37
8.2 Contact information .......................................................................................................... 37
Appendix 1 Technical Specifications .......................................................................................... 38
A1.1 Safety Specifications ...................................................................................................... 38
A1.2 Environment Specifications ........................................................................................... 38
A1.3 Physical Specifications ................................................................................................... 39
A1.4 Power Supply Specifications .......................................................................................... 39
A1.5 Performance Specifications ............................................................................................ 39
Appendix 2 EMC Information .................................................................................................... 41
Appendix 3 Abbreviation ............................................................................................................. 47
IV
Page 6
PADECG User Manual Safety Guidance
Chapter 1 Safety Guidance
This chapter provides important safety information related to the use of PADECG.
1.1 Intended Use/Indications for Use
The intended use of PADECG is to acquire resting ECG signals from adult and pediatric patients
through body surface ECG electrodes. It is only intended to be used in hospitals or healthcare
facilities by doctors and trained healthcare professionals. The cardiogram recorded by PADECG
can help users to analyze and diagnose heart disease. However, the interpreted ECG with
measurements and interpretive statements is offered to clinicians on an advisory basis only. It is
mainly used in ECG inpatient department of hospitals or healthcare facilities.
WARNING
1. This system is not designed for intracardiac use or direct cardiac application.
2. This system is not intended for home use.
3. This system is not intended for treatment or monitoring.
4. This system is intended for use on adult and pediatric patients only.
5. The results given by the system should be examined based on the overall clinical
condition of the patient, and they cannot substitute for regular checking.
1.2 Warnings and Cautions
To use the system safely and effectively, firstly be familiar with the operation method of
Windows and read the user manual in detail to be familiar with the proper operation method for
the purpose of avoiding the possibility of system failure. The following warnings and cautions
must be paid more attention to during the operation of the system.
1.2.1 General Warnings
WARNING
1. The system is intended to be used by qualified physicians or personnel professionally
trained. They should be familiar with the contents of this user manual before
operation.
- 1 -
Page 7

PADECG User Manual Safety Guidance
WARNING
2. Only qualified service engineers can install this equipment, and only service
engineers authorized by the manufacturer can open the shell. Otherwise, safety
hazards may happen.
3. EXPLOSION HAZARD - Do not use the system in the presence of flammable
anesthetic mixtures with oxygen or other flammable agents.
4. Only the patient cable and other accessories supplied by the manufacturer can be
used. Or else, the performance and electric shock protection cannot be guaranteed.
The system has been safety tested with the recommended accessories, peripherals,
and leads, and no hazard is found when the system is operated with cardiac
pacemakers or other stimulators.
5. Make sure that all electrodes are connected to the patient correctly before operation.
6. Ensure that the conductive parts of electrodes and associated connectors, including
neutral electrodes, do not come in contact with earth or any other conducting objects.
7. Disposable electrodes must be used during defibrillation.
8. Electrodes of dissimilar metals should not be used; otherwise it may cause a high
polarization voltage.
9. The disposable electrodes can only be used for one time.
10. Do not touch the patient, bed, table or the equipment while using the ECG together
with a defibrillator.
11. Do not touch accessible parts of electrical equipment and the patient simultaneously.
12. The use of equipment that applies high frequency voltages to the patient (including
electrosurgical equipment and some respiration transducers) is not supported and
may produce undesired results. Disconnect the patient data cable from the ECG
workstation, or detach the leads from the patient prior to performing any procedure
that uses high frequency surgical equipment.
13. Fix attention on the examination to avoid missing important ECG waves.
14. Do not connect any equipment or accessories that are not approved by the
manufacturer or that are not IEC/EN 60601-1 approved to the system. The operation
or use of non-approved equipment or accessories with the system is not tested or
supported, and system operation and safety are not guaranteed.
15. The use of patient cable and other accessories not supplied by the manufacturer may
result in increased emissions or decreased immunity of the equipment.
- 2 -
Page 8

PADECG User Manual Safety Guidance
WARNING
16. Any non-medical equipment (such as the external printer) is not allowed to be used
within the patient vicinity (1.5m/6ft.).
17. Accessory equipment connected to the analog and digital interfaces must be certified
according to the respective IEC/EN standards (e.g. IEC/EN 60950 for data processing
equipment and IEC/EN 60601-1 for medical equipment). Furthermore all configuration
shall comply with the valid version of the standard IEC/EN 60601-1. Therefore
anybody, who connects additional equipment to the signal input or output connector to
configure a medical system, must make sure that it complies with the requirements of
the valid version of the system standard IEC/EN 60601-1. If in doubt, consult our
technical service department or your local distributor.
18. Connecting any accessory (such as external printer) or other device (such as the
computer) to this system makes a medical system. In that case, additional safety
measures should be taken during installation of the system, and the system shall
provide:
a) Within the patient environment, a level of safety comparable to that provided by
medical electrical equipment complying with IEC/EN 60601-1, and
b) Outside the patient environment, the level of safety appropriate for non-medical
electrical equipment complying with other IEC or ISO safety standards.
19. All the accessories connected to system must be installed outside the patient vicinity,
if they do not meet the requirement of IEC/EN 60601-1.
20. You are recommended to purchase the iOS tablet from the manufacturer. Otherwise,
the manufacturer will not be held responsible for the maintenance of the hardware,
operating system and other accessories.
21. If multiple instruments are connected to a patient, the sum of the leakage currents
may exceed the limits given in the IEC/EN 60601-1 and may pose a safety hazard.
Consult your service personnel.
22. Connecting to other devices may decrease the antistatic gradation of the system
during operation.
23. Make sure that there is no intense electromagnetic interference source around when
using the wireless system of PADECG. Furthermore, Keep a unobstructed distance of
at most 3 meters between DX12(iOS) Transmitter and the iOS tablet.
- 3 -
Page 9

PADECG User Manual Safety Guidance
WARNING
24. Do not open the battery cover of DX12(iOS) Transmitter when using the wireless
system of PADECG.
25. The iOS tablet shall comply with the valid version of the standard IEC 60950 and be
used outside the patient environment (at least 2 meters away from the patient). The
iOS tablet shall be charged outside the patient environment, and no operations are
permitted during the charging.
26. The device shall not be serviced or maintained while in use with a patient.
27. The medical electrical equipment needs to be installed and put into service according
to Appendix 2 EMC information.
28. Portable and mobile RF communications equipment can affect medical electrical
equipment, refer to the recommended separation distances provided in Appendix 2
EMC Information.
29. The equipment should not be used adjacent to or stacked with other equipment, refer
to the recommended separation distances provided in Appendix 2 EMC Information.
30. Assembly of the ECG workstation and modifications during actual service life shall be
evaluated based on the requirements of IEC60601-1.
31. Check the gain and speed of the report thoroughly when confirming diagnosis.
32. The device is MR unsafe. It is not intended for use in an MRI environment.
33. Magnetic and electrical fields are capable of interfering with the proper performance
of the device. For this reason make sure that all external devices operated in the
vicinity of the device comply with the relevant EMC requirements. X-ray equipment or
MRI devices are a possible source of interference as they may emit higher levels of
electromagnetic radiation.
- 4 -
Page 10
PADECG User Manual Safety Guidance
1.2.2 Battery Care Warnings
WARNING
1. Improper operation may cause the internal battery to be hot, ignited or exploded, and
it may lead to the decrease of the battery capacity. It is necessary to read the user
manual carefully and pay more attention to warning messages.
2. Batteries of the same model and specification as manufacture configuration should be
used.
3. DANGER OF EXPLOSION -- Do not reverse the anode and the cathode when
installing the battery.
4. Do not heat or splash the battery or throw it into fire or water.
5. Do not destroy the battery; do not pierce battery with a sharp object such as a needle;
do not hit with a hammer, step on or throw or drop to cause strong shock; do not
disassemble or modify the battery.
6. When leakage or foul smell is found, stop using the battery immediately. If your skin or
cloth comes into contact with the leakage liquid, cleanse it with clean water at once. If
the leakage liquid splashes into your eyes, do not wipe them. Irrigate them with clean
water first and go to see a doctor immediately.
7. Properly dispose of or recycle the depleted battery according to local regulations.
8. Remove the battery from the transmitter if the system won’t be used for a long time.
9. Replace the depleted battery before use.
1.2.3 General Cautions
CAUTION
1. Avoid liquid splash and excessive temperature. The temperature must be kept
between 5 ºC and 40 ºC during operation, and it should be kept between -20 ºC and
55 ºC during transportation and storage.
2. Do not use the equipment in a dusty environment with bad ventilation or in the
presence of corrosive.
- 5 -
Page 11

PADECG User Manual Safety Guidance
No.
Symbol
Description
1
DEFIBRILLATION-PROOF TYPE CF APPLIED
PART
2
Caution
3
Operating instructions
4
Warning
(Background: Yellow; Symbol&Outline: Black)
5
Refer to User Manual
(Background: Blue; Symbol: White)
6
Non- ionizing electromagnetic radiation
CAUTION
3. Make sure that there is no intense electromagnetic interference source around the
equipment, such as radio transmitters or mobile phones etc. Attention: large medical
electrical equipment such as electrosurgical equipment, radiological equipment and
magnetic resonance imaging equipment etc. is likely to bring electromagnetic
interference.
4. The device and accessories are to be disposed of according to local regulations after
their useful lives. Alternatively, they can be returned to the dealer or the manufacturer
for recycling or proper disposal. Batteries are hazardous waste. Do NOT dispose of
them together with house-hold garbage. At the end of their lives hand the batteries
over to the applicable collection points for the recycling of waste batteries. For more
detailed information about recycling of this product or battery, please contact your
local Civic Office, or the shop where you purchased the product.
5. Federal (U.S.) law restricts this device to sale by or on the order of a physician.
1.3 List of Symbols
- 6 -
Page 12
PADECG User Manual Safety Guidance
7
General symbol for recovery/recyclable
8
Part Number
9
SERIAL NUMBER
10
Date of manufacture
11
MANUFACTURER
12
AUTHORISED REPRESENTATIVE IN THE
EUROPEAN COMMUNITY
13
CE marking
14
Disposal method
15
Caution: Federal (U.S.) law restricts this device to
sale by or on the order of a physician.
16
FCC ID: SMQDX12TREDAN
Federal Communications Commission:
FCC ID: SMQDX12TREDAN
17
MR Unsafe–Keep away from magnetic resonance
imaging (MRI) equipment
NOTE: The user manual is printed in black and white.
- 7 -
Page 13
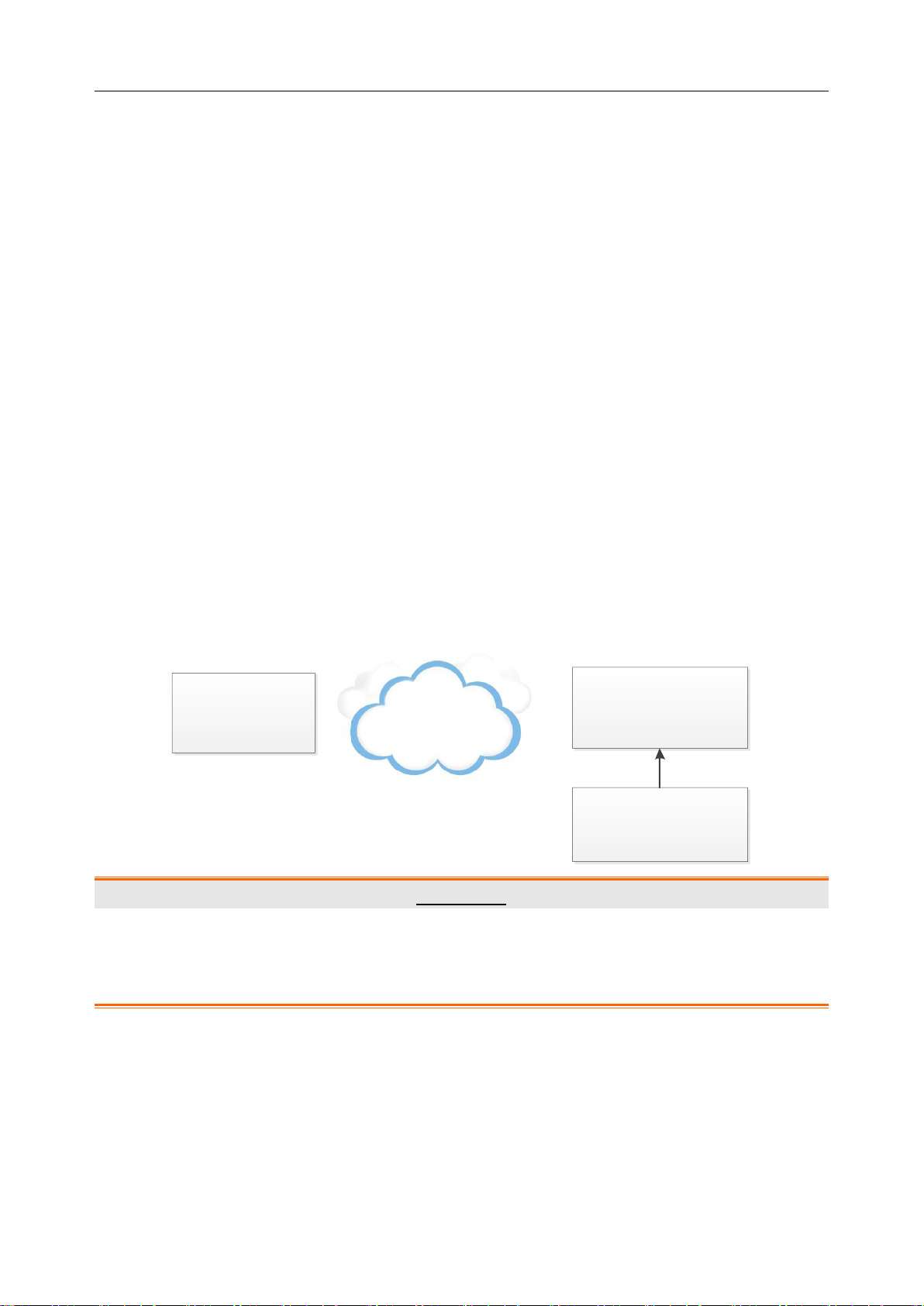
PADECG User Manual Introduction
iOS Tablet
DX12(iOS)
Transmitter
Patient
Patient Cable
Wireless
Chapter 2 Introduction
PADECG as mobile ECG Workstation has similar functions with an ordinary ECG Workstation.
ECG data can be sampled, analyzed and stored in a Pad, ECG waves can be reviewed. Auto
measurement and diagnosis are available, and the diagnosis template can be edited.
PADECG includes the following equipment, you can also purchase the iOS tablet.
DX12(iOS) Transmitter
Patient Cable
Electrodes
NOTE: The pictures and windows in this manual are for reference only.
2.1 Assembling the System
2.1.1 PADECG System
WARNING
DX12(iOS) Transmitter of the wireless system uses the Bluetooth technology, which
could make the patient with the pacemaker uncomfortable. Keep DX12(iOS) Transmitter
far away from the pacemaker when using the wireless system of PADECG.
- 8 -
Page 14

PADECG User Manual Introduction
WARNING
1. Accessory equipment connected to the analog and digital interfaces must be certified
according to the respective IEC/EN standards (e.g. IEC/EN 60950 for data
processing equipment and IEC/EN 60601-1 for medical equipment). Furthermore all
configuration shall comply with the valid version of the standard IEC/EN 60601-1.
Therefore anybody, who connects additional equipment to the signal input or output
connector to configure a medical system, must make sure that it complies with the
requirements of the valid version of the system standard IEC/EN 60601-1. If in doubt,
consult our technical service department or your local distributor.
2. If multiple instruments are connected to a patient, the sum of the leakage currents
may exceed the limits given in the IEC/EN 60601-1 and may pose a safety hazard.
Consult your service personnel.
- 9 -
Page 15
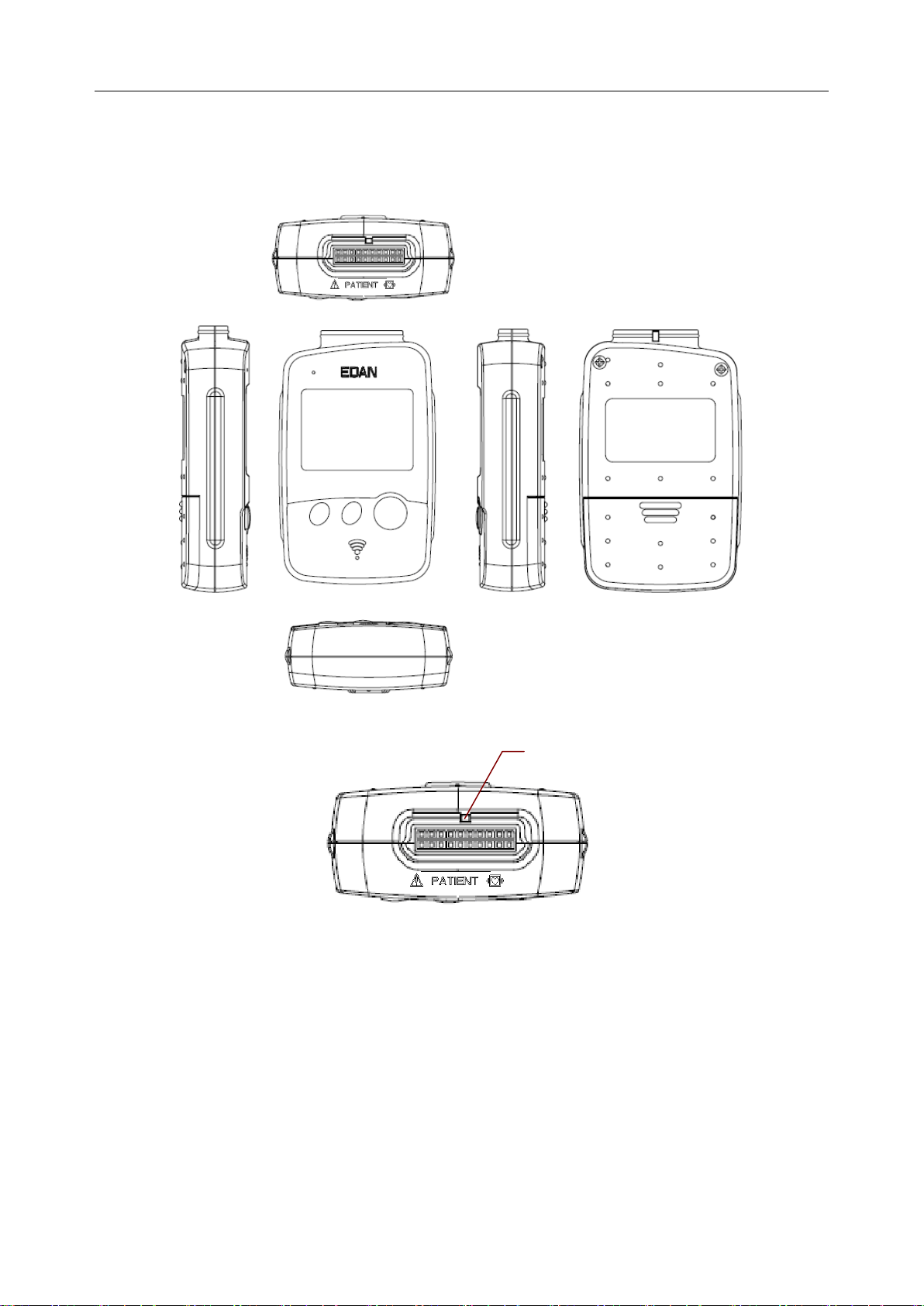
PADECG User Manual Introduction
Patient Cable Socket
2.1.2 DX12(iOS) Transmitter
DX12(iOS) Transmitter Appearance
Front Panel
- 10 -
Page 16

PADECG User Manual Introduction
Keys/Icons
Description
After batteries are installed, press this key to turn on/off DX12(iOS)
Transmitter.
When the Menu screen is displayed, press this key to return to the previous
screen.
When the Main or Menu screen is displayed, press this key to enter the next
menu.
Press this key, and then press in 1.2 seconds to lock / unlock the
keypad.
When the Main screen is displayed, press this key to switch the lead.
When the Menu screen is displayed, press this key to display an item in black.
Icon for Bluetooth Connection
If this icon is not displayed on the main screen, you need to match the device
manually.
Icon for Keypad Locked
If no operation is taken, the Main screen will be displayed and the keyboard
will be locked automatically in 8 seconds.
Icon for Battery Capacity
When the battery is weak, a hint will be displayed in PADECG software.
2.1.2.1 Keys and Icons
Main Screen Menu Screen
- 11 -
Page 17
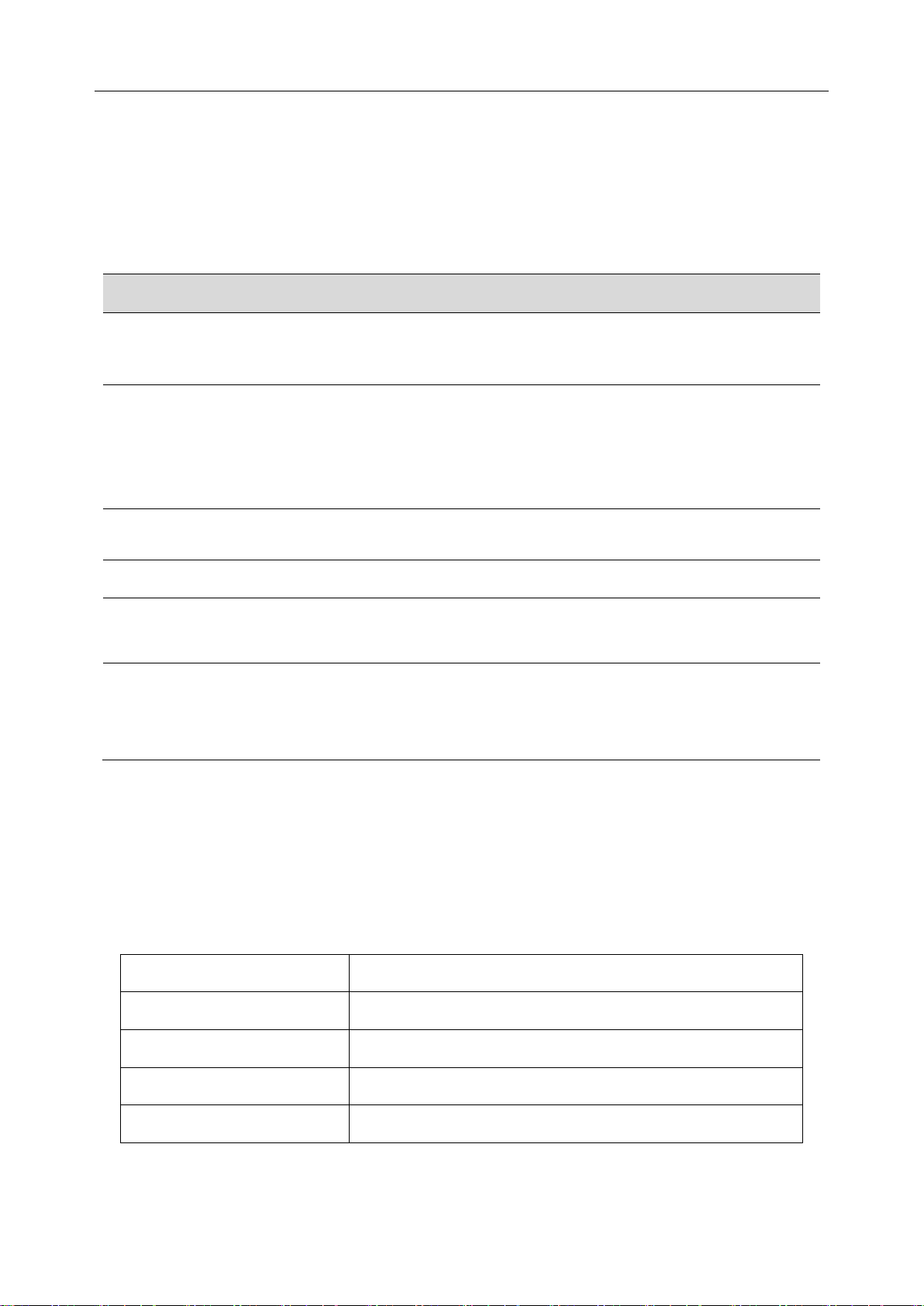
PADECG User Manual Introduction
Menu
Description
Back Light
Select On to turn on the backlight of LCD screen.
Select Off to turn off the backlight of LCD screen.
Auto Sleep
Select On to display Sleeping on the screen and make DX12(iOS)
Transmitter be in low power consumption mode after lead off for 5
minutes.
Select Off to turn off auto sleep function.
Language
You can set the system language.
Lead Electrode
You can select IEC or AHA.
Bluetooth
Device
You can see the Bluetooth name and address of the DX12(iOS)
Transmitter.
Device
Information
You can see the related information, such as software version, ID, address
of the device, manufacture and release time about the device.
NOTE: The device information is for reference only.
Applicable iOS Tablets:
iPad4, iPad air, iPad mini, iPad mini with Retina display
Operating System:
iOS 7.0 or above
CPU:
Apple A5 or above
Capacity:
16 G or above
Transmission
Built-in Bluetooth and WIFI
NOTE: ECG waveforms displayed on the DX12(iOS) Transmitter only indicates whether
the electrodes are attached properly. Diagnosis and evaluation of the ECG
should be done based on the display on the iPad.
2.1.2.2 Setting the Menu
2.2 Installing the Software
NOTE: This section is only for reference when the operating system of the iOS tablet
needs to be reinstalled or DX12(iOS) Transmitter is broken.
2.2.1 System Running Environment
- 12 -
Page 18

PADECG User Manual Introduction
WARNING
1. Use the PADECG system only on the tablet that is installed with the official operating
system (OS) versions released by the tablet's manufacturer.
2. When upgrading the tablet OS, consult EDAN service engineer if necessary.
2.2.2 About Installation
Enter the App Store of the iOS tablet, input PADECG to search for the software. The installation
is the same as that of other software available in the iOS tablet.
NOTE:
1. To uninstall the software, exit the software first. Local data will be lost after
uninstalling the software.
2. Please upgrade the software in time when a new version is available.
2.2.3 Matching DX12(iOS) Transmitter with iOS Tablet
For the first time to match the transmitter with the iOS tablet, perform the following operations:
1. Start the DX12(iOS) Transmitter and the iOS tablet.
2. Enable the Bluetooth function in iPad>Settings>Bluetooth.
3. Click the required DX12(iOS) Transmitter for connection.
In later use, when turned on, the transmitter will automatically search for and connect with the
iOS tablet of the previous match in 10s.
Operation for viewing device information of the required transmitter: turn on DX12(iOS)
Transmitter press press to display Device Information in black press .
NOTE:
1. Before matching DX12(iOS) Transmitter and the iOS tablet, ensure batteries of
DX12(iOS) Transmitter and iOS tablet are full.
2. You need to reconnect the DX12(iOS) Transmitter to the iOS tablet after changing
system language.
3. Before matching the transmitter, please ensure that the iOS tablet Bluetooth is in
unconnected state.
- 13 -
Page 19

PADECG User Manual Introduction
WARNING
This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions:
1) this device may not cause harmful interference, and
2) this device must accept any interference received, including interference that may
cause undesired operation.
NOTE:
1. This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential installation.
This equipment generates, uses and can radiate radio frequency energy and, if not
installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the equipment and receiver.
- Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
- Consult the dealer or an experienced radio/TV technician for help.
2. Any changes or modifications to this unit not expressly approved by the party
responsible for compliance could void the user's authority to operate the equipment.
2.3 Features
Reliable and handy data recording, suitable for doctors’ inspections and visits
iOS operating system, user friendly interfaces and easy operation
Supporting order downloading function
Perfect data management, sampled ECG data can be transmitted to SE-1515 over LAN
3-/6-/12-channel ECG waves are displayed simultaneously
Supporting amplifying ECG waves, providing manual measurement with an electronic
ruler of high precision
0.32 Hz/0.67Hz DFT filter greatly reduces the baseline fluctuations without affecting
ECG signals
Supporting auto measurement and diagnosis
Supporting editing the Diagnosis Template
12-lead normal ECG analysis
- 14 -
Page 20

PADECG User Manual Preparations Before Operation
Chapter 3 Preparations Before Operation
3.1 Preparing the Patient
3.1.1 Instructing the Patient
Before attaching the electrodes, greet the patient and explain the procedure. Explaining the
procedure decreases the patient’s anxiety. Reassure the patient that the procedure is painless.
Privacy is important for relaxation. When possible, prepare the patient in a quiet room or area
where others can’t see the patient. Make sure that the patient is comfortable. The more relaxed
the patient is, the less the ECG will be affected by noise.
3.1.2 Cleaning the Skin
Thorough skin preparation is very important. The skin is a poor conductor of electricity and
frequently creates artifacts that distort the ECG signals. By performing methodical skin
preparation, you can greatly reduce the possibility of noise caused by muscle tremor and baseline
drift, ensuring high-quality ECG waves. There is natural resistance on the skin surface due to dry,
dead epidermal cells, oils and dirt.
To clean the skin
1. Shave hair from electrode sites, if necessary. Excessive hair prevents a good connection.
2. Wash the area thoroughly with soap and water.
3. Dry the skin to increase capillary blood flow and to remove the dead, dry skin cells and oils.
4. Use the disposable frosting film in the standard accessory list to get good ECG waveform.
NOTE: Rub the skin with a gauze pad to increase capillary blood flow if you don’t operate
the steps above.
3.2 Connecting the Patient Cable
WARNING
The performance and electric shock protection can be guaranteed only if the original
patient cable and electrodes of the manufacturer are used.
- 15 -
Page 21

PADECG User Manual Preparations Before Operation
IEC
AHA
WILSON
FRANK
Identifier
Color Code
Identifier
Color Code
Right arm
Right arm
R
Red
RA
White
Left arm
Left arm
L
Yellow
LA
Black
Right leg
Right leg
N or RF
Black
RL
Green
Left leg
Left leg
F
Green
LL
Red
Chest 1
I
C1
White/Red
V1
Brown/Red
Electrode Connector
Lead Wires
Connecting to
DX12(iOS) Transmitter
Main Cable
The patient cable includes the main cable and lead wires which can be connected to electrodes.
1. Connect the patient cable to the socket of DX12(iOS) Transmitter.
2. Align all lead wires of the patient cable to avoid twisting, and connect the lead wires to the
electrodes. Firmly attach them.
3.3 Attaching Electrodes
WARNING
Make sure that the conductive parts of electrodes and associated connectors, including
neutral electrodes, do not come in contact with earth or any other conducting objects.
The identifiers and color codes of electrodes used comply with IEC/EN requirements. In order to
avoid incorrect connections, the electrode identifiers and color codes are specified in the
following table. Moreover the equivalent codes according to American requirements are given in
the following table too.
Table 3-1 Electrodes and Their Identifiers and Color Codes
- 16 -
Page 22

PADECG User Manual Preparations Before Operation
Chest 2
E
C2
White/Yello
V2
Brown/Yello
Chest 3
C
C3
White/Green
V3
Brown/Green
Chest 4
A
C4
White/Brow
V4
Brown/Blue
Chest 5
M
C5
White/Black
V5
Brown/Orang
Chest 6
H
C6
White/Violet
V6
Brown/Violet
IEC
AHA
Electrode Placement
C1
V1
Fourth intercostal space at the right border of the sternum
C2
V2
Fourth intercostal space at the left border of the sternum
C3
V3
Fifth rib between C2 and C4
C4
V4
Fifth intercostal space on the left midclavicular line
C5
V5
Left anterior axillary line at the horizontal level of C4
C6
V6
Left midaxillary line at the horizontal level of C4
L
LA
Left arm/Left deltoid
R
RA
Right arm/Right deltoid
3.3.1 Electrode Placement
Only for the Reusable Electrodes Only for the Disposable Electrodes
- 17 -
Page 23

PADECG User Manual Preparations Before Operation
F
LL
Left leg/Upper leg as close to torso as possible
N
RL
Right leg/Upper leg as close to torso as possible
Reed
Connecting to a Lead Wire
Clamp
Suction Bulb
Connecting to a Lead Wire
Metal Cup
3.3.2 Attaching the Reusable Electrodes
3.3.2.1 Attaching the Limb Electrodes
Limb Electrode
Limb Electrode Connection:
1) Ensure that the electrodes are clean;
2) Clean the electrode area which is a short distance
above the ankle or the wrist with 75% alcohol;
3) Daub the electrode area on the limb with gel evenly;
4) Place a small amount of gel on the metal part of the
limb electrode clamp;
5) Connect the electrode to the limb, and make sure that
the metal part is placed on the electrode area above
the ankle or the wrist;
6) Attach all limb electrodes in the same way.
3.3.2.2 Attaching the Chest Electrodes
Chest Electrode
- 18 -
Page 24

PADECG User Manual Preparations Before Operation
Chest Electrode Connection:
1) Ensure that the electrodes are clean;
2) Clean the electrode area on the chest surface with 75% alcohol;
3) Daub the round area of 25mm in diameter on each electrode site with gel evenly;
4) Place a small amount of gel on the brim of the chest electrode’s metal cup;
5) Place the electrode on the chest electrode site and squeeze the suction bulb. Unclench it
and the electrode is adsorbed on the chest;
6) Attach all chest electrodes in the same way.
NOTE: Long-time measurement with a strong negative pressure on the suction bulb
may cause reddening of the skin. When using the electrode on kids or
patients with delicate skin, squeeze the suction bulb lightly.
3.3.3 Attaching the Disposable Electrodes
CAUTION
The disposable electrodes can only be used for one time.
Connect the snap socket adapter to the disposable electrode.
The quality of ECG waveform will be affected by the contact resistance between the patient and
the electrode. In order to get a high-quality ECG, the skin-electrode resistance must be minimized
while connecting electrodes.
- 19 -
Page 25

PADECG User Manual Operation instructions
Chapter 4 Operation Instructions
1. Turn on the iOS tablet and enable the Bluetooth function in iPad>Settings>Bluetooth.
2. Connect to the required DX12(iOS) Transmitter.
3. Start the PADECG Analysis Software.
4. Input the user name and password, and then click Login.
The default password is ecg (case sensitive). Click Setting, you can change the password, or
enable or disable the Remember Password function.
If no DX12(iOS) Transmitter is detected and no data exists on the file screen, a dialog box
requiring password will be displayed. Enter the correct password and you will enter the login
screen.
NOTE: Do not run other applications when running PADECG, or the system response
speed will be affected.
4.1 Entering Patient Information
On the Patient screen, you can view or create patient orders.
1. Entering patient information
Press on the patient screen, and then the system will automatically enter the New
Patient screen.
Input the related patient information in the inputting area, click OK and the new patient
record will be listed on top of the information list.
If Sample now is enabled, the system will automatically enter the pre-sampling screen after
you click on the OK button.
NOTE: Patient ID is a must when entering patient information. You can use the
number generated by the system or input a number manually. Patient ID can
be a random character string excluding ‘/’, ‘\’, ‘:’, ‘*’, ‘?’, ‘<’, ‘>’ ,‘|’, '%' and
Chinese characters.
2. Searching, modifying and deleting patient information
Input the patient name in the search area, click on , and all the patient information
which meet the conditions will be displayed in the information list. Patient records in
emergent state will always be listed on the top.
NOTE: Fuzzy search by patient name is supported.
Long press the patient information in the information list, you can modify or delete patient
information.
- 20 -
Page 26

PADECG User Manual Operation instructions
Key
Description
STAT ECG
Press to enter the ECG sampling screen directly.
Options: Delete, Scan, Download, Download Setting
Delete: Press to delete one or multiple orders.
Scan: Press to input patient information by scanning a bar code.
Download: Press to download patient orders.
Download Setting: Press to set the filter conditions for
downloading patient information from the server.
Press to load orders from the server (SE-1515). For details about the order
settings, please refer to "Order Server" in Section 4.6.4 "Transmission
Setting".
NOTE:
1. 1000 patient records can be displayed in the information list, and
200 latest created orders in the server can be loaded at one
time.
2. This item only applies to the local mode.
3. Not all options related to SE-1515 are available.
If the number of orders exceeds the maximum of a page, press these keys
to turn pages.
NOTE: Each page contains 50 orders.
File
Press to enter the file manager screen.
For details about the file manager screen, refer to section 4.5 "Processing
Patient Records".
System Setting
Press to enter the system setting screen.
For details about the system setting screen, refer to section 4.6
"Configuring the System".
3. Description for buttons
4.2 Sampling ECG Data
Enable Sample now and then click OK on the New Patient screen, the system will automatically
enter the pre-sampling screen.
If Sample now is not enabled, click on OK and click on the patient record in the patient list to
enter the pre-sampling screen.
- 21 -
Page 27

PADECG User Manual Operation instructions
Key
Description
Start/Stop
Press this button to start/stop sampling ECG data.
NOTE: At most 180s ECG data can be sampled.
100Hz
EMG Filter: Off, 25Hz, 35Hz or 45Hz
Lowpass Filter: Off, 75Hz, 100Hz or 150Hz
NOTE: This setup modified on the pre-sampling screen is only effective
for the current patient.
10mm/mV
Gain: 2.5 mm/mV, 5 mm/mV, 10 mm/mV or 20 mm/mV
25mm/s
Speed: 5mm/s, 12.5mm/s, 25mm/s or 50mm/s
Patient
Press to enter the patient screen.
File
Press to enter the file manager screen.
NOTE: Long press the tablet screen during ECG data pre-sampling, the display mode of
12-channel ECG waves will be switched among 12×1, 6×2+1 and 3×4+1.
4.3 Analyzing ECG Data
The system will automatically enter the ECG Analysis screen after sampling ECG data. ECG data
can be displayed with the following style: 12×1, 6×2+1, and 3×4+1.
1. Viewing ECG waveform
Long press the rhythm waveform to display the Rhythm Lead window on the 6×2+1 or
3×4+1 ECG Analysis screen. You can view other rhythm waveform by selecting one lead in
the pop-up window.
You can view other waveforms by dragging or clicking on the rhythm waveform on the
6×2+1 or 3×4+1 ECG Analysis screen.
2. Amplifying and measuring ECG waveform
Multi-touch on the ECG waveforms of the ECG Analysis screen can be used to amplify or
minify the ECG waveforms.
If more than 10s ECG data has been sampled, a scrollbar will appear above the ECG
waveforms. You can slide the scrollbar to adjust the time period of the waveforms to be
displayed. During sliding, the start time and end time of the displayed 10s ECG waveforms
will be shown. You can click "+" or "-" to view the 10s ECG waveforms 5s later or earlier.
- 22 -
Page 28

PADECG User Manual Operation instructions
Designation
Description
HR (bpm)
Heart Rate
P (ms)
P-wave duration of the current lead
PR (ms)
P-R interval of the current lead
QRS (ms)
QRS complex duration of the current lead
QT/QTc (ms)
Q-T interval of the current lead/Normalized QT interval
P/QRS/T (° )
Dominant direction of the average integrated ECG vectors
RV5/SV1 (mV)
The amplitude of R wave of V5 lead/the amplitude of S wave of
V1 lead
RV5+SV1 (mV)
The amplitude of R wave of V5 lead plus the amplitude of S wave
of V1 lead
RV6/SV2 (mV)
The amplitude of R wave of V6 lead/the amplitude of S wave of
V2 lead
Long pressing the waveform on the ECG Analysis screen can amplify the ECG waveform.
Press on the Amplified Waveform screen to measure the ECG waveform. Press
this button again to cancel measuring.
3. Modifying measure information
Long press one parameter in the top left corner of the ECG Analysis screen, and the Modify
measure information window pops up. Make the related settings, and then click on the OK
button to save the modifications.
The common parameters are displayed as follows.
4. Modifying diagnosis results
Long press one diagnosis result in the top right corner of the ECG Analysis screen, and the
Auto Diagnosis window pops up.
Select one diagnosis result from the Diagnosis List or input diagnosis information directly
in the textbox, and click on the OK button to save the modifications.
5. Modifying Diagnosis List
Long press one diagnosis result in the Diagnosis List, and you can add, delete, or modify
the diagnosis result.
NOTE: If Diagnosis List is modified, you should click on the Save button in the
Auto Diagnosis window to save the related modifications.
- 23 -
Page 29

PADECG User Manual Operation instructions
6. Hiding or displaying measure information and diagnosis results
On the ECG Analysis screen, you can slide the ECG waveforms upward to hide measure
information and diagnosis results or downward to display them again.
7. Email
Click on on the analysis screen, and then you can Email files by clicking on the Send
Email button. The procedure is as follows:
1) Add an Email account.
If no Email account is set, add one in the following directory: iPad>Mail, Conatact,
Calendars.
2) Set default Email information in the following directory: PADECG>System
Settings>Transmission Setting>Email Settings.
3) Click on the ECG analysis screen and select Sending Email.
If no default Email information is set, a dialog box requiring report format selection will
be displayed. After the file format is selected, the current file will be added to the mail
editing window as an accessory. Input the receiver address before sending it.
8. Edit
Click on the analysis screen, and then you can click Edit to modify patient
information.
4.4 Printing Reports
We suggest that the report be printed out on a printer to diagnose and sign.
To print a report, the tablet should be connected to a PC which is installed with the SE-1515
analysis software or an FTP server by setting the IP address and FTP parameters of the PADECG
analysis software. For details, please refer to "FTP Setting" in section 4.6.4 "Transmission
Setting".
To use the FTP server, configure it before connecting to the PC. For details please contact the
local distributor or the manufacturer.
NOTE:
1. This function is available only under network connection.
2. Not all options related to SE-1515 are available.
WARNING
The printouts need to be done with no scaling or sizing done to fit the page.
- 24 -
Page 30

PADECG User Manual Operation instructions
Key
Description
Press this button to enter submenu.
Press this button to upload the selected examination records to the server.
If the checkbox before Select All is ticked, pressing this button can clear
the examination record list.
Set the search criteria for the records to be displayed.
4.5 Processing Patient Records
1. Search
Input the patient name in the search area, click on , and all the examination records
which meet the conditions will be displayed in the examination record list.
NOTE: Fuzzy search by patient name is supported in the search area.
2. Upload and Delete
Long press one examination record in the examination record list, and you can perform the
following operations on the selected record: view, upload, modify, or delete.
NOTE:
1. The patient ID cannot be modified.
2. The modification on the patient name and gender will be synchronized to other
data of the same patient ID.
If you need to delete or upload records in bulk, press and choose Upload or Delete.
- 25 -
Page 31

PADECG User Manual Operation instructions
Item
Description
ID Generating
Method
Choose from: Auto or Manual Input
Select Auto, the patient ID can be automatically generated according to
the examination date.
Select Manual Input, you should enter the patient ID manually on the
New Patient screen.
Patient Info Auto
Clear
Enabled:
The system automatically clears the current patient information
except Gender, Exam.Room, Technician, and Physician.
If ID Input Method is set to Manual Input, the patient ID will also be
cleared.
Disabled:
A Clear key will appear in the New Patient window.
If Sample now is on, the current patient information remains when
creating a new patient.
If Sample now is off, the current patient information will be
automatically cleared when creating a new patient.
4.6 Configuring the System
The system will automatically save the modifications after you make the related settings.
4.6.1 Patient Information Setting
- 26 -
Page 32
PADECG User Manual Operation instructions
First/Last Name
If enabled, the Name textbox on the New Patient screen will change into
the First Name and Last Name textboxes.
Age
Choose from: Manual or Date of Birth
Other Display
Settings
You can configure the items to be displayed on the New Patient window,
including: height, weight, blood pressure, race, medication, department,
room number, request number, exam. room, technician, physician, and
priority.
You can also add other items to be displayed by editing the customization
options.
Barcode Setting
NOTE: If unset, the scanned results may be incorrect.
Enter the start and end addresses, the male and female codes and encoding
mode, and then click on the OK button confirm.
Item
Description
Sampling Mode
Choose from: Real-time Sample, Pre-sample
Select Pre-Sample, 10s ECG data before pressing the Start key will
be saved.
Select Real-time Sample, 10s ECG data sampled after pressing the
Start key will be saved.
Sampling Time
Setting
It can be set to a value between 10s and 180s.
Auto Diagnosis
If selected, the system automatically generates diagnosis results after
sampling finishes.
Enter the Analysis
screen when
sampling finishes
If selected, the system automatically enters the wave analysis screen after
sampling finishes.
File Format
Choose from: SCP, FDA-XML, DICOM, PDF, JPG, BMP.
If selected, files in the selected format will be generated when sampling
finishes.
NOTE: This item only applies to the local mode.
Report Format
Choose from: 12*1, 6*2+1, 3*4+1.
4.6.2 Sampling Storage Setting
- 27 -
Page 33

PADECG User Manual Operation instructions
Rhythm Lead
Definition
It can be set to any one of the leads.
The default value is II.
Edit Analysis
Result Locally
If selected, you can modify the measurement information and diagnosis
results locally.
Item
Description
DFT Filter
DFT filter greatly reduces the baseline fluctuations without affecting ECG
signals. The purpose of this filter is to keep the ECG signals on the
baseline of the printout.
Choose from: 0.05Hz, 0.32Hz or 0.67Hz
The set value is the low limit of the frequency range.
EMG Filter
EMG filter suppresses the disturbance caused by strong muscle tremor.
The cutoff frequency can be set to Off, 25Hz, 35Hz or 45Hz.
Lowpass Filter
Lowpass Filter restricts the bandwidth of input signals.
The cutoff frequency can be set to Off, 75Hz, 100Hz or 150Hz.
All the input signals whose frequency is higher than the set cutoff
frequency will be attenuated.
NOTE: The Lowpass Filter is effective only when the EMG Filter is
set to Off.
AC Filter
AC filter suppresses AC interference without attenuating or distorting
ECG signals.
Choose from: Off, 50Hz and 60Hz.
Item
Description
Device No.
Type the Device No., within 30 ASCII characters.
4.6.3 Filter Setting
NOTE: To pass the distortion test, the ECG workstation has to be configured with the
highest bandwidth in filter settings. Otherwise, ECG signal may be distorted.
4.6.4 Transmission Setting
- 28 -
Page 34

PADECG User Manual Operation instructions
Auto Upload
Select this item, the system will automatically upload files to the server
when sampling finishes.
NOTE: This item only applies to the local mode. Under the network
mode, the system automatically uploads files when
sampling finishes.
Delete file
after uploading
Select this item, the system will automatically delete files from the
examination record list after they are uploaded.
Order Server
Address
Set it to IP address of the order server.
NOTE: For more information on configuring network settings,
consult your Network Administrator.
FTP Setting
Set the FTP address, port, user name, and password of the FTP server.
Email Settings
Set the default recipient, subject, and file format.
Item
Description
DEMO mode
Choose from: Off, Normal or Abnormal
Lead Electrode
Choose from: IEC, AHA
If IEC is selected, the hint information on the sampling screen will
show the IEC electrode name upon lead off.
If AHA is selected, the hint information on the sampling screen will
show the AHA electrode name upon lead off.
Hospital Name
Enter the hospital name, within 20 Chinese characters or within 60
English characters.
The configured hospital name will appear in the PDF/JPG/BMP report.
System Password
Type a password that allows you to access the System Setting screen.
Restore to factory
defaults
Press to restore the factory settings.
Device Info
View or edit the information of sampling devices.
NOTE: If a DX12(iOS) Transmitter is connected, its information
(name and address) will be displayed on the screen,
otherwise, the hint "No sampling device is connected" will
be displayed.
4.6.5 Other Setting
- 29 -
Page 35

PADECG User Manual Hint Information
Hint Information
Causes
Lead off: X
Electrodes fall off the patient or the patient
cable falls off the DX12(iOS) Transmitter.
Lead wire reversal
The limb leads are reversed.
Order server connection fails!
The order server is not enabled or the network
connection is abnormal.
FTP connection fails!
The FTP server is not enabled or the network
connection is abnormal.
Battery of sampling device is weak,
please change the battery after the test!
Battery of DX12(iOS) Transmitter is low.
Battery is weak, and the sampling device
will be powered off!
Battery of DX12(iOS) Transmitter runs out.
Chapter 5 Hint Information
Hint information and the corresponding causes provided by the system are listed as follows.
Table 5-1 Hint Information and Causes
- 30 -
Page 36

PADECG User Manual Cleaning, Care and Maintenance
Chapter 6 Cleaning, Care and Maintenance
Use only the EDAN-approved substances and methods listed in this chapter to clean or disinfect
your equipment. Warranty does not cover damage caused by using unapproved substances or
methods.
Edan Instruments has validated the cleaning and disinfection instructions provided in this User
Manual. It is the responsibility of the healthcare professional to ensure that the instructions are
followed so as to ensure adequate cleaning and disinfection.
6.1 General Points
Keep your DX12(iOS) Transmitter and accessories free of dust and dirt. To prevent the device
from damage, please follow the instructions:
Use only the recommended cleaning agents and disinfectants listed in this manual. Others
may cause damage (not covered by warranty), reduce product lifetime or cause safety
hazards.
Always dilute according to the manufacturer's instructions.
Unless otherwise specified, do not immerse any part of the equipment or any accessories in
liquid.
Do not pour liquid onto the equipment.
Do not allow liquid to enter the case.
Never use abrasive material (such as steel wool or silver polish).
Inspect the DX12(iOS) Transmitter and reusable accessories after they are cleaned and
disinfected.
CAUTION
If you spill liquid on the equipment or accessories, or they are accidentally immersed in
liquid, contact your service personnel or EDAN service engineer.
6.2 Cleaning
If the equipment or accessory has been in contact with the patient, then cleaning is required after
each use.
The validated cleaning agents for cleaning the DX12(iOS) Transmitter and patient cable are:
Mild near neutral detergent
Ethanol (75%)
Isopropanol (70%)
The validated cleaning agent for cleaning the reusable electrodes is:
Mild near neutral detergent
- 31 -
Page 37

PADECG User Manual Cleaning, Care and Maintenance
Cleaning agents should be applied or removed using a clean, soft, non-abrasive cloth or paper
towel.
6.2.1 Cleaning the DX12(iOS) Transmitter
WARNING
Turn off the power and take out the battery before cleaning.
1. Switch off the DX12(iOS) Transmitter and take out the battery.
2. Wipe the exterior surface of the equipment using a soft cloth dampened with the cleaning
solution until no visible contaminants remain.
3. Wipe off the cleaning solution with a fresh cloth or towel dampened with tap water after
cleaning until no visible cleaning agent remains.
4. Dry the DX12(iOS) Transmitter in a ventilated and cool place.
6.2.2 Cleaning the Patient Cable
1. Wipe the patient cable with a soft cloth dampened with the cleaning solution until no visible
contaminants remain.
2. Wipe off the cleaning solution with a fresh cloth or towel dampened with tap water after
cleaning until no visible cleaning agent remains.
3. Wipe off with a dry cloth to remove residual moisture.
4. Leave the patient cable to air dry.
CAUTION
Any remainder of cleaning solution should be removed from the DX12(iOS) Transmitter
and the patient cable after cleaning.
6.2.3 Cleaning the Reusable Electrodes
1. Wipe off with a soft cloth to remove residual gel.
2. Wipe the suction bulbs of chest electrodes and the clamps of limb electrodes with a soft cloth
dampened with the cleaning solution until no visible contaminants remain.
3. Wipe off the cleaning solution with a fresh cloth or towel dampened with tap water after
cleaning until no visible cleaning agent remains.
4. Wipe off with a dry cloth to remove residual moisture.
5. Leave the suction bulbs and clamps to air dry.
- 32 -
Page 38

PADECG User Manual Cleaning, Care and Maintenance
6.3 Disinfection
If the equipment or accessory has been in contact with the patient, then disinfection is required.
To avoid permanent damage to the equipment, it is recommended that disinfection is performed
only when it is considered as necessary according to your hospital' regulations.
Clean the equipment and reusable accessories before they are disinfected. The validated
disinfectants for disinfecting the DX12(iOS) Transmitter and patient cable are:
Ethanol (75%)
Isopropanol (70%)
The validated disinfectant for disinfecting the reusable electrodes is:
Isopropanol (70%)
If Ethanol or Isopropanol is used for both cleaning and disinfecting, then a new cloth is required
to be used for the disinfection step.
CAUTION
1. Do not use high-temperature, high-pressure vapour or ionizing radiation as
disinfection methods.
2. Do not use chloric disinfectant such as chloride, sodium hypochlorite etc.
3. Clean and disinfect reusable electrodes after each use.
6.3.1 Disinfecting the DX12(iOS) Transmitter
WARNING
Turn off the power and take out the battery before disinfection.
1. Switch off the DX12(iOS) Transmitter and take out the battery.
2. Wipe the exterior surface of the equipment using a soft cloth dampened with the disinfectant
solution.
3. Wipe off the disinfectant solution with a dry cloth after disinfection if necessary.
4. Dry the DX12(iOS) Transmitter for at least 30 minutes in a ventilated and cool place.
6.3.2 Disinfecting the Patient Cable
1. Wipe the patient cable with a soft cloth dampened with the disinfectant solution.
2. Wipe off the disinfectant solution with a dry cloth after disinfection.
3. Leave the patient cable to air dry for at least 30 minutes.
- 33 -
Page 39

PADECG User Manual Cleaning, Care and Maintenance
6.3.3 Disinfecting the Reusable Electrodes
1. Wipe the suction bulbs of chest electrodes and the clamps of limb electrodes with a soft cloth
dampened with the disinfectant solution.
2. Wipe off the disinfectant solution with a dry cloth after disinfection.
3. Leave the suction bulbs and clamps to air dry for at least 30 minutes.
6.4 Care and Maintenance
CAUTION
Besides the maintenance requirements recommended in this manual, comply with local
regulations on maintenance and measurement.
The following safety checks should be performed at least every 12 months by a qualified
person who has adequate training, knowledge, and practical experience to perform these
tests.
a) Inspect the equipment and accessories for mechanical and functional damage.
b) Inspect the safety related labels for legibility.
c) Verify that the device functions properly as described in the instructions for use.
d) Test the enclosure leakage current according to IEC/EN 60601-1: Limit: NC 100
μA, SFC 500 μA.
e) Test the patient leakage current according to IEC/EN 60601-1: Limit: NC a.c. 10
μA, d.c. 10 μA; SFC a.c. 50 μA, d.c. 50 μA.
f) Test the patient auxiliary current according to IEC/EN 60601-1: Limit: NC a.c. 10
μA, d.c. 10 μA; SFC a.c. 50 μA, d.c. 50 μA.
g) Test the patient leakage current under single fault condition with mains voltage
on the applied part according to IEC/EN 60601-1: Limit: 50 μA (CF).
h) Test the essential performance according to IEC/EN 60601-2-25, or methods
recommended by the hospital or local distributor.
The data should be recorded in an equipment log. If the equipment is not functioning
properly or fails any of the above tests, the equipment has to be repaired.
WARNING
Failure on the part of the responsible individual hospital or institution employing this
equipment to implement a satisfactory maintenance schedule may cause undue
equipment failures and possible health hazards.
- 34 -
Page 40

PADECG User Manual Cleaning, Care and Maintenance
1) iOS Tablet and DX12(iOS) Transmitter
♦ Avoid excessive temperature, sunshine, humidity and dirt.
♦ Put the dustproof coat on the DX12(iOS) Transmitter after use and prevent shaking it
violently when moving it to another place.
♦ Prevent any liquid from seeping into the equipment; otherwise the safety and the
performance of the ECG workstation cannot be guaranteed.
2) Patient Cable
♦ Integrity of the patient cable, including the main cable and lead wires, should be
checked regularly. Make sure that it is conductible.
♦ Do not drag or twist the patient cable with excessive stress while using it. Hold the
connector plug instead of the cable when connecting or disconnecting the patient cable.
♦ Align the patient cable to avoid twisting, knotting or crooking in a closed angle while
using it.
♦ Store the lead wires in a big wheel to prevent any people from stumbling.
♦ Once damage or aging of the patient cable is found, replace it with a new one
immediately.
3) Reusable Electrodes
♦ Electrodes must be cleansed after use and make sure there is no remainder gel on them.
♦ Keep suction bulbs of chest electrodes away from sunshine and excessive temperature.
♦ After long-term use, the surfaces of electrodes will be oxidized because of erosion and
other causes. By this time, electrodes should be replaced to achieve high-quality ECG
records.
CAUTION
The device and accessories are to be disposed of according to local regulations after
their useful lives. Alternatively, they can be returned to the dealer or the manufacturer for
recycling or proper disposal.
- 35 -
Page 41

PADECG User Manual Accessories
Accessory
Part Number
DX12 Patient Cable (IEC)
01.57.471030
DX12 Patient Cable (AHA)
01.57.471055
Patient Cable (IEC)
01.57.471278
Patient Cable (AHA)
01.57.471279
Chest electrodes
01.57.040163
Limb electrodes
01.57.040162
Excell Alkaline AA LR6 1.5V
01.21.064086
Portable Bag
01.56.465623
Adult Disposable Adhesive Electrodes
01.57.471056
Pediatric Disposable Adhesive Electrodes
01.57.471057
Clip/Snap/Banana Socket Adapter
01.57.040172
Alligator Clip/Banana Socket Adapters
01.57.040173
Snap/Banana Socket Adapters
01.13.107449
Disposable Resting Tab electrodes
01.57.471031
Chapter 7 Accessories
WARNING
Only the patient cable and other accessories supplied by the manufacturer can be used.
Or else, the performance and electric shock protection cannot be guaranteed.
Table 7-1 Accessory List
NOTE:
1. The chest electrodes and limb electrodes are not available in the U.S.
2. The part name may vary depending on context, but the part number is constant.
- 36 -
Page 42

PADECG User Manual Warranty and Service
Chapter 8 Warranty and Service
8.1 Warranty
EDAN warrants that EDAN’s products meet the labeled specifications of the products and will be
free from defects in materials and workmanship that occur within warranty period.
The warranty is void in cases of:
a) Damage caused by mishandling during shipping.
b) Subsequent damage caused by improper use or maintenance.
c) Damage caused by alteration or repair by anyone not authorized by EDAN.
d) Damage caused by accidents.
e) Replacement or removal of serial number label and manufacture label.
If a product covered by this warranty is determined to be defective because of defective materials,
components, or workmanship, and the warranty claim is made within the warranty period, EDAN
will, at its discretion, repair or replace the defective part(s) free of charge. EDAN will not provide
a substitute product for use when the defective product is being repaired.
8.2 Contact information
If you have any question about maintenance, technical specifications or malfunctions of devices,
contact your local distributor.
Alternatively, you can send an email to EDAN service department at: support@edan.com.cn.
- 37 -
Page 43

PADECG User Manual Technical Specifications
Comply with:
IEC 60601-1:2005/A1:2012
EN 60601-1:2006/A1:2013
IEC 60601-1-2:2007
EN 60601-1-2:2007/AC:2010
IEC 60601-2-25:2011
Anti-electric-shock type:
Internally powered equipment
Anti-electric-shock degree:
Type CF with defibrillation-proof
Degree of protection against
harmful ingress of water:
Ordinary equipment (Sealed equipment without liquid
proof)
Degree of protection against
falling:
Handheld device
Disinfection/sterilization method:
Refer to the user manual for details
Degree of safety of application in
the presence of flammable gas:
Equipment not suitable for use in the presence of
flammable gas
Working mode:
Continuous operation
EMC:
CISPR 11, Group 1, Class A
Patient Leakage
Current:
NC
<10μA (AC) / <10μA (DC)
SFC
<50μA (AC) / <50μA (DC)
Patient Auxiliary
Current:
NC
<10μA (AC) / <10μA (DC)
SFC
<50μA (AC) / <50μA (DC)
Transport & Storage
Working
Temperature:
-20ºC (-4ºF) ~ +55ºC (+131ºF)
+5ºC (+41ºF) ~ +40ºC (+104ºF)
Appendix 1 Technical Specifications
A1.1 Safety Specifications
A1.2 Environment Specifications
- 38 -
Page 44
PADECG User Manual Technical Specifications
Relative Humidity:
15%RH ~ 95%RH
Non-Condensing
15%RH ~ 95%RH
Non-Condensing
Atmospheric Pressure:
70 kPa ~ 106 kPa
70 kPa ~ 106 kPa
Dimensions
DX12(iOS) Transmitter:
63mm(L)×107mm(W) ×23mm(H) (2.5in×4.2in×0.9in)
Weight
DX12(iOS) Transmitter:
Approx. 113g (not including battery)
Power Supply:
DX12(iOS) Transmitter:
Input Power: 2×1.5V Excell Alkaline AA IEC LR6;
Operation life of battery≥12 hours
HR Recognition
HR Range:
30 bpm ~300 bpm
Accuracy:
1 bpm
Filter
(PADECG Analysis Software)
AC Filter: 50Hz/60Hz/Off
DFT Filter: 0.32Hz (weak) /0.67Hz (strong) /0.05Hz
EMG Filter: 25Hz/35Hz/45Hz/Off
Lowpass Filter: 150Hz/100Hz/75Hz/Off
DX12(iOS) Transmitter Performance
Leads Mode:
12 standard leads
Acquisition Mode:
Simultaneously 12 leads
A/D:
18 bits
Resolution:
2.52uV/LSB
Sample Frequency:
10,000 /sec/channel (sampling)
500 /sec/channel (analysis)
A1.3 Physical Specifications
A1.4 Power Supply Specifications
A1.5 Performance Specifications
- 39 -
Page 45

PADECG User Manual Technical Specifications
Time Constant:
≥3.2 s
Frequency Response:
0.05Hz ~ 150Hz
Gain:
2.5, 5, 10, 20 (mm/mV) (±5%)
Speed:
5mm/s, 12.5mm/s, 25mm/s or 50mm/s
Input Impedance:
≥20MΩ (10Hz)
Input Circuit Current:
≤0.05μA
Input Voltage Range
<±5mVp-p
Calibration Voltage:
1mV±2%
DC Offset Voltage:
±5 00mV
Minimum Amplitude:
20 μVp-p
Noise:
≤15μVp-p
Recovery Time After Defibrillation
<5 s
CMRR
≥100 dB
Pacemaker Detection (Single Channel Detection)
Amplitude
±2 mV to ± 500 mV
Width
0.1 ms to 2.0 ms
Sampling Frequency
10,000Hz, rhythm lead
DX12(iOS) Transmitter Bluetooth
Transmitting Frequency
2402 Hz ~ 2480Hz
Frequency Band
2402 Hz ~ 2480Hz
Modulation Type
FHSS, GFSK, DPSK, DQPSK
Transmitting Power
≥ 0 dBm
NOTE: Operation of the equipment below the minimum amplitude may cause inaccurate
results.
- 40 -
Page 46

PADECG User Manual EMC Information
Guidance and manufacture’s declaration - electromagnetic emission
The PADECG is intended for use in the electromagnetic environment specified below. The
customer or the user of the PADECG should assure that it is used in such an environment.
Emission test
Compliance
Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The PADECG uses RF energy only for its
internal function. Therefore, its RF emissions
are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emission
CISPR 11
Class A
The PADECG is suitable for use in all
establishments, other than domestic and those
directly connected to the public low-voltage
power supply network that supplies buildings
used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not applicable
Guidance and manufacture’s declaration - electromagnetic immunity
The PADECG is intended for use in the electromagnetic environment specified below. The
customer or the user of the PADECG should assure that it is used in such an environment.
Immunity test
IEC 60601 test level
Compliance level
Electromagnetic
environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
8 kV contact
15 kV air
8 kV contact
(only for
PADECG)
15 kV air (only
for PADECG)
Floors should be wood,
concrete or ceramic tile. If
floor are covered with
synthetic material, the
relative humidity should be
at least 30%.
Appendix 2 EMC Information
Electromagnetic Emissions
Electromagnetic Immunity
- 41 -
Page 47

PADECG User Manual EMC Information
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for power
supply lines
1kV for input/output
lines
Not applicable
Not applicable
Surge
IEC 61000-4-5
1 kV line to line
2 kV line to ground
Not applicable
Not applicable
Power frequency
(50Hz/60Hz)
magnetic field
IEC 61000-4-8
30A/m
30A/m
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
0 % UT; 0,5 cycle
At 0°, 45°, 90°, 135°,
180°, 225°, 270° and
315°
0 % UT; 1 cycle
and
70 % UT; 25/30
cycles )
Single phase: at 0°
0 % UT; 250/300
cycle
Not applicable
Not applicable
NOTE UT is the a.c. mains voltage prior to application of the test level.
Guidance and manufacture’s declaration - electromagnetic immunity
The PADECG is intended for use in the electromagnetic environment specified below. The
customer or the user of the PADECG should assure that it is used in such an environment.
Immunity
test
IEC 60601 test level
Compliance
level
Electromagnetic environment guidance
Portable and mobile RF
communications equipment should be
used no closer to any part of the
Electromagnetic Immunity
- 42 -
Page 48
PADECG User Manual EMC Information
PADECG, including cables, than the
recommended separation distance
calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance
Conducted RF
IEC61000-4-6
3 V
rms
150 kHz to 80 MHz
6Vrmsc) in ISM
bands between
0,15 MHz and 80
MHz
3 V
rms
150 kHz to
80 MHz
6Vrmsc) in
ISM bands
between
0,15 MHz
and 80 MHz
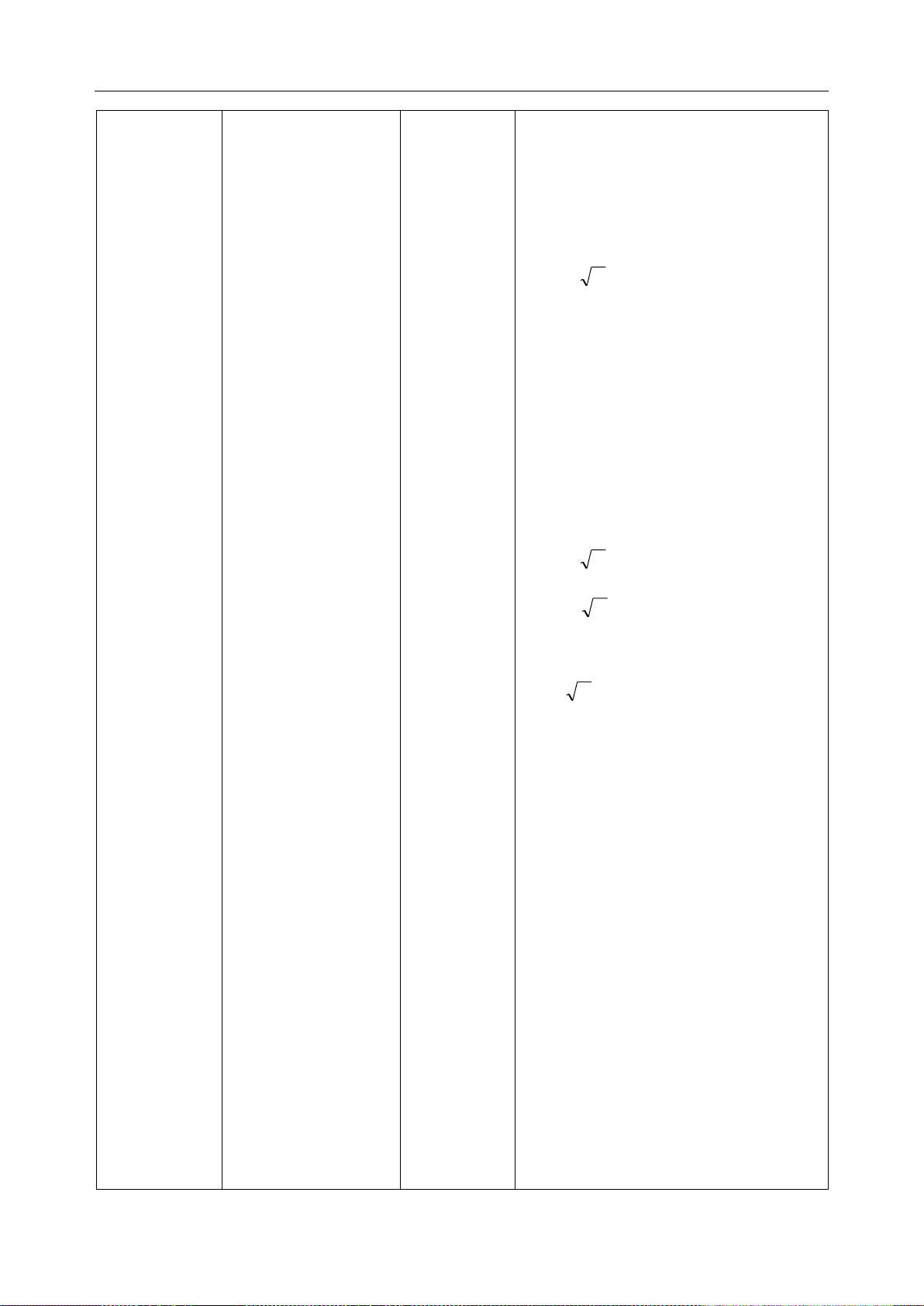
Pd 2.1
Radiated RF
IEC61000-4-3
3 V/m
80 MHz to 2.7 GHz
3 V/m
80 MHz to
2.7 GHz
Pd 2.1
80 MHz to 800 MHz
Pd 3.2
800 MHz to 2.5 GHz
See Table 1
See Table 1
E/6 Pd
at RF wireless
communications equipment bands
(Portable RF communications
equipment (including peripherals such
as antenna cables and external
antennas) should be used no closer than
30 cm (12 inches) to any part of the
PADECG, including cables specified
by the manufacturer).
Where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter
manufacturer and d is the
recommended separation distance in
meters (m).
Field strengths from fixed RF
transmitters, as determined by an
electromagnetic site survey,a should be
less than the compliance level in each
- 43 -
Page 49

PADECG User Manual EMC Information
frequency range.b
Interference may occur in the vicinity
of equipment marked with the
following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the PADECG is used
exceeds the applicable RF compliance level above, the PADECG should be observed to
verify normal operation. If abnormal performance is observed, additional measures may
be necessary, such as reorienting or relocating the PADECG.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
c The ISM (industrial, scientific and medical) bands between 0,15 MHz and 80 MHz are
6,765 MHz to 6,795 MHz; 13,553 MHz to 13,567 MHz; 26,957 MHz to 27,283 MHz;
and 40,66 MHz to 40,70 MHz. The amateur radio bands between 0,15 MHz and 80 MHz
are 1,8 MHz to 2,0 MHz, 3,5 MHz to 4,0 MHz, 5,3 MHz to 5,4 MHz, 7 MHz to 7,3
MHz, 10,1 MHz to 10,15 MHz, 14 MHz to 14,2 MHz, 18,07 MHz to 18,17 MHz, 21,0
MHz to 21,4 MHz, 24,89 MHz to 24,99 MHz, 28,0 MHz to 29,7 MHz and 50,0 MHz to
54,0 MHz.
Test
frequency
(MHz)
Band a)
(MHz)
Service a)
Modulation b)
Maximum
power
(W)
Distance
(m)
Immunity
test level
385
380-390
TETRA
400
Pulse
modulation b) 18
Hz
1,8
0,3
27
450
430-470
GMRS
460, FRS
460
FM c) ± 5 kHz
deviation
1 kHz sine
2
0,3
28
Table 1 Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless
communications equipment
- 44 -
Page 50

PADECG User Manual EMC Information
710
704-787
LTE Band 13,
17
Pulse
modulation b)
217 Hz
0,2
0,3
9
745
780
810
800-960
GSM
800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation b)
18 Hz
2
0,3
28
870
930
1720
1700-1990
GSM 1800;
CDMA 1900;
GSM 1900;
DECT; LTE
Band 1, 3, 4,
25; UMTS
Pulse
modulation b)
217 Hz
2
0,3
28
1845
1970
2450
2400-2570
Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse
modulation b)
217 Hz
2
0,3
28
5240
5100-5800
WLAN
802.11 a/n
Pulse
modulation b)
217 Hz
0,2
0,3
9
5500
5785
NOTE If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the
transmitting antenna and the ME EQUIPMENT or ME SYSTEM may be reduced to 1 m.
The 1 m test distance is permitted by IEC 61000-4-3.
a) For some services, only the uplink frequencies are included.
b) The carrier shall be modulated using a 50 % duty cycle square wave signal.
c) As an alternative to FM modulation, 50 % pulse modulation at 18 Hz may be used because
while it does not represent actual modulation, it would be worst case.
- 45 -
Page 51

PADECG User Manual EMC Information
Recommended separation distances between
portable and mobile RF communications equipment and the PADECG
The PADECG is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the PADECG can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile
RF communications equipment (transmitters) and the PADECG as recommended below,
according to the maximum output power of the communications equipment.
Rated
maximum
output power of
transmitter
(W)
Separation distance according to frequency of transmitter (m)
150 kHz to 80 MHz
Pd 2.1
80 MHz to 800 MHz
Pd 2.1
800 MHz to 2.7 GHz
Pd 3.2
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in metres (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
Recommended separation distances between portable and mobile
RF communications equipment and the EQUIPMENT or SYSTEM
- 46 -
Page 52

PADECG User Manual Abbreviation
Abbreviation
Statement
ECG
Electrocardiograph/Electrocardiogram
BP
Blood Pressure
HR
Heart Rate
P
P-wave Duration
PR
P-R Interval
QRS
QRS Complexes Duration
QT/QTc
Q-T Interval of the Current Lead / Normalized QT Interval
P/QRS/T
Dominant Direction of the Average Integrated ECG Vectors
aVF
Left Foot Augmented Lead
aVL
Left Arm Augmented Lead
aVR
Right Arm Augmented Lead
LA
Left Arm
LL
Left Leg
RA
Right Arm
RL
Right Leg
ID
Identification
AC
Alternating Current
USB
Universal Serial Bus
NC
Normal Condition
SFC
Single Fault Condition
Appendix 3 Abbreviation
- 47 -
Page 53

 Loading...
Loading...