
X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 1/7
INSTRUCTIONS FOR
USE
ECONOX
USA
600 Mogadore Road
Kent, OH 44240
T: +1 330 678-3889
www.econox.us
info@econox.us
The details in this document are provided for information purposes only. This manual may not, in any
event, be reproduced, dissociated or distributed to third parties without the consent of ECONOX SA.

X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 2/7
TABLE OF CONTENT
1.Operational principles ..................................................................... 3
Introduction ........................................................................... 4
Applications ............................................................................ 4
How does the air supply affect the firing? .................................... 5
The benefits of an oxygen probe ................................................ 5
How to install the oxygen probe ................................................. 5
Understanding an oxygen probe ................................................. 6
2.Checking temperature using the CarboProbe CP plus .......................... 6
3.Repairing the sensor ....................................................................... 7

X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 3/7
1. Operational principles
The purpose of ECONOX CarboProbe oxygen sensors is to measure and regulate
atmospheres. ECONOX uses two different types of electrolyte made of ZrO2 (zirconium
oxide) for its oxygen sensors:
1. A ball made of ZrO2, an ECONOX-patented system, which may only be obtained from
ECONOX. The ball is used in the CarboProbe ZI pro sensor.
2. A C-700 ZrO2 electrolyte.
This is used in the CarboProbe ZS, HT, CP and LTsensors.
An oxygen probe works by comparing the oxygen level in the kiln with the oxygen level
inside the alumina tube. This is why the air inside the tube must always be renewed. If the
temperature of the zirconia tip is over about 700 °C, it produces an electrical voltage. The
less oxygen in the kiln, the bigger the electrical voltage, so the voltage can be used as a
guide to the oxygen level.
There is no safety hazard - the maximum a probe can produce is less than a
battery!
X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 4/7
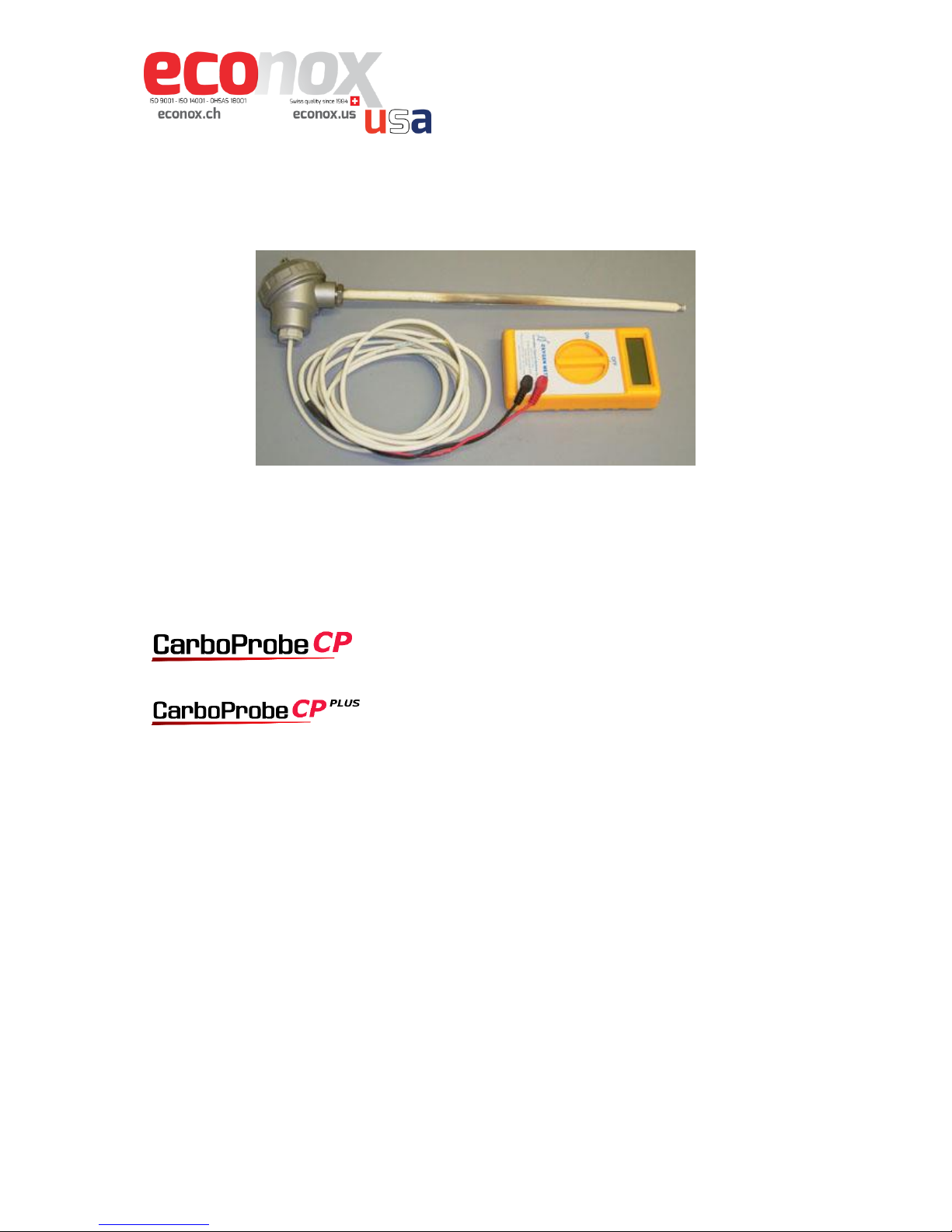
Introduction
The CP probe is a very simple, low cost oxygen probe with an easy-to-read digital meter
displaying oxidation/reduction. The CP Probe saves you money by omitting the
thermocouple pyrometer, so that you can continue to use cones. This probe is ideal to
control both gas and wood-fired kilns.
An oxygen probe works by comparing the oxygen level in the kiln with the oxygen level
inside the alumina tube. This is why the air inside the tube must always be renewed. If the
temperature of the zirconia tip is over about 700 °C, it produces an electrical voltage. The
less oxygen in the kiln, the bigger the electrical voltage, so the voltage can be used as a
guide to the oxygen level.
ECONOX supplies 2 different kinds of CP probes
For the measure of oxygen concentration only
For measure of oxygen concentration AND temperature
(includes an R-type thermocouple for temperature up
to 1700°C)
Applications
The advantages of measurement of oxidation/reduction include:
Fuel savings - an economical firing depends on supplying the right ratio air/fuel,
without waste of energy from heating excess air.
Reliable glaze colors - Knowing the right level of reduction every firing gives
you the colors you want, firing after firing.
Reduced air pollution - Use the CP Probe as a guide for stoking wood-fired kilns
to reduce wood consumption and unnecessary ash and smoke.

X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 5/7
How does the air supply affect the firing?
The right amount of air
will give the hottest flame and the fastest temperature rise. This is the most economical
heating.
Too much air
makes the flame cooler. The flow of excess air can help distribute the heat more
uniformly.
Not enough air
can still give a hot flame and can give interesting special effects in glaze colors.
The benefits of an oxygen probe
Efficient fuel use
To reach the highest temperature with the least fuel, use a neutral flame. The oxygen
probe will tell you if you have a good balance of fuel and air, because the reading will be
in the range of 100mV to 200mV.
Glaze control
Some glaze colors are affected by how heavily reducing the flame is. Sometimes there is
a difference in color between a slightly reducing flame with an oxygen reading of 0.35
and a heavy reduction of 0.6. In a reducing flame, the oxygen reading is usually much
more stable and it is easy to measure the degree of reduction quite accurately. The
potter can record the reduction conditions, then obtain the same glaze colors on later
burns.
How to install the oxygen probe
Fit the probe anywhere in the kiln or furnace where a pyrometer could be fitted. If the
probe is used at temperatures over 1100 degrees C / 2000 degrees F, put it through the
top, so the ceramic tube hangs vertically. If the probe is used horizontally at high
temperatures, it will gradually sag.
Seal the hole for the probe well enough to prevent air from flowing inwards and affecting
the oxygen reading.
When fitting or removing a probe into or out of a hot furnace, move the probe slowly to
prevent thermal shock.
Once the kiln is over 700 °C and the reference air is available, the probe is ready to use.

X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 6/7
Understanding an oxygen probe
Roughly speaking, a reading less than 0.1 represents oxidizing conditions and a reading
over 0.3 represents reduction. Heavy reduction might give a reading of 0.5 or even
more. In between 0.1 and 0.3 the temperature must be known for accurate
interpretation.
In any flame, the air and fuel never mix perfectly. As burning fuel blows past the tip of
the oxygen probe, some of the flame will have excess air and some will be gas rich. This
means that the oxygen reading will jump around as the flame flickers past. This is most
noticeable when there is just the right amount of air to give a neutral flame.
Chart of oxygen concentration from probe reading
mV
700°C
800°C
900°C
1000°C
1100°C
1200°C
1300°C
50
1.9 %
2.4 %
2.9 %
3.4 %
3.9 %
4.3 %
4.8 %
100
0.2 %
0.3 %
0.4 %
0.5 %
0.7 %
0.9 %
1.1 %
150
0.02 %
0.03 %
0.06 %
0.09 %
0.13 %
0.18 %
0.25 %
200
0.002 %
0.004 %
0.01 %
0.01 %
0.02 %
0.04 %
0.06 %
250
0.001 %
0.0004 %
0.001 %
0.002 %
0.004 %
0.01 %
0.01 %
300
0.00001%
0.00005%
0.0001%
0.0004%
0.001 %
0.002 %
0.003 %
Red= OXIDISING Green= NEUTRAL Blue= REDUCING
Air consists of 20.9% oxygen, about 78% nitrogen and some trace gases. In a flame, the
fuel combines with the oxygen in the air and burns, forming carbon dioxide and water
vapor (steam). Inside a kiln, there is a mixture of fuel, oxygen, carbon dioxide, steam
and nitrogen. The amount of each of these depends on the amount of fuel and air in the
flame.
Oxidizing flame
With excess air, there is typically over 2% oxygen in the exhaust gas, but it can be
almost up to the limit of 20.9%. This is called an "oxidizing" flame.
Neutral flame
With exactly the right amount of air for the fuel, there is a "neutral" flame. Even in ideal
conditions, there will be some fuel and some air that cannot find each other to burn
completely. A little unused fuel and air will be in the exhaust gas leaving the kiln. There
is typically anything from 0.02% to 2% unused oxygen in the exhaust.
Reducing flame
With too little air, there will be unburnt fuel in the exhaust gas. This is called a "reducing"
flame. Many people say that there is no oxygen under these conditions, but there will
always be some unused oxygen in the exhaust. It might be less than 0.02%. It might be
less than 0.000001%, but it is there and it can be measured.
There is no sharp distinction between oxidizing, neutral and reducing. There is a smooth
variation from one to the next, so the above figures are only guidelines.
Checking temperature using the CarboProbe CP plus
When the probe is ready to use, connect the probe to the meter. Select the "temperature"
position. The meter will display the thermocouple signal from the thermocouple in mV.

X:\Fiches tech. - Normalisation\ECONOX\Normalisation\Mode d'emploi\PDF\sonde 7/7
There is no cold junction ambient temperature compensation in the meter, so the reading
must be corrected for room temperature. This can be approximated by measuring room
temperature separately then adding the thermocouple voltage corresponding to the room
temperature.
Example, for an R-type thermocouple:
Ambient temperature is 25°C and meter indicates probe thermocouple signal 8.5 mV
Thermocouple chart (see section 4) shows 25°C corresponds to 0.14 mV
8.5 mV + 0.14 mV = 8.64 mV, which corresponds to 856°C from chart
Hence probe temperature = 856°C
Thermocouple test
Prolonged exposure (hundreds of hours) to high temperature (over 1500°C) causes
evaporation of platinum. The thermocouple can be tested for open circuit or thinning that
may lead to failure.
Swap the positions of the yellow and green wires in the meter. Select "TEST" position. The
reading should be less than 0.005. A reading of 1 indicates that the thermocouple is either
broken or may soon do so. This test should be done at room temperature.
3. Repairing the sensor
CarboProbe sensors are highly technical measuring instruments subjected to potentially
difficult work conditions. The lifespan of a given sensor depends, to a large extent, on the
conditions in which it is used.
When sending a sensor for repair, pack it carefully in its original packaging, mark it "Fragile
Instrument", and return it to:
ECONOX USA
600 Mogadore Road
Kent, Ohio 44240
T: +1 330-678-3889
www.econox.us
info@econox.us
 Loading...
Loading...