
PhysioTel®Digital Device
Surgical Manual
Surgical Implantation of the PhysioTel®Digital Blood
Pressure and Biopotential Telemetry Devices
Doc-To-Help Standard Template Error! No text of specified style in document. 1

Acknowledgments
Vetbond® and Bair Hugger® are registered trademarks of 3M.
PhysioTel®Digital Device Surgical Manual
Copyright 2012 Data Sciences International
All Rights Reserved
Printed in U.S.A.
Part Number
Rev. 01
Data Sciences International (DSI)
119 14th Street NW ● Suite 100 ● St. Paul, MN 55112
Telephone: (1-651) 481-7400 ● 1-800 262-9687
Doc-To-Help Standard Template Error! No text of specified style in document. 2
Fax: (1-651) 481-7417
Website: www.datasci.com

Introduction
PhysioTel®Digital telemetry devices are surgically implanted into large laboratory animals
to acquire multiple types of physiologic measurements, process the information and
transmit the data via radio-frequency signals. The PhysioTel®Digital device can measure
pressure (such as systemic blood pressure or intra-ventricular pressure), a biopotential
(such as ECG) temperature and physical activity. This manual contains detailed procedures
for implantation of the TS-L11 and TS-L21 PhysioTel®Digital telemetry devices. The
techniques described are designed for large laboratory animals including dogs, primates
and swine but may be applicable to other, similar sized animals.
The PhysioTel®Digital Device Surgical Manual is intended for use by laboratory personnel
who will perform or assist in surgical procedures to implant PhysioTel®Digital devices. The
surgical procedures written in this manual are at a level of detail appropriate for persons
who have previous experience with surgical procedures. These devices should only be
implanted by a person who has previous surgical experience.
WARNING: The PhysioTel®Digital implantable device is not intended for use in humans. It
is a misuse of this device, and a possible violation of law, to use these devices in humans.
This Manual Contains the Following Sections:
Required Supplies for the TS-L11 and TS-L21 Surgery
Anesthesia and Analgesia Guidelines
Peri-operative Antibiotics
Device Description
Surgical Preparation
Device Handling
Device Placement
o Intra-abdominal placement
Intraperitoneal
Subperitoneal
o Extra-abdominal placement
Intramuscular
Subcutaneous
Systemic Blood Pressure Catheter Placement
o Mesenteric Artery
o Medial Saphenous/Femoral Artery
o Iliac Artery
o Thoracic Aorta
Left Ventricular Pressure Catheter Placement
Doc-To-Help Standard Template Error! No text of specified style in document. 3
o Trans-diaphragmatic Approach
o Intercostal Thoracotomy Approach
Solid Tip ECG Lead Placement
o Internal Jugular Vein
o External Jugular Vein
Traditional ECG Lead Placement
o Lead II
o Base-Apex
Appendix A: Additional Device Information
Appendix B: Functional Specifications
Appendix C: Care and Use
Appendix D: Equipment and Supplies
Appendix E: Checking the Offset of a Pressure Device
Doc-To-Help Standard Template Error! No text of specified style in document. 4
Required Supplies for the PhysioTel®Digital Surgery
EQUIPMENT
Clippers
Supplemental heating
PhysioTel®Digital device
Ponemah 5.1 data collection system
Mechanical ventilator
INSTRUMENTS
Details contained in Appendix D
SUPPLIES
Surgical scrub (Chlorhexidine or Providine-Iodine scrub)
Sterile drapes
Sterile gloves, hair bonnet and face mask
Sterile surgical gown
Sterile gauze sponges-4 inches x 4 inches (10 cm x 10 cm)
Sterile saline
Sterile basin
2% Lidocaine
Elastic vessel loops
2-0 * to 4-0 (Smaller or larger suture may be needed depending on size and species
used) non-absorbable, non swaged suture[MES1]
2-0 to 4-0 (Smaller or larger suture may be needed depending on size and species
used) non-absorbable suture swaged on a tapered needle[MES2]
2-0 or 4-0 (Smaller suture may be needed depending on size and species used)
absorbable suture material swaged on a tapered needle[MES3]
2-0 to 4-0 (Smaller suture may be needed depending on size and species used)
absorbable surgical suture swaged on a cutting needle[MES4]
14-gauge hypodermic needle *
20-gauge hypodermic needle *
Catheter introducer (i.e. vein pick)*
Doc-To-Help Standard Template Error! No text of specified style in document. 5

Magnet *
Re-gel syringe
Vetbond®Tissue adhesive (if placing systemic blood pressure catheter in iliac artery)
Gel loading micropipette tip or insulin syringe (if placing systemic blood pressure
catheter in iliac artery)
* Contained in starter kit
Doc-To-Help Standard Template Error! No text of specified style in document. 6
Anesthesia and Analgesia Guidelines
Proper peri-operative pain control and anesthesia are critical to humane treatment of
laboratory animals. Each institution’s staff veterinarian should be contacted for proper
analgesic and anesthetic protocols and training before survival surgery is attempted.
The use of pre- and post-surgical analgesics is strongly encouraged for all surgical
manipulations performed on laboratory animals. “The proper use of anesthetics and
analgesics in research animals is an ethical imperative…The selection of the most
appropriate analgesic or anesthetic should reflect professional judgment as to which best
meets clinical and humane requirements without compromising the scientific aspects of the
research protocol.”
staff veterinarian.
Typically, the surgical procedure for the TS-L11 will require 60 minutes of surgical
anesthesia, and the surgical procedure for the TS-L21 device will require approximately
120 minutes of surgical anesthesia. Intermittent positive pressure mechanical ventilation is
required any time the thoracic cavity is opened, such as during placement of a left
ventricular pressure catheter. Appropriate use of this technique is essential, and should be
directed by the staff veterinarian. The surgical procedures described in this manual were
developed using inhalational anesthesia consisting of Isoflurane delivered in 100% Oxygen.
These recommendations are intended as a guide only and should be modified to the
individual animal and institution’s protocol.
1
Questions regarding the use of analgesics should be directed to your
Anesthetized animals are predisposed to hypothermia. The use of supplemental heat
sources such as warm water re-circulating heating pads or Bair Huggers® are important to
maintain baseline body temperature. Hypothermia will prolong the recovery period and may
result in animal loss.
For additional help in determining an appropriate anesthetic protocol, the staff veterinarian
should be contacted. DSI has also prepared an Anesthesia Reference Manual as a guide
to assist in choosing an appropriate anesthetic agent for a wide variety of common
laboratory species.
1
Guide for the Care and Use of Laboratory Animals, NRC, National Academy Press, 1996 [MES5]
Doc-To-Help Standard Template Error! No text of specified style in document. 7
Peri-operative Antibiotics and Antiarrhythmic
Medications
The use of antibiotics may be elected at the discretion of the investigator. The combination
of sterile device packaging and proper aseptic technique help increase the potential for
successful surgical outcomes. Investigators should follow the guidelines of their own
institution. Questions regarding the use of antibiotics should be directed to the institution’s
staff veterinarian.
Due to the manipulation of the heart, there is a potential to induce an arrhythmia, and the
anesthetist may wish to be prepared to deliver antiarrhythmic agents as appropriate. The
choice and dose of agents should be determined through consultation with the institution’s
veterinarian.
Device Description
It is important that you are familiar with the device and its function before you attempt
implantation (see Figure 1).
Figure 1. PhysioTel®Digital Device[MES6]
The PhysioTel®Digital device measures pressure, a biopotential signal, temperature, and
physical activity in primates, dogs and swine and is a rectangular shaped device.
The devices consist of the following major components:
Device Body - The titanium housing containing:
Pressure sensor: receives pressure fluctuations from the fluid-filled catheter
and sends the signals to the electronics module.
Reusable electronics module: translates the pressure fluctuations,
biopotential signal and temperature into digitized signals and transmits them
to a receiver. It also interprets signals received from the laboratory software
and contains a magnetically activated switch that allows the device to be
switched on or off.
Battery: provides the power supply for the electronics module.
Suture aid: allows the surgeon to suture the device securely in place at the
implant site.
Pressure Catheter(s) - Polyurethane tubing that extends (25, 35 or 40 cm) out of the
device body and contains:
Non-compressible fluid: relays pressure fluctuations to the sensor in the device
body.
Doc-To-Help Standard Template Error! No text of specified style in document. 8
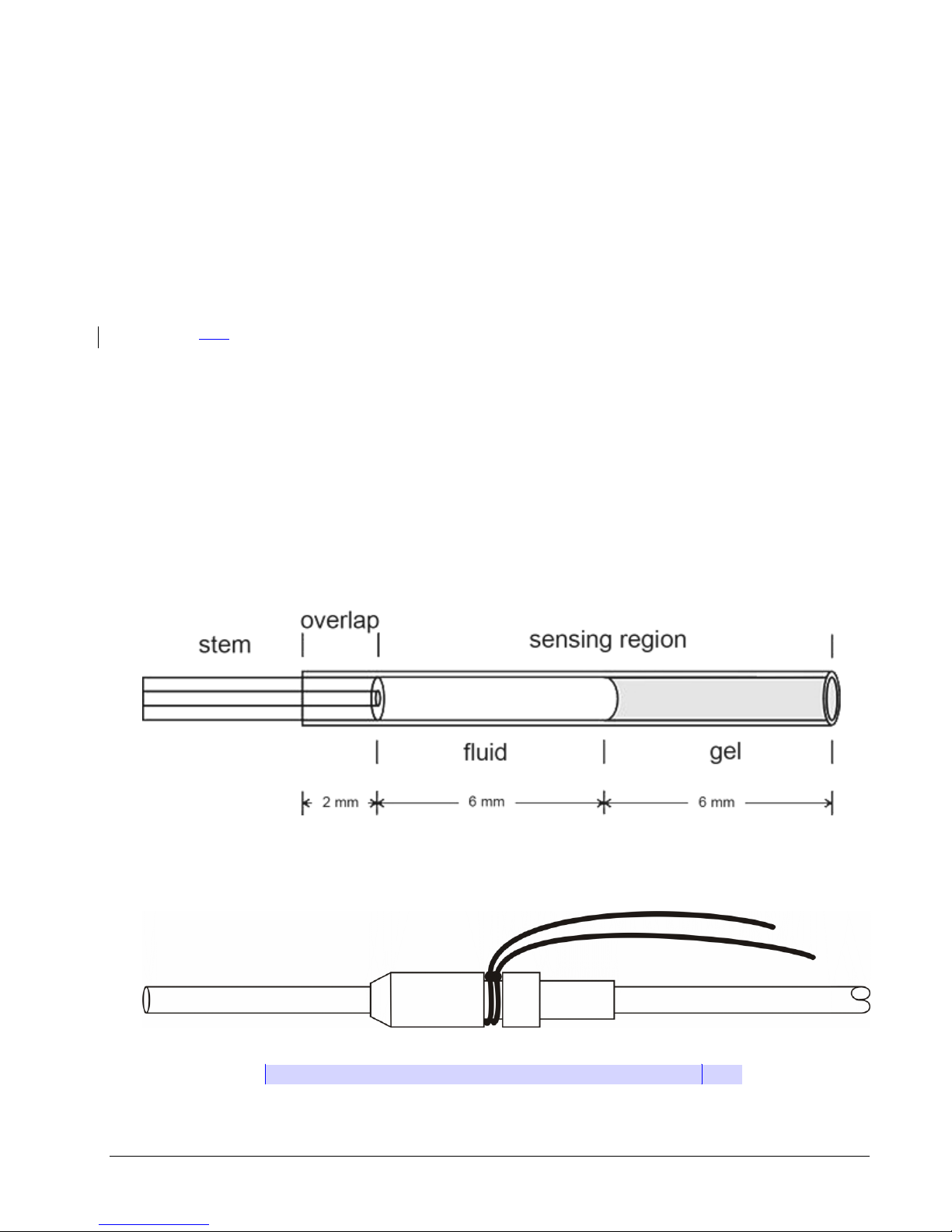
Thin-walled section: tip of the catheter farthest from the device body that senses the
dynamic portion of the pressure wave. It is designed to be completely inserted into
the vessel or space where the desired pressure can be sensed. It contains
biocompatible gel at the very tip, which prevents the non-compressible fluid from
leaving the catheter and blood from clotting in the catheter tip (see Figure2).
Tip cover: removable section of silicone tubing that protects the catheter tip until it is
actually inserted into the desired vessel. Must be removed prior to catheter insertion.
Systemic blood pressure catheter: containing a radio-opaque ring encircling the
distal end of the systemic blood pressure catheter (This is the channel 2 catheter on
the TS-L21 PhysioTel®Digital Device.)
Left ventricular pressure catheter: containing a plastic suture collar near the tip, with
only the thin-walled section protruding beyond. The white suture collar will be
inserted until the suture groove is flush with the heart wall (see Figure 3).
It is important to be familiar with the catheter and its features. See the figure below for a
detailed diagram of each catheter.
Figure 2. The PhysioTel®Digital catheter
Figure 3. The Left Ventricular Catheter Tip With Collar[MES7]
Doc-To-Help Standard Template Error! No text of specified style in document. 9

Biopotential Leads - Two silicon coated helices of medical grade stainless steel wire
extending out of the device body. The positive (red) lead is designed to be cut to a length
suitable for the biopotential signal to be monitored. The negative (clear) lead has a solid tip
and is NOT meant to be cut (unless you require traditional lead placement). It is designed
to be introduced into the right jugular vein and fed into the cranial vena cava to collect the
ECG signal.
Silicone tubing: provides insulation from external electrical activity.
Solid tip on the negative lead: senses ECG signal within the vena cava, near the
base of the heart.
Doc-To-Help Standard Template Error! No text of specified style in document. 10

Figure 4. [MES8]Solid Tip Biopotential Lead
Device Handling
ALWAYS handle the device with care supporting both the device body
and catheters from underneath when moving or placing the device.
Allowing the catheters to hit a solid surface can damage the pressure
sensor.
Before removing the device from its sterile package
Turn the device to the ON mode with a magnet and audibly verify proper device operation
with a DSI receiver.
1. Record the serial number of the device and ensure that the device has been identified
with the animal into which it will be implanted.
2. Measure and record the pressure offset. Refer to Appendix E for further information on
this process.
To Hydrate the Catheter
1. Open the sterile package by peeling back the white package cover from the clear plastic
tray. Do not discard the white package cover as it contains important device calibration
information. Also doDo not discard the sterile package as it can be used for eventual
return of the device to DSI.
2. Place the device and catheter into a sterile basin with sterile saline warmed to body
temperature. Do not heat the sterile saline higher than body temperature as this
can result in clotting at the catheter tip once it is placed in the animal.
3. The catheter should be hydrated for approximately 30 minutes before implantation.
Note: The catheter is very hydrophilic and, if not hydrated, will absorb water from
the blood. This can cause the gel to recede due to catheter expansion and leave a
void at the tip of the catheter, which could increase the risk of blood clot
formation.
WARNING: Do NOT use surgical electrocautery on the animal once the
device is on the surgical tableimplanted into the animal. Use of
electrocautery once the ECG leads are implanted will cause failure of the
device!
Doc-To-Help Standard Template Error! No text of specified style in document. 11

Preoperative Patient Preparation
1. Administer the appropriate surgical anesthesia.
2. Apply Artificial Tears eye ointment to each eye.
3. Remove the body hair liberally from all intended incision sites.
4. Surgically scrub the incision sites with Chlorhexidine or Providine-iodine scrub.
a. A series of at least three scrubs after all gross debris has been removed is
recommended.
b. Begin each scrub in the center of the scrubbed area, over the planned incision
site, and scrub in a ‘bulls eye’ pattern toward the periphery, never going back to
the center with the same gauze sponge. The skin preparation should be
thorough but gentle to avoid unnecessary skin trauma.
c. The final application of scrub may be allowed to remain on the skin.
5. Once the animal and the surgeon have been prepped for surgery and a sterile field has
been established, the surgery is ready to begin.
For intra-thoracic procedures, the animal must be placed on a ventilator to maintain
respiration.
Doc-To-Help Standard Template Error! No text of specified style in document. 12
Device Implantation
Device Body
Location
Dog
NHPs
Swine
Peritoneal cavity;
sutured to the inner
abdominal wall
Acceptable location
Acceptable location
(NHP must be > 2.5
kg)
Not recommended
Peritoneal cavity;
sutured between
the peritoneum and
the abdominal wall
muscles
Acceptable location
Acceptable location
(NHP must be > 2.5
kg)
Acceptable location
Intramuscularly
along the animal’s
flank
Acceptable location
Acceptable location
Acceptable location
Subcutaneously
along the dorsum or
flank
Acceptable location
Not recommended
Not recommended
Site Selection
The PhysioTel®Digital device can be implanted either intramuscularly, subcutaneously,
intraperitoneally or subperitoneally in animals weighing at least 2.5 kilograms. Possible
locations for placement of the device body vary with the species and size of animal that is
implanted and the physiologic parameters that will be measured.
If core body temperature measurements are desired, the device must be placed in the
peritoneal cavity or subperitoneally (between the peritoneum and the abdominal muscle).
Direct placement of the device body in the abdomen of swine is not recommended due to
rare cases of engulfment and ingestion of the device by the swine’s gastrointestinal tract,.
Ttherefore subperitoneal placement is recommended in this species.
Other possible locations for placement of the device body include intramuscularly along the
animal’s flank or subcutaneously along the dorsum or the flank. Subcutaneous placement
is not generally recommended in pigs and NHPs since they are prone to picking and
rubbing the device when it is placed in this location.
For subcutaneous and intramuscular locations, the animal must be large enough to allow
the antennae to lie perpendicular to the device body to preserve signal quality and in
location that is not directly over bone since this can lead to skin necrosis and irritation. The
device must lie flat under the skin in a pocket that is large enough to accommodate the
device comfortably. However excessive pocket size predisposes to seroma formation.
Doc-To-Help Standard Template Error! No text of specified style in document. 13

Intra-abdominal Placement: Intraperitoneal/Subperitoneal
Subperitoneal placement
Intraperitoneal placement
3a. Make an incision into the peritoneal
lining of the abdominal wall approximately 23 cm to the left animal’s left of the midline
incision (the surgeon’s right) large enough to
place the device portion of the device.
3b. Gently retract the left side of the
abdominal wall slightly to expose the internal
surface approximately 2-3 cm away from the
incision.
4a. Use a mayo scissors to create an
appropriately sized pocket using blunt
dissection. Place the device inside the
pocket with the catheters and biopotential
leads oriented cranially and the antennae
perpendicular to the device body, towards
the opposite side of the abdomen. (i.e. with
4b. Place the device inside the abdomen to
animal’s left of the midline incision (the
surgeon’s right). Orientate the catheters and
biopotential leads cranially and the antennae
perpendicular to the device body, towards
the opposite side of the abdomen. (i.e. with
device is placed on the left side of the linea
Intraperitoneal/subperitoneal placement is appropriate for canines and non-human primates
weighing ≥ 2.5 kg. Due to potential device engulfment by the intestines, intraperitoneal
placement is not recommended in swine; instead a modified subperitoneal approach is
favored. The intraperitoneal/subperitoneal placement is particularly useful when the transdiaphragmatic approach is used to access the heart for left ventricular pressure catheter
placement, since access to the peritoneal cavity will have already been established.
1. Make an incision through the skin and subcutaneous tissues between the xyphoid
process cranially and the umbilicus caudally (length will vary according to procedure, and
can be extended as needed).
2. Make a small incision in the body wall through the linea alba (tenting can prevent trauma
to underlying viscera), then insert a forceps or grooved director and use a scalpel with the
sharp edge facing externally to extend the incision. Keep The length of the incision in the
body wall should be lesssmaller than the skin incision to allow for closure (see figure 4).
Figure 5. Midline Abdominal Incision[MES9]
Doc-To-Help Standard Template Error! No text of specified style in document. 14

the device is placed on the left side of the
linea alba the antennae will run along the
abdominal wall, across the abdominal
incision, towards the right side of the
abdomen.)
alba the antennae will run along the
abdominal wall, across the abdominal
incision, towards the right side of the
abdomen.)
5a. Secure the device body to the inside of
the abdominal wall by suturing the suture
aids to the inner abdominal muscle using
non-absorbable suture, it may not be
possible to place a suture through the
deepest suture tab.
5b. Secure the device body to the inside of
the abdominal wall by suturing the suture
aids to the inner abdominal muscle using
non-absorbable suture. Be sure the device
body is secured away from the incision site
so that it will not interfere with healing once
the incision is closed.
6a. Close the subperitoneal pocket using
absorbable suture material in a simple
continuous pattern.
6b. N/A
7. Do NOT secure the antennae; the antennae will be secured just prior to closing the
abdomen (see below).
8. The negative (± positive) biopotential lead(s) will now need to be exteriorized from the
peritoneal cavity by passing a 14 gauge needle from outside of the abdomen to inside, next
to the incision. The lead can then be passed into the needle which is then withdrawn.
Figure 6. Cather and Biopotential Lead Exteriorization[MES10]
9. As soon as access to the abdominal cavity is no longer needed (after catheter(s) and
biopotential leads have all been exteriorized/placed) the abdominal incision should be
closed.
10. Before closing the abdominal wall, the antenna of the device must be secured. A small
incision (~1cm) should be made in the peritoneum just next to the right of the midline
incision (surgeon’s left), across from the body of the device.
Doc-To-Help Standard Template Error! No text of specified style in document. 15

Figure 7. PhysioTel®Digital Device Placed Intraperitoneally[MES11]
11. Next a small pocket should be tunneled in the subperitioneal space using blunt
dissection with a mayo scissors or a straight hemostat to provide a secure location for the
antenna to sit.
12. The body wall can be temporarily apposed near the antenna (without including the
antenna itself) using an interrupted suture or a towel clamp.
13. The body wall should be closed in 2-3 layers. The first layer is the muscular body wall
itself which should be closed in a simple interrupted pattern with an monofilament
absorbable suture of the appropriate size.
14. Next, the subcutaneous tissue should be closed in a simple continuous pattern using an
absorbable material, burying the knots.
15. Finally, skin should be closed in an intradermal/subcuticular pattern using an
absorbable suture material. This pattern is recommended to prevent post-operative
irritation. Tissue glue may be used to seal the incision if the surgeon chooses.
Extraperitoneal Placement: Intramuscular/Subcutaneous
Intramuscular or subcutaneous device placement is appropriate in laboratory animals
where the anatomy allows for a sufficiently sized pocket in the flank (paralumbar fossa
area) in which the device and antenna can lie flat and at 90 degrees to one another. The
device and antenna must also lie in a location that does not place either portion of the
device over bone. No implants should be placed directly underneath an incision, as this can
interfere with proper healing, but rather the overlying tissue should be undermined to create
a pocket that lies slightly distant from the incision. The intramuscular placement provides
additional soft tissue between the device body and the skin and has been noted to reduce
the incidence of rubbing or scratching in swine and non-human primates.
Figure 8. PhysioTel®Digital Device in SQ/IM Pocket with Antenna at 90 Degrees
Lateral Recumbency
1. Place a straight to curvilinear incision, slightly longer than the device body, in the
paralumbar fossa area, between the tuber ischii and the last rib. A second smaller stab
skin incision should be made at the point to which the antenna is expected to extend (as
determined by estimating approximate device location prior to surgery) at a 90 degree
angle to the device body.
Figure 9. Subcutaneous/Intramuscular Placement in Lateral Recumbency
Figure 10. Abdominal Wall Muscle Layers
Doc-To-Help Standard Template Error! No text of specified style in document. 16

Subcutaneous
Intramuscular
2a. Using a mayo scissors bluntly dissect
under the skin to form a pocket
approximately the size of the device body.
2b. Using a gridding technique, bluntly
separate the superficial external abdominal
oblique muscle along the fibers running
craniodorsal to caudoventrally. Be cautious
not to dissect too deeply and enter the
abdominal cavity.
4a. Pass a trochar and cannula can between
the small stab incision for antenna
placement and the larger incision for device
placement.
4b. See 4a.
3a. The device body should be placed in the
pocket created using blunt dissection, and
sutured to the underlying muscle using nonabsorbable suture material through the
suture aids. The antenna should be passed
through the cannula so it lies at a 90 degree
angle to the device body.
3b. The device body should be placed in this
space created between the external and
internal abdominal oblique muscles, with
their fibers running in opposite directions.
The device should be secured to the
underlying muscle using non-absorbable
sutures through the suture aids. The
antenna should be passed through the
cannula so it lies at a 90 degree angle to the
device body.
Subcutaneous
Intramuscular
a. First the muscle can be gently
approximated in a simple continuous suture
pattern using an absorbable suture material
on a tapered point needle. Subcutaneous
tissue can also be approximated similarly.
The skin can be closed using an
intradermal/subcuticular suture pattern with
a cutting needle. The stab incision for
b. Subcutaneous tissue can also be
approximated similarly. The skin can be
closed using an intradermal/subcuticular
suture pattern with a cutting needle. The
stab incision for placement of the antenna
should also be closed using an
intradermal/subcuticular pattern.
4. The catheters and biopotential leads now need to be routed to their implantation sites.
The first step to do this requires a skin incision be made over the planned implantation site
(i.e. left jugular furrow for negative solid tip lead, medial thigh for medial saphenous artery
etc.).
5. A cannula and trochar can then be passed between the two incisions, the trochar
removed, the catheter(s) and biopotential leads passed through the cannula and the
cannula removed. If necessary to navigate difficult anatomy, an incision can be made
partway between the origin and the planned implantation site, allowing for easier navigation
of corners, angles etc. The surgeon is likely to pass the cannual and trochar multiple times
to route the catheter(s) and biopotential leads to multiple different implantation locations.
6. AFTER the catheter(s), biopotential leads and antenna have been directed to their
appropriate locations, the incision can be closed in layers.
Doc-To-Help Standard Template Error! No text of specified style in document. 17

placement of the antenna should also be
closed using an intradermal/subcuticular
pattern.
Intramuscular
Subcutaneous
1a. Place a longitudinal incision just axial (to
the inside of) the fold of the flank. Make the
incision long enough to allow for insertion of
the device in whichever positioning will allow
the antenna to sit at 90 degrees to the
device body (with neither device body nor
antenna placed over any bony structure).
The incision is made through the skin and
superficial muscle layer (external abdominal
oblique), providing a natural separation
between muscle layers of the lateral body
wall.
1b. Place a longitudinal incision just abaxial
(to the outside of) the fold of the flank. Make
the incision long enough to allow for
insertion of the device in whichever
positioning will allow the antenna to sit at 90
degrees to the device body (with neither
device body nor antenna placed over any
bony structure). The incision is made
through skin only.
2a. Place a small stab incision through the
skin and external muscle layer at the level
that the antenna will extend to.
2a. Place a small stab incision through the
skin at the level that the antenna will extend
to.
3a. Blunt dissection between muscles is
used to create a pocket for the device. The
pocket should be large enough to
accommodate the device comfortably, but
not too large, as this can cause seroma
formation.
3b. Blunt dissection underneath the skin is
used to create a pocket for the device. The
pocket should be large enough to
accommodate the device comfortably, but
not too large, as this can cause seroma
formation.
Trans-diaphragmatic Approach
Thoracotomy Approach*
1a. After the abdominal wall incision has
been made (see 1 and 2 in intrabdominal
and subperitoneal device placement).
Retract the abdomen wall with an
appropriately sized Belfour retractor and
elevate the xiphoid process with an armynavy or malleable retractor to allow access
to the diaphragm.
1b. Counting backwards from the first or last
rib, locate the 5th intercostal space. Make
an incision through the skin, subcutaneous
tissue, and cutaneous trunci muscle midway
between the ribs, being careful to follow the
contour of the ribs closely from the
costovertebral junction to the sternum.
Incise the latissimus dorsi and pectinius
muscles parallel with the skin and then
incise the external abdominal oblique.
2a. Incise the diaphragm over the left apex
2b. During exhalation, cautiously make a
Dorsal Recumbency
Figure 11. Subcutaneous/Intramuscular Placement in Dorsal Recumbency
Left Ventricular Pressure Catheter Placement
Doc-To-Help Standard Template Error! No text of specified style in document. 18

of the heart. Remembering that during
dorsal recumbency the animal’s heart
shifts from its natural position so the
incision should be placed slightly
ventrally.
small nick in the intercostals muscles, being
very careful to center the incision midway
between the cranial and caudal rib; this
prevents trauma to the intercostals nerve
and blood vessels running along the caudal
aspect of the cranial rib and provides
adequate tissue for closure. Then extend
the incision using a push-cut method
dorsally to the tubercle of the rib and
ventrally past the costochondral arch to the
internal thoracic vessels (avoid cutting these
vessels).
3a. The diaphragm may be kept open by
placing stay sutures in the diaphragm on
each side of the incision using 3-0 or 4-0
suture with a taper needle. Secure the ends
of the stay suture with a hemostatic clamp
which can then be held by an assistant.
3b. Place wet laparotomy sponges or gauze
squares under the blades of a finochietto
retractor which can be used to expose the
heart and vessels.
*A rib resection may provide additional access to the thoracic cavity, especially in
swine, due to the anatomical difference of a wider rib.
Figure 12. Trans-diaphragmatic Approach to Left Ventricle[MES12]
Figure 13. Thoracotomy Approach to Left Ventricle[MES13]
1. Incise the pericardium to allow for access to the apex of the heart. Begin the incision by
tenting the pericardium over the apex and extend the incision cranially, stopping before
reaching the phrenic nerve that runs through the pericardium horizontally along the
base of the heart. Next, extend the incision to the right and left below the phrenic nerve,
exceess pericardial tissue can be excised.
2. Stay sutures using 3-0 or 4-0 suture on a tapered needle may be placed in the
pericardium on either side of the incision to improve access to the apex of the heart.
Secure the ends of the stay sutures with clamps which can be manipulated by an
assistant. Minimize cardiac retraction as it can cause poor flow into and out of the
heart, severe hypotension and arrhythmias. Monitor blood pressure and ECG
closely when manipulating the heart.
3. Identify the target area at the apex of the heart and install a purse-string suture usinig 34 partial-thickness bites (avoid entering the lumen) in the myocardium (see Figure
13). This should be done using 3-0 or 4-0 non-absorbable suture with a taper needle.
Figure 13: Placement of the purse-string suture
Doc-To-Help Standard Template Error! No text of specified style in document. 19

4. Carefully remove the tip cover from the LV catheter (Channel 1, see description above).
Removal of the tip cover should be done by alternating gentle traction and release.
Take care to prevent gel loss due to compression of the catheter or sudden
release of the tip cover. Always examine the catheter prior to implantation for gel
loss or bubbles. If there is gel loss or bubbles, the catheter will need to be regelled. For help with this process, refer to the Guidelines for the Re-gel of the PAC40 Device on our website: www.datasci.com. A video clip of this procedure is
also available on our website.
5. Tie a piece of non-absorbable suture around the suture aid on the catheter (see Figure
14). The size of the suture should be similar to that used for the purse string suture in
the heart, and using a different may be helpful.
Figure 14: Suture around the suture aid
The process of inserting the catheter into the left ventricle is an intricate
maneuver and needs to be performed quickly and efficiently in order to
prevent damage to the heart.
6. Using a hemostat or clamp, grasp the hub of a 14-gauge needle.
7. Puncture the heart in the center of the purse-string suture and verify perforation into the
left ventricle by the presence of blood in the needle (see Figure 15).
Doc-To-Help Standard Template Error! No text of specified style in document. 20

Figure 15: Puncture the heart wall
8. Withdraw the needle and insert a micro-mosquito hemostat into the hole. Open the
hemostat slightly to expand the hole.
9. Grasp the overlap section of the catheter using a Ddebakey forceps, vessel cannulation
forceps or gently using the hand.
10. Insert the tip of the catheter into the perforation in the heart wall. Advance the catheter
until the suture aid suture on the catheter is in direct contact with the heart wall (see
Figure 16). Releasing the catheter at this point may cause the catheter to withdraw
from the heart. Keep grasping the catheter until the purse-string suture is
tightened.
Figure 16. Catheter Inserted into Heart
11. Monitor the left ventricular pressure signal to verify proper placement (see Figure 17).
Doc-To-Help Standard Template Error! No text of specified style in document. 21

Figure 17: Left ventricular pressure signal
12. Once proper positioning is verified, draw the purse-string suture closed around the
catheter. Ensure this suture is tight and multiple square knots are tied to prevent
the catheter from withdrawing from the heart (see Figure 18).
Doc-To-Help Standard Template Error! No text of specified style in document. 22
Figure 18. Tying the purse-string suture

13. Tie one tail of each of the purse-string sutures to one tail of each of the suture aid
Trans-diaphragmatic Approach
Thoracotomy
16a. Prior to closing the diaphragm, any
blood should be gently removed from the
chest cavity. Stay sutures in the
pericardium and diaphragm itself should
also be removed.
16b. First gently remove any blood from the
chest cavity. Pericardial stay sutures should
also be removed if they were used.
17a. Begin at the dorsal-most aspect of the
incision and begin closing in a simple
continuous pattern using an absorbable
suture on a tapered needle. The catheter
should exit the thoracic cavity near the
ventral aspect of the incision to allow for
neutral positioning when the animal is
awake or in sternal recumbency. (Keep in
mind that the heart has shifted dorsally
during surgical recumbency).
17b. Prior to closing the thorax, a 12-20
French catheter should be placed through a
stab incision caudodorasl to the main
incision and then tunneled under the skin to
enter the thorax through the intercostal
space caudal to the one through which the
thoracotomy was performed.
18a. Just prior to completing the closure of
the diaphragm, place a 8 to 10 french sterile
urinary catheter through the incision to allow
for negative pressure to be restored in the
thoracic cavity. Attach a 3-way stopcock
and 20 ml syringe to the end to withdraw the
air until you begin to feel resistance.
Remove all retraction devices from the
abdomen and check again to be sure no
additional air can be removed from the
thoracic cavity (if air is not completely
removed the animal will have difficulty
breathing after manual ventilation is
discontinued). If this should occur,
thoracocentesis should be performed to
remove the air as needed. Once you are
satisfied all air has been removed, the
catheter can be removed, and the remainder
18b. Multiple pieces of large absorbable
suture (~0) should be passed around the rib
in front of and behind the thoracotomy to
approximate the ribs for closure of the
thoracotomy. The assistant will pull the ribs
together to allow the surgeon to tie each
suture individually if needed.
sutures (this is why using different colored sutures can be helpful) to further secure
the catheter in place.
14. Ensure that the catheter is placed securely in the heart wall and that all bleeding has
stopped, and cut the stay suture aid suture and purse string suture tails. If necessary,
an additional purse-string suture can be placed.
15. Optimize the orientation of the catheter so that the catheter is perpendicular to the heart
wall.
Closure of Thoracic Access
Doc-To-Help Standard Template Error! No text of specified style in document. 23

of the diaphragm closed.
19a. Make sure there are no leaks in the
diaphragmatic closure, and that the
diaphragm maintains its concave
appearance following catheter removal.
Place additional sutures as needed to
completely seal the diaphragm, and perform
repeat thoracocentesis if additional air
withdrawal is needed to correct
pneumothorax.
19b. Next the muscle layers of the incision
should be closed in multiple discrete layers
using 2-0 to 4-0 absorbable suture material
in a simple continuous suture pattern (first
the intercostals separately, next the serratus
ventralis and scalenus together, then the
latissimus dorsi separately, and finally the
cutaneous trunci separately).
20b. Finally, the skin should be closed using
2-0 to 4-0 absorbable material using an
intradermal/subcuticular pattern.
21b. Prior to discontinuation of intermittent
positive pressure manual ventilation and
discontinuation of anesthesia, The air should
be withdrawn from the chest until negative
pressure is achieved. Be sure to monitor
the animal carefully as it is weaned from
ventilator support and begins breathing on
its own. If it experiences difficulty breathing,
additional air may need to be removed from
the chest.
Systemic Blood Pressure Catheter Placement
There are multiple arteries where systemic blood pressure catheter (Channel 2 of an TS-
L21) can be placed. Selection of an artery dependends on animal size, conformation, and
surgeon preference. The mesenteric arteries are a good option in canines and non-human
primates where these vessels are large enough for placement of the catheter (animals ≥ 6
kg). In animals ≤ 6 kg direct cannulation of the iliac artery may be preferable due to its
larger size. Other arteries that can be cannulated for systemic blood pressure
measurement include the thoracic descending aorta, the medial saphenous artery or the
femoral artery. Use of the meseneteric, medial saphenous and femoral arteries requires
permanent vessel ligation, while use of the iliac and descending aorta require only
temporary occlusion.
Mesenteric or Medial Saphenous/Femoral Artery Systemic Pressure
Catheter Placement
Figure 19. Mesenteric Artery Exposure
Doc-To-Help Standard Template Error! No text of specified style in document. 24

Figure 20. Medial Saphenous Artery Exposure
Mesenteric Artery
Medial Saphenous/Femoral Artery
1a. Locate an intestinal artery running
through the mesentery closely associated
with the vein and lymphatic vessel. Choose
an artery that has nearby collateral blood
supply to avoid compromise to the intestine.
Using a fine tipped, curved forceps carefully
isolate at least 2.5 cm of the artery.
1b. The pulse of the medial saphenous
artery can be palpated on the inside of the
thigh with the hindlimb extended straight out
behind the animal and rotated externally so
the inside of the thigh is easily accessable.
A skin incision should be made over the
palpable pulse, pulling the skin to the side to
avoid damaging the underlying vessel.
1c. The femoral artery originates deeper
between muscle bellies. To find it, follow the
medial saphenous artery proximally and
sharply transect the fascia between muscle
bellies (avoid cutting or dissecting
through the muscle itself). Continue the
dissection proximally and deep to the
femoral artery. Once the fascia is cut, blunt
dissection can be used to isolate the vessel
and a Weitlaner retractor can be used to
provide better visualization.
[MES14]
2. Once the appropriate vessel is located, apply a few drops of 2% Lidocaine without
epinephrine on the artery to prevent vasospasm.
3. Pass three pieces of non-absorbable suture under the isolated section of artery. Tie the
distal-most suture to permanently occlude the blood vessel. The two more proximal
Doc-To-Help Standard Template Error! No text of specified style in document. 25

sutures can be tied in loose knots to allow the suture to pass as it is inserted into the
abdominal aorta. The proximal-most suture will be used to temporarily occlude blood flow
when the artery is punctured (see Figure 21).
Figure 21. Preparing Artery for Catheter Placement
4. Prepare a 20-gauge needle by bending the beveled tip (while holding the bevel up) to a
90 degree angle (see Figure 22). This will be used to puncture the vessel and can be used
to introduce the catheter.
Figure 22. Bending Needle for Catheter Placement
5. Place a length marking suture around the catheter body at the approximate site that you
wish to insert the catheter to; this is determined by estimating the distance needed for the
pressure-sensing tip to be placed in free flowing blood within the abdominal aorta. When
placing the length-marking suture use multiple passes of suture around the catheter to
more evenly distribute the pressure and avoid compromising the pressure catheter or
sensor (see Figure 23).
Doc-To-Help Standard Template Error! No text of specified style in document. 26
Figure 23. Length-marking Suture
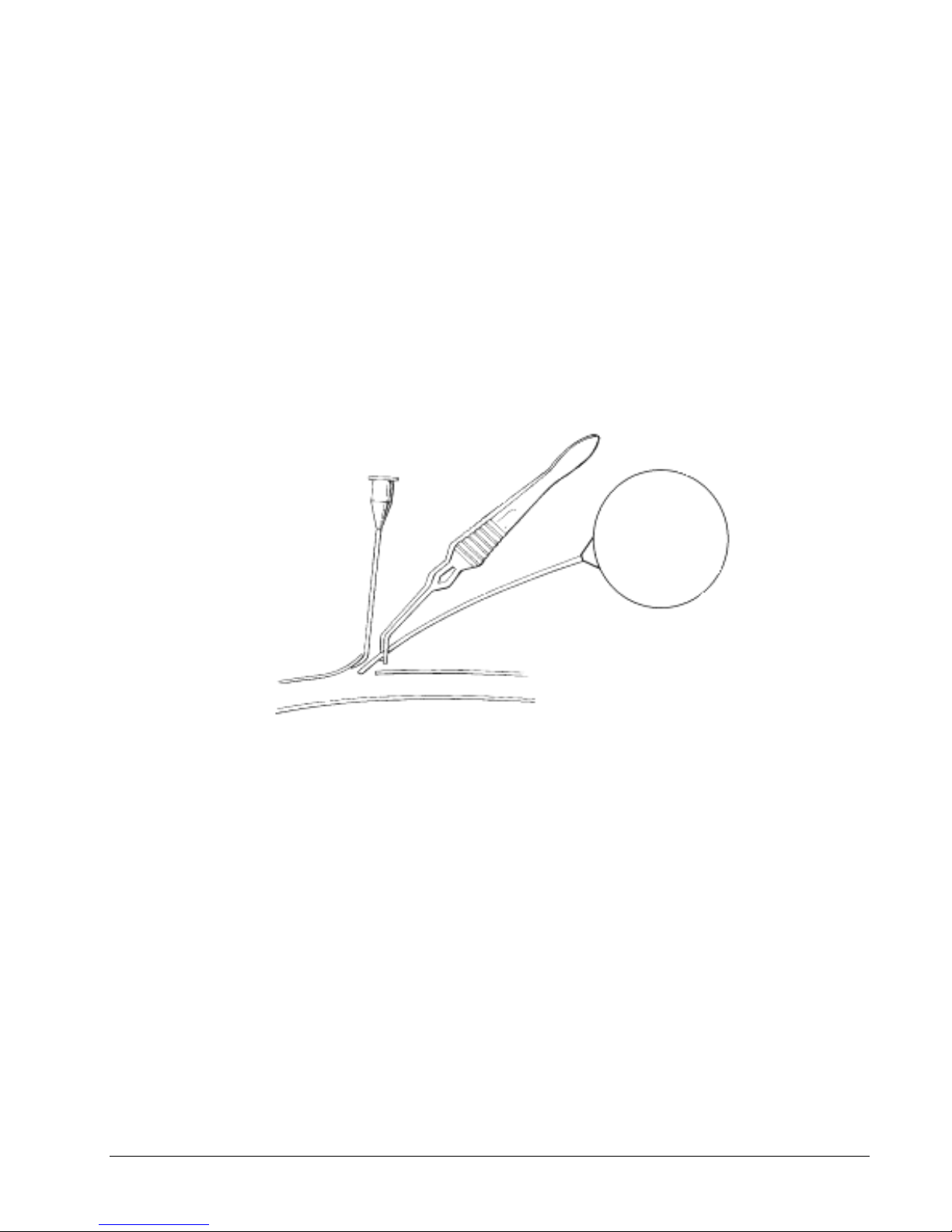
6. Gently and slowly remove the tip cover using gentle traction and release, without
touching the distal thin-walled sensing portion of the catheter. Take care to prevent gel
loss due to compression of the catheter or sudden release of the tip cover. Always
examine the catheter prior to implantation for gel loss or bubbles. If there is gel loss
or bubbles, the catheter will need to be re-gelled. For help with this process, refer to
the Guidelines for the Re-gel of the PA-C40 Device on our website:
www.datasci.com. A video clip of this procedure is also available on our website.
6. Apply gentle tension to the distal ligation suture and proximal temporary occlusion
suture. Grasp the catheter at the overlap section in your dominant hand and the pre-bent
20-gauge needle in the other hand. Pierce the artery using the needle and insert the
catheter under the tip of the needle as it is withdrawn. Alternatively, a vessel pick may be
used to dilate the vessel slightly before placing the catheter (see Figure 24).
Figure 24. Systemic Pressure Catheter Placement
7. Advance the catheter into the artery until it reaches the proximal occlusion suture and
then stop. Now gently tighten the middle suture around the artery containing the catheter
to secure the catheter in the vessel. Next release tension on the proximal occlusion suture
and continue passing the catheter until the length marking suture is at the level of the
artery. Now release tension on the distal ligation suture, and tighten both the proximal
temporary occlusion suture and middle sutures around the artery containing the catheter.
8. Each tail of the middle suture can now be tied to one of the length-marking suture tails to
further lock the catheter into place. Next the tails of the distal occlusion sutures can be
brought around the catheter and tied.
Iliac Artery Systemic Blood Pressure Catheter Placement
Doc-To-Help Standard Template Error! No text of specified style in document. 27

1. Carefully locate and isolate the iliac artery. The paired iliac arteries are located in the
caudal abdomen and branch directly off of the caudal abdominal aorta and can be
palpated by first detecting the aortic pulse and moving caudally (see Figure 25).
Figure 25. Iliac Artery
2. Using fine tipped, curved forceps, carefully isolate at least 2.5 cm of the iliac artery from
the surrounding tissue and the iliac vein.
3. Pass two elastic vessel loops or two pieces of non-absorbable suture underneath the
isolated artery section. Both sutures will be used to temporarily occlude blood flow to
allow for placement of the catheter (see Figure 26). Place the loops/sutures as far apart
as possible and secure with a hemostatic clamp. Do NOT occlude the vessel until
everything is prepared for vessel cannulation.
Figure 26. Temporary Occlusion of Iliac Artery
Doc-To-Help Standard Template Error! No text of specified style in document. 28
[MES15]

4. Fill four gel-loading micropipette tips with VetbondR tissue adhesive using capillary
action and set aside. They will be used to dispense a very small amount of adhesive to
seal the vessel. Using micropipettes to dispense the Vetbond will help control the
amount of Vetbond applied to the artery. Excessive Vetbond can encircle the artery
and compromise blood flow. This can result in hind limb paresis.
5. Prepare a 20-gauge needle to puncture the vessel, place a length-marking suture and
carefully remove the tip cover as described above (4-6).
6. Identify the catheter insertion site just proximal to the distal occlusion loops/suture.
Apply one drop of 2% Lidocaine to the iliac artery to fully dilate it, if necessary. Grasp
the catheter at the overlap section in your dominant hand and the pre-bent 20-gauge
needle in the other hand. Pierce the artery using the needle and insert the catheter
under the tip of the needle as it is withdrawn. Alternatively, a vessel pick may be used
to dilate the vessel slightly before placing the catheter.
7. Advance the catheter into the artery until it reaches the proximal occlusion suture and
then stop. Now release tension on the proximal occlusion suture temporarily to pass
the catheter beyond this point and then replace gentle tension. Continue passing the
catheter until the length marking suture is at the level of the artery.
8. Verify appropriate blood pressure signal has been achieved. Then thoroughly dry the
artery at the catheter entry site with cotton tip applicators and apply a very small amount of
Vetbond tissue adhesive using the gel-loading micropipette tips. If the area is not dried
effectively, there will be poor bonding of the tissue adhesive, resulting in leakage.
9. Once the Vetbond has visibly set, slowly release the tension on both of the occlusion
sutures and observe the catheter entry site for leakage. If leakage is observed, re-occlude
the vessel, clear the site of blood and apply only enough additional Vetbond to seal the
leak.
10. Anchor the catheter in place with a small fiber patch. The patch can be prepared by
cutting out a small 5 millimeter x 7 millimeter rectangle. Cut a wedge in the patch halfway
across the width of the patch. (see Figure 26). Place the fiber patch across the catheter
entry site with the catheter passing through the wedge. Secure the patch to the catheter,
vessel, and surrounding tissues by applying a few drops of Vetbond tissue adhesive using
the gel-loading micropipette tips.
Figure 26: Fiber Patch to Seal Venipuncture Site
Doc-To-Help Standard Template Error! No text of specified style in document. 29

11. Secure the catheter that is outside the vessel near the entry site to the lumbar muscles
in at least two locations using non-absorbable suture. If the catheter is not secured to
nearby muscles there is a high risk that the catheter will back out of the vessel postoperatively, which would result in internal bleeding and loss of the blood pressure
signal.
Thoracic Aorta Systemic Blood Pressure Catheter Placement
1. The descending thoracic aorta should be identified, and the thin layer of serosal covering
carefully dissected away from approximately 1 cm of the vessel’s surface.
2. Next a small purse-string suture should be placed and a knot loosely tied on the surface
of the vessel using 3-0 to 5-0 suture material on a tapered needle. Be careful not to take
full thickness bites of vessel wall or bleeding will occur. Should this occur apply gentle
pressure until bleeding stops. Leave the ends of the purse-string suture long (see Figure
27).
Figure 27. Preparation of Thoracic Aorta for Catheterization
3. Complete needle preparation, length marking suture placement and tip cover removal as
described above.
4. When you are fully prepared for catheter insertion, apply a Satinsky clamp to the area
around the purse string to temporarily occlude blood flow. This clamp should remain in
place for the minimum amount of time possible, so be sure everything is fully
prepared prior to placement.
5. Once the Statinsky clamp is in place, grasp the catheter at the overlap section in your
dominant hand and the pre-bent 20-gauge needle in the other hand. Pierce the artery
using the needle and insert the catheter under the tip of the needle as it is withdrawn.
Alternatively, a vessel pick may be used to dilate the vessel slightly before placing the
catheter.
6. Advance the catheter into the artery, the clamp may have to be temporarily released or
adjusted to allow passage of the catheter. Continue advancing the catheter until the length
marking suture is at the level of the artery. Now tighten the purse-string suture and ensure
no blood leaks around the catheter, if needed, you can place an additional purse string
suture around the outside of the first for hemostasis.
[MES16]
7. Each tail of the purse string suture can now be tied to one of the length-marking suture
tails to further lock the catheter into place and all sutures cut short[MES17](see Figure 28).
Figure 28. Purse-string Tightening
8. Secure the catheter that is outside the vessel near the entry site to the lumbar muscles in
at least two locations using non-absorbable suture. If the catheter is not secured to
Doc-To-Help Standard Template Error! No text of specified style in document. 30

nearby muscles there is a high risk that the catheter will back out of the vessel postoperatively, which would result in internal bleeding and loss of the blood pressure
signal.
Electrocardiogram (ECG) Lead Placement
Positive Lead Placement
The positive lead must be placed first so you can detect an ECG signal to guide
placement of the negative solid tip lead. The positive lead can be placed in a variety of
different locations including the abdominal side of the diaphragm, the epicardium, or
subcutaneously over the middle of the left ribcage approximately level with the xyphoid
process for a lead II ECG or on midline for a base-apex ECG. The choice of location for the
positive lead is often dictated by the other surgical approaches being used (i.e.
diaphragmatic ECG lead placement with intra-abdominal device placement etc.).
Regardless of the site chosen for implantation, the basic technique is the same and will be
described below.
1a. If the positive ECG lead will be placed on the diaphragm, it can be placed following
closure of the diaphragm following trans-diaphragmatic placement of the left ventricular
catheter. It should be placed over the apex of the heart (see Figure 29[MES18]).
Figure 29. Diaphragmatic Positive ECG Lead Placement
1b. If the positive ECG lead will be placed subcutaneously there are two different options
including a lead II configuration and a base-apex configuration. For the lead II
configuration, a small skin incision should be made over the middle of the left ribcage,
approximately level with the xyphoid process (see Figure 30). For the base-apex
configuration, a small skin incision should be made over the ventral midline at
approximately the level of the xyphoid process (see Figure 31). The lead will be
exteriorized as needed and passed through a cannula to the site of implantation (as
described above in the device body placement description).
Doc-To-Help Standard Template Error! No text of specified style in document. 31

Figure 30. Lead II ECG Placement
Figure 31. Base-apex ECG Lead Placement
1c. If the positive ECG lead will be placed epicardially, it will need to be directed to the
thoracic cavity and placed prior to closure of the chest following left ventricular pressure
catheter ± thoracic aorta systemic blood pressure catheter placement.
2. Cut the lead to the appropriate length to reach the incision, allowing for growth if needed.
Next make a circumferential cut around the silicone covering from the last few centimeters
of ECG lead, leaving the exposed wire. Form this wire into a loop with a diameter of
approximately 1 cm and secure the loop with a non-absorbable suture. Finally, place
another suture a few millimeters from the end of the silicone covering, just before the loop
of exposed wire in order to prevent fluid migration (see Figure 32). Cut the tail, but leave
the suture attached onto the ECG lead[MES19].
Doc-To-Help Standard Template Error! No text of specified style in document. 32

External Jugular Vein
Internal Jugular Vein
1a. A skin incision should be made in the
jugular furrow to expose the external jugular
vein, approximately 4 cm caudal to the
confluence of the maxillary and lingofacial
veins. The skin can be pulled to the side of
the vein so the surgeon isn’t cutting directly
over the vessel. The external jugular vein
can be used in the canine and swine, but the
internal jugular provides a more direct route
to the intended location of the solid tip in the
cranial vena cava. The external jugular vein
tends to be larger and located more
superficially (see Figure 33).
1b. The internal jugular vein can be exposed
through an incision next to the trachea
approximately 1/3 of the distance between
the sternum and the corner of the mandible.
Blunt dissection should be used and the
sternal and clavicular heads of the
sternocleidomastoid muscle can be
separated to expose the external jugular
vein and carotid artery (see Figure 34).
Figure 32. ECG Lead Modification
3. Take the suture still attached to the ECG lead and use this to tack the loop to the
underlying muscle/tissue. Anchor the exposed portion of the lead to the underlying muscle
using at least 3 simple interrupted knots using 2-0 to 0 3-0 non-absorbable suture. You can
also tack the lead along its course if you are concerned about tension.
Negative Solid Tip Lead Placement
The PhysioTel®Digital device will come with a solid tip negative lead. This has been
shown to provide accurate ECG signals in animals ≥ 2.5 kg while virtually eliminating
muscle noise and artifact. The right external jugular vein has been used in the canine and
swine. Due to differing anatomy, the internal juglar vein is recommended in non-human
primates.
Doc-To-Help Standard Template Error! No text of specified style in document. 33
[MES20]

Figure 33. External Jugular Vein
Figure 34. Internal Jugular Vein in Non-human Primate
2. Once the solid-tipped lead is exteriorized at the jugular incision via passing it through a
cannula (as described above in device placement description), it’s time to prepare the
vessel for cannulation. Pass 3 pieces of non-absorbable suture around the vessel. The
cranial-most suture will be used to permanently ligate the vessel. Loose knots can be
placed in the other two sutures and the tails left long (see Figure 35).
[MES21]
Figure 35. Sutures Placed for Solid Tip Negative ECG Lead Placement
3. Tension should then be placed on the caudal suture to temporarily occlude blood flow
and on the cranial ligation suture to hold the vessel in place during lead placement. Then
pierce the vessel cranial to the middle tie using the bent needle technique (described
above). You can then choose to use a vein pick to dilate the opening slightly and gently lift
upwards.
5. The solid tipped lead can then be inserted into the vein in a direction toward the heart.
Stop passing once the lead is near the caudal occlusion suture. At this point, the middle tie
can be gently tightened around the lead to secure it in the vessel and then continue
passing the lead into the vein (see Figure 36).
Figure 36. Placing Solid Tip Negative ECG Lead
Doc-To-Help Standard Template Error! No text of specified style in document. 34

6. It is imperative to monitor the ECG signal while passing the negative lead. The
appropriate location of the solid tip is dictated by the size of the P wave. The P wave will
start out small and grow increasingly larger as it approaches the heart and may become
negative when it is passed too far. The solid tip is in its optimal position when the P wave
is approximately 1/3 the height of the QRS complex. Also ensure the head and forelimbs
are in a relatively neutral position to ensure the ECG signal will remain consistent once the
animal is awake. Once you are satisfied with the signal you can tighten the two caudal
sutures around the vessel containing the lead and tie the tails of the cranial suture around
the lead to further secure it in place.
7. The lead should also be tacked to the surrounding tissue once or twice to minimize
tension and prevent it from being pulled out of the vessel (see Figure 37).
Figure 37. Solid Tip Negative ECG Lead in Place
Surgical Recovery
1. Discontinue surgical anesthesia.
2. Maintain supplemental warmth throughout the anesthetic recovery.
3. Administer post-surgical analgesia.
4. Monitor animal closely for the return of normal postures and behaviors.
This completes the surgery.
Doc-To-Help Standard Template Error! No text of specified style in document. 35

Appendix A: Additional Device Information
Device Explantation
When explanting DSI devices implanted intraperitoneally, intramuscularly or
subcutaneously, consider the following:
Carefully remove the device body.
Be careful not to drop the device.
If cutting the catheter is necessary, use only a new scalpel blade to cut the catheter at a 45degree angle away from the device body and approximately 3 cm from the device body. Do
not use any instrument other than a scalpel blade to cut the catheter. Cutting the
catheter with a pair of scissors or any other instrument could cause damage to the
pressure sensor and void the warranty. If the catheter must be cut, the device cannot
be reused.
Clean and sterilize the device with an approved enzyme detergent and sterilant before
returning the device to DSI. For complete information on products and techniques approved
for use with DSI devices, visit www.datasci.com.
Product Return Information
A detailed procedure for properly returning telemetry devices to DSI for exchange is
provided on our website, www.datasci.com. The following additional considerations should
be made:
To be covered under the manufacturer’s warranty, the devices must be returned for
exchange within the warranty period.
Contact DSI Technical Services with any concerns or comments regarding the
performance of the devices.
Ensure that the devices are well packed, preferably in their original packaging and
boxes.
Return the devices via a traceable shipping method to prevent losses in transit.
Complete product return information can also be found online at www.datasci.com.
Doc-To-Help Standard Template Error! No text of specified style in document. 36

Appendix B: Functional Specifications
Specifications
PhysioTel®Digital
Device
Weight
Volume
Height
Width
Length
Usable Catheter
Length
35 cm***
Catheter Diameter
Temperature Range
Pressure Range
Initial Accuracy
Battery Life
Intended Cage Size
* Standard catheter length. Also available in 10 cm and 15 cm lengths.
** The diameter is 5.5 cm and the thickness is 1.5 cm.
*** Standard catheter length. Also available in 25 cm and 40 cm lengths.
Doc-To-Help Standard Template Error! No text of specified style in document. 37

Appendix C: Device Care and Use
Operational Modes
TL implantable devices are equipped with two operational modes: ON and OFF.
Devices are shipped to you in the OFF mode. The battery in the device is not activated.
When switched to ON, the devices begin to sense and transmit data. The switch to change
between these two modes is in the interior of each device and is therefore not visible. The
switch is magnetically activated.
To switch operational modes:
1. Power on a PhysioTel®Digital reader
2. Bring the reader close to the packaged device.
It is important the devices remain in the sterile packages!
3. Momentarily bring a strong magnet within approximately one inch of the package.
A magnetically activated internal switch is moved. The order of modes is:
Off (You should hear no tone)
On (You should hear a tone)
On-Site Cleaning and Re-sterilization
All new and exchanged devices shipped to an investigator are sterile and ready for
implantation.
Prior to returning TL devices for refurbishment we ask that they are cleaned and sterilized.
For complete and current information on products and techniques approved for use with
DSI devices, visit www.datasci.com
Storage
Storage of New Devices
Carefully examine all devices when they arrive at your facility. Remove the packages
containing the devices from the shipping boxes. Save the shipping boxes to use when
returning used devices for the Device Exchange Program. Inspect each device package for
signs of damage. Using your AM radio on the low frequency setting, turn each device on
and off by scanning a magnet across the device to ensure that none of the devices were
damaged during shipping. Confirm that each device is turned off before storing. Although
each unit is checked just before shipping, the device may have been exposed to stray
magnetic fields during shipment. This can cause the unit to be turned on unintentionally.
New and exchanged units are sterile upon arrival. If the package remains undamaged, this
sterility is warranted according to the information on the package label. Devices in the OFF
mode may lose up to 10% of the battery life within 12 months after the manufacture date.
The devices should be stored in a cool (between 10 and 25 degrees Celsius), dry area
away from exposure to static discharge and magnetic fields. Never expose them to
temperatures above 60 degrees Celsius, as this will void all warranties. It is also important
to store them in an area where they will not be accidentally dropped or have items placed
on top of them. Storage in a refrigerator does not provide significant benefit in terms of
battery life.
Doc-To-Help Standard Template Error! No text of specified style in document. 38

Storage of On-Site Sterilized Devices
Occasionally there may be a delay between the device removal from the animal and return
to DSI for refurbishment. Proper storage of the on-site sterilized device is necessary to
ensure that the unit will not be damaged.
Thoroughly clean and sterilize each device according to DSI's On-Site Re-sterilization
procedure. If the original device sterile package was saved, place the device into the plastic
packaging. This will help to identify the device and the calibration values associated with it.
Do not store devices in saline or other liquid. Sterilization before storage is necessary to
prevent the spread of bacteria during handling.
The devices should be stored in a cool (between 10 and 25 degrees Celsius), dry area
away from exposure to static discharge and magnetic fields. Never expose them to
temperatures above 60 degrees Celsius, as this will void all warranties. It is also important
to store them in an area where they will not be accidentally dropped or have items placed
on top of them. Storage in a refrigerator does not provide significant benefit in terms of
battery life.
Using your PhysioTel®Digital reader, check each device to ensure that it is properly turned
off.
For complete and current information on products and techniques approved for use with
DSI devices, visit www.datasci.com.
Doc-To-Help Standard Template Error! No text of specified style in document. 39

Part Number
Description
11006-12
Adson Forceps-straight, serrated
11027-12
Adson-Brown Tissue Forceps-straight, with
teeth
13019-14
Kelly Hemostat Forceps, curved
13018-14
Kelly Hemostat Forceps, straight
13009-12
Halsted Mosquito Forceps, curved
13008-12
Halsted Mosquito Forceps, straight
11617-12
Debakey Forceps
14010-17
Mayo Scissors-straight, 15 cm
14019-14
Metzenbaum Scissors-curved, 14.5 cm
12002-14
Olsen-Hegar Needle Holder
11095-09
Backhaus Towel Clamps
Sterile permanent marker
Appendix D: Equipment and Supplies
The surgical instruments, along with their part numbers from Fine Science
Tools, are listed below.
A hollow cannula and trocar or skin tunneling needle is also helpful to tunnel
the intravenous lead subcutaneously from the device site. An excellent large
animal trocar is available through Chiron Bioscience Limited.
Chiron Bioscience Limited
Email: info@chironbioscience.com
Telephone: +44(7775-517302
Fax: 44-1233-221580
PO Box 979
Canterbury
Kent
CT1 9DW
United Kingdom
Fine Science Tools, Inc.
Telephone: (1-800) 521-2109 or (1-650) 349-1636
Fax: (1-800) 523-2109 or (1-650) 349-3729
Website: www.finescience.com (A list of offices in other countries can also be found here.)
A wound clip applier can be purchased from Fisher Scientific.
For large animals:
o Wound clip applier with wound clips-Part Number NC9154268
Doc-To-Help Standard Template Error! No text of specified style in document. 40

Gel-loading micropipette tips (Part Number 02-707-83) can also be purchased from Fisher
Scientific.
Fisher Scientific:
Telephone: (1-800) 766-7000
Fax: (1-800) 926-1166
Website: www.fishersci.com
\
Doc-To-Help Standard Template Error! No text of specified style in document. 41

Appendix E: Checking the Offset of a Pressure Device
The following protocol will allow you to verify that the pressure device is functioning
normally prior to surgical placement in an animal.
NOTE: Turn the device on approximately 1-4 hours before taking the pressure offset
measurement. This will allow the electronics time to warm up and stabilize. Pressure
offsets can be affected by temperature and intense light.
ASSUMPTION: The user is familiar with Dataquest ART configuration setup or
Ponemah protocol setup for configuring and assigning devices to receivers and also
for using appropriate acquisition settings. For more information please refer to the
Dataquest ART User Guide or Ponemah Physiology Platform User Guide.
Dataquest ART Users
1. In the Configuration module, assign each device to a receiver, enter the calibration
information, and assign an animal ID for each device.
2. After the device has been on for 1-4 hours, place the device in the packaging tray
onto its assigned receiver.
3. From the Acquistion window, select the animal icon, right-click and choose Start
Sampling – Continuous… to display the Start Continuous Sampling window.
4. Select Trace to display a waveform trace in the graph window. It will not save the
displayed graphs.
5. From the Real-Time graph window, select Data - Pause once a steady waveform
trace appears.
6. Click on the zoom icon depicted as a magnifying glass. This will change the display
of the pressure waveform in a Static Graphs window.
7. Righ-click on the pressure waveform and select Tracking. This option displays the X
and Y values on the Static Graphs window’s status bar. By moving the mouse on the
waveform, you will be able to see where the offset lies.
8. Click and drag the mouse to create a box around the portion of the waveform you
would like to magnify. Repeat as necessary.
9. Record the offset value on the Lab Sheet. If desired, a hard copy of a trace can be
made by right-clicking the mouse and selecting Print. This should be kept with the lab data
for the project as verification of initial accuracy.
Ponemah Physiology Platform Users
1. Assign each device to a receiver, enter the calibration information, and assign an
animal ID for each device through the Edit DSI Setup from the Hardware menu.
2. Select the appropriate animal ID from the Select DSI Sources from the Hardware
menu.
Doc-To-Help Standard Template Error! No text of specified style in document. 42

3. After the device has been on for 1-4 hours, place the device in the packaging tray
onto its assigned receiver.
4. From the Setup menu, choose PD Setup... and change the analysis module to BP
for the pressure channel in the Channel Input Setup screen.
5. Close the Setup window and start an acquisition from Acquisition – Start
Acquistion.
6. In the Status window, double-click on the blood pressure channel to open the Blood
Pressure Analysis Attributes window. Select the Offsets tab.
7. Under “Implant Pressure Offset”, click the Measure button to see an instantaneous
reading of the offset from 0. Clicking this again will give you an updated instantaneous
offset. Click Cancel to close this window without readjusting the pressure trace.
8. Record the offset value on the Lab Sheet. If desired, a hard copy of a trace can be
made by clicking on the print button in the graph page menu bar. This should be kept with
the lab data for the project as verification of initial accuracy.
Doc-To-Help Standard Template Error! No text of specified style in document. 43
 Loading...
Loading...