Dräger Medical C2000e, C2000 Service Manual

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Isolette® Infant Incubator
(Models C2000 and C2000e)
WARNING:
For a full understanding of the performance
characteristics of this equipment, the user
should carefully read this manual before
operating.
Emergency Care · OR/Anesthesia · Critical Care · Perinatal Care · Home Care
Operating Instr u ct ions
Because you care

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Table of Contents
Section 1: Definitions Inte nded Use and Disclaimer
Definit i o n s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-1
Symbol D efinitio n s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-1
Technical Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-5
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-6
Disclai m er . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
Section 2: Introduction, Fe a tures , and Sp ecifications
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 -1
System O v e rview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Humidity System (Op t i o n a l ) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Humidity Reservoir . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Manifo l d A ssembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
Evapo r a t o r A ssembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Oxygen Control System (Optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
Weighing System (Accessory) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
Uninterruptible Power Supply (UPS) (Optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
Functi onal Descrip t i o n . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 -3
Air Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
Skin Mo d e . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Standa rd F e atures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Option a l Fea t u r e s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
Specifi cations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Standa rd F e atures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6
Option s and Accessor i es . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7
Stands . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7
Humidity System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Oxygen System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Weigh in g S y stem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Regul a t i o n s, Standards, and Co d es . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-11
Electromagnetic Compatibility (EMC) Guidan ce and Manufacture r Declar ations . . . . . . 2-11
Devic e Cl a ssification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
i

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Section 3: Pr ecautions and Safety Tips
Precau t i o n s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-1
Electri c a l P re cautio ns . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 -1
Explos ion Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
EMC Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Oxygen Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Humidit y P recauti o n s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 -6
Safety Ti p s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-7
Section 4: Installation and Operational Checkout
Instal l a t i o n . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Unpacka g i n g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
C2000 Stand Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
C2000e Stand Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4- 2
Rail Assembly and Accessories Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Rail Ass e m b ly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Rail Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Hood, Shell, and Stand Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 -6
Mattress Restrain t S t ra p In stallation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
UPS Syste m In stalla t ion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Weigh in g S y stem (Scale Ass embly) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 -9
Humidit y S y st e m . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Oxygen Control System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Oxygen Sensor Cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Oxygen Calibration Fixture . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-12
Controller Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12
Hood/Shell Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14
VHA Stand Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-17
UPS System Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-18
Check On /Off/Test Swi t ch a n d Lo w Ba t t e ry Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . 4-18
Test Ba tt ery Back-up Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-19
Rail System Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19
Oxygen Control Module Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19
Humidity System Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-20
Weighing System Operational Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-20
ii

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Section 5: Instructi on s for Use
Controls, Indicators and Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Incub at o r . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Incuba t o r Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-1
Hardke y s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Softkey s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-2
Incuba t o r Indicators. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Incuba t o r Connectors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Controller Interface Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Serial P o rt . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Sensor Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
Sensor Module Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Sensor Module Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Sensor Module Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Stand . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
Stand Cont rols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Main Ci rc u i t B r e a k er . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
Foot Ped al Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
Stand In d i c a t o rs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
Stand Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
Convenience Outlet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
AC Input Connector. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
UPS Electronic Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-7
UPS Electronic Module Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-7
UPS Electronic Module Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-7
UPS Electronic Module Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-8
Display s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Temperature Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Trend/Alarm Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
Humidity Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-9
Oxygen Window . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
System D is p lays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-10
Temperature Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Trend Di sp l a y . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Weight Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Oxygen Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
Humidit y Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1 1
Factory D e fault Set t i n g s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12
iii

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-13
System A larms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-13
UPS Alar m s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-17
System Pr o mpts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-17
Infant Pl a cement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-19
Operat i n g In st ructio n s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-20
Syste m S t a r t-Up and Shut-D o w n . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-20
System St a r t -u p . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-20
Initial Start-up for systems without UPS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-20
Initial Start-up for systems with UPS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-20
Recovery from Power Failure (non-UPS systems) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-21
Recov ery from Power Failure (UPS sys t e m s) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-21
System Sh u t D o w n . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-21
System C o n fi g u r a t i o n . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-22
Variab l e H eight Adjustment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-23
Tempera t u re Settin g s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-23
Air Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-23
Skin Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-24
Skin Prob e A t t a c h m en t . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2 5
Singl e Te m p e rature Monit o rin g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-25
Dual Te m p e rature Monit ori n g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2 5
Data Tren d s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-26
Scale Mea sureme n t s . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 -2 7
Initial Weigh . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-27
Re-weig h . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-28
Oxygen Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-28
Oxygen Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-28
Oxygen Control Set Point . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-29
Humidity Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-29
Humidit y Mo d e . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-29
Humidity Control Set P o i n t . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3 0
VueLink™ Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-30
X-Ray Tr a y Usa g e . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-31
Non-Servo Control Oxygen Usage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-31
Calibr at ion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-32
Scale Cal i b ration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-32
Oxygen Sensor Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-33
Oxygen Sensor Calibration to Room Air (21%) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-33
iv

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Oxygen Sensor Calibration to 100% Oxygen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-34
Patien t Tran sport . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-35
Section 6: Cleaning, Maintenance, and Replacement P arts
Cleanin g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 -1
General C l e an i n g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Steam Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Stain Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-2
Disinf ec t i n g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 -2
Disassembly for Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-2
Mattress Tray, X-Ra y Tra y , Main Deck, Sca l e (optional) . . . . . . . . . . . . . . . . . . . . . . . 6-3
Heate r an d Im p e l l e r . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-3
Humidity Tray and Reservoir . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Access Door Gaskets, Tubing, Iris Entry Port Sleeves, Cuffs . . . . . . . . . . . . . . . . . . . . 6-3
Air Intake Microfilter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Cleanin g P ro cedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-4
Reusab l e Skin Tempera t u re Probe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-4
Access Door Gaskets and Tubing Access Ports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Controller, Shell, and Stand . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Sensor Module, Hood, and Inner Walls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
Heate r Radiator and F an Im p eller . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-5
Humidity Reservoir . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-5
Air Intake Microfilter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 -6
Base Covers, Rail and Accessories, Drawers, Tank Mounts, Monitor
Shelf , a n d I.V. Pole . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
Mattress, Mattress Tray, X-Ray Tray, Main Deck, Heater/Im pel l er Cover,
Scale (Optional), and Mattress Tilt Bars . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-6
Uninterruptible Power Supply (UPS) Air Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Reass embly After Cle a n i n g . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Mainten ance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6- 9
UPS Bat t e ry P ac k Ma i n t e n ance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6- 9
UPS Electronics Module Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-10
Air Intake Microfilter Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-10
Replac e m en t Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-11
Section 7: Troubleshooting
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-1
Sympt o m , Cause, and Remed y . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
v

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Notes:
vi
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Section 1
Definitions, Intended Use,
and Disc laimer
Definitions
This manual contains different typefaces and icons designed to improve readability and increase
understanding of its content. Note the following examples:
• Standard text—used for regular information.
• Boldface text—emphasizes a word or phrase.
• NOTE:—sets apart special information or important instruction clarification.
The Definitions subsection contains label symbol definitions and technical definitions. Additional
definitions of system symbols and icons are located in Section 5 (see “Controls, Indicators and
Connectors” on page 5-1).
Some of the warnings contained in this user manual include number tags or bracketed wording (
example [6.8.2.9] or IHA025])
purposes.
. These are requirements, which are used solely for internal documentation
Symbol Definitio ns
• The symbol below highlights a WARNING or CAUTION:
Warning and Caution
– A WARNING identifies situations or ac tions that may affect patient or user safety . Disregarding a
warning could result in patient or user injury.
– A CAUTION points out special procedures or precautions that personnel must follow to avoid
equipment damage.
• The symbol below highlights an ELECTRICAL SHOCK HAZARD WARNING:
Electrical Shock Hazard Warning
for
1 - 1
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
• The symbol below indicates “Attention: Consult accompanying documents:”
Attention: Consult Accompanyi ng Documents
• The symbol below indicates a “Type BF applied part:”
Type BF Applied Part
– The instrument provides a specified degree of protection against electric shock, particularly the
leakage current and reliability of the protective ground connection with a BF-type applied part.
– A BF-type applied part indicates an applied part isolated from all other parts of the instrument to
such a degree that the patient leakage current allowable in a single-fault condition is not
exceeded.
• The symbol below indicates “AC power:”
AC Power
• The symbol below indicates “Protective earth (ground):”
Protective Earth (Ground)
• The symbol below indicates “Caution: Hot surface:”
Caution: Hot Surface
• The symbol below indicates “Weight limit:”
Weight Limit
1 - 2
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
• The symbol below indicates “Consult Accompanying Document on the Battery Weight:”
Battery Weigh t
• The symbol below indicates “Consult Accompanying Document on Battery Pack Orientation:”
Battery Pack Orie n tation
• The symbol below indicates “Power Failure:”
Power Failure
• The symbol below indicates “Lock casters when parked on an incline:”
Lock Casters
• The symbol below indicates an ELECTROSTATIC DISCHARGE (ESD) sensitive part:
Electrostatic Discharg e (ESD) Sensit i ve Part
1 - 3
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
• The symbol below indicates “Consult accompanying documents on latch lock/unlock and rail
loading:”
Latch Lock/Unlock and Rail Loading
• The symbol below indicates “Electromagnetic interference:”
Electromagnetic Interference
Interference can occur in the vicinity of the equipment marked with the Electromagnetic
Interference symbol.
• The symbol below indicates “Consult accompanying document on the large tray loading:”
large Tray Loading
• The symbol below indicates a “Communication port”
Communication Port
1 - 4

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Technical Definitions
• Incubator temp e rature—Air temperature at a point 4" (10 cm) above and centered over the mattress
surface.
• Control temperature—The temperature controller set point selected by the user.
• Average incubator temperature—The average of the maximum and minimum incubator
temperatures achieved during temperature equilibrium.
• Incubator temp e rature equilibrium—The condition reached when the average temperature of the
incubator does not vary more than 1°C over a period of 1 hour.
• Temperature uniformity—The amount by which the average temperatures at each of four points 4"
(10 cm) above the mattress surface differs from the average incubator temperature at incubator
temperature equilibrium.
• Temperature var iability—The variability of the incubator temperature that will be obser ved over a 1-
hour period after incubator temperature equilibrium has been reached.
• Temperature rise time—The time required for the incubator temperature to rise 20°F (11°C), when
the air control temperature is at least 22°F (12°C) above the ambient temperature.
• Temperature overshoot—The amount by which the incubator temperature exceeds the average
incubator temperature at incubator temperatur e equilibrium as a result of an increase in control
temperature.
• Temperature correlation—Temperature indicator versus incub ator temperature—The amount
the air tempe rature indicator at incubator temperature equilibrium differs from the incubator
temperature.
• Temperature correlation—Incubator temperature versus control temperature—The amount the
average incubator temperature in Air mode at incubator temperature equilibrium differs from the
control temperature.
• Temperature correlation—Temper ature indicator versus control temperature—The amount the
air temperature indicator in Air mode at incubator temperature equilibrium diff e rs from the control
temperature.
• Measurement points—Measurements are taken at five points in a plane parallel to and 4" (10 cm)
above the mattress surface. One point is 4" (10 cm) above the center of the mattress, the remaining four
points are the centers of the four areas formed by lines that divide both the width and length in two
parts.
1 - 5

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Intended Use
This manual provides an overall functional description and the instructions for use of the Isolette® Infant
Incubator, Models C2000 and C2000e.
The Isolette® Infant Incubator, Models C2000 and C2000e should be used only by appropriately trained
personnel and under the direction of qualified medical personnel.
1 - 6

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Disclaimer
Dräger Medical cannot be responsible for the performance of the incubator if the user does not operate
the unit in accordance with the instructions, fails to follow the maintenance recommendations, or makes
any repairs with unauthorized components. Only qualified service personnel should calibrate and repair
it. Technical information is available through local distributors.
All personnel working with the unit should read, thoroughly understand, and have ready access to this
manual. Store the manual with the i ncubator when not i n use. Please contact your local r epresentative for
clarity or further information.
1 - 7

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Notes:
1 - 8

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Section 2
Introduction, Features,
and Specifications
Introduction
The Introduction subsection provides a system overview and functional description of the Isolette®
Infant Incubator, Models C2000 and C2000e.
System Overview
The Isolette® Infant Incubator, Models C2000 and C2000e is a modular controller-based incubator,
which enables simultaneous control of temperature, oxygen and humidity parameters affecting the infant.
The incubator hood and shell assemblies are mounted on a variable height adjustable (VHA) stand or
fixed height (FH) stand.
Humidity Sys tem (Optio nal )
When installed, the built-in humidifier provides humidification of the incubator fr om 30% to 95%
relative humidity (RH) in 1% increments. When the humidity system senses an absence of water an
audible and visual Low Humidi ty alarm occurs. The humidifier is a three -part system consisting of a
humidity reservoir, manifold assembly and evaporator assembly.
Humidity Reservoir
The humidity reser voir has a 1 liter capacity. The reservoir perm its visual inspection of the water level. It
is located in a drawer in the front of the incubator shell. When the drawer is closed and the latching
handle is engaged, the reservoir is connected to a manifold.
Manifold Assembly
The manifold assembly allows the water to flow into the metering valve and the evaporator assembly.
Evaporator Assembly
The metering valve regulates the flow of water to the evaporator chamber to maintain a constant level of
water, ensuring optimum responsiveness of the evaporator heater. The evaporator as sembly raises the
temperature of the water to the boiling point, causing vaporization. Any waterborne bacteria are killed,
preventing transfer into the patient compartment. The rate of vaporization is dete rmined by the level of
power transmitted to the evaporator heater. The sensor module located within the hood environment
houses the humidity sensor, which sends information to the controller module. The controller module
regulates the output of the evaporator .
Oxygen Control System (Optional)
When installed, the oxygen (servo) control system adjusts the flow of oxygen within the incubator hood
with a valve and an oxygen sensor module. Th e sensor module hous es two independent oxygen fuel cells.
When the sensor module is outside of the hood during Oxygen Control mode, audible and visual alarms
are enabled, and the flow of oxygen is interrupted.
2 - 1
NOTE:
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Weighing System (Accessory)
The description in this section does not apply to the EU (Type NAWI) weighing system. Refer to the
Isolette® Infant Incubator, Type - NAWI, Weighing System Quick Reference Guide.
When installed, the weighing system is located in a platform under the mattress. The scale contains two
load beams, which perform the actual w eighing function. The controller processes the load beam
information and displays the weight in kilograms or pounds in the Trend/Alarm window.
The Weight softkey allows for repeated re-weighing of the infant after the weighing routine has been
initiated.
System prompts are displayed in the Trend/Alarm window, during the weighing procedure.
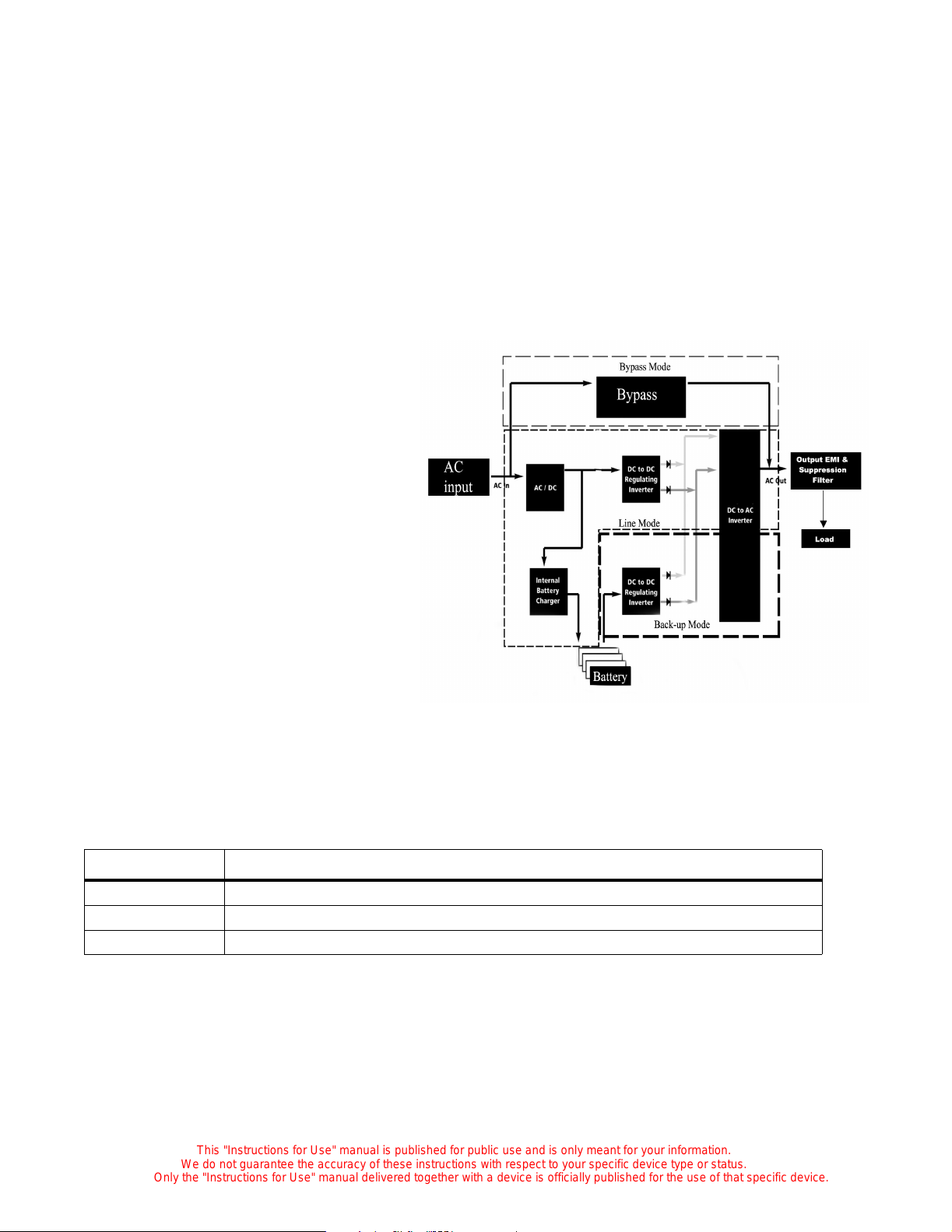
Uninterruptible Power Supply (UPS)
(Optional)
When installed, the UPS system provides
an on-line uninterruptible back-up power
supply to the incubator, which can also be
used for intra-facility transport.
With batteries fully charged, the power
available from battery backup is sufficient
to maintain a C2000e incubator in
operation for 30 min in a 20°C ambient at a
set point of 39°C in the Air mode, without
oxygen or humidity control, or additional
loads drawn from the accessory outlets.
Battery back-up usage occurs during power
failure or while transporting an unplugged
incubator within the facility.
The UPS system consists of two main components: the electronics control module and battery pack
module. The battery pack module consists of 3 sealed gel cell batteries that are charged by the electronics
module. The electronics module is responsible for monitoring, distributing and controlling the power
delivered to the incubator .
The UPS system operates in three modes: line, back-up and bypass. Refer to the table below:
Modes Operating Conditions
Line AC input normal; load range acceptable; inverter (DC to AC) operational
Back-up Loss of AC input; load range acceptable; inverter operational
Bypass Loss of inverter output; acceptable load; power supplied from AC only
2 - 2
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
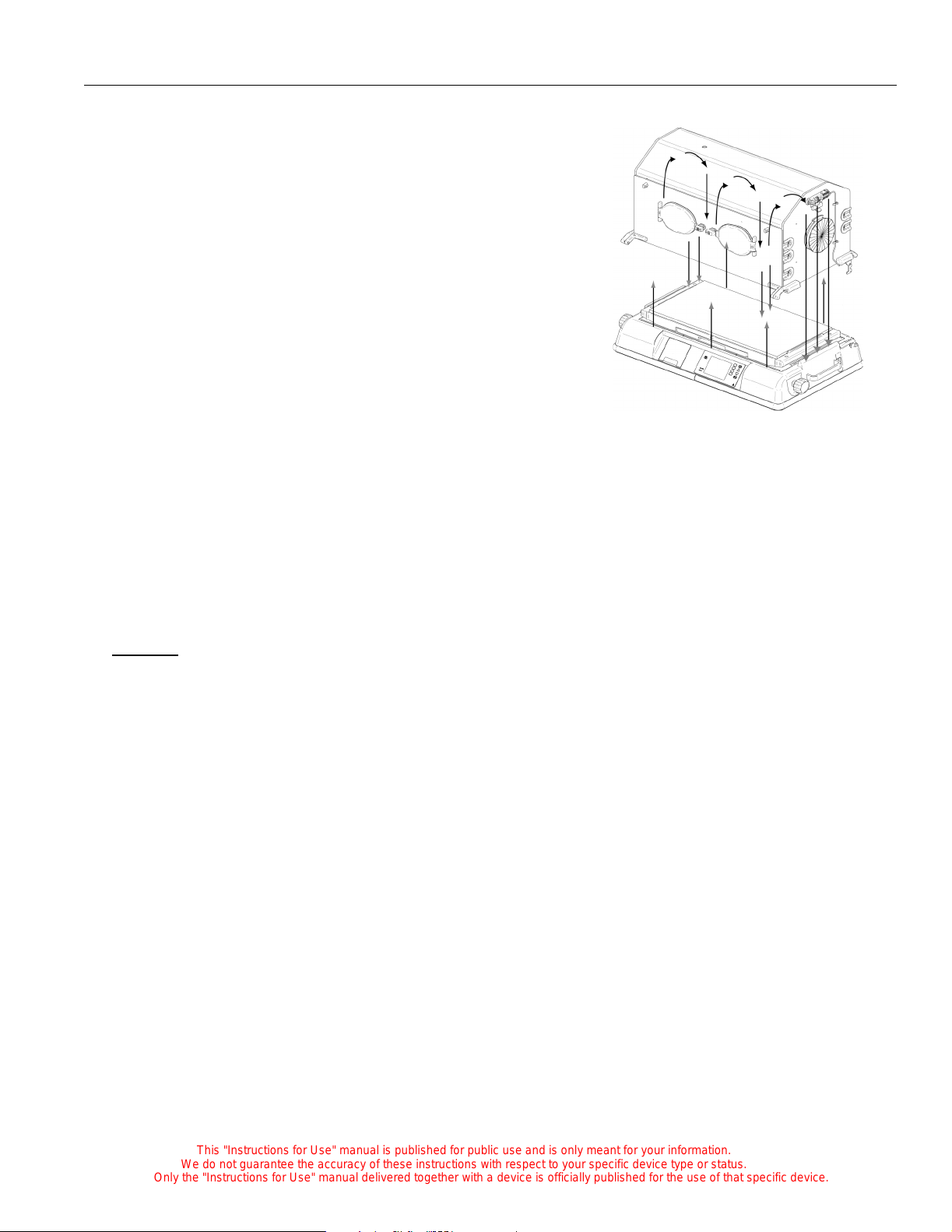
Functional Description
The temperature, humidity, and oxygen concentration is
controlled by the forced air circulation system. A controlled
amount of room air, approximately 7 lpm, is drawn through
the air intake filter by the motor-driven impeller loca ted in
the shell.
The impeller int ernally r ecirculates air at a much gr eater f low
than that of the fre sh gas inflow. The total infl ow of fresh and
re-circulated air is directed around the heater. The air enters
the infant compartment up through the slots at the front and
rear of the main deck. It then passes between the front and
rear inner walls. The air circulates past the sens or module
containing the temperature sensing probe, which
encapsulates the air temperature control ther mis tor and a
high air temperature alarm thermistor. After circulating
within the infant compartment, the air is then recirculated down through a slot in the right end of the main
deck, and back to the impeller. When the front and/or rear access panel(s) of the hood is/are open, the air
continues to flow upward past the opening, creating a warm air curtain. This curtain minimizes the drop
in air temperature in the incubator.
Temperature is regulated by using either incubator air or sk in temperature. The front panel keys enable
the user to select the desired Air or Skin mode.
In either mode of operation, the heater output is proportional to the amount of heat required to maintain
the desired temperature. The Air and Skin modes are described below .
Air Mode
In the Air mode, the air temperature can be maintained from 20.0°C (68.0°F) to 37.0°C (98.6°F) as
selected by the Up and Down Arrow keys on the front panel. In Temperature Override mode, the
temperature can be maintained from 37.0°C (98.6.0°F) to 39.0°C (102.2°F).
The incubator air temperature is monitored by a probe located in the sensor module and compared with
the air set temperature parameter. The information from this probe is supplied to the heater control
circuitry, which regulates the heater output to maintain the air temperature setting. The actual air
temperature is shown in the Temperature window. A second sensor within the air temperature probe
serves as a backup to limit the maximum incubator temperature. If the high temperature limit is reached,
the heater shuts off.
The infant temperature is a function of: 1) the air temperature and 2) the ability of the infant to establish
and maintain his/her own temperature. A sma ll infant, or one with underdeveloped homeostatic control,
may not be able to maintain a stable temperature at the desired level.
2 - 3

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Skin Mode
In the Skin mode, the Up and Down Arr ow keys on the controller front panel are used to select the infant
temperature from 34.0°C (93.2°F) to 37. 0°C (98.6°F). In Temperatur e Override mode, the temperature
can be selected from 37.0°C (98.6°F) to 38.0°C (100.4°F).
A temperature sensing probe is attached directly to the s kin of the infant. The information from the probe
is supplied to the heater control circuitry, which proportions the heater output to maintain the skin set
temperature.
The air temperature is still shown in Skin mode, but for information purposes only. If the Air mode is
selected while the skin probe remains connected, the skin temperature parameter continues to display the
actual skin temperature. However, it does not control the incubator temperature.
The sensor module is equipped to accept two skin probes. To control the incubator temperature in the skin
mode, insert a skin probe into the skin probe 1 connector (see “Controls, Indicators and Connectors” on
page 5-1). When a second skin probe is connected to the sensor module while operating in the skin mode,
an alarm sounds and the message Remove Skin 2 Probe is displaye d. To connect a second skin probe,
select the Air mode first. The controller then displays the respective Skin 1 and Skin 2 temperatures
monitored by the skin probes .
If Probe 1 is disconnected from its receptacle while in the Skin mode, the skin temperature parameter
goes blank on the display, an alarm sounds, and the heater turns off.
2 - 4
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Features
The Features subsection provides a list of the standard and optional features and available accessories for
the Isolette® Infant Incubator, Models C2000 and C2000e.
Standard Features
Standard features include:
• Oval access doors with a quiet latch
• Trendelenberg mattress tilt mechanism (0° to 12°)
• Pedestal base cover (C2000e, only)
• Rail system (C2000e, only)
Optional Features
Options include:
• Oxygen control system
• Humidity system
• Fixed height stand (without UPS system)
• VHA stand (without UPS system)
C2000e only
• Fixed height stand (with UPS system)
• VHA stand (with UPS system)
Accessories
Accessories include:
• Weighing system
• Ventilation support
• Temperature probes
• Port sleeves and cuffs
• 5 kg weight
• Gas tank mounts and tanks (E and D sizes)
C2000e only
• Rail mounted accessories
C2000 only
• Non-rail mounted accessories
2 - 5
≤
≤
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
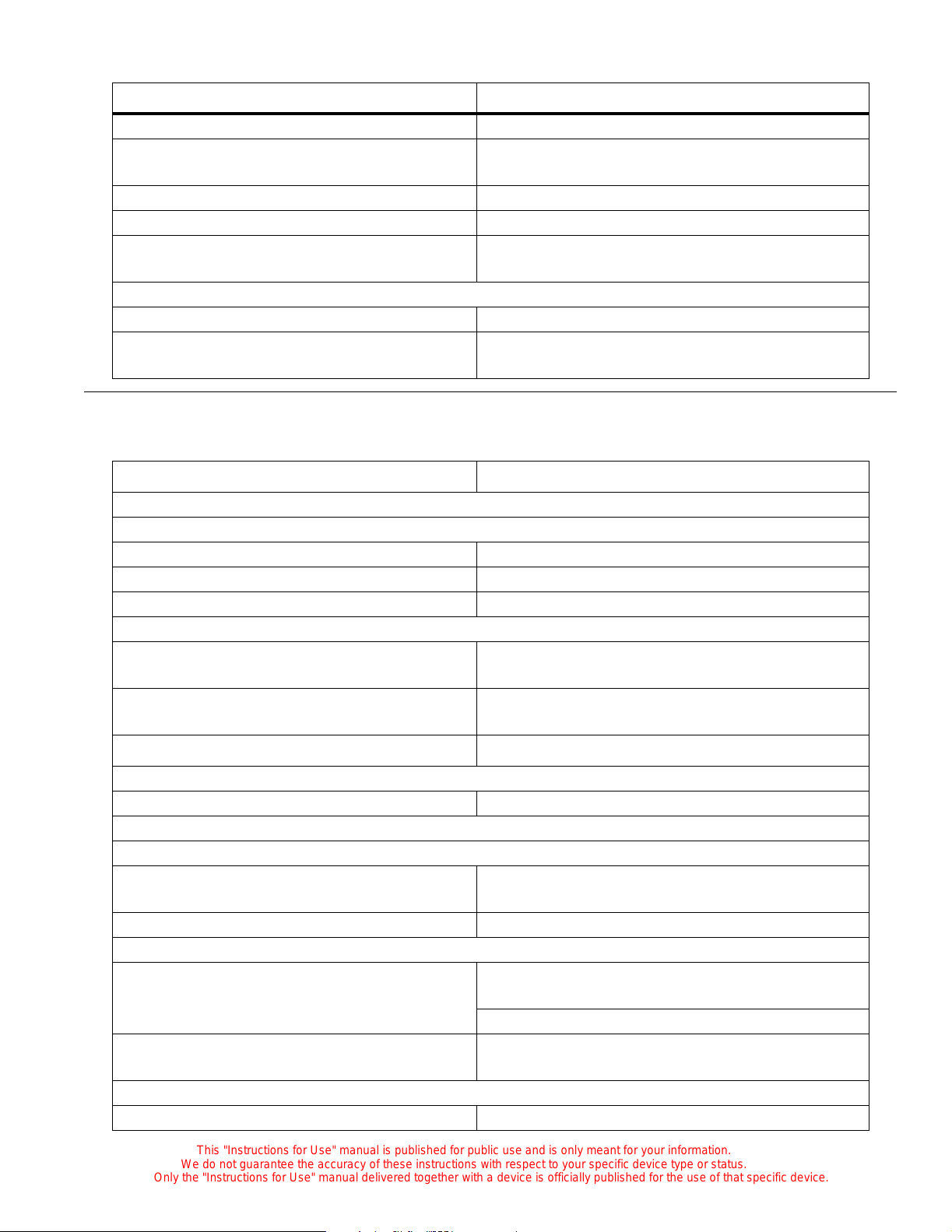
Specifications
The Specifications subsection provides specifications for the standard and optional features and
available accessories of the Isolette® Infant Incubator, Models C2000 and C2000e. It also includes
regulation, standards and code information for the system.
Standard Features
Physical
C2000
Depth 67.31 cm (26.5)
Width 102.9 cm (40.5'')
C2000e
Depth 67.31 cm (26.5")
Width 120 cm (47.25'') (including rail width)
Incubator Weight 49 kg (108 lb)
Mattress tray width 79 cm (31")
Mattress tray depth 41 cm (16")
Mattress Trendelenburg/Re verse
Trendelenburg tilt
Electrical
Convenience outlets
(Model C2000, 100V, only)
Convenience outlets (120V) 120V, 50/60 Hz, 300 W maximum
Convenience outlets (230V) 230V, 50/60 Hz, 300 W maximum
Chassis current leakage (100V and 120V) 300 µA
Feature Dimension
≥
Continuously variable to 12° ± 1°
100V, 50/60 Hz, 300 W maximum
Chassis current leakage (230V) 500 µA
Environmental
Air mode control temperature range 20.0°C (68.0°F) to 37.0°C (98.6°F)
Air mode control override temperature range 37.0°C (98.6°F) to 39.0°C (102.2°F)
Skin mode control temperature range 34.0°C (93.2°F) to 37.0°C (98.6°F)
Skin mode control override temperature range 37.0°C (98.6°F) to 38.0°C (100.4°F)
Temperature rise time at 22°C (72°F) ambient < 35 min
Temperature variability < 0.5°C
Temperature overshoot < 0.5°C maximum
Temperature uniformity with a level mattress < 0.8°C
Correlation of the indicated air temperature to
≤
0.8°C
the actual incubator temperature (after the
incubator temperature equilibrium is reached)
Environment temperature operating range 20°C (68°F) to 30°C (86°F)
Operating temperature— RH sensor 20°C (68°F) to 41°C (106°F)
Operating temperature— oxygen sensor 20°C (68°F) to 41°C (106°F)
Operating humidity range 5% to 99% RH non-condensing
2 - 6
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Feature Dimension
Noise level within the hood environment < 47 dBa with 37 dBa or less ambient
Air velocity over the mattress < 4"/second (10 cm/s econd); average of five points
at 4" (10 cm) above the mattress
Storage temperature -25°C (-13°F) to 60°C (140°F)
Storage humidity range 0% to 99% relative humidity non-condensing
Carbon Dioxide (CO2) Level
<0.5%
(per EN60601-2-19, Clause 105)
Operational
Set point data retention (non-UPS systems) power failures lasting <10 min
Set point data retention (UPS systems) power failures lasting <(batte ry charge depletion
time + 10 min)
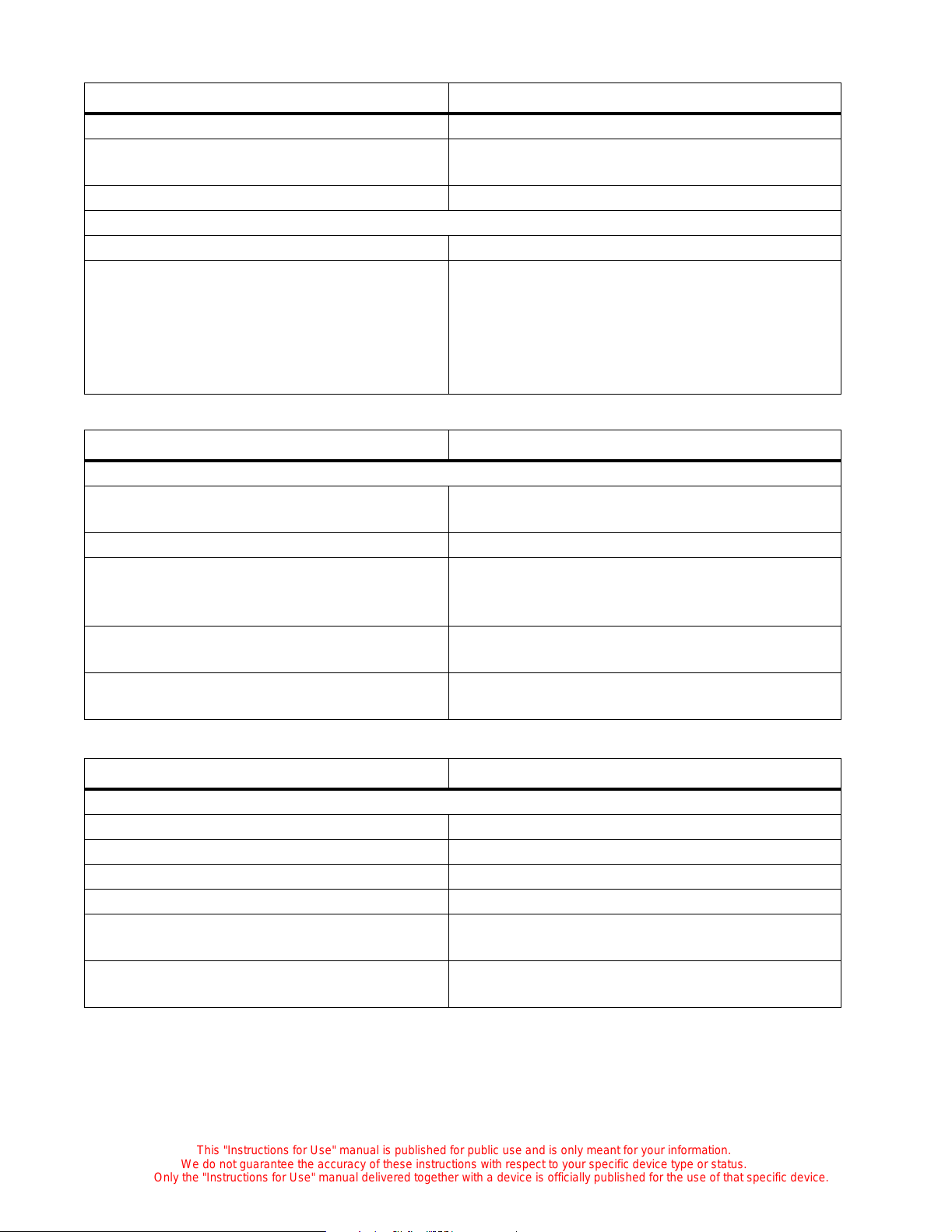
Options and Accessories
Stands
Feature Dimension
Physical
Fixed Height Stand
Top of hood to floor (incubator with FH stand) 142 cm (56") ± 12.7 mm (½")
Mattress to floor (incubator with FH stand) 100.97 cm (39¾") ± 12.7 mm (½")
Weight (without UPS system and accessories) 49.4 kg (109 lb)
Variable Height Stand
Top of hood to floor (incubator with VHA
stand)
Mattress to floor (incubator with VHA stand) 90.17 cm (35½") ± 12.7 mm ( ½") to 1 10½" (43½")
Weight (with UPS system and accessor i es )
133.35 cm (52½") ± 12.7 m m (½") to 152 cm (60")
± 12.7 mm (½")
± 12.7 mm (½")
97 kg (214 lb)
≤
UPS
Battery pack weight 9.5 kg (20.9 lb)
Electrical
Fixed Height Stand
Power requirements for 100V/120 FH stand
100 V/120 V, 50/60 Hz, 9.9 A maximum
(100V for Model C2000, only)
Power requirements for 230V FH stand 230 V, 50/60 Hz, 9.9 A maximum
Variable-Height Stand
Power requirements for 100/120V VHA stand
(100V for Model C2000 only)
Without UPS: 100 V/120 V, 50/60 Hz,
9.9 A maximum
With UPS: 120 V, 50/60 Hz, 11 A maximum
Power requirements for 230V VHA stand
model
With and without UPS: 230V, 50/60 Hz, 9.9 A
maximum
UPS
Input current breaker rating 15 A for 120 V; 7A for 230 V
2 - 7
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Feature Dimension
Output circuit breaker rating 10 A for 120 V; 5 A for 230 V
Input frequency 50/60 Hz (auto sensed by the microprocessor)
45-65 Hz (inverter phase-lock frequency range)
Battery type gel cell
Operational
UPS charge time Full capacity after 8 hours
UPS operating time With batterie s fully charged, the power available
from battery backup is suffic ient to maintain a
C2000e incubator in operation for 30 min in a
20°C ambient at a set point of 39°C in the Air
mode, without oxygen or humidity control, or
additional loads drawn from the accessory outlets.
Humidity System
Feature Dimension
Humidity System
Humidity control operating time without
refilling
24 hours maximum @ 85% RH and 36°C, in Air
mode
Humidity control reservoir capacity 1000 ml
Humidity control range 30% to 95% in 1% increments (at high ambient
humidity levels, low level humidity settings may
not be attainable)
Humidity control accuracy between 10% and
± 6% RH
90% @ 68°F (20°C) to 104°F (40°C)
Maximum humidity levels >85% (incubator set temp at 39°C, with at least
30% RH at ambient)
Oxygen System
Feature Dimension
Oxygen Control System
Oxygen inlet pressure 40 psi to 150 psi
Oxygen inlet flow rate 30 liters/min
Oxygen control range 21% to 65%
Oxygen display resolution 1% increments
Oxygen control accuracy at 100%
± 3%
calibration
Oxygen control accuracy at 21%
± 5%
calibration
2 - 8

NOTE:
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Weighing System
The procedures described in this section do not apply to the EU (T ype NAWI) weighing system. Refer to
the Isolette® Infant Incubator, Type -NAWI, Weighing System Quick Reference Guide.
Feature Dimension
W ei ghing Syst em
Weight display range 0 kg (0 lb) to 7 kg (15 lb)
Weight display resolution 1.0 g or 0.04 oz
Weight display accuracy 0 - 2 kg; 2 g ± ½ digit
> 2 kg; ±5 g ± ½ digit
Maximum tare weight 4.0 kg (8.82 lb)
Rail Accessory Weight Limitations
Feature Dimension
Rail System Assembly T otal rail accessory weight not to exceed 36.3 kg (80
lb); 13.1 kg (40 lb) per side rail or 6.8 kg (15 lb) per
front and rear rail (with remaining weight distributed
along side rails)
Monitor shelf assembly,
11 kg (25 lb)
high/low, C2000e
I.V. pole assembly,
5 kg (11 lb)
C2000e
Basket 18.0 W x 9.5 D x
4.4 kg (10 lb)
4.0 H
Basket 6.5 W x 4.0 D x
2.2 kg (5 lb)
5.0 H
Basket 11.0 W x 4.0 D x
2.2 kg (5 lb)
4.0 H
Basket - pivoting 2.2 kg (5 lb)
Chart holder
Hinged mayo tray, 13.5
2.2 kg (5 lb)
W x 9.75 D
Hinged mayo tray, 17.0
2.2 kg (5 lb)
W x 11.5 D
Cable organizer 2.2 kg (5 lb)
Horizontal cord wrap 2.2 kg (5 lb)
Standard cam adapter 2.2 kg (5 lb)
Ball action adapter 2.2 kg (5 lb)
Double cam adapter 2.2 kg (5 lb)
Cam adapter, threaded
2.2 kg (5 lb)
mount
Utility hook assembly 2.2 kg (5 lb)
2 - 9

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
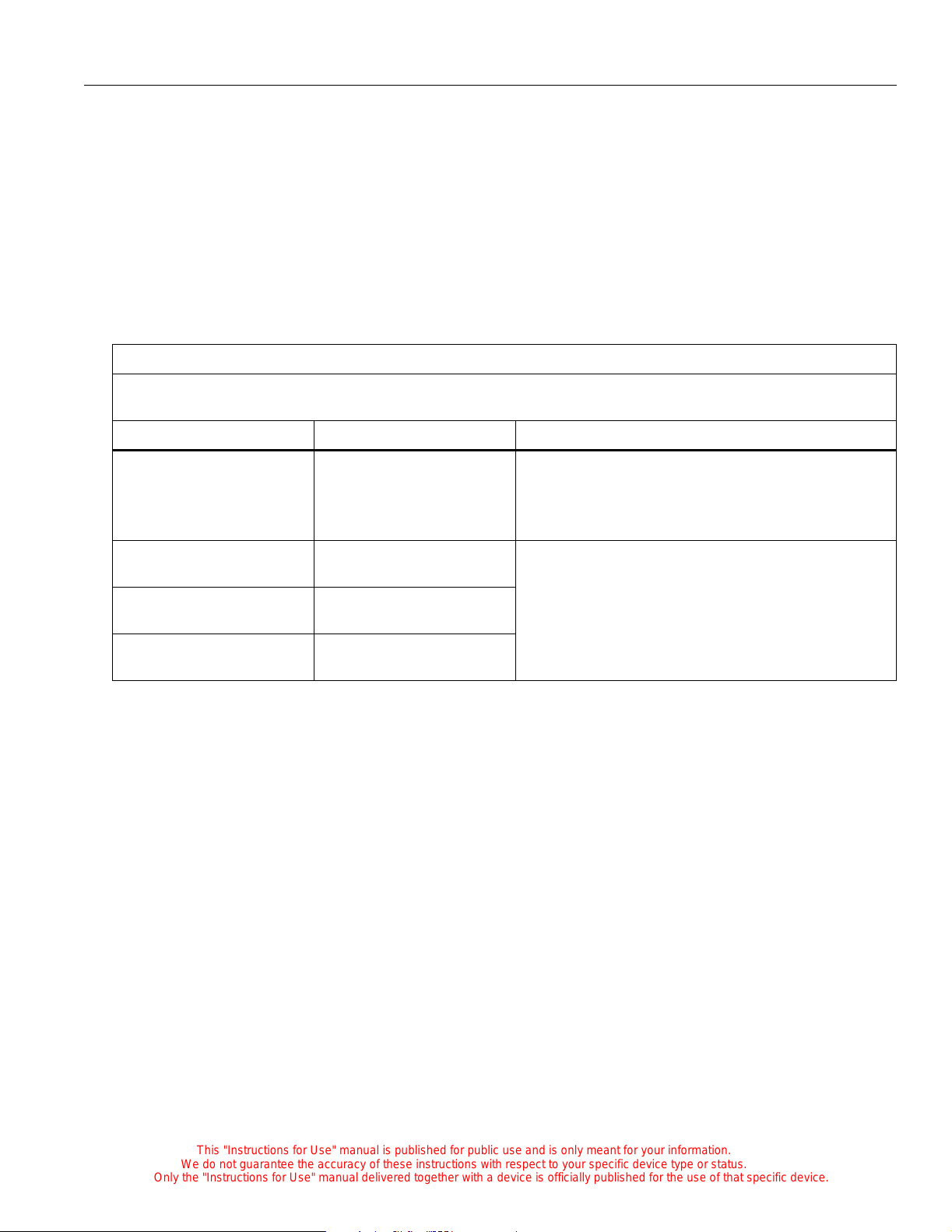
Non-Rail Accessory Weight Limitations
Feature Dimension
Monitor shelf assembly,
11 kg (25 lb)
high, C2000e
Monitor shelf assembly,
11 kg (25 lb)
low , C2000e
I.V. pole assembly,
5 kg (11 lb)
C2000e
Tray assembly, large 17.6 kg (40 lb)
Swivel drawer assembl y,
large
Swivel drawer assembl y,
small
Swivel drawer assembl y,
small, C2000e
Drawer housing assem-
Tray - 0.91 kg (2 lb)
Drawer - 4.5 kg (10 lb)
Tray - 0.91 kg (2 lb)
Drawer - 2.2 kg (5 lb)
Tray - 0.91 kg (2 lb)
Drawer - 2.2 kg (5 lb)
2.2 kg (5 lb)
bly, shallow, short
Drawer housing assem-
2.2 kg (5 lb)
bly, shallow, long
Swivel drawer assembl y,
large, C2000e
Drawer housing assem-
Tray - 0.91 kg (2 lb)
Drawer - 4.5 kg (10 lb)
4.5 kg (10 lb)
bly, deep, short
Drawer housing assem-
4.5 kg (10 lb)
bly, deep, long
2 - 10
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Regulations, Standards, and Codes
The Isolette® Infant Incubator, Models C2000 and C2000e complies with the following safety standards
and performance standards:
• EN 60601-1—1990, Medical Electrical Equipment, Part 1: General Requirements for Safety ,
including Amendments 1, 2, 12 and 13
• EN 60601-1-2—2002, Collateral Standard: Electromagnetic Compatibility—Requirements and Test s
• EN 60601-2-19—1996, Particular Requirements for the Safety of Baby Incubators, including
Amendment 1
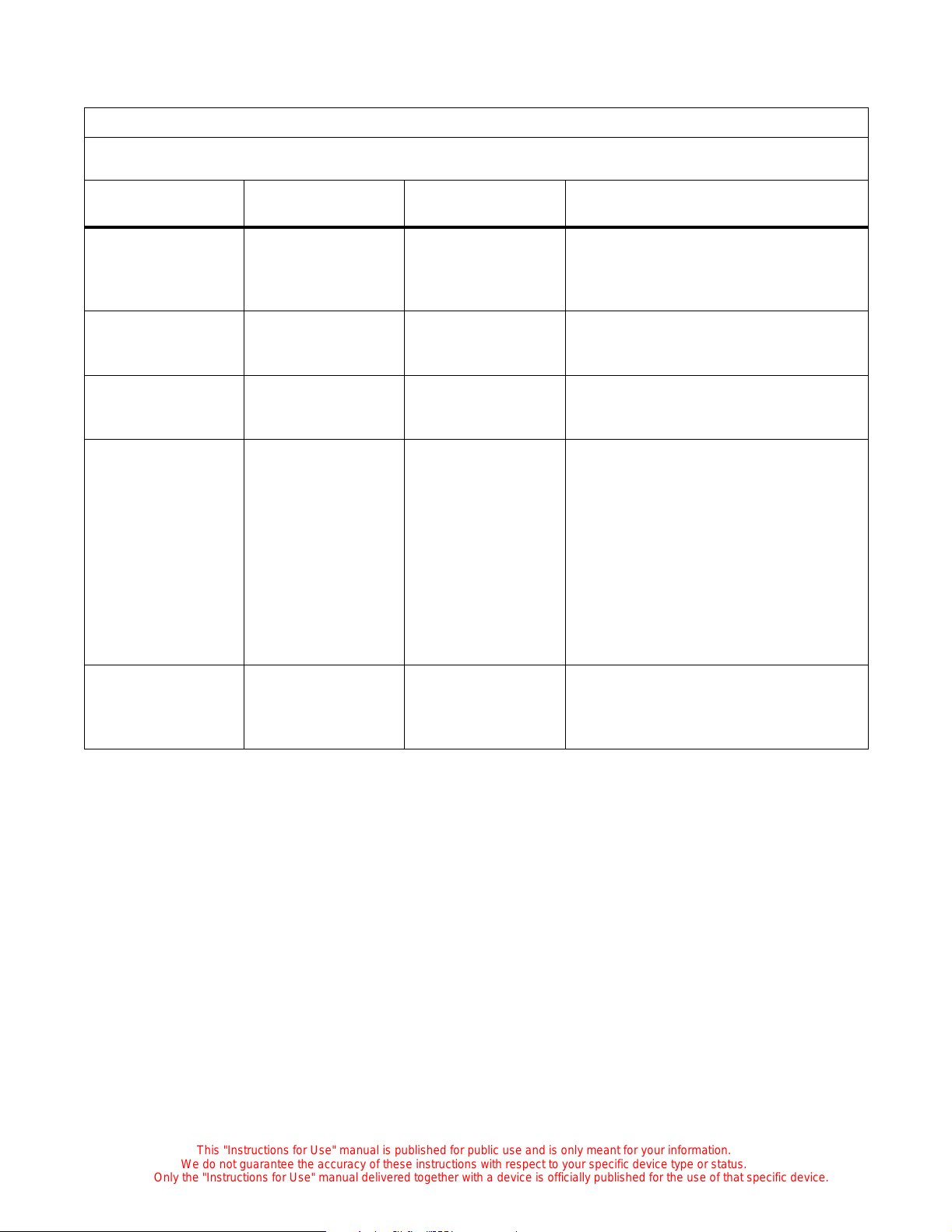
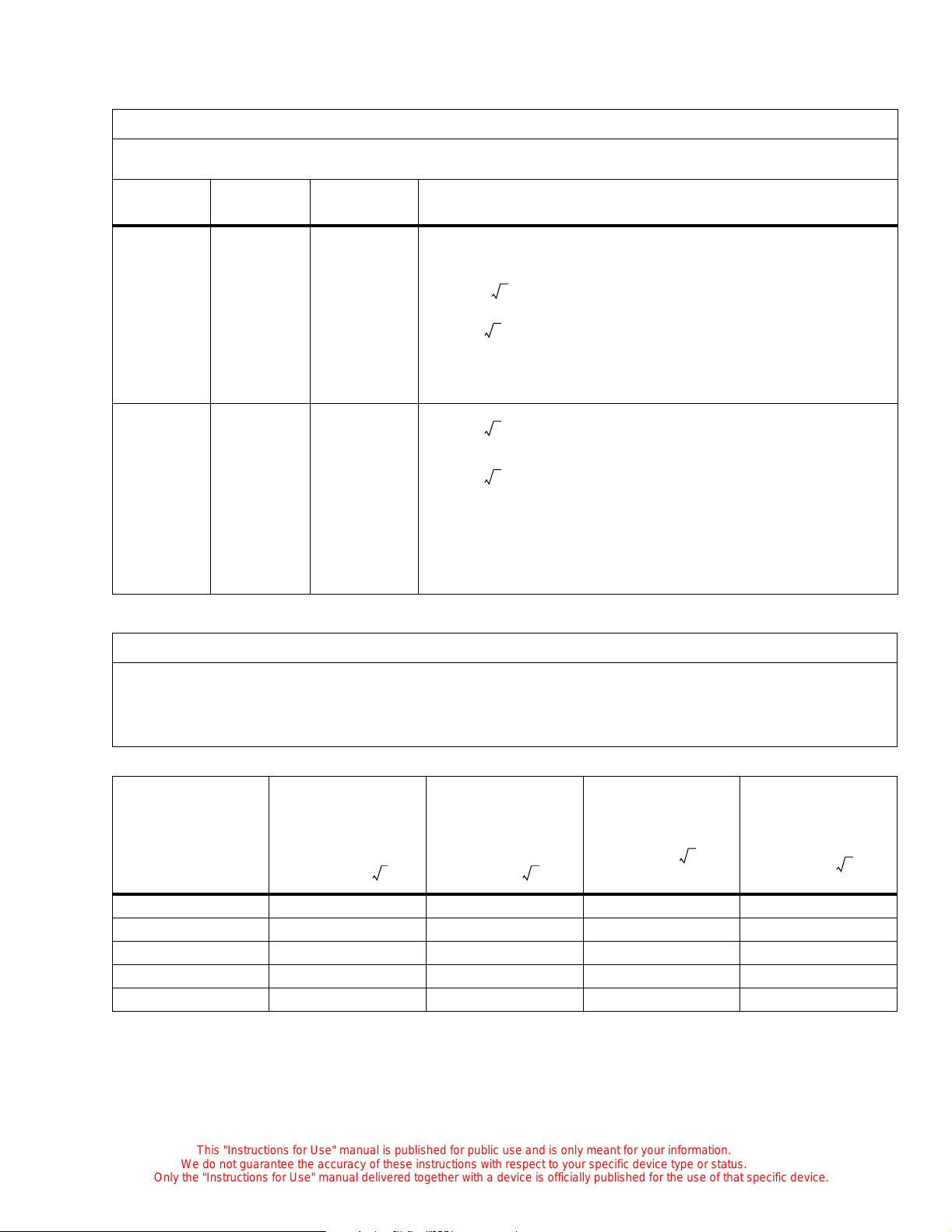
Electromagnetic Compatibility (EMC) Guidance and Manufacturer Declarations
Guidanc e and Manu facturer’s Declarat ion — Emiss ion s
The C2000 is intended for use in the electromagnetic environment specified below. The customer or
user of the unit should ensure that the unit is used in such an environment.
Emissions Test Compliance Electromagnetic Environment—Guidance
Radio frequency (RF)
emissions—CISPR 11
RF emissions
CISPR 11
Harmonics
IEC 61000-3-2
Flicker
IEC 61000-3-3
Group 1 The C2000 uses RF energy only for its internal
function. Therefore, its RF emissions are very
low and are not likely to cause interference with
nearby electronic equipment.
Class A The C2000 is suitable for use in all establish-
ments, including domestic, and those directly
Class A
connected to the public low-voltage power supply network that supplies buildings used for
Class A
domestic purposes.
2 - 11
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Guidance and Manufacturer’s Declaration—Immunity
The C2000 is intended for use in the electromagnetic environment specified below. The customer or user of the
C2000 should ensure that the unit is used in such an environm ent.
Immunity Test
ESD
IEC 61000-4-2
EFT
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage Dips/Dropout
IEC 61000-4-1 1
IEC 60601 Test
Level
± 6 kV contact
± 8 kV air
± 2 kV Mains
± 1 kV I/Os
± 1 kV Differential
± 2 kV Common
> 95% dip for 0.5
cycle
60% dip for 5 cycle
30% dip in for 25
cycles
> 95% dip for 5 seconds
Compliance Level
± 6 kV contact
± 8 kV air
± 2 kV Mains
No I/Os
± 1 kV Differential
± 2 kV Common
> 95% dip for 0.5
cycle
60% dip for 5 cycle
30% dip in for 25
cycles
> 95% d ip for 5 seconds
Electromagnetic Environment—
Guidance
The floors should be wood, concrete, or
ceramic tile. If floors are covered with
synthetic, the relative humidity should be
at least 30%.
Mains power quality should be that of a
typical commercial or hospit al
environment.
Mains power quality should be that of a
typical commercial or hospit al
environment.
Mains power quality should be that of a
typical commercial or hospit al
environment. If the user of the C2000
requires continued operatio n during power
mains interruptions, it is recommended
that the C2000 be powered from an
uninterruptible power supply or battery.
Power frequency
50/60 Hz
Magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should
be that of a typical commercial or hospital
environment.
2 - 12
D
P
P
P
D
P
P
P
P
This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Guidance and Manufacturer’s Declaration— Immunity
The C2000 is intended for use in the elect romagnetic environment specified below. The customer or us er of the C2000
should ensure that the unit is used in such an environment.
Immunity
Test
Conducted
RF
IEC 61000-46
IEC 6060 1
Test Level
3 Vrms (outside ISM)
20Vrms (in
ISM bands)
150 KHz to
80 MHz
Compliance
Level
V1=3 Vrms
V2=10 Vr m s
Electromagneti c En vironmen t—Guid an ce
Recommended Separation Distance
Portable and mobile comm unications equipment should be sepa rated from
the C2000 by no less than the distances calculated/listed below:
1.167 P=
D 1.2
=
outside ISM
in ISM
Radiat ed RF
IEC 61000-43
10 V/m E1=10 V/m
80 MHz to
2.5 GHz
D 1.2
=
D 2.3
=
where P is the maximum power in watts and D is the recommende d
separation distance in meters.
Field strengths from fixed RF transmitters, as determ ined by an
electromagnet ic site survey, should be less than the c omp liance levels (V1,
V2, and E).
80 MHz to 800 MHz
800 MHz to 2.5 GHz
Recommended Separations Distances for the C2000
The C2000 is intended for use in the electromag netic environment in which radiated RF disturbances are controlled. The customer or user of the C2000 can help preve nt electromagnetic interference by maintaining a minimum distance between portable and mobile RF Communications Equipment and the C2000 as recommended,
according to the maximum output power of the communica tions equipment.
Separation (m)
150 kHz to
80 MHz
ISM
=
Separation (m)
80 to 800 M H z
D 1.2
=
Separation (m)
800 MHz to
2.5 GHz
D 2.3
=
Maximum Output
Power
(Watts)
Separation (m)
150 kHz to
80 MHz
Non-ISM
1.167
= D 1.2
0.01 0.1167 m 0.12 m 0.12 m 0.23 m
0.1 0.369 m 0.38 m 0.38 m 0.73 m
1 1.167 m 1.2 m 1.2 m 2.3 m
10 3.69 m 3.8 m 3.8 m 7.3 m
100 11.67 m 12 m 12 m 23 m
2 - 13

This "Instructions for Use" manual is published for public use and is only meant for your information.
We do not guarantee the accuracy of these instructions with respect to your specific device type or status.
Only the "Instructions for Use" manual delivered together with a device is officially published for the use of that specific device.
Device Classification
The Isolette® Infant Incubator, Models C2000 and C2000e meets the requirements for the following
classifications:
•Class I
• Type BF
• IPX0—Ordinary equipment
•Not AP
• Continuous operation
2 - 14
 Loading...
Loading...