AV-S Ventilator
User Manual
This manual contains information for software upgrade v.1.87.01

DATASHEET
USER
AV-S Ventilator User Manual
Software Version 1.87.01
Addendum to the User Manual
Keep this Datasheet with the User Instruction Manual for AV-S at all times
Introduction
AVS software V. 1.87.01 introduces a new
user interface. However, if you are familiar
with the AV-S, please note that the basic
operation and calibration procedures remain
as per v.1.86.
Modifications to the User Interface
Setting up the Ventilator for use
1. Introduction Screen
1.1 Start-up
At start-up, the introduction screen
allows the user to select one of three
default settings:
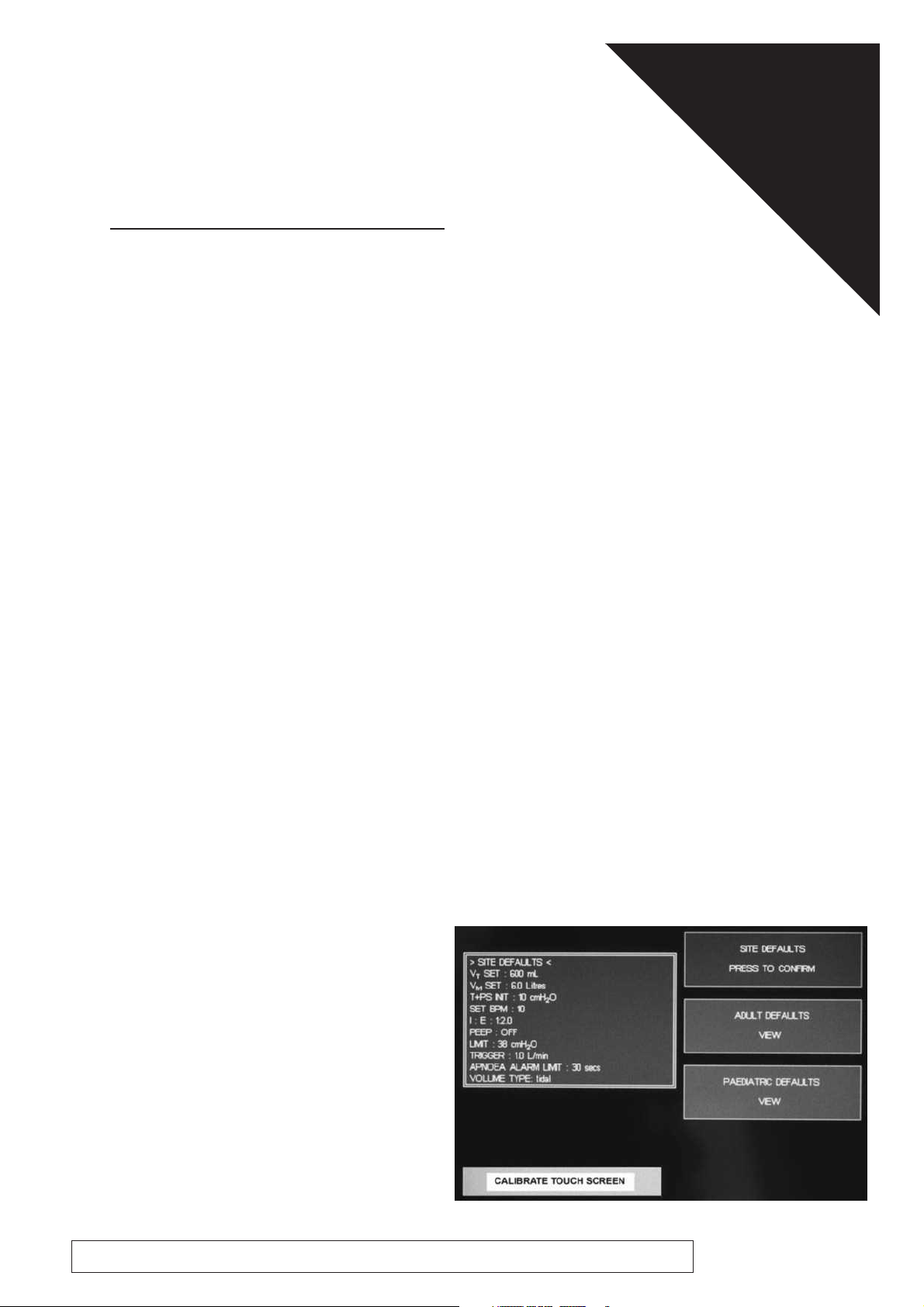
SITE DEFAULTS
ADULT DEFAULTS
PEDIATRIC DEFAULTS
NOTE
a) The user must select one of the
above default groups before the
ventilator will switch to standby in that
default mode
b) SITE DEFAULT is editable in
standby mode (see section 1.2, below)
c) Settings can be saved via the
service menu to create a new site
default
1.2 Default Settings
1.2.1 Selection
The user can select ADULT, or
PEDIATRIC, or SITE, and view the
default parameter settings.
The options will remain, even after the
ventilator is turned off.
1.2.2 Site Default Settings
Adjust the parameter values from within
the Service menu (SITE DEFAULTS)
Press to confirm the new settings for
site defaults.
1.3 Calibrate Touchscreen
The introduction screen allows the
user to calibrate the screen

2. Parameter Display Identification
2.1 Active Parameters
Active parameters that can be set for use
in the current mode are displayed as:
White Text on Blue
2.2 Inactive Parameters
Inactive parameters that can be set for
any non-current mode are displayed as:
White Text on Blue Label
White values on Black
2.3 Measured Parameters
Yellow values on Black
2.4 T+PS INIT (target and pressure support
initial value)
The initial pressure value can be changed
so that when entering either PRESSURE
or PSV modes the TARGET value or
PSUPP value are pre-selected.
NOTE
Changing either of these limits in their
active modes will maintain the value when
changing between PSV, PRESSURE, and
STANDBY modes.
3. Gas Mixture
The Gas Mixture window is an active
touch-selectable area (in any mode), with
a drop down menu.
Gas Mixture is also available through the
menu structure.
Selection of the required mixture is in the
normal way with the scroll wheel.
Using the Ventilator - description of
modes and functions
4. Modes
4.1 Access to Support Modes
Access is available in Standby mode
(depending on the support mode options
on the ventilator).
Support Mode
a) PSV
b) SIMV
c) SMMV
d) SIGH ENABLE
SIGH TO BREATH RATIO
e) INSP PAUSE
INSP PAUSE %
WARNING
Modes a, b, and c are only available
when Spirometry is enabled.
2

4.2 Standby Mode
a) Standby mode at ventilator start-up:
The last used Volume mode settings will
be displayed
b) Standby mode selected while the
ventilator is in use:
The screen will display the previous
ventilation mode, highlighted in yellow,
within the relevant box. The last used
parameters will also be displayed.
4.3 Spontaneous Mode
a) Spontaneous mode at ventilator start-
up:
Default values will be displayed in white
on a black background if the ventilator
has just been powered ON.
b) Spontaneous mode selected while the
ventilator is in use:
The last used ventilation mode
(underlined) will be displayed, with the
last used set values in white on a black
background
4.4 Sigh
Sigh is settable from 1:n, where n has a
range of 10 to 100.
The Sigh menu can also be accessed by
touching the icon area of the screen.
NOTE
1:10 is one sigh to ten normal breaths.
4.5 Inspiratory Pause
Inspiratory pause can be varied in the
menu from 0 - 60%.
The inspiratory pause menu can also be
accessed by touching the icon area of
the screen.
WARNING
This can affect the maximum Tidal
Volume.
3
5. Apnoea Alarm Mute - Spontaneous
mode only
NOTE
The occurrence of another alarm event
will override this feature
In spontaneous mode the mute button
acts both to silence an existing apnoea
alarm and inhibit new apnoea alarms for
a given period (provided that no other
alarm events are present) .
This time period is selectable (choose
from 15, 30, 60, 120, or 180 seconds)
through the alarm settings menu, or
accessed by touching the alarm area of
the screen.
To adjust the default setting, use the SITE
DEFAULT menu option.
6. Touchscreen Access to Mode
Configuration Options
Touch the screen in the area containing
the green icons to access mode
configuration options (including INSP
PAUSE, SIGH, and APNOEA ALARM
mute/inhibit).
7. Waveform Pause and Print
Waveform pause and print icons are
located to the left hand side of the
waveform displays.
Ensure that a compatible printer is
connected, and switched On (see section
5.1.8).
To print the waveform information, press
the pause icon. The print icon will be
displayed. Press the icon to print.
Press the pause icon to unfreeze the
waveform.
8. Waveform Freeze Loop
The FREEZE LOOP icon is located at the
left hand side of the top waveform.
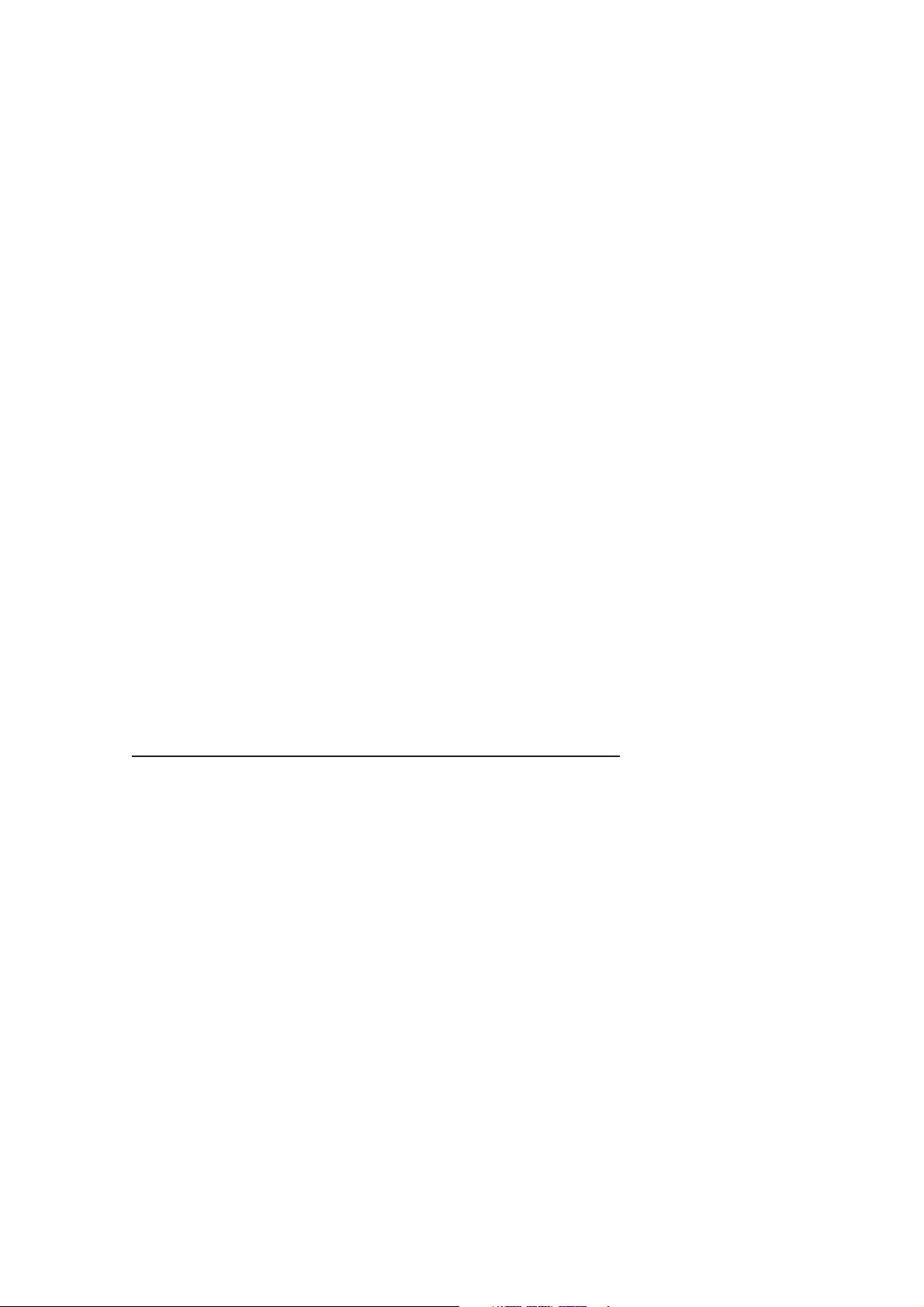
9. Leak Test
a) Select LEAK TEST through the Menu
in Standby Mode.
b) With the bag/vent switch in VENT
position, this checks for a leak using an
occluded breathing system
The leak test procedure is given in
section 5.1.12 in the user manual.
4
Modifications to Operational Envelope
(sections 4.5 and 4.6 in user manual)
Flow Range: 2 – 70 Litres per min.
Volume Range: 20 ml – 1.6 Litres (tidal)
2 – 50 Litres per min (minute vol.)
Rate 4 – 100 bpm
I:E Ratio 1:0.2 – 1:8.0 (normal)
1:2.0 – 1:8.0 (effective in support modes)
Inspiratory Time 0.3 – 10 seconds (normal)
0.3 – 5 seconds (effective in support modes)
ExpiratoryTime 0.3 – 10 seconds
(effective dependent on Inspiratory time)
Additional information on Sigh and Inspiratory Pause
(section 3.7.2.4)
Inspiratory Pause
Inspiratory Pause has a range of 0 to 60% of the inspiratory time.
Sigh
This function must be enabled in the mode menu but is only
operational in volume ventilation.
The sigh ratio is 1 to n (1:n) with n giving a range of 10 – 100 breaths
between sighs.
5
EXIT MENUS
O2 MONITOR & SPIROMETRY
LEAK TEST
FRESH GAS COMPENSATION:ON
MODES
WAVEFORM
ALARM SETTINGS
GAS MIXTURE: O2+AIR
SERVICE MENU
O2 Monitor & Spirometry
ESCAPE FROM MENU
O2 MONITOR: on
CALIBRATION: 100%
HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min
off / on (Toggle option
21 / 100% (Toggle option)
19 -105 (Integer)
18 - 99 (Integer)
off / on (Toggle option)
0 L/min / 10 L/min (Toggle option)
off/on (Toggle option)
Fresh Gas Compensation
ON / OFF
Special Modes
See next page
Waveform
ESCAPE FROM MENU
SECOND WAVEFORM: off
Second waveform pick list
off
vol. vs time
vol. vs press.
Alarm settings
ALARM MENU
ESCAPE FROM MENU
ALARM MODE : default
HIGH TIDAL VOLUME: off
VM MIN: 3 L
VM MAX: 9 L
VT MIN: 300 mL
VT MAX: 900 mL
APNOEA ALARM LIMIT: 15 secs
ALARM VOLUME: 50%
default / user (Toggle option)
off / on (Toggle option)
0.0 - 7.4 (Integer)
0.1 - 7.5 (Integer)
10 - 1600 (Integer)
20 - 2400 (Integer)
0.3 - 3.5 (Integer)
50 - 100% (Integer)
Gas mixture: O2+Air
O2+AIR
O2+N2O
Leak Test
ESCAPE FROM MENU
<START/STOP LEAK TEST>
LEAK STATUS: unknown
LEAK LEVEL: 0 mL/min
BSYS COMP 7.0 mL/cmH2O
Service
See page 62
Menu Structure
Main Menu
6
The SPECIAL MODES menu is context sensitive, with the contents
dependent on current mode.
In STANDBY the SPECIAL MODES menu is:
ESCAPE FROM MENU
SUPPORT MODE: SIMV, SMMV, PSV
(1)
VOLUME TYPE: Tidal
SIGH ENABLE:
(2)
SIGH TO BREATH RATIO:
INSP. PAUSE% : 0%
APPLY: SITE DEFAULT
In SPONT mode and VOLUME mode, and SIMV/ SMMV, the SPECIAL
MODES menu is:
ESCAPE FROM MENU
VOLUME TYPE: Tidal
SIGH ENABLE:
(2)
SIGH TO BREATH RATIO:
INSP. PAUSE% : 0%
In PRESSURE mode and PSV modes the SPECIAL MODES menu is:
ESCAPE FROM MENU
SIGH ENABLE:
(2)
SIGH TO BREATH RATIO:
INSP. PAUSE% : 0%
Notes
(1) Support mode depends on configuration options.
The SUPPORT MODE option will be missing from the SPECIAL
MODE menu if:
a) Options are not enabled
b) ‘’SPIROMETRY: off’’ is displayed.
The support mode sub menu can include:
none / PSV / SIMV / SMMV
(2) The options here are: 0 - 60%
(3) The options here are:
on - off
1:10 to 1:100
Note
1:10 indicates 1 breath with sigh, then 10 breaths without sigh
(2) The TRIGGER values are L/min with SPIROMETRY enabled, or
cmH
2O when SPIROMETRY disabled.
Spirometry enabled Spirometry disabled
0.7 L/min 0.5 cmH
2O
0.8 L/min 0.6 cmH
2O
0.9 L/min 0.7 cmH
2O
1.0 L/min 0.8 cmH
2O
1.5 L/min 0.9 cmH
2O
2.0 L/min 1.0 cmH
2O
2.5 L/min 1.2 cmH
2O
3.0 L/min 1.5 cmH
2O
3.5 L/min 1.7 cmH
2O
4.0 L/min 2.0 cmH
2O
SPECIAL MODES MENU
ESCAPE FROM MENU
SUPPORT MODE: SIMV, SMMV, PSV
VOLUME TYPE: Tidal
SIGH ENABLE:
SIGH TO BREATH RATIO:
INSP. PAUSE% : 0%
APPLY: SITE DEFAULT
7
UPGRADE MENU
ESCAPE FROM MENU
I/O HARDWARE: 2
I/O FIRMWARE: vx.xx [Build xx]
MAIN FIRMWARE: vx.xx [Build xx]
REGISTRATION KEY: unknown
UPGRADE FIRMWARE: unavailable
ADD NEW FEATURE: unavailable
DISPLAY HISTORY
ESCAPE FROM MENU
MANUFACTURER DATE : 03/03/05
TOTAL HOURS RUN: 100
LAST SERVICE DATE: 13/08/04
HOURS SINCE SERVICE: 100
DRIVE VALVE CYCLES: 1253
PATIENT VALVE CYCLES: 822
CUTOFF VALVE CYCLES: 72
Service
ESCAPE FROM MENU
LANGUAGE: ENGLISH
PATIENT LOG MENU
SITE DEFAULTS
SERIAL MODE: none
ABSORBER SWITCH; ON
CLOCK MENU
UPGRADE MENU
AMBIENT PRESSURE: 988 mBar
DISPLAY HISTORY
*SERVICE PIN: 0
*ENGINEER MENU
CLOCK MENU
ESCAPE FROM MENU
YEAR: 2005
MONTH: 3
DATE: 16
HOUR: 9
MINUTE: 57
UPDATE CLOCK
DAYLIGHT SAVING: off
PATIENT LOG MENU
ESCAPE FROM MENU
PRINT PATIENT DATA
LOGGING: off
LOG STATUS: disabled
CLEAR LOG DATA
LOGGING WINDOW: 10 min
Clock pick list (integer)
2005 - 2099 (integer)
1 - 12 (integer)
1 -31 (integer)
0 - 23 (integer)
0 - 59 (integer)
off / on (toggle
option)
*NOTE
Sub-menus for Service PIN and
Engineer Menu are not accessible
by users.
SERVICE MENU
SITE DEFAULTS
ESCAPE FROM MENU
SAVE TO SITE
VIEW: SITE DEFAULTS
VOLUME TYPE : tidal
Vt SET: 550 ml
Vm SET: 5.5 Litres
T+PS INIT: 10 cmH2O
SET BPM : 10
I : E : 1:1.0
PEEP : OFF
LIMIT : 38 cmH2O
TRIGGER : 10 L/min
APNOEA ALARM LIMIT : 15 Sec
BACK LIGHT LEVEL : 50 %
Doc No. AVS 0408DS (U)
September 2008
DRE, Inc. 1800 Williamson Court Louisville, KY 40223 USA
Tel: (502) 244-4444
Fax: (502) 244-0369
Web: www.dremed.com

IMPORTANT
(i)
Servicing and Repairs
In order to ensure the full operational life of this
ventilator, servicing by an engineer trained by
the manufacturer should be undertaken
periodically.
The ventilator must be serviced to the following
schedule:
(a) Six monthly service - inspection and
function testing.
(b) Annual / two year / four year service -
inspection and function testing, and
component replacement.
Details of these operations are given in the
Service Manual for the AV-S, available only for
engineers trained by the manufacturer.
For any enquiry regarding the servicing or
repair of this product, contact DRE, Inc.
Technical Support
DRE, Inc.
1800 Williamson Court
Louisville, KY 40223
USA
Tel: (502) 244-4444
Fax: (502) 244-0369
Web: www.dremed.com
Always give as much of the following
information as possible:
1. Type of equipment
2. Product name
3. Serial number
4. Approximate date of purchase
5. Apparent fault

FOREWORD
(ii)
Parameter / Device Relevant Standard
Pressure Measuring ISO 8835-2
Pressure Limitation Device EN 60601-2-13:2006 - 51.101.1
Exhaled Volume Monitor EN 60601-2-13:2006 - 51.101.4
Breathing System Integrity Alarm System EN 60601-2-13:2006 - 51.101.5
Continuing Pressure Alarm EN 60601-2-13:2006 - 51.101.6
Oxygen Monitor ISO 7767
Carbon Dioxide Monitor ISO 9918
Breathing Circuit ISO 8835-2
Agent Monitor ISO 11196
Gas Scavenging ISO 8835-3
For information on installing and connection of any of these systems or devices, please refer to the relevant manufacturer’s instructions.
This manual has been produced to provide
authorized personnel with information on the
function, routine performance and
maintenance checks applicable to the AV-S
Anesthesia Ventilator.
Information contained in this manual is
correct at the date of publication.
The policy of the manufacturer is one of
continued improvement to its products.
Because of this policy, the manufacturer
reserves the right to make any changes
which may affect instructions in this manual,
without giving prior notice.
Personnel must make themselves familiar
with the contents of this manual and the
machine’s function before using the
apparatus.
The Importance of
Patient Monitoring
WARNING
Anesthetic systems have the capability to
deliver mixtures of gases and vapours to the
patient which could cause injury or death
unless controlled by a qualified anesthetist.
There can be considerable variation in the
effect of anesthetic drugs on individual
patients so that the setting and observation of
control levels on the anesthesia systems
does not in itself ensure total patient safety.
Anesthesia system monitors and patient
monitors are very desirable aids for the
anesthetist but are not true clinical monitors
as the condition of the patient is also
dependent on his respiration and the
functioning of his cardio-vascular system.
IT IS ESSENTIAL THAT THESE ELEMENTS
ARE MONITORED FREQUENTLY AND
REGULARLY AND THAT ANY OBSERVATIONS
ARE GIVEN PRECEDENCE OVER MACHINE
CONTROL PARAMETERS IN JUDGING THE
STATE OF A CLINICAL PROCEDURE.
Before using any monitoring system or
device, the user must check that it conforms
to the relevant standard, as listed in the table
below.

CONTENTS
(iii)
Page No.
USER RESPONSIBILITY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
1. WARNINGS AND CAUTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
2. PURPOSE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
3. DESCRIPTION
3.1 General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
3.2 Ventilation Cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
3.3 Pneumatic System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
3.3.1 System Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
3.4 Electrical System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 14
3.5 Control Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
3.5.1 Touchscreen Operation and Navigator wheel / push-button . . . . . . . . . . . . 15
3.5.2 User Adjustable Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
3.5.3 Operational capability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3.5.4 Output Compensation Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
3.6 Interface with Integra AV-S and A200SP . . . . . . . . . . . . . . . . . . . . . . 20
3.7 Ventilation Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.7.1 Standby Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.7.2 Volume Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.7.3 Pressure Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
3.7.4 Spontaneous Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
3.7.5 Advanced Spontaneous Breathing Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
3.7.5.1 SIMV (Synchronised Intermittent Mandatory Ventilation) . . . . . . . . . . . . . 26
3.7.5.2 SMMV (Synchronised Mandatory Minute Ventilation) . . . . . . . . . . . . 27
3.7.5.3 PSV (Pressure Supported Ventilation) . . . . . . . . . . . . . . . . . . . . . . 28
3.7.5.4 PEEP ( Positive End Expiratory Pressure) . . . . . . . . . . . . . . . . . . . . . . . . . 29
3.8 On-screen Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
3.9 Spirometry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
3.10 Display Waveforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
3.11 Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
3.12 Oxygen Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
3.12.1 System Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
3.12.2 The Oxygen Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
3.12.3 Menus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
3.12.4 Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
3.12.5 Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
3.12.6 Alarm Mute. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
4. SPECIFICATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Ventilator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Oxygen Monitor . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40

CONTENTS
(iv)
5. PRE-OPERATION PROCEDURES
5.1 Ventilator Set-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
5.1.1 Mounting the Ventilator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
5.1.2 Electrical Power Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
5.1.3 Ventilator Gas Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
5.1.4 Breathing System Schematic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
5.1.5 Bellows Drive Gas . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
5.1.6 Anesthetic Gas Scavenging System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
5.1.7 Remote Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
5.1.8 Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
5.1.9 Breathing System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
5.1.10 Spirometer Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
5.1.11 Pressure Monitor Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
5.1.12 Leak Test / Compliance value Compensation . . . . . . . . . . . . . . . . . . . . . . . . . . 52
5.1.13 Bellows Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
5.2 Pre-use Checklists . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
5.2.1 Daily Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
5.2.1.1 Alarm System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
5.2.1.2 Ventilator Internal Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
5.2.1.3 Function Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
5.2.2 Weekly Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
5.3 Oxygen Monitor Set-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
5.3.1 Installation . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
5.3.2 Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
5.3.3 Sensor Low Indication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
5.3.4 Setting the High and Low O
6. MAINTENANCE
6.1 Service Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
6.2 Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
6.2.1 Outside Surfaces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
6.2.2 Bellows Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
6.2.3 Spirometer Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
6.2.4 Oxygen Monitor Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
6.2.5 Patient Connector Block . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
6.3 Sterilization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
6.4 Oxygen Monitor Sensor Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
7. APPENDIX
1. Back-up Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
2. Menu System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
3. Ventilator Spirometry System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
4. Disposal after use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
5. Approved Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
2 Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60

Statements in this manual preceded by the
following words are of special significance:
WARNING means there is a
possibility of injury to the
user or others.
CAUTION means there is a possibility
of damage to the apparatus
or other property.
NOTE indicates points of
particular interest for more
efficient and convenient
operation.
Always take particular notice of the
warnings, cautions and notes provided
throughout this manual.
USER RESPONSIBILITY
1
This anesthesia ventilator has been built to
conform with the specification and operating
procedures stated in this manual and/or
accompanying labels and notices when
checked, assembled, operated, maintained
and serviced in accordance with these
instructions.
To ensure the safety of this device it must be
checked and serviced to at least the
minimum standards laid out in this manual.
A defective, or suspected defective, product
must not under any circumstances be used.
The user must accept responsibility for any
malfunction which results from noncompliance with the servicing requirements
detailed in this manual.
Additionally, the user must accept
responsibility for any malfunction which may
result from misuse of any kind or noncompliance with other requirements detailed
in this manual.
Worn, broken, distorted, contaminated or
missing components must be replaced
immediately. Should such a repair become
necessary it is recommended that a request
for service advice be made to DRE, Inc.
This device and any of its constituent parts
must be repaired only in accordance with
written instructions issued by the
manufacturer and must not be altered or
modified in any way without the written
approval of the manufacturer. The user of
this equipment shall have the sole
responsibility for any malfunction which
results from improper use, maintenance,
repair, damage or alteration by anyone other
than the manufacturer.
USA and Canada:
Federal Law restricts the sale and use of this
device to, or on the order of, a licensed
practitioner.

1. WARNINGS AND CAUTIONS
2
The following WARNINGS and CAUTIONS
must be read and understood before using
this ventilator.
WARNINGS
General Information
1. Personnel must make themselves
familiar with the contents of this
manual and the machine’s function
before using the ventilator.
Before Using the Ventilator
2. Before the AV-S ventilator is used
clinically for the first time a Calibration
Check and Output Check must be
successfully completed.
Calibration and output checks must be
carried out by a DRE-trained
technician, following the procedure in
Appendix 6 in the AV-S Service Manual.
3. Before the ventilator is used clinically
for the first time, verify that the hospital
engineering department has carried out
an earth continuity test.
If the integrity of the protective
conductor is in doubt, the ventilator
must not be used.
Before the ventilator is used clinically
for the first time, the commissioning
engineer must confirm that the
air/oxygen selection is set correctly for
the drive gas that is to be used.
The use of any other gas will cause
inaccurate operation and may damage
the ventilator, resulting in potential
injury to the patient.
8. The driving gas is discharged through
the opening in the back of the ventilator
control unit.
The discharged gas may contaminate
the environment, and should therefore
be extracted using a gas scavenging
system.
9. The bellows can only support
approximately 1 kPa (10 cmH
differential positive pressure, above
which it may be dislodged from the
mounting ring, resulting in dangerous
malfunction of the ventilator.
Do not connect a positive end
expiratory pressure (PEEP) valve or
other restrictive device to the exhaust
port on the bellows base.
This would increase the pressure inside
the bellows and the bellows could
detach from the base, causing serious
malfunction.
2O)
4. Excessive electronic noise caused by
other poorly regulated devices, such as
an electrocautery unit, may adversely
interfere with the proper functioning of
the ventilator.
To avoid this problem, do not connect
the ventilator’s power cord into the
same electrical wall outlet or adaptor
strip into which an electrocautery unit
is connected.
5. If used with a mains extension cord, the
unit may be subject to electro-magnetic
interference.
6. The driving gas supply must be clean
and dry to prevent ventilator
malfunction.
7. This ventilator is designed to be driven
by oxygen or medical air only. The
drive gas is set during manufacture
and the ventilator is calibrated for that
gas.
10. Breathing System
The breathing system which conveys
gases from the anesthetic machine to
the patient, and disposes of expired
gases, must conform to the
requirements of ISO 8835-2.
Because breathing systems require
frequent cleaning and disinfection they
are not a permanent part of the
anesthetic ventilator and therefore
cannot be directly under the control of
the anesthetic ventilator manufacturer.
However, we strongly recommend that
only breathing systems which have
been approved and authorized by the
manufacturer for use with AV-S should
be employed.
Do not use conductive breathing
system hoses.
When mechanical ventilation is
employed the patient breathing system
must be connected directly to a
pressure relief valve to prevent the
possibility of barotrauma.

WARNINGS AND CAUTIONS
3
11. The spirometer sensors are mounted
within the A200SP absorber. Do not fit a
spirometer sensor to any other
location.
The device will not measure exhaled
volumes in any other position.
12. The operation of each alarm function
should be verified daily.
Periodically check the alarms at
clinically suitable intervals. If the
audible alarm or the visual indicator of
any alarm function fails to activate
during any alarm condition or fails to
reset after the alarm has been cleared,
refer the unit to an authorized service
technician.
13. Before using the ventilator check that
all connections are correct, and verify
that there are no leaks.
Patient circuit disconnects are a hazard
to the patient. Extreme care should be
taken to prevent such occurrences.
It is recommended that Safelock
fittings are used throughout the
breathing circuit.
Any problem arising from an
improperly functioning scavenging
system is solely the user’s
responsibility.
Do not use a scavenging system that
restricts drive gas flow when negative
pressure is exerted on it.
18. When the ventilator is connected to a
patient, it is recommended that a
qualified practitioner is in attendance
at all times to react to an alarm or other
indication of a problem.
19. In compliance with good anesthesia
practice, an alternative means of
ventilation must be available whenever
the ventilator is in use.
20. It is recommended that the patient
oxygen concentration should be
monitored continuously.
21. If the drive gas supply pressure drops
below a nominal 241 kPa (35 psi), the
LOW DRIVE GAS SUPPLY alarm will
activate both audibly and visually.
Patient minute volume may be reduced
due to lowered flow rates
14. Check that the cable between the
control unit and remote display screen
unit is connected before use.
Always use a cable type recommended
by the manufacturer.
Using the Ventilator
15. The AV-S ventilator is not intended for
use in intensive care applications.
16. This apparatus must not be used with,
or in close proximity to, flammable
anesthetic agents.
There is a possible fire or explosion
hazard.
17. Anesthesia apparatus must be
connected to an anesthetic gas
scavenging system (AGSS) to dispose
of waste gas and prevent possible
health hazards to operating room staff.
This requirement must be observed
during test procedures as well as
during use with a patient.
The scavenging transfer and receiver
system must conform to ISO 8835-3.
22. An audible alarm indicates an
anomalous condition and should never
go unheeded.
23. The characteristics of the breathing
circuit connected between the
ventilator and the patient can modify or
change patient ventilation.
To assist the maintenance of the
delivered patient tidal volume, the
ventilator control system software
includes:
A) a compliance compensation
algorithm,
B) a fresh gas compensation
algorithm.
However, patient ventilation must be
monitored independently from the
ventilator.
It is the responsibility of the user to
monitor patient ventilation.
24. Care must be taken to ensure that the
flow sensors are connected correctly
to the inspiratory and expiratory ports
of the absorber.

WARNINGS AND CAUTIONS
4
25. The Vent Inop (ventilator inoperative)
alarm indicates that one of the
following conditions has occurred:
a) The drive gas solenoid has failed.
b) The flow control valve has failed.
c) Internal electronic fault.
d) Internal electrical fault.
e) Software error.
Note that if a ventilator error is
detected, ‘Ventilator Inoperative’ will be
displayed on the front control panel
display.
26. The High and Low Airway Pressure
Alarms are important for patient care.
It is important that the sensor is
properly located in the expiratory limb
of the circuit - refer to section 5.1.10.
27. The patient must be continuously
attended and monitored when
Advanced Breathing Modes are in use.
User Maintenance
28. User maintenance is restricted to
cleaning the outside surfaces of
the ventilator, see section 6.
Other procedures detailed in this
manual must be carried out by
trained technicians.
Service and repair operations must
only be carried out by an engineer
trained by the manufacturer.
The warranty for this product is
void if the product is not
maintained in accordance with the
service schedule detailed in
section 6.1, and the procedures
published in the Service Manual for
this product.
Control Unit
29. Opening the control unit by
unauthorized personnel automatically
voids all warranties and specifications.
Prevention of tampering with the
control unit is exclusively the user’s
responsibility. If the control unit seal is
broken, the manufacturer assumes no
liability for any malfunction or failure of
the ventilator.
30. For continued protection against fire
hazards, any replacement fuses must
be the identical type and rating as the
original components. Replacement
must be carried out by trained
technician.
See section 4 for fuse rating.
31. If the internal battery is fully
discharged, the ventilator will not
function in the event of mains power
failure. The battery must be recharged
before the ventilator is used clinically,
otherwise backup cannot be
guaranteed.
See Appendix for battery maintenance.
See also CAUTION No. 7.
Used or defective batteries must be
disposed of according to hospital,
local, state, and federal regulations.
32. No oil, grease or other flammable
lubricant or sealant must be used on
any part of the ventilator in close
proximity to medical gas distribution
components.
There is a risk of fire or explosion.
33. Exterior panels must not be removed
by unauthorized personnel and the
apparatus must not be operated with
such panels missing.
There is a possible electric shock
hazard.
Bellows Assembly
34. The valve seat on the patient gas
exhalation diaphragm valve in the base
of the bellows assembly must be
cleaned regularly. Note that the bellows
assembly is built into the A200SP
Absorber - please refer to User Manual
for this product.
Failure to keep the valve seat clean
could result in the diaphragm sticking,
thus preventing exhalation.
Great care must be taken not to
damage the precision surface of the
valve seat on the patient gas exhalation
diaphragm valve in the base of the
bellows assembly.
Never use any hard object or abrasive
detergent to clean it; use only a soft
cloth.
If the valve seat is damaged, the valve
will leak and may cause serious
ventilator malfunction.

10. Circuit compliance is not activated until
Fresh Gas Compensation is switched
OFF.
NOTES
1. The term ‘cycle’ is used to designate the
transition to the exhalation phase.
2. The term ‘trigger’ is used to indicate the
transition to the inhalation phase.
WARNINGS AND CAUTIONS
5
CAUTIONS
1. Do not sterilize the ventilator control unit.
The patient block assembly must be
removed from the control unit before
sterilization ( see section 6.2.5).
All other internal components are not
compatible with sterilization techniques
and damage may result.
2. For ventilator components which require
sterilization, peak sterilization
temperatures should not exceed 134oC
(275oF) to prevent possible damage.
(See section 6).
3. Care must be taken not to let any liquid
run into the control unit; serious damage
may result.
4. The exhalation valve located in the
bellows base assembly and the pediatric
bellows adaptor must be cleaned and
sterilized separately. Note that the bellows
assembly is built into the A200SP
Absorber - please refer to User Manual for
this product.
5. Always check for correct fitment, and carry
out a full function test before clinical use, if
the bellows has been removed and
refitted for any reason. Note that the
bellows assembly is built into the A200SP
Absorber - please refer to User Manual for
this product.
6. Always check for correct fitment, and carry
out a full function test before clinical use, if
the bellows has been removed and
refitted for any reason. See section 6.
7. Damage may occur to the battery if it is
allowed to remain in a discharged state.
Check the battery frequently if the
ventilator is in storage (see Appendix 1).
8. Fresh gas compensation is disabled if :
a) The spirometry system is turned OFF
through the menu system, or
b) The spirometry system is not functioning
correctly.
9. Fresh gas mixture compensation is disabled
if :
a) The spirometry system is turned OFF
through the menu system, or
b) The spirometry system is not functioning
correctly.
c) The O2 monitor is switched OFF.

6
WARNINGS AND CAUTIONS - Oxygen Monitor
Oxygen Monitor
Note that the sensor for the oxygen
monitor is built into the A200SP
Absorber - for additional information,
please refer to the A200SP User
Manual.
WARNINGS
1. We recommend calibration of the
oxygen monitor every time the system
is turned on, as a safety precaution.
2. Do not attempt to open the fuel cell.
The sensor contains small quantities
of :
a) electrolyte, classified as a harmful
irritant which is potentially hazardous,
and
b) lead.
Used or defective cells must be
disposed of according to hospital,
local, state, and federal regulations.
CAUTIONS
1. Do not sterilize any oxygen monitor
component.
2. Do not autoclave or expose the sensor to
high temperatures.
3. If the sensor shows signs of being affected
by condensation, dry the sensor with soft
tissue.
Do not use heat to dry the sensor.
NOTES
1. The O2 SENSOR FAULT alarm indicates
that one of the following conditions has
occurred.
a) Internal electrical fault
b) Software/electronics fault
c) Oxygen sensor fault.
2. The concentration read-out may, in
certain conditions of excess pressure,
show a value above 100%.
To accommodate these conditions it is
possible to set the high alarm value up to
105% (see section 5).
3. ALWAYS check the integrity of the
sensor assembly before use.
4. Once exhausted, the sensor must be
disposed of according to hospital,
local, state and federal regulations.
5. The sensor measures oxygen partial
pressure, and its output will rise and
fall due to pressure change.
An increase in pressure of 10% at the
sensor inlet will produce a 10%
increase in sensor output.
6. The oxygen sensor is not suitable for
sterilization.
If contamination is suspected, fit a new
sensor (see section 6.4) and dispose of
the contaminated unit according to
hospital, local, state and federal
regulations.
3. To maintain maximum sensor life:
i) always switch off the anesthetic
machine after use, to ensure that the basal
flow ceases.
ii) disconnect the breathing circuit after
use.
4. The accuracy of flow and volume
measurements may be reduced if the
oxygen monitor is not in use.
5. Fresh gas mixture compensation is disabled
if the oxygen monitor is switched OFF.

2. PURPOSE
7
The AV-S Ventilator is a software controlled,
multi-mode ventilator, designed for
mechanical ventilation of adult and
pediatric patients under general
anesthesia.
In addition, in spontaneous mode, it can be
used to monitor spontaneously breathing
patients
It is designed for use in closed-circuit
anesthesia.
Indications for use of the device:
The AV-S Ventilator is intended to provide
continuous mechanical ventilatory support
during anesthesia. The ventilator is a
restricted medical device intended for use by
qualified trained personnel under the
direction of a physician. Specifically the
ventilator is applicable for adult and
pediatric patients.
The ventilator is intended for use by health
care providers, i.e. Physicians, Nurses and
Technicians with patients during general
anesthesia.
The AV-S ventilator is not intended for use in
intensive care applications.
Oxygen Monitor
The Oxygen Monitor is intended to
continuously measure and display the
concentration of oxygen in breathing gas
mixtures used in anesthesia, and is
intended for adult and pediatric patients.
The oxygen monitor is an integral part of the
ventilator.
The oxygen monitor is intended for use by
health care providers, i.e. Physicians,
Nurses and Technicians for use with patients
during general anesthesia.
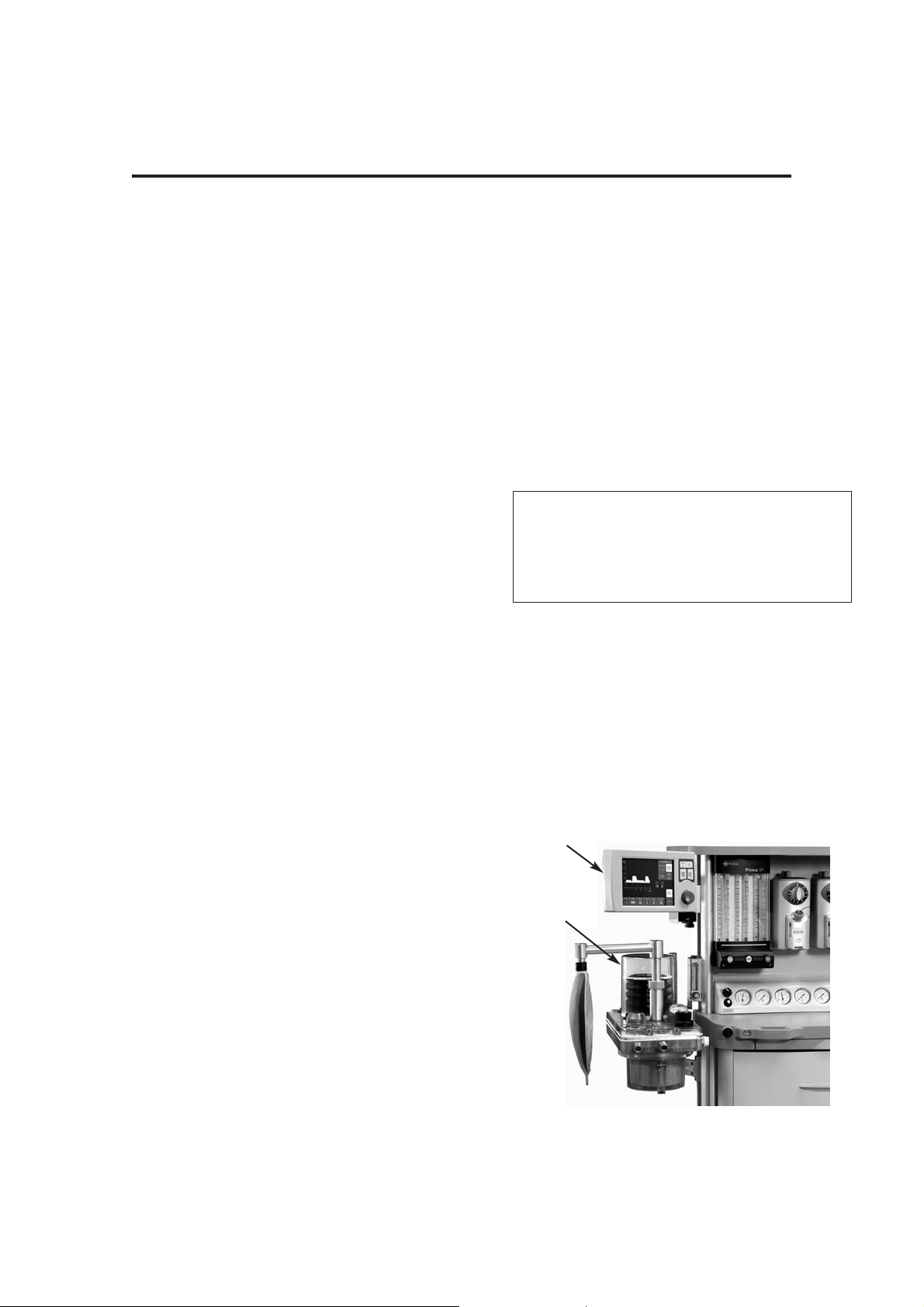
3. DESCRIPTION
8
1
2
Spontaneous Mode Patient Support
SIMV - Synchronised Intermittent Mandatory Ventilation
SMMV - Synchronised Mandatory Minute Ventilation
PSV - Pressure Supported Ventilation
PEEP - Positive End Expiratory Pressure
3.1 General Description
The AV-S Ventilator is a pneumatically driven, software
controlled, multi-mode ventilator.
The ventilator is a time-cycled, volume/pressure
controlled, and pressure limited.
The ventilator has compliance compensation and a
user selectable option of an inspiratory pause fixed at
25% of the inspiratory time.
In addition, fresh gas compensation and user
selectable gas mixture compensation is a standard
feature.
Ventilation Modes
Volume Mode - continuous mandatory ventilation
Pressure Mode - pressure controlled ventilation
Spontaneous, with advanced patient support -
SIMV, SMMV, PSV, PEEP
Patient Monitoring
Airway pressure, measured from the expiratory limb of
the breathing circuit.
Tidal Volume and Minute Volume measurement is
provided by a dual spirometry system
An integral oxygen monitor system measures oxygen
concentration in the breathing circuit inspiratory limb.
The print function provides a permanent record of
function activity for up to eight hours during a
procedure, or can be used to record waveforms.
Screen
210 mm (8.4 inch) high definition, colour TFT screen,
with single/dual waveform display.
Mounting:
Remote, arm-mounted as illustrated (1) or optional
combined control unit / screen (see section 5.1.1).
Bellows unit
The bellows unit (2) is built into the A200SP absorber.
A pediatric bellows assembly is available as an
option
Drive gas supply
The drive gas supply can be oxygen or air.
The supply must be at 310 to 689 kPa (45 to 100 psi).
Note that the drive gas is specified by the customer,
and set during manufacture. Conversion from one
drive gas to another must only be carried out by an
authorized service engineer trained by the
manufacturer.
9
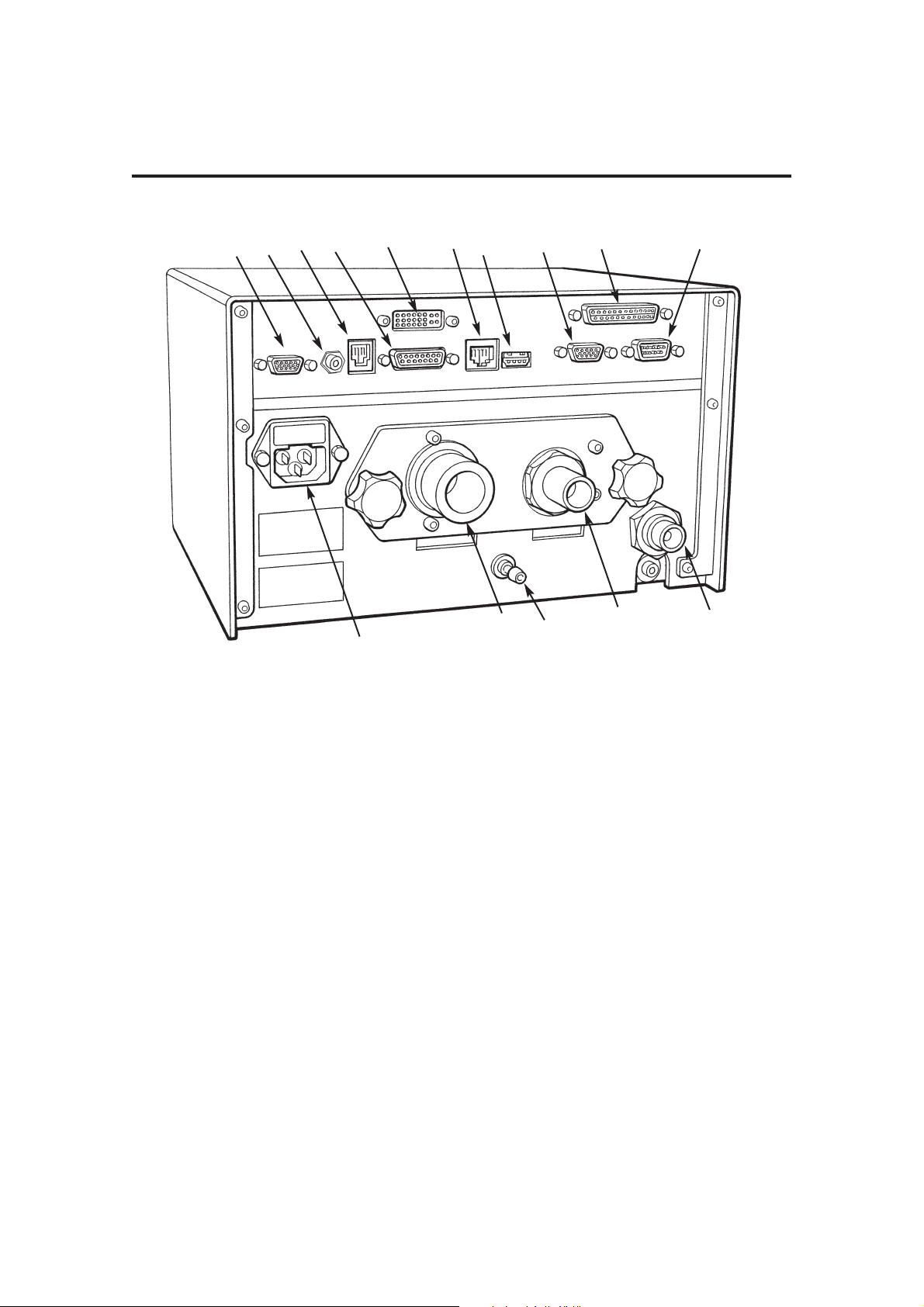
DESCRIPTION
2
7
3
13 14
15
12
1110
9
8
6
5
4
1
Control Unit
Rear Panel
Gas Connections
1. Ventilator drive gas inlet
- connect to anesthetic machine
auxiliary gas outlet
2. Bellows Drive Gas Output
- connect to bellows via A200SP
absorber - see section 5.1.5
3. Outlet - Exhaust Valve
- connect to scavenge system - see
section 5.1.6
Electrical Connection
4. Electrical mains input and fuse unit
Interface and Parameter inputs
5. A200SP Absorber Bag/Vent
switch interface, and
Spirometer connector
6. Integra AV-S Interface
connector - (primary on/off
switch)
7. Pressure Monitor Port
8. Input socket - Oxygen monitor
sensor
Data and Printer Ports
9. Data Output
10. Output to remote display
11. Ethernet
12. USB
13. VGA
14. Printer port
15. RS232 (manufacturer’s use only)
NOTE
USB port is for access only by engineers
trained by the manufacturer.
All other data ports are read only.
For further information, please contact
your distributor’s service department, or
the manufacturer.

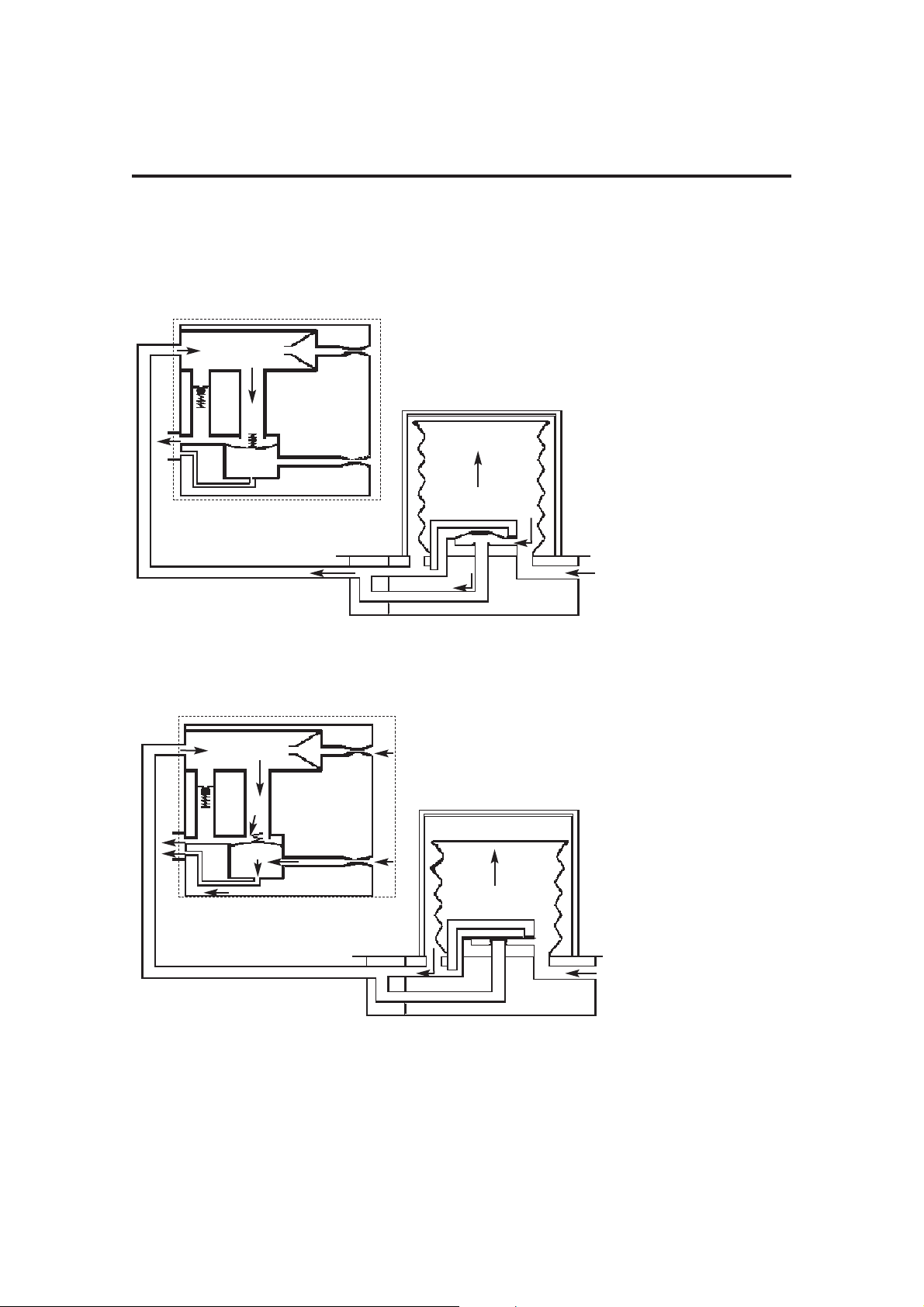
3.2 Ventilation Cycle
This section provides a simplified description of the ventilation cycle.
1. Inspiratory Phase
The drive gas proportional
valve (1) in the control unit
opens.
Drive gas is delivered to the
bellows housing (2).
The patient proportional
valve (3) opens, and gas
flows through the bleed
valve. The back pressure
ensures that the exhaust
valve (4) is kept closed.
Drive gas pressure builds
up above the bellows (5),
which starts to move down.
The diaphragm (6) in the
bellows assembly base is
held closed, and patient gas
is forced out of the bellows
base (7) into the breathing
system.
2. Beginning of
Expiratory Phase
The drive gas proportional
valve (1) closes.
The patient proportional
valve (3) closes.
The exhaust valve (4) opens.
Patient gas returns to the
bellows (5).
As the bellows rises,
redundant drive gas is
pushed out through the
exhaust valve.
DESCRIPTION
10
1
4
4
6
7
5
5
2
3
3
1
3
1
4
DESCRIPTION
3. End of
Expiratory Phase
With the bellows at the top
of its housing fresh gas
continues to flow.
To prevent a high pressure
build up the exhalation
diaphragm (6) lifts and
allows gas to exit through
the exhaust valve (4).
4. PEEP
Positive End
Expiratory
Pressure
(user selectable)
The patient proportional
valve (3) applies PEEP
pressure plus 20 cmH2O to
the exhaust valve, which
remains closed at this stage.
As fresh gas flows in the
patient circuit, any pressure
increase above PEEP
pressure in the bellows (5)
will cause gas to bleed past
the exhaust valve (4).
If there is a fall in pressure in
the breathing circuit, the
continuous flow from the
drive gas proportional valve
(1) helps maintain the set
PEEP pressure.
11
6
4
5
5
12
DESCRIPTION
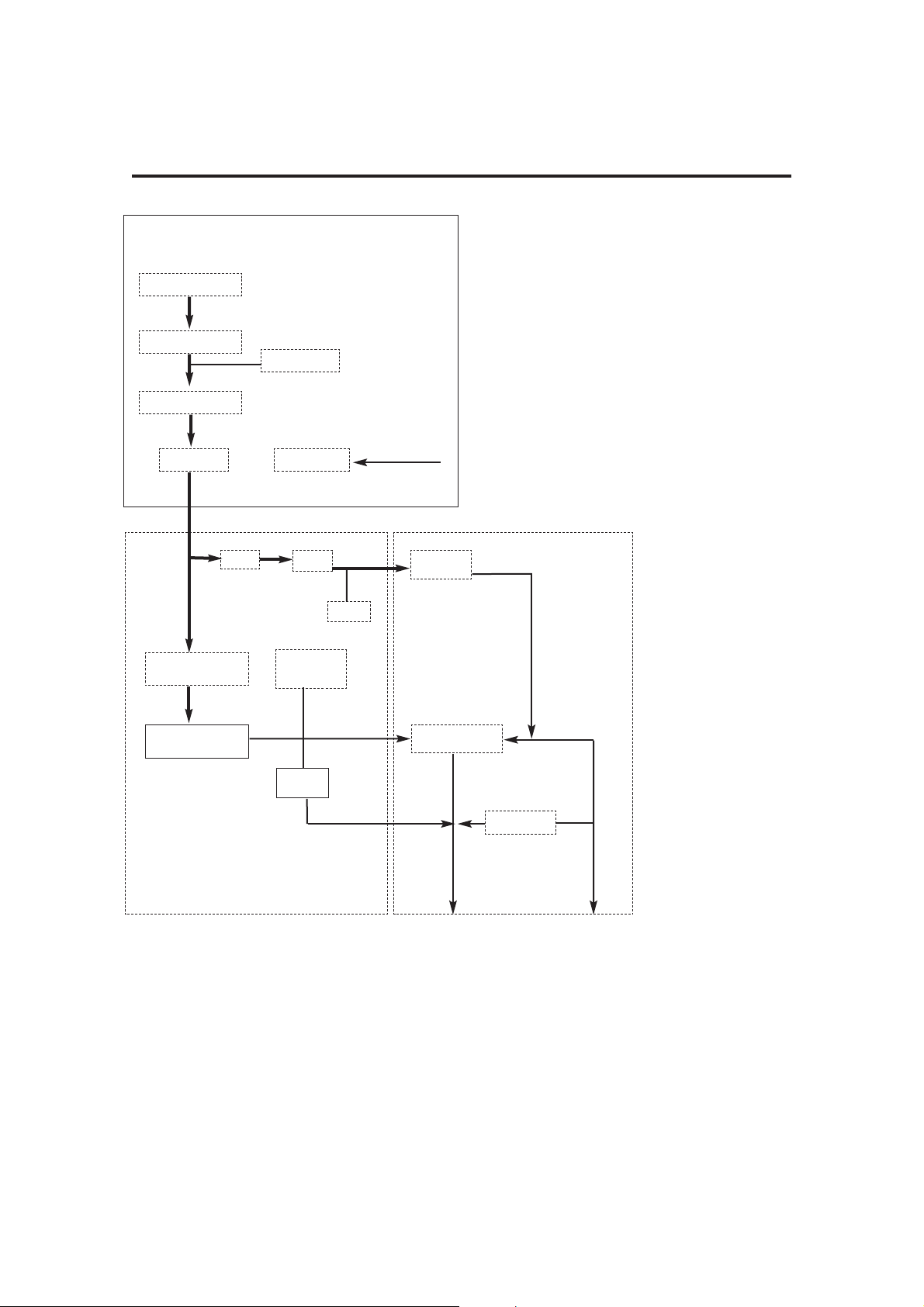
A
Pneumatic Flow
Diagram
C
1817
5
8
14
9
12
6
16
13
15
11
7
1
2
10
4
3
B
0 - 80 cmH2O
100 cmH
2O
0 - 90 cmH2O
241 kPa (35 psi)
3 to 7 bar

3.3 Pneumatic System
3.3.1 System Operation
Refer to the pneumatic system diagram on the previous page.
A) Gas inlet manifold block
The AV-S Ventilator is designed to operate on a 310 - 689 kPa (45 -100 psi)
drive gas supply (oxygen or air - to customer’s requirement).
1. DISS Connector
The gas source is connected to the DRIVE GAS SUPPLY fitting on the
rear of the ventilator control unit.
The gas supply should be capable of a flow rate of 80 L/min while
maintaining a minimum pressure in excess of 310 kPa (45 psi).
2. Filter
The drive gas is filtered with a 40-micron Input Gas Filter which protects
the pneumatic components from incoming particulate matter.
3. The Low Supply Pressure Detector
The pressure switch is set at a predetermined level to detect a loss or
reduction of the input gas source pressure.
When the pressure falls below 235 kPa (35 psi ± 1 psi), the LOW
SUPPLY PRESSURE indicator will be displayed and the high priority
audible alarm will activate.
4. Input Pressure Regulator
Regulates the input drive gas to 260 kPa ± 21 kPa (38 psi ± 3 psi).
5. Cut-off Valve
The valve isolates the the gas supply :
a) when the ventilator is switched off
b) when a fault condition occurs.
6. Airway Pressure Sensor
Connected to expiratory limb of breathing circuit.
B) Pneumatic Control Manifold Block
7. Drive Gas Proportional Valve
8. Drive Gas Flow Sensor
9. Drive Gas Pressure Sensor
10. Low Pressure Regulator
11. Patient Proportional Valve
12. PEEP pressure sensor
13. Restrictor
The restrictor allows a flow of up to 2 L/min (<2 L/min bleeding)
C) Exhaust Manifold Block
14. Check Valve
15. Diaphragm Valve
16. Pressure Relief valve - Set to 100 cmH
2O
17. Exhaust Port ( to AGSS)
18. Bellows drive gas outlet (to bellows assembly)
DESCRIPTION
13

3.4 Electrical System
Mains Supply
The mains supply inlet is designed for
connection to the following mains voltage
supplies:
100 to 120 VAC, 50 to 60 Hz
200 to 240 VAC, 50 to 60 Hz
Note that the ventilator adjusts automatically to
the supply voltage range.
The connector is a standard IEC type.
Back-up Battery
In the event of mains electrical failure, the backup battery cuts in automatically.
Standard battery:
A fully charged battery will power the ventilator
for approximately 30 minutes.
High-power battery (option):
A fully charged battery will power the ventilator
for approximately one hour.
See Appendix for battery care procedures.
DESCRIPTION
14

DESCRIPTION
3.5 Control Panel
3.5.1 Touchscreen and Navigator Wheel / Push Button
3.5.1.1 Control Panel
1. On/Off control
Switch On: Short internal test sequence
Switch Off: Power down sequence with progress indicator
2. Status indicators for electrical power (mains/battery supply)
Yellow indicator - illuminated whenever power is applied to the unit and internal battery is being
charged
Green indicator - illuminates when the unit is switched on
3. Menu switch
The menu function provides access to user and service pages, including alarm settings.
4. Alarm mute switch
30 second or 120 second alarm silence, depending on alarm status.
Note also that some alarms are not mutable (see 3.11).
5. Navigator Wheel and Press Button
Turn the wheel to select a function or parameter, or to alter the value of an active parameter.
Press to confirm the setting.
6. Active Tabs
Touch the screen at the appropriate tab area to activate the required function/parameter.
15
VmSET
mL
600
VmMEAS
Litres
3.6
SET
BPM
10
SET
I:E
2
PEEP
cmH2O
OFF
LIMIT
cmH2O
38
VOLUME
CONTROL
SPONT
MODE
STANDBY
%O2 105
21 18
PRESSURE
CONTROL
cm H2O
secs
AV-S
1
4
3
5
6
2
Gas Mixture
O
2 + air
.
.
IO
o
o

16
DESCRIPTION
3.5.1.2 Selecting Functions and Parameters
The functions/parameters shown on the screen can be
activated as follows:
a) touch the screen at the appropriate tab area.
b) rotate the navigator wheel and press it when the
indicator arrow is on the required parameter tab
Note that parameters default to factory-set values when
the ventilator is switched on and no further user
selection is made.
3.5.2 User Adjustable Parameters
Variable parameters can be altered by rotating
the navigator wheel.
When the required value is displayed, press
the active tab or
the wheel to confirm the
setting.
Tidal Volume Range 20-1600 ml
Rate 4-100 bpm
I:E Ratio 1:0.3 to 1:8
PEEP 4-20 cmH2O
Can be set to OFF
Pressure Limit
Volume mode: 10-80 cmH2O
Pressure mode: 10-50 cmH2O
Alarm limits (user adjustable alarms only - see 3.11)

3.5.3 Operational Capability
Tidal Volume, Rate, and I:E ratio settings are
all limited by a maximum inspiratory flow of
75 L/min, and a minimum flow of 2 L/min.
DESCRIPTION
17
0.1
0.2
0.4
0.3
0.6
0.5
1.6
1.5
1.4
1.3
1.2
1.1
1.0
0.9
0.8
0.7
0 10 20 30 40 50 60 70 80
1:6 1:5 1:4 1:3 1:2 1:1 1:0.3
Rate (bpm)
Tidal
Volume
(litres)
(Vt)
I:E Ratio
The ventilator is capable of operating at the volumes and rates below
each I:E ratio curve.
Example
1. Select required volume: Vt = 1.0 L
2. Select rate = 20 bpm
In this example, the point of intersection X on the graph shows that an I:E ratio can be set
from 1:0.3 to 1:4, as these curves are all above the intersection point.
Similarly, a ratio of 1:5 cannot
be set, as this is below the intersection point.
X

3.5.4 Output Compensation
Functions
WARNING
The AV-S automatically compensates for
fresh gas (spirometry On), fresh gas mixture
(spirometry and oxygen monitor On), and
altitude.
However, the actual tidal volume delivered to
the patient may be different to the ventilation
parameters set by the user, due to:
A) an extreme compliance condition,
B) a substantial system leak,
C) patient circuit pressure effects, or
D) extreme fresh gas flows
In addition, high fresh gas flows will lead to an
increased Vt being delivered to the patient.
The patient must
be monitored independently
from the ventilator.
It is the responsibility of the user to monitor
the patient for adequate ventilation.
Fresh Gas Compensation
Adjusts delivered volume up to 60%
An alarm is triggered if the measured
volume varies by 50% from the set volume.
This function is user adjustable
NOTE
Fresh gas compensation is disabled if :
a) The spirometry system is turned OFF through
the menu system, or
b) The spirometry system is not functioning
correctly.
Fresh Gas Mixture Compensation
- models with Spirometry
The spirometry system compensates for fresh
gas mixture - the user must access the menu
system and select the gas mixture that will be
used for each clinical procedure.
NOTE
Fresh gas mixture compensation is disabled if :
a) The spirometry system is turned OFF through
the menu system, or
b) The spirometry system is not functioning
correctly.
If the O2 monitor is switched OFF, a 40% / 60%
mixture of O2/N2O is assumed.
DESCRIPTION
18

Compliance compensation
The ventilator will apply compliance
compensation to account for compliance
loss in the breathing system in cases where:
i) Fresh gas compensation is disabled, or
ii) Spirometry is unavailable or disabled
IMPORTANT
For correct operation the value of the
breathing system compliance must be
established first, by completing the ventilator
leak-test as part of the Pre-operation
Procedure.
Refer to section 5.1.12, noting that breathing
system compliance is displayed as
‘Bsys.comp’
If the leak test is not carried out, the default
value will be used.
NOTE
In compliance compensation mode any fresh
gas used will be in addition to the set tidal
volume.
Altitude Compensation
This function monitors ambient pressure,
and adjusts the delivered volume
accordingly
NOTE Altitude compensation is automatically
applied during calibration of the oxygen monitor see section 5.3.2.
19
DESCRIPTION

A
B
20
DESCRIPTION
D
C
3.6 Interface to Integra AV-S and A200SP Absorber
The AV-S is designed to interface with the Integra AV-S
Anesthetia Machine and the A200SP Absorber.
3.6.1 Integra AV-S Interface
The interface cable links the socket (A) on the control panel to a
socket on the rear panel of the anesthetic machine.
a) Turn the anesthetic machine Gas Delivery Switch to ON.
The ventilator will power-up.
b) While the anesthetic machine power is ON, the Ventilator
can be turned OFF and ON, using the ventilator On/Off
switch, as described in section 3.5.1.
c) Turn the anesthetic machine Gas Delivery Switch to OFF.
The ventilator will power-down.
3.6.2 A200SP Absorber Interface
The interface cable links the socket (B) on the control panel to a
socket (C) at the rear of the absorber.
a) The A200SP is fitted with a sensor that detects the
position of the absorber bag/vent control (D). The sensor
signal cabling is routed internally to connector (C), and a
second cable runs to the the rear of the AV-S control unit.
b) Operation of the Bag/Vent control will trigger automatic
Mode switching on the AV-S ventilator, as follows:
i) Ventilator in Volume or Pressure mode
Switching the absorber Bag/Vent control from Vent
to Bag
- the ventilator will change from Volume Mode, or
Pressure Mode, into Spontaneous Mode.
ii) Ventilator in Spontaneous Mode
Switching the absorber Bag/Vent control from Bag
to Vent
Note that the mode switching operation is dependant on the
original selection process used to reach Spontaneous Mode:
A) If the ventilator was previously in Volume, or
Pressure, or Special Mode, and Spontaneous Mode was
automatically selected by the operation of the bag/vent
control (from Vent to Bag, as described above):
- the ventilator will now revert to that previous mode.
B) If the ventilator was in Standby Mode, and
Spontaneous Mode was selected on-screen:
- the ventilator will revert to Volume Mode.
NOTE
a) operation of the absorber Bag/Vent control will have no effect
on the ventilator unless the above conditions are met.
b) This function can be enabled/disabled through the on-screen
Service sub-menu (see appendix).

DESCRIPTION
21
Volume Mode Parameters
Tidal volume 20 - 1600 mL
Rate 4 -100 bpm
I:E ratio 1:0.3 - 1:8
PEEP 'Off' or adjustable 4 -20 cmH
2O
Inspiratory pressure limit 10 to 100 cmH
2O
Inspiratory pause 25%
(does not affect I:E ratio)
Sigh Approximately 1.5 x Set Vt is
delivered once, twice, three times
or four times every 50 breaths
(user selects frequency)
VmSET
mL
600
VmMEAS
Litres
3.6
SET
BPM
10
SET
I:E
2
PEEP
cmH2O
OFF
LIMIT
cmH2O
38
VOLUME
CONTROL
SPONT
MODE
STANDBY
%O2 105
21 18
PRESSURE
CONTROL
Gas Mixture
O2 + air
3.7 Ventilation Modes
3.7.1 Standby Mode
Allows parameters to be set.
Some patient alarms are active:
High airway pressure
(at 80 cmH
High/Low Oxygen
Negative pressure
Incorrect Rate/Ratio
Continuous high pressure
2O)
3.7.2 Volume Mode
The ventilator delivers a mandatory set
volume of gas at preset, fixed breath
intervals.
The Patient is making no respiratory
effort.
3.7.2.1 Fresh Gas Compensation
The delivered volume is adjusted by up
to 60%.
This delivered volume will consist of the
volume delivered from the ventilator
bellows, plus the fresh gas flow from the
anesthetic machine fresh gas supply,
minus any compliance loss and minus
any leak.
This gives a total actual inspired tidal
volume.
An alarm is triggered if the measured
volume is 50% above or below the set
volume.
This function is user adjustable.
Compliance Compensation
Please refer to section 3.5.4
Altitude Compensation
This function monitors ambient
pressure, and adjusts the delivered
volume accordingly.

3.7.2.2 Select Volume Mode
Volume Mode selected from Standby Mode:
1. Press the screen tab: ‘VOLUME CONTROL’
Volume Mode selected from Pressure Mode:
1. Press the screen tab: ‘VOLUME CONTROL’
The ventilator continues to ventilate in
Pressure Mode.
2. The Volume Set display shows the previous
setting, or default setting.
3. A new Volume value can be set if required.
WARNING
Set appropriate values for the clinical procedure
in progress. Take note of all on-screen symbols
and display messages.
4. Press to confirm change of mode and new
setting.
NOTE
Pressure limit will default to the previous
Pressure Target value + 5 cmH
2O
5. At confirmation, the ventilator will switch to
Volume Mode.
NOTE
Volume Mode will commence at the beginning
of an exhalation phase.
3.7.2.3 Volume Type Selection
Use the menu to switch between Tidal
Volume and Minute Volume.
NOTE Minute Volume is derived from a rolling average
during a 30 second period.
3.7.2.4 Volume Mode Operating Functions
Inspiratory Pause function:
This function creates a plateau that equates
to 25% of the inspiratory time.
Select Inspiratory Pause
Press the Menu switch
Select Special Modes
Select Insp pause on/off
Exit menus
The symbol for Inspiratory Pause
will appear on the display:
Note that Inspiratory Pause function is cancelled
when Standby is selected
DESCRIPTION
22
Menu Switch
Turn the wheel
to scroll through
the menus.
Press to enter
sub-menu
EXIT MENUS
O2 MONITOR & SPIROMETRY
FRESH GAS COMPENSATION: ON
> SPECIAL MODES
WAVEFORM
ALARM SETTINGS
GAS MIXTURE: O2+AIR
USER SETTINGS
SERVICE MENU

Sigh function:
When the ventilator is in Volume Cycle mode
the "Sigh" option is available.
When selected, this option provides extra
volume for 1 to 4 breaths in 50 (the user can
select 1, 2, 3, or 4 breaths).
The extra volume will be approximately 50%
above the tidal volume set by the user.
Note that the High Volume Alarm is not
triggered when ‘Sigh’ is selected.
Select Sigh function:
Press the Menu switch
Select Special Modes
Select Sigh Enable on/off
Select Sigh to Breath Ratio
Rotate the wheel to select required value
Press wheel to confirm
Exit menus
The legend for Sigh will
appear on the display:
Note that sigh function is cancelled when Standby is
selected
Volume measurement:
Volumes are measured if the Spirometry
function is selected.
Automatic High or Low volume alarms are
triggered if the measured volume is 50%
above or below the set volume.
User adjustable option
If the maximum pressure limit is achieved,
the ventilator cycles to the expiratory phase.
SIGH
23
DESCRIPTION

DESCRIPTION
24
PRESS
CONTROL
AV-S
.
.
IO
o
o
3.7.3 Pressure Mode
3.7.3.1 Parameters
In pressure mode the ventilator delivers a flow of gas
to achieve a set pressure at fixed breath intervals.
The Patient is making no respiratory effort.
This is a common mode for the ventilation of small
pediatric patients.
Inspiratory pressure 10 - 70 cmH
2O
Rate 4 - 100 bpm
I:E ratio 1:0.3 - 1:8
PEEP 'Off' or adjustable: 4 - 20 cmH2O
Inspiratory decelerating flow is controlled by the
ventilator according to the pressure setting.
There is no Inspiratory Pause function in pressure
mode.
3.7.3.2 Selecting Pressure Mode
Pressure Mode selected from Standby Mode:
1. Select by touching the screen tab: ‘PRESS CONTROL’.
Pressure Mode selected from Volume Mode:
1. Select by touching the screen tab: ‘PRESS CONTROL’.
The ventilator continues to ventilate in Volume Mode.
2. The target pressure button flashes (the display shows
the previous setting of target pressure, or default setting).
3. The user can set a new Target Pressure if required.
WARNING
Set appropriate values for the clinical procedure in
progress. Take note of all on-screen symbols and
display messages.
4. Press to confirm change of mode and new target
pressure.
5. At confirmation of the new mode, the ventilator will switch
to Pressure Mode.
NOTE
Pressure Mode will commence at the beginning of an
exhalation phase.
3.7.3.3 Pressure Mode Operating Functions
Pressure mode defaults to a target pressure of 10
2O at switch on.
cmH
A high Inspiratory Flow is used to achieve and maintain
the target pressure.
The exhaust valve operates to prevent excess
pressure.

DESCRIPTION
25
3.7.4 Spontaneous Mode
3.7.4.1 Parameters
The ventilator monitors the following patient parameters:
Rate
I:E ratio
Pressure
Tidal volume
In spontaneous mode the waveform displays are active,
and inspiratory oxygen levels are measured
3.7.4.2 Spontaneous Mode Operating Functions
Selection during Ventilation
Move the absorber Bag/vent switch to ‘Bag’ - the ventilator will
switch from Pressure Mode or Volume Mode to Spontaneous
Mode (see 3.6.2 - Absorber Interface).
Functions
No mechanical ventilation
No Inspiratory Pause function
Patient Monitoring (Bag mode and Ventilator mode):
Airway pressures
FiO2,
Tidal volume,
Rate
I:E ratio,
Supply pressures
Advanced Ventilation Modes
Patient support modes are selectable from this mode see below, and section 3.7.5.
3.7.4.3 Patient Support Modes
The following support modes are selectable from the
'Special Modes' menu, and must be pre-selected from the
main menu, whilst in Standby.
SIMV - Synchronised Intermittent Mandatory Ventilation
SMMV - Synchronised Mandatory Minute Ventilation
PSV - Pressure Supported Ventilation
CAUTION
The required patient support mode must be pre-selected in
Standby Mode (select from main menu), before it can be
activated during the ventilation of a patient.
Please refer to sections 3.7.5.1, 3.7.5.2, 3.7.5.3.
Note that if the system fails to detect an absorber
bag/vent switch, a confirm message will be displayed.

26
DESCRIPTION
3.7.5 Advanced Spontaneous
Breathing Modes
3.7.5.1 SIMV
Synchronised Intermittent
Mandatory Ventilation
SIMV provides a minimum level of tidal
volume.
SIMV allows spontaneous breaths and a set
mandatory breath, synchronised with the
start of a patient breath
SIMV must be pre-selected in Standby
Mode
Select Standby
Select Menu
Select Special Modes
Select Support Mode
Select SIMV
Escape Menu
SIMV will be displayed on the main screen
when Spontaneous mode is selected or
triggered.
NOTE
1. The trigger window is pre-set to 60% of
the BPM cycle time.
2. The trigger is flow activated.
3. If Spirometry is disabled then SIMV is
not available
4. If the pressure limit and alarm are
activated the inspiratory phase is
terminated
Activate SIMV during Ventilation
NOTE
SIMV will not function unless already preselected in Standby Mode
1. Select ‘Special Mode’ on the display.
If the absorber Bag/Vent switch is not
detected, a message will appear:
‘SET ABSORBER TO VENT’
Press the navigator wheel / push
button to confirm.
2. Move the absorber Bag/vent switch to
‘Ventilator’.
3. Check that SIMV is functioning
correctly.
SIMV Default Settings
The ventilator will default to pre-set values
for Tidal volume (Vt), Rate, Inspiratory Time
and Trigger Level, after selecting 'SIMV'.
Note:
1. Vt can be adjusted before SIMV is
confirmed.
2. The trigger setting is adjustable
between 0.7 and 4.0 L/min.
PEEP
0 cmH2O
Pmax
A
SIMV - Spontaneously Breathing Patient
A = Cycle Time (set from BPM)
B = Trigger Window
C = Spontaneous Breath
D = Trigger
E = Mandatory breath at the set tidal volume (Vt)
Inspiratory flow in the Trigger Window (generated by the
patient’s spontaneous breath) results in a synchronised
mandatory breath at a preset volume and rate
SIMV - No breathing effort by Patient
A = Cycle Time (set from BPM)
B = Trigger Window
C = Flat Pressure Trace (no breathing effort)
D = Mandatory breath at the end of the Trigger Window at the set Vt
If the patient makes no effort to breathe during a cycle, a
mandatory breath, at the end of the trigger window, will still be
delivered at the preset volume and rate.
A
B
B
C
D
E
PEEP
0 cmH2O
Pmax
A A
B
B
C
D

27
DESCRIPTION
3.7.5.2 SMMV
Synchronised Mandatory
Minute Ventilation
SMMV provides a set level of minute
volume ventilation.
SMMV allows spontaneous breaths,
combined with a synchronised mandatory
breath, to achieve the set minute volume
SMMV must be pre-selected in Standby
Mode
Select Standby
Select Menu
Select Special Modes
Select Support Mode
Select SMMV
Escape Menu
SMMV will now be displayed on the main
screen when Spontaneous mode is
selected or triggered.
NOTE
1. The trigger window is pre-set to 60% of
the BPM cycle time.
2. The trigger is flow activated.
3. If the Spirometry is disabled then
SMMV is not available
4. If the pressure limit and alarm are
activated the inspiratory phase is
terminated.
Activate SMMV during Ventilation
NOTE
SMMV will not function unless already preselected in Standby Mode
1. Select ‘Special Mode’ on the display.
If the absorber Bag/Vent switch is not
detected, a message will appear:
‘SET ABSORBER TO VENT’
Press the navigator wheel / push
button to confirm.
2. Move the absorber Bag/vent switch to
‘Ventilator’.
3. Check that SMMV is functioning
correctly.
SMMV Default Settings
The ventilator will default to pre-set values
for minute volume (Vm), Rate, Inspiratory
Time and Trigger Level, after selecting
'SMMV'.
Note:
1. Vm can be adjusted before SMMV is
confirmed.
2. The trigger setting is adjustable
between 0.7 and 4.0 L/min.
PEEP
0 cmH2O
Pmax
A
SMMV - Spontaneously Breathing Patient
A = Cycle Time (set from BPM)
B = Trigger Window
C = Spontaneous Breath
D = Trigger
E = Mandatory Breath tidal volume.
This is equal to Vm/BPM, minus the volume spontaneously
breathed during the cycle (this maintains the set Vm)
Inspiratory flow in the Trigger Window (generated by the
patient’s spontaneous breath) results in a synchronised
mandatory breath, ensuring that the set minute volume is
achieved
A
B
B
C
D
E
PEEP
0 cmH2O
Pmax
A
SMMV - No breathing effort by Patient
A = Cycle Time (set from BPM)
B = Trigger Window
C = Flat Pressure Trace (no breathing effort)
D = Mandatory breath at the end of the Trigger Window (at the set Vm)
If the patient makes no effort to breathe during a cycle, a
mandatory breath, at the end of the trigger window, will still be
delivered at the preset volume and rate
A
B
B
C
D

DESCRIPTION
28
3.7.5.3 PSV
Pressure Supported
Ventilation
PSV assists each spontaneous breath to
achieve a preset pressure, thus reducing the
effort required to breathe.
Inspiratory flow (generated by the patient’s
spontaneous breath) results in synchronised
pressure support.
PSV must be pre-selected in Standby
mode
Select Standby Mode
Select Menu
Select Special Modes
Select Support Mode
Select PSV
Escape Menu
PSV will be displayed on the main screen
when Spontaneous mode is selected or
triggered.
Activate PSV during Ventilation
NOTE
PSV will not function unless already preselected in Standby Mode
1. Select ‘Special Mode’ on the display.
If the absorber Bag/Vent switch is not
detected, a message will appear:
‘SET ABSORBER TO VENT’
Press the navigator wheel / push
button to confirm.
2. Move the absorber Bag/vent switch to
‘Ventilator’.
3. Check that PSV is functioning
correctly.
NOTE
1. The trigger window is pre-set to 60% of
the BPM cycle time.
2. The trigger pressure is PEEP referenced.
3. If the Spirometry system is disabled, then
PSV is not available.
4. If the pressure limit and alarm are
activated the inspiratory phase is
terminated.
PSV Default Settings
The ventilator will default to pre-set values
for Support Pressure, Inspiratory Time, and
Trigger Level after selecting 'PSV' .
Note:
1. Support Pressure can be adjusted
before PSV is confirmed.
2. The trigger setting is adjustable
between 0.7 and 4.0 L/min.
PEEP
0 cmH2O
A
PSV Pressure Supported Ventilation
A = Set Inspiratory Time
B = Pressure Support Level
C = Spontaneous Breath results in a synchronised pressure supported breath
PSV is used to support spontaneously breathing patients ONLY
If the patient makes no attempt to breathe, the ventilator will not
provide support and the apnoea alarm will be activated
A
B
C
C

3.7.5.4 PEEP ( Positive End Expiratory Pressure)
The AV-S ventilator includes a microprocessor-controlled,
electronically integrated PEEP system, regulated by the
secondary pressure on the exhaust diaphragm ( see 3.2).
The ventilator controls PEEP by allowing flow from, or
delivering flow into the bellows drive circuit, thereby
maintaining the set pressure
NOTE
1. PEEP is electronically controlled
2. PEEP is variable from 4 - 20 cmH
2O, in increments of 1
cmH
2O
3. The display shows “OFF” when PEEP is not in use
4. PEEP is switched off when the ventilator is switched off.
5. PEEP is switched off during 'Spont' mode to minimise
patient’s breathing effort.
Selecting PEEP
1. Select by touching the screen tab PEEP, or using the
navigator wheel
The setting will flash.
2. Rotate the navigator wheel to set the required PEEP
pressure.
A confirm message will be displayed.
3. Press the Screen Tab, or Wheel to confirm.
Note that Electronic PEEP does not function in
Spontaneous Mode.
PEEP on/off sequence
Using the A200SP Absorber Interface - Ventilator
Mode Selection
1. Switch the ventilator to Volume Ventilation Mode
2. Select PEEP, and set pressure to the required level.
The PEEP display indicates pressure.
3. Switch the A200SP Absorber Bag/Vent control (A) to
the ‘Bag’ position.
The ventilator automatically switches to Spontaneous
Mode.
PEEP is automatically switched off (does not function
in Spontaneous Mode)
PEEP display is blank.
4. Reset the Bag/Vent control ‘Vent’ position.
The ventilator automatically switches to the mode
previously set by the user.
PEEP is Off.
PEEP display indicates Off.
5. Set the ventilator to Volume Ventilation Mode.
PEEP remains Off.
Select PEEP if required.
DESCRIPTION
29
A

DESCRIPTION
3.8 On-Screen Menus
To Access:
Press the menu switch on the front panel to access
the following functions and parameters via dropdown menus:
EXIT MENUS
O2 MONITOR & SPIROMETRY
LEAK TEST MENU
FRESH GAS COMPENSATION: ON
SPECIAL MODES
WAVEFORM
ALARM SETTINGS
GAS MIXTURE: O2+AIR
USER SETTINGS
SERVICE MENU
To Exit:
Press the menu switch on the front panel, or, select
EXIT MENUS and press the wheel.
NOTE
The menu window will not be displayed if:
A) Control parameters (VT MEAS, BPM, I:E, PEEP, or
LIMIT) are enabled but not confirmed.
B) A display window is active
To Operate:
1. Rotate the navigator wheel clockwise to scroll
through the menu options - the cursor ( > )
aligns with each parameter in turn.
2. Press the wheel to enter the required submenu.
3. Rotate the navigator wheel to change any
displayed values, and press to confirm.
4. To exit the menu display:
A) Press the menu switch on the front panel
B) Scroll to EXIT MENUS,
and press the navigator wheel.
NOTE
A) If confirmation does not take place within 8
seconds, the parameter reverts to its previous
value.
B) If another parameter is selected using the
touchscreen, the menu is de-selected.
C) While any menu is selected:
- the alarms are active,
- the ventilator can be switched off.
See Appendix 2 for a further information on the
Menu system.
Menu Switch
30
Turn the wheel
to scroll through
the menus.
Press to enter
sub-menu

3.9 Spirometry
Spirometry can be enabled or disabled via the
on-screen menu system.
NOTE
If the spirometry system is turned OFF:
a) Fresh gas / fresh gas mixture
compensation is disabled.
b) Special Modes are disabled.
See Appendix 3 for a detailed description of the
spirometry system.
3.10 Display Waveforms
NOTE
1. The default waveform is always Pressure
v Time (cmH
2O v seconds)
2. Wave Freeze is available when
ventilation is in progress
Second waveform
The second waveform can be displayed by
using the menu control or by touching the
waveform on screen.
Select from:
Volume v Time (litres v seconds)
Volume v Pressure (litres v cmH
2O)
Compliance loop waveform
- First loop can be frozen
- Subsequent loops overlaid
Display Functions - Automatic Scale
adjustment
Y axis
a) The scale adjusts as Plimit is changed
(-20 to 40, 60, 80 cmH
2O)
b) In Vol. v Time mode the scale adjusts as
Vt is changed
(0 to 0.5 L, 1.0 L, 2.0 L)
X axis
a) The scale adjusts as Rate is changed
(0 to 15 sec, 5 sec, 3 sec)
b) In Vol. v Pres. mode the scale adjusts as
Plimit is changed (-20 to 40, 60, 80
cmH
2O)
DESCRIPTION
31

DESCRIPTION
Alarm Priority Trigger Mute Set by:
time
Ventilator Inoperative (vent inop) High Internal failure or Battery failure zero Automatic
Outlet blocked High Positive pressure exceeds 120 cmH2O, due to blocked exhaust valve outlet zero Automatic
Power About to Fail High Ventilator is using the battery, and the battery voltage is less than 10.2 v zero Automatic
Low Drive Gas Supply Pressure High Less than 235 kPa (35 psi +/-1 psi) zero Automatic
Low Bellows Drive Gas Pressure High Fails to reach target level 30 s Automatic
High Bellows Drive Gas Pressure High Exceeds calculated target level 30 s Automatic
High Continuous Airway Pressure High Breathing system pressure fails to return to below 30 cmH
2O 120 s Auto
by the start of the next inspiratory phase
High Airway Pressure High Pressure reaches set limit (10 to 80 cmH2O adjustable) 30 s User / Default
Low Airway Pressure High Breathing system pressure fails to reach minimum level (4 to 14 cmH
2O) 120 s Automatic
Negative Airway Pressure High Breathing system pressure exceeds 10 cmH
2O 120 s Automatic
Low Tidal Volume (Vt) High a) Measured Vt less than 50% of volume set 120 s User / Default
b) Spirometer disconnected
Low Minute Volume (Vm) High Calculated volume lower than 50% of volume set 120 s User / Default
Apnoea High In Spontaneous mode, no breath detected within 15 seconds 120 s Automatic
High Tidal Volume (Vt) High Measured value exceeds 150% of set value 120 s User/Default
High Minute Volume (Vm) High Calculated value exceeds 150% of set value 120 s User / Default
High O2 Concentration High Measured O2 % exceeds set value 120 s User / Default
Low O2 Concentration High Measured O2 % lower than set value 120 s User / Default
O2 Sensor low output Low Sensor life exhausted zero Automatic
O2 sensor fault High Sensor disconnected 120 s Automatic
Incorrect Rate or Ratio Medium Settings outside 75 L/min 120 s Automatic
Mains Failure Low Mains power fails zero Automatic
Fully charged battery gives 30 mins use (60 mins with high power battery)
Battery Power Fail Medium Battery disconnected, or missing, or totally discharged 120 s
Low Battery Low Battery voltage has dropped below 11.2 v zero
Absorber cable fault (A200SP) Medium Disconnection or short circuit 120 s Automatic
Printer not available Low Printer disconnected, or has no power, or has no paper zero
Priority identification:
High Priority: Five ascending tones - repeated Medium Priority: Three ascending tones - repeated Low Priority: Single tone - repeated
32
3.11 Alarms
NOTE User Adjustable Alarms - use the menu system to set the required limits - press the menu switch on
the front panel (see 3.8), and select ALARM SETTINGS.

DESCRIPTION - O2 Monitor
3.12 Oxygen Monitor
The oxygen monitor continuously measures and indicates
the concentration of oxygen in the breathing system, and
triggers an alarm when the concentration varies from the
set levels.
3.12.1 System Description
The Oxygen Monitor uses a fast-responding, oxygenspecific, self powered sensor that achieves 90% of final
value in less than 10 seconds.
An external probe (1) is supplied with a 2 m (6 ft)
extendable cable.
The system has user-adjustable high-level and low-level
alarms with visual and audible indication of alarm
conditions.
Bacterial Filter
Always use a breathing system bacterial filter in the
expiratory limb of the breathing circuit to protect the
oxygen sensor and breathing system components
from contamination (see section 5).
CAUTION
Replacement/Disposal - always follow the instructions
supplied with the filter, and always replace at the
recommended interval.
O2 Sensor Location
Integra AV-S with A200SP Absorber
3.12.2 The Oxygen Sensor
The oxygen sensor offers quick response, linear output
over the entire 0-100% oxygen range, and long service
life.
The sensor is a self-powered galvanic cell that generates
a current proportional to oxygen concentration.
The cell has a highly stable output over its operating life.
Significant output loss is only shown at the very end of its
life.
Typical sensor drift rates are less than 1% per month
when the sensor is exposed to gas in typical applications.
Sensor life:
approximately 1500000 O2% hours at 20oC
(minimum one year in most normal applications).
Sensor lifetime is governed by the mass of lead available
to react with the oxygen and its rate of consumption. High
oxygen partial pressure and high temperature will increase
the sensor output current, thus shortening the operation
life.
At the point where all lead has been consumed, the output
will fall very quickly to zero over a period of two to three
weeks.
1
33

3.12.3 O2 Monitor sub-menu
ON/OFF
Turn the navigator wheel to switch between
ON and OFF.
Press to confirm.
Scroll to EXIT MENUS and press the wheel
to exit.
NOTE
The oxygen monitor automatically switches ON
and defaults to the previous values for high and
low alarm settings when the ventilator is
switched on.
Fresh gas mixture compensation is disabled if the
O2 monitor is switched OFF.
CALIBRATION
Press the navigator wheel to initiate the
calibration procedure (see section 5.3.2 for
full procedure).
To exit the menu, scroll to EXIT MENUS
and press the wheel.
HIGH ALARM SET
LOW ALARM SET
Scroll to the required parameter and press
the navigator wheel to activate.
Rotate the navigator wheel again to change
the displayed value.
(see section 5.3.4 for full procedure).
High Alarm range: 19% to 105%
Low Alarm range 18% to 99%
The displayed figure will flash on and off.
Press to confirm.
Scroll to EXIT MENUS and press the wheel
to exit.
O2 Monitor sub-menu
O2 Monitor sub-menu - calibration
O2 Monitor sub-menu - alarms
DESCRIPTION - O2 Monitor
34
O2 Monitor & Spiro
ESCAPE FROM MENU
> O2 MONITOR: on
CALIBRATION: 100%
HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min
O2 Monitor & Spiro
ESCAPE FROM MENU
O2 MONITOR: on
> CALIBRATION: 100%
HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min
O2 Monitor & Spiro
ESCAPE FROM MENU
O2 MONITOR: on
CALIBRATION: 100%
> HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min

DESCRIPTION - O2 Monitor
3.12.4 Display
High-set, low-set, and oxygen concentration
percentage readings are displayed on screen.
Touch the tab to activate O2 menu
Oxygen Concentration
The display provides a direct readout of measured
oxygen concentrations in the range 0-100%.
Low Alarm Set - limited within 18-99%
The oxygen percentage, set by the user, at which the
low alarm will be activated.
To set the low oxygen alarm, see section 5.3.4.
High Alarm Set - limited within 19-105%
The oxygen percentage, set by the user, which the
high alarm will be activated.
Note that in certain conditions of excess pressure,
the readout may show a value above 100%.
To set the high alarm, see section 5.3.4.
3.12.5 Oxygen Monitor Alarms
HIGH O2 ALARM
The high O2 alarm is triggered when the oxygen
concentration is 1% above the set value.
a) The High O
2 Alarm visual indicator will
illuminate.
b) A high priority audible alarm will sound.
To cancel this alarm, the high alarm setting must be
equal to, or above the oxygen concentration.
The alarm can be muted for 120 seconds.
Measured O2
concentration
High Alarm Set Value
Low Alarm Set Value
35
%O2 100
33 20
.
.
IO
o
o

LOW O2 ALARM
The low alarm is triggered when the oxygen
concentration is 1% below the set value.
a) The Low O
2 Alarm visual indicator will
illuminate.
b) A high priority audible alarm will sound.
To cancel this alarm, the low alarm setting must
be equal to, or below the oxygen concentration.
The alarm can be muted for 120 seconds.
O2 SENSOR FAULT
The alarm is triggered:
i) when either the oxygen sensor is
disconnected or approaching the end of its life.
ii) if the O
2 concentration exceeds 110%.
a) The message O2 SENSOR FAULT will be
displayed.
b) A high priority audible alarm will sound.
To cancel this alarm, check the sensor
connection or replace the sensor.
The alarm can be muted for 120 seconds.
O2 SENSOR LOW
This alarm indicates the sensor has approached
the end of its life.
The legend O
2 SENSOR LOW will be displayed,
and a low priority alarm (single note) will sound.
The sensor must be replaced as the output will
fall very quickly to zero within two to three weeks
of normal usage.
See section 6.5 for sensor replacement.
3.12.6 Oxygen Monitor Alarm Mute
In an alarm condition, pressing the ALARM
MUTE button will deactivate the audible alarm,
but the alarm message display will remain on
screen.
The switch will illuminate, and a single note will
sound.
The alarm mute can not be operated:
a) Until the mute time is over, or the alarm
condition has been rectified.
b) When O
2 concentration drops below 18%.
DESCRIPTION - O2 Monitor
36

4. SPECIFICATION
37
4.1 Application Ventilation for use in anesthesia.
4.2 Internal Compliance
Adult bellows 3 ml/cmH
Pediatric bellows 2 ml/cmH
4.3 Physical
Size (mm)
- control unit only 290 wide x 300 deep x 185 high
- with adult bellows 290 wide x 300 deep x 385 high
Screen Size 210 mm (8.4") TFT
Weight - control unit only 7.6 kg
- with adult bellows 9 kg
Bellows
Adult (Latex free): 20 ml - 1600 ml
Pediatric : 20 - 350 ml
(Note - latex free pediatric available as option)
2O (nominal)
2O (nominal)
Power 100 - 120 VAC, 50 - 60 Hz, 0.6 A max.
200 - 240 VAC, 50 - 60 Hz, 0.3 A max.
(automatic ranging)
Fuse (mains supply) Two fuses, Type T 2AH
2 A, 250 V rating, 20 mm, anti surge, ceramic.
Battery Back-up: Standard battery: 12 V, 1.2 Ah, sealed lead-acid battery
Fully charged battery provides 30 minutes (nominal) backup
High power battery (option): 12 V, 2 Ah, sealed lead-acid battery
Fully charged battery provides one hour (nominal) backup
Fuse (battery) 3 A mini-blade type
Drive Gas Oxygen or Air (dry, and oil free) at 45 to 100 psi (310 to 689 kPa).
4.4 Alarms
Alarm Mute 30 or 120 seconds (see 3.11)
Apnoea Flow referenced (no breath detected within 15 seconds)
Low Drive Gas Pressure Less than 235 kPa (35 psi)
High Continuous Airway Pressure Above 30 cmH
Volume Mode: alarms after 10 seconds
Standby Mode: alarms after 30 seconds
Low Pressure 4 - 14 cmH2O PEEP referenced
Incorrect Rate or Ratio
Mains Failure Fully charged standard battery provides 30 mins (nominal) backup
Fully charged high-power battery (option) provides one hour
(nominal) backup
Low Battery 5 minutes Use
Ventilator Inoperative Internal or Battery Failure
Outlet Blocked Exhaust valve outlet blocked
2O at start of cycle

SPECIFICATION
38
Alarms - User Adjustable
Low Tidal Volume Measured value is below 50% of volume set
Range: 0 - 1600 ml
High Tidal Volume Measured value exceeds 150% of volume set
Range: 20 - 1600 ml
Low Minute Volume Calculated value is 50% below volume set
Range: 0 - 10 L
High Minute Volume Calculated value exceeds 150% of volume set
Range: 0 - 30 L
Low and High O2 Concentration 18% - 105%
High Airway Pressure 10 - 80 cmH2O adjustable
4.5 Functional
Tidal Volume
Adult bellows 20 to 1600 ml (±10%)
Pediatric bellows 20 to 350 ml (±10%)
At ambient temperature of 20oC (+/-10%) and ambient atmosphere of 101.3 kPa (+/-10%).
Minute Volume 0 to 30 L
Rate 4 - 100 bpm
I:E Ratio 1:0.3 - 1:8
Pressure Limit 10 - 100 cmH2O
Fresh Gas Compensation Automatic Tidal Volume adjustment
Modes Off
Standby
Volume Cycle
Pressure Controlled
Spontaneous (includes advanced breathing modes)
Pressure Control 10 - 70 cmH
Inspiratory Flow 2 - 70 L/min
Spontaneous Mode Active Volume and Pressure Alarms,
Advanced Breathing Modes selectable (see section 4.6)
Electronic PEEP 4 - 20 cmH
Spirometry - Resolution ±10 ml
Ventilator Performance - accuracy of delivered volumes
2O
2O
≤ 100 ml ± 50%.
> 100 ml ± 20%
NOTE
The ventilator is designed for use with Spirometry ON.
Accuracy of delivered volumes with Spirometry OFF may vary from the figures given above.

SPECIFICATION
39
4.6 Advanced Spontaneous Breathing Modes (SIMV, SMMV, PSV)
Trigger (PEEP Referenced) 0.7 to 4 L/min
Trigger Window Set 60% of Expiratory Time
Vt and Vm As Volume Mode
Insp Time (Ti) 0.5 to 5 secs
Support Pressure 3 to 20 cmH2O
Default settings
Volume Vt BPM I:E Pmax
Adult 600 ml 10 1:2 38 cmH2O
Pediatric 150 ml 15 1:2 38 cmH2O
Pressure Vt BPM I:E P-target
Adult 600 ml 10 1:2 10 cmH2O
Pediatric 150 ml 15 1:2 10 cmH2O
SIMV Vt BPM Insp time Trigger
Adult 600 ml 6 2 sec 1 L/min
Pediatric 200 ml 10 1 sec 1 L/min
SMMV Vm BPM Insp time Trigger
Adult 3.6 L 6 2 sec 1 L/min
Pediatric 2 L 10 1 sec 1 L/min
PSV Support Pressure Trigger
Adult 10 cmH2O 1 L/min
Pediatric 10 cmH2O 1 L/min
4.7 Disinfection and Sterilization Patient Block assembly can be sterilized if
necessary - section 6.
NOTE : The bellows assembly, oxygen monitor
sensor, and spirometer sensors are built into the
A200SP Absorber - for information, please refer to
the User Manual for A200SP.
4.8 Bacterial Filter None (always use a bacterial filter in the
breathing system to protect the oxygen sensor see section 5.1.4)
4.9 Fail Safe Mechanism Battery back-up in case of mains electricity
failure
Gas shut-off in the event of electronic failure
4.10 Reliability Not applicable
4.11 Waveform Tests Not applicable
4.12 Volume Tests Not applicable
4.13 Mobility and Mounting
(A) Mobility Secure mounting required
(B) Mounting Control unit and remote screen are mounted on
anesthetic machine .
The bellows assembly is built into the A200SP
Absorber.

4.14 Environmental
Operating:
Temperature 15 to 30oC (59 to 86oF)
Humidity 10 - 95% RH (relative humidity), non-condensing
Altitude Up to 2775 m (9000 feet)
Air Pressure 70 - 110 kPa
Storage and Transport:
Temperature -5 to 40oC (23 to 104oF)
Note: For battery care during storage, refer to Appendix 1.
Humidity 10 - 95% RH (relative humidity), non-condensing
Air Pressure 11.5 - 110 kPa
Electro-magnetic compatibility:
The AV-S meets the requirements of EN60601-1-2 (Electromagnetic compatibility - requirements
and tests).
MRI compatibility: The AV-S is not suitable for use in an MRI environment
4.15 Device Classification and Labelling
Type B Applied Part
Degree of protection against electric shock
This symbol denotes: Type B equipment:
Class 1 Classification
Type of protection against electric shock
Class 1 with internal electrical power source (battery backup)
IPX0 Ingress protection
Classification according to the degree of protection against ingress of water
IPX0 (not protected)
Labelling
This symbol denotes: Refer to the User Manual
4.16 Oxygen Monitor
Measurement Range: 0 - l00%
Resolution: ± 1%
Accuracy and Linearity: ± 2% of full scale (at constant temperature and pressure)
Response Time: 90% of final value in approximately 10 seconds
(air to 100% O
2)
Operating Temperature: 15 to 30oC (59 to 86oF)
Storage Temperature: -5 to 40oC (23 to 104oF)
Relative Humidity Range: 10 - 95% (non-condensing)
SPECIFICATION
40

Oxygen monitor, continued
Battery Back-up: As per ventilator
High Priority Alarm: Flashing, 2x 5 audio pulses with 6 seconds repeat time.
Medium Priority Alarm: Flashing, 3 audio pulses with 24 seconds repeat time
Low Priority Alarm: Static with single beep sound
Alarm Mute: 30 seconds for high priority alarm
120 seconds for medium priority alarm
Low Alarm Set Range: 18%-99% (
± 1%)
High Alarm Set Range: 19%-105% (± 1%)
Cable length: 2 m (6 ft), fully extended
Sensor
Type: Galvanic fuel cell sensor (0-100%)
Life: 1500000 O
2% hours
(One year minimum in typical applications)
Interference Gases and Vapours (in 30% Oxygen, 70% Nitrous Oxide)
Interference Volume % Dry Interference in O2%
Nitrous Oxide 80% <1%
Carbon Dioxide 5% <1%
Halothane 5% <1%
Enflurane 5% <1%
lsoflurane 5% <1%
Sevoflurane 5% <1%
Humidity Effects
Sensor output is relatively unaffected by prolonged operation in either high or very low relative
humidity.
If the sensor shows signs of being affected by condensation, dry the sensor with soft tissue.
CAUTION DO NOT use heat to dry the sensor.
Temperature Effects
The sensor has a built-in temperature compensation circuit, and is relatively unaffected by
temperature changes within the operating temperature range given above.
Pressure Effects
The sensor measures O
2 partial pressure, and its output will rise and fall due to pressure change
(e.g. changes in barometric pressure, or breathing system pressure).
An increase in pressure of 10% at the sensor inlet will produce a 10% increase in sensor output.
NOTE
Altitude compensation is automatically applied during calibration.
SPECIFICATION
41

5. PRE-OPERATION PROCEDURES
42
5.1 Ventilator Set-up
WARNING
Before the AV-S ventilator is used clinically for the
first time a Calibration Check and Output Check
must be successfully completed.
Calibration and output checks must be carried out
by a DRE-trained technician, following the
procedure in Appendix 6 in the AV-S Service
Manual.
5.1.1 Mounting the Ventilator
The remote screen is mounted on an adjustable
arm, with the control unit mounted at the rear or
side of the anesthetic machine.
Location for optional control unit / screen
Preferably, mount the unit permanently on the
shelf of the anesthesia machine or on a strong
bracket.
This will protect the unit from accidental fall and
disconnection of hoses and cables.
To fit the unit permanently on a mounting
bracket:
1. Align the four mounting feet over the mating
holes in the bracket.
2. Use the four M4 screws supplied with the
mounting bracket kit, inserted through the
bracket and rubber feet and screwed into the
threaded inserts in the base of the
ventilator.
Only use the screws supplied with the kit.
Pole-mount type mounting brackets and side
frame brackets are available from the
manufacturer.
Bellows unit
The bellows unit is built into the A200SP absorber.
5.1.2 Electrical Power Connection
Before connecting the ventilator to the mains
supply, check that the power supply is within the
correct rating as stated on the label on the rear of
the control unit.
WARNING
Excessive electronic noise caused by other, poorly
regulated devices, such as electrocautery, may
adversely interfere with the proper functioning of
the ventilator.
To avoid this problem, do not connect the ventilator
power cord into the same electrical wall outlet or
strip into which an electrocautery unit is connected.

43
PRE-OPERATION PROCEDURES
5.1.3 Ventilator Gas Supply
1. Verify the drive gas specified for the
ventilator (oxygen or air).
Always use the correct drive gas.
2. Connect the drive gas inlet port on the
rear of the control unit to a dry, oil free
supply.
Supply pressure range:
45 to 100 psi
(3.1 - 6.9 bar, 310 - 689 kPa)
OXYGEN SUPPLY:
a) O
2 cylinder,
b) Anesthetic machine O
outlet,
c) O
2 pipeline supply from a wall outlet.
AIR SUPPLY:
a) Air cylinder,
b) Anesthetic machine Air auxiliary gas
outlet
c) Air pipeline supply from a wall outlet.
2 auxiliary gas
Supply pressure should be monitored by
a separate means, e.g. pressure gauge on
anesthetic machine or supply line.
NOTE: It is possible to reconfigure the
ventilator for use with a different drive gas to
the gas originally specified.
This work must be carried out by an engineer
trained by the manufacturer.
5.1.4 Breathing System Schematic
The next page contains a schematic showing
the cables and tubing for an AV-S ventilator
mounted on a Integra SP I/SP II anesthesia
machine with an integral A200SP Absorber.

44
Hoses and Cables Schematic
AV-S (with remote screen) and
A200SP Absorber
PRE-OPERATION PROCEDURES
1
2
3
6
7
8
12
13
16
14
26
17
15
12
19
18
20
24
25
23
28
27
29
31
30
26
21
22
9
1011
4
5
Note
1. AV-S has spirometry and oxygen monitor.
2. Interface cabling is shown for connection
to Integra AV-S On/Off switch and
A200SP Bag/Vent switch.

PRE-OPERATION PROCEDURES
45
1. Bellows
2. Ventilator Control Unit
3. Outlets to Anesthetic Gas Scavenging System (AGSS)
4. Bacterial Filter
5. Absorber valve block
6. Heat and moisture exchanger
7. Patient
8. CGO Block on anesthetic machine (Fresh Gas Supply)
9. Auxiliary Outlet on anesthetic machine (Drive Gas Supply)
10. Flow sensor - expiratory
11. Flow sensor - inspiratory
12 Connectors - sensor - pressure monitor
13. Expiratory Valve - Absorber
14. Inspiratory Valve - Absorber
15. Inlet - from Ventilator Bellows
16. Connector - Reservoir Bag
17. Inlet - Absorber - Fresh Gas Supply
18. Drive Gas Inlet - Ventilator
19. Drive gas Outlet - ventilator control unit to bellows
20. Outlet - Exhaust Valve
21. Inlet - Bellows Drive Gas
22. Outlet - to breathing system
23. Input socket - Oxygen monitor sensor
24. Input socket - Integra AV-S interface
(SP on/off switch)
25. Input socket:
(i) A200SP Absorber Bag/Vent control position
(ii) Spirometer sensor signal
26. Interface connections on Integra AV-S and A200SP
27. APL Valve
28. Outlet from APL Valve to AGSS
29. Oxygen sensor
30. Remote screen unit
31. Cable - control unit to screen
(a combined unit with a bacterial filter can be used - see 5.1.9)

PRE-OPERATION PROCEDURES
46
2
7
3
13 14 15
12
1110
9
8
6
5
4
1
Control Unit
Rear Panel
Gas Connections
1. Ventilator drive gas inlet
- connect to anesthetic machine
auxiliary gas outlet
2. Bellows Drive Gas Output
- connect to bellows
( on Integra AV-S with A200SP
absorber
- connect to absorber - see section
5.1.5)
3. Outlet - Exhaust Valve
- connect to scavenge system
Electrical Connection
4. Electrical mains input and fuse unit
Interface and Parameter inputs
5. A200SP Absorber Bag/Vent switch
interface, and
Spirometer connector
6. Integra SP I/SP II Interface connector
- (primary on/off switch)
7. Pressure Monitor Port
8. Input socket - Oxygen monitor
sensor
Data and Printer Ports
9. Data Output
10. Output to remote screen
11. Ethernet
12. USB
13. VGA
14. Printer port
15. RS232 (manufacturer’s use only)
NOTE
USB port is for access only by engineers
trained by the manufacturer.
All other data ports are read only.
For further information, please contact your
distributor’s service department, or the
manufacturer.

PRE-OPERATION PROCEDURES
47
1
5.1.5 Bellows drive gas hose
1. Integra AV-S with A200SP absorber:
Connect a 16 mm diameter corrugated
hose between the ventilator control unit
drive gas outlet (labelled: DRIVE GAS)
and the outlet (1) at the rear of the
A200SP absorber.
2. All other AV-S configurations:
Connect a 16 mm diameter corrugated
hose between the control unit drive gas
outlet (labelled: DRIVE GAS) and the
bellows base DRIVE GAS inlet port.
5.1.6 Anesthetic Gas Scavenging
System
1. Connect the EXHAUST valve port on
the control unit to a properly functioning
scavenging system.
Use a 19 mm hose.
2. Fit a 10 cmH
between the exhaust valve port and the
inlet port of the AGSS receiver.
Note that the diaphragm valve under
the bellows is connected internally to
the EXHAUST port to facilitate the
discharge of excess breathing gas at
the end the expiratory phase.
2O pressure relief valve
WARNING
Do not use a scavenging system that
restricts drive gas flow when negative
pressure is exerted on it.
Applying negative or positive pressure to the
bellows exhaust port results in positive
pressure in the patient breathing system.
Therefore, the scavenging system must not
generate more than 0.5 cmH2O positive or
negative pressure when connected to the
ventilator.
Any problem arising from an improperly
functioning scavenging system is solely the
user`s responsibility.

1
2
5.1.7 Remote Screen
48
Attach the DVI cable supplied with the
screen between the interface connectors
(1) on the rear of the control unit and
screen.
WARNING
Check that the cable between the control unit
and remote display screen unit is securely
connected before use.
Always use a cable type recommended by the
manufacturer.
5.1.8 Printer
Attach a printer to the printer port (2) if a
printed output of the ventilator function is
required.
5.1.9 Breathing System
1. Connect the ventilator bellows base
BREATHING SYSTEM port to the
breathing system.
2. a) Use a breathing system bacterial
filter in the expiratory limb of the
breathing circuit to protect the oxygen
sensor.
b) Use a heat and moisture exchanger
(HME) at the patient Y piece.
(a combined HME / bacterial filter can
also be used, but note that the
expiratory limb bacterial filter is still
required)
CAUTION
Replacement/Disposal - always follow the
instructions supplied with the filter or HME.
Fit new components at the recommended
interval.
3. Connect a 2-litre breathing bag to the
patient connection as a test lung.
4. Close the anesthetic machine APL
valve.

A
B
49
PRE-OPERATION PROCEDURES
5.1.10 Spirometer
5.1.10.1 Flow sensors fitted to an
A200SP Absorber mounted
on a Integra AV-S
1. Use a breathing system bacterial filter
- see section 5.1.9, operation 2.
CAUTION
Replacement/Disposal - always follow the
instructions supplied with the filter.
Always renew components at the
recommended interval.
2. The two spirometry flow sensors are
mounted within the A200SP Absorber
in the inspiratory and expiratory
airways.
3. Connect the cable assembly between
the connector at the rear of the
A200SP Absorber (A) and the the
socket (B) at the rear of the Ventilator
control unit.
4. Check that the cable connections are
secure.
NOTE
A) If the connections are incorrectly made, the
ventilator will alarm LOW TIDAL VOLUME
or HIGH TIDAL VOLUME.
B) To allow the ventilator to be used in the
event of damage, or non-functioning of the
spirometer heads, turn off the spirometry
function - see MENU function, section 3.5.
If the spirometer is switched OFF:
a) Fresh gas compensation is disabled
b) Fresh gas mixture compensation is
disabled.
c) Patient support function is disabled.

PRE-OPERATION PROCEDURES
1
On/Off Switch
Alarm Mute
Menu Switch
Navigator
Wheel and
Press Button
3
2
O2 Monitor & Spiro
ESCAPE FROM MENU
O2 MONITOR: on
CALIBRATION: 100%
HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
> SPIRO CALIBRATION: 0 L/min
.
.
IO
o
o
5.1.10.2 Spirometer Zero Calibration
Flow sensors fitted to an A200SP
Absorber mounted on a Integra AV-S
The Spirometry sensor heads must be calibrated with
zero flow going through them.
The individual spirometers must be matched to the
specific AVS ventilator by a qualified service
engineer as part of commissioning or a subsequent
service visit.
The following procedure defines the zero calibration
which should be performed by the user as part of the
daily check procedure.
1. Turn the anesthetic machine gas flow off at the
Gas Delivery on/off switch. This will stop all gas
flows (including the AHD basal flow).
This will also turn the AV-S off.
2. Turn the AV-S on at the ventilator (Do not use the
Integra AV-S Gas Delivery switch).
or,
Disconnect the fresh gas hose from the CGO
block on the anesthetic machine.
3. Remove the breathing circuit hoses from the
inspiratory and expiratory connectors (1) on the
absorber.
4. Disconnect the hose that connects the APL valve
outlet (2) at the rear of the manifold block to the
AGSS receiver (or disconnect at receiver).
5. a) Remove the bag, and set the Bag/Vent
control (3) to Bag position.
or,
b) Ensure that the ventilator bellows is empty,
6. Calibrate the spirometer via the ventilator menu
procedure.
7. Press the menu switch on the front panel.
8. Scroll down the main menu and select O2
MONITOR & SPIROMETRY.
9. Select SPIRO CALIBRATION.
10. Press the wheel to initiate calibration.
11. Calibration is completed.
12. Scroll to ESCAPE FROM MENUS.
13. Press the wheel to confirm.
50

PRE-OPERATION PROCEDURES
51
B
X
C
5.1.11 Pressure Monitor Connections
WARNING
The High and Low Airway Pressure Alarms are
important for patient care.
The connection point must be properly
located in the expiratory limb of the breathing
system.
1. PATIENT PRESSURE port (A) on the
rear panel of the control unit:
Use the tubing assembly supplied by
the manufacturer to connect to the
expiratory limb of the breathing system,
close to the circle system expiratory
valve.
2. Push-fit, self sealing connectors (B)
Push in the tube as far as possible
Do not use excessive force.
The connector end piece ‘X’ will also
move inwards.
Pull the tube carefully outwards.
The end piece ‘X’ will be pulled
outwards to the ‘locked’ position.
3. Connect the tubing (with adaptor, Part
No 053049) to the push-fit, self-sealing
connector (C) at the rear of the
A200SP Absorber.
NOTE
Disconnection of airway pressure
tubing, or pressure sensor
malfunction
Message displayed:
‘’Check pressure sensing’’
Action by user:
Check the condition of the tubing, and the
connection at the ventilator (A) and the rear
of absorber (C).
If the tubing is undamaged and the
connections are secure, the operation of the
sensors must be checked by a service
engineer
CAUTION
The ventilator will continue to function,
although the target pressure may be exceeded
by up to 10 cmH
2O.
A

PRE-OPERATION PROCEDURES
52
AV-S
.
.
IO
o
o
Leak Test
ESCAPE FROM MENU
<START/STOP LEAK TEST>
LEAK STATUS: unknown
LEAK LEVEL: 0 mL/min
BSYS COMP 7.0 mL/cmH
2O
1
2
3
5.1.12 Leak Test / Compliance Value
Calculation
This procedure checks the breathing system for
pressure leakage, and calculates and displays
Leak Status, Leak Level, and Breathing
System Compliance.
. Occlude the breathing circuit at the
1
patient Y piece
2. Ensure that the absorber is switched to
vent mode and the bellows are fully
inflated.
3. Either (a) disconnect the fresh gas
hose from the CGO and connect it to
the end of the bag arm,
or
(b) Turn the anesthetic machine gas
flow off at the gas delivery on/off switch
(1).
This will stop all gas flows (including
oxygen basal flow) and will also turn
the AVS off.
Then switch the AV-S on (2) at the
ventilator (Do not use the anesthetic
machine gas delivery switch.
4. Press the menu switch (3).
Select LEAK TEST from the main
menu.
Select <START/STOP LEAK TEST> to
start the leak test.
The ventilator will now drive gas into
the absorber until a pressure of 30
2O is obtained, and then hold that
cmH
pressure for approximately 25 seconds
before releasing the pressure and
completing the test.
5. The menu will display the leak test
results :
(i) Leak Status
Excellent: under 50 ml/min
Good: between 50 and 149 ml/min
Poor: between 150 and 349 ml/min
Bad: 350 ml/min or more
NOTE
The ventilator will still operate, irrespective
of the displayed Leak Status.

(ii) Leak Level
Indication of leak rate is displayed
NOTE
During the test, any pressure drop discovered
once the 30 cmH
2O level is reached will be
displayed as a possible leak by the ventilator
self-test. This includes pressure drop due to
the relaxation of any elastic components in
the breathing system (e.g a breathing bag)
(iii) BSys Comp
BSys Comp is the compliance of the
breathing system and is the value that
will be used in providing the default
compliance compensation for volume
delivery.
NOTE
This value has an upper limit of 18 cmH
2O
(sufficient for normal breathing system
capacities).
53
PRE-OPERATION PROCEDURES

PRE-OPERATION PROCEDURES
54
1
2
4
5
7
6
3
Note that the bellows
assembly is built into the
A200SP Absorber.
For additional information,
please refer to the user
manual for that product.
5.1.13 Bellows Assemblies
CAUTION
Always ensure correct fitment of bellows (see illustration
above), and carry out a full function test before clinical use, if
a bellows is removed and refitted.
1. Remove the bellows housing (1).
Twist carefully counterclockwise until the bayonet
tabs become free, then lift up from the base (2).
2. Remove the bellows (3).
3. Refit the bellows and check for correct assembly,
as illustrated (4).
4. Fit the bellows housing by pushing down, then
twisting clockwise until the bayonet tabs
completely engage.
5. Function test the ventilator - section 5.3.1.
NOTE
If there is any malfunction, the ventilator must NOT be used.
If the problem cannot be rectified, the ventilator must be
checked by an engineer trained by the manufacturer
.
Pediatric Bellows Assembly
1. Remove the adult bellows housing (1) - twist
carefully counterclockwise until the bayonet tabs
become free, then lift up from the base (2).
Remove the bellows (3).
2. Fit the pediatric adaptor (5) - press the adaptor
into the ventilator bellows assembly base (2).
3. Fit the pediatric bellows (6) to the adaptor.
Check for correct assembly, as illustrated (4).
4. Fit the pediatric bellows housing (7) to the base
by pushing down, then twisting clockwise until the
bayonet tabs completely engage.
5. Function test the ventilator - section 5.3.1.

55
PRE-OPERATION PROCEDURES
1
.
.
IO
o
o
Back-up Battery
WARNING
If the internal battery is fully
discharged, the ventilator will not
function.
Recharge the battery before the
ventilator is used clinically.
Charging the battery for 14 hours from
a discharged state will allow a
minimum of 30 minutes of continuous
operation.
Connect the ventilator to a mains
power supply.
The mains power indicator will
illuminate to show that the battery is
being charged (it is not necessary to
turn on the ventilator).
A
B
A
B
5.2 Pre-use Checklist
5.2.1 Daily Checklist
The following tests must be carried out at the beginning
of every working day:
5.2.1.1 Alarm System
WARNING
The operation of each alarm function should be verified
daily.
If the audible alarm or the visual display for any alarm
function fails to activate during any alarm condition or
fails to reset after the alarm has been cleared, refer the
unit to an authorized service technician.
5.2.1.2 Ventilator Internal Test
Press the ON/OFF switch (1).
A three-second internal test is initiated:
1. The ‘power -up’ screen is displayed.
2. The audible alarm sounds.
3. The ventilator reverts to STANDBY mode if no
selection is made.
NOTE special operating system on ventilators interfaced with
Integra AV-S (see section 3.5.2).
a) Turn the anesthetic machine Gas Delivery Switch to
ON - the ventilator will power-up.
While machine power is ON, the Ventilator can be
turned OFF and ON, using the ventilator On/Off switch.
Test Display
Two small boxes (A and B) appear on the left hand side
of the screen, showing either:
Top box BLACK, lower box GREEN
Indicates the initial system self-test has been
completed successfully.
The boxes will be removed from the screen as soon
as the ventilator is taken out of standby mode.
Top box RED, lower box BLACK
Indicates that the initial system self test has
detected that a service calibration is required.
A full system calibration must be carried out by
qualified service personnel.
The boxes will remain visible until calibration is
completed.
The ventilator may still be used, but with
reduced accuracy.

56
PRE-OPERATION PROCEDURES
5.2.1.3 Function Test
1. Set the AIRWAY PRESSURE LIMIT to 50 cmH2O.
2. PRESSURE TRANSDUCER connection
Check that the port on the rear of the control unit
is correctly connected to the port on the rear of
the absorber assembly (see section 5.1.10).
3. Connect a 2-litre breathing bag to the patient
connection as a test lung.
4. Adult bellows only:
Set the tidal VOLUME to 600 ml; RATE to 10
bpm, and I:E RATIO to 1:2.0.
5. Use the O2 flush button on the anesthetic
machine to fill the bellows.
6. Select VOLUME CYCLE mode.
7. The delivered tidal volume indicated on the scale
printed on the bellows housing should be
approximately 600 ml.
If the delivered tidal volume is less than 500 ml or
greater than 700 ml, refer the ventilator to an
engineer trained by the manufacturer.
8. Set a basal flow only on the anesthetic machine.
Check the bellows after 10 breaths - the bellows
should return to the top of the housing.
Failure to return to the top of the housing
indicates a leak in the breathing circuit.
Rectify the leak before clinical use.
9. Occlude the patient ‘Y’ -piece.
The HIGH AIRWAY PRESSURE alarm should be
activated.
The peak pressure read on the breathing system
pressure gauge is the maximum working airway
pressure limit and should agree with the setting.
10. Open the patient ‘Y’ -piece to ambient pressure.
At the second cycle, the LOW AIRWAY
PRESSURE alarm should be activated.
11. Select STANDBY mode
Before using the ventilator clinically, check that all
connections are correct, and verify that there are
no leaks.
NOTE
If there is any malfunction, the ventilator must NOT be used.
If the problem cannot be rectified, the ventilator must be
checked by an engineer trained by the manufacturer.

5.2.2 Weekly Checklist
At least every week, in addition to the daily
function test, the following checks must be
carried out:
Alarms
1. Select STANDBY MODE.
2. Unplug the mains power cable from
the AC outlet.
The MAINS FAILURE alarm should
activate.
3. Reconnect the mains power cable to
the AC outlet. The alarm should turn
off.
4. Disconnect the drive gas supply hose.
The LOW SUPPLY PRESSURE
alarm should activate.
NOTE
If there is any malfunction, the ventilator must
NOT be used.
If the problem cannot be rectified, the ventilator
must be checked by an engineer trained by the
manufacturer.
Bellows
Check the condition of the bellows and
exhalation diaphragm valve.
Note that the bellows assembly is built into
the A200SP Absorber - please refer to the
user manual for this product.
PRE-OPERATION PROCEDURES
57

PRE-OPERATION PROCEDURES - O2 Monitor
58
A
B
5.3 O2 Monitor System Set-up
5.3.1 Installation
Fit the probe (A) to the A200SP absorber.
Connect the cable to the input socket (B) on
the back of the AV-S ventilator control unit
NOTE The anesthetic machine gas control switch
must be in the ON position for gas delivery.
WARNING
The sensor contains a small quantity of
electrolyte, classified as a harmful irritant which
is potentially hazardous.
Do not attempt to open a cell.
ALWAYS check the integrity of the sensor
assembly before use.
Once exhausted, the sensor must be disposed
of according to hospital, local, state and federal
regulations.
NOTE
To maintain maximum sensor life:
i) always disconnect the breathing circuit after use.
ii) Switch off the anesthetic machine to cut-off the
basal flow through the system.
Bacterial Filter
Use a breathing system bacterial filter in the
expiratory limb of the breathing circuit to
protect the oxygen sensor (see section 5.1.9).
CAUTION
Replacement/Disposal - always follow the
instructions supplied with the filter, and always
replace at the recommended interval.
5.3.2 Calibration
The new unit must be calibrated before clinical
use.
Thereafter, as a safety precaution, we
recommend calibration of the unit every
time the system is switched on.
Calibration must also be performed:
A) when the sensor is replaced
B) when point-of-use elevation changes by more
than 160 m (500 ft).
NOTE
Altitude compensation is automatically applied
during calibration.
We recommend calibration with a 100%
oxygen standard source, at a pressure and
flow similar to your application.

PRE-OPERATION PROCEDURES - O2 Monitor
2
1
59
3
5
6
4
%O2 100
21 20
.
.
IO
o
o
O2 Monitor & Spiro
ESCAPE FROM MENU
O2 MONITOR: on
> CALIBRATION: 100%
HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min
5.3.2.1 Calibration - Using 100% Oxygen
AV-S ventilator mounted on a Integra AV-S
anesthesia machine fitted with a A200SP
absorber
Calibrate with the sensor in position within the absorber.
1. Detach the absorbent canister (1).
2. Remove the breathing circuit hoses from the
inspiratory and expiratory connectors (2) on
the absorber.
This will give a free flow of oxygen through the
sensor.
3. Switch on the ventilator (3) and the
anesthetic machine gas delivery switch.
The oxygen monitor automatically switches
ON when the ventilator is switched on.
Ensure that all vaporizers are OFF.
4. Apply 100% oxygen only, at 5 L/min, from the
anesthetic machine flowmeter.
5. Allow the oxygen to flow until the oxygen
monitor readout (4) stabilises.
6. Calibrate the sensor, using the AV-S ventilator
menu procedure, as follows.
7. Press the menu switch (5) and select the O
monitor sub-menu.
8. Scroll to CALIBRATION.
If the menu shows 21% (which indicates
calibration using air), press the navigator
wheel / button (6) to switch to 100%
(calibration using oxygen).
2
9. A message will flash on the screen:
O2 AT 100% ?
Press the button (5) to confirm
NOTE
The message:
OXYGEN SENSOR LOW OUTPUT
will appear on screen if the user attempts to
calibrate at 21% in 100% oxygen.
10. Scroll to ESCAPE FROM MENUS and press
the button (6) to exit.
11. Turn off the flow of oxygen.
12. Refit the absorbent canister (1).

5.3.3 Sensor Low Indication
The unit automatically detects when sensor
life is low.
The message:
OXYGEN SENSOR LOW OUTPUT
will appear on screen to indicate that the
sensor must be replaced.
The sensor output will fall very quickly to
zero over a period of two to three weeks
from the first time that the alarm is
activated.
Sensor replacement - see section 6.5.
5.3.4 Setting the O2 Alarms
5.3.4.1 Set High Alarm
The high alarm value cannot be set below
19% or above 105% (Note that in certain
conditions of excess pressure, the readout
may show a value above 100%.).
1. Touch the O2 concentration display, or
Press the menu switch on the
ventilator front panel and select the O
2
monitor sub-menu.
2. Scroll to HIGH ALARM SET and
press the navigator wheel.
3. Rotate the wheel to change the
displayed alarm figure to the desired
value.
4. Press the wheel to confirm.
5. Scroll to ESCAPE FROM MENUS
and press the wheel to exit.
5.3.4.2 Set Low Alarm
The low alarm value cannot be set lower
than 18%, or above 99%.
1. Touch the O2 concentration display, or
Press the menu switch on the
ventilator front panel and select the O
2
monitor sub-menu.
2. Scroll to LOW ALARM SET and
press the navigator wheel.
3. Rotate the wheel to change the
displayed alarm figure to the desired
value.
4. Press the wheel to confirm.
5. Scroll to ESCAPE FROM MENUS
and press the wheel to exit.
PRE-OPERATION PROCEDURES - O2 Monitor
60
Low Alarm
Set Value
High Alarm
Set Value
O2 Monitor & Spiro
ESCAPE FROM MENU
O2 MONITOR: on
CALIBRATION: 100%
> HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min
On/Off Switch
Alarm Mute
Menu Switch
Navigator
Wheel and
Press Button
%O2 100
21 20
.
.
IO
o
o

6. MAINTENANCE
WARNING
1. User maintenance is restricted to cleaning the
outside surfaces of the device, as detailed in this
section.
2. Other procedures detailed in this section must be
carried out by trained technicians only.
3. Service and repair operations must only be
carried out by an engineer trained by the
manufacturer.
The warranty for this product is void if the
product is not maintained in accordance with the
service schedule detailed below, and the
procedures published in the Service Manual for
this product.
6.1 Service Schedule
At 6 months, 12 months, 2 years and 4 years, the
ventilator must be serviced by an engineer trained
by the manufacturer, following the schedule given
below, and the procedures given in the AV-S
Service Manual.
Every day:
Pre-use function check
Every week:
Check the condition of the bellows assembly
diaphragm valve, and clean as required.
Test the Mains Failure Alarm and the Low Supply
Pressure Alarm
Every 6 months:
Inspection and Function Check.
Remove patient block assembly and clean.
Check condition of bellows.
Every 12 months:
Repeat six month procedure, plus:
Replace O-seals and drive gas inlet filter.
Replace exhaust diaphragm valve
Preventive maintenance kit available.
Every 2 years:
Repeat 12 month service, plus:
Replace 12 v battery.
Every 4 years:
Repeat 2 year service, plus:
Replace PCB battery.
Replace bellows diaphragm valve
Details of these service operations are given in the
Service Manual.
Always ensure that a record is kept of any
service or repair work.
61

62
MAINTENANCE
1
6.2 Cleaning
6.2.1 Outside surfaces
CAUTION
a) Care must be taken not to allow liquids to run into the control
unit; serious damage may result.
b) Check that the unit is disconnected from the electrical supply
before cleaning.
c) Do not use harsh abrasive cleaning agents.
To clean the outside surface of the ventilator, use a damp cloth
that has been immersed in a cleaning solution and thoroughly
wrung out.
Use cleaning agents as recommended by your hospital infection
control department:
Use a warm, mild detergent solution to remove resistant grime.
To remove blood etc, clean as above then use an antiseptic
solution, or anti-microbial wipes.
Make sure that all cleaning agent residues are fully removed
after cleaning.
Touchscreen
Use a soft cloth only. Never use any harsh abrasive
cleaning agent.
6.2.2 Bellows Assembly
The bellows assembly is built into the A200SP absorber.
For further information please refer to the user
instructions supplied with the A200SP.
6.2.3 Spirometer Sensors
The sensors are built into the A200SP absorber, and
cleaning and sterilization can only be carried out when
the absorber assembly is removed for cleaning.
For further information please refer to the user
instructions supplied with the A200SP.
6.2.4 Oxygen Monitor Sensor
The sensor (1) is built into the A200SP absorber.
For further information please refer to the user
instructions supplied with the A200SP.
WARNING
The sensor is not suitable for sterilization. If
contamination is suspected, fit a new sensor (see section
6.4).
Dispose of the contaminated unit according to hospital,
local, state and federal regulations.

63
MAINTENANCE
1
1
2
3
4
4
3
3
2
6.2.5 Control Unit Patient Block
Assembly
These operations must be carried out by
suitably trained technicians only.
6.2.5.1 Inspection: frequency and
indications
On a regular basis (in line with hospital
procedures for infection control), and at least
every six months, the patient block (1) must be
removed, cleaned and sterilized.
6.2.5.2 Disassembly
1. Detach the hoses from the outlets (2).
Note the different diameters for correct
refitment.
2. Undo the securing knobs (3).
3. Carefully detach the assembly (1) from
the control unit.
Note that resistance will be felt until the
metal tubes (4) disengage.
Do not disassemble the patient block
before cleaning and sterilization.
6.2.5.3 Cleaning
1. Pre-cleaning - submerge the patient
block in an enzymatic solution within an
ultrasonic tank for a period of 20 minutes.
2. Clean the patient block in a
washer/disinfector unit that incorporates
an initial cold rinse, a detergent wash, a
decontamination stage at 92oC, followed
by a final drying stage.
6.2.5.4 Sterilization
1. Sterilize, as recommended in section 6.3.
Do not disassemble.
6.2.5.5 Reassembly
1. Position the patient block and push fully
into the control unit, ensuring that the
metal tubes (4) are engaged in their
unions.
2. Fit the securing knobs (3).
6.2.5.6 Pre-use Checks
1. Function test the ventilator before
clinical use - see section 5.2.1.

MAINTENANCE
64
6.3 Sterilization
These operations must be carried out by suitably
trained technicians only.
CAUTION
To prevent possible damage to components, peak
sterilization temperatures must not exceed 134oC
(275oF) for steam autoclave.
Do not sterilize the ventilator control unit. Apart from the
patient block assembly the internal components are not
compatible with sterilization techniques and may be
damaged.
6.3.1 Recommended Sterilization
Parameters
6.3.1.1 Control Unit Patient Block Assembly
1. Clean, as described in section 6.2.5.3
2. Autoclave at 134oC, for a holding time of 3.5
minutes, using packaging and equipment as listed
below:
Packaging
Pack the control unit with material which is
permeable to air and steam but has an effective
maximum pore size which is small enough to
exclude microbial contamination.
All wrapping materials must comply with EN 868:
Packaging Materials for Sterilization of Wrapped
Goods.
Processing Equipment
The sterilizer must comply with the stated
performance class BS 3970 and HTM 2010 and with
additional requirements stated in Section D.
If a porous-load sterilizer is used it must conform to
the specifications in EN 285 and the safety
specifications in EN 61010: Part 2-041.
Sterilization must be achieved by direct contact with
good quality saturated steam .
Post-processing
Following reprocessing the patient block must be
kept in a sterile plastic pouch to avoid being recontaminated prior to being fitted to the ventilator.
Refit in accordance with section 6.2.5.5. Function
test the ventilator before clinical use - see section
5.2.1.

MAINTENANCE
65
6.4 Oxygen Sensor Replacement
These operations must be carried out by suitably
trained technicians only.
WARNING
The sensor contains:
A) A small quantity of electrolyte, classified as a harmful
irritant which is potentially hazardous.
B) Lead
Do not attempt to open a cell.
ALWAYS check the integrity of the sensor assembly
before use.
Once exhausted, the sensor must be disposed of
according to hospital, local, state and federal
regulations.
6.4.1 Sensor Unit - Remove and Refit
Replacement parts
102714 Sensor
1. Detach the cable connector (1) from the
sensor (2).
2. Unscrew the sensor from the A200SP
Absorber, and discard.
3. Discard the expired sensor.
4. Screw the new sensor (2) into the absorber.
5. Attach the cable connector (1).
6. Fit the assembly into the absorber.
7. Calibrate the new sensor - see section 5.2.1.
8. Dispose of the used components according
to hospital, local, state and federal
regulations.
1
2

7. APPENDIX
APPENDIX 1
Care of Back-up Battery
CAUTION
Damage may occur if the battery is allowed to remain in a discharged state.
A. Battery installed in ventilator
The battery must be charged before the machine is released for use
with an 14 hour charge from the ventilator’s internal power supply
(ventilator connected to the mains supply, but not running).
Note that the mains power indicator on the front panel will show a
yellow light during charging.
Subsequently the recharge periods for a battery on a ventilator in
store are similar to those in B, below.
Batteries in machines in normal use will be kept charged by the
internal power supply.
Note that the Low Battery Alarm indicator may be displayed if
automatic recharging is taking place as the ventilator is in use.
B. Battery care/storage requirements.
During storage, batteries will require a periodic recharge, the
frequency of which is determined by the storage temperature, which
must not exceed 50oC (120oF).
Storage Recharge
temperature period
38 to 50oC (100 to 122oF) 1 month
21 to 38oC (70 to 100oF) 3 months
7 to 21oC (45 to 70oF) 6 months
0 to 7oC (32 to 45oF) 9 months
-5 to 0oC (23 to 32oF) 12 months
Recharge duration - a charging cycle of at least 12 hours, to ensure
that the battery is kept at full capacity.
It is recommended that at each charge an updated label is affixed to
the unit to indicate date of the last charge.
C. Disposal of used batteries
Do not dispose of in landfill, refer to an approved recycling facility.
Follow your hospital, local, state and federal regulations.
Note
Removal/replacement of battery must only be undertaken by a trained
technician
66

On-screen Menus
67
APPENDIX 2
APPENDIX
NOTE:
1. All selection or changes in the menu are
followed by a "CONFIRM" message
prompt on the screen, and accompanied
by a "BEEP" (user volume set)
2. The selected text or option will invert in
colour
3. User settings menus only activate in
Standby mode.
4. Clock menu, Upgrade menu, Diagnostic
menu only activate in Standby mode.
5. Special Modes on-screen tab only
activates in Spontaneous mode
6. Adult default settings
VT=600 mL
RATE=10 bpm
IE RATIO=1:2
Plimit=38 cmH2O
Ptarget=10 cmH2O
7. Pediatric default settings
VT=150 ml
RATE=15 BPM
IE RATIO=1:2
Plimit=38 cmH2O
Ptarget=10 cmH2O

68
EXIT MENUS
O2 MONITOR & SPIROMETRY
LEAK TEST
FRESH GAS COMPENSATION: ON
SPECIAL MODES
WAVEFORM
ALARM SETTINGS
GAS MIXTURE: O2+AIR
USER SETTINGS
SERVICE MENU
O2 Monitor & Spirometry
ESCAPE FROM MENU
O2 MONITOR: on
CALIBRATION: 100%
HIGH ALARM SET: 105
LOW ALARM SET: 18
SPIROMETER: on
SPIRO CALIBRATION: 0 L/min
off / on (Toggle option
21 / 100% (Toggle option)
19 -105 (Integer)
18 - 99 (Integer)
off / on (Toggle option)
0 L/min / 10 L/min (Toggle option)
off/on (Toggle option)
Fresh Gas Compensation
ON / OFF
Special Modes
See next page
Waveform
ESCAPE FROM MENU
SECOND WAVEFORM: off
Second waveform pick list
off
vol. vs time
vol. vs press.
Alarm settings
ALARM MENU
ESCAPE FROM MENU
ALARM MODE : default
HIGH TIDAL VOLUME: off
VM MIN: 0.3 L
VM MAX: 0.9 L
VT MIN: 300 mL
VT MAX: 900 mL
APNOEA ALARM LIMIT: 0.3 cmH2O
ALARM VOLUME: 50%
default / user (Toggle option)
off / on (Toggle option)
0.0 - 7.4 (Integer)
0.1 - 7.5 (Integer)
10 - 1600 (Integer)
20 - 2400 (Integer)
0.3 - 3.5 (Integer)
50 - 100% (Integer)
Gas mixture: O2+Air
O2+AIR
O2+N2O
O2+Xe
O2+He
User Settings
ESCAPE FROM MENU
SELECT SETTINGS
SAVE SETTINGS
BACK LIGHT LEVEL: 50%
VOLUME TYPE: tidal
Leak Test
ESCAPE FROM MENU
<START/STOP LEAK TEST>
LEAK STATUS: unknown
LEAK LEVEL: 0 mL/min
BSYS COMP 7.0 mL/cmH2O
Service
See page 62
Menu Structure
Main Menu
Select settings
ESCAPE FROM MENU
USER1: CCT1
USER2: CCT2
USER3: CCT3
USER4: CCT4
USER5: CCT5
ADULT DEFAULT
PEDIATRIC DEFAULT
Save settings
ESCAPE FROM MENU
USER1: CCT1 CONFIRM: CCT1
USER2: CCT2 CONFIRM: CCT2
USER3: CCT3 CONFIRM: CCT3
USER4: CCT4 CONFIRM: CCT4
USER5: CCT5 CONFIRM: CCT5
Backlight level
0 - 100% (integer)
Volume type
tidal/minute (toggle)

69
In STANDBY the SPECIAL MODES menu is:
ESCAPE FROM MENU
SUPPORT MODE: none
(1)
TRIGGER: 1.0 L/min
(2)
SIGH ENABLE: off
(3)
SIGH TO BREATH RATIO: 1:50
(4)
INSP. PAUSE: off
(3)
In SPONT mode the SPECIAL MODES menu is:
ESCAPE FROM MENU
TRIGGER: 1.0 L/min
(2)
In VOLUME Control the SPECIAL MODES menu is:
ESCAPE FROM MENU
SIGH ENABLE: off
(3)
SIGH TO BREATH RATIO: 1:50
(4)
INSP. PAUSE: off
(3)
In PRESSURE, SIMV, SMMV, or PSV modes:
The SPECIAL MODES menu is unavailable.
Notes
(1) Support mode depends on configuration options.
The SUPPORT MODE option will be missing from the SPECIAL
MODE menu if:
a) Options are not enabled
b) ‘’SPIROMETRY: off’’ is displayed.
The support mode sub menu can include:
none
PSV
SIMV
SMMV
(2) The TRIGGER values are L/min with SPIROMETRY enabled, or
cmH
2O when SPIROMETRY disabled.
Spirometry enabled Spirometry disabled
0.7 L/min 0.5 cmH
2O
0.8 L/min 0.6 cmH
2O
0.9 L/min 0.7 cmH
2O
1.0 L/min 0.8 cmH
2O
1.5 L/min 0.9 cmH
2O
2.0 L/min 1.0 cmH
2O
2.5 L/min 1.2 cmH
2O
3.0 L/min 1.5 cmH
2O
3.5 L/min 1.7 cmH
2O
4.0 L/min 2.0 cmH
2O
(3) The options here are: on or off.
(4) The options here are:
1:50
2:50
3:50
4:50
SPECIAL MODES MENU
NOTE
The SPECIAL MODES menu is
context sensitive, with the contents
dependent on current mode.

70
Upgrade
ESCAPE FROM MENU
I/O HARDWARE: 2
I/O FIRMWARE: v0.47 [Build 68]
MAIN FIRMWARE: v0.92 [Build 32]
REGISTRATION KEY: unknown
UPGRADE FIRMWARE: unavailable
ADD NEW FEATURE: unavailable
History Display
ESCAPE FROM MENU
MANUFACTURER DATE : 03/03/05
TOTAL HOURS RUN: 100
LAST SERVICE DATE: 13/08/04
HOURS SINCE SERVICE : 100
DRIVE VALVE CYCLES: 1253
PATIENT VALVE CYCLES: 822
CUTOFF VALVE CYCLES: 72
Service
ESCAPE FROM MENU
LANGUAGE: ENGLISH
PRINT PATIENT DATA
SERIAL MODE: none
CLOCK MENU
UPGRADE MENU
AMBIENT PRESSURE: 988 mBar
DISPLAY HISTORY
*SERVICE PIN: 0
*ENGINEER MENU
Language pick list
ENGLISH
ITALIANO
TURKCE
POLSKI
ESPANOL
Serial mode pick list
NONE
PHILIPS
SPACELABS
Clock
ESCAPE FROM MENU
YEAR: 2005
MONTH: 3
DATE: 16
DOW: 3
HOUR: 9
MINUTE: 57
UPDATE CLOCK
DAYLIGHT SAVING: off
Clock pick list (integer)
2005 - 2099 (integer)
1 - 12 (integer)
1 -31 (integer)
1 - 7 (1 = Monday) (integer)
0 - 23 (integer)
0 - 59 (integer)
off / on (toggle option)
*NOTE
Service PIN
Engineer Menu
Sub-menus are not accessible by users.
SERVICE MENU

71
A similar measurement method is used for the
exhaled volume. During the exhalation period
the measured exhaled volume is subtracted from
the inspired volume, and again at the end of
exhalation.
A negative (more gas coming out) volume
indicates that fresh gas has increased the
delivered volume.
A positive volume (less gas coming out)
indicates a leak in the circuit.
The ventilator control system will then adjust the
next delivered tidal volume (up to a maximum of
100 ml). This will bring the delivered volume to
exactly as set.
If the variation between set and delivered is
greater than the maximum rate of change
allowed, the adjustment will occur gradually over
several breaths.
The displayed volume is the average of the
inspiratory and expiratory volumes. If this value
is less or more than 50% of set volume, a low or
high volume alarm is given.
Breathing System Gas Composition
Gas flow measurements are affected by the
composition of the breathing system gas.
To compensate for these effects the
ventilator has
a) a gas composition setting whereby the
user is able to select the gases being
delivered, i.e. oxygen/air, oxygen/nitrous
oxide etc,
b) an oxygen monitor;
Thus the ventilator knows the overall
oxygen concentration and the majority of
the remaining gas composition.
Altitude Effects
Gas flow measurements are also affected by
atmospheric pressure, in a linear relationship.
To compensate for altitude effects an ambient
pressure sensor is used. When the spirometers
are calibrated for zero flow the ambient pressure
is recorded so that the measured volume may
be adjusted. The measured volume is multiplied
by the ratio of Pamb to Pcal; where Pamb is the
latest ambient pressure and Pcal is the ambient
pressure recorded when the spirometers were
calibrated at zero flow.
Carrier Gas Effects
The effect of air as the dilutent gas is
different to that of nitrous oxide and as the
ventilator includes only an oxygen monitor,
the additional information of gas being
ventilated is included to increase available
accuracy.
APPENDIX
APPENDIX 3
AV-S Ventilator Spirometry System
Ventilator Spirometry Measurement
The AV-S ventilator drive gas system and
spirometry system uses a total of three mass
flow gas sensors. The sensors monitor, and then
independently measure the gas flows within the
ventilator and breathing system.
This ensures that correct volumes are delivered
to the patient.
The sensors are measuring firstly in the
ventilator delivery control system, and secondly
in the patient breathing system.
During use of the ventilator the user will set a
required tidal volume and at the first breath the
ventilator will use its pre- calibrated delivery flow
rate valve settings to set the proportional
delivery valve position to deliver the requested
tidal volume.
To confirm that the correct flow rate (tidal
volume) is being delivered by the ventilator
delivery system an internal flow sensor (a
Honeywell AWM43300V mass flow sensor),
monitors the delivered flow rate and makes
adjustments every 30 ms using proportional
regulation.
As this sensor is always measuring the known
drive gas rather than breathing system gas the
volumes measured will always be independent
of breathing system gas composition. This
method ensures accurate delivery volume from
the ventilator control unit.
To monitor for correct delivery volumes in the
breathing system there are two breathing system
mass flow sensors (Honeywell AWM 720P1
spirometers).
One sensor is located in the inspiratory limb,
and one in the expiratory limb.
Measurements are taken from these sensors to
determine the actual delivered and exhaled gas
volumes in the breathing system. This enable
measurements to be made to compensate for
fresh gas flow, compliance losses and possible
breathing system leaks.
During the inspiratory cycle the inspiratory flow
sensor measures the gas volume delivered to
the patient.
The flow sensor output is read at least every 2
msec. Five sets of readings are averaged and
the averaged value is sent every 10 ms to the
processor for calculation of the volume delivered
to the patient.
This delivered volume will consist of the volume
delivered from the ventilator bellows, plus the
fresh gas flow from the anesthetic machine
fresh gas supply, minus any compliance loss
and minus any leak.
This gives a total actual inspired tidal volume.

The microbridge mass airflow sensor uses
temperature-sensitive resistors deposited within
a thin film of silicon nitride. They are suspended
in the form of two +bridges over an etched cavity
in the silicon.
The chip is located in a precisely dimensioned
airflow channel to provide a repeatable flow
response.
Highly effective thermal isolation for the heater
and sensing resistors is attained by etching the
cavity space beneath the flow sensor bridges.
The small size and thermal isolation of the
microbridge mass airflow sensor are responsible
for the extremely fast response and high
sensitivity to flows.
Dual Wheatstone bridges control airflow
measurement - one provides closed loop heater
control, the other contains the dual sensing
elements.
The heater circuit minimizes shift due to ambient
temperature changes by providing an output
proportional to mass flow.
The circuit keeps the heater temperature at a
constant differential (160°C) above ambient air
temperature which is sensed by a heat-sunk
resistor on the chip.
The ratiometric voltage output of the device
corresponds to the differential voltage across the
Wheatstone bridge circuit.
Sensor flow characteristics
The graph shown below is a typical flow versus
resistance graph for the Honeywell spirometer
head units for the flow range showing typical
hysteresis between up and down flow
measurements (and repeatability).
72
APPENDIX
0 20 40 60 80 100 120 140
5
4
3
2
1
0
Flow (L/min)
Resistance
(cmH
2O)
Anesthetic Agent Effects
The addition of anesthetic agent is known also
to increase the spirometry readings (by up to
approximately 2%) depending on the agent and
its concentration. Again, this minor volume
measurement variation is of no known clinical
disadvantage and is therefore not compensated
for other than that due to oxygen variation due to
the percentage change.
Water Vapour Effects
Water vapour volumes in the breathing gas are
not detectable in normal breathing system
dynamics.
Additional Features
Additional spirometry features available for
selection by the user are the ability to turn off
the automatic compliance and fresh gas
compensation, and also the feedback provided
by the oxygen monitor.
In this event, the ventilator relies on the basic
delivery look up table and the internal flow
sensor to confirm delivery volumes as near as
possible, under the circumstances.
Accuracies for spirometry measurement are
≤100 ml ± 50%.
>100 ml ± 20%
Flow sensor description
The microbridge mass airflow sensor operates
on the theory of heat transfer. Mass airflow is
directed across the surface of the sensing
elements.
Output voltage varies in proportion to the mass
airflow (or other gas flow) through the inlet and
outlet ports of the unit.
The specially designed housing precisely directs
and controls the airflow across the
microstructure sense element.
The microbridge mass airflow sensor has a
unique silicon chip based on advanced
microstructure technology. It consists of a thinfilm, thermally isolated bridge structure
containing a heater and temperature sensing
elements. The bridge structure provides a
sensitive and fast response to the flow of air or
other gas over the chip.
Dual sensing elements positioned on both sides
of a central heating element indicate flow
direction as well as flow rate.
Laser trimmed thick film and thin film resistors
provide consistent interchangeability from one
device to the next.

APPENDIX
APPENDIX 4
Disposal at end of useful life - risk assessment
Do not dispose of in landfill, refer to an approved recycling facility.
Follow your hospital, local, state and federal regulations.
EC territories: Follow the requirements of Directive 2002/96/EC.
Note Disposal of used batteries - see Appendix 1.
73
APPENDIX 5
Approved Accessories
WARNING
Only use accessories approved by DRE, Inc.
57655 Compact Pressure Tee
57523 Pressure sensing tube
57545 Adult Bellows and base canister
57551 Adult Canister
57550 Adult Bellows
57548 Bellows Base
57656 Bellows base manifold block
dditional to bellows base, use on stand-alone unit
A
57553 Pediatric Canister
57552 Pediatric Bellows
57554 Pediatric Bellows Adaptor
Sales
Tel: (502) 244-4444
Fax: (502) 244-0369
Web: www.dremed.com

Copyright © DRE, Inc., 2008
All rights reserved.
Cat No 52967
Doc No AV-S (A2) 0208UI
September 2008
Sold by:
DRE, Inc.
1800 Williamson Court
Louisville, KY 40223
USA
Technical Support & Sales
Tel: (502) 244-4444
Fax: (502) 244-0369
Web: www.dremed.com
 Loading...
Loading...