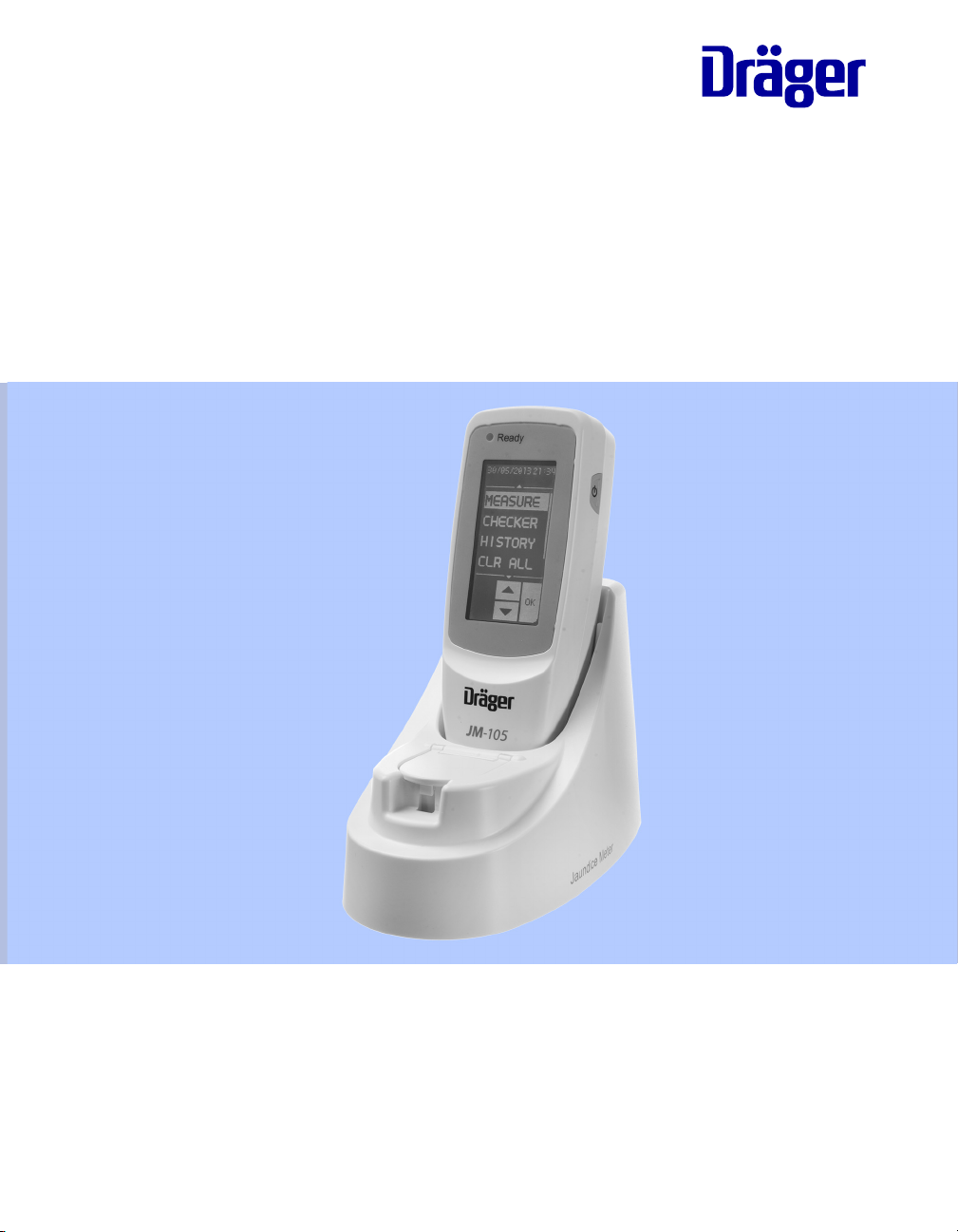
Instructions for Use
JM-105
WARNING
To properly use this medical device,
read and comply with these instructions for use.
Jaundice Meter
Software 1.20

This page intentionally left blank.
Instructions for Use JM-105

Typographical conventions
1 Consecutive numbers indicate steps of action,
with the numbering restarting with "1" for each
new sequence of actions.
Bullet points indicate individual actions or differ-
ent options for action.
– Dashes indicate the listing of data, options, or
objects.
(A) Letters in parentheses refer to elements in the
related illustration.
A Letters in illustrations denote elements referred
to in the text.
> The greater-than symbol indicates the naviga-
tion path in a dialog window.
Bold, italicized text indicates labels on the device and texts that are displayed on the screen.
Trademarks
Trademarks owned by third-party manufacturers
Illustrations
Illustrations of products and screen content in this
document may differ from the actual products
depending on configuration and design.
Use of terms
Dräger uses the term "accessories" not only for accessories in the sense of IEC 60601-1, but also for
consumables, removable parts, and attached parts.
Trademark Trademark owner
Actichlor Ecolab USA
Oxycide Ecolab USA
Adobe
Reader
Klorsept 17 Medentech
Peridox RTU Bio Med Protect
Windows Microsoft Corporation
Windows
Vista
Pentium Intel Corporation
Dismozon
pur
Instructions for Use JM-105 i
Adobe Corporation
BODE Chemie
Safety information definitions
WARNING
A WARNING statement provides important
information about a potentially hazardous
situation which, if not avoided, could result in
death or serious injury.
CAUTION
A CAUTION statement provides important information about a potentially hazardous situation
which, if not avoided, may result in minor or moderate injury to the user or patient or in damage to
the medical device or other property.
NOTE
A NOTE provides additional information intended
to avoid inconvenience during operation.
ii Instructions for Use JM-105

Contents
Contents
Typographical conventions. . . . . . . . . . . . . . . . i
Trademarks . . . . . . . . . . . . . . . . . . . . . . . . . . . i
Safety information definitions . . . . . . . . . . . . . . ii
Contents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . iii
For your safety and that of your patients. . . 1
General safety information . . . . . . . . . . . . . . . . 2
Target groups . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Product-specific precautions . . . . . . . . . . . . . . 7
Application . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Intended use. . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Indications/contraindications . . . . . . . . . . . . . . 12
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Device views. . . . . . . . . . . . . . . . . . . . . . . . . . . 16
External devices . . . . . . . . . . . . . . . . . . . . . . . . 19
Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Operating concept . . . . . . . . . . . . . . . . . . . . . 25
Screen layout for device. . . . . . . . . . . . . . . . . . 26
Screen layout for data transmission software. . 28
Trends and Data. . . . . . . . . . . . . . . . . . . . . . . 63
Viewing measurements stored in the data
log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Deleting individual measurements in the
data log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Deleting all measurements in the data log . . . 65
Transmitting data to electronic charts . . . . . . . 66
Viewing the transmission log from the JM-
S1w software. . . . . . . . . . . . . . . . . . . . . . . . . . 68
Configuration. . . . . . . . . . . . . . . . . . . . . . . . . 69
Changing settings on the JM-105 . . . . . . . . . . 70
System and default settings for the JM-105 . . 70
Problem solving . . . . . . . . . . . . . . . . . . . . . . 75
Fault – Cause – Remedy. . . . . . . . . . . . . . . . . 76
Reprocessing. . . . . . . . . . . . . . . . . . . . . . . . . 81
Safety information . . . . . . . . . . . . . . . . . . . . . . 82
Information on reprocessing . . . . . . . . . . . . . . 82
Classifications for reprocessing. . . . . . . . . . . . 83
Before reprocessing . . . . . . . . . . . . . . . . . . . . 84
Validated reprocessing procedures . . . . . . . . . 85
Other agents and reprocessing procedures . . 87
After reprocessing . . . . . . . . . . . . . . . . . . . . . . 88
Assembly and preparation . . . . . . . . . . . . . . 33
Charging the battery. . . . . . . . . . . . . . . . . . . . . 34
Unpacking the data transmission software . . . . 36
Getting started . . . . . . . . . . . . . . . . . . . . . . . . 37
Switch on and pre-set the device for the
first time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Pre-use checkout . . . . . . . . . . . . . . . . . . . . . . . 40
Data transmission software . . . . . . . . . . . . . . . 42
Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Ensuring correct measurement . . . . . . . . . . . . 52
Choosing settings for measurement. . . . . . . . . 53
Measuring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Storing the device. . . . . . . . . . . . . . . . . . . . . . . 60
Switching off the device . . . . . . . . . . . . . . . . . . 61
Measuring quick guide . . . . . . . . . . . . . . . . . . . 62
Instructions for Use JM-105 iii
Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 89
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 90
Definition of service terminology . . . . . . . . . . . 90
Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Performing service . . . . . . . . . . . . . . . . . . . . . 92
Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 93
Disposal of the product . . . . . . . . . . . . . . . . . . 94
Technical data . . . . . . . . . . . . . . . . . . . . . . . . 95
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . 96
Ambient conditions . . . . . . . . . . . . . . . . . . . . . 98
EMC declaration . . . . . . . . . . . . . . . . . . . . . . . 99
Principles of operation . . . . . . . . . . . . . . . . . 101

Contents
Measuring principle . . . . . . . . . . . . . . . . . . . . . 102
List of accessories . . . . . . . . . . . . . . . . . . . . . 105
Appendix A Clinical performance
summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 107
Investigating The Agreement Between
Transcutaneous Bilirubin Measurements
Using the JM-105 and Total Serum Bilirubin
Measurements By Site, Before, During and
After Phototherapy in an ethnically diverse
population of infants ≥ 24 weeks gestation. . . . 108
Tables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 112
References . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
Index. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 115
iv
Instructions for Use JM-105

For your safety and that of your patients
General safety information . . . . . . . . . . . . . . 2
Strictly follow these instructions for use . . . . . . 2
Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Connected devices. . . . . . . . . . . . . . . . . . . . . . 3
Not for use in areas of explosion hazard . . . . . 3
Safe connection with other electrical
equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Connection to other devices. . . . . . . . . . . . . . . 3
Patient safety . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Patient monitoring. . . . . . . . . . . . . . . . . . . . . . . 4
Electromagnetic compatibility (EMC) . . . . . . . . 4
Storing the instructions for use. . . . . . . . . . . . . 5
Training. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Tar get gro ups . . . . . . . . . . . . . . . . . . . . . . . . . 6
Duties of the operating organization. . . . . . . . . 6
Description of target groups . . . . . . . . . . . . . . . 6
For your safety and that of your patients
Product-specific precautions . . . . . . . . . . . . 7
Electrical precautions . . . . . . . . . . . . . . . . . . . . 7
General precautions . . . . . . . . . . . . . . . . . . . . . 8
Storage and transportation precautions . . . . . . 9
Restrictions for use. . . . . . . . . . . . . . . . . . . . . . 9
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Instructions for Use JM-105 1
For your safety and that of your patients
General safety information
The following WARNING and CAUTION statements apply to general operation of the medical
device.
WARNING and CAUTION statements specific to
subsystems or particular features of the medical
device appear in the respective sections of these
instructions for use or in the instructions for use of
another product being used with this medical
device.
Strictly follow these instructions for use
WARNING
Risk of incorrect operation and of incorrect
use
Any use of the medical device requires full
understanding and strict observation of all
sections of these instructions for use. The
medical device must only be used for the purpose specified under Intended use on page 12
and in conjunction with appropriate patient
monitoring (see page 4).
Strictly observe all WARNING and CAUTION
statements throughout these instructions for
use and all statements on medical device
labels. Failure to observe these safety information statements constitutes a use of the
medical device that is inconsistent with its
intended use.
Maintenance
WARNI NG
Risk of medical device failure and of patient
injury
The medical device must be inspected and
serviced regularly by service personnel.
Repair and complex maintenance carried out
on the medical device must be performed by
specialized service personnel.
If the above is not complied with, medical
device failure and patient injury may occur.
Observe chapter "Service".
Dräger recommends that a service contract is
obtained with DrägerService and that all
repairs are performed by DrägerService. For
maintenance Dräger recommends the use of
authentic Dräger repair parts.
Service
WARNI NG
Risk if service is not performed regularly
If service is not performed regularly, malfunctions may occur, which can result in personal
injury and property damage.
Perform the service in accordance with the
chapter "Service".
2
Instructions for Use JM-105
For your safety and that of your patients
Connected devices
WARNING
Risk of electric shock and of device malfunc-
tion
Any connected devices or device combinations not complying with the requirements
mentioned in these instructions for use can
compromise the correct functioning of the
medical device and lead to an electric shock.
Before operating the medical device, strictly
comply with the instructions for use of all connected devices or device combinations.
Not for use in areas of explosion hazard
WARNING
Risk of fire
The medical device is not approved for use in
areas where combustible or explosive gas
mixtures are likely to occur.
Safe connection with other electrical equipment
CAUTION
Risk of patient injury
Electrical connections to equipment not listed in
these instructions for use or these assembly
instructions must only be made when approved
by each respective manufacturer.
Connection to other devices
If a device combination is not approved by Dräger,
proper operation of the devices can be compromised.
The operator must ensure that the device combination meets the applicable standards.
Strictly observe instructions for use and assembly
instructions of all connected devices.
Patient safety
The design of the medical device, the accompanying documentation, and the labeling on the medical
device are based on the assumption that the purchase and the use of the medical device are
restricted to persons familiar with the most important inherent characteristics of the medical device.
Instructions and WARNING and CAUTION statements are therefore largely limited to the specifics
of the Dräger medical device.
The instructions for use do not contain any information on the following points:
– Risks that are obvious to users
– Consequences of obvious improper use of the
medical device
– Potentially negative effects on patients with dif-
ferent underlying diseases
Medical device modification or misuse can be dangerous.
Instructions for Use JM-105 3
For your safety and that of your patients
Patient monitoring
WARNING
Risk of patient injury
Do not make therapeutic decisions based solely
on individual measured values and monitoring
parameters.
WARNING
Risk of patient injury
The device is not intended as a stand-alone
screening device for diagnosis of hyperbilirubinemia. It is used as a screening device with
other clinical signs and laboratory measurements.
The user of the medical device is responsible for
choosing a suitable patient monitoring system that
provides appropriate information on medical device
performance and patient condition.
Patient safety can be achieved by a wide variety of
means ranging from electronic surveillance of medical device performance and patient condition to
direct observation of clinical signs.
The responsibility for selecting the best level of
patient monitoring lies solely with the user of the
medical device.
Electromagnetic compatibility (EMC)
Medical electrical equipment is subject to special
precautionary measures concerning
electromagnetic compatibility. During installation
and before initial operation, follow the information in
section: "EMC declaration" (page 99).
This device can be affected by other electrical
devices.
WARNI NG
Risk due to electrostatic discharge
Malfunctions that endanger the patient may
occur if no protective measures against electrostatic discharge are employed in the following situations:
– When touching the pins of connectors that
carry the ESD warning symbol.
– When establishing connections with these
connectors.
To prevent malfunctions, observe the following measures and train the relevant personnel:
– Observe the ESD protective measures.
Such measures may include wearing antistatic clothing and shoes, touching a potential equalization pin before and while
making the connection, or using electrically insulating and antistatic gloves.
– Observe the requirements for the electro-
magnetic environment. Observe the following section: "EMC declaration"
(page 99).
WARNI NG
Risk due to electromagnetic disturbance
Wireless communication devices (e.g., cellular phones) and medical electrical equipment
(e.g., defibrillators, electrosurgical devices)
emit electromagnetic radiation. When such
devices are operated too close to this device
or its cables, the functional integrity of this
device may be compromised by electromagnetic disturbances. As a result, the patient
could be put at risk.
Maintain a distance of at least 0.3 m (1.0 ft)
between this device and wireless communication devices, to ensure that the essential performance of this device is fulfilled.
Maintain an adequate distance between this
device and other medical electrical equipment.
4
Instructions for Use JM-105
Storing the instructions for use
CAUTION
Risk of incorrect use
Instructions for use must be kept accessible to the
user.
Training
Training for users is available via the Dräger organization responsible (see www.draeger.com).
Service
WARNING
Risk if service is not performed regularly
If service is not performed regularly, malfunctions may occur, which can result in personal
injury and property damage.
Perform the service in accordance with the
chapter "Service".
For your safety and that of your patients
Instructions for Use JM-105 5
For your safety and that of your patients
Target groups
Duties of the operating organization
The tasks described in this document specify the
requirements that have to be met by each respective target group.
The operating organization of this product must ensure the following:
– The target group has the required qualifications
(e.g., has undergone specialist training or acquired specialist knowledge through experience).
– The target group has been trained to perform
the task.
– The target group has read and understood the
chapters required to perform the task.
Description of target groups
The target groups may only perform the following
tasks if they meet the corresponding requirements.
User
Service personnel
Task Requirement
Installation Specialist knowledge in
Basic service work
(inspection, maintenance according to
the "Maintenance"
chapter)
Dräger recommends arranging a service contract
with DrägerService.
electrical engineering and
mechanics
Experience in the servicing
of medical devices
Task Requirement
Use of the product in
accordance with the
intended use
Use of the product in
accordance with the
intended use
Reprocessing personnel
Task Requirement
Reprocessing Specialist knowledge in the
6
Specialist medical knowledge in neonatology
Specialist medical knowledge in the use of the product
reprocessing of medical
devices
Instructions for Use JM-105
Product-specific precautions
For your safety and that of your patients
Electrical precautions
WARNING
Risk of fire, electric shock, or equipment dam-
age
Using a docking station or AC adapter other
than the one provided with the device could
damage the device.
Use only the docking station JM-A33 and the
AC adapter JM-A32 with the device.
WARNING
Risk of fire, electric shock, or equipment dam-
age
Connecting to a power source without a protective earth ground could damage the device.
Connect the device only to a power source
with a protective earth ground.
WARNING
Risk of fire, electric shock, or equipment dam-
age
Pulling the power cable by the cable could
damage the cable and cause fire or electric
shock.
Hold the AC power cable by the plug-end
when disconnecting from a power source or
the AC adapter.
WARNING
Risk of fire
Dust or water could collect at the plug of the
power cable.
Disconnect the power cable when the device
is not being used or charged for any length of
time.
WARNING
Risk of electric shock
Touching the AC power cable with wet hands
could cause electric shock.
Do not connect or disconnect the AC power
cable with wet hands.
WARNING
Risk of electric shock or device malfunction
Penetrating metal objects may damage the
device or docking station, causing malfuntion
of the device, which may endanger the patient.
Do not allow metal objects to penetrate into
the device or docking station.
WARNING
Risk of fire
Operating the device and its accessories
when they are damaged could cause a fire.
Do not operate the device or its accessories if
any of them are damaged, or if there is smoke
or an odd odor.
WARNING
Risk of patient injury
Strong ambient light, electromagnetic interference, and mobile telephone use can interfere
with accurate measurement of data.
Do not use the device in strong ambient light,
or near electronic devices or mobile telephones.
Instructions for Use JM-105 7
For your safety and that of your patients
General precautions
WARNING
Risk due to modifications
Modifications to the product may lead to malfunctions and unforeseen risks. This may result in injury to the patient or the user or in
property damage.
Do not modify this product.
WARNING
Risk of injury
Operating the device while the probe is
directed at the eyes can cause eye damage.
Do not press the measuring probe when it is
directed at the eyes.
WARNING
Risk of patient injury
Pathologic or other skin conditions that may
affect light scattering or absorption could
result in incorrect TcB measurements.
Do not conduct measurements in patients with
early jaundice and pathologic jaundice.
Do not conduct measurements when skin
conditions may violate the assumption made
concerning light scattering and light absorption.
Use device only on healthy skin of the patient.
Do not conduct measurements on birthmarks
and hairy areas. Avoid areas of thickness that
in the opinion of the physician would preclude
or interfere with the use of the TcB meter.
WARNI NG
Risk of delayed therapy
Using the device without checking the accuracy of the measurements could cause incorrect measurements.
To check the measurement reliability of the
system, compare the transcutaneous bilirubin
value (TcB) determined by the device and the
total serum bilirubin (TSB) determined on the
basis of blood samples. Follow your hospital
guideline to determine the frequency of
checks (e.g. after repair or calibration of JM105 or TSB lab equipment, or following a
change to clinical processes.
CAUTION
Risk of equipment damage
The device or docking station could overturn or
fall.
Do not place the device on an unstable or sloped
surface.
CAUTION
Risk of equipment damage
Do not drop the device or place heavy objects on
top of the device.
CAUTION
Risk of equipment damage
The device is not waterproof or liquid proof.
Do not expose the device to rain, water, blood, or
other liquids.
CAUTION
Risk of equipment damage
Excessive vibration or impact could damage the
device.
Handle the device gently, and avoid excessive
impact or vibration.
8
Instructions for Use JM-105
For your safety and that of your patients
NOTE
Ensure that the device is placed near the AC
power source. Also ensure the AC power cable
can be easily connected and disconnected.
Storage and transportation precautions
CAUTION
Risk of equipment damage
Do not store the device in areas where direct sunlight, pressure, temperature, humidity, ventilation,
dust, strong magnetic fields, or saline or sulphurous atmospheres affect the device.
Do not store the device where it is exposed to
water.
Do not store the device in areas where chemicals
are stored or where gas is emitted.
CAUTION
Risk of equipment damage
The device or docking station could overturn or
fall.
Do not store the device on an unstable or sloped
surface, or a surface subject to vibration or physical shock.
Restrictions for use
CAUTION
Device for use in health care facilities only and
exclusively by persons with specific training and
experience in its use.
Accessories
WARNING
Risk due to incompatible accessories
The use of incompatible accessories may adversely affect the functional integrity of the
product. Personal injury and property damage
may occur as a consequence.
Use only compatible accessories. The accessories that are compatible with this product
are listed in the list of accessories supplied
with the product.
CAUTION
Risk of equipment damage
Avoid vibration and physical shock during transportation.
NOTE
Thoroughly clean the device and accessories
before storing.
Instructions for Use JM-105 9

This page intentionally left blank.
10 Instructions for Use JM-105

Application
Intended use . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Indications/contraindications . . . . . . . . . . . . 12
Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Contraindications/limitations. . . . . . . . . . . . . . . 12
Application
Instructions for Use JM-105 11

Application
Intended use
The Jaundice Meter is a non-invasive transcutaneous bilirubinometer. It measures yellowness of subcutaneous tissue in newborn infants. The unit
provides a visual digital measurement that has
been shown to correlate with serum bilirubin in
newborn infants.
The device is intended for use in hospitals or doctors offices under a physician’s supervision, or at
their direction. It helps clinicians to monitor newborn infants. The device is a screening device for
the detection of neonatal hyperbilirubinemia in the
early stages. The measurement data provided by
the device should be used in conjunction with other
clinical symptoms and laboratory measurements
for diagnosis and therapy decisions.
Indications/contraindications
Indications
The Jaundice Meter is indicated for use in neonatal
patients born 24 weeks gestation who have not
undergone exchange transfusion. The device is
indicated for use before, during, and after phototherapy treatment.
Newborn infants whose Jaundice Meter test results
are indicative of hyperbilirubinemia should be evaluated by their physicians for appropriate patient
management. Specific neonatal patient bilirubin
levels should be confirmed by other methods, such
as serum bilirubin, before treatment determinations.
The Jaundice Meter is not intended for home use.
The JM-105 is a prescription medical device.
The JM-105 may only be used at the sternum measurement site for Physician's office applications.
Contraindications/limitations
The Jaundice Meter is not intended as a diagnostic
device. It is a screening device for the detection of
neonatal hyperbilirubinemia in the early stage that
shall be used in conjunction with other clinical
symptoms and laboratory measurements for diagnosis and therapy decisions.
Do not use this device on infants with pathologic
jaundice. If there is a possibility that the infant is
suffering from pathologic jaundice, as a result of an
incompatible blood type or hemolytic jaundice, then
total serum bilirubin should be measured.
Do not use this device on patients with hydrops
fetalis major, congenital malformations, diseases or
skin conditions or thickness that in the opinion of
the physician would preclude or interfere with the
use of the TcB meter (e.g. skin infections, purpura,
etc.)
12
Instructions for Use JM-105

Limitations (During phototherapy)
Before beginning measurements, ensure that all
phototherapy lights are shut off.
Limitations (Doctors Office Use)
Use only on infants up to 14 days of age.
Please be aware, performance in doctors offices
may vary from performance in hospitals.
Measuring Point
Typically clinicians measure the TCB on either the
sternum, or the forehead or both. Some studies
reported the sternum to be more accurate than the
forehead in term and near term infants [17, 18].
Some studies show the forehead had a stronger
correlation with TSB that the sternum. In other studies looking at the agreement between TCB and
TSB measurements, the site of TCB measurement
has not been indicated [19, 20].
Therefore, measuring site (forehead or sternum)
shall be at the clinician’s discretion in a hospital setting (avoid birthmarks and hairy areas). However,
only sternum measurements are recommended at
the doctor’s office, as there is a possibility that the
difference may be more pronounced for infants that
have been exposed to sunlight.
Application
Instructions for Use JM-105 13

This page intentionally left blank.
14 Instructions for Use JM-105

Overview
Device views . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Jaundice meter JM-105 - Front . . . . . . . . . . . . 16
Jaundice meter JM-105 - Rear. . . . . . . . . . . . . 16
Docking station JM-A33 - Front . . . . . . . . . . . . 17
Docking station JM-A33 - Rear. . . . . . . . . . . . . 17
AC adapter JM-A32 . . . . . . . . . . . . . . . . . . . . . 18
External devices . . . . . . . . . . . . . . . . . . . . . . . 19
Valid device combinations . . . . . . . . . . . . . . . . 19
Interfaces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Device software . . . . . . . . . . . . . . . . . . . . . . . . 19
Data transmission software . . . . . . . . . . . . . . . 19
Abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . 20
Symbols. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Symbols on device . . . . . . . . . . . . . . . . . . . . . . 21
Symbols on touch screen . . . . . . . . . . . . . . . . . 22
Symbols on the PC. . . . . . . . . . . . . . . . . . . . . . 23
Overview
Instructions for Use JM-105 15
Overview
002
A
B
C
D
003
A
B
C
D
E
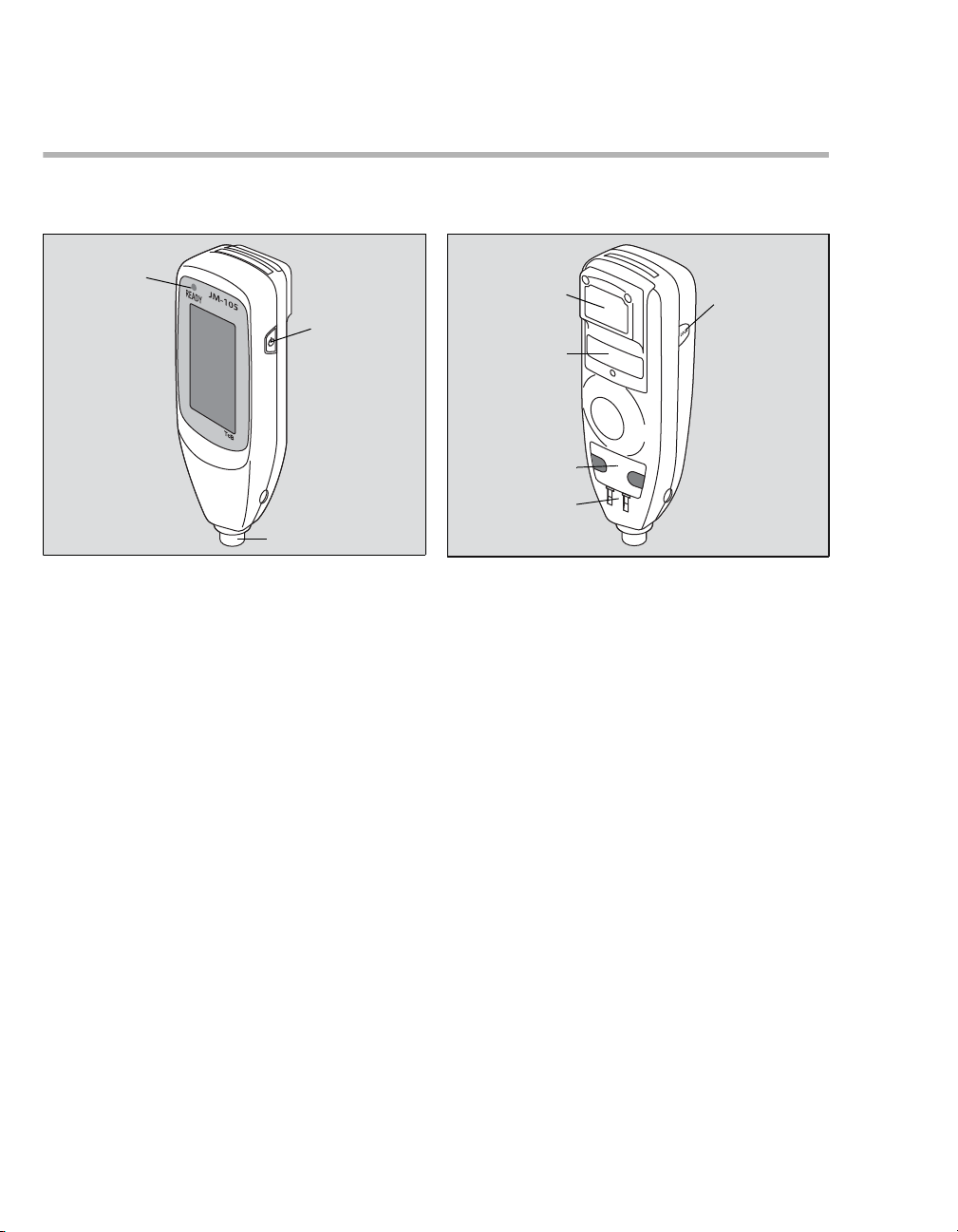
Device views
Jaundice meter JM-105 - Front Jaundice meter JM-105 - Rear
A
Power button
B
Measuring probe
C
Display/Touch panel
D
READY lamp
16
A
Screen LOCK button
B
Charging contact
C
Communication port
D
Battery cover
E
Barcode reader
Instructions for Use JM-105
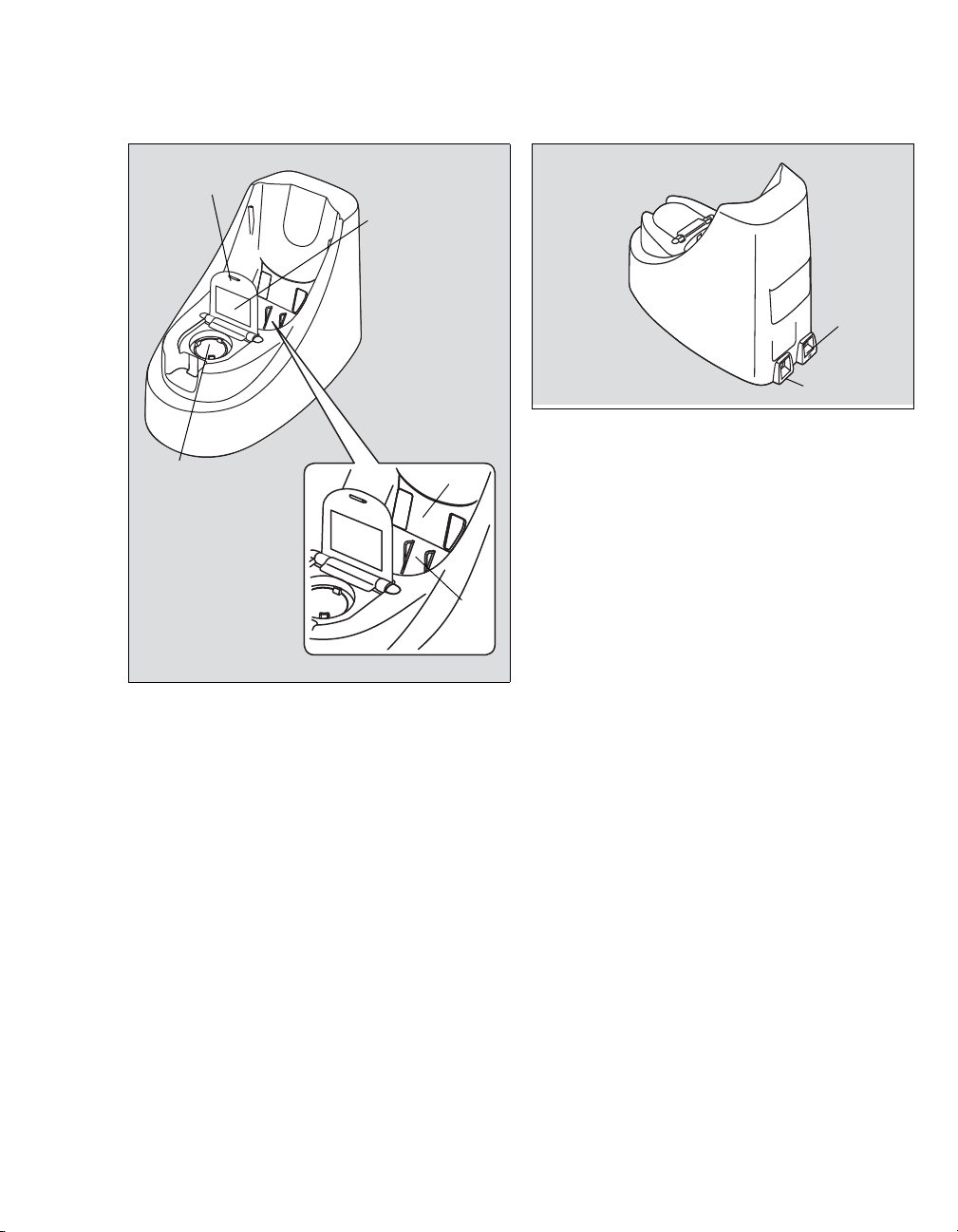
Docking station JM-A33 - Front Docking station JM-A33 - Rear
004
A
B
C
D
E
005
A
B
A
USB connector
B
DC jack
Overview
A
Checker cover
B
Standard checker values
C
Reading checker
D
Communication window
E
Charger jack
Instructions for Use JM-105 17
Overview
006
A
B
C
D
AC adapter JM-A32
A
AC power cable plug
B
AC power cable
C
AC adapter
D
DC plug
18
Instructions for Use JM-105

External devices
Overview
Valid device combinations
The JM-105 can only be combined with the JM-A33
docking station and the JM-A32 AC adapter to
measure bilirubin. It can also be connected to a
computer to transmit data from the device to an
electronic health records system.
Software
Device software
The JM-105 is a non-invasive transcutaneous bilirubinometer. It uses the digital data generated by
converting the amount of light reflected from human
tissue. The device displays the results on the LCD
display. The software is installed in the device
ROM. The device becomes operable when the batteries supply power and then the release signal is
released.
Interfaces
The USB port provides a connection for transmitting data to electronic health records systems. It
also provides an alternate method to charge the
device.
Data transmission software
The data transmission software, SW JM-S1w,
enables the JM-105 to transmit measurement data
to a PC and send it to an electronic health record
system (EHR). It also enables saving the data to a
CSV file.
Instructions for Use JM-105 19

Overview
Abbreviations
Abbreviation Meaning
AC Alternating current
AP Applied part
CD Compact Disc
CSA Canadian Standards Association
DC Direct current
DVD Digital Video Disc
EHR Electronic Health Record
EMC Electromagnetic compatibility
ESD Electrostatic discharge
GMDN Global Medical Device Nomencla-
ture
IEC International Electrotechnical Com-
mission
LCD Liquid crystal display
PC Personal computer
RH Relative humidity
ROM Read only memory
UMDNS Universal Medical Device Nomen-
clature System
USB Universal Serial Bus
20
Instructions for Use JM-105
Symbols
Overview
The following symbols appear on labels on the JM105 jaundice meter, on the screen, and in these
instructions for use. These standards apply as
noted in the table.
Symbols on device
Warning
Caution
Degree of protection against electric shock: Type BF
Refer to instructions for use
USB port
Standby or On/Off
Input
Circuit output terminal
AC power
DC Power
Do not discard with regular waste
Date of manufacture
Instructions for Use JM-105 21
Overview
…
DEL
BACK
EDIT
CLEAR
NEXT
BABY
N
B
L
S
Symbols on touch screen
Rechargeable battery
Priority flag - to show baby has high
bilirubin level and may need further
evaluation.
Lock
Phototherapy flag - to show baby
has been treated with phototherapy
Menu
Key
Go BACK to previous screen
EDIT the selected item
CLEAR the entry
Proceed to the NEXT BABY
Nurse ID
Baby ID
22
DEL
Scan
Sent to chart
Delete
Delete
L Value (long)
S Value (short)
Delta Value (difference between
long value and short value)
Instructions for Use JM-105
CANCEL the entry or stop the task
CANCEL
OK
Confirm the entry
Busy
Symbols on the PC
JM-S1w
Overview
JM-S1w error
Instructions for Use JM-105 23

This page intentionally left blank.
24 Instructions for Use JM-105

Operating concept
Screen layout for device . . . . . . . . . . . . . . . . 26
Main screen . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
MENU screen . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Measured data screen . . . . . . . . . . . . . . . . . . . 27
Averaging screen . . . . . . . . . . . . . . . . . . . . . . . 27
Screen layout for data transmission
software. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
JM-S1w menu. . . . . . . . . . . . . . . . . . . . . . . . . . 28
Common screen . . . . . . . . . . . . . . . . . . . . . . . . 28
Network screen. . . . . . . . . . . . . . . . . . . . . . . . . 29
HL-7 screen . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Edit custom message screen . . . . . . . . . . . . . . 30
Transmission log screen. . . . . . . . . . . . . . . . . . 31
Barcode reader screen . . . . . . . . . . . . . . . . . . . 32
Operating concept
Instructions for Use JM-105 25

Operating concept
007
OK
2012/08/0110:00
MENU
A
B
C
D
E
F
E
MEASURE
CHECKER
HISTORY
CLR ALL
008
A
C
D
E
E
B
MEASURE
CHECKER
HISTORY
CLR ALL
Screen layout for device
Main screen
The main screen contains these fields.
A Date
B Time
C Available selections
– MEASURE
– CHECKER
–HISTORY
–CLR ALL
D OK
E Arrows
F MENU
MENU screen
2012/08/0110:00
BACK OK
The MENU screen contains these buttons.
A Date
B Time
C Available selections
– MEASURE
– CHECKER
–HISTORY
–CLR ALL
–CONFIG
D OK
E Arrows
26
Instructions for Use JM-105

Operating concept
046
A
B
C
D
E
F
G
043
MENU
CLEAR
2012/08/0110:00
NEXT
BABY
N
NURSE001
B
BABY0001
004/100
AVE.3
12. 4
12. 3
!
12.3
mg/dL
B
A
C
D
K
J
I
H
G
F
E
Measured data screen
2012/08/0110:00
****
N
0001
B
BABY0002
01/12
11:23AM
01/12
11:23AM
01/12
11:23AM
!
MENU
The Measured data screen contains these fields.
A NURSE ID (nurse ID)
B BABY ID (baby ID)
C Date of measurement
D Time of measurement
E/
F Dots - Indicates no flags
are set. Push button to begin setting
Priority or Phototherapy flags.
E Priority flag - Push button to show baby
has high bilirubin level and may need
further evaluation.
F Phototherapy flag - Push button to set
flag to show baby has been treated with
phototherapy.
G Sent to electronic chart
…
002/004
DEL
2. 2
2. 4
2. 3
mg/dL
OK
Instructions for Use JM-105 27
Averaging screen
The Averaging screen contains these fields.
A Display lock
B Battery indicator
C Measurement result
D Touch to proceed to next baby
E Number of measurements for averag-
ing
F Data log capacity
G Number of measurements stored in
data log
H/
I Dots - Indicates no flags
are set. Push button to begin setting
Priority or Phototherapy flags.
H Priority flag - Push button to show baby
has high bilirubin level and may need
further evaluation.
I Phototherapy flag - Push button to set
flag to show baby has been treated with
phototherapy.
J BABY ID (baby ID) (9 trailing charac-
ters if ID is 10 or more characters long.)
K NURSE ID (nurse ID) (9 trailing charac-
ters if ID is 10 or more characters long.)

Operating concept
067
Preferences
Exit
A
B
069
A
B
C
D
E
F
G
H
I
Screen layout for data transmission software
JM-S1w menu
The JM-S1w menu screen contains these selections.
A Preferences - Opens the JM-S1w dia-
log where preferences are set.
B EXIT
Common screen
C Save data on PC - Enables/disables
saving data to CSV (comma-separated
value) text files on the computer.
D CSV Folder - Shows the selected folder
in which to save the CSV file.
E Open - Opens a window for selecting
the CSV folder.
F COM port (Docking Station) - Shows
a list of available COM ports.
G Allow changes. - Check if you want to
change preferences on any of the preferences screens.
H OK - Saves the selections and closes
the dialog.
I CANCEL - Cancels the transaction.
The Common screen contains these fields.
A Send HL7 Message - Enables/disables
communication with an electronic
health records system.
B Retry Interval - Sets the time to wait
before resending data after an error
occurs.
Available selections:
No retry
28
1 Min.
10 Min.
30 Min.
Instructions for Use JM-105

Operating concept
070
A
B
C
DE
071
A
B
C
D
E
F
G
H
I
J
K
L
M
NO
Network screen
The Network screen contains these fields.
A Server address - Enter the IPv4
address or alphanumeric host name of
the electronic health records server.
Restrictions:
Proxy cannot be used.
A PC on the LAN should be set as
the destination.
B Server port - Enter the communication
destination port on the server. The
default is the HL-7 default port (2575).
C Allow changes. - Check if you want to
change preferences on any of the preferences screens.
D OK - Saves the selections and closes
the dialog.
E CANCEL - Cancels the transaction.
Instructions for Use JM-105 29
HL-7 screen
The HL-7 screen contains these fields.
A HL7 version - Select Ver. 2.3.1 or Ver.
2.5.1.
B Message structure - JM-105 can take
up to 3 measurements per baby ID.
Select how JM-S1w handles such
grouped measurements.
C Send 1 message per measurement -
JM-S1w creates 1 message for each
measurement taken. If 3 measurements were taken for a baby ID, then 3
messages are created.
D Send 1 message per baby (up to 3
measurements) - JM-S1w creates 1
message for each baby ID. If 3 measurements were taken for a baby ID,
then 1 message is created containing
all 3 measurements. A maximum of 3
measurements per baby is allowed.
E Send 1 message with highest mea-
surement per baby - JM-S1w creates
1 message containing only the highest
measurement taken for each baby ID
(maximum of 3 measurements per baby
ID). If the highest value occurs for more
than 1 measurement, the message contains the first highest measurement
recorded.
F Swap ORC - OBR field - When
checked, switches the sending order of
the ORC and OBR fields.

Operating concept
072
A
B
C
D
G Swap AR - AE - When checked,
switches AR and AE in sent and
received messages.
H Add ’0B’ before message - When
checked, adds OB to the beginning of
sent messages.
I Use custom message - Enables the
use of a custom message. When
checked, the "Edit custom message"
button is enabled.
When this button is checked, the settings for "HL7 version" and " Swap
ORC - OBR field" is ignored.
J Edit custom message - When
enabled, clicking this button opens the
"Edit custom message" dialog.
K Transmission log - Opens the trans-
mission log dialog.
NOTE: This field only applicable for JMS1w version 1.40 or higher.
L Sending facility - User can edit this
field as needed.
NOTE: This field only applicable for JMS1w version 1.40 or higher.
M Allow changes. - Check the radio but-
ton if you want to change preferences
on any of the preferences screens.
N OK - Saves the selections and closes
the dialog.
O CANCEL - Cancels the transaction.
Edit custom message screen
The Edit custom message dialog appears when
the Edit custom message button on the HL7
screen is clicked. The Edit custom message
screen contains these fields.
A Pick list - lists fields available for cus-
tomizing.
B Insert - Inserts the selected field at the
end of the current message or wherever
the cursor is placed.
C OK - Saves the custom message for-
mat and closes the dialog.
D CANCEL - Cancels the transaction.
30
Instructions for Use JM-105

Transmission log screen
JM-S1w
Failed transmission data
Successfull transmission data
Allow changes. Delete All
Transmission log
Close
Transmission Date/Time Date/Time 1
Value 1
Value 2
2017/02/28/12:34:56
:::::: :::
:
:::
2017/02/28/12:34:56
2017/02/28/12:00:00
2017/02/28/12:00:00
Date/Time 2
2017/02/28/12:00:10
2017/03/01/12:00:15
JOHN DOE
NURSE ID
JOHN DOE
NURSE_01
BABY ID Instrument ID
NURSE_01
3501001
3051000
12.1
12.3
12.2
12.4
Value 3 Error
Date/Time 2
2017/02/28/12:00:20
2017/03/01/12:01:00
12.1
12.2
Transmission Date/Time
Value 1
Value 2
2017/02/28/12:34:56
:::: :::
:
::
2017/02/28/12:34:56
2017/02/28/12:00:00
2017/02/28/12:00:00
Date/Time 2
2017/02/28/12:00:10
2017/03/01/12:00:15
JOHN DOE
NURSE ID
JOHN DOE
NURSE_01
BABY ID Instrument ID
NURSE_01
3501001
3051000
12.1
12.3
12.2
12.4
Value 3
Date/Time 2
2017/02/28/12:00:20
2017/03/01/12:01:00
12.1
12.2
AE
AR
Retry Delete
Retry Delete
A
B
C
D
EF
G
H
I
J
K
L
M
N
O
B
C
D
E
F
G
HI
J
K
P
Q
R
Operating concept
076a
The Transmission log dialog appears when the
Transmission log button on the HL7 screen is
clicked. The Transmission log screen contains
these fields.
L Error - Shows the type of error. When
you click on the error, a pop-up message appears with details of the error.
M Retry - Attempts the transmission
again.
A Failed transmission data - Lists all
transmissions that failed or had errors.
B Transmission Date/Time - Shows the
date and time of the transmission.
C Nurse ID - (Editable)
D Baby ID - (Editable)
E Instrument ID (instrument ID) -
F Date/Time1 - Shows the date and time
of the first averaged measurement.
G Value1 - Shows the value of the first
averaged measurement.
H Date/Time2 - Shows the date and time
of the second averaged measurement.
I Value2 - Shows the value of the second
averaged measurement.
J Date/Time3 - Shows the date and time
of the third averaged measurement.
K Value3 - Shows the value of the third
Instructions for Use JM-105 31
averaged measurement.
NOTE: This field may be hidden if contents of other fields are long.
N Delete - Deletes the failed transmis-
sion.
NOTE: This field may be hidden if contents of other fields are long.
O Successful transmission data - Lists
all successful transmissions.
P Allow changes. - Check if you want to
edit fields or delete/retry transmissions.
Q Delete All - Deletes all transmissions.
R Close - Closes the dialog.

Operating concept
A
B
E
F
G
H
I
C
D
Barcode reader screen
The Barcode reader screen contains these fields.
NOTE
The Barcode reader screen is only applicable to
JM-S1w version 1.30 or higher and requires
device firmware 1.10 or higher.
A Code 39 check digit Disable - dis-
ables the check digit.
B Code 39 check digit Enable - enables
the check digit.
C Code 39 full ASCII conversion Dis-
able - disables the ASCII conversion.
D Code 39 full ASCII conversion
Enable - enables the ASCII conversion.
E Write - applies the selection of enable
or disable.
F Read current setting - shows current
barcode settings.
G Allow changes. - Check if you want to
change preferences on any of the preferences screens.
H OK - Closes the dialog.
I CANCEL - Closes the dialog.
074
32
Instructions for Use JM-105

Assembly and preparation
Charging the battery . . . . . . . . . . . . . . . . . . . 34
Alternate charging method using AC
adapter (optional) . . . . . . . . . . . . . . . . . . . . . . . 35
Unpacking the data transmission
software. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Assembly and preparation
Instructions for Use JM-105 33

Assembly and preparation
012
011
Charging the battery
Before using the instrument, charge and inspect
the instrument.
When using the instrument for the first time, ensure
that it is fully charged. To always maintain a full
charge, place the instrument on the charger unit
when it is not being used for measurements. When
the battery charge is low, the Battery display blinks.
NOTE
The power of the battery diminishes when the
device is left uncharged for a long time. Ensure
that the battery is charged before use.
1 Before charging the JM-105 using a USB con-
nector, ensure the USB port meets these minimum specifications:
– USB connection must be USB 2.0 or later
– USB connection must supply 5V, 500mA or
more of power to the JM-105
– USB connection must have passed electri-
cal safety certifications for CE and UL
2 Plug the USB cable into the USB connector of
the docking station.
3 Plug the USB cable into a USB port on a com-
puter.
4 Place the device into the docking station.
Ensure that the display faces forward.
R
E
ADY
NOTE
When the device is placed in the docking station,
the power switches on and the READY lamp turns
orange. When charging is completed, the READY
lamp switches off.
The device charges in 2 hours. Two hundred fifty
measurements can be performed with a fully
charged new battery.
NOTE
Do not connect the JM-105 to bus-powered or selfpowered USB hubs that are connected to a PC
USB port. They do not provide sufficient power to
charge the JM-105. The JM-105 may not charge
fully or may not charge at all.
34
Instructions for Use JM-105

Alternate charging method using AC
009
010
060
011
R
EADY
adapter (optional)
The AC adapter can be used to charge the device
instead of the USB cable.
1 Plug the power cable into the AC adapter.
WARNING
Risk of fire, electric shock, or equipment dam-
age.
Using a docking station or AC adapter other
than the one provided with the device could
damage the device.
Use only the docking station JM-A33 and the
AC adapter JM-A32 with the device.
2 Plug the AC adapter into the DC jack of the
docking station.
Assembly and preparation
WARNING
Risk due to incorrect mains voltage or missing
protective ground
If the device is connected to a power socket
with incorrect mains voltage or a power socket without a protective ground, an electric
shock may occur.
Connect the device only to power sockets with
correct mains voltage and a protective
ground.
3 Plug the power cable into an appropriate AC
source.
4 Place the device into the docking station.
Ensure that the display faces forward.
NOTE
When the device is placed in the docking station,
the power switches on and the READY lamp turns
orange. When charging is completed, the READY
lamp switches off.
Instructions for Use JM-105 35

Assembly and preparation
NOTE
When the docking station is plugged into both the
AC adapter and the USB port, the device takes
power from the AC adapter.
Unpacking the data transmission software
WARNING
Risk of fire, electric shock, or equipment dam-
age.
Connecting to a power source without a protective earth ground could damage the device.
Connect the device only to a power source
with a protective earth ground.
The JM-105 device is accompanied by data transmission software. Software SW JM-S1w enables
the PC to receive measurement data from a JM105 and send it to an electronic health record system (EHR). It also enables saving the data to a file.
Please note that this manual assumes that the user
is familiar with basic Windows operations.
Package contents
Installation CD-ROM of data transmission soft-
ware for jaundice meter, SW JM-S1w
USB Cable
36
Instructions for Use JM-105

Getting started
Switch on and pre-set the device for the
first time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Configuring Code 39 barcode format . . . . . . . . 39
Checking Out of range message format. . . . . . 39
Pre-use checkout . . . . . . . . . . . . . . . . . . . . . . 40
Data transmission software. . . . . . . . . . . . . . 42
Notes on Use . . . . . . . . . . . . . . . . . . . . . . . . . . 42
System requirements . . . . . . . . . . . . . . . . . . . . 43
Preparations . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
Installation of Microsoft Visual C++ 2015
Redistributable Package. . . . . . . . . . . . . . . . . . 44
Installation of Microsoft .NET Framework
4.6.2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Installation of JM-S1w . . . . . . . . . . . . . . . . . . . 46
Getting started
Instructions for Use JM-105 37

Getting started
013
R
E
A
DY
014
ENGLISH
日本語
中文
言語
OK
2012/08/0110:00
015
016
A
Switch on and pre-set the device for the first time
When switching on the device for the first time, you
can select display language and date format, and
set the date and time.
1 Press the POWER button and hold for 1 s. The
language screen appears.
2 Select display language by touching the desired
language or by using the UP/DOWN arrows.
Selected language is highlighted.
5 Select date format.
2012/08/0110:00
M/D/Y
D/M/Y
Y/M/D
言語
BACK
6 Touch OK to save selection.
7 Set date/time screen appears.
8 Set date and time by touching the item you want
to change.
9 Touch EDIT button (A).
OK
3 Touch OK to save selection.
4 Date format screen appears.
38
EDIT
10 To change value, touch UP/DOWN arrows.
Instructions for Use JM-105
OK

Getting started
11 Touch OK to save selection.
12 Touch OK again when you complete changes.
OPENING and the software version appear on
the display.
Configuring Code 39 barcode format
1 If using Code 39 barcode format, set prefer-
ences in the data transmission software (refer
to Setting preferences - Barcode on page 50.)
NOTE
Requires device firmware version 1.10 or higher.
Checking Out of range message format
1 To check the current format, Touch MENU but-
ton, if needed.
2 Touch the CONFIG button or press UP/DOWN
arrows.
3 Touch the UNITS button. The device shows the
current setting as either HI:>20 or HI:>340.
Instructions for Use JM-105 39

Getting started
018
BACK OK
2012/08/0110:00
MEASURE
CHECKER
HISTORY
CLR ALL
019
020
Pre-use checkout
WARNING
Risk of delayed therapy
Using the device without checking the accuracy of the measurements could cause incorrect measurements.
To check the measurement reliability of the
system, compare the transcutaneous bilirubin
value (TcB) determined by the device and the
total serum bilirubin (TSB) determined on the
basis of blood samples. Follow your hospital
guideline to determine the frequency of
checks (e.g. after repair or calibration of JM105 or TSB lab equipment, or following a
change to clinical processes.
NOTE
Do not touch the checker surface. If the checker
surface is dirty, wipe with a soft cloth dampened
with alcohol. To dry it, wipe with a soft cloth.
1 Switch on device.
2 Select CHECKER.
4 Open checker cover.
Y
AD
E
R
5 Place the measuring probe perpendicular to the
checker and push gently until a flash occurs.
NOTE
Do not take measurements with the device slanted
on the checker.
3 Touch OK to save selection.
40
6 Review check results.
– L value (measured value of long optical
path)
– S value (measured value of short optical
path)
– Delta value (difference between L
and S values)
NOTE
If values are repeatedly out of range, contact
DrägerService.
Instructions for Use JM-105

Getting started
017
021
– All values should fall within the ranges
shown on the checker cover.
– If any value is out of range, clean the
checker and probe. Repeat the measurement.
2012/08/0110:00
L 2.4
S 2.2
0.2
㸊
NOTE
Product labels shall be inspected for legibility
before use.
MENU
7 Close checker cover.
NOTE
If a device check has not been performed during
the current day, the MEASURE READING
CHECKER message appears for 3 s. when you
switch on the device.
To clear the message, check the device.
NOTE
If 12 months or more have passed since the last
device calibration, the TIME FOR PERIODIC
CALIB. message appears for 3 s. when you switch
on the device.
To clear the message, calibrate the device.
Instructions for Use JM-105 41

Getting started
066
Data transmission by
software JM-S1w
JM-105
Docking
station
CSV text file
Electronic health
records system
(HL7 protocol)
Data transmission software
After the user sets the JM-105 MEMORY to LINK
ON and the device is set into the docking station, it
sends data to the docking station. Through the USB
port, the docking station then sends data to the data
transmission software SW JM-S1w. The software
sends data to an electronic health records system
using the HL-7 Application Protocol for Electronic
Data Exchange in Healthcare Environments using
TCP/IP. Or it sends a CSV file to a folder on the PC
as identified during set-up.
Block diagram of the system
Notes on Use
No operating system is included with this soft-
ware.
Operating system must be installed on the PC
before this software can be installed.
When inserting the CD-ROM into the CD-ROM
drive, note the correct orientation of the disc
and insert it gently.
Keep the CD-ROM clean and free from
scratches. If the recorded surface becomes
dirty or the label surface is scratched, a read
error may result.
42
Avoid exposing the CD-ROM to rapid tempera-
ture changes and condensation.
Avoid leaving the CD-ROM in locations where it
is exposed to high temperatures from direct
sunlight or heaters.
Do not drop the CD-ROM or subject it to strong
impact.
Keep the CD-ROM away from water, alcohol,
paint thinners, and other such substances.
Remove the CD-ROM from the DVD-ROM drive
while the computer is turned on.
Use only Data Transmission SW 1.50 or higher.
Instructions for Use JM-105

Getting started
System requirements
Operating Systems Windows 7 Professional SP1 32-bit
Windows 7 Professional SP1 64-bit
Windows 8.1 Professional 32-bit
Windows 8.1 Professional 64-bit
Windows 10 Professional 64-bit (Creators update)
Screen resolution 1024 X 768 or higher
CPU Pentium III 1.6 GHz or higher
Memory 1 GB or more
Hard disk At least 3.5 GB of available disk space (Of this disk
space, at least 300 MB must be on the system
drive.)
Other CD-ROM drive (for installation)
USB port For connecting docking station
Languages (CE1) English, French, German, Italian, Spanish
Languages (CE2) English, Dutch, Portuguese, Russian, Swedish
Languages (CE3) English, Croatian, Polish, Serbian, Turkish
Languages (CE4) English, Czech, Hungarian, Norwegian, Slovakian
Languages (CE5) English, Danish, Finnish, Greek, Romanian
Instructions for Use JM-105 43

Getting started
A
B
Preparations
Software license compliance
The license agreement terms of the data transmission software for Jaundice Meter, SW JM-S1w are
provided in the Software License Agreement dialog
box displayed on-screen during installation. This
software can be installed only if you agree to all the
terms of the agreement.
Introduction
Three setup files are needed to set up the JM-S1w
(version 1.50) software. These three files must be
installed in the following order:
1 setup1.exe: Microsoft Visual C++ 2015 Redis-
tributable Package Installer
2 setup2.exe: Microsoft .NET Framework 4.6.2
Installer
3 setup3.exe: JM-S1w Installer
NOTE
Use only Data Transmission SW 1.50 or higher.
NOTE
If CSV file storage was used with the old software,
it might be necessary to manually adjust the location to an appropriate directory. The default location is: C:\users\public\documents\jm-s1w\CSV.
Installation of Microsoft Visual C++ 2015
Redistributable Package
NOTE
When the Microsoft Visual C++ 2015 Redistributable package has already been installed on your
PC, you should move to the next step (Installation
of Microsoft .NET Framework 4.6.2).
NOTE
In case the login user who executes JM-S1w
installer does not have administrative right of windows, the "User Account Control" dialog will
appear.
1 Run the setup1.exe.
NOTE
If a previous software version of JM-S1w is
installed, do not uninstall it. Installing JM-S1w (version 1.50) will automatically uninstall the previous
version of software and inherit the previous settings.
NOTE
For JM-S1w (version 1.30) or older, settings were
allocated to each Windows login user. JM-S1w
(version 1.50) has only one set of settings for all
users.
44
000
2 Check the I agree to the license terms and
conditions check box (A).
3 Click the Install button (B).
4 Click the Yes button (C).
Instructions for Use JM-105

Getting started
C
D
A
B
C
D
5 Click the Close button (D).
Installation of Microsoft .NET Framework
4.6.2
NOTE
When the later version of .NET Framework has
already been installed on your PC, you should
move to the next step (Installation of JM-S1w).
001002
3 Check the I have read and accept the license
terms. check box (B).
4 Click the Install button (C).
5 If the dialog below appears, click the Yes button
(D).
003004005
1 Run the setup2.exe.
2 Click the Yes button (A).
Instructions for Use JM-105 45

Getting started
E
F
A
B
D
C
6 Click the Finish button (E).
7 Click the Restart Now button (F) to restart the
PC.
1 Run the setup3.exe.
2 Click the Yes button (A).
008009010
3 Open the drop-down list (B).
006007
4 Select the language that you want to install (C).
5 Click the OK button (D).
Installation of JM-S1w
NOTE
Use only Data Transmission SW 1.50 or higher.
46
Instructions for Use JM-105

Getting started
E
F
G
H
I
J
K
6 Click the Next button (E).
7 Select the I accept the terms in the license
agreement button (F).
8 Click the Next button (G).
10 Click the Next button (I).
011012
11 Click the Install button (J).
013014015
12 Click the Install button (K) to install the USB
driver.
9 To install the software in another location, click
the Change... button (H).
Instructions for Use JM-105 47

Getting started
L
13 Click the Finish button (L).
Installing the USB driver for the docking station
Before using the software, it is necessary to connect the docking station to the computer.
When the docking station is connected to a computer for the first time, installation of the USB driver
is required.
1 Ensure MEMORY is set to LINK ON.
2 Plug the USB cable, TA-15, into the docking sta-
tion and the USB port of the computer.
3 The Found New Hardware Wizard starts on the
PC, prompting for installation of the driver for
the Jaundice Meter. Accept the software license
agreement.
4 Check that Install the software automatically
(Recommended) is selected, and click Next >.
5 If a warning message stating that the software
has not passed the Windows logo test appears,
click Continue or OK. Then continue the installation of the USB driver.
6 When the dialog with the message that driver
installation has been completed, click Close to
close the dialog.
Checking the COM port
To check the COM port that has been assigned to
the docking station, follow the procedure.
1 Open Control Panel.
2 Double-click System.
3 Select the Hardware tab, and click Device
Manager.
4 Click the + next to Ports (COM & LPT). The list
of connected devices appears.
5 Jaundice meter appears in the list, followed by
the assigned COM port in parentheses.
If Jaundice meter is not shown in the list under
016
Ports (COM & LPT), then the driver has not been
installed correctly. If Jaundice meter is shown
somewhere else on the list, select it and uninstall
the driver. Then unplug the docking station from the
computer and plug it back into the computer and
reinstall the driver.
Verifying software load
1 Restart PC.
2 Once JM-S1w has been installed on a PC, JM-
S1w starts when Windows starts. The JM-S1w
runs in the background. A symbol appears
in the task tray when JM-S1w is in progress.
NOTE
While JM-S1w in progress, the computer is prevented from automatically entering Sleep mode. If
the computer is set to Sleep mode manually, communication errors may occur when Sleep mode is
canceled.
3 If JM-S1w does not start automatically, it can be
started by clicking Start > All Programs >
Draeger
> JM-S1w.
48
Instructions for Use JM-105

Getting started
067
Preferences
EXIT
ABCD
F
G
H
E
Exiting JM-S1w software
1 Right-click on the task tray symbol for JM-
S1w.
2 Select Exit. JM-S1w shuts down.
When Windows is restarted, JM-S1w also restarts.
Notes on CD-ROM Storage
After using the CD-ROM, return it to its case
and store in a safe place.
Do not leave the CD-ROM in locations that are
exposed to high temperatures from direct sunlight or heaters.
Do not store the CD-ROM in areas of high
humidity.
Setting preferences
1 Access the JM-S1w menu by clicking or right-
clicking on the JM-S1w symbol on the task
tray.
This action opens the JM-S1w menu.
NOTE
All users can change preferences. However, preferences apply to all users. Users cannot set individual preferences.
1 Select Preferences.
Setting preferences - Common
1 A dialog box opens that shows tabs for each of
4 setting categories: Common (A), Network
(B), HL7 (C), and Barcode (D). The Common
tab is in front.
Instructions for Use JM-105 49
069
2 Check the box (E) to allow changes to settings.
3 Determine if you want to save to CSV text file or
to EHR.
4 If CSV text file is preferred, change Common
settings, by checking the Save data on PC box
(F).
5 Click on the OPEN button (G) to select CSV file
storage location.

Getting started
A
B
A
B
C
D
E
F
A
B
C
D
E
F
G
6 Click OK (H) to save settings.
Setting preferences - Network
1 If EHR is preferred, select the Network tab.
2 Change Network settings to input the server
address (A) and the server port (B).
Setting preferences - HL7
1 Select the HL7 tab.
Setting preferences - Barcode
NOTE
This function is only available using JM-S1w version 1.30 or higher and device firmware 1.10 or
higher.
1 If Code 39 barcode is preferred, select the Bar-
code tab.
070071
2 Check the appropriate radio button to disable
(A) or enable (B) the Code 39 check digit.
3 Check the appropriate radio button to disable
(C) or enable (D) full ASCII.
4 Press the Write button (F) to apply the setting.
5 Click OK (
G) to save.
074
2 To allow changes to settings, check the radio
button (A) to allow changes to settings.
3 Change HL7 settings: HL7 version (B) and
Message structure options (C) and (D).
4 Click the Edit custom message button (E), if
desired.
5 Edit messages as desired.
6 Edit the Sending facility field (F), if desired.
7 Click OK to save.
50
NOTE
To see the current settings, click on the Read current setting button (E).
Instructions for Use JM-105

Operation
Ensuring correct measurement . . . . . . . . . . 52
Measuring point . . . . . . . . . . . . . . . . . . . . . . . . 53
Choosing settings for measurement . . . . . . 53
Setting the number of average
measurements . . . . . . . . . . . . . . . . . . . . . . . . . 53
Selecting whether to store measurements . . . . 54
Measuring . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Removing from docking station . . . . . . . . . . . . 56
Measuring bilirubin (not storing
measurements in data log). . . . . . . . . . . . . . . . 57
Measuring bilirubin (storing measurements
in data log) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
Storing the device . . . . . . . . . . . . . . . . . . . . . 60
Switching off the device . . . . . . . . . . . . . . . . 61
Operation
Measuring quick guide. . . . . . . . . . . . . . . . . . 62
Instructions for Use JM-105 51

Operation
022
023
SINGLE
2TIMES
3TIMES
4TIMES
Ensuring correct measurement
WARNING
Risk of injury.
This device emits intense light.
Never allow device to emit light into the eyes.
Always take measurements from either the
sternum or the forehead.
WARNING
Risk of patient injury
Pathologic or other skin conditions that may
affect light scattering or absorption could
result in incorrect TcB measurements.
Do not conduct measurements in patients with
early jaundice and pathologic jaundice.
Do not conduct measurements when skin
conditions may violate the assumption made
concerning light scattering and light absorption.
Use device only on healthy skin of the patient.
Do not conduct measurements on birthmarks
and hairy areas. Avoid areas of thickness that
in the opinion of the physician would preclude
or interfere with the use of the TcB meter.
1 Before beginning measurements, ensure that
all phototherapy lights are shut off.
2 Place measuring probe perpendicular to mea-
suring point.
3 Push down gently. Do not lean the measuring
probe.
4 Configure the device to the desired number of
measurements (refer to Choosing settings for
measurement on page 53). Dräger recommends that the device is set to average 2 times
to 5 times. This setting minimizes measurement
errors due to leaning measuring probe.
2012/08/0110:00
NOTE
Clean measuring probe before use.
NOTE
Incorrect position of measuring probe can result in
erroneous measurements. Ensure that measuring
probe is perpendicular to measuring point.
NOTE
Ensure that patient is calm before taking measurements. Movement can interfere with correct probe
placement.
NOTE
There is a minor risk of administering phototherapy
when not clinically indicated.
52
OK
Instructions for Use JM-105

Operation
024
J
M
-
1
0
5
READ
Y
J
M
-
1
0
5
READ
Y
025
J
M
-
1
0
5
READY
J
M
-
1
0
5
READY
Measuring point
WARNING
Risk of patient injury
Pathologic or other skin conditions that may
affect light scattering or absorption could
result in incorrect TcB measurements.
Do not conduct measurements in patients with
early jaundice and pathologic jaundice.
Do not conduct measurements when skin
conditions may violate the assumption made
concerning light scattering and light absorption.
Use device only on healthy skin of the patient.
Do not conduct measurements on birthmarks
and hairy areas. Avoid areas of thickness that
in the opinion of the physician would preclude
or interfere with the use of the TcB meter.
Measurements must be taken only on the sternum
(at hospital sites or physicians offices) or forehead
(at hospital sites only) where enough blood is circulated.
Choosing settings for measurement
This device performs single measurements and
average measurements. Single measurements
take the results from each measurement as the
measured value. Average measurements take the
average results from 2 to 5 individual measurements as the measured value.
The device also allows the user to select whether to
store the measurements. Set-up of the device
depends on the measuring point and conditions.
Instructions for Use JM-105 53
Setting the number of average measurements
1 Touch MENU button, if needed.
2 Using UP/DOWN arrows, scroll through the list
until CONFIG appears.

Operation
026
BACK OK
2012/08/0110:00
CONFIG
CLR ALL
HISTORY
CHECKER
027
MENU
OK
2012/08/0110:00
UNITS
AVERAGE
MEMORY
NURSE ID
028
SINGLE
2TIMES
3TIMES
4TIMES
029
CONFIG
CLR ALL
HISTORY
CHECKER
3 Select CONFIG.
4 SETTING screen appears.
5 Select AVERAGE.
2TIMES to 5TIMES: Shows the average of
the results from 2 measurements to 5 measurements. AVE indicates that multiple
measurements were selected.
2012/08/0110:00
OK
8 Touch OK to save selection.
Selecting whether to store measurements
1 Touch the MENU button, if needed.
2 Select CONFIG.
6 Touch OK to save selection.
7 Select the number of measurements.
SINGLE: Shows the result form one mea-
54
surement.
3 Touch OK to save selection.
4 SETTING screen appears.
2012/08/0110:00
BACK OK
Instructions for Use JM-105

Operation
030
UNITS
AVERAGE
MEMORY
NURSE ID
031
OK
2012/08/0110:00
OFF
MEM ONLY
LINK ON
5 Select MEMORY. Previous selection is high-
lighted (OFF, MEM ONLY, or LINK ON).
2012/08/0110:00
MENU
OK
6 Select desired option
OFF: No measured data is stored in data
log.
MEM ONLY: Measured data is stored in
data log.
LINK ON: Measured data is stored in data
log and sent to PC.
Instructions for Use JM-105 55

Operation
032
MEASURE
CHECKER
HISTORY
CLR ALL
Measuring
WARNING
Risk due to incorrect reading
Screening measurements must be compared
to measurements from collected blood samples.
– Compare TcB value (Transcutaneous Bili-
rubin) measured by the device and TSB
value (Total Serum Bilirubin) measured
from collected blood samples.
NOTE
For quality control purposes, periodically compare
the JM-105 to serum bilirubin results. This checks
that the instrument maintains consistent performance over time and that the operators are using
the instrument properly.
Removing from docking station
1 Before beginning measurements, ensure that
all phototherapy lights are shut off.
2 Remove device from docking station.
3 Clean measuring probe.
4 Switch on device.
6 Select MEASURE.
2012/08/0110:00
MENU
OK
NOTE
After a few s. the READY lamp turns green. This
action indicates that the device is ready.
NOTE
If not operated for 1 min or longer, touch screen
goes blank. If needed, touch the display to activate
it.
5 Touch MENU button, if needed.
56
Instructions for Use JM-105

Operation
033
034
MENU
CLEAR
2012/08/0110:00
mg/dL
12.1
12.
3
12.2
AVE.3
A
Measuring bilirubin (not storing measurements in data log)
WARNING
Risk of patient injury
Pathologic or other skin conditions that may
affect light scattering or absorption could
result in incorrect TcB measurements.
Do not conduct measurements in patients with
early jaundice and pathologic jaundice.
Do not conduct measurements when skin
conditions may violate the assumption made
concerning light scattering and light absorption.
Use device only on healthy skin of the patient.
Do not conduct measurements on birthmarks
and hairy areas. Avoid areas of thickness that
in the opinion of the physician would preclude
or interfere with the use of the TcB meter.
1 Before beginning measurements, ensure that
all phototherapy lights are shut off.
2 Ensure that MEMORY is set to OFF (refer to
Selecting whether to store measurements on
page 54).
3 Ensure that the READY lamp is on.
4 Place measuring probe perpendicular to mea-
suring point (on forehead or sternum).
5
5
0
0
1
1
-
-
M
M
J
J
Y
Y
D
D
REA
REA
If AVERAGE is selected, display shows
remaining number of measurements
needed for averaging.
6 If averaging, wait for green READY lamp and
repeat measurement for the number of times
selected (2TIMES or 5TIMES). Display shows
the average calculated from the multiple measurements.
7 Read measurements.
Display shows up to 3 measured values.
If >20 or >340 blinks in a measured value
field, the measured value is outside the
measurement range (>20.0 mg/dL/>340
mol/L).
To take another measurement, touch
CLEAR button (A) and repeat step 2
through step 6.
NOTE
Touching CLEAR once deletes the last measured
5 Push gently until it flashes.
If AVERAGE is not selected, display shows
value. Touching and holding CLEAR deletes all
displayed measured values.
the measured data.
Instructions for Use JM-105 57

Operation
036
BABYID
2012/08/0110:27
MENUKEY
SCAN
OK
ABC
123
038
039
Measuring bilirubin (storing measurements in data log)
Ensure that MEMORY is set to MEM ONLY
or LINK ON (refer to Selecting whether to
store measurements on page 54).
Maximum 15 characters
Entering NURSE ID (nurse ID) and BABY ID (baby ID) using barcode reader
1 Touch SCAN button. Barcode reader emits
light.
2 Scan NURSE ID (nurse ID) (optional), and
touch OK.
3 Scan BABY ID (baby ID), and touch OK.
4 Ensure that the READY lamp is on. Go to Per-
forming measurements on page 59.
3 When in numeric mode, touch the number
desired.
NURSEID
1234567890
2 31
5 64
8 97
ABC
123
When in alphabet mode, touch the appropri-
ate button for the desired letter. To move to
the next letter, touch the right arrow button .
NURSEID
1234567890AB
CD
DEL
0
OK
MENU
ABC DEF
JKL MNOGHI
Entering NURSE ID (nurse ID) and BABY ID (baby ID) using touch screen
1 To select letter/number input mode, touch KEY
button.
NOTE
Touching the BARCODE button switches you back
to barcode input mode.
2 To switch between alphabet mode and numeric
mode, touch ABC/123 button.
58
TUV WXYZPQRS
ABC
123
OK
Example of alphabet mode:
To enter BR, touch ABC button twice.
Touch the right arrow button.
Touch the PQRS button 3 times.
4 Touch OK to save the ID entered. Device
returns to measurement screen.
5 Ensure that the READY lamp is on.
Instructions for Use JM-105
DEL
MENU

Operation
040
041
MENU
CLEAR
2012/08/0110:00
NEXT
BABY
N
NURSE001
B
BABY0001
004/100
AVE.3
12. 4
12. 3
!
12.3
mg/dL
A
MENU
CLEAR
2012/08/0110:00
mg/dL
>20
MENU
CLEAR
2012/08/0110:00
umol
>340
Performing measurements
WARNING
Risk of patient injury
Pathologic or other skin conditions that may
affect light scattering or absorption could
result in incorrect TcB measurements.
Do not conduct measurements in patients with
early jaundice and pathologic jaundice.
Do not conduct measurements when skin
conditions may violate the assumption made
concerning light scattering and light absorption.
Use device only on healthy skin of the patient.
Do not conduct measurements on birthmarks
and hairy areas. Avoid areas of thickness that
in the opinion of the physician would preclude
or interfere with the use of the TcB meter.
1 Before beginning measurements, ensure that
all phototherapy lights are shut off.
2 Place measuring probe perpendicular to mea-
suring point (on forehead or sternum).
5
5
0
0
1
1
-
-
M
M
J
J
Y
Y
D
D
REA
REA
4 If averaging, wait for green READY lamp and
repeat measurement for the number of times
selected (2TIMES or 5TIMES). Display shows
the average calculated from the multiple measurements.
5 Read measured data.
Display shows up to 3 measured values.
If >20 or >340 blinks in a measured value
field, the measured value is outside the
measurement range (>20.0 mg/dL/>340
mol/L).
3 Push gently until it flashes.
If AVERAGE is not selected, display shows
the measured data.
If AVERAGE is selected, display shows
Instructions for Use JM-105 59
remaining number of measurements
needed for averaging.
042a

Operation
N
NURSE001
B
BABY0001
004/100
AVE.3
12. 4
052
053
6 To attach flags, perform the following:
To attach a priority flag, touch
the dots button (the priority
flag appears which indicates
high bilirubin level in the patient).
To attach a phototherapy flag, touch the
button again (the
phototherapy flag appears
which indicates that patient has been
treated with phototherapy).
To attach both flags, touch the button again
(both flags appear ).
To remove flags, touch the button again
(both flag disappear and the dots
return .
WARNI NG
Risk of delayed phototherapy
Set flags must be considered when intepreting test measurements. Otherwise there is an
increased risk of incorrect therapy.
– Follow hospital procedures on the assess-
ment of phototherapy needed or of any further
evaluation needed.
7 To proceed to the next patient, touch NEXT
BABY button (A), enter NURSE ID (optional)
and BABY ID, and repeat steps 6 through 9.
NOTE
When the number of measurements stored in the
data log reaches 100, measuring is disabled.
Storing the device
1 Clean the measuring probe with a wipe soaked
in alcohol.
While the device is in the docking station,
power remains on. After 1 min, the screen
60
2 Place the device in the docking station.
R
EADY
3 Leave the device in the docking station when
not in use.
goes blank. The power remains on.
Instructions for Use JM-105

If the device is left out of the docking station
for 1 min, the screen goes blank. After
9 mins, the power switches off.
Switching off the device
To switch off power when the device is not in the
docking station, press the power button and hold it
down for 1 s.
Operation
Instructions for Use JM-105 61

Operation
Switch on power.
Perform initial settings (if switching
on device for the first time).
Change settings if needed.
Check device.
Take measurement.
If internal memory is
deactivated:
-Take measurement.
Check data.
Check data.
If internal memory is activated:
-Enter nurse ID.
-Enter baby ID.
-Take measurement.
Place device into docking
station.
Place device into docking
station.
Transfer data to electronic
medical chart system (MEMORY must be set to LINK ON).
Refer to Switch on and pre-set the
device for the first time on page 38.
Refer to "Choosing settings for measurement" on page 53.
Ensure phototherapy is shut off.
Refer to Pre-use checkout on page 40.
Refer to "Measuring" on page 56.
Refer to "Measured data
screen" on page 27.
Refer to "Transmitting
data to electronic charts"
on page 66.
Refer to "Transmitting
data to electronic charts"
on page 66.
Measuring quick guide
62
Instructions for Use JM-105

Trends and Data
Viewing measurements stored in the
data log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Deleting individual measurements in the
data log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Deleting all measurements in the data log. . 65
Transmitting data to electronic charts . . . . . 66
Data transmission errors . . . . . . . . . . . . . . . . . 67
Transmission log . . . . . . . . . . . . . . . . . . . . . . . 67
Viewing the transmission log from the
JM-S1w software . . . . . . . . . . . . . . . . . . . . . . 68
Trends and Data
Instructions for Use JM-105 63

Trends and Data
044
BACK OK
2012/08/0110:00
HISTORY
CLR ALL
CHECKER
MEASURE
045
1:
...
Y0001
2:
...
Y0002
3:
...
Y0003
4:
...
Y0004
MENU
OK
2012/08/0110:00
!
046
Viewing measurements stored in the data log
1 Touch MENU button, if needed.
2 Touch HISTORY.
3 Touch OK to save selection. Display shows list
of baby IDs (5 trailing characters if ID is 6 or
more characters long), measurement numbers,
and flags.
4 Select desired baby ID.
5 Touch OK to save selection. Display shows
detailed measured data.
2012/08/0110:00
****
N
0001
B
BABY0002
01/12
11:23AM
01/12
11:23AM
01/12
11:23AM
!
MENU
…
002/004
DEL
2. 2
2. 4
2. 3
mg/dL
OK
WARNI NG
Risk due to incorrect reading
Screening measurements must be compared
to measurements from collected blood samples.
– Compare TcB value (Transcutaneous Bili-
rubin) measured by the device and TSB
value (Total Serum Bilirubin) measured
from collected blood samples.
64
Instructions for Use JM-105

Deleting individual measurements in the data log
035
047
2012/08/0110:00
N
****
0001
B
BABY0002
01/12
11:23AM
01/12
11:23AM
01/12
11:23AM
2. 2
2. 4
!
2. 3
mg/dL
MENU
OK
DEL
002/004
…
A
048
CLR ALL
HISTORY
CHECKER
MEASURE
Trends and Data
1 Touch MENU button, if needed.
4 Select the desired measurement.
2 Touch HISTORY.
3 Select the desired baby ID.
2012/08/0110:00
...
1:
Y0001
2:
3:
4:
...
Y0002
...
Y0003
...
Y0004
OK
5 Touch DELETE (A).
!
MENU
6 Touch OK to save selection.
Deleting all measurements in the data log
1 Touch MENU button, if needed.
2 Touch CLR ALL.
2012/08/0110:00
3 Touch CLEAR on the confirm screen.
4 Touch OK to save selection. All measurements
in the data log are deleted.
BACK OK
Instructions for Use JM-105 65

Trends and Data
A
B
049
050
CONFIRM
SENDING
Transmitting data to electronic charts
If Send HL7 Message is enabled in the Common
tab of the JM-S1w dialog, measurements from JM105 are automatically sent to the electronic health
records system. If Save data on PC in the
Common tab is enabled, then measurements from
the JM-105 are sent to a CSV log file on the PC.
Refer to Setting preferences - Common on page 49
to confirm settings.
1 On the jaundice meter, navigate to the settings
menu: MENU>CONFIG.
2 Select MEMORY from the menu items.
3 Select LINK ON from the menu items.
4 Connect the docking station to the PC.
5 Open the SW JM-S1w on the PC.
NOTE
Ensure the Common screen shows (docking station) (A) above the list of COM ports and that there
is at least 1 COM port in the list.
6 Confirm COM port (B) on Common screen.
7 Place the device in the docking station that is
connected to the PC.
R
E
ADY
8 Display shows the CONFIRM screen.
2012/08/0110:00
NOTE
Ensure the COM port is selected before placing
the JM-105 into the docking station.
66
CANCELOK
069
Instructions for Use JM-105

Trends and Data
051
9 Touch OK to transmit data. Display shows
SENDING screen.
2012/08/0110:00
SENDING
…
001/004
CANCEL
Display shows the number of data transferred
and the number of data in memory to be transferred. It also shows the progress of the transfer
of all data in memory.
NOTE
Touching CANCEL during data transmission stops
data transmission. The data already transmitted is
considered transmitted.
Data transmission errors
If data transmission could not be completed, for
example, if the wrong COM port was selected or no
COM port was selected, data transfer times out on
the JM-105. JM-105 display shows SEND FAILED
and a balloon with the error message appears
above the JM-S1w tray symbol on the PC.
When an error message appears on the JM-105,
press OK to continue. When the balloon with the
error message appears on the PC, close it.
If data transmission to the electronic health records
system fails, JM-S1w saves the data in a file. It later
attempts to resend the data after the period specified by the Retry Interval setting. If No retry is set,
no attempt to resend is performed. Resend
attempts are performed at the specified interval
until success is achieved or until Send HL7 Mes-
sage is disabled.
Transmission log
If Send HL7 Message is enabled in the Common
tab of the JM-S1w dialog, the results of sending
measurements to the HL-7 system are saved in the
transmission log.
Refer to Viewing the transmission log from the JMS1w software on page 68.
Instructions for Use JM-105 67

Trends and Data
A
B
C
JM-S1w
Failed transmission data
Successfull transmission data
Allow changes. Delete All
Transmission log
Close
Transmission Date/Time Date/Time 1
Value 1
Value 2
2017/02/28/12:34:56
:::::: :::::::
2017/02/28/12:34:56
2017/02/28/12:00:00
2017/02/28/12:00:00
Date/Time 2
2017/02/28/12:00:10
2017/03/01/12:00:15
JOHN DOE
NURSE ID
JOHN DOE
NURSE_01
BABY ID Instrument ID
NURSE_01
3501001
3051000
12.1
12.3
12.2
12.4
Value 3 Error
Date/Time 2
2017/02/28/12:00:20
2017/03/01/12:01:00
12.1
12.2
Transmission Date/Time
Value 1
Value 2
2017/02/28/12:34:56
:::: ::::::
2017/02/28/12:34:56
2017/02/28/12:00:00
2017/02/28/12:00:00
Date/Time 2
2017/02/28/12:00:10
2017/03/01/12:00:15
JOHN DOE
NURSE ID
JOHN DOE
NURSE_01
BABY ID I nstrument ID
NURSE_01
3501001
3051000
12.1
12.3
12.2
12.4
Value 3
Date/Time 2
2017/02/28/12:00:20
2017/03/01/12:01:00
12.1
12.2
AE
AR
Retry Delete
Retry Delete
D
E
F
G
H
I
J
K
Viewing the transmission log from the JM-S1w software
1 Select the HL-7 tab (A).
2 Edit the Sending facility (B), if desired.
3 Select the Transmission log button (C).
4 The Transmission log dialog opens.
5 Use the scroll bars (D) to navigate through the
data (left and right/ up and down).
6 Check the Allow changes. radio button (E) to
allow editing of the transmission log.
7 To view error details, click on the AE/AR/CN/UN
link (F).
8 To resend a failed transmission, click on the
Retry button (G).
10 Sort data as desired by date or by name by
clicking the arrow (I) in the column header
(ascending or descending order). Data can be
sorted by fields: Transmission Date/Time,
Nurse ID, Baby ID, Date/Time1.
NOTE
The default sort order shows the newest transmission on top.
071076a
11 Edit data as desired in fields: Nurse ID, Baby
ID.
NOTE
The transmission log can hold 500 entries. When
the log reaches 500 entries, new entries push out
the oldest entries in the log.
12 To clear all data from the transmission log, click
on the Delete All button (J).
13 A confirmation message appears, Are you
sure you want to delete all items?.
14 Select yes or no.
15 To close the window, click on the Close button
(K).
080
081
NOTE
The Retry and the Delete buttons may be hidden
to the right if data in other fields is long. Use the
scroll bars to find these buttons.
9 To delete a failed transmission, click on the
Delete button (H).
68
Instructions for Use JM-105

Configuration
Changing settings on the JM-105 . . . . . . . . . 70
System and default settings for the JM-
105 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
Configuration
Instructions for Use JM-105 69

Configuration
054
BACK OK
2012/08/0110:00
CONFIG
CLR ALL
HISTORY
CHECKER
055
UNITS
AVERAGE
MEMORY
NURSE ID
Changing settings on the JM-105
1 Switch on device.
2 Touch MENU button, if needed.
3 To select CONFIG, touch CONFIG button or
press UP/DOWN arrows.
4 Touch OK to save selection.
5 Settings screen appears.
6 Touch item you want to change.
7 To change value, touch UP/DOWN buttons.
8 Touch OK to save selection.
2012/08/0110:00
MENU
OK
9 Touch OK again when you have finished chang-
ing settings.
System and default settings for the JM-105
Setting Description Options Description
UNITS Select unit for measuring and view-
ing data log.
AVERAGE Select number of measurements for
averaging.
70
mg/dL Default
mol/L
SINGLE Default
2TIMES
3TIMES
4TIMES
5TIMES
Instructions for Use JM-105

Configuration
Setting Description Options Description
MEMORY Select whether to store data in
device or transfer to PC.
OFF Default
No data stored in data log.
MEM ONLY Data stored in data log.
LINK ON Data stored in data log.
Data transmitted to PC (USB
cable and communication
software required).
NURSE ID Select whether to enter nurse ID. NONE No nurse ID entered.
BARCODE
1)
Default
1)
Enter nurse ID using barcode reader.
TOUCH Enter nurse ID using touch
screen.
BABY ID Select how to enter baby ID. BARCODE
1)
Default
1)
Enter baby ID using barcode
reader.
TOUCH Enter baby ID using touch
screen.
BUZZER Select whether to activate beeper. OFF No beep sound.
ON Default
Beep sound.
ALERT ON Beep sounds for alerts only.
SET TIME Set date and time. Must be set or selected
when device is switched on
for the first time.
2)
DATE FMT
Select date format. M/D/Y
D/M/Y
Y/M/D
TIME FMT Select format of time stamp for mea-
12-HOUR
sured data in data log.
(Time in upper right of touch screen
is always 24-hour format.)
24-HOUR Default
Instructions for Use JM-105 71

Configuration
Setting Description Options Description
LANGUAGE2)
Select display language. ENGLISH Default
for CE1
DEUTSCH
ESPAÑOL
FRANÇAIS
ITALIANO
2)
LANGUAGE
Select display language. ENGLISH Default
for CE2
NEDERL.
SVENSKA
РУС.
PORTUG.
2)
LANGUAGE
Select display language. ENGLISH Default
for CE3
POLSKI
TÜRKÇE
HRVATSKI
SRPSK
2)
LANGUAGE
Select display language. ENGLISH Default
for CE4
ČEŠTINA
MAGYAR
NORSK
SLOVENSK
2)
LANGUAGE
Select display language. ENGLISH Default
for CE5
SUOMI
DANSK
ROMÂNĂ
CONTRAST Select contrast of touch screen. 1 Darkest
2
3 Default
4
5 Lightest
72
Instructions for Use JM-105

Setting Description Options Description
TOUCHSCR Adjust touch screen. Touch the cen-
ter of X.
COM T.O. Select the time after which data
1-MIN Default
transmission is canceled.
5-MIN
S/W VER. Shows software version.
INITIAL Initialize device.
(Allows you to select display language, date format and to set date
and time.)
Data transmission software
Code 39
check digit
Select whether or not the barcode
1)
contains a check digit.
Disable
Enable Default
Code 39 full
ASCII conver-
1)
sion
Select whether or not to enable full
ASCII.
This page intentionally left blank.
Disable
Enable Default
Sending facil-
ity3) for HL-7
Displays the facility sending the
data during data transmission. This
Nursery Default
field is editable by the user.
1) Only applies to devices with firmware 1.10 or higher and barcode feature using JM-S1w version 1.30 or higher.
2) Must be set or selected when device is switched on for the first time.
3) Requires JM-S1w version 1.40 or higher.
Instructions for Use JM-105 73

This page intentionally left blank.
74 Instructions for Use JM-105

Problem solving
Fault – Cause – Remedy . . . . . . . . . . . . . . . . 76
Error messages . . . . . . . . . . . . . . . . . . . . . . . . 76
Battery indications . . . . . . . . . . . . . . . . . . . . . . 77
Other problems. . . . . . . . . . . . . . . . . . . . . . . . . 78
JM-S1w errors . . . . . . . . . . . . . . . . . . . . . . . . . 79
Problem solving
Instructions for Use JM-105 75

Problem solving
Fault – Cause – Remedy
The table shows possible causes for a fault and corresponding remedies. Causes and remedies must be
worked through in the order listed until the fault has been resolved.
Error messages
Fault Cause Remedy
ERROR01 Measured value is abnormal. For
averages, the difference between
measurements is excessively
large.
ERROR03 RAM error. Abnormalities or cor-
rupted data in RAM.
ERROR03
ERROR04
ERROR06
ERROR04 Memory error. Abnormalities or
ERROR05 Insufficient charge, circuit error. Charge the device.
ERROR06 Calibration data error. Calibration
ERROR07 Communication error between PC
JIG MODE Ver. 1.x Battery exhausted. Charge device for 32 hours.
Averaging failure Switch OFF power.
Hardware failure Switch OFF power.
corrupted data in EEPROM.
data in the EEPROM is corrupted.
and electronic clinical record system.
Repeat the measurement.
Switch off the device and remove it
from service.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
Switch off the device and remove it
from service.
If the failure continues, switch off
the device and remove it from service.
Switch off the device and remove it
from service.
Review setup of PC and repeat
data transmission.
If failure continues, replace battery
76
Instructions for Use JM-105

Battery indications
Fault Cause Remedy
Battery indicator is on. Battery power is low. Charge battery.
Backlight of touch screen
illuminates for 5 s and
then a shutdown occurs.
Battery power depletes
quickly.
READY lamp blinks red
during charging
Battery power is depleted. Charge battery.
Switch OFF power.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
Touch screen is used frequently. Establish measurement proce-
dures that reduce activation of the
backlight of the touch screen.
Backlight of touch screen consumes a lot of power.
Battery often recharged before
power was fully discharged.
Battery is overdischarged when
placed on the docking station.
Battery temperature too high. Allow battery to cool. Charging
No battery connected to the JM-
105.
Allow battery to discharge completely and then recharge. Repeat
this procedure a few times.
Switch OFF power.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
Wait for a few minutes. The battery
charge increases to 1.2 V or
higher. The red READY lamp illuminates continuously.
starts automatically when battery
has cooled.
If the failure continues, switch off
the device and remove it from service.
Connect battery.
Problem solving
Instructions for Use JM-105 77

Problem solving
Other problems
Fault Cause Remedy
Blank screen Power switched OFF. Switch ON power.
Battery power depleted. Charge battery.
Slow response of touch
screen
Charger lamp does not
illuminate even when
device is placed on the
docking station
Device has not been used for
1 min or more after power was
switched ON.
Touch on touch screen is too light. Increase pressure of touch on
Docking station not connected to
AC adapter or not connected correctly.
AC adapter not connected to AC
power or not correctly connected
to AC power.
USB cable not connected to the
docking station or not correctly
connected to the docking station.
USB cable not connected to the
PC or not correctly connected to
the PC.
Device is not seated correctly in
the docking station.
Touch any part of the touch screen.
Switch OFF power.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
touch screen.
Switch OFF power.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
Correctly connect docking station
to AC adapter.
Correctly connect AC adapter to
AC power.
Correctly connect USB cable to
docking station.
Correctly connect USB cable to
PC.
Reseat the device in the docking
station.
Switch OFF power.
Wait 10 s.
Switch ON power.
78
Instructions for Use JM-105

Fault Cause Remedy
If the failure continues, switch off
the device and remove it from service.
Not possible to take mea-
Battery power is depleted. Charge battery.
surements
Touch screen is locked. To unlock the touch screen, press
the display lock button.
Touch screen is frozen or has
failed.
Press the On/Off switch and the
display lock button simultaneously
and HOLD for 5 s. Power switches
OFF. Then, switch ON power.
Switch OFF power.
Wait 10 s.
Switch ON power.
If the failure continues, switch off
the device and remove it from service.
Problem solving
JM-S1w errors
When a data transfer error occurs, a balloon with an error message appears above the JM-S1w tray symbol. The symbol changes to include a red line through it. If a data transfer error occurs and the JM-S1w
error symbol appears, check the JM-S1w settings, exit from the software, and restart the software.
Error symbol:
Fault Cause Remedy
1)
Couldn't save Log data.
Check HDD space.
Server couldn't receive
data. Check Server setting or Message setting.
JM-S1w failed to save or delete
cache file for sending/re-sending.
Hard disk could be full or cache
folder set to "read-only."
Settings for message or server are
wrong and HL-7 server could not
receive the data. The data are not
stored on the server, and JM-S1w
does not retry sending the data
since the server does not accept
the messages.
Check hard disk, cache folder
CSV folder. Clear space on hard
disk or reset cache folder to
read/write.
Correct the server setting or message setting.
, or
Instructions for Use JM-105 79

Problem solving
Fault Cause Remedy
Server's response may
be wrong. Check Server
setting.
The format is wrong for the message received. Server may or may
not have accepted the message,
Correct the server settings.
but JM-S1w cannot know the
result and does not resend the
data
Cannot connect to server.
Check Network setting or
Server setting.
JM-S1w cannot connect to the
HL-7 server via the network:
-Wrong server address or port
Correct network setting or server
setting.
-Connection is physically down.
The data is resent.
Some data were not
received. JM-S1w will
retry with the same settings.
Server could not accept the message because server was processing. Server may accept the
message later, so JM-S1w retries
Wait a little while and try again.
automatically.
1)
Couldn't save Log data.
Check HDD space.
JM-S1w could not save log data.
HDD is full or cache folder is set to
Reset cache folder
space on HDD.
or clear
"Read-only".
The Log files could not be created,
but connections are OK and data
transfer to the HL-7 server was
successful.
When performing barcode settings, select only
one COM port (Docking
Station).
COMx Failure
(where x is the number of
the communication port)
This message appears on the JMS1w Barcode screen when more
than one port was selected on the
Common JM-S1w screen.
An attempt was made to read current barcode 39 data, but there is
no JM-105 connected or the connected JM-105 does not support
Select only one COM port on the
Common JM-S1w screen.
Connect a JM-105 that supports
the barcode 39 format, and
attempt the reading again.
the barcode 39 format.
1) Cache folder: On Windows Vista, Windows 7, Windows 8, Windows 10 C:\ProgramData\Draeger\JM-S1w\
80
Instructions for Use JM-105

Reprocessing
Safety information . . . . . . . . . . . . . . . . . . . . . 82
Information on reprocessing. . . . . . . . . . . . . 82
Classifications for reprocessing. . . . . . . . . . 83
Classification of medical devices . . . . . . . . . . . 83
Classification of device-specific components . . 83
Before reprocessing. . . . . . . . . . . . . . . . . . . . 84
Device-specific components. . . . . . . . . . . . . . . 84
Visual inspection. . . . . . . . . . . . . . . . . . . . . . . . 84
Validated reprocessing procedures . . . . . . . 85
Overview of the reprocessing procedures of
the components . . . . . . . . . . . . . . . . . . . . . . . . 85
Surface disinfection with cleaning . . . . . . . . . . 85
Storage and transport. . . . . . . . . . . . . . . . . . . . 86
Reprocessing
Other agents and reprocessing
procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Disinfectants. . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Reprocessing procedures. . . . . . . . . . . . . . . . . 88
After reprocessing . . . . . . . . . . . . . . . . . . . . . 88
Assembling and fitting device-specific
components . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
Preparation before next use of device . . . . . . . 88
Instructions for Use JM-105 81

Reprocessing
Safety information
WARNING
Risk due to inappropriately reprocessed prod-
ucts
Reusable products must be reprocessed, otherwise there is an increased risk of infection.
– Follow the infection prevention policies
and reprocessing regulations of the
health-care facility.
– Follow the national infection prevention
policies and reprocessing regulations.
– Use validated procedures for reprocess-
ing.
– Reprocess reusable products after every
use.
Follow the manufacturer's instructions for
cleaning agents, disinfectants, and reprocessing devices.
Information on reprocessing
Follow the national infection prevention policies
and reprocessing regulations.
Follow the infection prevention policies and reprocessing regulations of the health-care facility (e.g.,
concerning the reprocessing cycles).
CAUTION
Risk due to faulty products
Signs of wear, e.g., cracks, deformation, discoloration, or peeling, may occur with reprocessed
products.
Check the products for signs of wear and replace
them if necessary.
CAUTION
Risk due to faulty accessories
Even reusable accessories have a limited service
life. External signs of wear can occur, e.g., cracks,
deformations, discolorations, or peeling.
If there are external signs of wear, exchange
affected accessories.
82
Instructions for Use JM-105

Reprocessing
Classifications for reprocessing
Classification of medical devices
The classification depends on the intended use of
the medical device. The risk of infection transmission through the application of the product to the
patient without proper reprocessing is the basis of
the Spaulding classification.
Classification Explanation
Non-critical Components that come only into contact with skin that is intact
Semi-critical Components that carry breathing gas or come into contact with mucous mem-
branes or pathologically altered skin
Critical Components that penetrate skin or mucous membranes or come into contact with
blood
Classification of device-specific components
The following classification is a recommendation
from Dräger.
Non-critical
– Device components
– JM-105 main device
– Chargers
– JM-A33 docking station
Instructions for Use JM-105 83

Reprocessing
Before reprocessing
Observe before disassembly
WARNING
Risk of serious injury or equipment damage.
Disassembling or modifying the device or
accessories could cause electric shock or fire.
Do not disassemble or modify the device or
accessories.
WARNING
Risk of serious injury or equipment damage.
Cleaning device while it is connected to power
could cause electric shock or fire.
Disconnect all parts and accessories from AC
outlet.
1 Switch off the device.
2 Disconnect all power plugs.
Device-specific components
The device-specific components must be removed
from the device and, if necessary, disassembled.
Removing the [AC adapter]
1 Disconnect AC adapter from AC outlet.
2 Disconnect DC plug from device.
Disassembling the [docking station]
1 Remove the jaundice meter from the docking
station.
Visual inspection
Check all items for damage and external signs of
wear, such as cracking, embrittlement, or pronounced hardening, and residual dirt.
84
Instructions for Use JM-105

Validated reprocessing procedures
Overview of the reprocessing procedures of the components
Reprocessing
Components Surface disin-
fection with
cleaning
JM-105 main device
Yes No No No See page 85
Manual cleaning followed
by disinfection by immersion
Machine
cleaning with
thermal disinfection
Steam
sterilization
Description of the
procedure
Surface disinfection with cleaning
Components:
– Device components
– JM-105 main device
Surface disinfectant Manufacturer Concentration Contact time
Dismozon pur Bode 1.6 % 15 min
Prerequisites:
– The surface disinfectant has been prepared in
accordance with the manufacturer's instructions.
– The manufacturer's instructions, e.g., regarding
shelf life or application conditions, are observed.
– An uncontaminated, lint-free cloth soaked in
surface disinfectant is used for the cleaning surface disinfection.
WARNING
Risk due to penetrating liquid
Penetrating liquid may cause the following:
– Damage to the device
– Electric shock
– Device malfunctions
Ensure that no liquid penetrates the device.
WARNING
Risk of cross contamination
Reusable products must be reprocessed, otherwise there is an increased risk of infection.
– Disinfect the measuring probe before and
after every use on a patient.
Cleaning
1 Wipe off obvious soiling with a disposable cloth
soaked in surface disinfectant. Dispose of the
cloth.
2 Wipe all surfaces. After that, there must no lon-
ger be any soiling visible.
Surface disinfection
3 Wipe cleaned surfaces again to visibly wet all
surfaces to be disinfected with surface disinfec-
tant.
4 Wait for the surface disinfectant contact time.
Instructions for Use JM-105 85

Reprocessing
5 At the end of the contact time, moisten a new
uncontaminated and lint-free cloth with water
(at least drinking water quality).
6 Wipe all surfaces until no remains of the surface
disinfectant, such as foam residues or streaks,
are visible.
7 Wait until the surfaces are dry.
8 Check the surfaces for visible damage and, if
necessary, replace the product.
Supplementary information
9 Wipe clean the measuring probe with alcohol
before and after taking a measurement on the
next patient. Wipe dry with a dry cloth.
Storage and transport
After reprocessing, there are no special requirements for storage and transport of the product.
However, the following must be observed:
– Store dry and free of dust
– Avoid recontamination and damage during
transport
All further information on storage and transport
included in the accompanying documents must be
observed.
86
Instructions for Use JM-105

Other agents and reprocessing procedures
Disinfectants
WARNING
Risk of equipment damage.
Benzene, solvents, and thinners may dissolve the
case of the JM-105.
Do not use benzene, solvents, or thinners.
Use disinfectants that are nationally approved and
are suitable for the particular reprocessing procedure.
Surface disinfectants
The manufacturers of the surface disinfectants
have verified at least the following spectra of activity:
– Bactericidal
– Yeasticidal
– Virucidal or virucidal against enveloped viruses
Follow the manufacturer's instructions for surface
disinfectants.
The following surface disinfectants were compatible with the material at the time of testing:
Reprocessing
Class of active ingredient
Chlorine-releasing
agents
Oxygen-releasing
agents
Alcohol Alcohol, ethanol, 70% Various All
1) United States Environmental Protection Agency
Dräger states that oxygen-releasing agents and
chlorine-releasing agents may cause color change
in some materials. Color change does not indicate
that the product is not functioning correctly.
Instructions for Use JM-105 87
Surface disinfectant Manufacturer Listing
Klorsept 17 Medentech EPA
Actichlor plus Ecolab USA EPA
Dismozon pur BODE Chemie CE
Oxycide Ecolab USA EPA
Periodox RTU Bio Med Protect CE
Other surface disinfectants are used at one's own
risk.
1)

Reprocessing
Reprocessing procedures
Surface disinfection with cleaning
Components:
– Chargers
– JM-A33 docking station
Cleaning
1 For docking station, remove dust and dirt from
the surface and from the bottom.
2 Wipe off obvious soiling with a disposable cloth
soaked in surface disinfectant. Dispose of the
cloth.
3 Wipe all surfaces. After that, there must no lon-
ger be any soiling visible.
After reprocessing
Assembling and fitting device-specific components
Prerequisites:
– All components have been reprocessed and are
dry.
Assembling the JM-105
1 Prepare the device so that it is ready for use,
see chapter Assembly and preparation.
Preparation before next use of device
Checking the operational readiness
Prerequisites:
– The device has been assembled and prepared
so that it is ready for operation.
Procedure:
Check the operational readiness, see chapter
"Getting started“.
Clean measuring probe with alcohol before tak-
ing a measurement on the next patient. Wipe
dry with a dry cloth.
88
Instructions for Use JM-105

Service
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 90
Definition of service terminology . . . . . . . . . 90
Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Performing the inspection. . . . . . . . . . . . . . . . . 91
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . 92
Performing service . . . . . . . . . . . . . . . . . . . . . 92
Repair. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 92
Service
Instructions for Use JM-105 89

Service
Overview
This chapter describes the maintenance measures
required to maintain the proper functioning of the
medical device. Maintenance measures must be
performed by the personnel responsible.
WARNING
Risk due to inappropriately reprocessed prod-
ucts
The product may be contaminated with infectious agents.
Before service is performed and before the
product is sent back for repair, reprocess the
product in accordance with the chapter
"Reprocessing".
WARNING
Risk when the housing is being opened
Under the housing, there are live electrical
components, which may cause an electric
shock.
– The housing may only be opened by those
target groups that are assigned to that particular measure.
WARNI NG
Risk if service is not performed properly
Personal injury and property damage may occur if service is not performed properly.
Service must be performed by those target
groups that are assigned to the particular
measure.
WARNI NG
Risk if maintenance is not performed properly
If the device is connected to the power supply
or the gas supply during maintenance, there is
a risk of personal injury and property damage.
Before performing maintenance, disconnect
all electrical connections from the power supply and all gas connections from the gas supply.
Definition of service terminology
Concept Definition
Service All measures (inspection, maintenance, repair) intended to maintain or re-
store the functional integrity of a product
Inspection Measures intended to determine and assess the current state of a product
Maintenance Regular specified measures intended to maintain the functional integrity of a
product
Repair Measures intended to restore the functional integrity of a product after a fail-
ure
90
Instructions for Use JM-105

Inspection
Checks Interval Personnel responsible
Inspection Every 1 year Service personnel
Service
Performing the inspection
1 Check that the respective instructions for use
are present.
2 Perform a functional test of the following func-
tions according to the instructions for use:
– Light measurement
– Internal battery
3 Check that the product is in good condition:
– All labels are complete and legible
– There is no visible damage
4 Observe the instructions for use and check that
all components and accessories needed to use
the product are present.
5 Check the electrical safety in accordance with
the IEC 62353 standard.
Maintenance
WARNING
Risk if service is not performed regularly
Wear and material fatigue of the components
may lead to device failure and malfunctions.
Perform service at the specified intervals.
Component Interval Task Personnel responsible
Internal battery Every 2 years Exchange Service personnel
WARNING
Risk of electric shock
Before performing any maintenance work, disconnect all electrical connectors from power
supply.
Instructions for Use JM-105 91

Service
Calibration
Dräger recommends that calibration is performed
by DrägerService every 12 months. Return the
device to DrägerService for calibration.
The initial calibration is valid for one year from the
date of manufacture. Subsequent calibrations are
valid for one year from the date of the prior calibration.
Performing service
Removing battery
1 Remove battery cover (refer to Jaundice meter
JM-105 - Rear on page 16).
2 Replace battery.
3 Charge battery for 2 hours.
4 Perform operational checkout.
Repair
Dräger recommends that all repairs are performed
by DrägerService and that only authentic Dräger
repair parts are used.
WARNING
Risk of injury or equipment damage.
This device has a built-in battery.
Ensure only properly trained personnel open
device or attempt to replace battery.
WARNING
Risk if the battery is not replaced properly
If the battery is not replaced properly, short
circuits and high temperatures leading to explosion or fire may occur.
The battery must be replaced by the assigned
target groups.
92
Instructions for Use JM-105

Disposal
Disposal of the product . . . . . . . . . . . . . . . . . 94
Disposal
Instructions for Use JM-105 93

Disposal
Disposal of the product
At the end of its useful life, dispose of the prod-
uct in accordance with the applicable legal provisions.
For countries subject to the EU Directive 2002/96/EC
This device is subject to EU Directive 2002/96/EC
(WEEE). In order to comply with its registration
according to this directive, this device may not be
disposed of at municipal collection points for waste
electrical and electronic equipment. Dräger has
authorized a company to collect and dispose of this
device. To initiate collection or for further information, visit Dräger on the Internet at www.draeger.com. Use the Search function with the keyword
"WEEE" to find the relevant information. If access
to Dräger website is not possible, contact the local
Dräger organization.
Disposing of batteries
WARNING
Risk of explosion and of chemical burns
Improper handling of batteries can result in
explosions and chemical burns.
Do not throw batteries into fire. Do not force
batteries open.
Observe the applicable laws and regulations for
battery disposal.
94
Instructions for Use JM-105
 Loading...
Loading...