DMC Therapy EC, Therapy ILIB, Therapy XT User Manual

User Manual
Therapy EC
0843
ENG

Aiming at the practicality and ease of access to the information contained in the
User Manual of our products, DMC® provides the documents for download at the
following electronic address www.dmcgroup.com.br, in the “USE INSTRUCTIONS”
page.
Check the revision of the User Manual indicated on the User Guide to correctly
identify the desired file.
21-MAN-227 Rev. 02
Review Date: 09/10/2017

Therapy EC is a high-technology equipment which meets the most recent national manufacturing
standards required by ANVISA - Agência Nacional de Vigilância Sanitária [National Agency for Sanitary Vigilance].
Therapy EC was created to be used by medical and dental professionals. This professional must
be able to apply the techniques related to the product. Its inappropriate use may bring irreversible
injuries.
EQUIPMENT FUNCTIONS
Therapy EC releases red and infrared laser.
EQUIPMENT WORKING
By means of a small display and three keys, the operator views and performs all settings and features
of the equipment.
INDICATIONS
LASER THERAPY
Soft Tissue Recovery (Red Laser): Aphthae and traumatic ulcer, oral manifestation of systemic
diseases, lichen planus, lupus erythematosus, pemphigus vulgaris, gingival hyperplasia (diabetes),
cheilitis angular, gingivitis, postoperative, ATM dysfunction, acne, healing, combats free radicals
(Ilib Technique), lower limb ulcers, venous ulcers, arterial ulcers, diabetic ulcers, cutaneous ulcers
of different etiologies, venous stasis ulcer, skin scores, vascular microcurrent diabetes, post-surgery,
contact dermatitis, burns, pressure ulcer, diabetic ulcer and varicose ulcer.
Bone Tissue Recovery (Infrared Laser): Orthodontia, implantodonty, periodontitis, extraction,
traumatic injury, biostimulation of bones, Left Femur Fracture and Middle Third Closed Fracture,
Faster Consolidation of Fractured Bone, Cartilage and Bone Regeneration and Sjögren’s Syndrome.
Dental Tissue Recovery (Red and Infrared Laser): sensitivity following cavity preparation, sensitivity
following scaling, amelogenesis imperfecta, dentine hypersensibility, decubitus ulcers and acute inflammation.
Nerve Recovery (Infrared Laser): Neuralgias, paresthesias, paralysis and pain syndrome, hand
articulation, arthritis, rheumatoid arthritis, biomodulation, neck pain, DMED pain, epicondylitis,
fasciitis plantar, fibromyalgia, gonarthrosis, rotator cuff injury, muscle injuries, cartilage injuries, peripheral nerve injuries, low back pain, osteoarthritis, facial paralysis, paresthesias, post-surgery, nerve
repair, carpal tunnel syndrome, myofascial pain syndrome, calcaneal tendinitis, patellar tendinitis
and tendinopathies.
3
Therapy EC | User’s Manual
Other Applications (Infrared and Red Laser): Alveolitis, edema, xerostomia, pericoronitis, anesthesia, benign migratory glossitis, simple herpes and zoster herpes.
PHOTODYNAMIC THERAPY
Note: DMC provides scientific information about the subject as described in the flyer attached to
the product.
CONTRAINDICATIONS
Find below the following cases in which this equipment should not be used:
• Pregnancy uterus;
• Neoplasia on irradiated region;
• Clinical lesions without diagnosis.
• ILIB technique during pregnancy period.
CLASSIFICATION
Standard Classification
IEC 60601-1 Internally Powered Equipment and Applied Part Type B
MDD 93/42 (European Union) IIa
RDC 185/2001 (ANVISA) III
IEC 60825-1 3R
SPECIFICATIONS
Infrared Laser Features
Wavelength 808 nm ± 10 nm
Useful power of sender unit 100 mW ± 20%
Red Laser Features
Wavelength 660 nm ± 10 nm
Emitter net power 100 mW ± 20%
Red Laser (Laser Indicator) Features
Wavelength 660 nm ± 10 nm
Emitter net power 0,5 mW - 2,5 mW
4
GENERAL FEATURES
Features Specifications
Supply voltage 100-240 V~
Input current Alternating current
Output current Continuous current
Input power 25 VA
Working mode Continuous
Lasers working mode Continuous
Voltage frequency 50/60 Hz
Protection against shock Internally Powered
Protection degree regarding water and solid
object penetration
Uncertainty of time (seconds) ± 4%
Uncertainty of energy (Joules) ± 20.4%
Applied part Polymeric spacer
Handpiece size 3 cm (length) x 5 cm (width) x 21 cm (height)
Handpiece support size 6 cm (length) x 19 cm (width) x 11 cm (height)
Handpiece weight 0,18 Kg
Handpiece support weight 0,12 Kg
Optic fiber diameter 1000 µm each fiber
Battery Li-ion 3,7V 18650 with protection circuit
Manufactured and tested according to: IEC 60601-1, IEC 60601-1-2, IEC 62034 and IEC 60685-1.
Software version 11
Use temperature 10° - 30°C
IP20
SAFETY - IMPORTANT PRECAUTIONS
• Please read the whole manual before using the equipment.
• Attention - Using other controls, setting or running not specified in this manual may result in the
exposure to hazardous radiation.
• Attention - Laser smoke may present living tissue particles.
• As the equipment will be used by qualified professional, an additional specific training for its using
is not required. However, the reading of whole manual before its using is recommended.
• If the equipment is not used for some time, the battery shall be removed.
5

Therapy EC | User’s Manual
• The replacement of the battery by a personnel with inadequate training might result in a danger
(such as excessive temperature, fire or explosion).
• Laser lights may cause eye injuries. People who are on site should protect themselves against laser
lights emission. Therapy EC supplies three green glasses (one for operator, the other for assistant
and the other one for patient). Only glasses supplied by DMC should be used with the equipment.
• Never look directly to laser light. Never direct it for any person, except he/she is on treatment.
• Bright surfaces may reflect laser light towards the eyes.
• Never radiate tumor processes directly, the laser can stimulate them.
• Never radiate the infectious processes directly, the laser can stimulate them.
• Never radiate an undiagnosed injury.
• Never make extraoral application into patients who use photosensitizers medications; any high-intensity light may interact with drug and spot irradiation site.
• Only skilled professionals should handle the equipment. An inappropriate use may bring irreversible injuries.
• Only components mentioned in this manual should be used together with equipment.
• This equipment cannot be used with power cable not supplied by DMC because it may provoke an
increase of emission or decrease of immunity of equipment.
• To avoid risks of shocks, the equipment should be connected only in grounded outlets.
• Do not connect power cable in difficult-to-access outlets.
• Disconnect the power cable from outlet by plug.
• No change should be made in the equipment.
• Do not obstruct equipment air input/output.
• Do not apply any protector film on handpiece in order to obstruct air input/output.
• Do not install the equipment directly on sunlight, dusty places or mechanical vibration.
• There are risks of fire and/or explosion when laser exit is used with flammable materials, solutions
or gases or in an environment full of oxygen. Avoid using flammable or oxidizable anesthetic gases,
such as nitrous oxide (N20). Due to high temperatures, some materials, like cotton, when saturated
with oxygen, may be ignited. Adhesive solvents and flammable solutions used for cleaning and disinfection should be evaporated before using equipment. Pay attention to the ignition of endogenous
gases.
• The user should be exposed to the equipment noise during a maximum period of 8 hours/day.
• Due to the reduced size of the equipment, some safety items could not be inserted, such as: means
of protection against non-authorized use, control switch and emergency stop for the laser. Thus, the
user shall keep the equipment in a safe and protected place.
EQUIPMENT SAFETY ITEMS
Laser Beam
The equipment uses a low intensity laser beam (red laser) to indicate the infrared laser irradiation
point of the laser therapy handpiece.
As the target beam passes through the same delivery system as the working beam, it offers a good
6
way to check the integrity of the delivery system. If the target beam is not present in the distal end
of the delivery system, if its integrity is reduced or if it seems diffuse, this is a possible indication that
the delivery system is damaged or not working properly.
Alarms
For operator’s safety, during emission of laser light, the equipment sounds an alarm.
Spacer
A spacer is sent to be inserted into the tip of the optical fiber, so as to have a contact application,
i.e., the spacer is an applied part of the product. To replace the spacer, simply pull out the one that
has already been used, clean the equipment and the new spacer, according to item “CLEANING/
DISINFECTING”, and insert it into the end of the optical fiber.
Handpiece Led
When the led of the handpiece is blinking fast, the laser has been activated.
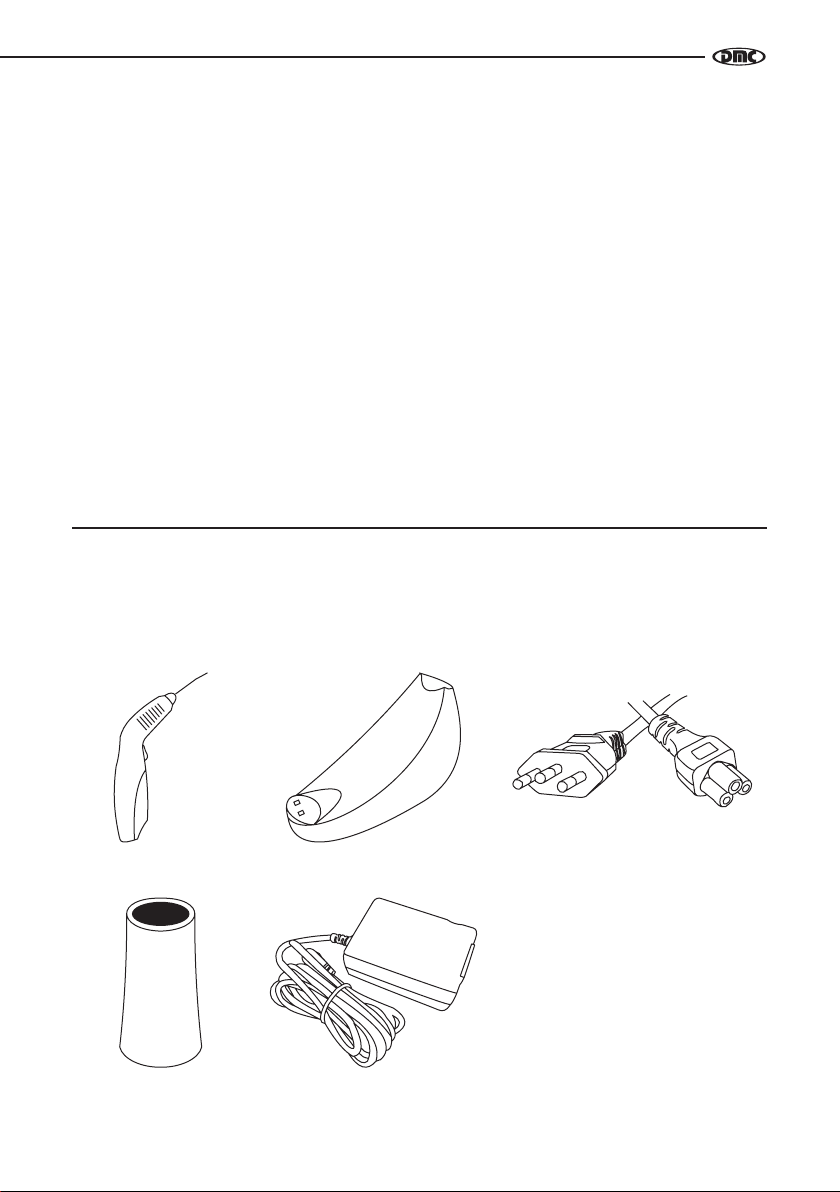
LIST OF COMPONENTS
Therapy EC is composed by:
PIECES:
Handpiece Handpiece Support Power Cable
Spacer Power Supply
7
Therapy EC | User’s Manual
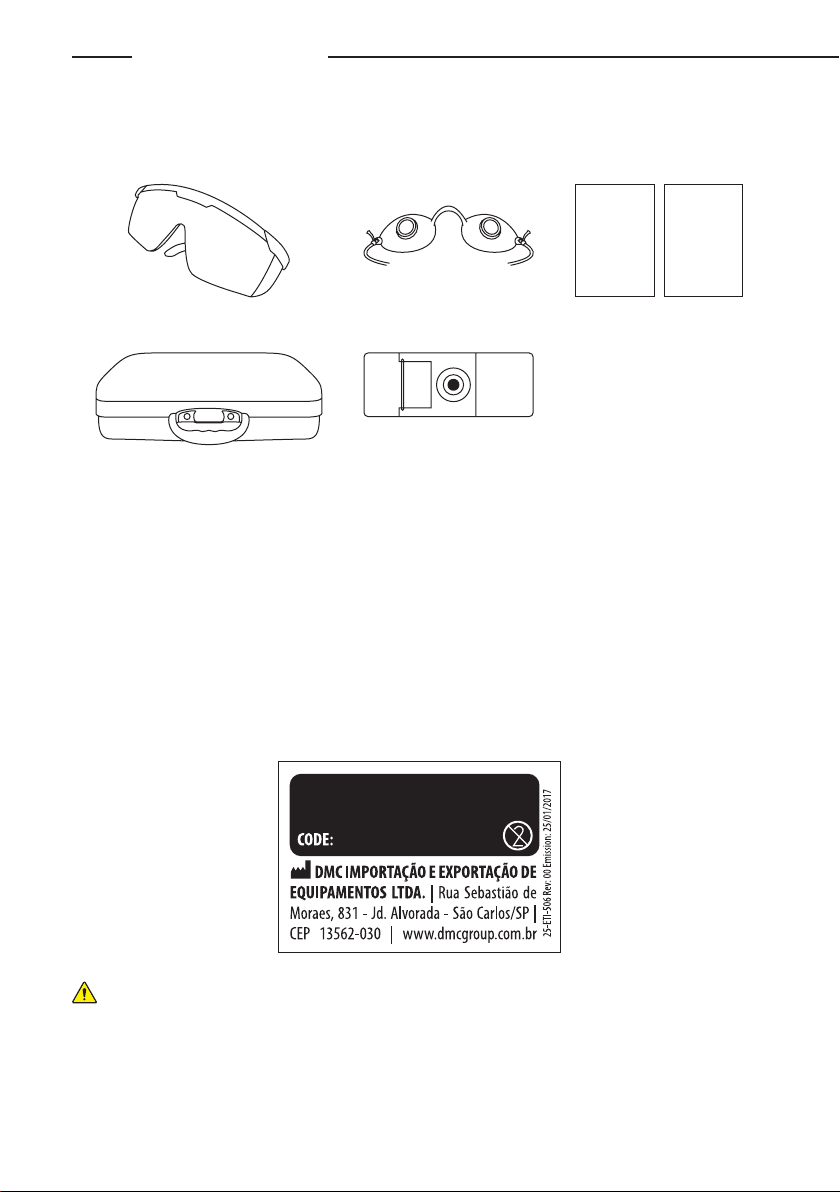
ACCESSORIES:
02 safety glasses 01 eye protector User’s Manual / Warranty term
Suitcase for transportation ILIB bracelet
MANUAL
DO
USUÁRIO
TERMO
DE
GARANTIA
In case you need to buy an accessory, it may be purchased by DMC Imp. e Exp. de Equipamentos
Ltda by the following codes:
• Power cable: 010130131;
• Power supply: 010990317;
• Safety glasses: 050020001;
• Battery: 110030182;
• ILIB bracelet: 121040002;
• Eye protector: 050020004;
• Spacer: 110010371 - The spacer has an individual packaging with the product code, with the
do not reuse symbol and with the manufacturer’s information, that is, DMC Importação e Exportação de Equipamentos Ltda, as follows:
All accessories and pieces described above are for exclusive use of Therapy EC.
8
 Loading...
Loading...