Direct Supply CX4, EX4 User Manual

USER MANUAL
Operation Instructions For:
Direct Supply EX4
(Electrotherapy)
Direct Supply CX4
(Combination — Electrotherapy & Ultrasound)
Direct Supply® CX4/EX4

Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 2 - - 3 -
THIS USER MANUAL IS VALID FOR THE
DIRECT SUPPLY CX4 & EX4 DEVICES
This user manual is published by Direct Supply Manufacturing Inc.
Direct Supply Manufacturing Inc. reserves the right to improve and amend it at any time
without prior notice. Amendments will however be published in a new edition of this manual.
All Rights Reserved. 42-DS-DQ8000-MAN_01 © 2017
Conformity to safety standards
Direct Supply Manufacturing Inc. declares that the
Direct Supply EX4 and CX4 comply with following normative documents:
IEC60601-1, IEC60601-1-2, IEC60601-2-10, IEC60601-2-5, ISO7010
IEC61689, ISO14971, ISO10993-1, ISO10993-5, ISO10993-10
Complies with MDD 93/42/EEC and Amended by directive 2007/47/EC requirements
TABLE OF CONTENTS
Forward ...............................................5
Safety Precautions And Warnings .....................5 – 10
Do Not Use With These Medical Devices ..................6
Do Not Use This Device Under These Conditions ...........6
Electrode Warnings...................................7
General Warnings ....................................8
Electrotherapy Warnings...............................8
Ultrasound Warnings...............................8 – 9
Cautions & General Precautions .....................9 – 10
Intended Use..........................................11
Electrotherapy Indications & Contraindications ............11
Ultrasound Indications & Contraindications ...............12
Adverse Effects........................................13
Applicator Movement Of Ultrasound ....................13
Potential Adverse Effects Of Ultrasound..................13
Patient Susceptibility.................................13
Coupling ..........................................13
Parameter Definitions ..............................13 – 22
Waveform Specifications............................14 – 22
IF-4P: IFC (Interferential) Traditional (4 Pole)...............14
IFC (Interferential) Premodulated (2 Pole) .................15
TENS: Biphasic — Asymmetrical & Symmetrical ...........16
RAAS: Biphasic.....................................17
Russian ...........................................18
High Volt ..........................................19
Microcurrent .......................................20
NMS .............................................21
Ultrasound Specications.........................22 – 23
Package Contents .....................................24
Operating Instructions..............................25 – 28
EX4 – Operating Controls .........................25 – 26
CX4 – Operating Controls .........................27 – 28
Product Description ................................29 – 33
Home Screen Display (EX4/CX4) .......................30
Main Therapy Display ................................30
Touchscreen Display.................................31
Electrotherapy Treatment Screen .......................31
Ultrasound Treatment Screen..........................32
Combination Therapy Treatment Screen .................32
Navigation ........................................33 – 45
Application Info (Electrotherapy)..................33 – 34
Before Treatment ................................33
Rubber Electrodes (Flexible) .......................33
Self-Adhesive Electrodes..........................33
Connection and Disconnection Reactions.............33
Electrotherapy Set-Up — Clinical Protocols...............34
Channel Selection ...............................35
Treatment Time Adjustment........................35
Start Therapy ...............................35 – 36
Storing Favorites ................................36
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 4 - - 5 -
INTENDED USER/OPERATOR
This manual has been written for the users of the Direct Supply,
CX4 and EX4 medical devices. It contains general information on the
operation, precautionary practices, and maintenance information.
In order to maximize its use, efciency, and the life of the system,
please read this manual thoroughly and become familiar with the
controls, as well as the accessories before operating the system.
These devices may not be appropriate for all individuals and are
designed to only be used by or under the supervision of persons
using the medical device in the course of their work and in the
framework of a professional healthcare activity, who understand the
benets and limitations of electrotherapy and ultrasound therapy.
WARNING (USA ONLY):
U.S.A. Federal Law restricts these devices to sale by, or on the
order of a physician or licensed practitioner. This device should
be used only under the continued supervision of a physician or
licensed practitioner.
These devices have been thoroughly tested and inspected to assure
proper performance and operation.
Specications put forth in this manual were in effect at the time of
publication. However, to ensure continual improvement measures,
changes to these specications may be made at any time without
obligation on the part of manufacturer.
IMPORTANT SAFETY PRECAUTIONS
AND WARNINGS
It is important that you read all the warnings and
precautions included in this manual because they
are intended to keep the patient safe, prevent injury
and avoid a situation that could result in damage to
the device.
SAFETY SYMBOLS USED IN THIS MANUAL
DANGER
Indicates a potentially hazardous situation which,
if not avoided, could result in death or serious injury.
WARNING
Indicates a potentially hazardous situation which,
if not avoided, could result in serious injury and
equipment damage.
CAUTION
Indicates a potentially hazardous situation which,
if not avoided, may result in minor or moderate injury
to the user or patient or damage to the device or
other property.
Navigation (Cont.)..................................33 – 44
Electrotherapy Set-Up — Manual Operation ..............37
Waveform Selection..............................37
Channel Selection ...............................37
Treatment Time Adjustment........................38
Start Therapy ...................................38
Storing Favorites ................................38
Ultrasound Therapy — Application . . . . . . . . . . . . . . . . . . . .39
Contact Control .................................39
Contact Medium ................................39
Before Treatment ................................39
During Treatment ................................39
After Treatment .................................39
Ultrasound Therapy Set-Up — Clinical Protocols...........40
Parameters.....................................41
Treatment Time Adjustment........................41
Start Therapy ...................................41
Storing Favorites ................................42
Ultrasound Therapy Set-Up — Manual Operation ..........42
Parameters.....................................43
Treatment Time Adjustment........................43
Start Therapy ...................................43
Combination Therapy Set-Up — Manual Operation.........44
Parameters — Electrotherapy ......................44
Treatment & Parameter Adjustment..................44
Parameters — Ultrasound .........................45
Treatment Time Adjustment........................45
Storing Favorites ................................45
Operating Details ......................................46
System Settings ....................................46
Setting Adjustment ..................................46
Adjusting Current Amplitude...........................46
CC/CV Mode.......................................46
Current Polarity .....................................46
Maintenance ......................................47 – 48
Cleaning Device ....................................47
Cleaning Display Panel ...............................47
Cleaning Electrodes .................................47
Cleaning Lead Wires and Cables .......................48
Cleaning Ultrasound Applicator ........................48
Troubleshooting ...................................49 – 51
Error Codes........................................49
Technical Maintenance ...............................50
End Of Life ........................................51
Safety And Performance Standards .......................51
EMC Table........................................52 – 54
Warranty .........................................55 – 56
Notes ..........................................57 – 59
Forward
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 6 - - 7 -
DANGER
THIS STIMULATOR MUST NOT BE USED IN COMBINATION
WITH THE FOLLOWING MEDICAL DEVICES:
1. Internally transplanted electronic medical devices,
such as a pacemaker.
2. Electronic life support equipment, such as respirators.
3. Electronic medical devices attached to the body,
such as electrocardiographs.
Using this stimulator with other electronic medical devices may cause
erroneous operation of those devices.
Do not use other RF equipment near the Direct Supply
CX and EX devices.
Do not use RFID systems near the Direct Supply CX and EX devices.
Do not use electrical stimulation in conjunction with high frequency
surgical equipment or microwave or shortwave therapy systems.
This device needs special precautions regarding EMC and
needs to be installed and put into service according to the
EMC information provided on pages 51 – 53.
These devices are contraindicated for use in an MRI
environment and should be removed prior to an MRI exam
or MRI exposure. Keep the device away from strong magnetic elds.
These devices are MR unsafe.
WARNING
DO NOT USE THIS DEVICE UNDER THESE CONDITIONS:
• If a patient has a cardiac pacemaker, implanted debrillator, or other
implanted metallic or electronic device. Such use could cause electric
shock, burns, electrical interference, or death.
• Together with a life-supporting medical electronic device such as an
articial heart or lung or respirator.
• In the presence of electronic monitoring equipment (e.g., cardiac
monitors, ECG alarms), which may not operate properly when the
electrical stimulation device is in use.
• On open wounds or rashes, or over swollen, red, infected, or inamed
areas or skin eruptions (e.g., phlebitis, thrombophlebitis, varicose veins);
or on top of, or in proximity to, cancerous lesions.
• Over areas of skin that lack normal sensation.
• Do not use while patient is in bath or shower.
• Do not use while patient is sleeping.
• Patients with arterial or venous thrombosis or thrombophlebitis are at risk
of developing embolisms when electrical stimulation is applied over or
adjacent to the vessels containing the thrombus. If a patient has a history
of deep vein thrombosis, even many years past, the affected area should
not be stimulated.
• Fresh fractures should not be stimulated in order to avoid unwanted
motion.
• Stimulation should not be applied immediately following trauma or
to tissues susceptible to hemorrhage.
WARNING (cont.)
• The electrodes are already pre-gelled and will adhere to clean skin.
• To avoid damage to the adhesive surface of the electrodes, put them
only on the skin or on the plastic lm provided.
• Make sure the components are connected well and the electrodes are
xed on the part of the body you wish to treat or the therapy may not be
effective.
DO NOT USE ELECTRODES THIS WAY:
• The electrodes are intended ONLY for use with the Direct Supply
CX4/EX4. Do NOT use the electrodes with any other device
• Self-adhesive electrodes are for single-patient use only.
Do not use electrodes on several different patients to avoid
transferring any contamination.
• Electrodes should not touch each other when placed onto patient’s skin.
Keep them at least 11∕2" apart during treatment. Electrodes too close
together or touching could result in improper stimulation or skin burns.
• Do not place on patient’s spine or backbone.
• Electrodes should not touch any metal object, such as a belt buckle
or necklace.
• Electrodes should not be placed simultaneously on the soles
of both feet.
• Electrodes should not be placed simultaneously on the calves
of both legs.
• Do not place or relocate the electrodes while the device is on.
• Always turn the power off before removing or changing the
electrode location.
• Do not leave electrodes attached to the skin after treatment.
• Do not lean against or lay on electrodes while administering
electrotherapy as this could cause an increase in stimulation.
WARNING (cont.)
DO NOT USE ON THESE INDIVIDUALS:
• Pregnant women, because the safety of electrical stimulation during
pregnancy has not been established.
• Children or infants, because the device has not been evaluated for
pediatric use.
• Persons incapable of expressing their thoughts or intentions.
NEVER APPLY THE ELECTRODES TO:
• The head or any area of the face. The effects of stimulation
of the brain are unknown.
• Any area of the throat because this could cause severe
muscle spasms resulting in closure of the airway, difculty
in breathing, or adverse effects on heart rhythm or
blood pressure.
• Both sides of the thorax simultaneously (lateral or front and
back), or across the patient’s chest because the introduction
of electrical current may cause rhythm disturbances which
could be lethal.
WARNINGS AND PRECAUTIONS REGARDING
THE ELECTRODES:
• Apply electrodes to normal, healthy, dry, clean skin (of adult patients)
because it may otherwise disrupt the healing process.
• If the patient experiences any skin irritation or redness after a session,
do not continue stimulation in that area of the skin. Do not bend or
fold the electrode because it may not function properly. Place the
self-adhesive electrodes onto the plastic lm and then store into the
sealed package when not in use.
• Do not apply ointment or any solvent to the electrodes or to the patient’s
skin because it will disrupt the electrodes from functioning properly.
Safety Precautions Safety Precautions
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 8 - - 9 -
WARNING (cont.)
ELECTROTHERAPY WARNINGS
• TENS therapy has not been established for pain of central origin.
• This device is to be used as a symptomatic treatment for pain and
has no curative value. Patients should be cautioned and their activities
regulated if pain that would otherwise serve as a protective mechanism
is suppressed.
• The long-term effects of chronic electrical stimulation are unknown.
• Safety has not been established for the use of therapeutic electrical
stimulation during pregnancy.
• Stimulation should not be applied over swollen, infected, or inamed
areas of skin eruptions (e.g., phlebitis, thrombophlebitis, varicose
veins, etc.).
ULTRASOUND WARNINGS
• Precaution should be taken when using therapeutic ultrasound on
patients with hemorrhagic diathesis.
• Ultrasound treatment presents a potential safety hazard in patients
whose pain response has been decreased because of disease, previous
surgery, ionizing radiation therapy, chemotherapy, general or regional
anesthesia. It may cause burns. Do not use on insensitive areas or in
the presence of poor circulation.
• Large thermal doses may result in regions of thermal aseptic necrosis
which may not be apparent on inspection of the skin.
• Patients who have cardiac pacemakers should be protected from
direct ultrasound exposure over the thorax to protect the lead wires
and pacemaker from such exposure.
• If a patient complains of periosteal pain (deep, achy pain) during
ultrasonic treatment, intensity should be reduced to a comfortable level.
WARNING (cont.)
GENERAL WARNINGS:
• Make certain the unit is electrically grounded by connecting only to
a grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
• To avoid the risk of electric shock, this equipment must only be
connected to a grounded outlet.
• The Direct Supply CX and EX devices are not suitable for use
in the presence of ammable anesthetics mixture with air, oxygen,
or nitrous oxide.
• These devices should be kept out of the reach of children.
• Care must be taken when operating this equipment around other
equipment. Potential electromagnetic or other interference could
occur to this or to the other equipment. Try to minimize this
interference by not using other equipment in conjunction with it.
• Before administering any treatment to a patient you should become
acquainted with the operating procedures for each mode of treatment
available, as well as the indications, contraindications, warnings and
precautions. Consult other resources for additional information
regarding the application of electrotherapy and ultrasound.
• To prevent electrical shock, disconnect the unit from the power source
before attempting any maintenance procedures.
• The use of accessories, transducers and cables than those specied,
with the exception of transducers and cables sold by the manufacturer
as replacement parts for internal components, may result in increased
emissions or decreased immunity of the device.
• Make certain there are no cracks or damage to any wires attached
to the device.
WARNING (cont.)
ULTRASOUND WARNINGS (CONT.)
• Moving technique of the applicator should be used when applying
therapeutic ultrasound at intensities greater than 0.5 W/cm² to assure
even exposure of tissues to ultrasound.
• Heating of the joint capsule in acute or subacute arthritis should be
avoided.
• Additional precautions should be used when ultrasound is used on
patients with the following conditions:
1. Laminectomy, i.e., when major covering tissues have been removed
2. Over anesthetic areas
3. On patients with hemorrhagic diathesis
• Ultrasound should be routinely checked before each use to determine
that all controls function normally. Especially if the intensity control
does properly adjust the ultrasonic power output in a stable manner.
Also, determine that the treatment time control does actually terminate
ultrasonic power output when the timer reaches zero.
• Use the ultrasound applicator with care. Inappropriate handling of the
ultrasound applicator may adversely affect its characteristics.
• Before each use, inspect the ultrasound applicator for cracks, which
may allow conductive uid to seep through.
• Ultrasound therapy is not designed to be water tight. Entrance of water
or liquid could cause malfunction of internal components and therefore
create risk of severe injury to the patient.
• Any bleeding tendency is increased by heating because of the increase
in blood ow and vascularity of the heated tissues. Care, therefore,
should be used in treating patients with therapeutic ultrasound who have
hemorrhagic diathesis or bleeding disorders.
• Do not use a conductive medium with an alcohol based content.
CAUTION
CAUTION WHILE USING THE STIMULATOR:
• If the stimulator is not functioning properly or the patient feels
discomfort, immediately stop using the device.
• Do not use for any other purpose except for what it is intended for.
• Do not pull on the electrodes or lead wires during treatment.
• Patients should remove all metal accessories (i.e. necklace, watch,
ring(s), etc.) prior to administering therapy as these items may cause
damage to the device.
• Do not use near a cell phone as this may cause the stimulator
to malfunction.
• Do not bend or pull the end of the cord.
• When pulling out the cord from the device, hold the plug and pull.
• Replace the lead wires when broken or damaged.
• Dispose of the device, batteries, and components according to
applicable legal regulations. Unlawful disposal may cause
environmental pollution.
• The size, shape and type of electrodes may affect the safety and
effectiveness of electrical stimulation. Please read instructions for
which electrodes should be used (specically for combination therapy
and High Volt).
• The electrical performance characteristics of electrodes may affect the
safety and effectiveness of electrical stimulation.
• Using electrodes that are too small or incorrectly applied, could result
in discomfort or skin burns.
• Keep yourself informed of the contraindications.
• DO NOT operate this unit in an environment where other devices are
being used that intentionally radiates electromagnetic energy in an
unshielded manner.
Safety Precautions Safety Precautions
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 10 - - 11 -
GENERAL PRECAUTIONS
• The long-term effects of electrical stimulation are unknown.
• Apply stimulation to only normal, intact, clean, dry, and healthy skin.
• Electrotherapy is not effective in treating the original source or cause of
the pain, including headache.
• Electrotherapy is not a substitute for pain medications and other
pain management therapies.
• Electrotherapy devices do not cure disease or injuries.
• Effectiveness is highly dependent upon patient selection by a practitioner
qualied in the management of pain.
• Patients may experience skin irritation or hypersensitivity due to the
electrical stimulation or electrical conductive medium (gel).
• If patients have suspected or diagnosed epilepsy, proceed with caution.
• Use caution if patient has a tendency to bleed internally, such as
following an injury or fracture.
• Using the device after a recent surgical procedure may disrupt the
healing process.
• Use caution if stimulation is applied over areas of skin that lack
normal sensation.
GENERAL PRECAUTIONS
• Keep unit out of the reach of young children. The unit contains
small pieces that may be swallowed. The electrode cord can cause
strangulation. Immediately DIAL 911 should any of these things occur.
• It is highly recommended that only clinical grade electrodes be used
with these devices. Economic electrodes used with other portable
electrotherapy devices could cause uneven dispersion and/or cause
shocking sensation or possible burns to the area being treated.
• If a patient is injured during treatment, discontinue use immediately
and contact your dealer about the injury.
CAUTION
CAUTION WHILE USING THE STIMULATOR (CONT.)
• Inspect applicator cables and associated connectors before each use.
• This device should not be used adjacent to or stacked with other
equipment and if adjacent or stacked use is necessary, the equipment
should be observed to verify normal operation in the conguration in
which it will be used.
ELECTROTHERAPY INDICATIONS & CONTRAINDICATIONS
Indications for TENS, EMS, NMS, NMS Burst, Russian (RUSS),
High Voltage Pulsed Current (HVPC), Interferential,
Pre-modulated Interferential and Microcurrent waveforms:
• Pain relief of chronic intractable pain
• Pain associated with post-traumatic or postoperative
conditions
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increase local blood circulation
• Muscle re-education
• Maintaining or increasing range of motion
• Immediate post-surgical stimulation of calf muscles to
prevent venous thrombosis (do not stimulate calf muscle
simultaneously)
CONTRAINDICATIONS
• This device should not be used for symptomatic pain relief
unless etiology is established or unless a pain syndrome has
been diagnosed.
• This device should not be used on patients with demand-type
cardiac pacemakers.
• This device should not be used over cancerous lesions.
• Electrode placements that apply current to the carotid sinus
region (anterior neck) must be avoided.
• Electrode placements that apply current transcerebrally
(through the head) must be avoided.
• Electrode placements that apply current transthoracically
(the introduction of electrical current into the heart may cause
cardiac arrhythmias) must be avoided.
• Stimulation should not be applied over swollen, infected,
inamed area or skin eruptions (e.g. phlebitis,
thrombophlebitis, varicose veins, etc.).
• Other contraindications are patients suspected of carrying
serious infectious disease and/or disease where it is
advisable, for general medical purposes, to suppress heat
or fevers.
• Safety has not been established for the use of therapeutic
electrical stimulation during pregnancy.
Safety Precautions Intended Use

Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 12 - - 13 -
ULTRASOUND INDICATIONS & CONTRAINDICATIONS
INDICATIONS FOR ULTRASOUND
• Relief of pain, muscle spasms and joint contractures
that may be associated with:
1. Adhesive capsulitis
2. Bursitis with slight calcication
3. Myositis
4. Soft tissue injuries
5. Shortened tendons due to past injuries and scar tissues
6. Ligament sprains
• Relief of sub-chronic, chronic pain and joint contractures
resulting from:
1. Capsular tightness
2. Capsular scarring
CONTRAINDICATIONS
The established contraindications to heat therapy itself, for example:
• In an area of the body where a malignancy is known
to be present
• Over or near bone growth centers until bone growth
is complete
• Over the thoracic area at all
• This device should not be used over a healing fracture
• In the presence of metal implants of any type
• Patients with sensory loss on the area to be treated
• Therapeutic ultrasound should not be applied over the
pregnant or potentially pregnant uterus. Therefore,
therapeutic ultrasound should not be applied over the
uterus unless specic assurance can be attained from
the patient that she is not pregnant.
• Areas of thrombophlebitis should not be treated with
therapeutic ultrasound due to the increased possibility of
clotting or dislodging a thrombus. Conditions where this
might occur are deep vein thrombosis, emboli and severe
atherosclerosis.
• Tissues previously treated by deep x–ray or other radiation
should not be exposed to therapeutic ultrasound.
• Ultrasonic treatment over the stellate ganglion, the spinal
cord after laminectomy, subcutaneous major nerves and
the cranium should be avoided.
• This device should not be used over the gonads/testicles
or to the developing fetus.
• This device should not be used over the heart.
• This device should not be used on the brains.
• This device should not be used on ischemic tissues in
individuals with vascular disease where the blood supply
would be unable to follow the increase in metabolic demand
and tissue necrosis might result.
• This device should not be used over or applied to the eyes.
• This device should not be use on the facial sinus as this
exposes the eyes to the same hazards.
• Ultrasound should not be used on unconscious patients
or over anesthetic areas.
• On the head or near the pharynx or larynx.
APPLICATOR MOVEMENT OF ULTRASOUND
If movement of the applicator is too slow, the patient may feel
periosteal pain characterized by a deep ache or pain. If motion is
too fast, or if the applicator does not maintain good contact with
the skin, the therapeutic effect of the sound waves will be reduced
and the applicator may overheat.
POTENTIAL ADVERSE EFFECTS OF ULTRASOUND
• Cataracts
• Male Sterility
• Enhanced Drug Activity
• Thermal Stress
PATIENT SUSCEPTIBILITY
Some patients are more sensitive to ultrasound output and may
experience a reaction similar to a heat rash. Be sure to inspect the
treatment area during and following treatment, and discontinue if
an adverse reaction does occur.
COUPLING
Coupling is described as contact between the applicator and
the treatment site and may be accomplished through the use of
a coupling agent, such as gel or lotion. Anything used as a coupling
agent must be highly conductive. Air is a very poor conductor of
ultrasonic waves. DO NOT use a conductive medium with an
alcohol based content or that is not approved specically for
ultrasound conductivity.
PARAMETER DEFINITIONS
C.C. Constant Current Output Mode
C.V. Constant Voltage Output Mode
F.M. Frequency Modulation
Freq. Frequency
C.F. Carrier Frequency
Duty Duty Cycle
Beat H. Sweep High Beat Frequency
Beat L. Sweep Low Beat Frequency
A.M. Amplitude Modulation
P. Dur. Phase Duration
Cycle Cycle Time
Ramp Ramp Time
Intended Use Adverse Effects
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 14 - - 15 -
ELECTROTHERAPY
IF-4P: IFC (Interferential) Traditional (4 Pole)
Interferential Current is a medium frequency waveform distributed
through two channels (four electrodes)*. The currents cross each
other in the body at the area requiring treatment. The two currents
interfere with each other at this crossing point, resulting in a
modulation of the intensity (the current intensity increases and
decreases at a regular frequency).
PARAMETERS:
Carrier Frequency Carrier frequency is the base frequency
of the alternating current.
Beat Frequency
(High & Low)
Occurs when two waveforms are in and out
of phases. The difference between the two
frequencies produces the modulated effect
(i.e. Beat H. of 4000 and Beat L. of 4150 will
yield a 150 pps beat frequency).
Vector-Auto Vector-Auto is a form of amplitude modulation
and is a percentage of the set interferential
amplitude (intensity) and will decrease from its
maximum level over 6 seconds.
Vector-Manual Vector-Manual is a form of amplitude
modulation. When Vector-Manual is set to a
different angle, the output intensities of two
channels are different. The rhythmical change
in position of the interference pattern, results in
the modulation of the amplitude of one or both
input currents.
Stimulator Output Parameters
Waveform Type Sinewave
Output Mode Electrodes
Mode Selection CC (Constant Current) or
CV (Constant Voltage)
Vector Scan Auto: 20% – 100%, Stepping 20%;
Manual: 0° – 90°, Stepping 15°
Carrier Frequency 2 – 10 KHz, Stepping 0.5 KHz
Beat High (Beat L.) – 200 Hz, Stepping 1Hz
Beat Low 1 – (Beat H.) Hz, Stepping 1Hz
Intensity CC: 0 – 100mA, Stepping 0.5mA;
CV: 0 – 100V, Stepping 0.5V
Treatment Time 1 – 60 Minutes
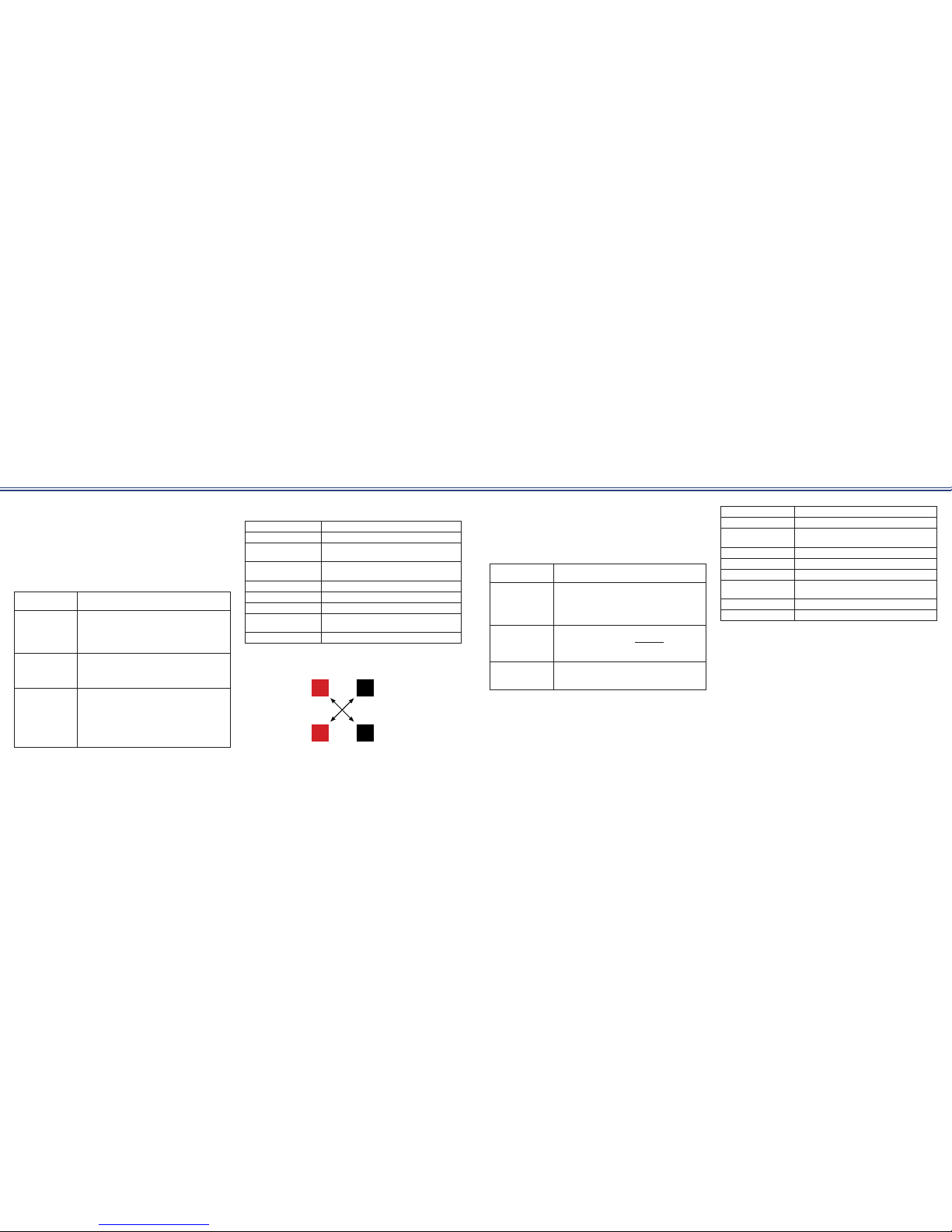
* IF-4P requires the use of at least four (4) electrodes at all times, as
well as criss-crossing the electrodes as indicated below.
IFC (Interferential) Premodulated (2 Pole)
Premodulated Current is a medium frequency waveform. Current
comes out of one channel (two electrodes). A bipolar technique in
which the two frequencies are “mixed” inside the machine prior to
tissue delivery.
PARAMETERS:
Carrier Frequency Carrier frequency is the base frequency
of the alternating current.
Beat Frequency Occurs when two waveforms are in and out
of phases. The difference between the two
frequencies produces the modulated effect
(i.e. Beat H. of 4000 and Beat L. of 4150 will
yield a 150 pps beat frequency).
Cycle Time
Cycle time refers to the time that the current is
On and Off (in seconds). Example: For a cycle
time of 10/50, the current will be owing for 10
seconds and resting for 50 seconds.
Ramp Time
Ramp time is used to set a gradual increase in
intensity during the “on-time”. Ramps occur at
the beginning and ending of a cycle.
Stimulator Output Parameters
Waveform Type Sinewave
Output Mode Electrodes
Mode Selection CC (Constant Current) or
CV (Constant Voltage)
Carrier Frequency 2 – 10 KHz, Stepping 0.5KHz
Beat High (Beat L.) – 200 Hz, Stepping 1Hz
Beat Low 1 – (Beat H.) Hz, Stepping 1Hz
Intensity CC: 0 – 100mA, Stepping 0.5mA;
CV: 0 – 100V, Stepping 0.5V
Treatment Time 1 – 60 Minutes
Ramp 2s
Waveform Specifications Waveform Specifications
1
3 2
4
Ch 1
Ch 2
Ch 2
Ch 1
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 16 - - 17 -
BIPHASIC (TENS)
The Asymmetrical Biphasic and the Symmetrical Biphasic waveforms
are often used in TENS (Transcutaneous Electrical Nerve Stimulation)
applications. TENS is low frequency waveform and has a short pulse
duration. The Alternating Rectangular waveform is an interrupted
biphasic current with a rectangular pulse shape. This waveform is
commonly used as a pain management application.
PARAMETERS:
Phase Duration Expressed in µs, is the time it takes to
complete one phase of a pulse. The length
affects the type of nerve recruited.
Frequency In a pulsed current, the Frequency refers to
the number of pulses that occur in a one
second period of time and is denoted in (Hz)
or Pulses Per Second (pps).
Frequency Modulation
Expressed in Hz, varies the frequency to
reduce accommodation. Example: When the
pulse frequency is set to 80 Hz and the
frequency modulation is set to 40 Hz, the
nal frequency will vary from 80 – 120 Hz.
Amplitude Modulation Amplitude Modulation is rhythmical
uctuation of the intensity to prevent
accommodation.
Stimulator Output Parameters
TENS Asymmetrical Biphasic
Output Mode Electrodes
Mode selection CC (Constant Current) or CV (Constant voltage)
Intensity CC: 0 – 200mA, Stepping 0.5mA;
CV: 0 – 200V, Stepping 0.5V
Phase Duration 20μs – 1,000μs, Stepping 5μs
Frequency 1 – 250 Hz, Stepping 1 Hz
Cycle Time Continuous, 10/10, 10/20, 10/30,
10/50, Custom
Ramp 1s, 2s, 5s
Treatment Time 1 – 60 Minutes
TENS Symmetrical Biphasic
Output Mode Electrodes
Mode selection CC (Constant Current) or CV (Constant voltage)
Intensity CC: 0 – 200mA, Stepping 0.5mA;
CV: 0 – 200V, Stepping 0.5V
Phase Duration 20μs – 1,000μs, Stepping 5μs
Frequency 1 – 250 Hz, Stepping 1 Hz
Cycle Time Continuous, 10/10, 10/20, 10/30,
10/50, Custom
Ramp 1s, 2s, 5s
Treatment Time 1 – 60 Minutes
Waveform Specifications Waveform Specifications
BIPHASIC (RAAS)
Rapid Agonist Antagonist Sequencing (RAAS) is proposed to mimic
muscle-ring patterns of healthy individuals. It uses the electrical
stimulation of sensory and motor nerves to achieve a skeletal muscle
contraction using an electromyogram — derived functional pattern.
It is used extensively for neuromuscular reeducation and treatment of
muscle disuse atrophy.
PARAMETERS:
Phase Duration Expressed in μs, is the time it takes to
complete one phase of a pulse. The length
affects the type of nerve recruited.
Frequency In a pulsed current, the Frequency refers to
the number of pulses that occur in a one
second period of time and is denoted in (Hz)
or Pulses Per Second (pps).
Burst Duration The time elapsed from the beginning to the
end of one burst, and is denoted in (ms)
Pattern Frequency The Pattern Frequency refers to the num-
ber of the sequential pulse train pattern be
repeated in a one second period of time and
is denoted in (Hz).
Stimulator Output Parameters
RAAS Asymmetrical/Symmetrical Biphasic
Output Mode Electrodes
Mode selection CC (Constant Current) or CV (Constant voltage)
Intensity CC: 0 – 200mA, Stepping 0.5mA;
CV: 0 – 200V, Stepping 0.5V
Phase Duration 20μs – 1,000μs, Stepping 5μs
Frequency 1 – 250 Hz, Stepping 1 Hz
Burst Duration 100ms – 5,000ms, Stepping Adaptive
Pattern Frequency 0.7 Hz (Burst Duration 100ms-500ms)
0.3 Hz (Burst Duration 500ms-1,000ms)
0.15 Hz (Burst Duration 1,000ms-2,000ms)
0.07 Hz (Burst Duration 2,000ms-5,000ms)
Treatment Time 1 – 60 Minutes
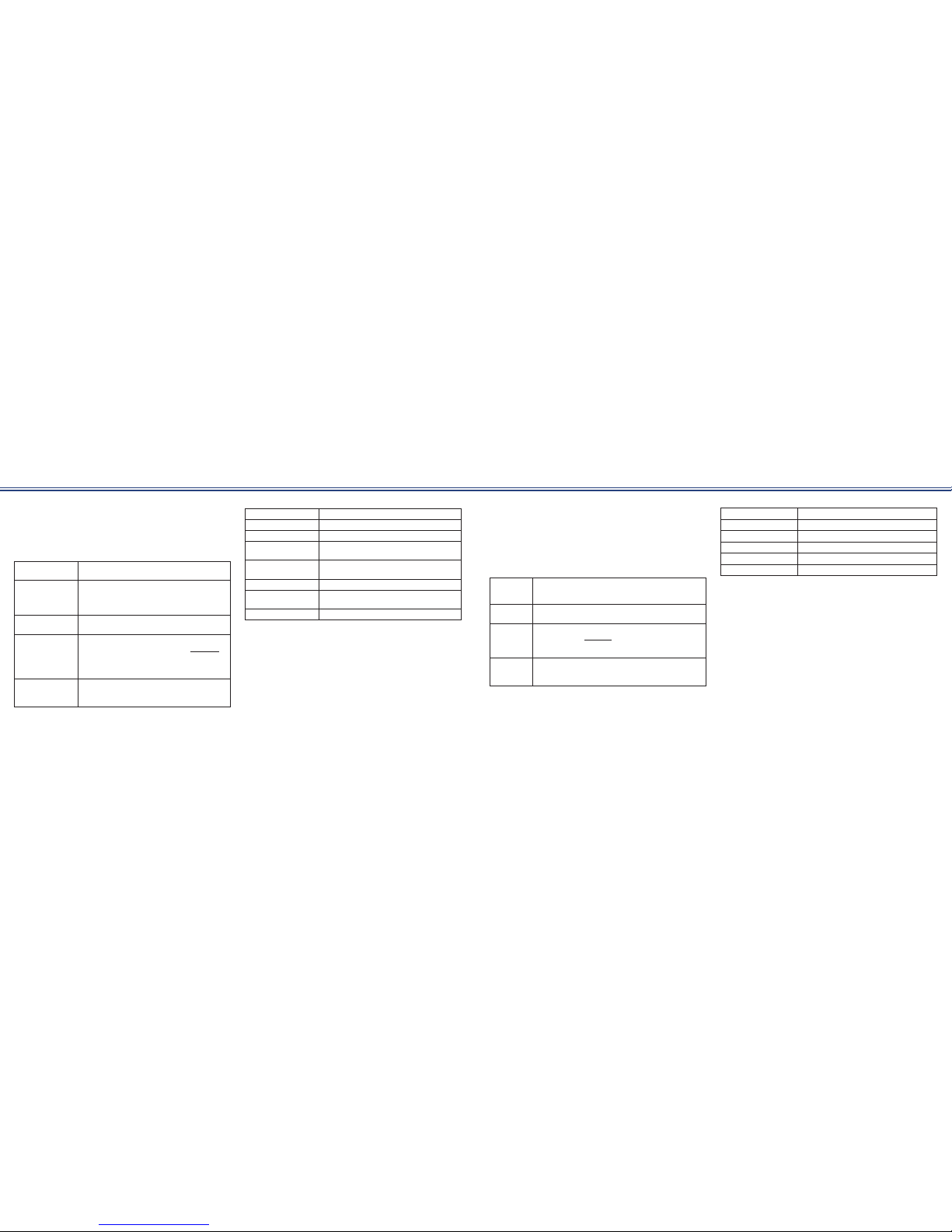
Burst Duration
Phase Duration
Ch
1
Ch
2
Direct Supply — CX4/EX4 Direct Supply — CX4/EX4
- 18 - - 19 -
RUSSIAN STIMULATION
Russian Current is a medium frequency rectangle waveform,
delivered in bursts or series of pulses. This method was claimed
by its author (Kots) to produce maximal muscle strengthening
effects without signicant discomfort to the patient.
PARAMETERS:
Carrier Frequency Carrier frequency is the base frequency of the
alternating current.
Frequency In a pulsed current the Frequency refers to the
number of pulses that occur in a one second
period of time and is denoted in (Hz) or Pulses
Per Second (pps).
Duty
Duty is the percentage of the total treatment
time that the current is actually owing.
Cycle Time
Cycle time refers to the time that the
current is On and Off (in seconds). Example:
for a Cycle Time of 10/50, the current will be
owing for 10 seconds and resting for
50 seconds.
Ramp
Ramp is used to set a gradual increase in
intensity during the “on-time”. Ramps occur at
the beginning and ending of a cycle.
Stimulator Output Parameters
Carrier Frequency 2.5 KHz
Frequency 20 – 100 Hz, Stepping 5 Hz
Duty cycle 10% – 50%, Stepping 10%
Mode selection CC (Constant Current) or
CV (Constant voltage)
Intensity CC: 0 – 100mA, Stepping 0.5mA;
CV: 0 – 100V, Stepping 0.5V
Treatment Time 1 – 60 Minutes
Cycle time Continuous, 10/10, 10/20, 10/30,
10/50, Custom
Ramp 1s, 2s, 5s
Waveform Specifications
HIGH VOLT
The High Volt waveform has a very brief pulse duration characterized
by 2 distinct peaks delivered at high voltage. High voltage causes a
decreased skin resistance making the current comfortable and easy
to tolerate. A monophasic twin-peaked waveform, with a short phase
duration and a long interpulse interval, eliminates the formation of
any appreciable chemical or thermal effects in the tissue.
PARAMETERS:
Frequency In a pulsed current, the Frequency refers to the number
of pulses that occur in a one second period of time and
is denoted in (Hz) or Pulses Per Second (pps).
Polarity This refers to the polarity (+/-) of the red lead wire;
connect the lead wire to the active electrode.
Cycle Time
Cycle Time refers to the time that the current is on and
off (in seconds). Example: for a Cycle Time of 10/50,
the current will be owing for 10 seconds and resting
for 50 seconds.
Ramp
Ramp is used to set a gradual increase in intensity
during the “on-time”. Ramps occur at the beginning
and ending of a timed on cycle.
NOTE: When administering High Volt Therapy (whether in
combination or only electrotherapy stim) you should use the
large dispersive electrode measuring 3" x 5" at the least. This
will reduce adverse reactions to the skin as well as evenly
distribute the stimulation.
Stimulator Output Parameters
Frequency 1 – 120 Hz, Stepping 1 Hz
Polarity Positive, or Negative,
Phase Duration. 100μs
Intensity CV: 0 – 500V, Stepping 5V
Treatment Time 1 – 60 Minutes
Ramp 1s, 2s, 5s
Waveform Specifications
 Loading...
Loading...