Page 1
MSQ
Document No. 031871
Hardware
Revision 02
October 2003
™
Page 2

©2003 by Dionex Corporation
All rights reserved worldwide.
Printed in the United States of America.
This publication is protected by federal copyright law. No part of this publication may be copied or
distributed, transmitted, transcribed, stored in a retrieval system, or transmitted into any human or computer
language, in any form or by any means, electronic, mechanical, magnetic, manual, or otherwise, or disclosed
to third parties without the express written permission of Dionex Corporation, 1228 Titan Way, Sunnyvale,
California 94088-3603 U.S.A.
DISCLAIMER OF WARRANTY AND LIMITED WARRANTY
THIS PUBLICATION IS PROVIDED “AS IS” WITHOUT WARRANTY OF ANY KIND. DIONEX
CORPORATION DOES NOT WARRANT, GUARANTEE, OR MAKE ANY EXPRESS OR
IMPLIED REPRESENTATIONS REGARDING THE USE, OR THE RESULTS OF THE USE, OF
THIS PUBLICATION IN TERMS OF CORRECTNESS, ACCURACY, RELIABILITY,
CURRENTNESS, OR OTHERWISE. FURTHER, DIONEX CORPORATION RESERVES THE
RIGHT TO REVISE THIS PUBLICATION AND TO MAKE CHANGES FROM TIME TO TIME IN
THE CONTENT HEREINOF WITHOUT OBLIGATION OF DIONEX CORPORATION TO
NOTIFY ANY PERSON OR ORGANIZATION OF SUCH REVISION OR CHANGES.
TRADEMARKS
Chromeleon is a registered trademark of Dionex Corporation.
Cone Wash, M-Path, MSQ, and Xcalibur are trademarks of Thermo Electron Corporation.
Microsoft is a registered trademark of Microsoft Corporation.
Q-tip is a trademark of Chesebrough-Pond’s, Inc.
Teflon, Vespel, and Viton are registered trademarks of E.I. du Pont de Nemours and Company.
PRINTING HISTORY
Revision 01, July 2002
Revision 02, October 2003
The products of Dionex Corporation are produced under ISO 9001 accredited quality management systems.
Published by Technical Publications, Dionex Corporation, Sunnyvale, CA 94086.
Page 3

Chapter 1
Introducing the MSQ 1.
Introducing the MSQ.................................................................................................................1-i
Introduction ....................................................................................................................................1-1
System Overview............................................................................................................................1-2
What Is Mass Detection?...................................................................................................1-4
Exterior Features of the MSQ............................................................................................1-5
The Source–An Introduction to API Techniques ...........................................................................1-8
Electrospray.......................................................................................................................1-9
Atmospheric Pressure Chemical Ionization.....................................................................1-13
Source Fragmentation......................................................................................................1-16
Cone Voltage Ramping ...................................................................................................1-18
Polarity Switching ...........................................................................................................1-19
Application of API Techniques .......................................................................................1-20
The Self-Cleaning Source: Cone Wash ........................................................................................1-23
Introduction .....................................................................................................................1-23
Functional Description ....................................................................................................1-24
The Reference Inlet System..........................................................................................................1-25
Introduction .....................................................................................................................1-25
Functional Description ....................................................................................................1-25
The Mass Analyzer and Detector .................................................................................................1-26
The Vacuum System.....................................................................................................................1-27
The Data System...........................................................................................................................1-28
Software...........................................................................................................................1-28
Raw Data .........................................................................................................................1-30
Raw Data Types...............................................................................................................1-31
____________________________MSQ Hardware Manual _____________________________ 1-i
Page 4

Introducing the MSQ
Introduction ____________________________________________________________________________
1-ii____________________________ MSQ Hardware Manual ____________________________
Page 5

Introducing the MSQ
___________________________________________________________________________ Introduction
Introduction
The MSQ™ MS detector has been specifically designed and engineered for
liquid chromatographic detection using Atmospheric Pressure Ionization
(API) and Mass Spectrometry (MS) technology. These technologies can
provide sensitive and selective detection of organic molecules.
Interfacing High Performance Liquid Chromatography (HPLC or LC) and
MS provides the separation scientist with one of the most powerful
analytical tools available. Both LC and MS have developed to a point
whereby they represent two of the most important techniques in
characterizing and detecting organic compounds. Although the potential
benefits of interfacing LC to MS have been clearly recognized for many
years, producing a truly automated “connect-and-use” interface has proven
to be a challenging task.
Atmospheric Pressure Ionization (API) techniques now provide highly
sensitive detection using conventional to capillary LC flow rates on benchtop MS detector systems. LC/MS works with typical solvent compositions,
whether the separation is achieved by isocratic or gradient elution.
Historically, LC/MS has been compatible only with volatile buffer systems
using modifiers such as trifluoroacetic acid, formic acid, and acetic acid.
Phosphate buffers, although extensively used in LC separations, were not
suited to LC/MS due to rapid blocking of the ion sampling region caused by
the deposition of involatile phosphate salts. The self-cleaning API source
allows for extended periods of operation in LC/MS with chromatographic
buffers such as phosphates or ion-pairing agents and samples in dirty
matrices.
API using Electrospray (ESI) or Atmospheric Pressure Chemical Ionization
(APCI) interfaces has proved to be invaluable in meeting sensitivity
requirements in quantitative methods. It can also provide structural
information, which is complementary to techniques such as NMR and infra
red spectroscopy.
This introduction focuses on the principal components of the system.
___________________________MSQ Hardware Manual ____________________________ 1-1
Page 6
Introducing the MSQ
System Overview ________________________________________________________________________
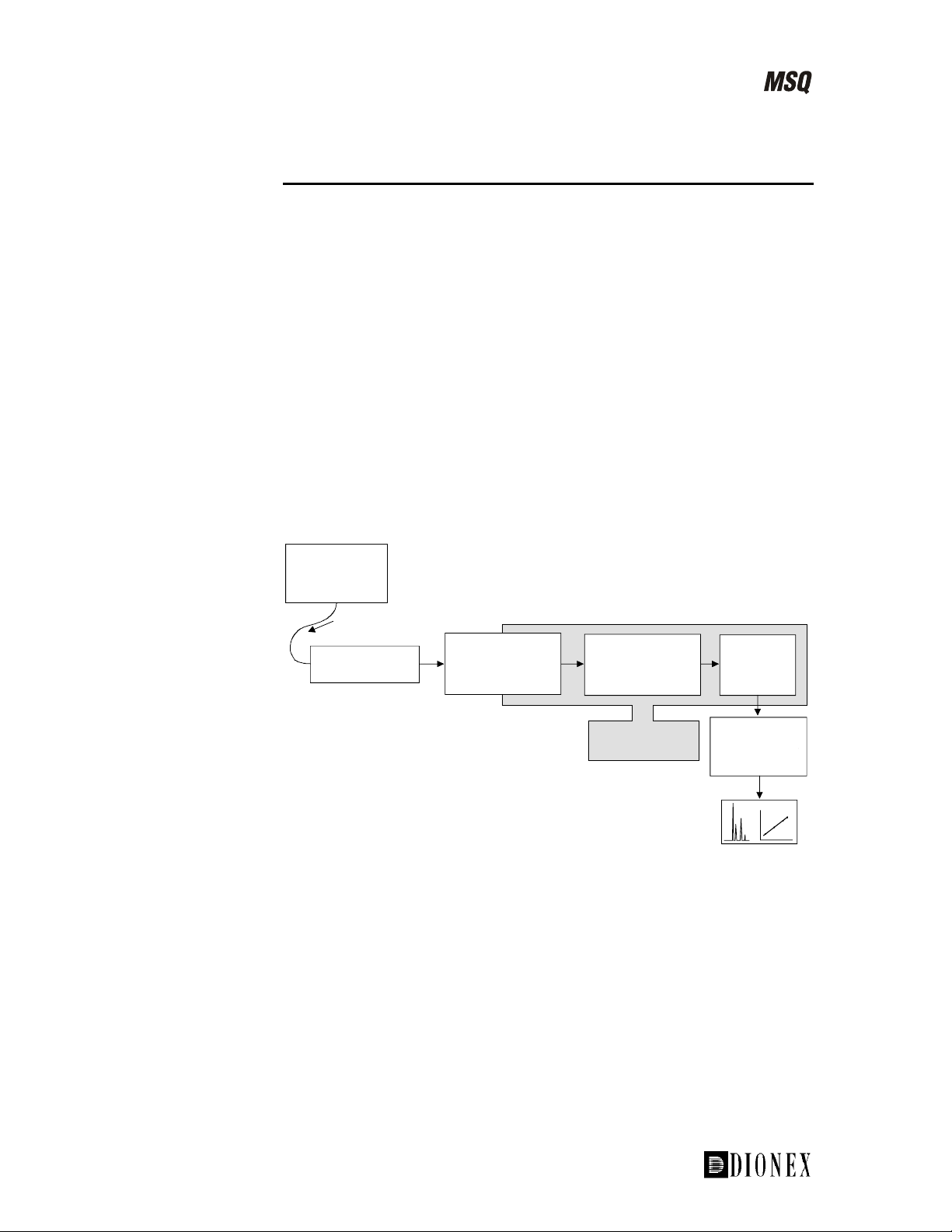
System Overview
The MSQ MS detector is an integral part of the LC detection system. Key
points of the system are:
The sample is introduced into the ion source using an LC system,
•
possibly through a column.
•
In an API MS detector, the part of the source where ionization takes
place is held at atmospheric pressure, giving rise to the term
Atmospheric Pressure Ionization (API).
•
In ESI, the sample is ionized in the liquid phase, while in APCI,
ionization occurs in the gas phase. In both cases, efficient desolvation is
needed to remove the solvents from the sample.
•
Ions, now in the gas phase, are passed through the mass analyzer and are
collected at the detector.
•
The detected signal is sent to the data system and stored ready for
processing.
LC System
Sample
introduction
LC Column
Separation
Figure 1-1. The key components of the MSQ API LC detection system
Ion Source
Ionization &
transmission
Mass Analyzer
Sorting of ions
Turbomolecular
& rotary pumps
Molecular weight information
Structural information
Positive identification
Quantitative information
Detector
Detection
of ions
Data System
Windows NT
1-2 ___________________________ MSQ Hardware Manual ____________________________
Page 7
Introducing the MSQ
_______________________________________________________________________System Overview
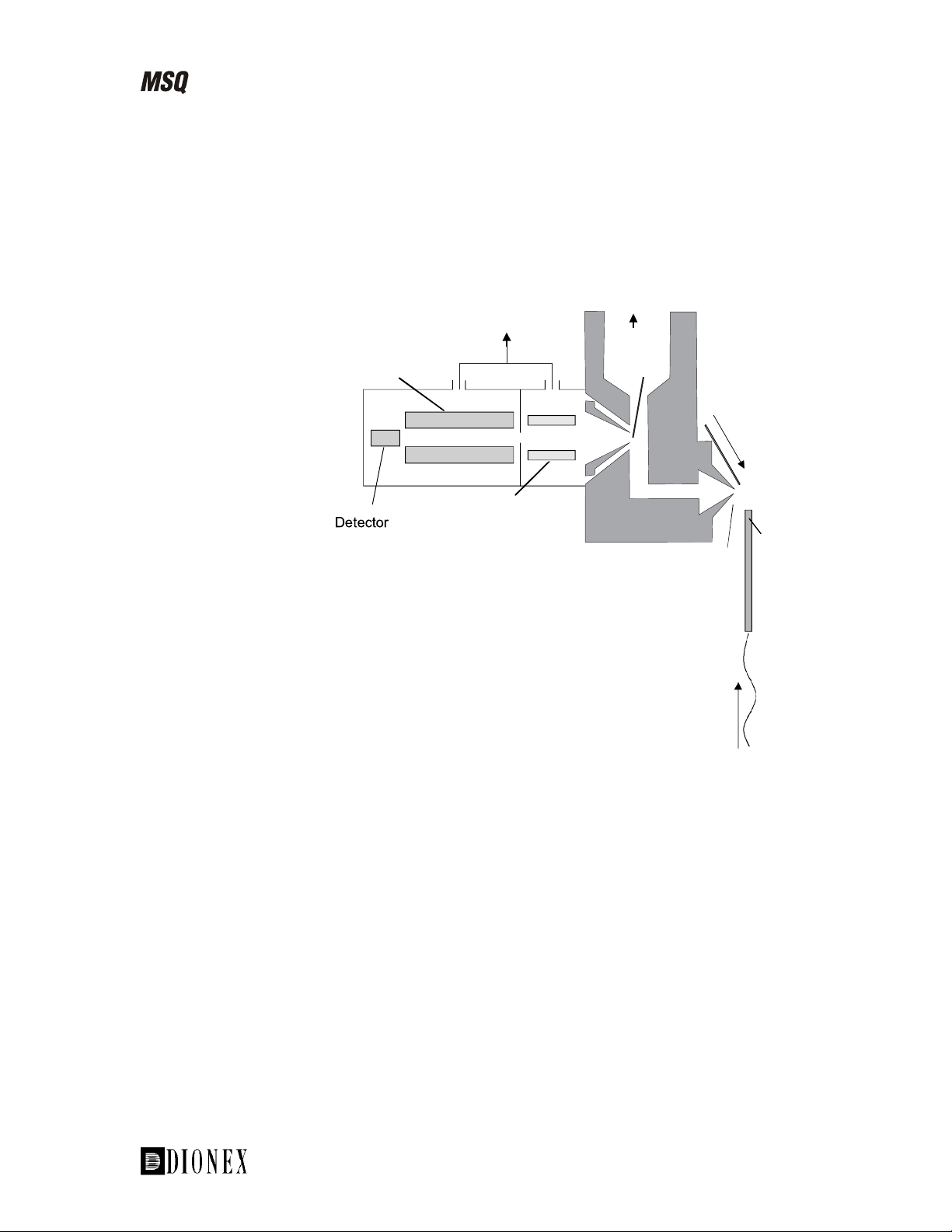
The main features of the MSQ MS detector are:
Dual ESI/APCI orthogonal probe •
• • Self-cleaning API-LC/MS interface
M-Path™ triple orthogonal source
Quadrupole
mass analyzer
Split flow
turbomolecular
pump
Square
quadrupole
RF lens
Rotary
pump
Exit cone
Entrance cone
From HPLC
Cone wash
Orthogonal sample
introduction probe
Figure 1-2. Schematic diagram of the MSQ API inlet, analyzer, and
detector system
The LC eluent is ionized at the API probe and the resulting ions are focused
into a square quadrupole RF lens. The quadrupole mass analyzer filters the
ions before detection.
___________________________MSQ Hardware Manual ____________________________ 1-3
Page 8

Introducing the MSQ
System Overview ________________________________________________________________________
What Is Mass Detection?
Mass detection is a very powerful analytical technique used in a number of
fields, including:
Identification of unknown compounds •
• • Quantitation of known compounds
Determination of chemical structure
The basic function of an MS detector is to measure the mass-to-charge ratio
of ions.
The unit of mass used is the Dalton (Da). One Dalton is equal to 1/12 of the
mass of a single atom of carbon-12. This follows the accepted convention
that an atom of carbon-12 has exactly 12 atomic mass units (amu). The MS
detector does not directly measure molecular mass, but the mass-to-charge
ratio of the ions. Electrical charge is a quantized property and so can exist
only as an integer; that is, 1, 2, 3, and so on. The unit of charge used here (z)
is that which is on an electron (negative) or a proton (positive). Therefore,
the mass-to-charge ratio measured can be denoted by m/z. Most ions
encountered in mass detection have just one charge. In this case, the massto-charge ratio is often spoken of as the “mass” of the ion.
1-4 ___________________________ MSQ Hardware Manual ____________________________
Page 9

Introducing the MSQ
_______________________________________________________________________System Overview
Exterior Features of the MSQ
This section highlights the exterior features of the MSQ. The parts labeled
here may be referred to in later chapters of this manual or other manuals
supplied with the MSQ.
Status light
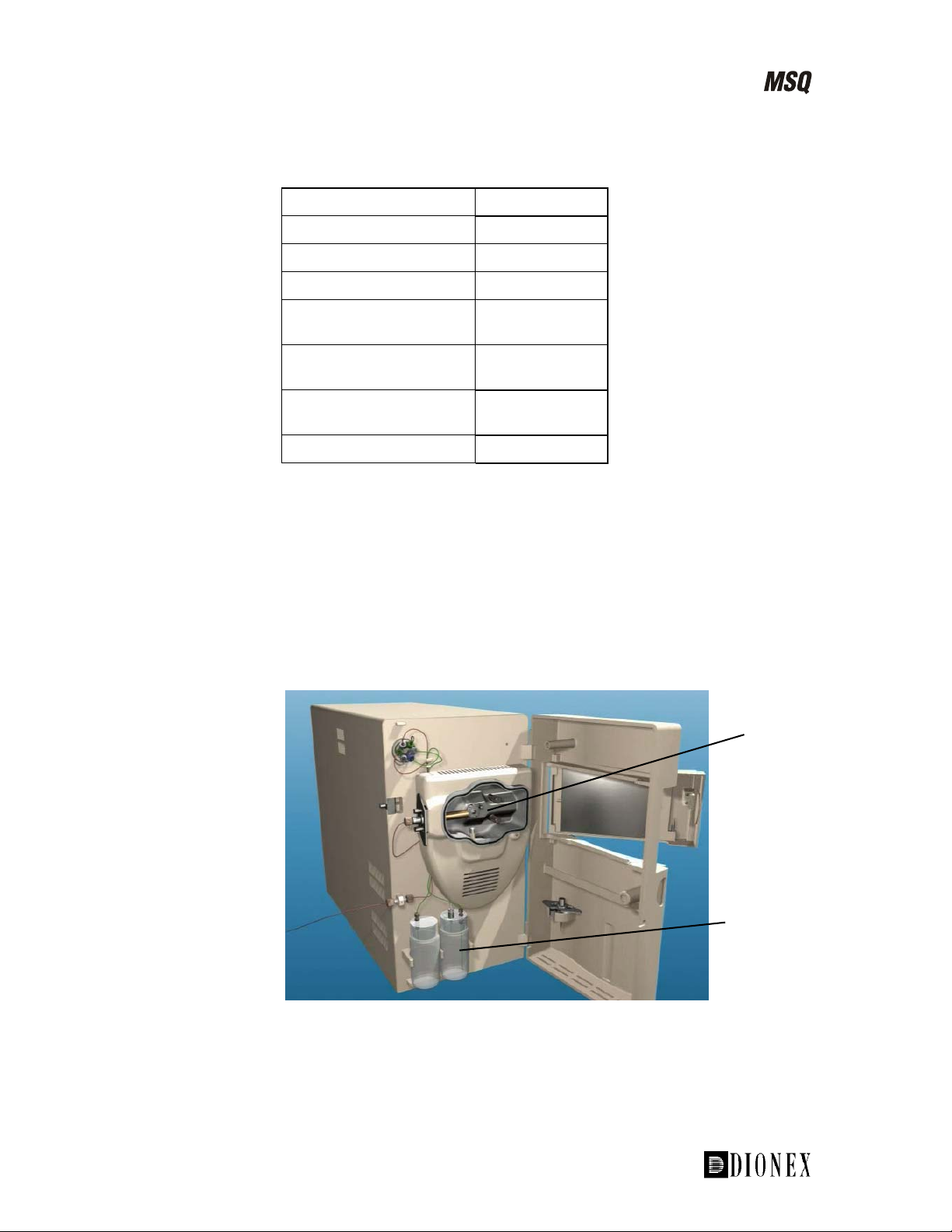
Figure 1-3. Front view of the MSQ
Figure 1-3 shows the front view of the MSQ. The main feature is the status
light.
___________________________MSQ Hardware Manual ____________________________ 1-5
Page 10
Introducing the MSQ
System Overview ________________________________________________________________________
Table 1-1. Instrument status light
Instrument Status Light
Vented Red
Venting Red
Pumping down Flashing yellow
Under vacuum (above
vacuum trip)
Under vacuum (ready
for use)
Operate on (MSQ in
use)
Source enclosure open Red
Red
Yellow
Green
The vacuum trip is the pressure below which it is safe to switch on the
voltages in the source. When the instrument is functioning normally, the
status light will go from flashing yellow to solid yellow and Operate can be
switched On. If the pressure in the instrument rises above the operating
pressure, the status light turns red to indicate that the pressure is above a
safe level. See the chapter Shutting Down and Restarting the System for
information on pumping down the MSQ.
Figure 1-4 shows the MSQ with the doors open. The source enclosure and
reference inlet are now visible.
Source
enclosure
Figure 1-4. The MSQ with the doors open
1-6 ___________________________ MSQ Hardware Manual ____________________________
Reference
inlet
Page 11
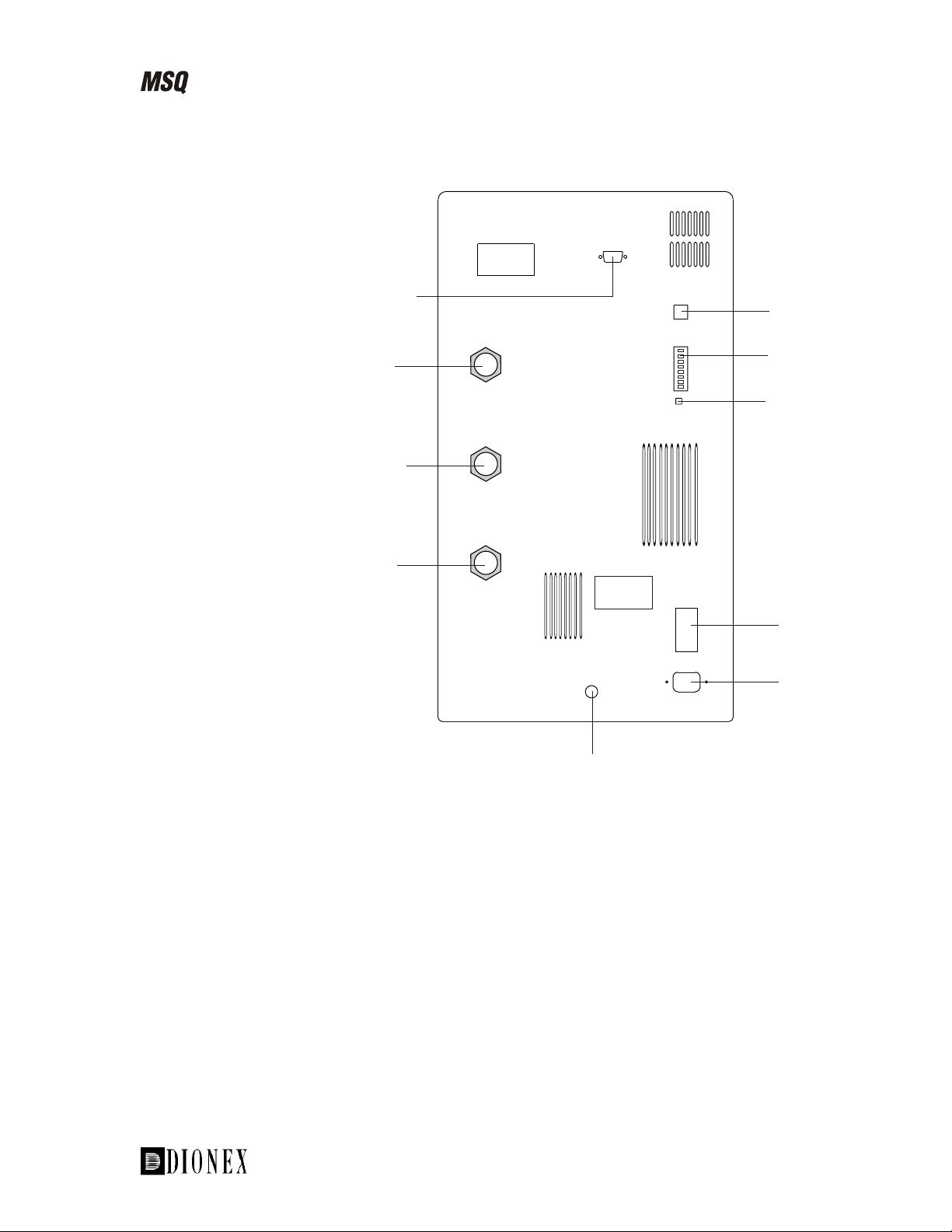
Introducing the MSQ
_______________________________________________________________________System Overview
Figure 1-5 is a schematic of the rear view of the MSQ.
PUMP RELAY
Rotary pump power
To P C
Contact closure and
analog inputs
To rotary pump
USB
SOURCE
USER I/O
EXHAUST
Exhaust from API
source
BACKING
To rotary pump
Figure 1-5. Rear view of the MSQ
RESET
MODEL:
RATING: 220-240v
GAS IN
6 BAR MAX
50/60 Hz
1000 VA
MAINS ON/OFF
Gas inlet for nebulizer
and sheath gas
Reset communications
Power switch
MAINS IN
Power supply
___________________________MSQ Hardware Manual ____________________________ 1-7
Page 12

Introducing the MSQ
The Source–An Introduction to API Techniques ________________________________________________
The Source–An Introduction to API Techniques
The source, or interface, performs four main functions:
Separates the analytes from the solvent and buffer systems used in LC •
• • Ionizes the analyte molecules
Allows efficient transfer of ions into the mass analyzer for detection
LC eluent enters the source through the orthogonal sample introduction
probe. The primary objective of an orthogonal probe is to direct any
involatile components present in the LC eluent, such as those from buffers,
ion-pairing agents, or matrices, away from the entrance orifice. Under
operating conditions, however, both the sample ions and the charged liquid
droplets (containing any involatile components, if they are present) are
deflected by the electric field towards the entrance orifice. This leads to a
gradual buildup of involatiles and a concomitant loss in sensitivity with
time. The self-cleaning source delivers a constant, low flow of solvent (the
cone wash™) to the edge of the inlet orifice, helping to prevent a buildup of
involatiles during an LC/MS run.
1-8 ___________________________ MSQ Hardware Manual ____________________________
Page 13
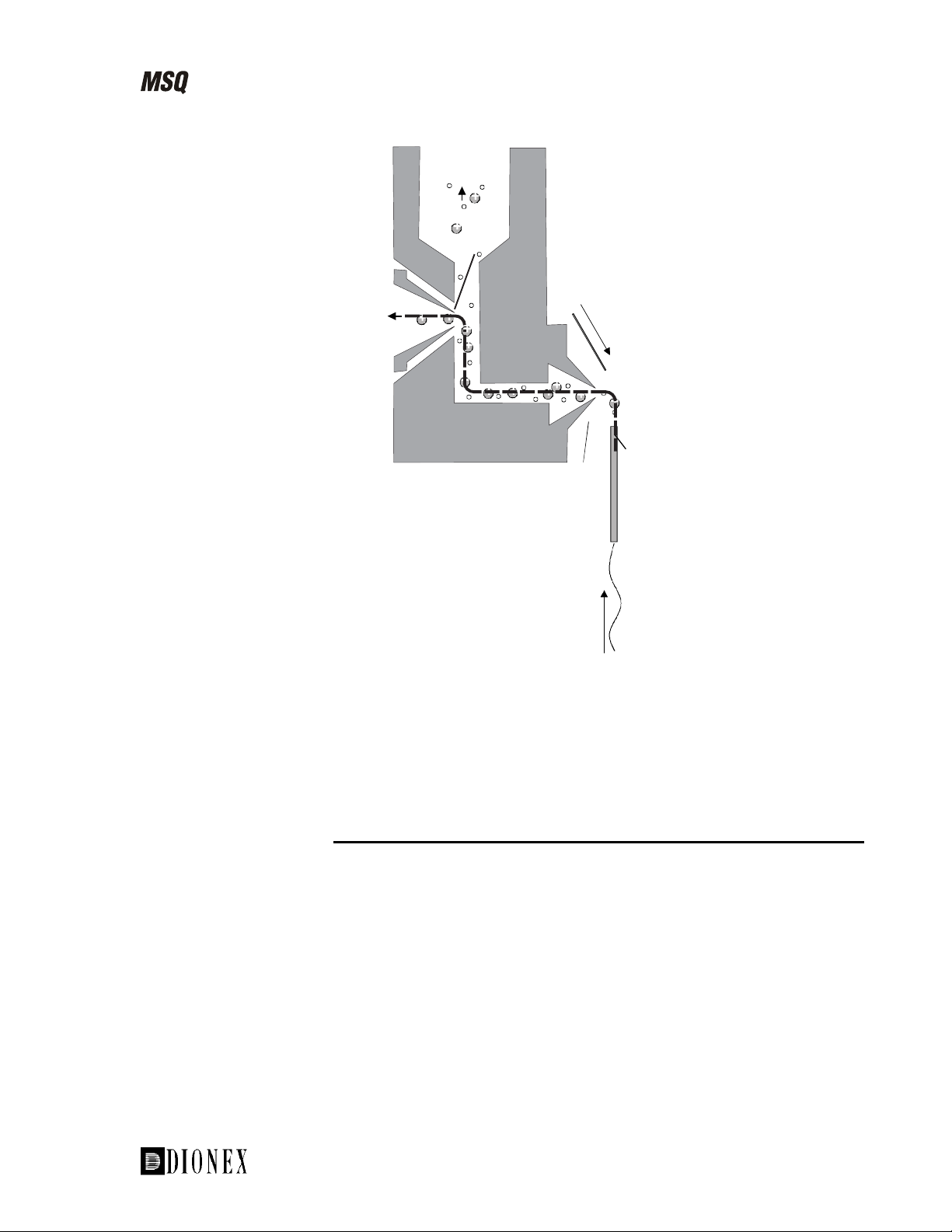
Introducing the MSQ
_______________________________________________ The Source–An Introduction to API Techniques
Rotary
pump
Exit cone
To the mass
analyzer
Entrance cone
From HPLC
Figure 1-7. Schematic of the MSQ source showing the cone wash
Cone wash
Orthogonal sample
introduction probe
Two types of API are commonly encountered. These are Electrospray
Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI).
The following sections discuss the mechanism of ion generation in each.
Electrospray
Electrospray Ionization (ESI) is regarded as a soft ionization technique
providing a sensitive means of analyzing a wide range of polar molecules.
Since the first combined ESI LC/MS results were announced in 1984, and
its first application to protein analysis four years later, the technique has
become an established analytical tool in separation science.
When applied to smaller molecules up to 1000 Daltons in molecular mass,
electrospray ionization results in either a protonated, [M+H]
1-8) or deprotonated, [M-H]
-
, molecule. Choice of ionization mode is
governed by the functional chemistry of the molecule under investigation. In
ESI, fragmentation is generally not apparent; however, increased source
voltages can induce fragmentation to provide structural information.
___________________________MSQ Hardware Manual ____________________________ 1-9
+
(see Figure
Page 14
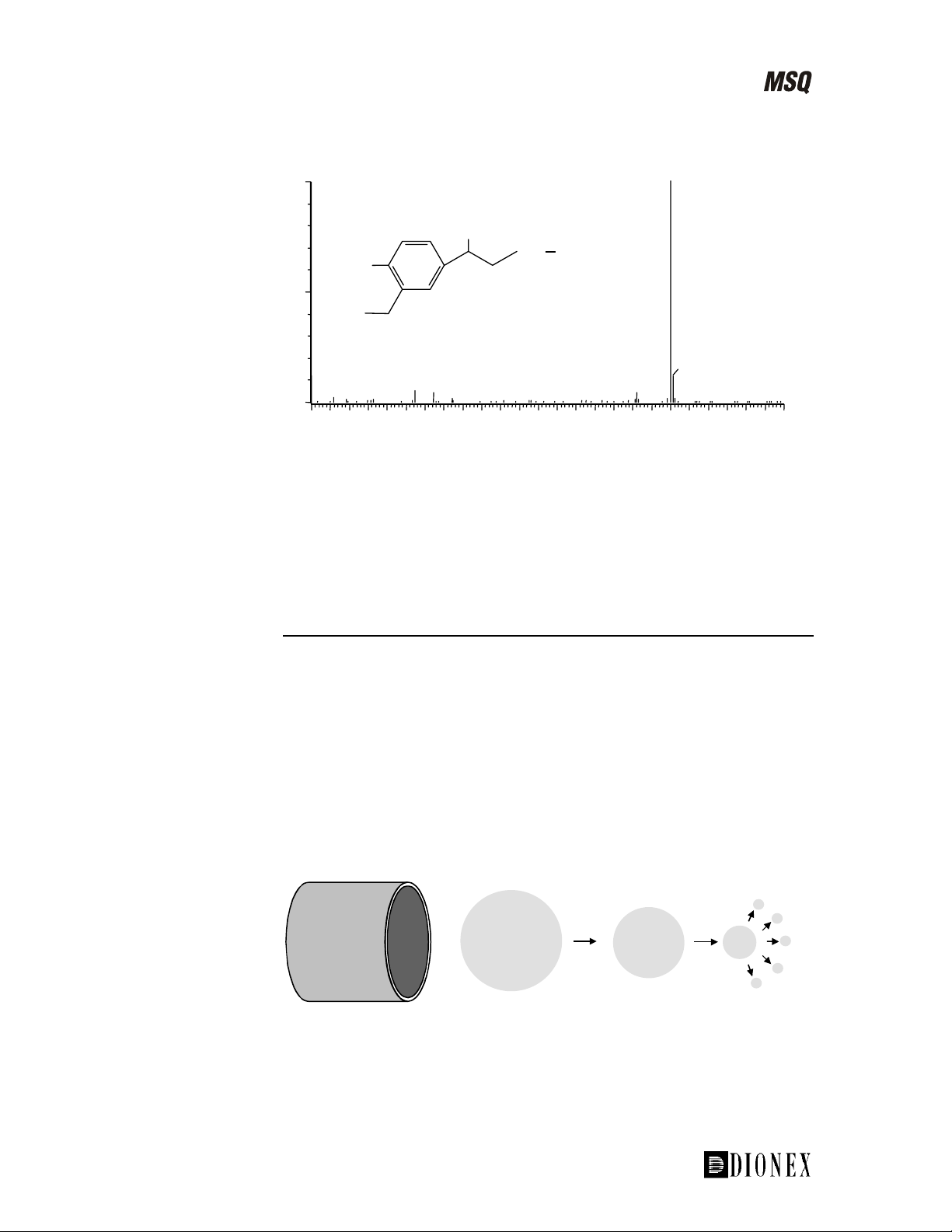
Introducing the MSQ
z
The Source–An Introduction to API Techniques ________________________________________________
100
240
OH
NH
tBu
HO
%
HO
Chemical structure of salbutamol,
(molecular weight 239)
0
60 80 100 120 140 160 180 200 220 240 260 280 300
241
m/
Figure 1-8. Electrospray mass spectrum of salbutamol in positive ion
mode
The base peak at m/z 240 (see Figure 1-8) corresponds to the protonated
salbutamol molecule. It is notable that ESI results in a prominent base peak
with minimal fragmentation, quite dissimilar from the results often achieved
with GC/MS.
Mechanism of Ion Generation
Electrospray ionization operates by the process of emission of ions from a
droplet into the gas phase, a process termed Ion Evaporation. A solvent is
pumped through a stainless steel insert capillary that carries a high potential,
typically 3 to 5 kV (see Figure 1-9). The strong electric field generated by
this potential causes the solvent to be sprayed from the end of the insert
capillary (hence, electrospray), producing highly charged droplets. As the
solvent is removed by the desolvation process, the charge density on the
surface of the droplets increases until the Rayleigh limit is exceeded; after
this, a multitude of smaller droplets are formed by coulombic explosion.
This process is repeated until charged sample ions remain. These ions are
then available for sampling by the ion source.
-
+
Insert capillary +3-5 kV
+
+
+
+
+
Droplet
containing
ions
+
+
+
+
-
+
+
+
+
-
+
+
+
+
+
+
+
As the droplet
evaporates, the
electric field
increases and ions
move towards
the surface
Figure 1-9. Positive ion electrospray mechanism
-
+
+
+
-
+
+
+
+
+
-
+
-
+
+
+
-
+
+
++
+
+
+
Ions evaporate
from the surface
1-10 __________________________ MSQ Hardware Manual ____________________________
Page 15
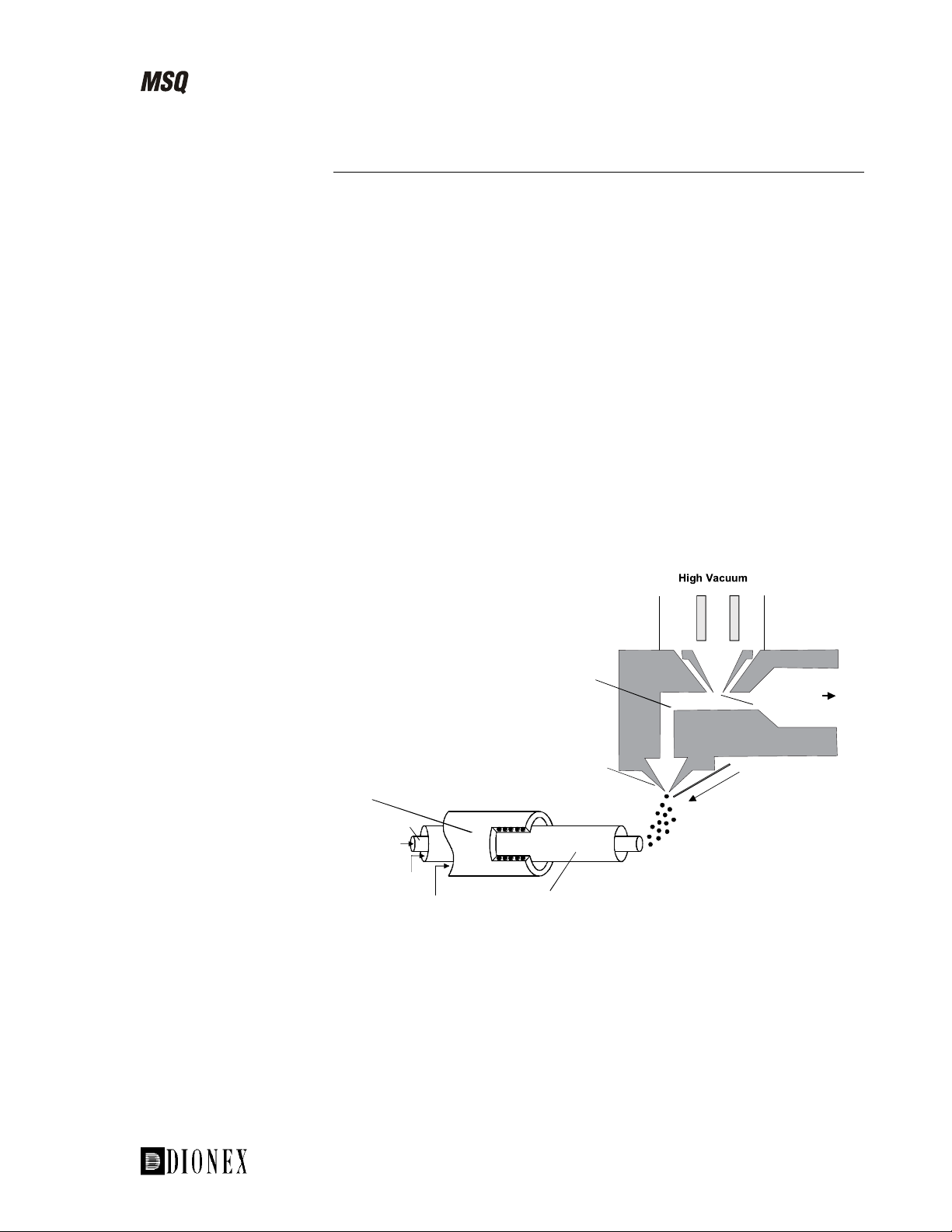
Introducing the MSQ
_______________________________________________ The Source–An Introduction to API Techniques
Electrospray Ionization Using the MSQ Source
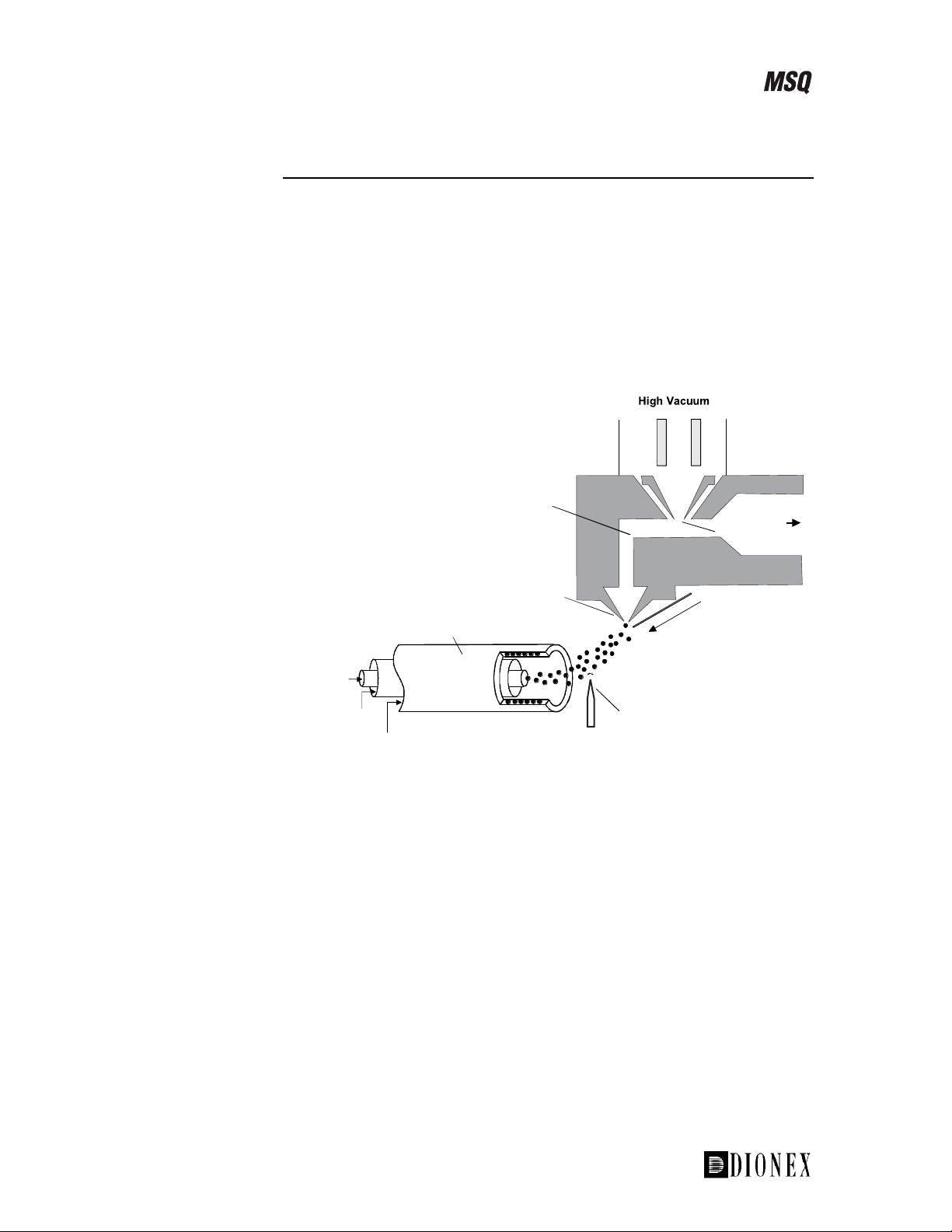
The sample, in solution, enters the source via a stainless steel insert capillary
held at a voltage of 3 to 5 kV. The insert capillary is surrounded by a tube
that directs a concentric flow of nitrogen nebulizing gas past the droplets of
liquid forming at the probe tip. The action of the nebulizing gas, high
voltage, and heated probe produces an aerosol of liquid droplets containing
ions of the sample and solvent. The ion evaporation process is assisted by a
second concentric flow of heated nitrogen gas. This is the sheath gas. This
highly efficient evaporation process close to the entrance cone enables the
routine use of high LC flow rates (up to 2.0 mL/min) in ESI mode.
The newly formed ions then enter the focusing region through the entrance
cone. This is due to the following:
The high electric field. The insert capillary is at 3 to 5 kV with respect
•
to the rest of the source, which is typically at 20 to 30 V.
• The gas flow into the focusing region.
Ions then exit the focusing region and pass into the RF lens. The RF lens
(square quadrupole) helps to focus the ions before they enter the mass
analyzer region.
Region
Intermediate
Pressure
Region
Exit cone
Entrance cone
Probe
Insert capillary
LC eluent
Nebulizing gas, N
Sheath gas, N
2
2
Insert
Cone Wash
Atmospheric
Pressure
Region
Figure 1-10. Schematic of the ESI source on the MSQ, showing the
principal components and pressure regions
Rotary
pump
___________________________MSQ Hardware Manual ___________________________ 1-11
Page 16
Introducing the MSQ
The Source–An Introduction to API Techniques ________________________________________________
Spectral Characteristics
Polar compounds of low molecular weight (<1000 amu) typically form
singly charged ions by the loss or gain of a proton. Basic compounds (for
example, amines) can form a protonated molecule [M+H]
analyzed in positive ion mode to give a peak at m/z M+1. Acidic
compounds (for example, sulphonic acids) can form a deprotonated
molecule [M-H]
-
, which can be analyzed in negative ion mode to give a
peak at m/z M-1. As electrospray is a very soft ionization technique, there is
usually little or no fragmentation and the spectrum contains only the
protonated or deprotonated molecule.
Some compounds are susceptible to adduct formation if ionization takes
place in the presence of contamination or additives such as ammonium or
sodium ions. The spectra will show other ions in addition to, or instead of,
the quasi-molecular ion. Common adducts are ammonium ions NH
[M+18]
100
+
, sodium ions Na+ [M+23]+, and potassium ions K+ [M+39]+.
+
, which can be
+
4
[M+H]
322
+
%
+
100
103
80
99
0
60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360
141
145
181
187
241
244
261
279
282
[M+Na]
344
363
m/z
Figure 1-11. Electrospray spectrum showing a sodium adduct
The singly charged ions arising from samples of relatively low molecular
masses can be interpreted directly, as they represent the protonated or
deprotonated molecule. Electrospray, however, can produce multiply
charged ions for analytes that contain multiple basic or acidic sites, such as
proteins and peptides. As an MS detector measures mass-to-charge ratio
(m/z), these ions appear at a m/z value given by the mass of their protonated
molecule divided by the number of charges:
n
+
nHM
+
n
zm
=
Where, M = actual mass, n = number of charges, and H = mass of a proton.
Electrospray allows molecules with molecular weights greater than the mass
range of the MS detector to be analyzed. This is a unique feature of
electrospray.
1-12 __________________________ MSQ Hardware Manual ____________________________
Page 17

Introducing the MSQ
_______________________________________________ The Source–An Introduction to API Techniques
Flow Rate
The electrospray source can be used with flow rates from 5.0 µL/min to
2.0 mL/min.
Atmospheric Pressure Chemical Ionization
Atmospheric Pressure Chemical Ionization (APCI) is also a very soft
ionization technique and has many similarities to electrospray ionization.
Ionization takes place at atmospheric pressure and the ions are extracted into
the MS detector in the same way as in electrospray.
Similarly, as observed in ESI, [M+H]
providing molecular weight information. Fragmentation can be induced in
the source by increasing the source voltage to give structural information.
Mechanism of Ion Generation
+
and [M-H]- ions are usually formed
In APCI, the liquid elutes from an insert capillary, surrounded by a coaxial
flow of nitrogen nebulizing gas into a heated region. The combination of
nebulizing gas and heat form an aerosol that evaporates quickly to yield
desolvated neutral molecules (see Figure 1-12).
At the end of the probe is a corona pin held at a high potential (typically 2.0
to 3.5 kV). This produces a high-field corona discharge that causes solvent
molecules eluting into the source to be ionized. In the atmospheric pressure
region surrounding the corona pin, a series of reactions occur that give rise
to charged reagent ions. Any sample molecules, which elute and pass
through this region of reagent ions, can be ionized by the transfer of a proton
to form [M+H]
+
or [M-H]-. This is a form of chemical ionization; hence the
name of the technique, Atmospheric Pressure Chemical Ionization.
Heated nebuliser
N
2
Liquid
N
2
Solvent molecules
An aerosol
is formed
Sample molecules
Solvent and
sample molecules
are desolvated
Corona pin
Collisions and
+
+
+
proton transfer
+
+
+
+
Solvent molecules
are ionized
+
+
Sample
+
[M+H]
ions formed
Figure 1-12. Positive ion APCI mechanism
___________________________MSQ Hardware Manual ___________________________ 1-13
Page 18
Introducing the MSQ
The Source–An Introduction to API Techniques ________________________________________________
APCI Using the MSQ Source
The sample is carried to a spray region via a stainless steel insert capillary.
The action of both the nebulizing gas and the heated probe leads to the
formation of an aerosol. The desolvation process is assisted by a second
concentric flow of nitrogen gas, the sheath gas.
In contrast to electrospray, APCI is a gas phase ionization technique.
Ionization occurs as the aerosol leaves the heated nebulizer region. A corona
pin, mounted between the heated region and the entrance cone, ionizes the
sample molecules with a discharge voltage of approximately 3.0 to 3.5 kV
in positive ion mode and 2.0 to 3.0 kV in negative ion mode.
Region
Intermediate
Pressure
Region
Exit cone
Rotary
pump
Entrance cone
Pressure
Region
Cone Wash
LC eluent
Nebulizing gas, N
Sheath gas, N
Probe
2
2
Atmospheric
Corona pin
Figure 1-13. Schematic of the APCI source on the MSQ, showing the
principal components and pressure regions
The newly formed ions then enter the focusing region through the entrance
orifice and pass into the RF lens region. The RF lens (square quadrupole)
helps to focus the ions before they enter the mass analyzer region.
1-14 __________________________ MSQ Hardware Manual ____________________________
Page 19

Introducing the MSQ
_______________________________________________ The Source–An Introduction to API Techniques
Spectral Characteristics
Like electrospray, APCI is a soft ionization technique and forms singly
charged ions–either the protonated, [M+H]
+
, or deprotonated, [M-H]-,
molecule–depending on the selected ionization mode. Unlike electrospray,
however, APCI does not produce multiply charged ions and so is unsuitable
for the analysis of high molecular weight compounds such as proteins or
peptides.
Although a high temperature is applied to the probe, most of the heat is used
in evaporating the solvent, so the thermal effect on the sample is minimal. In
certain circumstances (for example, with very thermally labile (unstable)
compounds), the heated probe may cause some thermal fragmentation.
Flow Rate
Flow rates of 0.2 to 2.0 mL/min can be used with APCI.
___________________________MSQ Hardware Manual ___________________________ 1-15
Page 20
Introducing the MSQ
The Source–An Introduction to API Techniques ________________________________________________
Source Fragmentation
Both electrospray and APCI are regarded as soft ionization techniques.
Ionization generally results in spectra dominated by either the protonated
molecule [M+H]
(negative ion mode), depending on whether positive or negative ionization
mode has been selected. Choice of ionization mode is governed by the
functional chemistry of the molecule under investigation.
Source fragmentation can be induced to give additional information on a
compound, such as diagnostic fragment ions for structural determination or
an increased response on a particular confirmatory ion for peak targeting.
Formation of Diagnostic Fragment Ions
The MSQ allows the simultaneous acquisition of MS data at a number of
different source voltages. For example, the MSQ can be programmed to
acquire data at source voltages of 20, 40, and 60 V on an alternating scan
basis within a single acquisition. The benefits of setting up acquisitions in
this way are:
+
(positive ion mode) or deprotonated molecule [M-H]-
The optimum source voltage for a particular ion can be determined in
•
one acquisition for compounds where sample volume is at a premium.
• The intensity of fragment ions can be maximized to gain structural
information.
Fragmentation at increased source voltages is useful for most compounds.
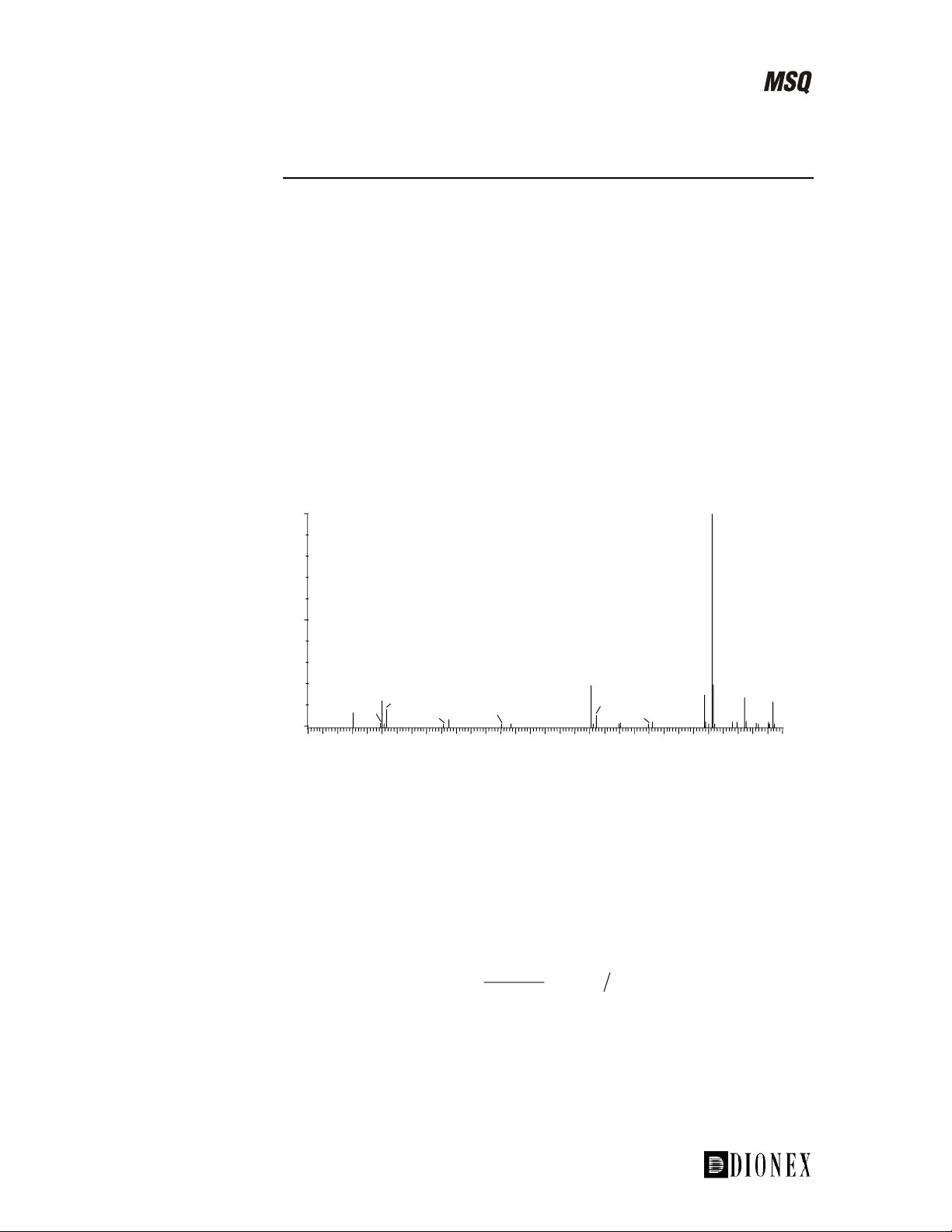
For example, using source fragmentation of salbutamol in electrospray
ionization, a number of confirmatory fragment ions can be generated and
their intensity maximized (see Figure 1-14).
The mechanism for the formation of the fragment ions is characteristic for
not only salbutamol, but also for related β-agonists such as clenbuterol,
terbutaline, and metaproterenol. It involves loss of water (-18 amu; resulting
in the fragment ion at m/z 222 (middle trace)) and an additional loss of the
tert-butyl group (-56 amu; resulting in the fragment at m/z 166 (lower
trace)).
Note. The MSQ uses the term “cone voltage” to represent source voltage.
1-16 __________________________ MSQ Hardware Manual ____________________________
Page 21
Introducing the MSQ
+
+
+
u
_______________________________________________ The Source–An Introduction to API Techniques
100
Source voltage 10V
%
0
50 75 100 125 150 175 200 225 250 275 300
100
Source voltage 25V
%
0
50 75 100 125 150 175 200 225 250 275 300
100
Source voltage 35V
%
0
50 75 100 125 150 175 200 225 250 275 300
148
166
240
241
240
222
241
240
222
241
m/z
m/z
m/z
Protonated molecule at m/z 240
OH
+
tBu
NH
HO
HO
2
Fragment ion at m/z 222
NH
tBu
HO
HO
2
Fragment ion at m/z 166
NH
HO
HO
3
[M+H]
[M+H]+-H2O
[M+H]+-H2O-tB
Figure 1-14. Source fragmentation of salbutamol in electrospray
ionization
Optimum Response for Confirmatory Ions
When acquiring fragment ions for confirmation purposes, the applied source
voltage per compound would require some optimization to maximize the
intensity of these ions. It is generally observed that small changes to the
source voltage result in only small intensity changes; thus, fine-tuning of
this voltage is usually not critical (typically +/- 5 V is adequate).
___________________________MSQ Hardware Manual ___________________________ 1-17
Page 22
Introducing the MSQ
The Source–An Introduction to API Techniques ________________________________________________
Cone Voltage Ramping
Source voltage ramping can be used in Full Scan operation (see page 1-32)
in electrospray when the compound of interest forms multiply charged ions.
A spectrum similar to that in Figure 1-15 will be produced, showing the
multiply charged envelope.
771
808
848
893
942
944
998
999
1060
1131
1212
1305
m/z
100
%
738
707
694
0
700 800 900 1000 1100 1200 1300
Figure 1-15. Electrospray spectrum of horse heart myoglobin
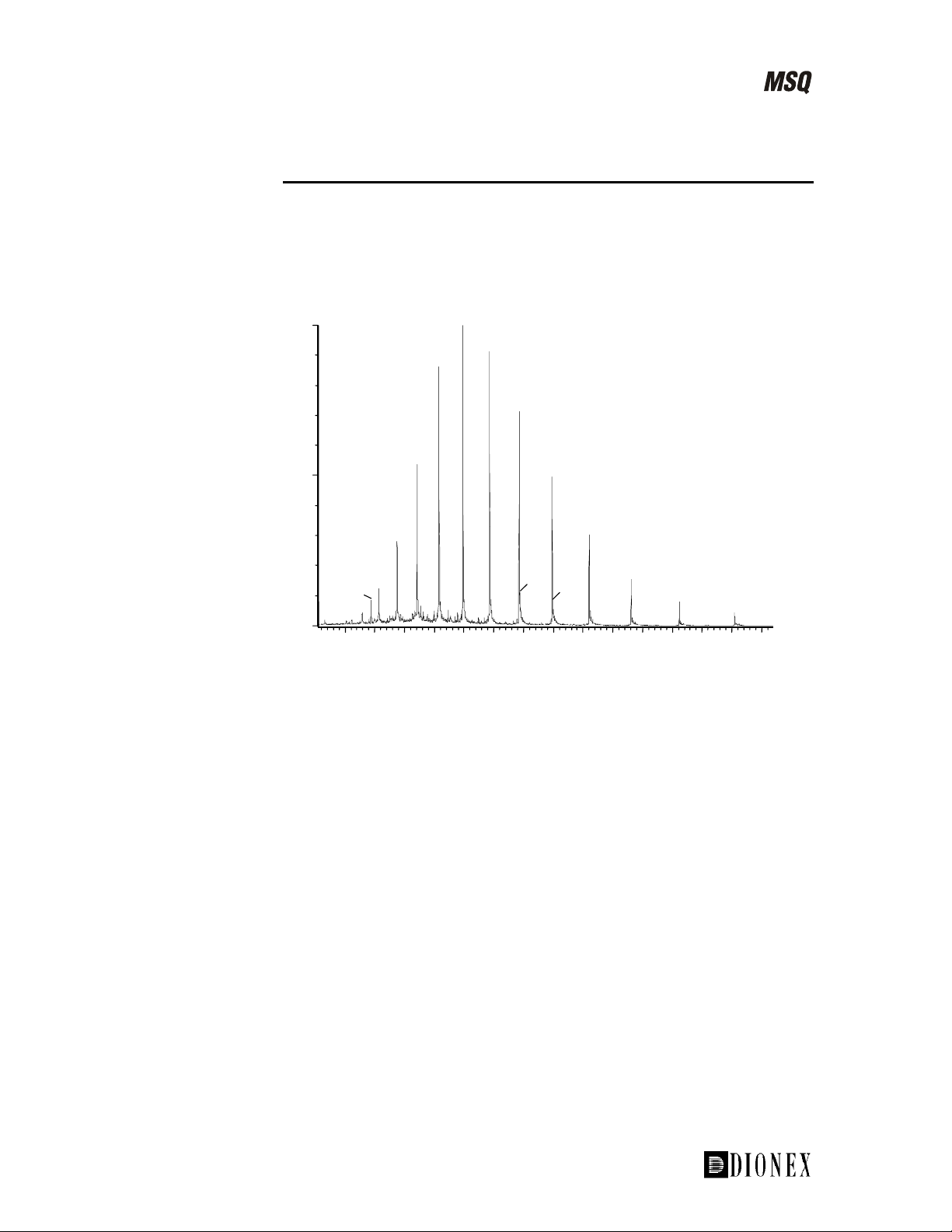
This envelope is represented in diagrammatic form in Figure 1-16. The first
diagram shows the envelope at a source voltage of 30 V. Next, the envelope
is shown at 60 V. There is no ramping applied here. The envelope has the
same shape but has moved to a higher m/z value. This is due to the charge
stripping that occurs at high source voltages, which lowers the charge state
and produces an apparent higher mass distribution. If the source voltage is
ramped between 30 and 60 V, the charge distribution envelope is extended
and it resembles the lower diagram.
1-18 __________________________ MSQ Hardware Manual ____________________________
Page 23
Introducing the MSQ
f
_______________________________________________ The Source–An Introduction to API Techniques
Intensity
Without s ource voltage ramping
Source voltage 30V
Increasing
number of
charges
Intensity
Decreasing
number o
charges
With source voltage ramping 30-60V
m/z
Intensity
Without s ource voltage ramping
Source voltage 60V
m/z
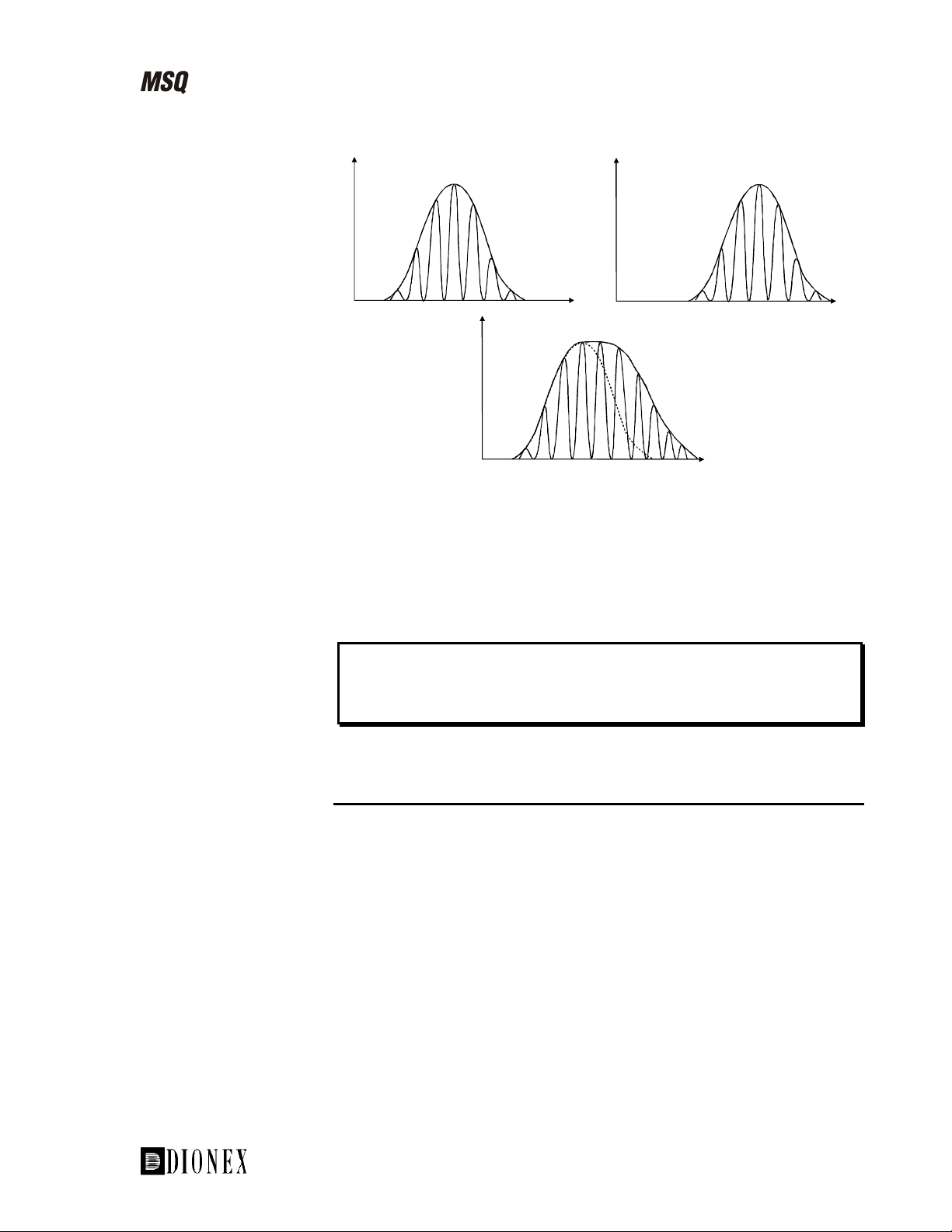
Figure 1-16. Cone voltage ramping
Cone voltage ramping is often used for proteins and peptides during:
Calibration (to extend the calibration range) •
• Analysis (to increase the number of charge states leading to greater
accuracy of molecular weight determination)
m/z
Note. Cone voltage ramping will not perform source fragmentation because
the same cone voltage corresponds to the same m/z value each time.
Polarity Switching
Switching between positive and negative ionization modes in a single
analytical run is supported by the MSQ. Rapid polarity switching is a
technique that is applied to several important areas of MS analysis; for
example:
Quantitation of different chemistries within the same run. In drug
•
metabolism studies, certain compounds preferentially ionize in positive
ion mode because they may contain a primary amino group. Other
metabolites, such as glucuronide metabolites, are likely to lose a proton
and respond in the negative ion mode.
• Rapid screening of unknown analytes; for example, in combinatorial
chemistry. If the compound has a carboxylic acid group that is sterically
unhindered, it is likely that the compound will lose a proton in negative
ion mode and not respond in positive ion mode.
___________________________MSQ Hardware Manual ___________________________ 1-19
Page 24
Introducing the MSQ
A
The Source–An Introduction to API Techniques ________________________________________________
Application of API Techniques
Both electrospray and APCI are ideal for online liquid chromatography
detection, providing an additional dimension of information. With many
compounds, it is possible to analyze them by both APCI and electrospray. It
may be difficult to decide which is the more appropriate technique,
especially when the compounds of interest lack polar functionalities.
Points to note:
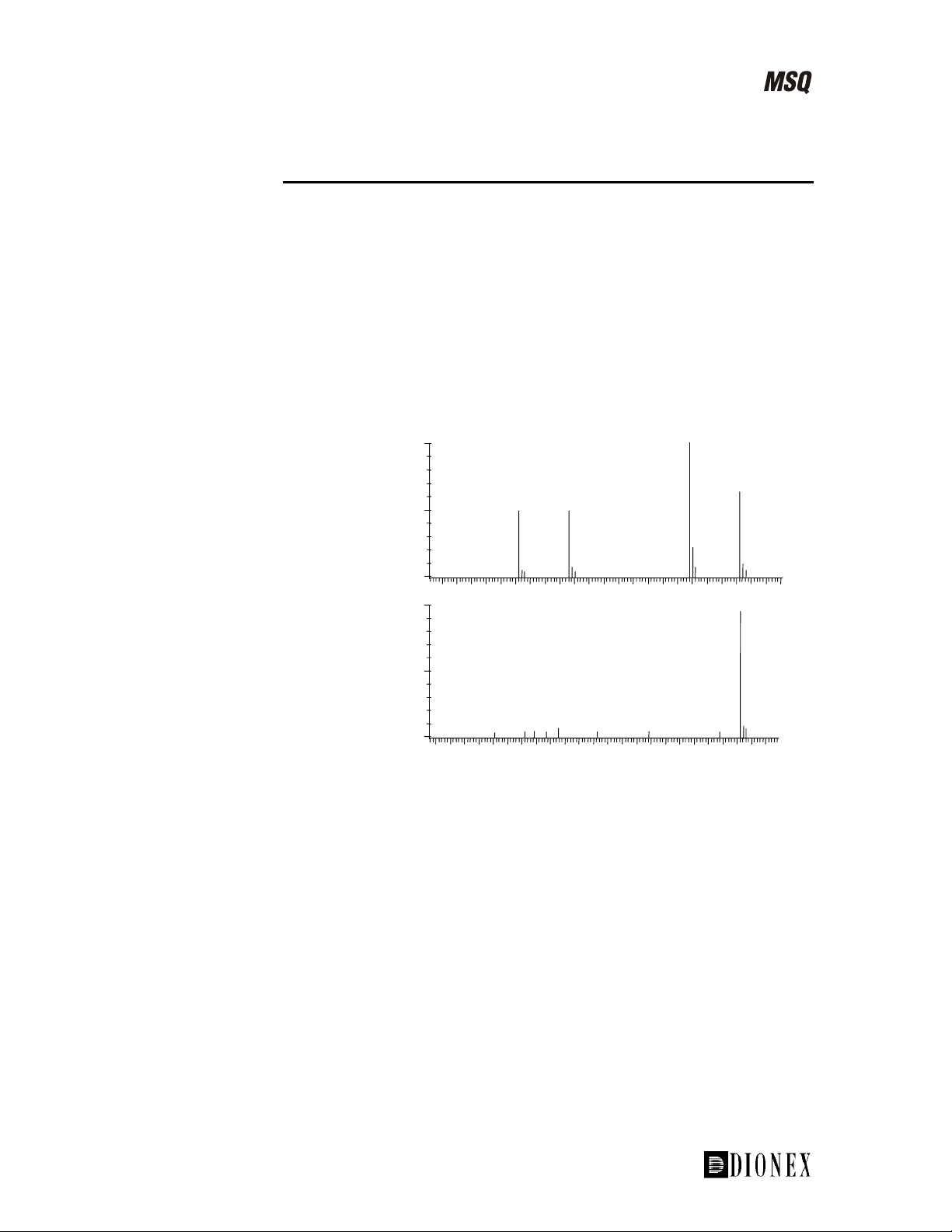
Electrospray is one of the softest ionization methods available, whereas
•
APCI, although also a soft ionization technique, may not be suitable for
some very thermally labile compounds as there may be thermal
fragmentation (see Figure 1-17).
214
231
231
PCI mass spectrum,
source voltage 25V.
Electrospray mass
spectrum, source
voltage 25V.
100
%
0
130 140 150 160 170 180 190 200 210 220 230 240
100
%
0
130 140 150 160 170 180 190 200 210 220 230 240
156
173
Figure 1-17. Thermal fragmentation of the herbicide asulam in APCI
APCI does not yield multiply charged ions like electrospray, and so is
•
unsuitable for the analysis of high molecular weight compounds such as
proteins.
m/z
m/z
• Both APCI and electrospray generally provide data from which it is
simple to infer molecular weight values.
In many cases, with the correct conditions, only one major peak is
observed in the spectrum: either the protonated molecule [M+H]
ion) or deprotonated molecule [M-H]
-
(-ve ion). However, some
+
(+ve
compounds are more susceptible to fragmentation than others, so
different degrees of fragmentation may be seen from compound to
compound. When determining molecular weights, always take account
of possible adduct ions. Common adducts are [M+18]
+
NH
+
4
(ammonium adducts seen in the presence of buffers such as ammonium
acetate), [M+23]+ Na+ (sodium adducts), and [M+39]+ K+ (potassium
adducts).
1-20 __________________________ MSQ Hardware Manual ____________________________
Page 25

Introducing the MSQ
_______________________________________________ The Source–An Introduction to API Techniques
Source fragmentation is used in both APCI and electrospray to give
•
structural information.
In general, increasing the voltage applied to the source block (the cone
voltage) yields increasing amounts of fragmentation, depending on the
nature of the compound. The optimum source voltage required to give
the maximum intensity of the protonated or deprotonated molecule is
compound-dependent, as is the source voltage required for
fragmentation. The energies involved in source fragmentation are low,
so usually only weaker bonds such as C-N and C-O are broken.
Since there are many similarities between electrospray and APCI, there are
many applications common to both.
Compounds suitable for analysis by electrospray are polar and of molecular
weight less than 100,000 amu. The higher molecular weight compounds,
such as proteins, can produce multiply charged ions. As it is the mass-tocharge ratio (m/z) that is measured by the MS detector, these can often be
seen at lower masses. For example, if the molecular weight is 10,000, a
doubly charged ion (2+ in +ve ion) would be seen at m/z 5001, 10+ at m/z
1001, etc.
Typical electrospray applications are: peptides, proteins, oligonucleotides,
sugars, drugs, steroids, and pesticides.
Compounds suitable for analysis by APCI are generally polar (although less
polar than electrospray) and of molecular weight <1000 amu.
Typical APCI applications are: pesticides, drugs, azo dyes, and steroids.
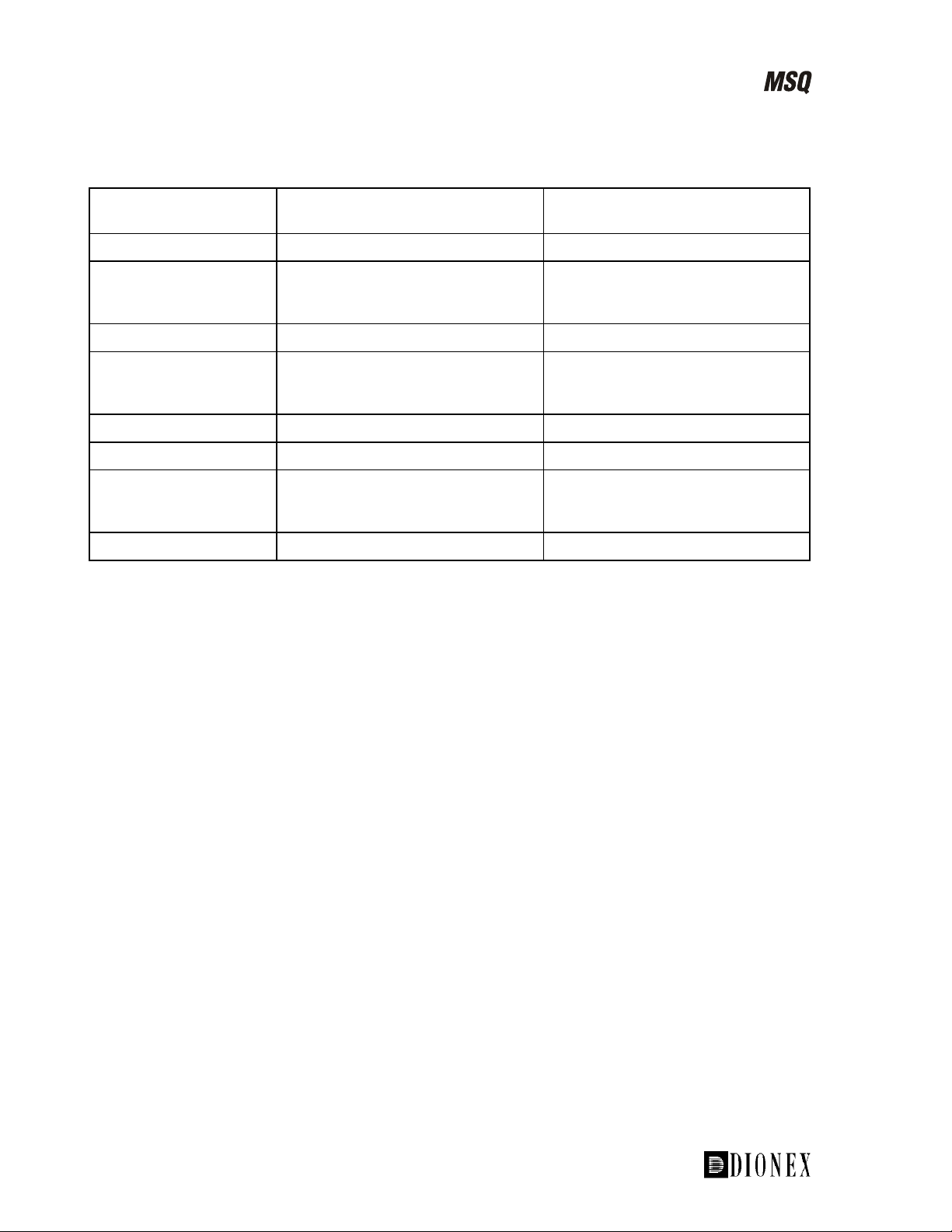
A summary comparing electrospray and APCI is shown in Table 1-2.
___________________________MSQ Hardware Manual ___________________________ 1-21
Page 26
Introducing the MSQ
The Source–An Introduction to API Techniques ________________________________________________
Table 1-2. Comparison of ESI and APCI
LC/MS technique Electrospray (ESI) Atmospheric Pressure Chemical
Ionization (APCI)
Compound polarity Polar Polar, some non-polar
Examples Drugs, proteins, biopolymers,
oligonucleotides, steroids, and
pesticides
Pesticides, azo dyes, drugs,
metabolites, agrochemicals, and
steroids
Sensitivity fg to pg (compound-dependent) fg to pg (compound-dependent)
Type of spectra [M+H]+ for +ve ion mode, [M-H]- for
-ve ion mode, fragmentation via
source voltage
+
[M+H]
for +ve ion mode, [M-H]- for
-ve ion mode, fragmentation via
source voltage
Flow rates 2.0 µL/min to 2.0 mL/min 0.2 to 2.0 mL/min
LC columns Capillary to 4.6-mm ID columns 2.1- to 4.6-mm ID columns
Mobile phases H2O, CH3CN, CH3OH are most
frequently used.
H2O, CH3CN, CH3OH are most
frequently used. Non-polar
solvents can be used.
Typical mass range <100,000 <1000
1-22 __________________________ MSQ Hardware Manual ____________________________
Page 27
Introducing the MSQ
______________________________________________________The Self-Cleaning Source: Cone Wash
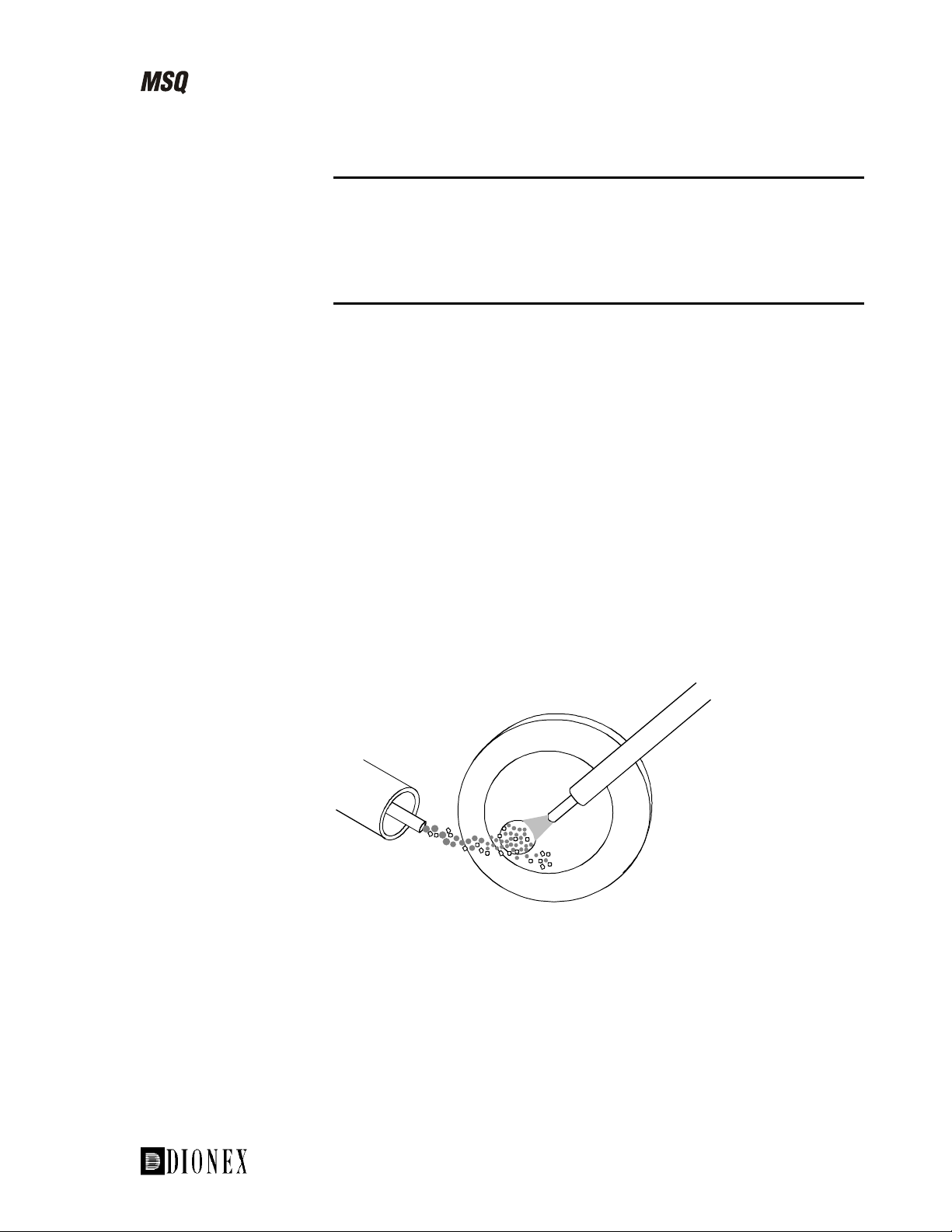
The Self-Cleaning Source: Cone Wash
This section introduces the cone wash. Information on how and when to use
it is provided in the chapter LC/MS and the Cone Wash.
Introduction
The API source on the MSQ includes a self-cleaning solvent delivery
system (the cone wash). This makes the source extremely robust and
productive and greatly increases the number of samples that can be analyzed
before maintenance is required.
The orthogonal API probe serves to direct the LC eluent away from the inlet
orifice. However, under typical LC/MS conditions, both the ions and the
charged liquid droplets (containing involatile components) are deflected by
the electric field towards the inlet orifice. This effect leads to a gradual
buildup of involatile components and an associated loss in sensitivity with
time.
The self-cleaning API source delivers a constant, low flow of solvent to the
edge of the inlet orifice (see Figure 1-18). This prevents the buildup of
involatile components during LC/MS analysis with typical chromatographic
buffers (for example, phosphates and ion-pairing agents). This greatly
improves the quantitation precision of analysis without the need to
compromise the LC method, and more importantly, dramatically extends the
length of time possible for analysis.
Figure 1-18. Dispersion of involatile components from the inlet orifice
___________________________MSQ Hardware Manual ___________________________ 1-23
Page 28
Introducing the MSQ
The Self-Cleaning Source: Cone Wash _______________________________________________________
Functional Description
As shown in Figure 1-18, the cone wash nozzle consists of a stainless steel
capillary, which is fed to the edge of the inlet orifice of the entrance cone.
The capillary is attached to PEEK tubing, internal to the instrument, which
when connected to an LC pump delivers the solvent (typically HPLC-grade
water or organic solvents such as methanol) at a controlled flow rate.
Note. It is necessary to use the cone wash only for compounds in dirty
matrices or when involatile buffers are used. Choose the cone wash solvent
to give the most effective solubility for the expected contaminants.
1-24 __________________________ MSQ Hardware Manual ____________________________
Page 29

Introducing the MSQ
______________________________________________________________ The Reference Inlet System
The Reference Inlet System
This section introduces the reference inlet system. Information on how to set
up the system is provided in the chapter Reference Inlet System.
Introduction
The recommended way to introduce a sample for tuning and mass
calibration in electrospray is to use the reference inlet system.
Functional Description
As shown in Figure 1-20, the reference inlet system consists of a pressurized
reservoir containing the reference sample and a PEEK delivery tube. One
end of the PEEK delivery tube is inserted into the reference inlet reservoir,
while the other end is attached to the switching valve. When the pressure in
the reservoir is increased, the sample is forced through the PEEK tube and
infused directly into the 100 µL sample loop.
Figure 1-20. The reference inlet system
___________________________MSQ Hardware Manual ___________________________ 1-25
Page 30
Introducing the MSQ
The Mass Analyzer and Detector____________________________________________________________
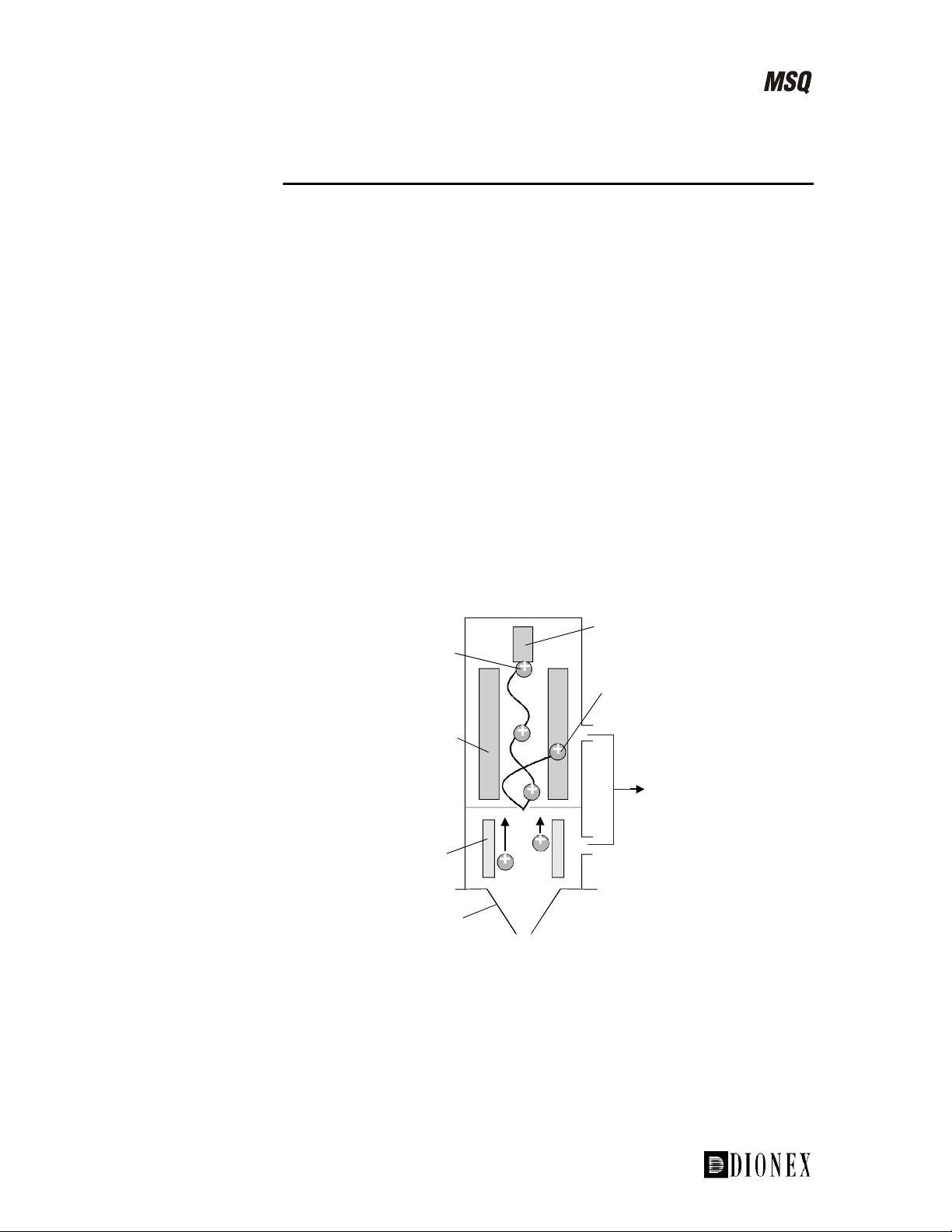
The Mass Analyzer and Detector
The mass analysis and detection system comprises two main components:
A quadrupole mass analyzer •
• A detector whose main component is a channeltron electron multiplier
The square quadrupole RF lens helps to focus the ions before they are
filtered, according to their mass-to-charge ratio in the mass analyzer.
The analyzer in the MSQ is a quadrupole. This is one of the most widely
used types of analyzer and can be easily interfaced to various inlet systems.
By applying carefully controlled voltages to the four rods in the quadrupole,
only ions of a specific mass-to-charge ratio are allowed to pass through at
any one time.
The ions then reach the detector, whose main component is a channeltron
electron multiplier. In positive ion mode, the ions exit the analyzer and
strike a conversion dynode, which results in the emission of electrons. The
electrons are accelerated towards the channeltron electron multiplier, which
then creates an electron cascade. The current is then converted and amplified
into a voltage signal that is analyzed and processed by the MSQ’s on-board
data acquisition system. The resultant peak information is sent to the data
system.
Ion successfully transmitted
by the mass analyzer
Quadrupole
mass analyzer
Square
quadrupole
RF lens
Source exit cone
Figure 1-21. Schematic of the MSQ analyzer and detector
Detector
Ion trajectory incorrect
for transmission trough
the mass analyzer
Split flow
turbomolecular
pump
1-26 __________________________ MSQ Hardware Manual ____________________________
Page 31

Introducing the MSQ
____________________________________________________________________ The Vacuum System
The Vacuum System
The main challenge in interfacing MS with LC is the introduction of a liquid
mobile phase at flow rates of up to 2.0 mL/min into a system that operates
under vacuum. The transition between atmospheric pressure and high
vacuum is achieved by using several different stages of pressure controlled
by the vacuum system. This arrangement effectively removes the mobile
phase, leaving the analytes to travel as ions through the mass analyzer.
It is important to remember that an MS detector must be under high vacuum
in order to operate. In the case of the MSQ system, not all of the MS
detector is under high vacuum. The ion source is held at atmospheric
pressure, while the region between the entrance and exit cones is held at an
intermediate pressure to step down to the high vacuum region in the mass
analyzer and detector. The intermediate pressure region is pumped by one
rotary pump. The high vacuum in the mass analyzer and detector region is
achieved by using a split-flow turbomolecular pump. All the pumps are
controlled by the data system.
___________________________MSQ Hardware Manual ___________________________ 1-27
Page 32

Introducing the MSQ
The Data System ________________________________________________________________________
The Data System
The data system has complete control of the MSQ system and runs on a
Microsoft® Windows platform.
Software
Xcalibur™ software controls the MSQ MS detector. When Xcalibur is run,
the Home page is displayed (see Figure 1-22). Xcalibur also runs the MSQ
server (see Figure 1-23).
The Home Page
The Home page opens to show a ‘road map’ view of the data system.
Figure 1-22. The Xcalibur Home page “road map” view
1-28 __________________________ MSQ Hardware Manual ____________________________
Page 33

Introducing the MSQ
_______________________________________________________________________The Data System
The icons shown on the road map provide an easy way to access all the
major modules of the data system. These modules are:
Instrument Setup •
Use Instrument Setup to configure the MSQ and all your LC equipment for
acquisition. This information is saved as an instrument method.
•
Processing Setup
Use Processing Setup to specify all parameters for processing, reporting,
and manipulation of acquired data. This information is saved as a processing
method.
•
Sequence Setup
Use Sequence Setup to enter the details of the samples to be examined,
including instrument and processing methods, and to control the acquisition
of data.
•
Qual Browser
Use Qual Browser to examine acquired data, both chromatograms and
spectra, in order to obtain more information about the compounds in the
sample.
•
Quan Browser
Use Quan Browser to examine acquired data in order to obtain an accurate
determination of the amounts of individual components present in a sample.
•
Library Browser
Use Library Browser to create your own libraries of spectra and to perform
searches of those libraries.
The Server
The server is displayed as an icon on the Windows taskbar. In Figure 1-23,
it is shown just to the left of the time display. The light will be red, yellow,
or green, depending on the status of the system.
Figure 1-23. The taskbar showing the Xcalibur Home page and server
The server is shown as one light. When the light is green, the MSQ is under
vacuum with Operate On and the API gas flowing. When the MSQ is
pumping down, the light is yellow and flashing.
Use the server to tune and calibrate the MSQ and to pump or vent the
system.
___________________________MSQ Hardware Manual ___________________________ 1-29
Page 34

Introducing the MSQ
The Data System ________________________________________________________________________
Right-click on the server to display a menu:
Choose Instrument Tune and Calibration to display the Instrument
•
Tuning and Calibration Wizard.
Figure 1-24. The MSQ Instrument Tuning and Calibration Wizard
Choose Tune to display the Tune page. •
• • Choose Pump to pump down the MSQ or Vent to vent the MSQ.
Choose Exit to close the server. If Xcalibur is still running, this will not
be allowed and an error message will be displayed.
Raw Data
Xcalibur acquires data in a “raw” file. Raw data can be viewed as
chromatograms and mass spectra (see Figure 1-25).
The term mass spectrum refers to a plot of mass-to-charge ratio (m/z)
versus relative abundance information. The mass spectrum at a particular
time in an analytical run will reveal a “snapshot” of the data at that time.
The chromatogram is a plot of relative abundance versus time. Xcalibur
produces the following types of chromatograms: total ion current (TIC)
chromatogram, base peak chromatogram, mass range chromatogram, and
analog UV chromatogram.
1-30 __________________________ MSQ Hardware Manual ____________________________
Page 35

Introducing the MSQ
_______________________________________________________________________The Data System
RT: 1.09 - 6.11
10 0
80
60
40
1. 2 4 1. 5 5 2 . 2 5
Relat ive Abundanc e
20
0
1. 5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0
carb mix pmix03#102 RT: 2.66 AV: 1 SB: 38 1.42-2.07, 2.87-3.15 NL: 1.02E5
T: + c ESI Ful l ms [ 150.00-500.00]
100
80
60
40
20
Relative Abundance
181.5 387.0
0
150 200 250 300 350 400 450 500
2.35
229.4
2.66
240.3
2.82
3.28
281.2
3.59
Time (min)
282.5
3.72
3.87
346.8 403.2
m/z
4.75
5.174.18 5.32
454.1
5.42
459.1
NL:
2.49E5
TIC carb
mix pmix03
461.0256.2
482.1
Figure 1-25. A mass spectrum taken at retention time 2.66 minutes
(lower trace) from a TIC chromatogram (upper trace)
Raw Data Types
The data can be collected and stored by the data system in two different
ways: Full Scan and Selected Ion Monitoring (SIM). The main difference
between these two modes is:
In Full Scan mode, data is collected across the whole scan range. •
• In SIM mode, data is acquired only at specific mass-to-charge ratios.
___________________________MSQ Hardware Manual ___________________________ 1-31
Page 36

Introducing the MSQ
The Data System ________________________________________________________________________
Full Scan Mode
There are three different types of Full Scan acquisition. These are:
Centroid •
• • Profile
MCA (Multi Channel Analysis)
In all full scan acquisitions, raw data is collected over the whole scan range
defined by the start and end mass.
Centroid
During centroid acquisitions, Xcalibur automatically determines and saves
the mass center of the acquired profile peak returned from the detector.
Hence, the previously large number of points that described the mass
spectral peak are reduced to a single centroid stick (see Figure 1-26) for
each ion mass recorded. This has the advantage of reducing the quantity of
data recorded to the hard disk and improving processing speeds.
100
%
0
120 140 160 180 200 220 240 260 280
265
267
263
Figure 1-26. Full scan centroid spectrum of pentachlorophenol
m/z
1-32 __________________________ MSQ Hardware Manual ____________________________
Page 37

Introducing the MSQ
_______________________________________________________________________The Data System
Profile
With profile acquisition, data is not “centroided” into sticks. Instead, the
signal received by the interface electronics is stored to give an analog
intensity profile of the data being acquired for every scan (see Figure 1-27).
Profile acquisition enables mass spectral peak width and resolution to be
examined and measured. For example, the resolution between an ion and its
isotope(s) or multiply charged ions can be seen and measured, if necessary.
This is most useful in the case of protein and peptide analysis, where
multiply charged ions are formed.
As data is being written to disk at all times (even when there are no peaks
being acquired), profile data acquisition places an extra burden on the
acquisition system in comparison to centroided acquisition. Profile data files
tend to be significantly larger than centroided ones and the scan speeds used
tend to be slower than when acquiring centroided data.
100
%
281
283
0
275 300 325 350 375 400 425 450 475 500 525
325
Figure 1-27. Full scan profile spectrum of D-raffinose
503
504
m/z
___________________________MSQ Hardware Manual ___________________________ 1-33
Page 38

Introducing the MSQ
The Data System ________________________________________________________________________
MCA
The third type of full scan acquisition is MCA. Such data can be thought of
as “summed profile,” with only one intensity-accumulated scan being
written to disk for a given experiment (see Figure 1-28). As each scan is
acquired, its intensity data is added to the accumulated summed data of
previous scans.
An advantage of MCA is that although noise will accumulate at the same
rate as sample-related data, noise is random, and therefore its effects will be
reduced over a number of scans. This will emphasize the sample-related
data and improve signal to noise. A further advantage of MCA is that data is
written to disk only at the end of an experiment; therefore, significantly less
storage space is required.
MCA cannot be used for time-resolved data because an MCA raw file
contains only one scan. Therefore, MCA is not used during a
chromatographic run. Generally, it is used to acquire infusion or loop
injected samples of fairly weak concentration (the signal can then be
enhanced). The real-time spectrum can be viewed and the acquisition
stopped when the required results are obtained. MCA is often used to
acquire raw data from the infusion of proteins and peptides.
771
808
848
893
942
944
998
999
1060
1131
1212
1305
100
%
738
707
694
0
700 800 900 1000 1100 1200 1300
Figure 1-28. Full scan MCA spectrum of horse heart myoglobin
m/z
1-34 __________________________ MSQ Hardware Manual ____________________________
Page 39

Introducing the MSQ
_______________________________________________________________________The Data System
SIM Mode
This acquisition mode is used when only one or a few specific masses are to
be monitored during the acquisition. Since most of the acquisition time is
spent on these masses, the SIM technique is far more sensitive (typically
greater than a factor of ten) than full scan techniques. However, this
sensitivity does depend on the number of masses being monitored
simultaneously.
SIM is also a highly selective technique. Impurities present in the sample
that co-elute with the compound of interest will not affect the analysis as
long as they do not produce ions at the same m/z value being monitored.
SIM does not produce spectra that can be used for library searching routines.
___________________________MSQ Hardware Manual ___________________________ 1-35
Page 40

Introducing the MSQ
The Data System ________________________________________________________________________
1-36 __________________________ MSQ Hardware Manual ____________________________
Page 41

Chapter 2
Changing Ionization Modes 2.
Changing Ionization Modes ......................................................................................................2-i
Introduction ....................................................................................................................................2-1
Switching from ESI to APCI ..........................................................................................................2-2
Switching from APCI to ESI ..........................................................................................................2-4
____________________________MSQ Hardware Manual _____________________________ 2-i
Page 42

Changing Ionization Modes
Introduction ____________________________________________________________________________
2-ii ____________________________ MSQ Hardware Manual ____________________________
Page 43

Changing Ionization Modes
____________________________________________________________________________ Introduction
Introduction
Use the information in this chapter in conjunction with the animations on
the MSQ CD shipped with your system. The chapter is divided into the
following sections:
Switching from ESI to APCI •
• Switching from APCI to ESI
____________________________MSQ Hardware Manual ____________________________ 2-1
Page 44

Changing Ionization Modes
Switching from ESI to APCI ________________________________________________________________
Switching from ESI to APCI
The starting point for this procedure is the source setup for ESI operation,
with the LC and gas flows off, and the probe cooled.
WARNING. Allow the source block and probe heater assembly to cool
before changing ionization modes.
1.
Unscrew and remove the PEEK finger-tight fitting from the ESI probe
assembly (P/N FM102595).
2.
Turn the locking plate clockwise to the open position, and remove the
ESI probe assembly (P/N FM102595).
PEEK fitting
Figure 2-5. ESI probe assembly
3.
Swap the ESI probe assembly (P/N FM102595) with the APCI probe
Locking plate
assembly (P/N FM102587), located in the holder in the door.
4.
Turn the locking plate on the APCI probe assembly clockwise into the
open position, insert the APCI probe assembly (P/N FM102587), and
turn the locking plate counterclockwise into the closed position.
5.
Change the APCI blank plug (P/N FM101437) with the APCI corona
pin (P/N FM101433), located in the holder in the source enclosure door.
2-2 ___________________________ MSQ Hardware Manual ____________________________
Page 45

Changing Ionization Modes
________________________________________________________________Switching from ESI to APCI
Figure 2-6. APCI corona pin and APCI blank plug
6. Insert the PEEK finger-tight fitting into the APCI probe assembly (P/N
FM102587) and screw it into place.
____________________________MSQ Hardware Manual ____________________________ 2-3
Page 46

Changing Ionization Modes
Switching from APCI to ESI ________________________________________________________________
Switching from APCI to ESI
The starting point for this procedure is the source setup for APCI operation,
with the LC and gas flows off, and the probe cooled.
WARNING. Allow the source block and probe heater assembly to cool
before changing ionization modes.
1.
Unscrew and remove the PEEK finger-tight fitting from the APCI probe
assembly (P/N FM102587).
2.
Change the APCI blank plug (P/N FM101437) with the APCI corona
pin (P/N FM101433), located in the holder in the source enclosure door.
Figure 2-7. APCI blank plug and APCI corona pin
3. Turn the locking plate clockwise to the open position, and remove the
APCI probe assembly (P/N FM102587).
2-4 ___________________________ MSQ Hardware Manual ____________________________
Page 47

Changing Ionization Modes
________________________________________________________________Switching from APCI to ESI
PEEK fitting
Figure 2-5. ESI probe assembly
4.
Swap the APCI probe assembly (P/N FM102587) with the ESI probe
Locking plate
assembly (P/N FM102595), located in the holder in the door.
5.
Turn the locking plate on the ESI probe assembly clockwise into the
open position, insert the ESI probe assembly (P/N FM102595), and turn
the locking plate counterclockwise into the closed position.
6.
Insert the PEEK finger-tight fitting into the ESI probe assembly (P/N
FM102595) and screw it into place.
____________________________MSQ Hardware Manual ____________________________ 2-5
Page 48

Changing Ionization Modes
Switching from APCI to ESI ________________________________________________________________
2-6 ___________________________ MSQ Hardware Manual ____________________________
Page 49

Chapter 3
LC/MS and the Cone Wash 3.
LC/MS and the Cone Wash ......................................................................................................3-i
Introduction ....................................................................................................................................3-1
LC/MS Considerations ...................................................................................................................3-2
Flow Rates .........................................................................................................................3-2
HPLC Solvents and Mobile Phase Additives ....................................................................3-3
Setting Up the Cone Wash..............................................................................................................3-7
Flow Splitting .................................................................................................................................3-9
___________________________ MSQ Hardware Manual _____________________________ 3-i
Page 50

LC/MS and the Cone Wash
Introduction ____________________________________________________________________________
3-ii____________________________ MSQ Hardware Manual ____________________________
Page 51

LC/MS and the Cone Wash
____________________________________________________________________________ Introduction
Introduction
Historically, LC/MS has been compatible only with volatile buffer systems
using modifiers such as trifluoroacetic acid, formic acid, and acetic acid.
Phosphate buffers, although extensively used in LC separations, were not
suited to LC/MS due to the rapid blocking of the ion sampling region caused
by the deposition of involatile phosphate salts. The self-cleaning API source
allows routine LC/MS with chromatographic buffers such as phosphates or
ion-pairing agents and samples in dirty matrices.
This chapter contains the following information:
Details of HPLC solvents and mobile phase additives that focus on
•
LC/MS applications using the MSQ.
• • Instructions on how to set up the cone wash and information on when to
use it.
Instructions on flow splitting for use with hyphenated detection
applications.
____________________________MSQ Hardware Manual ____________________________ 3-1
Page 52

LC/MS and the Cone Wash
LC/MS Considerations ____________________________________________________________________
LC/MS Considerations
This section discusses the considerations to be taken into account when
choosing solvents and additives. It also provides guidance on how to
optimize LC/MS analyses to produce high quality data using the MSQ.
Flow Rates
In general, the column in use determines the flow rate. Each column has an
optimum flow rate. The guidelines in Table 3-1 apply.
Table 3-1. LC columns and flow rates
Column ID Flow Rate
4.6 mm 1.0 mL/min
3.9 mm 0.5 mL/min
2.1 mm 0.2 mL/min
1.0 mm 40-50 µL/min
Capillary <10 µL/min
The different ionization modes require different flow rates and column IDs.
The following guidelines apply when using the MSQ:
Electrospray can operate at all the flow rates described in Table 3-1.
•
Therefore, the full range of column IDs can be used without splitting the
flow.
• APCI cannot operate at flow rates below 0.2 mL/min; therefore, suitable
column IDs are 2.1 mm, 3.9 mm, and 4.6 mm.
3-2 ___________________________ MSQ Hardware Manual ____________________________
Page 53

LC/MS and the Cone Wash
____________________________________________________________________LC/MS Considerations
HPLC Solvents and Mobile Phase Additives
The following section is a guide for the choice of solvent and mobile phase
additives to use. The choice of solvents for LC will be dictated primarily by
the separation requirements, but there are some guidelines that need to be
followed. These guidelines take the form of selected examples, which have
been divided into three categories: most compatible, least suitable, and other
less common ones. In all cases, degassed solvents are necessary for LC/MS
operation. Sonication, helium sparging, or vacuum membrane degassing
achieves this. Helium sparging and vacuum membrane degassing are the
more efficient techniques.
Most Compatible Solvents
Most compatible solvents are:
Water •
• • Acetonitrile
Methanol
These common reverse phase LC solvents are ideal for LC/MS. When using
high percentages of water, the probe temperature usually needs to be raised
to aid desolvation in the source.
Most Compatible Additives
The most compatible additives are:
Acetic acid or formic acid •
LC separations can be enhanced by reducing the pH of the mobile phase.
Suitable additives for this are acetic acid or formic acid. (Formic acid is
stronger than acetic acid and therefore, less needs to be added to reach a
required pH.) Addition of acids can suppress ionization in negative ion
analysis and weakly acidic compounds may not form [M-H]- ions in acidic
conditions.
•
Ammonium hydroxide
Ammonium hydroxide (ammonia solution) is suitable for increasing the pH
of the mobile phase, which can enhance LC separations. When analyzing
weakly acidic compounds, in negative ion mode, it is unlikely that there will
be any suppression of ionization.
____________________________MSQ Hardware Manual ____________________________ 3-3
Page 54

LC/MS and the Cone Wash
LC/MS Considerations ____________________________________________________________________
Ammonium acetate or ammonium formate •
These volatile salts are often used to buffer mobile phases. Use as little
ammonium acetate or ammonium formate as possible, keeping the
concentration below 100 mM. Ensure that the cone wash is running when
using high concentrations.
•
Non-volatile salts
When using non-volatile salts, ensure that the cone wash is running as they
can crystallize in the source, block the entrance cone, and prevent the mass
spectrometer from functioning. The most common non-volatile salts used
are phosphates.
•
Ion pairing agents
Ensure that the cone wash is running when using ion-pairing agents (for
example, sodium octanesulfonic acid). Many ion-pairing agents suppress
electrospray ionization.
Least Suitable Additives
Least suitable additives are surface-active agents/detergents.
These can suppress the ionization of other compounds. Detergents, by their
very nature, are concentrated at the surface of a liquid. This causes problems
with electrospray, as the ionization relies on the evaporation of ions from the
surface of a droplet. The detergent therefore suppresses the evaporation of
other ions. Use surfactants only when they are being analyzed themselves,
not as additives to HPLC mobile phases.
Other Solvents
Other solvents are:
Normal phase solvents •
Normal phase solvents such as dichloromethane, hexane, and toluene are
most suitable for use in APCI.
•
Propan-2-ol (IPA), 2-methoxyethanol, ethanol, and so on
These have all been used with LC/MS, but their use tends to be applicationspecific.
•
Dimethyl sulfoxide (DMSO)
This solvent is commonly used by synthetic chemists for primary dilution.
3-4 ___________________________ MSQ Hardware Manual ____________________________
Page 55

LC/MS and the Cone Wash
____________________________________________________________________LC/MS Considerations
Other Additives
•
Trifluoroacetic acid (TFA)
This is frequently used for peptide and protein analysis. High levels,
>0.1% v/v, can cause suppression of sensitivity in positive ion mode. TFA
may completely suppress ionization in negative ion mode.
•
Triethylamine (TEA)
This may suppress the ionization of less basic compounds in positive ion
mode (as it also is readily ionized to give a [M+H]
+
ion at m/z 102). TEA
enhances ionization of other compounds in negative ion mode because it is
basic. This is a particularly useful additive for the analysis of nucleic acids.
•
Tetrahydrofuran (THF)
In ESI, use of THF can reduce sensitivity. This effect can be counteracted
by post-column addition of ammonium acetate. It has no effect in APCI.
Caution. Do not use a concentration of THF greater than 5% with PEEK
tubing. THF causes swelling in the PEEK tubing and consequently presents
a risk of the LC tubing bursting.
• Inorganic acids
Inorganic acids (for example, sulfuric acid or phosphoric acid) can be used.
Check the suitability of the LC column to low pHs.
Caution. After using phosphoric acid, thoroughly clean the source, source
enclosure, and hexapole RF lens to minimize the physical damage.
____________________________MSQ Hardware Manual ____________________________ 3-5
Page 56

LC/MS and the Cone Wash
LC/MS Considerations ____________________________________________________________________
Summary of Additives
Table 3-2. Summary of additive use
Avoid
Use
Positive ion Trifluoroacetic acid (TFA) (>0.1% v/v),
surfactants.
Negative ion Surfactants, organic acids; for example,
acetic acid, formic acid, trifluoroacetic
acid (TFA).
Positive ion Acetic acid, formic acid, ammonium
acetate (<0.1M).
Negative ion Triethylamine (TEA), ammonium
hydroxide (ammonia solution),
ammonium acetate (<0.1M).
3-6 ___________________________ MSQ Hardware Manual ____________________________
Page 57

LC/MS and the Cone Wash
________________________________________________________________ Setting Up the Cone Wash
Setting Up the Cone Wash
Use the following information in conjunction with the setup and
maintenance animations on the MSQ CD shipped with the instrument.
Note. It is necessary to use the cone wash only for dirty matrices or with
involatile buffers. Choose the cone wash solvent to give the most effective
solubility for the expected contaminants.
A pump (such as the optional AXP-MS pump) delivers solvent to the cone
wash. The PEEK tubing (green stripe is recommended) is fed through the
door and attached to a PEEK fitting which screws into the instrument on the
right of the source block cover (see Figure 3-1). The recommended flow rate
is 100 µl/min with 50:50 methanol:water.
From LC pump
to cone wash
Figure 3-1. Cone wash solvent delivery
Turn the cone wash nozzle counterclockwise until the tip of the nozzle just
touches the top of the entrance cone, and then turn on the LC pump.
____________________________MSQ Hardware Manual ____________________________ 3-7
Page 58

LC/MS and the Cone Wash
Setting Up the Cone Wash_________________________________________________________________
Figure 3-2. Cone wash nozzle in the on position
Caution. Do not leave the cone wash running when the source heater is
turned off; this could lead to cone wash solvents condensing on the RF
lens.
3-8 ___________________________ MSQ Hardware Manual ____________________________
Page 59

LC/MS and the Cone Wash
___________________________________________________________________________ Flow Splitting
Flow Splitting
Due to the MSQ’s source design, flow splitting of the LC eluent is not
usually required. However, if hyphenated detection is required, flow
splitting can be achieved in the following way.
Zero dead volume
T-piece
HPLC column
PEEK LC tubing
PTFE sleeve
Fused silica
to waste or UV
Figure 3-7. Schematic of a split
Fused silica
to insert
and source
PTFE sleeve
A simple and effective way to make a post-column split for use with the
MSQ is shown in Figure 3-7.
1.
Connect a zero dead volume T-piece to the exit of the column, using the
normal PEEK or stainless steel LC tubing (PEEK tubing is used in the
figure).
2.
Connect one of the exits of the T-piece to the source enclosure, using
narrow bore PEEK tubing or, as shown in the diagram, fused silica. Use
a PTFE, or orange stripe PEEK tubing, sleeve to secure the fused silica
into the T-piece.
3.
Connect a length of the same tubing to the other exit (the split stream).
The amount of liquid directed through the split stream is determined by the
backpressure exerted at this exit, and hence by the internal diameter and the
length of the tubing attached. As a general rule, the longer the piece of
tubing attached to the split, the greater the flow to the source and the smaller
the split. To reduce the flow to the source and increase the split, shorten the
length of tubing at the split stream exit.
There are a number of ways to measure the amount of liquid flowing into
the source and hence measure the split ratio. Two methods are provided
here; one gives a rough estimate and the other a more accurate figure.
____________________________MSQ Hardware Manual ____________________________ 3-9
Page 60

LC/MS and the Cone Wash
Flow Splitting ___________________________________________________________________________
To achieve a rough estimate of the flow rate into the source:
1.
Remove the probe from the source enclosure.
2.
Connect the probe directly to the column outlet and set the flow rate of
the LC pump to the desired flow rate into the source; for example,
0.2 mL/min.
3.
Count the drops that fall off the end of the insert in one minute. Make a
note of this figure.
Connect the split as shown in Figure 3-7.
4.
Now set the LC pump to the flow rate that is required through the
5.
column, for example, 1.0 mL/min.
Count the drops that fall off the end of the insert in one minute.
6.
If the split is set up correctly (in this case, to give a split ratio of 4:1), then
the number of drops recorded in Step 6 will be the same as that in Step 3. If
the number of drops is greater in Step 6, shorten the length of the tubing
connected to the waste (or UV detector) stream. If there are fewer drops in
Step 6, then a longer length of tubing is required. Continue this until the
number of drops in Step 6 is the same as that recorded in Step 3.
Note. There is no need to measure the split ratio accurately. Even
significant changes in split ratio have only a minimal effect on
chromatographic peak shape.
To accurately measure the split and therefore know the exact flow rate into
the source, use the following method:
1.
Remove the probe from the source enclosure.
2.
Connect the split to the probe and LC pump as shown in Figure 3-7.
3.
Set the flow rate of the pump to the flow required through the column;
for example, 1.0 mL/min.
Collect and weigh the liquid that emerges from the insert in one minute.
4.
Follow the same procedure with the liquid that emerges from the waste
5.
(or UV detector) stream.
Calculate the ratio of the two masses of the liquid to give the split ratio,
6.
and hence calculate the exact flow rate into the source.
For example, if 200 mg emerges from the insert and 800 mg from the waste
stream in one minute, then the split ratio is 4:1 and the flow rate into the
source can be calculated as 0.2 mL/min.
3-10 __________________________ MSQ Hardware Manual ____________________________
Page 61

Chapter 4
Reference Inlet System 4.
Reference Inlet System ..............................................................................................................4-i
Introduction ....................................................................................................................................4-1
Overview ........................................................................................................................................4-2
Setting Up the Reference Inlet System...........................................................................................4-3
____________________________MSQ Hardware Manual _____________________________ 4-i
Page 62

Reference Inlet System
Introduction ____________________________________________________________________________
4-ii ____________________________ MSQ Hardware Manual ____________________________
Page 63

Reference Inlet System
____________________________________________________________________________ Introduction
Introduction
The MSQ’s built-in reference inlet system allows easy calibration and
tuning.
This chapter provides instructions on how to set up the reference inlet
system.
____________________________MSQ Hardware Manual ____________________________
4-1
Page 64

Reference Inlet System
Overview ______________________________________________________________________________
Overview
The recommended way to introduce a sample for tuning and mass
calibration is to use the reference inlet system. This method provides a
steady flow of sample directly into the source (typically at flow rates of
<50 µL/min).
Note. Calibration should be performed in positive electrospray mode.
4-2 ___________________________ MSQ Hardware Manual ____________________________
Page 65

Reference Inlet System
_______________________________________________________ Setting Up the Reference Inlet System
Setting Up the Reference Inlet System
Use the following information in conjunction with the setup and
maintenance animations on the MSQ CD shipped with the instrument.
These instructions show how to configure the reference inlet system to
introduce tuning and mass calibration samples into the source. They assume
that the source is set up for electrospray ionization. If your source is not set
up for ESI, refer to the chapter Changing Ionization Modes.
1. Ensure that there is calibrant in the calibration reference bottle. If not,
unscrew the calibration reference bottle (P/N FM102771), fill it with
calibrant, and screw it back into place.
Figure 4-2. Calibration reference bottle
2. Check the waste bottle (P/N FM102770). If it is full, unscrew it, empty
it, and then screw it back into place.
Figure 4-2. Waste bottle
3.
Check that liquid is emerging into the waste bottle.
4.
Ideally, the instrument should already be in operate with the gas flow
on. If it is not, the software will turn on the gas and put the instrument
into operate.
5.
Click, with the right mouse button, on the server icon and select
Instrument Tune and Calibration… from the menu displayed. The
Instrument Tuning and Calibration dialog box is displayed.
____________________________MSQ Hardware Manual ____________________________
4-3
Page 66

Reference Inlet System
Setting Up the Reference Inlet System _______________________________________________________
Figure 4-2. Instrument Tuning and Calibration dialog box
6. Select Full System Autotune and click the Next button. A complete
system tune will be performed, followed by a mass scale calibration. A
series of messages will be displayed, informing you of the progress. To
print a report, click the Print Report button.
Note. Allow time for the probe heater to warm up and stabilize at the
required temperature before beginning autotune and calibration.
4-4 ___________________________ MSQ Hardware Manual ____________________________
Page 67

Reference Inlet System
___________________________________________________________ Standard Mass Scale Calibration
Standard Mass Scale Calibration
If there is a significant change in the observed masses, you may wish to
perform a standard mass scale calibration.
1. Ensure that there is calibrant in the calibration reference bottle. If not,
unscrew the calibration reference bottle (P/N FM102771), fill it with
calibrant, and screw it back into place.
Figure 4-2. Calibration reference bottle
2. Check the waste bottle (P/N FM102770). If it is full, unscrew it, empty
it, and then screw it back into place.
Figure 4-2. Waste bottle
3.
Check that liquid is emerging into the waste bottle.
4.
Ideally, the instrument should already be in operate with the gas flow
on. If it is not, the software will turn on the gas and put the instrument
into operate.
5.
Click, with the right mouse button, on the server icon and select
Instrument Tune and Calibration… from the menu displayed. The
Instrument Tuning and Calibration dialog box is displayed.
____________________________MSQ Hardware Manual ____________________________
4-5
Page 68

Reference Inlet System
Standard Mass Scale Calibration____________________________________________________________
Figure 4-2. Instrument Tuning and Calibration dialog box
6. Select Standard Mass Scale Calibration and click the Next button. A
series of messages will be displayed, informing you of the progress. To
print a report, click the Print Report button.
Note. Allow time for the probe heater to warm up and stabilize at the
required temperature before beginning autotune and calibration.
4-6 ___________________________ MSQ Hardware Manual ____________________________
Page 69

Chapter 5
5. Routine and Preventive Maintenance
Routine and Preventive Maintenance ..................................................................................... 5-i
Introduction.................................................................................................................................... 5-1
Maintenance Schedule ................................................................................................................... 5-2
The Electrospray and APCI Probes ............................................................................................... 5-3
Flushing the Capillaries .................................................................................................... 5-3
ESI Probe Removal........................................................................................................... 5-4
ESI Probe Disassembly..................................................................................................... 5-5
Cleaning the ESI Probe..................................................................................................... 5-6
Replacing the Capillary..................................................................................................... 5-6
ESI Probe Assembly ......................................................................................................... 5-7
APCI Probe Removal........................................................................................................ 5-8
APCI Probe Disassembly.................................................................................................. 5-9
Cleaning the APCI Probe.................................................................................................. 5-9
Replacing the Capillary................................................................................................... 5-10
APCI Probe Assembly .................................................................................................... 5-10
The Source Block Assembly........................................................................................................ 5-11
Cleaning the Entrance Cone............................................................................................ 5-11
Cleaning the Cone Wash Nozzle .................................................................................... 5-13
Cleaning the Source Block Assembly............................................................................. 5-13
The Heater.................................................................................................................................... 5-21
Heater Removal .............................................................................................................. 5-21
Cleaning the Heater......................................................................................................... 5-21
Heater Replacement........................................................................................................ 5-22
The Rotary Pump ......................................................................................................................... 5-23
Checking the Rotary Pump Oil Level and Color ............................................................ 5-23
Changing the Rotary Pump Oil....................................................................................... 5-25
___________________________ MSQ Hardware Manual_____________________________ 5-i
Page 70

Routine and Preventive Maintenance
Introduction ____________________________________________________________________________
5-ii____________________________ MSQ Hardware Manual ____________________________
Page 71

Routine and Preventive Maintenance
____________________________________________________________________________ Introduction
Introduction
The MSQ has been designed to be a low maintenance instrument. Apart
from fairly light periodic preventive maintenance, the MSQ requires only
simple source cleaning and inspection on a “loss of performance” basis.
This chapter contains details of how to perform all the user maintenance
tasks on the MSQ, both routine and as required. Use this information in
conjunction with the setup and maintenance animations on the MSQ CD
shipped with the instrument.
The chapter begins with a maintenance schedule that indicates how often
you should perform each routine maintenance task.
The chapter then describes maintenance of the following parts of the MSQ:
The electrospray and APCI inserts •
•
The source block
•
The RF lens
•
The heater
•
The rotary pump
____________________________MSQ Hardware Manual ____________________________
5-1
Page 72

Routine and Preventive Maintenance
Maintenance Schedule____________________________________________________________________
Maintenance Schedule
Table 5-1 is a list of routine maintenance procedures that should be carried
out on the MSQ at the intervals specified.
The maintenance schedule provides only a rough guide to the frequency of
maintenance tasks. The appropriate frequency depends on instrument usage
and the level of system-induced contamination from samples and matrices.
Table 5-1. Maintenance schedule
Frequency Action
Weekly Clean the source if a drop in sensitivity is seen during
analysis, or if there is a drop in sensitivity when
performing the sensitivity verification test (refer to the
chapter Preparing for Daily Operation in the
MSQ Getting Started manual); see page 5-12.
Clean and flush the capillaries; see page 5-4.
Every 3-6
months
Clean the RF lens; see page 5-16.
Every 6
months
Check the oil level and color in the rotary pump and
add oil if necessary; see page 5-23.
Replace the rotary pump oil; see page Error!
Bookmark not defined..
Refer to the PC user guide supplied for cleaning the PC fan filters.
5-2 ___________________________ MSQ Hardware Manual ____________________________
Page 73

Routine and Preventive Maintenance
__________________________________________________________The Electrospray and APCI Probes
The Electrospray and APCI Probes
To extend their lifetime, regularly flush both the ESI and APCI probes with
solvent, especially after prolonged use with buffers. The probes can also be
stripped down and cleaned. Replace the capillaries if they become blocked.
Flushing the Capillaries
To prevent blockage, flush the capillaries with [50:50] acetonitrile:water or
methanol:water after use with phosphates, ion-pairing agents, acids, or other
additives. The probe does not need to be disassembled for this procedure,
but it should be removed from the source block enclosure. Remove the
probe from the source block, attach the probe to the pump (with the column
removed), and flush the probe.
Note. To prevent blockage, flush each capillary after using buffers.
____________________________MSQ Hardware Manual ____________________________
5-3
Page 74

Routine and Preventive Maintenance
The Electrospray and APCI Probes __________________________________________________________
ESI Probe Removal
WARNING. Allow the source block and probe heater assembly to cool
before changing ionization modes.
1.
Unscrew and remove the PEEK finger-tight fitting from the ESI probe
assembly (P/N FM102595).
2.
Turn the locking plate clockwise to the open position and remove the
ESI probe assembly (P/N FM102595).
PEEK fitting
Figure 5-5. ESI probe assembly
Locking plate
5-4 ___________________________ MSQ Hardware Manual ____________________________
Page 75

Routine and Preventive Maintenance
__________________________________________________________The Electrospray and APCI Probes
ESI Probe Disassembly
The starting point for this procedure is the ESI probe removed from the
instrument.
1.
Using the 2.5 mm Allen key, unscrew the M3 x 10 cap head stainless
steel screw (P/N FM103046).
2.
Remove the probe clamp and locking plate.
3.
Using the 10 mm spanner, unscrew the capillary retaining nut.
4.
Remove the graphite ferrule (P/N 6070119), PEEK insert (P/N
FM102591), and ESI probe capillary (P/N FM102698).
Caution. Exercise care when handling the ESI probe capillary because it is
fragile and may be damaged easily.
5.
Using the 2.5 mm Allen key, unscrew the two M3 x 8 cap head stainless
steel screws (P/N 5313020).
6.
Remove the ESI probe mount and O-rings.
7.
Remove the ESI ceramic sleeve (P/N FM103394) from the ESI ceramic
probe assembly.
____________________________MSQ Hardware Manual ____________________________
5-5
Page 76

Routine and Preventive Maintenance
The Electrospray and APCI Probes __________________________________________________________
Cleaning the ESI Probe
The ESI probe can be stripped down for thorough cleaning after prolonged
use with additives.
1.
Wipe the surface of the stainless steel insert capillary with [50:50]
methanol:water.
2.
Sonicate the ceramic sleeve in concentrated nitric acid. Then boil it in
distilled water for 5 to 10 minutes.
WARNING. Exercise extreme caution when using concentrated nitric
acid. Always wear protective clothing and use only in a fume hood.
Observe appropriate disposal requirements when discarding the used acid.
Replacing the Capillary
Replace the capillary if it has become blocked or partially blocked during
operation. A significant increase in LC pump backpressure (that is, up to
300 psig at 1 mL/min added to the total LC system backpressure) or
instability in the signal could be symptoms of a partially blocked capillary.
5-6 ___________________________ MSQ Hardware Manual ____________________________
Page 77

Routine and Preventive Maintenance
__________________________________________________________The Electrospray and APCI Probes
ESI Probe Assembly
The starting point for this procedure is a disassembled ESI probe.
1.
Insert the ESI ceramic sleeve (P/N FM103394) into the ESI ceramic
probe assembly.
2.
Place the O-rings in position and insert the ESI probe mount.
3.
Screw in the two M3 x 8 cap head stainless steel screws (P/N 5313020)
and tighten, using the 2.5 mm Allen key.
4.
Insert the ESI probe capillary (P/N FM102698), graphite ferrule (P/N
6070119), and PEEK insert (P/N FM102591).
Caution. Exercise care when handling the ESI probe capillary because it is
fragile and may be damaged easily.
5.
Screw in the capillary retaining nut until finger-tight.
6.
Use the setting fixture depth plate to align the end of the ESI probe
capillary and tighten the capillary retaining nut, using the 10 mm
spanner.
7.
Place the locking plate and probe clamp in position.
8.
Screw in the M3 x 10 cap head stainless steel screw (P/N FM103046)
and tighten, using the 2.5 mm Allen key.
____________________________MSQ Hardware Manual ____________________________
5-7
Page 78

Routine and Preventive Maintenance
The Electrospray and APCI Probes __________________________________________________________
APCI Probe Removal
WARNING. Allow the source block and probe heater assembly to cool
before changing ionization modes.
1.
Unscrew and remove the PEEK finger-tight fitting from the APCI probe
assembly (P/N FM102587).
2.
Turn the locking plate clockwise to the open position and remove the
APCI probe assembly (P/N FM102587).
PEEK fitting
Figure 5-5. APCI probe assembly
Locking plate
5-8 ___________________________ MSQ Hardware Manual ____________________________
Page 79

Routine and Preventive Maintenance
__________________________________________________________The Electrospray and APCI Probes
APCI Probe Disassembly
The starting point for this procedure is the APCI probe removed from the
instrument.
1.
Using the 2.5 mm Allen key, unscrew the M3 x 10 cap head stainless
steel screw (P/N FM103046).
2.
Remove the probe clamp and locking plate.
3.
Using the 10 mm spanner, unscrew the capillary retaining nut.
4.
Remove the graphite ferrule (P/N 6070119), PEEK insert (P/N
FM102591), and APCI capillary tube (P/N FM102594).
Caution. Exercise care when handling the APCI probe capillary because it
is fragile and may be damaged easily.
5.
Using the 2.5 mm Allen key, unscrew the two M3 x 8 cap head stainless
steel screws (P/N 5313020).
6.
Remove the APCI probe mount and O-rings.
Cleaning the APCI Probe
The APCI probe can be stripped down for thorough cleaning after prolonged
use with additives.
1. Wipe the surface of the stainless steel insert capillary with [50:50]
methanol:water.
____________________________MSQ Hardware Manual ____________________________
5-9
Page 80

Routine and Preventive Maintenance
The Electrospray and APCI Probes __________________________________________________________
Replacing the Capillary
Replace the capillary if it has become blocked or partially blocked during
operation. A significant increase in LC pump backpressure (that is, up to
300 psig at 1 mL/min added to the total LC system backpressure) or
instability in the signal could be symptoms of a partially blocked capillary.
APCI Probe Assembly
The starting point for this procedure is a disassembled APCI probe.
1.
Place the O-rings in position and insert the APCI probe mount.
2.
Screw in the two M3 x 8 cap head stainless steel screws (P/N 5313020)
and tighten, using the 2.5 mm Allen key.
3.
Insert the APCI capillary tube (P/N FM102594), graphite ferrule (P/N
6070119), and PEEK insert (P/N FM102591).
Caution. Exercise care when handling the APCI probe capillary because it
is fragile and may be damaged easily.
4.
Screw in the capillary retaining nut until finger-tight.
5.
Use the setting fixture depth plate to align the end of the APCI probe
capillary and tighten the capillary retaining nut, using the 10 mm
spanner.
6.
Place the locking plate and probe clamp in position.
7.
Screw in the M3 x 10 cap head stainless steel screw (P/N FM103046)
and tighten, using the 2.5 mm Allen key.
5-10 __________________________ MSQ Hardware Manual ____________________________
Page 81

Routine and Preventive Maintenance
A
_______________________________________________________________ The Source Block Assembly
The Source Block Assembly
The source block assembly can be removed from the instrument (see page 5-
12) and disassembled into its component parts for cleaning. Typically, the
entrance cone needs to be cleaned most frequently because it becomes dirty
with use over a long period. The cone can be reached without the need to
remove the source block from the instrument.
WARNING. Allow the source block and probe heater assembly to cool
before carrying out any maintenance.
Cleaning the Entrance Cone
Probe
Figure 5-4. Exposing the entrance cone
1.
Vent the instrument if it is currently under vacuum. Right-click on the
server and choose Vent.
2.
Remove the PEEK fitting from the APCI or ESI probe assembly, turn
the locking plate clockwise into the open position, and remove the probe
assembly. See ESI Probe Removal or APCI Probe Removal.
3.
If the cone wash is in use, switch the cone wash pump off and turn the
cone wash nozzle until it is clear of the entrance cone.
4.
If in APCI mode, change the APCI corona pin with the APCI blank plug
(located in the source enclosure door).
____________________________MSQ Hardware Manual ___________________________
PCI corona pin Cone wash nozzle
5-11
Page 82

Routine and Preventive Maintenance
The Source Block Assembly _______________________________________________________________
5.
Turn the entrance cone assembly (P/N FM103412) clockwise and pull
forward to remove it.
6.
Using a 2.5 mm screwdriver, remove the entrance cone O-ring
(P/N FM100231).
Caution. Exercise great care when handling the entrance cone. Always
store with the cone facing upwards.
Note. To prevent the cone being damaged, it is recommended that you
place a clean lint-free cloth in the beaker and use tweezers to handle the
entrance cone.
7. Sonicate the cone, first in a 10% v/v solution of formic acid and then in
methanol.
Caution. Do not sonicate the O-ring, as the solvent and acid may damage
them.
8.
Replace the O-ring.
9.
For heavy buildup that is not removed by the steps above, use the 12
micron lapping paper.
10.
Take a small piece of the lapping paper and fold over, but do not crease
it to a point. This prevents too much pressure being applied to the cone
orifice.
11.
Rub the outside of the cone with the lapping paper until the buildup has
been removed.
12.
Replace the entrance cone assembly and turn counterclockwise until
locked in place.
13.
Reinsert the probe and PEEK fitting.
5-12 __________________________ MSQ Hardware Manual ____________________________
Page 83

Routine and Preventive Maintenance
_______________________________________________________________ The Source Block Assembly
Cleaning the Cone Wash Nozzle
The cone wash nozzle (P/N FM102521) should require cleaning only if it
becomes blocked. When the pump supplying the cone wash with solvent is
switched on, check the backpressure; if it rises, this could indicate a
blockage in the nozzle.
1.
Remove the entrance cone. See Cleaning the Entrance Cone on page 5-
11.
2.
Remove the cone wash nozzle and sonicate, first in a 10% v/v solution
of formic acid and then in methanol.
3.
Replace the nozzle and entrance cone.
Cleaning the Source Block Assembly
It is good practice to clean the entire source block assembly on a weekly
basis, particularly if complex sample matrices or chromatographic buffers
are routinely used.
Carry out the procedure for removing the source block assembly and then
the procedure for disassembling and cleaning the source block if the source
has become completely blocked.
From the animations main menu, choose RF Lens Disassembly, Cleaning,
and Assembly. Follow the procedure for RF Lens Removal and then RF
Lens Disassembly, or follow the Full RF Lens Cleaning Procedure.
Removing the Source Block Assembly
The starting point for this procedure is the source setup for ESI or APCI
operation, with the LC and gas flows off, and the probe cooled.
WARNING. Allow the source block and probe heater assembly to cool
before carrying out any maintenance.
1.
Vent the instrument if it is currently under vacuum. Right-click on the
server and choose Vent.
2.
Unscrew and remove the PEEK finger-tight fitting from the ESI probe
assembly (P/N FM102595) or APCI probe assembly (P/N FM102587).
3.
Turn the locking plate clockwise to the open position and remove the
ESI probe assembly (P/N FM102595) or APCI probe assembly (P/N
FM102587).
____________________________MSQ Hardware Manual ___________________________
5-13
Page 84

Routine and Preventive Maintenance
The Source Block Assembly _______________________________________________________________
PEEK fitting
Locking plate
Figure 5-5. ESI probe assembly and source block enclosure
4.
If in APCI mode, change the APCI corona pin (P/N FM101433) with
APCI blank plug
Entrance cone
Cone wash nozzle
the APCI blank plug (P/N FM101437), located in the holder in the
source enclosure door.
5.
If the cone wash is in use, switch off the cone wash pump and turn the
cone wash nozzle until it is clear of the entrance cone.
6.
Turn the entrance cone assembly (P/N FM103412) clockwise and pull
forward to remove it.
7.
Remove the cone wash nozzle (P/N FM102521).
8.
Loosen the thumbscrews on the source block and pull out the source
block assembly (P/N FM102573).
Source block
Thumbscrews
Figure 5-5. Removing the source block assembly
5-14 __________________________ MSQ Hardware Manual ____________________________
Page 85

Routine and Preventive Maintenance
_______________________________________________________________ The Source Block Assembly
Disassembling the Source Block Assembly
The starting point for this procedure is the source block assembly removed
from the MSQ.
WARNING. Allow the source block and probe heater assembly to cool
before carrying out any maintenance.
Figure 5-6. Source block assembly
1.
Unscrew the three spring screws at the base of the RF lens and remove
the RF lens.
2.
Remove the hexapole screw insulator (P/N FM102248), extraction cone
(P/N FM102263), and extraction cone insulator (P/N FM102264).
3.
Remove the three O-rings: top left O-ring 7 x 3 Viton® degas (P/N
FM102655), bottom left O-ring BS207 Viton degas (P/N FM101417),
and right O-ring BS225 Viton degas (P/N FM103048).
4.
Using the 6 mm screwdriver, unscrew the two sealing plugs
(P/N FM102277) and remove the two O-rings (one at each side of the
source block).
5.
Using the sump lever tool, unscrew the source block sealing plug
(P/N FM101460).
____________________________MSQ Hardware Manual ___________________________
5-15
Page 86

Routine and Preventive Maintenance
The Source Block Assembly _______________________________________________________________
Cleaning the Source Block
1. Sonicate the disassembled component parts (except the O-rings) first in
a 1% v/v solution of formic acid, then in water, and then in methanol.
Caution. Ensure that the parts do not become damaged in this process. If
necessary, sonicate each part separately. In particular, ensure that the cones
are face-up at all times.
Caution. Do not sonicate the O-rings, as the solvent and acid may damage
them.
Cleaning the RF Lens
Under normal working conditions, this procedure needs to be carried out
approximately once every 3 to 6 months. Refer to the Maintenance
Schedule topic on page 5-2.
WARNING. Allow the source block and probe heater assembly to cool
before carrying out any maintenance.
1. Totally immerse the RF lens in a measuring cylinder or beaker of
[50:50] methanol:water and sonicate.
5-16 __________________________ MSQ Hardware Manual ____________________________
Page 87

Routine and Preventive Maintenance
_______________________________________________________________ The Source Block Assembly
Figure 5-7. Cleaning the RF Lens
Caution. To prevent the RF lens from touching the bottom of the vessel
and hence becoming damaged, either suspend it on a wire or place a tissue
at the bottom of the vessel.
2.
If necessary, clean the differential aperture plate with a cotton bud
(Q-tip) soaked in [50:50] methanol:water.
3.
Rinse the RF lens with methanol and dry with nitrogen gas.
____________________________MSQ Hardware Manual ___________________________
5-17
Page 88

Routine and Preventive Maintenance
The Source Block Assembly _______________________________________________________________
Assembling the Source Block Assembly
The starting point for this procedure is the disassembled source block
assembly.
Figure 5-8. Source block assembly
1.
Screw in the source block sealing plug (P/N FM101460). Tighten, using
the sump lever tool.
2.
Insert the sealing plug O-rings and screw in the two sealing plugs
(P/N FM102277), using the 6 mm screwdriver. (There is one plug and
O-ring at each side of the source block.)
3.
Replace the three O-rings: top left O-ring 7 x 3 Viton degas (P/N
FM102655), bottom left O-ring bs207 Viton degas (P/N FM101417),
and right O-ring bs225 Viton degas (P/N FM103048).
4.
Replace the extraction cone insulator (P/N FM102264), extraction cone
(P/N FM102263), and hexapole screw insulator (P/N FM102248).
5.
Replace the RF lens and screw in the three spring screws at the base of
the RF lens.
5-18 __________________________ MSQ Hardware Manual ____________________________
Page 89

Routine and Preventive Maintenance
k
_______________________________________________________________ The Source Block Assembly
Replacing the Source Block Assembly
The starting point for this procedure is the source block assembly removed
from the MSQ.
Source bloc
Thumbscrews
Figure 5-5. Replacing the source block assembly
1.
Push the source block assembly (P/N FM102573) into place in the
source block enclosure and tighten the thumbscrews on the source
block.
2.
Replace the cone wash nozzle (P/N FM102521).
3.
Replace the entrance cone assembly (P/N FM103412) and turn
counterclockwise.
4.
Turn the locking plate clockwise to the open position and insert the ESI
probe assembly (P/N FM102595) or APCI probe assembly (P/N
FM102587).
5.
Turn the locking plate counterclockwise into the closed position.
6.
Screw the PEEK fitting finger-tight into the ESI probe assembly (P/N
FM102595) or APCI probe assembly (P/N FM102587).
____________________________MSQ Hardware Manual ___________________________
5-19
Page 90

Routine and Preventive Maintenance
The Source Block Assembly _______________________________________________________________
PEEK fitting
Locking plate APCI blank plug Entrance cone Cone wash nozzle
Figure 5-5. ESI probe assembly and source block enclosure
Cleaning the Extraction Cone
The starting point for this procedure is the source block assembly removed
from the MSQ and disassembled.
Caution. Exercise great care when handling the extraction cone. Always
store with the cone facing upward.
Note. To prevent the cone being damaged, it is recommended that you
place a clean lint-free cloth in the beaker and use tweezers to handle the
extraction cone.
1. Sonicate the cone, first in a 10% v/v solution of formic acid and then in
methanol.
5-20 __________________________ MSQ Hardware Manual ____________________________
Page 91

Routine and Preventive Maintenance
_____________________________________________________________________________The Heater
The Heater
If the instrument is used primarily in APCI mode, it is recommended that
the heater be cleaned on a monthly basis. If used in ESI mode, the heater
will need cleaning less frequently.
Heater Removal
The starting point for this procedure is the source setup for ESI or APCI
operation, with the LC and gas flows off, and the probe cooled.
WARNING. Allow the source block and probe heater assembly to cool
before performing any maintenance.
1.
Remove the ESI or APCI probe. See ESI Probe Removal or APCI Probe
Removal.
2.
Remove the source block cover.
3.
Disconnect the heater connectors.
4.
Using the 2.5 mm Allen key, unscrew the two M3 x 10 cap head
stainless steel screws (P/N FM103046). Remove the screws and the
screw insulators.
5.
Remove the heater assembly (P/N FM102576).
Cleaning the Heater
1.
Clean the inside of the heater tube with a cotton bud (Q-tip™) soaked in
[50:50] methanol:water.
2.
For heavy contamination, the heater tube can be sonicated in a 1% v/v
solution of formic acid.
Caution. If you are sonicating the heater, you must immerse only the tube
part of the heater. Damage will occur if any other part of the heater comes
into contact with the formic acid solution.
____________________________MSQ Hardware Manual ___________________________
5-21
Page 92

Routine and Preventive Maintenance
The Heater _____________________________________________________________________________
Heater Replacement
The starting point for this procedure is the heater assembly removed from
the MSQ.
1.
Insert the heater assembly (P/N FM102576).
2.
Screw in the M3 x 10 cap head stainless steel screw (P/N FM103046)
and tighten, using the 2.5 mm Allen key
3.
Insert the screw insulators and the two M3 x 10 cap head stainless steel
screws (P/N FM103046). Tighten, using the 2.5 mm Allen key.
4.
Push in the heater connectors.
5.
Replace the source block cove.
6.
Insert the ESI or APCI probe.
5-22 __________________________ MSQ Hardware Manual ____________________________
Page 93

Routine and Preventive Maintenance
_____________________________________________________________________ The Vacuum System
The Vacuum System
The vacuum system consists of two types of vacuum pumps: a
turbomolecular pump and a rotary pump. The turbomolecular pump is
housed within the MSQ and must be serviced only by a trained Dionex
Service Representative. The rotary pump is external to the MSQ and
requires routine maintenance to keep it running at its optimum performance
level.
Maintaining the Fore Pump
The fore pump (the rotary or backing pump) is external to the MSQ and
requires routine maintenance to keep it running at its optimum performance
level. The pumps used for this purpose come from two manufacturers–
Busch and Edwards. The type of pump on the system will depend on when
the instrument was purchased. The Busch pump has external and internal
filters that should be serviced at regular intervals. The Edwards pump does
not have filters that require routine maintenance.
The following maintenance procedures are covered here:
Checking the rotary pump oil level and color •
•
Adding oil to top-up the oil level
•
Changing the rotary pump oil
•
Replacing the external oil filter (Busch pumps only)
Note: More information on operating and maintaining the rotary pump can
be found in the Rotary Pump User Manual, supplied with the MSQ system.
Checking the Rotary Pump Oil Level and Color
Check the rotary pump oil level and color at least once a week.
If the oil level in the rotary pump gets low before the scheduled oil change,
use the procedure in the next section to add oil.
To check the oil:
1.
Look through the oil sight glass at one end of the rotary pump. The oil
level should be between the upper and lower marks positioned next to
the window.
2.
If the oil level is near or below the lower mark, add more oil. Refer to
the procedure for adding oil on page 5-24.
____________________________MSQ Hardware Manual ___________________________
5-23
Page 94

Routine and Preventive Maintenance
The Vacuum System _____________________________________________________________________
If a six-monthly service is due, it may be more convenient to drain and
replace the oil. In this case, refer to the procedure for changing the rotary
pump oil on page 5-25.
Note: If the oil has turned dark in color, it should also be replaced. (Clean
rotary pump oil is a clear straw color [Anderol 555] or almost colorless
[UltraGrade 19].)
Adding Oil
WARNING: Vent the instrument before adding oil. Attempting to add oil
while the pump is running could result in serious personal injury from hot
rotary pump oil.
NOTE: Do not mix oil types
The oil used in the Edwards Rotary Pump is type Ultragrade 19 (P/N
062746 or 062747).
The oil used in the Busch Rotary Pump is type Anderol 555 (P/N 060124
or 060125).
Turn Operate Off and vent the instrument. Refer to the chapter Shutting
3.
Down and Restarting the System for information on turning Operate
Off and venting.
4. Remove the oil filler plug located above the oil sight glass, marked
, from the pump.
5. Pour the oil into the pump until the oil level in the oil sight glass is close
to, but not above, the upper mark.
5-24 __________________________ MSQ Hardware Manual ____________________________
Page 95

Routine and Preventive Maintenance
_____________________________________________________________________ The Vacuum System
oil filter
oil filler plug
oil sight glass
oil drain plug
Figure 5-6. Busch Rotary pump
6. Replace the oil filler plug.
Changing the Rotary Pump Oil
The oil in the rotary pump should be replaced at least once every six
months.
The oil used in the Busch Rotary Pump is Anderol 555 (P/N 061024 and
061025).
The oil used in the Edwards Rotary Pump is type Ultragrade 19 (P/N
062746 or 062747).
WARNING: Wear gloves when changing the oil. Avoid contact with the
pump oil; it may contain dissolved toxic residues from analyzed samples.
Observe appropriate disposal requirements when discarding the used oil.
7.
Turn Operate Off and vent the instrument.
8.
Elevate the rotary pump to gain access to the oil drain plug.
WARNING: The rotary pump is a heavy item and requires at least two
people to lift and move it safely.
9. Place a container under the oil drain plug on the rotary pump (below the
oil sight glass, marked ).
____________________________MSQ Hardware Manual ___________________________
5-25
Page 96

Routine and Preventive Maintenance
The Vacuum System _____________________________________________________________________
10. Remove the oil filler plug located above the oil sight glass, marked
, from the pump.
11.
Remove the drain plug from the pump and allow the old oil to drain out
until it reaches a trickle.
12.
If the oil filter is to be replaced (Busch pump only), remove the old one
(using the type of tool used for automobile filter removal) and fit a new
one (FM103825).
13.
Replace the drain plug.
14.
Fill the pump reservoir up to, but not above, the upper mark.
15.
Replace the filler plug.
16.
Pump down the instrument to prepare for operation.
!
Note: Leave the system pumping for at least half an hour before
beginning operation.
5-26 __________________________ MSQ Hardware Manual ____________________________
Page 97

Chapter 6
Troubleshooting 6.
Troubleshooting .........................................................................................................................6-i
Introduction ....................................................................................................................................6-1
Troubleshooting Help .....................................................................................................................6-2
Resolving Common Problems ........................................................................................................6-3
Checking the MSQ Power Supply Requirements..............................................................6-3
Restarting the PC...............................................................................................................6-3
HPLC System Troubleshooting......................................................................................................6-4
Buffers and Additives........................................................................................................6-4
Mobile Phase Reservoir.....................................................................................................6-4
Bubble Problems and Degassing ....................................................................................... 6-4
Contamination in the LC System.......................................................................................6-5
Tubing and Fittings............................................................................................................6-5
Injection Valves.................................................................................................................6-7
UV Detectors .....................................................................................................................6-7
Preventive Maintenance and Spares ..................................................................................6-9
____________________________MSQ Hardware Manual _____________________________ 6-i
Page 98

Troubleshooting
Introduction ____________________________________________________________________________
6-ii____________________________ MSQ Hardware Manual ____________________________
Page 99

Troubleshooting
____________________________________________________________________________ Introduction
Introduction
This chapter helps you to diagnose and resolve problems that may occur
from time to time with the MSQ LC/MS system.
If you encounter a problem that is not described here, or have a problem that
is not resolved by the remedy suggested, contact your local Dionex Service
Representative for assistance.
This chapter is divided into the following sections:
Troubleshooting Help
•
An introduction to the troubleshooting help system that assists in
identifying the precise nature of the problem and the action to be taken.
• • Resolving Common Problems
This section provides procedures to help remedy some of the more
easily resolved problems you might encounter.
HPLC System Troubleshooting
This section provides a general guide to HPLC system troubleshooting.
____________________________MSQ Hardware Manual ____________________________ 6-1
Page 100

Troubleshooting
Troubleshooting Help _____________________________________________________________________
Troubleshooting Help
The troubleshooting help system is designed to help you identify problems
in the following areas:
General system problems
•
Problems relating to the basic operation of the system; for example,
breaks in power supply.
•
Low sensitivity
Problems relating to low sensitivity. The symptoms are divided into
excessive noise, low signal, and no signal.
•
Inlet system/chromatography
Problems relating to liquid chromatography and the HPLC system.
•
Spectral problems
Problems relating to mass spectral data.
•
Calibration
Problems relating to calibration.
•
Tuning
Problems relating to tuning.
•
Communication
Problems relating to communications among the various parts of the
system and with Xcalibur.
•
Vacuum
Problems relating to the vacuum system.
•
MS resolution
Problems relating to MS resolution.
The troubleshooting help system describes the visible symptoms, the
probable cause of the problem, and suggestions on how to remedy it.
To open the troubleshooting help, double-click on the troubleshooting help
desktop icon.
6-2 ___________________________ MSQ Hardware Manual ____________________________
 Loading...
Loading...