Dionex 063000, 062998, DNAPAC PA200 Product Manual
DNAPac PA200 Document No. 065036 Page 1 of 25
PRODUCT MANUAL
DNAPAC PA200 ANALYTICAL COLUMN
(4 x 250mm, P/N 063000)
DNAPAC PA200 GUARD COLUMN
(4 x 50mm, P/N 062998)
©DIONEX Corporation
Document No. 065036
Revision 01
20 August 2004

DNAPac PA200 Document No. 065036 Page 2 of 25
TABLE OF CONTENTS
SECTION 1 - INTRODUCTION ...................................................................................................................4
1.1 DNAP
1.2 BIOLC S
1.3 G
1.4 DNAPAC PA200 A
1.5 DNAP
AC
PA200 ................................................................................................................................ 4
YSTEM (WITHOUT COLUMNS
UARD COLUMN USE
NION EXCHANGE COLUMNS
AC COLUMN FAMILY
) .............................................................................................. 4
........................................................................................................................ 5
............................................................................... 5
............................................................................................................... 5
SECTION 2 - OPERATION AND SYSTEM REQUIREMENTS ..............................................................6
2.1 S
2.2 S
2.3 DNAPAC PA200 C
YSTEM REQUIREMENTS
YSTEM OPERATION REQUIREMENTS
.................................................................................................................... 6
OLUMN OPERATIONAL PARAMETERS
................................................................................................ 6
................................................................. 7
SECTION 3 - PURITY REQUIREMENTS FOR CHEMICALS............................................................... 8
3.1 D
3.2 I
3.3 S
EIONIZED WATER
NORGANIC CHEMICALS
OLVENTS
.......................................................................................................................................... 8
............................................................................................................................ 8
..................................................................................................................... 8
SECTION 4 - QUALITY ASSURANCE.......................................................................................................9
4.1 C
4.2 P
ERTIFICATE OF PERFORMANCE – RESIN BATCH TESTING
RODUCTION TEST CHROMATOGRAMS
........................................................................................... 10
............................................................... 9
SECTION 5 - METHODS DEVELOPMENT............................................................................................. 11
5.1 S
5.2 E
5.3
AMPLE CLEANUP
LUTION ORDER
E
FFECT OF SALT TYPE ON OLIGONUCLEOTIDE ELUTION
............................................................................................................................ 11
............................................................................................................................... 11
................................................................ 11
5.3.1 Eluent Strength ...................................................................................................................... 11
5.3.2 Loading Capacity................................................................................................................... 11
5.4
G
5.5 E
RADIENT SLOPE
FFECT OF PH AND SOLVENT ON OLIGONUCLEOTIDE CHROMATOGRAPHY
.............................................................................................................................. 12
.................................... 12
5.5.1 Effect of pH on Hydrogen Bond Interactions ........................................................................ 12
5.5.2 Effect of pH on Retention...................................................................................................... 13
5.5.2 Effect of pH on Retention...................................................................................................... 13
5.5.3 Effect of Solvent on Retention............................................................................................... 13
5.5.4 Effect of pH on Selectivity .................................................................................................... 14
5.6 E
5.7
FFECT OF TEMPERATURE ON OLIGONUCLEOTIDE RETENTION
E
FFECT OF TERMINAL BASE ON SELECTIVITY
................................................................................. 16
...................................................... 15
5.7.1 Selectivity in Sodium Chloride (NaCl) Gradients. ................................................................ 16
5.7.2 Selectivity in Sodium Perchlorate (NaClO4) Gradients......................................................... 17
5.8 A
PPLICATION-SPECIFIC MOBILE PHASE RECOMMENDATIONS
........................................................ 18
5.8.1 For synthetic ODNs where the goal is to evaluate purity. ..................................................... 18
5.8.2 When multiple possible ODNs of similar length in the same solution must be resolved...... 18
SECTION 6 - APPLICATIONS................................................................................................................... 19
6.1 D
6.2 E
6.3 P
ENATURING CONDITIONS FOR CONTROL OF SECONDARY STRUCTURE
FFECT OF HIGH TEMPERATURE AND HIGH PH ON COLUMN LIFETIME
HOSPHODIESTER ANALYSIS
........................................................................................................... 20
........................................ 19
.......................................... 19
6.3.1 Sodium Perchlorate Eluent Systems ...................................................................................... 20
6.3.2 Sodium Chloride Eluent Systems .......................................................................................... 21
SECTION 7 - DNAPac™ PA200 RESOURCES.........................................................................................22

DNAPac PA200 Document No. 065036 Page 3 of 25
Section 8 - TROUBLESHOOTING GUIDE ...............................................................................................23
8.1 F
8.2 B
8.3 D
8.4
8.5
8.6
8.7
8.8 C
8.9 P
8.10 S
INDING THE SOURCE OF HIGH SYSTEM BACK PRESSURE
ACKPRESSURE ON COLUMN HAS INCREASED
ECREASING PEAK RETENTION TIMES
D
ECREASING PEAK EFFICIENCY AND RESOLUTION
P
OOR PEAK EFFICIENCY AND RESOLUTION
U
NIDENTIFIED PEAKS APPEAR
D
ECREASED DETECTION SENSITIVITY
OLUMN PROBLEMS
EAK EFFICIENCY AND RESOLUTION ARE DECREASING
YSTEM PROBLEMS
........................................................................................................................ 24
.......................................................................................................................... 24
......................................................................................................... 24
............................................................................................ 23
..................................................................................... 24
............................................................................................. 24
.............................................................. 23
................................................................................ 23
......................................................................... 23
................................................................. 24
8.10.1 High Detection Background Caused by the System .............................................................. 24
8.10.2 No Peaks, Poor Peak Area Reproducibility or Unexpectedly Small Peak Area.................... 25
8.10.3 Incorrect or Variable Retention Times .................................................................................. 25
8.11 C
OLUMN CLEANUP
........................................................................................................................... 25
8.11.1 High Salt Wash to Remove Ionic Components ..................................................................... 25
8.11.2 Organic Solvent Wash to Remove Non-Ionic Components .................................................. 25
DNAPac PA200 Document No. 065036 Page 4 of 25
SECTION 1 - INTRODUCTION
1.1 DNAPac PA200
The DNAPac PA200 is a pellicular anion exchange column designed specifically to provide high-resolution
separations of single stranded nucleic acids. The DNAPac PA200 provides n, n-1 resolution over a wide range of
oligomer lengths and can perform separations under a variety of denaturing conditions:
• High temperature, pH 8 or below
• High pH (12.4) at 30° or below
Because of the unique pH stability of the packing material, elevated pH conditions can be used to optimize
selectivity for specific oligonucleotides.
The packing material inside the DNAPac PA200 is composed of 130 nm quaternary amine functionalized
MicroBeads™ bound to an 8 µm solvent compatible, non-porous substrate. The non-porous substrate design
provides rapid mass transport resulting in narrow high efficiency peaks. The low column capacity, typical of nonporous packings, is avoided by agglomerating functionalized MicroBeads to the surface of the substrate particle,
resulting in higher loading capacity than is possible with conventional non-porous materials, and good durability.
This produces a column with oligonucleotide resolution superior to columns using 2 to 3 µm resins.
Resin Characteristics:
Particle Size: 8 µm
Pore Size: non porous
Cross-linking: 55%
Ion exchange capacity: ~40 µeq/column
Latex Characteristics:
Functional Group: quaternary ammonium ion
Latex Diameter: ~130 nm
Latex Cross-link: 5 %
Typical Operating Parameters:
pH range: 4-10 unrestricted eluents
2.5-4 and 10-12.5, (Operation at these pH values require co-ion concentration to be
at least equimolar with hydroxide at high pH or H
Temperature: ≤85°C
Pressure: 3,000 psi
Organic Solvent Limit: 100% acetonitrile or methanol for cleaning
Typical eluents: High purity water (18.2 megohm-cm), sodium chloride, sodium perchlorate, buffers,
sodium acetate and sodium hydroxide
1.2 BioLC System (without Columns)
+
at low pH)
Table 1: System Components Recommended for DNA Analysis
Basic Gradient System Standard Gradient System
BioLC gradient pump, with degas BioLC gradient pump (degas recommended)
Chromatography oven with injection valve
and regulator assembly
Column Oven
Absorbance detector (D2 lamp for UV) Absorbance detector (D2 lamp for UV)
EO1 eluent organizers EO1 Eluent organizers
Autosampler
DNAPac PA200 Document No. 065036 Page 5 of 25
1.3 Guard Column Use
A guard column is usually placed before the analytical column to prevent contaminants in the sample from eluting
onto the analytical column. The addition of the guard column increases the net column capacity, which translates
into an increase of about 20% in the retention times for isocratic runs. If a guard is added to a system running a
gradient method that was initially developed for an analytical column alone, the analytes will elute slightly later and
usually with slightly better resolution.
1.4 DNAPAC PA200 Anion Exchange Columns
Part Number Product Description
063000 DNAPac PA200, Analytical (4 x 250mm)
062998 DNAPac PA200, Guard (4 x 50mm)
1.5 DNAPac Column Family
There are two varieties of columns in the DNAPac column family. Both columns are non-porous anion exchangers
that provide high-resolution oligonucleotide separations. The choice of column depends upon the goal of the
separation. The DNAPac PA100 consists of a 13 µm substrate particle with 100 nm functionalized MicroBeads.
This column is available in a variety of formats and should be used when higher capacity is required and if scale-up
to semi-preparative scale separations is anticipated.
The DNAPac PA200 consists of an 8 µm substrate particle with 130 nm functionalized MicroBeads. This column
provides higher resolution than the DNAPac PA100. The DNAPac PA200 is operated at a lower flow rate than the
DNAPac PA100, thus less eluent is consumed during a run. In addition, the DNAPac PA200 has been
manufactured to provide greater stability to high pH at elevated temperature, although this combination is not
recommended.
Assistance is available for any problem that may be encountered during the shipment or operation of
DIONEX instrumentation and columns through the DIONEX North America Technical Call Center at 1800-DIONEX-0 (1-800-346-6390) or through any of the DIONEX offices listed in “DIONEX Worldwide
Offices.”
DNAPac PA200 Document No. 065036 Page 6 of 25
SECTION 2 - OPERATION AND SYSTEM REQUIREMENTS
2.1 System Requirements
Oligonucleotide separations with the DNAPac PA200 columns are optimized for use with NON-METALLIC
systems, such as the Dionex BioLC. The key issue is that the eluent flow path from reservoir to detector is metalfree, because the salts used for oligonucleotide elution attack the metallic components of metallic pumps and
tubing. The released metals will irreversibly foul the column.
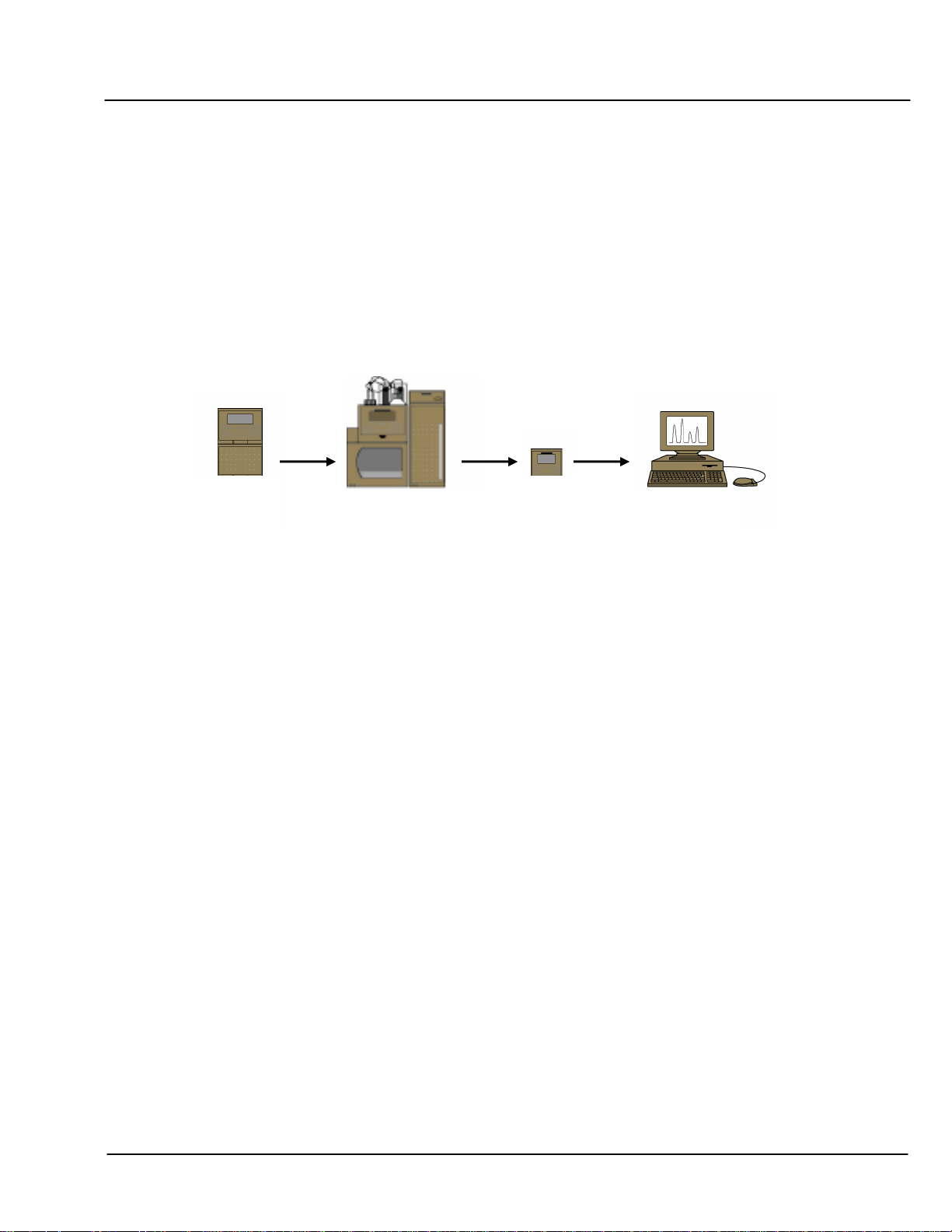
Each of the possible configurations offers multiple sampling options; however, consistently reproducible
quantification and an absence of disturbing artifacts are achieved best using an autosampler and “full loop”
injection mode. Reproducibility of retention time results can be enhanced by regulating the temperature of the
column using a column oven or thermal compartment.
Gradient
Pump
Autosampler
Thermal
Compartment
Absorbance
Detector
Data System
Figure 1 Oligonucleotide System Configuration
2.2 System Operation Requirements
The oligonucleotide analysis systems should be configured with Dionex modules to provide the following
attributes:
a) All components of the fluid path are non-metallic, to eliminate column poisoning.
b) Mobile phase components are kept under helium or nitrogen to minimize out-gassing (bubble formation)
in the detector cell. On-line degassing of eluents may be provided with the eluent degas option on Dionex
pump modules.
c) Accurate reproducible flow and gradient generation at settings between 0.20 and 2.0 mL/min.
d) Minimal contribution to the background signal by contaminants from the system and reagents.
e) Thermostated column compartment for consistent temperature control of the guard and separation
columns.
f) Minimal system volumes (employ low volume unions and minimal tubing length).
• 4-mm operation, liquid line inside diameter (I.D.) should be between .007” and 0.01”.
• 2mm operation, liquid line inside diameter (I.D.) should be between .003” and 0.005”.
In both operations, PEEK tubing is preferred as it does not contribute to metal leaching.
DNAPac PA200 Document No. 065036 Page 7 of 25
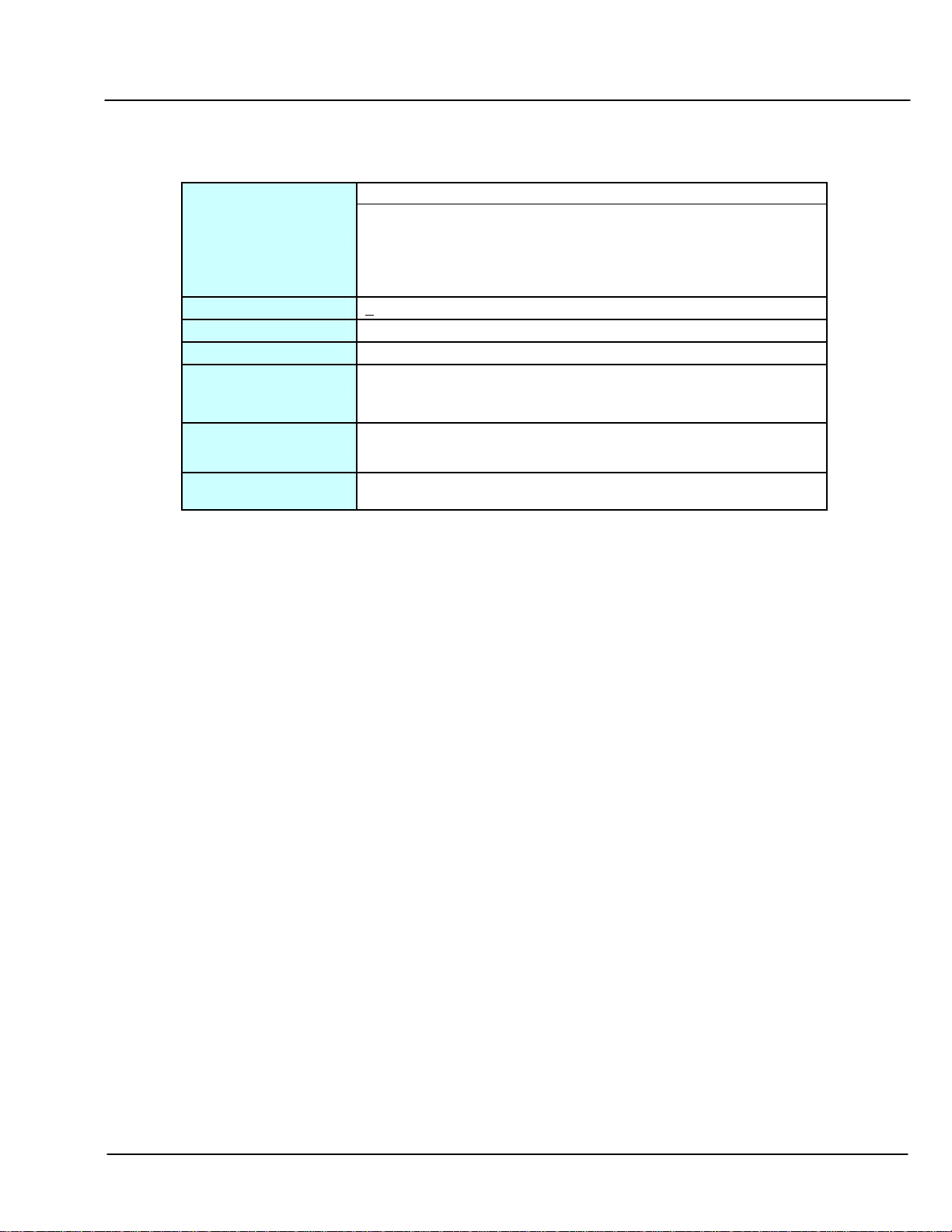
2.3 DNAPAC PA200 Column Operational Parameters
TABLE 2 Column Operational Parameters
pH
= 4-10 (unrestricted eluents)
pH
pH Range:
Temperature Limit:
Pressure Limit:
Organic Solvent Limit:
Chaotrope Limit:
Typical Eluents:
Detergent
Compatibility:
CAUTION:
Do not use anionic detergents. Anionic detergents will bind irreversibly to the column.
= 2.5- 4, and 10-12.5: Operation at these pH values require co-ion
concentration (e.g., Cl
pH) to be at least equimolar with hydroxide at high pH or H
-
or ClO
-
at high pH and Na+ or NH
4
+
at low
4
+
at
low pH.
<
85°C
4,000 psi
100% Acetonitrile, or methanol, if required for cleaning.
30% formamide, 6 M Urea.
Note:
Use of these chaotropes will increase back pressure, and
reduce column lifetime.
High purity water (18 megohm-cm), sodium chloride, sodium
perchlorate, buffers, sodium acetate and sodium hydroxide.
Nonionic, cationic or zwitterionic detergents.

DNAPac PA200 Document No. 065036 Page 8 of 25
SECTION 3 - PURITY REQUIREMENTS FOR CHEMICALS
Reliable and reproducible results require eluents that are prepared consistently and are free from impurities.
3.1 Deionized Water
The de-ionized (DI) water, used to prepare eluents, should be Type I reagent grade water with a specific resistance
of 18 megohm-cm. The water should be free from ionized impurities, organics, microorganisms, and particulate
matter. Ultra Violet (UV) treatment in the water purification unit is recommended. Follow the manufacturer’s
instructions regarding the replacement of ion exchange and adsorbent cartridges. All filters used for water
purification must be free from UV absorbing components. Contaminated water in eluents causes high background
signals, gradient artifacts, and even sample degradation due to nucleases arising from microbial contamination.
3.2 Inorganic Chemicals
Inorganic chemicals of reagent grade or better should be used to prepare ionic eluents. Whenever possible,
inorganic chemicals that meet or surpass the latest American Chemical Society standard for purity should be used.
These products will include detailed lot analyses on their labels.
3.3 Solvents
Solvents can be added to the ionic eluents used in DNAPac PA200 columns to modify the ion exchange process.
The solvents used must be free from ionic impurities; however, since most manufacturers of solvents do not test for
ionic impurities, it is important that the highest grade of solvents available be used. Currently, several
manufacturers are making “ultra high” purity solvents that are compatible with HPLC and spectrophotometric
applications. These “ultra high” purity solvents will usually be of sufficient purity to ensure that your
chromatography is not affected by ionic impurities in the solvent. At Dionex, we have obtained consistent results
using High Purity Solvents manufactured by Burdick and Jackson or Optima Solvents by Fischer Scientific.
When using an ionic eluent with solvent, column generated back pressure will depend on the solvent used, the
concentration of the solvent, the ionic strength of the eluent, and the flow rate applied. The column backpressure
will also vary if the composition of the water-solvent mixture varies. The practical backpressure limit for the
DNAPac PA200 is 4,000 psi (27.6 MPa). The DNAPac PA200 can withstand common HPLC solvents in a
concentration range of 0-100%. Solvents and water should be premixed in concentrations which allow proper
mixing by the gradient pump and to minimize out-gassing. Ensure that all of the inorganic chemicals are soluble in
the highest solvent concentration to be used during the analysis.
Solvent-Water mixtures are usually specified with a volume to volume basis. If a procedure requires an eluent of
90% acetonitrile; prepare the eluent by adding 900 mL of acetonitrile to an eluent reservoir. Then add 100 mL of
deionized water, or eluent concentrate, to the acetonitrile in the reservoir. Using this procedure to mix solvents with
water will ensure that a consistent true volume/volume eluent is obtained. Premixing water with solvent will also
minimize the possibility of out gassing which causes bubble formation in the detector cell. If you choose to mix
eluents containing solvents with those that do not – the eluent degas option for the pump is highly recommended.
As a second choice, pre-degassing the eluents and covering the eluent reservoir with Helium gas to limit gas
dissolution into the eluents will help limit out-gassing.
 Loading...
Loading...