Page 1

Defibtech DDU-100
Semi-Automatic
External Defibrillator
AHA /ERC 2010
User Manual
ELECTRONIC
DISTRIBUTION
DAC-510E-EN-BG
Page 2

Page 3
iii
DAC-510E-EN-BG
Page 4

Notices
Defibtech shall not be liable for errors contained herein or for incidental or
consequential damages in connection with the furnishing, performance, or use of
this material.
Information in this document is subject to change without notice. Names and data
used in the examples are fictitious unless otherwise noted.
Limited Warranty
The “Limited Warranty” shipped with Defibtech AED products serves as the sole
and exclusive warranty provided by Defibtech, LLC with respect to the products
contained herein.
Copyright
Copyright © 2014 Defibtech, LLC.
All rights reserved. Copyright questions should be directed to Defibtech.
For contact information, refer to the “Contacts” section of this manual.
Tracking
U.S.A. federal regulations require Defibtech to maintain records for each AED it
distributes (reference 21 CFR 821, Medical Device Tracking). These requirements
also apply anytime there is a change in the AED’s location, including if you move,
sell, donate, give away, export or even throw it away. We depend on AED owners/
users/holders to contact us when these things happen to ensure the tracking
information remains accurate in the event we need to share important product
notices. If your location is outside the U.S.A., we ask you share your information
for exactly the same reasons. To keep your information up to date, please inform
Defibtech using the information in the “Contacts” section contained in this
document.
iv
DAC-510E-EN-BG
CAUTIO N: Federal law (USA) restricts this device to
sale by or on the order of a physician.
Page 5

Contents
1 Introduction to the DDU-100 Series AED .....................................................1
1.1 Overview ..................................................................................................................1
1.2 The Defibtech DDU-100 AED ................................................................................... 2
1.3 Indications ................................................................................................................ 4
1.4 Contraindications ....................................................................................................4
1.5 Operator Training Requirements ............................................................................ 4
2 Dangers, Warnings and Cautions ..................................................................5
2.1 Shock, Fire Hazard, Explosion ................................................................................5
2.1.1 Electricity ............................................................................................................ 5
2.1.2 Battery Pack ........................................................................................................ 5
2.1.3 Usage Environment ........................................................................................... 6
2.1.4 Defibrillation/Shock Delivery ............................................................................ 6
2.1.5 Maintenance ...................................................................................................... 7
2.2 Improper Device Performance ................................................................................7
2.2.1 Usage Environment ........................................................................................... 7
2.2.2 Pads ....................................................................................................................8
2.2.3 Patient Analysis .................................................................................................. 8
2.2.4 Shock Delivery ................................................................................................... 9
2.2.5 Maintenance ...................................................................................................... 9
2.3 General ................................................................................................................... 10
3 Setting up the DDU-100 AED ......................................................................11
3.1 Overview ................................................................................................................ 11
3.2 Installing the Data Card ........................................................................................ 12
3.3 Installing the Active Status Indicator 9V Battery ...............................................12
3.4 Installing and Removing the Battery Pack ..........................................................13
3.5 Connecting the Pads ............................................................................................. 14
3.6 Performing Manually Initiated Self-Tests ............................................................15
3.7 Storing the DDU-100 AED .....................................................................................15
4 Using the DDU-100 AED .............................................................................17
4.1 Overview ................................................................................................................17
4.2 Checking DDU-100 AED Status ............................................................................18
4.3 Turning on the DDU-100 AED................................................................................18
v
DAC-510E-EN-BG
Page 6

4.4 Preparation .............................................................................................................19
4.4.1 Call for Help ..................................................................................................... 19
4.4.2 Preparing the Patient .......................................................................................19
4.4.3 Opening the Pad Package ............................................................................... 19
4.4.4 Connecting Defibrillation Pads to the DDU-100 AED .................................... 19
4.4.5 Applying Pads to the Patient...........................................................................20
4.4.6 Follow DDU-100 AED Prompts .......................................................................21
4.5 Heart Rhythm Analysis .........................................................................................22
4.6 Delivering the Shock .............................................................................................23
4.7 No Shock Required ................................................................................................25
4.8 Post-Shock CPR .....................................................................................................26
4.9 Post Use Procedures .............................................................................................27
4.10 Operational Environment .....................................................................................27
5 Maintaining and Troubleshooting the DDU-100 AED ..................................29
5.1 Self-Tests ................................................................................................................ 29
5.2 Routine Maintenance ............................................................................................ 30
5.2.1 Checking Active Status Indicator ....................................................................30
5.2.2 Checking the Condition of the Unit and Accessories ....................................31
5.2.3 Running a Manually Initiated Self-Test .......................................................... 32
5.2.4 Replacing Pads ................................................................................................32
5.2.5 Checking Pad and Battery Pack Expiration Dates ..........................................33
5.2.6 Checking the DDC If One Was Installed ..........................................................33
5.3 Replacing the Lithium 9V ASI Battery .................................................................34
5.4 Cleaning .................................................................................................................35
5.5 Storage ...................................................................................................................35
5.6 Operator’s Checklist .............................................................................................. 36
5.7 Troubleshooting ..................................................................................................... 37
5.8 Repair .....................................................................................................................39
vi
DAC-510E-EN-BG
6 DDU-100 AED Accessories .........................................................................41
6.1 Defibrillation/Monitoring Pads .............................................................................41
6.2 Battery Packs .........................................................................................................41
6.2.1 Battery Pack Active Status Indicator ...............................................................42
6.2.2 Active Status Indicator Battery .......................................................................42
6.3 Data Cards .............................................................................................................. 42
6.4 Recycling Information ...........................................................................................43
6.4.1 Recycling Assistance ....................................................................................... 43
6.4.2 Preparation .......................................................................................................43
6.4.3 Packaging .........................................................................................................43
6.4.4 Notice to European Union Customers ..........................................................44
Page 7

7 Event Viewing .............................................................................................45
7.1 Defibtech Data Cards ............................................................................................45
7.2 Downloading the Internal Data Log .....................................................................46
8 Technical Specifications .............................................................................47
8.1 Defibtech DDU-100 AED ........................................................................................47
8.1.1 Physical.............................................................................................................47
8.1.2 Environmental..................................................................................................47
8.1.3 Defibrillator ......................................................................................................48
8.1.4 Waveform Specifications ................................................................................48
8.1.5 Patient Analysis System .................................................................................. 49
8.1.5.1 Shockable Rhythm Criteria ......................................................................50
8.1.5.2 Patient Analysis System Performance .....................................................51
8.1.6 Clinical Summary ............................................................................................51
8.1.6.1 Background..............................................................................................51
8.1.6.2 Methods .................................................................................................. 52
8.1.6.3 Results ....................................................................................................52
8.1.6.4 Conclusion ..............................................................................................52
8.1.7. Guidance and Manufacturer’s Declaration –
Electromagnetic Emissions and Immunity ....................................................53
8.2 Battery Packs .........................................................................................................56
8.2.1 High-Capacity Lithium Battery Pack ...............................................................56
8.2.2 Standard Lithium Battery Pack .......................................................................56
8.3 Self-Adhesive Defibrillation/Monitoring Pads ....................................................57
8.4 Defibtech Data Cards (DDCs) ................................................................................57
8.5 DefibView ...............................................................................................................58
9 Glossary of Symbols ...................................................................................59
10 Contacts ....................................................................................................63
11 Warranty Information .................................................................................65
vii
DAC-510E-EN-BG
Page 8

viii
DAC-510E-EN-BG
Page 9

1 Introduction to the DDU-100 Series AED
This User Manual provides information to guide trained operators in the use and maintenance of the
Defibtech DDU-100 series Semi-Automatic External Defibrillator (“AED”) and its accessories. This
chapter includes an overview of the AED, a discussion of when it should and should not be used, and
information on required operator training.
1.1 Overview
The DDU-100 AED is a Semi-Automatic External Defibrillator (“AED”) that is designed to be easy to
use, portable and battery powered. It has only two user controls: the ON/OFF and SHOCK buttons.
Voice prompts and visual indicators provide a simple interface for the operator. The DDU-100 AED is
capable of recording event information including ECG, audio data (optional), and SHOCK/NO-SHOCK
recommendations.
When connected to a patient who is unconscious and not breathing, the DDU-100 AED performs the
following tasks:
• Prompts the operator to take necessary actions to enable analysis.
• Automatically analyzes the patient’s ECG.
• Determines whether a shockable rhythm is present.
• Charges the defibrillation capacitor and arms the SHOCK button if the AED
detects a shockable rhythm.
• Prompts the operator to press the SHOCK button when the device is ready and a shock is
recommended.
• Delivers a shock once the device has determined a shock is required and the SHOCK button has
been pressed.
• Repeats the process if additional shocks are required.
The Defibtech DDU-100 AED will NOT shock a patient automatically; it will only advise the operator.
The SHOCK button is enabled only when a shockable rhythm is detected and the device is charged
and ready to shock. Charging occurs automatically when the device detects a shockable rhythm. The
operator must press the SHOCK button to initiate defibrillation.
The DDU-100 AED uses two self-adhesive defibrillation/monitoring pads to monitor ECG signals and,
if necessary, to deliver defibrillation energy to the patient. These pads (also known as electrodes) are
provided in a single-use, disposable package.
The DDU-100 AED determines proper pad-to-patient contact by monitoring the impedance between
the two pads (impedance varies with the electrical resistance of the patient’s body). Visual and audio
prompts inform the operator of possible problems with patient contact. Voice prompts and visual
indicators communicate the status of the AED and of the patient to the operator. The DDU-100 AED
has two push-button controls and several LED indicators.
DAC-510E-EN-BG
1
Page 10

Defibrillation energy is delivered as an impedance compensated biphasic truncated exponential
waveform. The device delivers 150 Joules into a 50-ohm load when using adult pads or when
using attenuated child / infant pads, 50J of defibrillation energy into a 50-ohm load. Energy
delivered does not change significantly with patient impedance, although the duration of the
generated waveform will vary. The Defibtech AED is designed to deliver up to 150J of defibrillation
energy through a patient impedance range of 25 – 180 ohms or 50J of defibrillation energy when
using the child / infant pads.
Defibrillation and AED operating power is supplied by a replaceable (non-rechargeable) lithium
battery pack that provides for long standby life and low maintenance operation. Battery packs are
available in several configurations optimized for use in specific applications. Each pack is marked
with an expiration date.
The DDU-100 AED records event documentation internally and, optionally, on Defibtech Data Cards
(“DDC”). The optional DDC plugs into a slot in the AED and enables the AED to record event
documentation, and audio (audio enabled cards only) if sufficient space is available on the card.
Audio recording is available only for units with installed audio-enabled Defibtech Data Cards. Event
documentation stored internally can be downloaded onto a DDC for review.
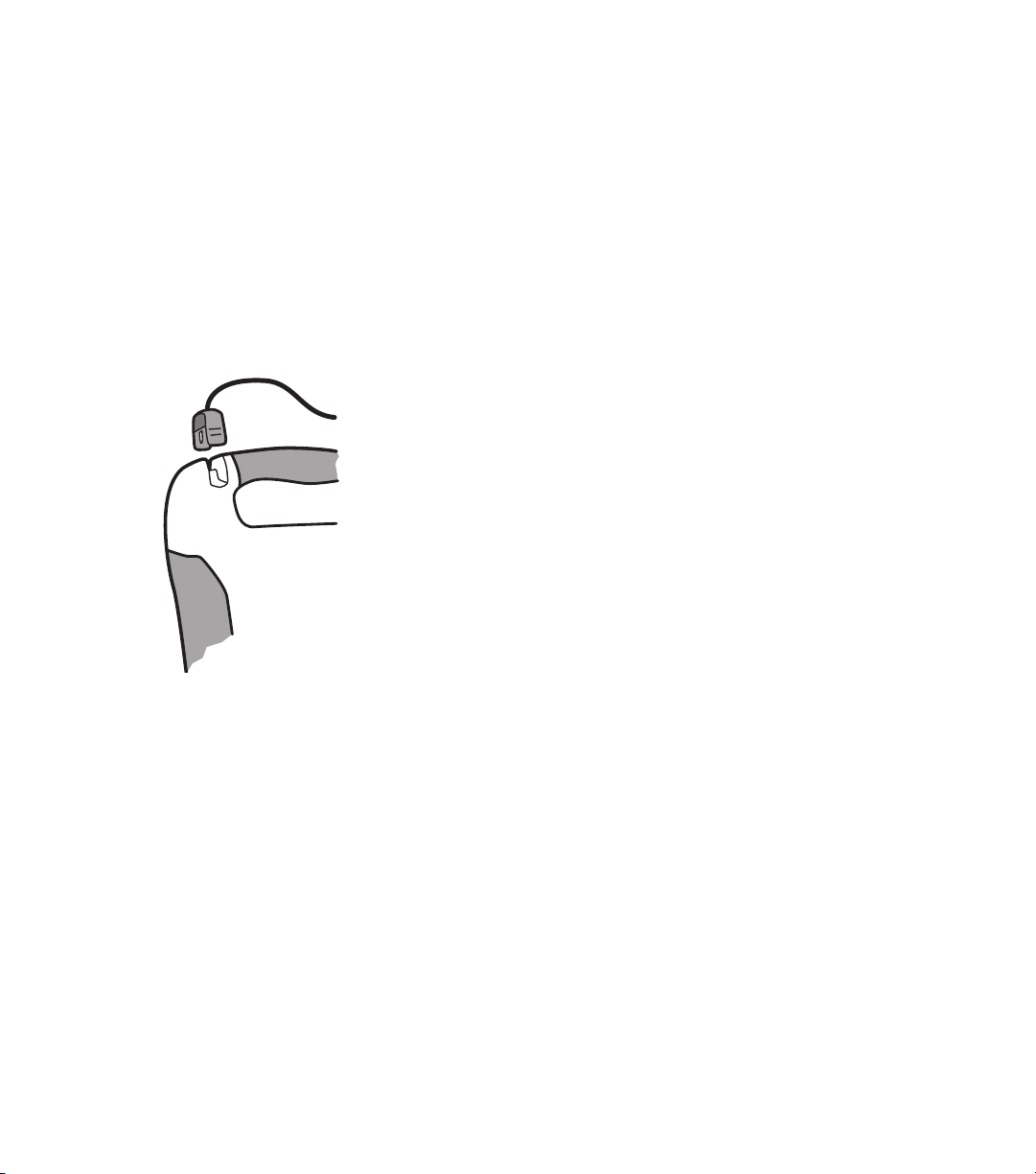
1.2 The Defibtech DDU-100 AED
A. Speaker. The speaker projects the voice prompts when the DDU-100 AED is on. The
speaker also emits a “beep” when the unit is in standby mode and has detected a condition
that requires operator attention.
2
DAC-510E-EN-BG
B. SHOCK button. This button will flash when a shock is recommended - push this button to
deliver the shock to the patient. This button is disabled at all other times.
C. “analyzing” LED (Light Emitting Diode). This green LED flashes when the DDU-100 AED is
analyzing the patient’s ECG rhythm.
D. “do not touch patient” LED. This red LED flashes when the DDU-100 AED detects motion
or other interference that prevents analysis of the signal or when the user should not be
touching or moving the patient.
E. “check pads” LED. This red LED flashes when the DDU-100 AED detects that the pad
connection to the patient is poor or pads are not applied.
F. ON/OFF button. Push button to turn the DDU-100 AED on. Push again to disarm and turn
the AED off.
G. Pads connector port. Insert Patient Pads Connector (item O) into this port to connect pads
to DDU-100 AED.
Page 11
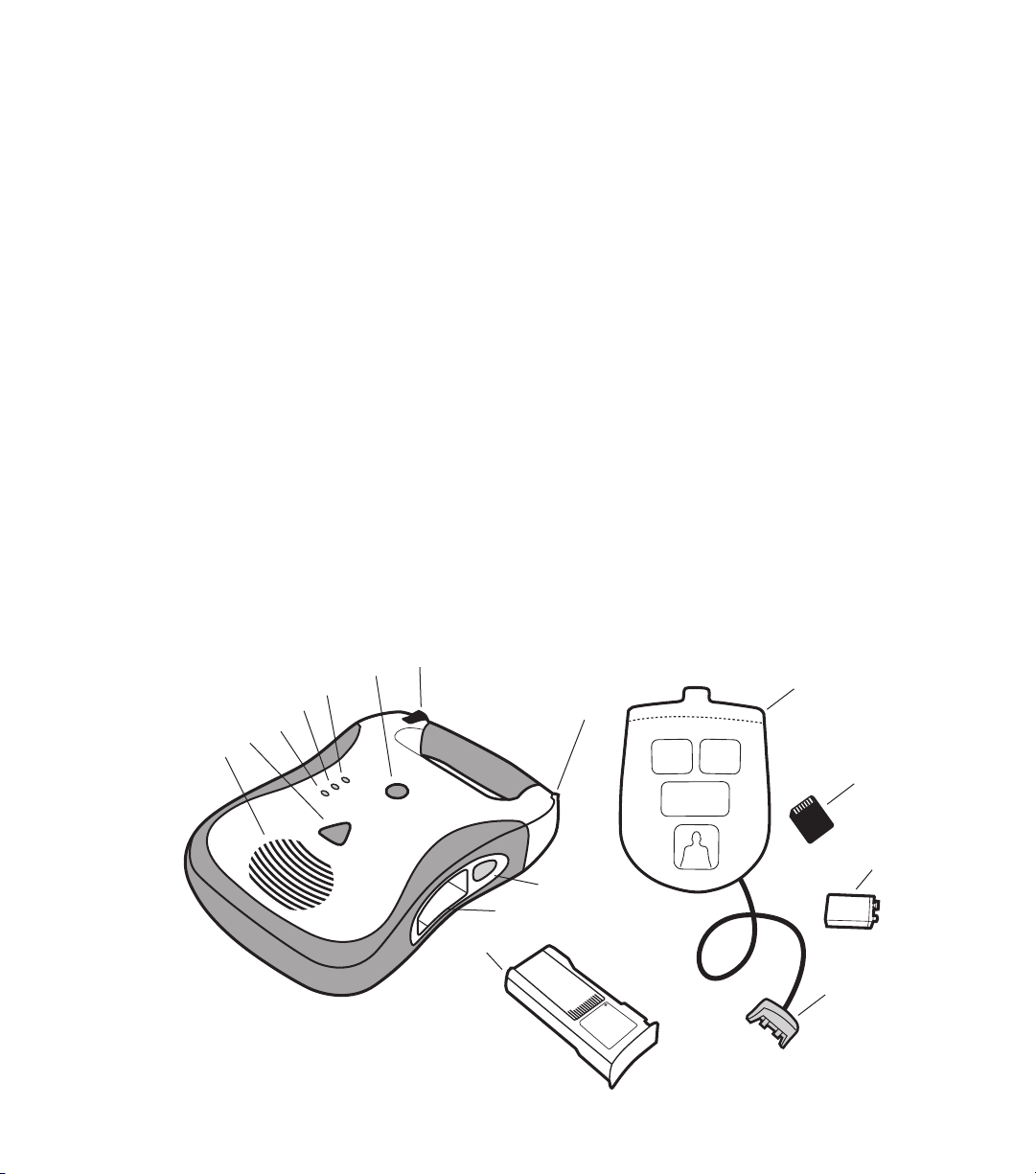
H. Battery pack. The battery pack provides a replaceable main power source for the DDU-100 AED.
I. Battery pack opening. Insert the battery pack firmly into this opening until the latch clicks
into place.
J. Battery pack eject button. This button releases the battery pack from the DDU-100 AED. To
remove the battery pack, push the button until the battery pack is partially ejected from the unit.
K. Active Status Indicator (ASI). When the unit is off, this indicator blinks green to indicate the
unit is fully operational and blinks red to indicate unit needs attention from the user or servicing.
L. Patient pads. The defibrillation/monitoring pads that are placed on the patient. The pads
may be stored in the pad storage area on the back of the unit.
M. Defibtech Data Card (DDC). This optional plug-in card provides enhanced storage
capabilities to the DDU-100 AED.
N. Active Status Indicator (ASI) battery. This is a 9V lithium battery that provides power to
the Active Status Indicator. It is inserted into a compartment in the battery pack.
O. Patient pads connector. Insert into Pads Connector Port (item G) to connect pads to the
DDU-100 AED.
G
F
E
D
C
B
A
K
L
M
N
J
I
H
O
3
DAC-510E-EN-BG
Page 12

1.3 Indications
The DDU-100 AED is indicated for use on victims of sudden cardiac arrest (“SCA”) when the
patient is:
• Unconscious and unresponsive.
• Not breathing.
For patients under 8 years old, use child/ infant electrode pads. Do not delay therapy
to determine exact age or weight.
The DDU-100 AED must be used by or on the order of a physician.
1.4 Contraindications
The DDU-100 AED should not be used if the patient shows any of the following signs:
• Conscious and/or responsive.
• Breathing.
• Has a detectable pulse.
1.5 Operator Training Requirements
In order to safely and effectively operate the DDU-100 AED, a person shall have met the following
requirements:
4
DAC-510E-EN-BG
• Defibtech DDU-100 AED and/or defibrillation training as required by local, state, provincial,
or national regulations.
• Any additional training as required by the authorizing physician.
• Thorough knowledge and understanding of the material presented in this User Manual.
Page 13

2 Dangers, Warnings and Cautions
This chapter includes a list of danger, warning, and caution messages that relate to the Defibtech
DDU-100 AED and its accessories. Many of these messages are repeated elsewhere in this
User Manual and on the DDU-100 AED or accessories. The entire list is presented here for
convenience.
DANGER: Immediate hazards that will result in serious personal injury
or death.
WARNING: Conditions, hazards, or unsafe practices that may result in
serious personal injury or death.
CAUTION: Conditions, hazards, or unsafe practices that may result in
minor personal injury, damage to the DDU-100 AED, or loss
of data.
2.1 Shock, Fire Hazard, Explosion
2.1.1 Electricity
DANGER
2.1.2 Battery Pack
CAUTION
WARNING
WARNING
Hazardous electrical output. This equipment is for use only by qualified
personnel.
Follow all battery pack labeling instructions. Do not install battery packs after
the expiration date.
Lithium battery packs are not rechargeable. Any attempt to recharge a lithium
battery pack may result in fire or explosion.
Do not immerse battery pack in water or other liquids. Immersion in fluids may
result in fire or explosion.
5
DAC-510E-EN-BG
Page 14

Do not attempt to recharge, short-circuit, puncture, or deform battery. Do not
WARNING
CAUTION
2.1.3 Usage Environment
DANGER
DANGER
CAUTION
expose battery to temperatures above 50°C (122°F). Remove battery when
depleted.
Recycle or dispose of lithium battery packs in accordance with local, state,
provincial, and/or national regulations. To avoid fire and explosion hazard, do not
burn or incinerate the battery.
Possible fire or explosion. Do not use in the presence of flammable gases
or anesthetics. Use care when operating this device close to oxygen sources
(such as bag-valve-mask devices or ventilator tubing). Turn off gas source or
move source away from patient during defibrillation, if necessary.
The DDU-100 AED has not been evaluated or approved for use in hazardous
locations as defined in the National Electric Code standard. In compliance
with IEC classification the DDU-100 AED is not to be used in the presence of
flammable substance/air mixtures.
Do not immerse any portion of this product in water or other fluids. Do not
allow fluids to enter the device. Avoid spilling any fluids on this device or
accessories. Spilling fluids into the DDU-100 AED may damage it or present a
fire or shock hazard. Do not autoclave or gas sterilize the DDU-100 AED or its
accessories.
2.1.4 Defibrillation/Shock Delivery
6
DAC-510E-EN-BG
CAUTION
WARNING
The DDU-100 AED should be stored and used only within the range of
environmental conditions specified in the technical specifications.
Defibrillation current can cause operator or bystander injury. Do not touch the
patient during defibrillation. Do not touch equipment connected to the patient
or metal objects in contact with the patient during defibrillation. Disconnect
other electrical equipment from the patient before defibrillating. Disconnect
the DDU-100 AED from the patient prior to use of other defibrillators.
Page 15

Improper use can cause injury. Use the DDU-100 AED only as instructed in the
WARNING
WARNING
CAUTION
2.1.5 Maintenance
WARNING
2.2 Improper Device Performance
User Manual. The DDU-100 AED delivers electrical energy that can potentially
cause death or injury if it is used or discharged improperly. Do not discharge
with defibrillation pads touching or gel surface exposed.
Disconnect all non-defibrillator proof equipment from the patient before
defibrillation to prevent electrical shock hazard and potential damage to that
equipment.
Avoid contact between parts of the patient’s body and conductive fluids such
as water, gel, blood or saline, and metal objects, which may provide unwanted
pathways for defibrillating current.
Electrical shock hazard. Dangerous high voltages and currents are present.
Do not open unit, remove covers, or attempt repair. There are no user
serviceable components in the DDU-100 AED. Refer servicing to qualified
service personnel.
2.2.1 Usage Environment
Radio frequency (RF) interference from RF devices such as cellular phones and
WARNING
CAUTION
two-way radios can cause improper AED operation. In accordance with IEC
801.3, a distance of 2 meters (6 feet) between RF devices and the DDU-100
AED is recommended.
Although the DDU-100 AED is designed for a wide variety of field use
conditions, rough handling beyond specifications can result in damage to the
unit.
7
DAC-510E-EN-BG
Page 16

2.2.2 Pads
Use only Defibtech disposable self-adhesive defibrillation/monitoring pads,
WARNING
CAUTION
WARNING
2.2.3 Patient Analysis
WARNING
battery packs, and other accessories supplied by Defibtech or its authorized
distributors. Substitution of non-Defibtech approved accessories may cause
the device to perform improperly.
Follow all defibrillation pad label instructions. Use defibrillation pads prior to
their expiration date. Do not re-use defibrillation pads. Discard defibrillation
pads after use (in the event of suspected pad malfunction, return pads to
Defibtech for testing).
The defibrillation pads are intended for one time use only and must be
discarded after use. Reuse can lead to potential cross infection, improper
performance of the device, inadequate delivery of therapy and/or injury to the
patient or operator.
Aggressive or prolonged CPR to a patient with defibrillation pads attached can
cause damage to the pads. Replace the defibrillation pads if they become
damaged during use.
8
DAC-510E-EN-BG
WARNING
WARNING
WARNING
WARNING
CPR during analysis can cause incorrect or delayed diagnosis by the patient
analysis system.
Do not place adult defibrillation pads in the anterior-posterior (front-back)
position. A shock or no shock decision may be inappropriately advised. The
DDU-100 AED requires that the adult defibrillation pads be placed in the
anterior-anterior (front-front) position.
Some very low amplitude or low frequency rhythms may not be interpreted
as shockable VF rhythms. Also some VT rhythms may not be interpreted as
shockable rhythms.
Handling or transporting the patient during ECG analysis can cause incorrect or
delayed diagnosis, especially if very low amplitude or low frequency rhythms
are present. During analysis and from “Shock Advised” until “Shock Delivered,”
patient movement and vibration must be minimized.
Page 17

In patients with cardiac pacemakers, the DDU-100 AED may have reduced
WARNING
2.2.4 Shock Delivery
WARNING
WARNING
2.2.5 Maintenance
WARNING
sensitivity and not detect all shockable rhythms. If you know the patient has
an implanted pacemaker, do not place electrodes directly over an implanted
device.
Do not allow defibrillation pads to touch each other, or to touch other ECG
electrodes, lead wires, dressings, transdermal patches, etc. Such contact can
cause electrical arcing and patient skin burns during defibrillation and may divert
defibrillating energy away from the heart.
During defibrillation, air pockets between the skin and defibrillation pads can
cause patient skin burns. To help prevent air pockets, make sure self-adhesive
defibrillation pads completely adhere to the skin. Do not use dried out or
expired defibrillation pads.
Periodic user-initiated and automatic self-tests are designed to assess the
DDU-100 AED’s readiness for use. However, no degree of testing can assure
performance or detect abuse, damage, or a defect that occurred after the most
recent test is completed.
WARNING
CAUTION
WARNING
Use of damaged equipment or accessories may cause the device to perform
improperly and/or result in injury to the patient or operator.
Improper maintenance can cause the DDU-100 AED not to function. Maintain
the DDU-100 AED only as described in this User Manual. The AED contains
no user serviceable parts – do not take the unit apart.
No modification of this equipment is allowed.
9
DAC-510E-EN-BG
Page 18

2.3 General
CAUTION
Federal law (USA) restricts this device to sale by or on the order of a physician.
10
DAC-510E-EN-BG
Page 19

3 Setting up the DDU-100 AED
This chapter describes the steps required to make your Defibtech DDU-100 AED operational. The
DDU-100 AED is designed to be stored in a “ready” state. This chapter tells you how to make the
device ready, so that if and when you need it, few steps are required to begin using the device.
3.1 Overview
The following components and accessories are included with your DDU-100 AED. Replacement
and other accessories are detailed in the “DDU-100 AED Accessories” section. Before getting
started, identify each component and ensure that your package is complete.
• DDU-100 AED • Battery pack
• Defibtech Data
• 9V lithium battery
Card (DDC)
(optional)
• Defibrillation pad
package
• User Manual
Defibtech DDU-100
Semi-Automatic
External Defibrillator
User Manual
11
DAC-510E-EN-BG
Page 20
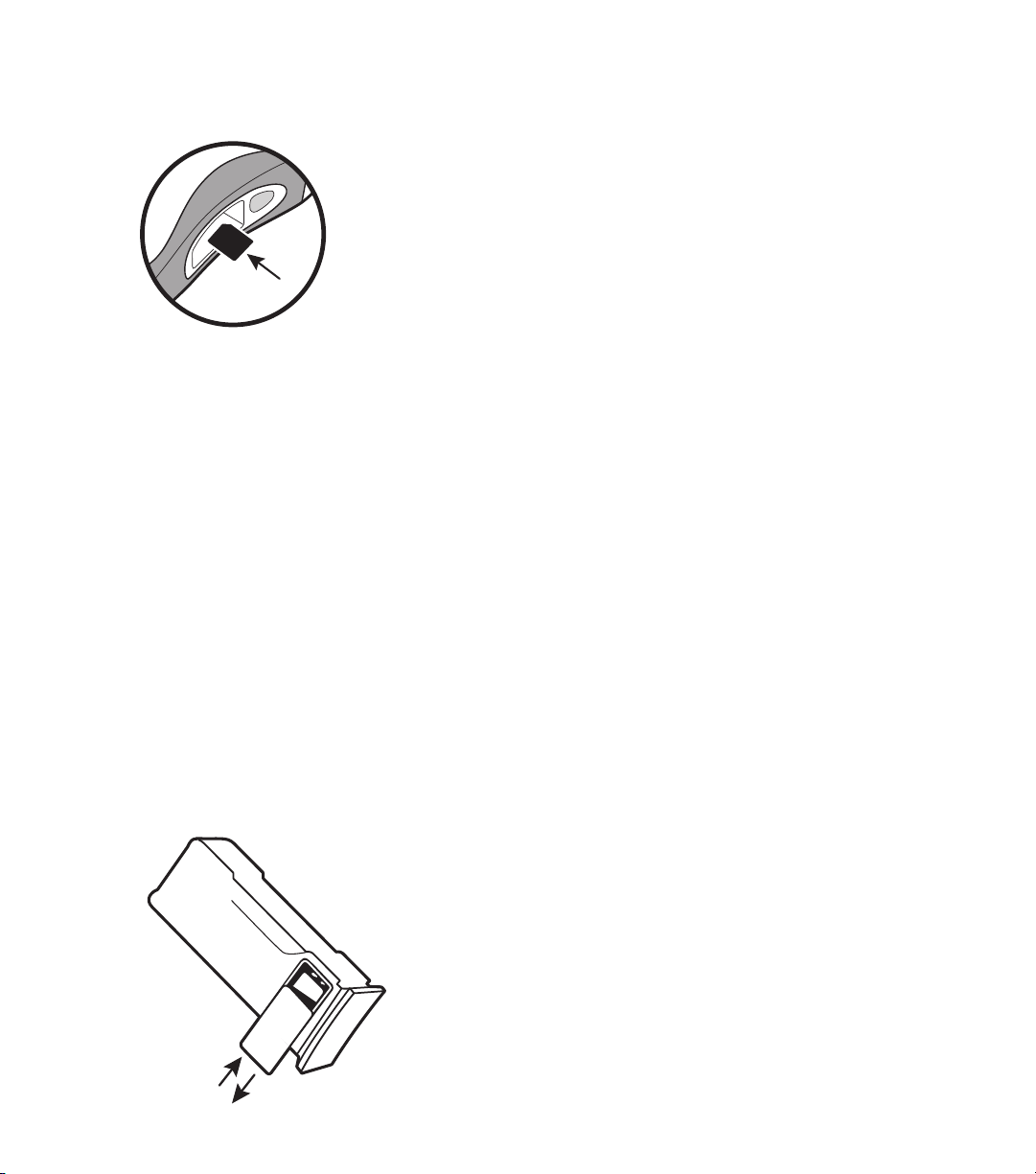
3.2 Installing the Data Card
The Defibtech Data Card (“DDC”) is used to store event and audio
information collected by the AED. All DDU-100 AEDs will operate
without DDCs and will still store critical event information internally.
Different DDC versions store different amounts of information. DDCs
are available in versions that store and don’t store audio information.
Refer to the DDC technical specification for exact storage capabilities.
DDCs may be reviewed with a separate PC based software package see “Event Viewing” section.
To install the DDC, remove the battery pack and push the DDC, label side up, into the thin slot in
the side of the AED centered over the battery pack opening. The card should click into place and
be flush with the surface of the slot. If the card does not push in all the way, it may have been
inserted upside down. In that case, remove the card, flip it over and try inserting it again.
To remove the DDC, press the card in all the way and then let go. The DDC will be partially ejected
and can be removed by pulling it the rest of the way out.
3.3 Installing the Active Status Indicator 9V Battery
A user-replaceable lithium 9V battery, located inside the battery pack, provides Active Status
Indicator (“ASI”) power. This auxiliary battery is used to provide standby indicator power
independently of the main lithium battery (contained in the battery pack) allowing the main battery
pack to have a significantly longer shelf and standby life.
The unit will operate without a 9V battery installed in the battery pack, but active status indication
will not be provided. If no 9V battery is installed, status can still be checked by turning the unit
on. Only a fresh 9V lithium battery should be used as a replacement. Refer to the Maintenance
section for more information on replacement batteries.
12
DAC-510E-EN-BG
The 9V battery is installed into the battery pack in the 9V battery
compartment. To install, remove the cover covering the 9V
battery compartment by pushing on it sideways. The cover will
slide and detach from the battery pack. Insert the 9V battery
into the 9V battery compartment so that the contacts on the
battery touch the contacts in the battery pack. The orientation of
the battery contacts is shown in a picture on the inside bottom
of the 9V battery compartment. Replace the 9V battery
compartment door by placing it in the almost closed position and
then sliding it closed.
Page 21
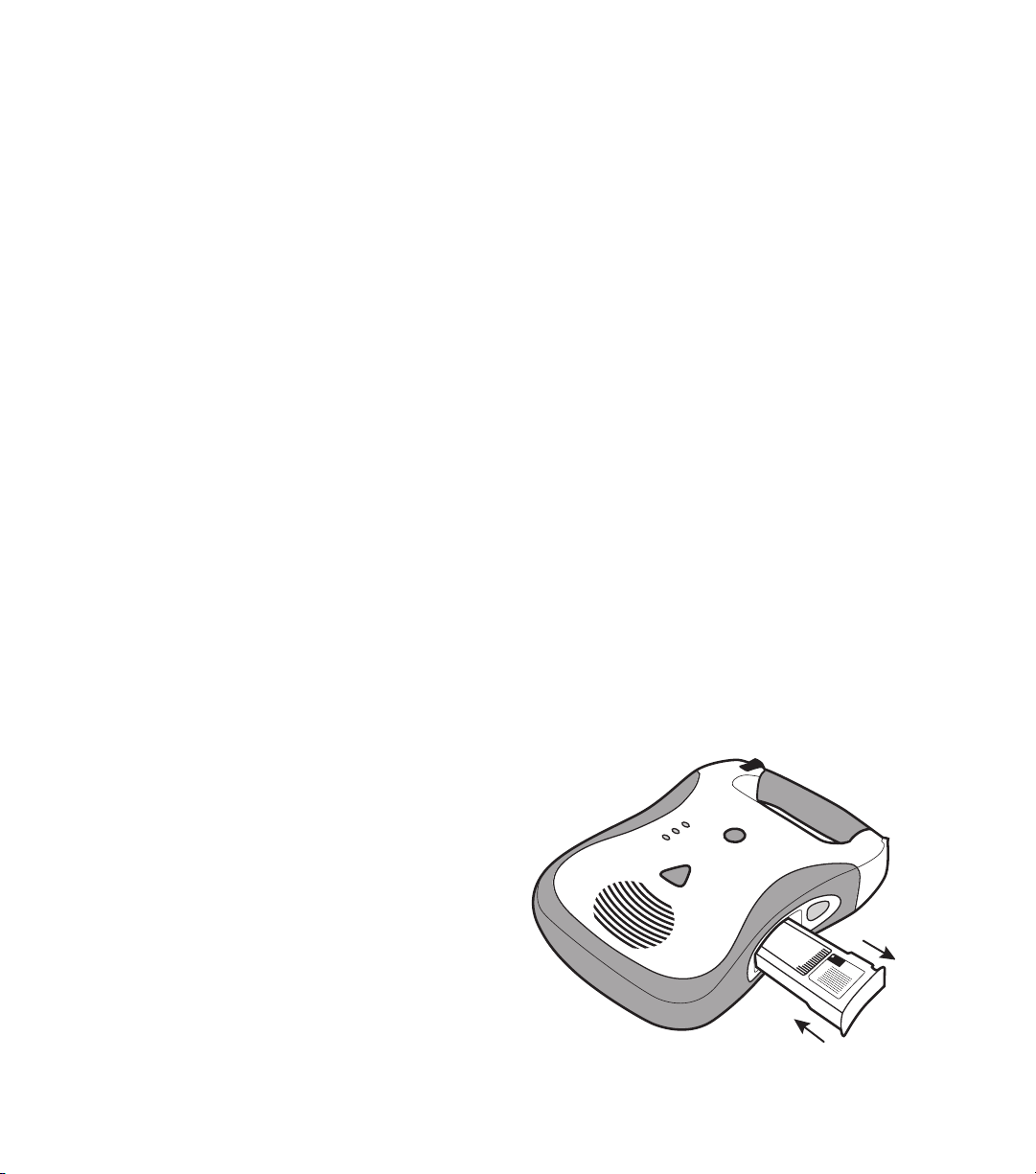
If the battery pack is stored outside the unit for an extended period of time, removal of the 9V
battery will extend the 9V battery’s life. Note that in an emergency situation, the battery pack may
be used without a 9V battery. If needed, a non-lithium based 9V battery may also be used, but
standby status indication life will be reduced.
Once the fresh 9V battery is installed, the battery pack status LED should periodically flash green
to indicate a ready state. If the indicator does not flash, either the battery pack is defective or
the 9V battery is discharged. Once the battery pack is installed into the unit, the DDU-100 AED’s
Active Status Indicator should flash green every five seconds.
3.4 Installing and Removing the Battery Pack
The lithium battery pack provides power to the DDU-100 AED. Before inserting the battery pack
into the AED, the 9V lithium battery should be installed in the battery pack itself as described in the
previous section.
In an emergency, the battery pack can be used without a 9V battery, but under normal operating
conditions the 9V battery should be installed. Do not install the battery pack after the expiration
date printed on the label. The battery pack is non-rechargeable.
A green Active Status Indicator on the label side of the battery pack will blink periodically to
indicate that the battery pack is ready for use. If the status indicator is not blinking, either the 9V
status battery has discharged or the battery pack is not suitable for use. If the indicator does not
blink after a new 9V battery has been installed, the battery pack should no longer be used and
should be removed from service. When the battery pack is in the AED, a “beep” will provide
notice that the 9V battery’s capacity is low and that the 9V battery should be replaced.
To insert the battery pack into the DDU-100 AED,
orient the battery pack so that the label faces
up. Make certain that the battery opening in the
side of the AED is clean and clear of any foreign
objects. Insert the battery pack into the opening in
the side of the AED. Slide the pack all the way in
until the latch clicks. If the pack does not slide all
the way in, it is most likely inserted upside down.
Once fully inserted, the battery pack surface
should be flush with the side of the AED.
To remove the battery pack, push the battery eject
button on the side of the AED. After the battery
pack is partially ejected, pull the battery pack out.
13
DAC-510E-EN-BG
Page 22
Within moments of insertion (if a non-discharged 9V ASI battery is installed) the DDU-100 will turn
on and run a battery pack insertion self-test. The unit will automatically shut off after the test is
run. Afterwards, the Active Status Indicator on the top corner of the DDU-100 AED will periodically
flash (if a nondischarged 9V ASI battery was previously installed in the battery pack). If the
indicator flashes green, the AED and battery pack are functioning properly, if the indicator flashes
red, there is a problem. Refer to the “Checking DDU-100 AED Status” section for more details on
the meaning of the indicator.
3.5 Connecting the Pads
The DDU-100 AED defibrillation/monitoring pads are supplied sealed in
a pouch with the connector and part of the cable exposed. This allows
the pads to be stored in a pre-connected state for rapid deployment
during an emergency.
Caution: DO NOT remove the defibrillation pads from the sealed
package until the pads are to be used. The packaging should be
opened only immediately prior to use, otherwise the pads may dry out
and become non-functional.
Note: The DDU-100 AED is designed to be stored with the pads
connector already installed. This simplifies the procedure for setting up
and operating the device in an emergency.
their expiration date should not be used and should be discarded.
Insert the connector end of the defibrillation pad cable into the pads connector port on the top-left
corner of the DDU-100 AED as shown. Insert pads connector firmly until it is fully seated in the unit.
The connected pad package can then be stored in the pad storage slot in the back of the DDU-100
AED. After connecting the pads connector to the unit, push the pad package, with the pictures
on the package facing out, rounded end first, into the pad holder compartment on the back of the
AED. When the pad package is fully inserted, press the pad cable into the groove in the back of
the unit to hold it in place and tuck any excess cable behind the pad package.
Caution: The pads are intended for one time use only and must be discarded after use or if the
package has been opened.
14
DAC-510E-EN-BG
First, check to ensure that the pad package has not expired. Pads past
Page 23

3.6 Performing Manually Initiated Self-Tests
Upon initial setup, perform a manually initiated Self-Test as described in the following paragraph.
To perform a manual Self-Test, begin with the unit powered off. Press and hold the ON/OFF
button until the unit announces that it is performing a Self-Test – this should take approximately
5 seconds. Once you hear the announcement, release the ON/OFF button. The unit will then
prompt you to “press flashing shock button.” When prompted, press and release the flashing
SHOCK button. The unit will run a series of internal tests, including charge and shock tests. The
manually initiated Self-Test can be aborted by pressing the ON/OFF button again to turn the
unit off. When the Self-Test is complete, the unit will announce its status and power off.
If the Self-Test passes: The unit will announce “UNIT OK” and power off. The unit may then be
immediately used by pressing the ON/OFF button again.
If the Self-Test fails: The unit will announce the symptom. The user should refer to the
“Troubleshooting” section in Chapter 5 of this manual for appropriate action.
Note: Every time the manually initiated Self-Test is run, the unit does an internal shock test.
This test reduces the capacity of the battery pack by one shock.
In addition, every time a battery pack with a non-depleted 9V battery is inserted, the unit runs a
Battery Pack Insertion Self-Test to test the battery pack. When the test is completed, the unit
reports the status of the battery pack and powers off. The unit may then be immediately used
by pressing the ON/OFF button again.
3.7 Storing the DDU-100 AED
The DDU-100 AED (preferably with pads attached) should be stored in environmental
conditions within range of the specifications - refer to the “Environmental” section of
“Technical Specifications”. The unit should also be stored so that the Active Status Indicator
can be readily seen.
The Active Status Indicator should periodically blink with a green light. If it blinks with a red
light or does not blink at all, the DDU-100 AED needs servicing – refer to the “Checking Active
Status Indicator” section for more information.
Defibtech recommends storing your AED in an easily accessible location.
15
DAC-510E-EN-BG
Page 24

16
DAC-510E-EN-BG
Page 25

4 Using the DDU-100 AED
chec k pad s
do n ot to uch pa tient
anal yzing
This chapter describes how to use the DDU-100 AED. The DDU-100 AED was designed for simple
operation, allowing the operator to focus on the patient. There are only two control buttons and
four light emitting diode (LED) indicators. Concise and easily understandable voice messages and
prompts guide the operator through the use of the unit.
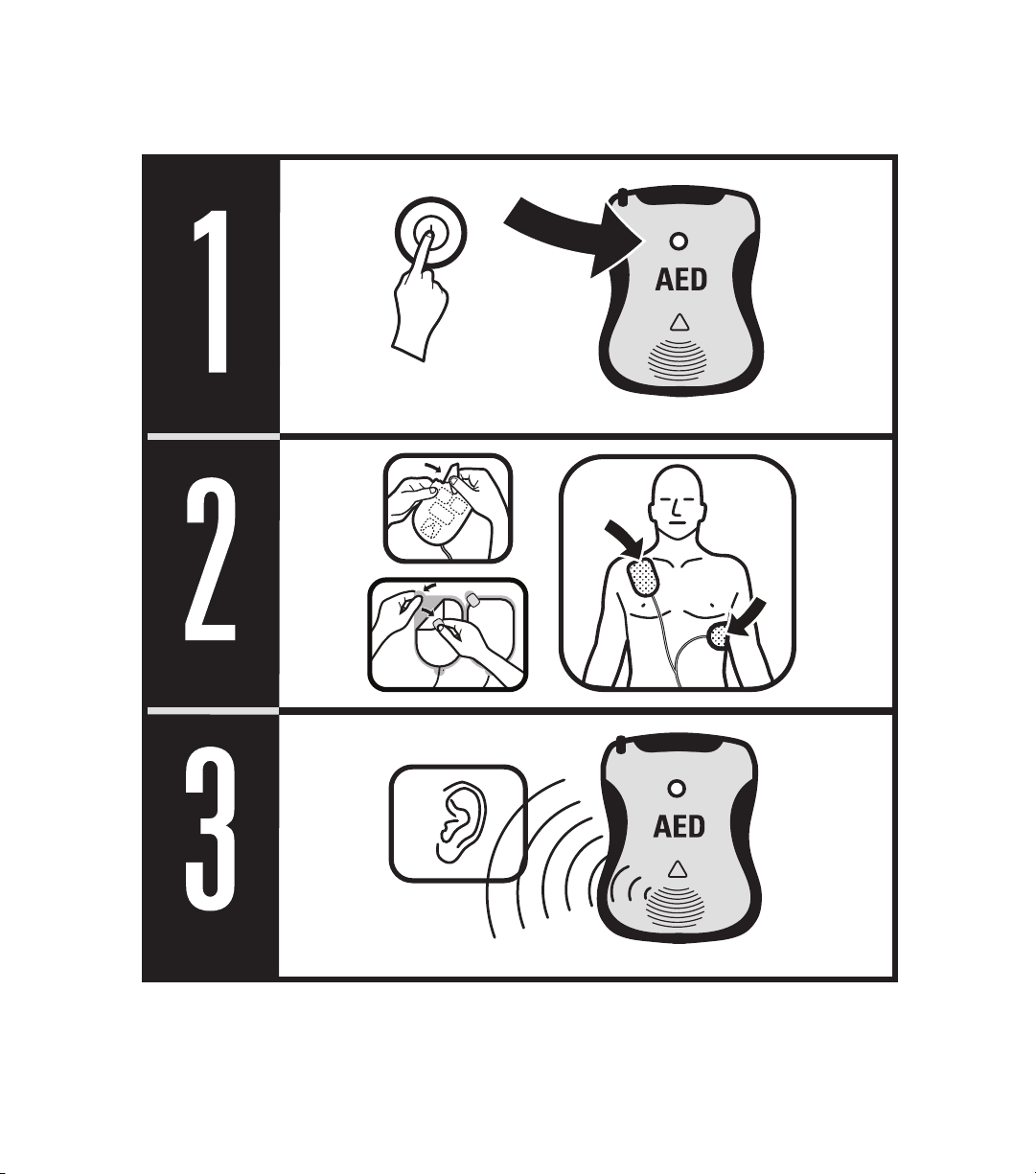
The following sections describe in detail how to use the DDU-100 AED. The basic steps for use are:
• Turn the DDU-100 AED ON by pressing the ON/OFF button.
• Connect pads to AED if not yet connected.
• Place pads on patient (follow instructions on pad package).
• Follow voice prompts.
• Press SHOCK button if instructed by the AED.
4.1 Overview
Pad
Connector
ON/OFF
Button
Indicator
LEDs
Speaker
Active Status
Indicator
Shock Button
17
DAC-510E-EN-BG
Page 26

4.2 Checking DDU-100 AED Status
Once a fully functional battery pack with a non-discharged 9V battery is installed in the DDU-100
AED, an LED indicator located in the corner of the unit actively indicates unit status. If the unit is
fully operational, the Active Status Indicator (“ASI”) will blink green and if the unit needs attention,
the ASI will blink red. Anytime the ASI blinks red and a good 9V battery is installed, the unit will
also “beep” periodically to call attention to itself.
The indicator is powered by a replaceable 9V battery in the battery pack. If the 9V battery has
discharged, active status indication will not be available. In this case, the 9V battery should be
immediately replaced to restore active status indication. If only the 9V battery is depleted, the
DDU-100 AED will still be fully functional when turned on and can be used in the on-state normally.
• Off: Battery pack not installed, AED is defective, or the
9V battery is discharged. Install functional battery pack or
replace the 9V battery in the battery pack.
• Steady-On green: The DDU-100 AED is ON and operating
Active
Status
Indicator
normally.
• Blinking green: The DDU-100 AED is OFF and ready to
operate normally.
• Blinking red: The DDU-100 AED is OFF and the AED or
battery pack needs attention.
• Steady-On red: The DDU-100 AED is ON and has
detected an error.
4.3 Turning on the DDU-100 AED
Press the ON/OFF button to turn the DDU-100 AED on. The unit will emit a “beep” and all the
LEDs will light up temporarily. The ON/OFF button will illuminate green anytime the AED is on.
Voice prompts will guide the operator in the use of the unit. To turn the unit off, press the button
again. The Active Status Indicator (“ASI”) will indicate the state of the unit.
18
DAC-510E-EN-BG
ON-OFF/
DISARM
• ASI off or blinking: The device is OFF.
Press green ON/OFF button to turn the device ON.
• ASI on (green): The device is ON. Press green ON/OFF
button to turn device OFF.
• ASI on (red): An error has been detected and unit will
turn off automatically.
Page 27

4.4 Preparation
4.4.1 Call for Help
As soon as the AED is turned on the unit will prompt the user to “call for help”. This indicates that
the first step in a rescue should always be to contact professional emergency services.
If another person is available, the user should direct that person to call for help and then continue
the rescue without delay.
4.4.2 Preparing the Patient
Prepare the patient by removing any clothing from the patient’s chest. Wipe away moisture
from the chest if necessary (the defibrillation pads will stick better on dry skin). If necessary,
shave excessive chest hair, which can prevent effective patient-electrode contact. To ensure that
electrode pads fully contact the patient’s skin, check that no jewelry or other objects are directly
underneath where the pads will be placed.
4.4.3 Opening the Pad Package
Remove the pad package from the pad storage slot at the back of the AED. Open the pad package
by tearing along the dotted line, starting at the black arrow (follow directions on the package). Pull
the protective backing from the pads and check that the pads are:
• Free from obvious signs of damage.
• Clean of excessive debris (for example, dirt if the pad was dropped).
• Not dried out, and that the gel is sticky and will adhere to the patient.
• Not expired. Do not use pads after the expiration date printed on the package.
If any of these conditions is found, use a new set of pads.
4.4.4 Connecting Defibrillation Pads to the DDU-100 AED
The DDU-100 AED is designed to be stored with the defibrillation pad connector
attached to the unit, while the pads themselves remain sealed in their package.
This reduces the time needed to setup and start treatment in an emergency.
The Defibtech AED should be stored with the pad connector plugged into the
unit. However, if pads were damaged or not properly connected, you may need
to substitute a new set of pads during an emergency. The pad connector is on
the corner of the AED.
To remove an old set of pads, pull firmly on the pad connector. Do not reuse used pads. Insert
the connector for the new pads as shown. The connector will only fit in one way – if the connector
does not fit, rotate the connector before trying again. Insert connector firmly until it is completely
seated in the unit.
DAC-510E-EN-BG
19
Page 28

If not needed for immediate use, the pad package can then be
stored in the pad storage slot in the back of the DDU-100 AED.
After connecting the pads connector to the unit, push the pad
package, with the pictures on the package facing up and out,
rounded end first, into the pad holder compartment on the back of
the AED. When the pad pack is fully inserted, press the pad cable
into the groove in the back of the unit to hold it in place and tuck any
excess cable behind the pad package.
4.4.5 Applying Pads to the Patient
Correct pad placement is essential for effective analysis of the patient’s cardiac rhythm and
subsequent shock delivery (if required). Remove the pads from the pad package by tearing the
package along the dotted line near the top of the package. Remove the pads from the package
and follow the directions and diagram showing proper defibrillation pad placement located on the
defibrillation pad package. Peel off the protective backing from each pad before placing it as shown
on the picture on the pad. Peel the backing off only when the pad is ready to be placed. Place the
pads with the sticky side of the pad on the patient’s skin. Pad placement on infants or children
under 8 years is different than placement for adults or children 8 years or older. Place the pads as
shown in the diagram.
20
DAC-510E-EN-BG
For adults and children 8 years or older use adult
pads: Place one pad just below the patient’s right
collar bone as shown in the picture. Place the second
pad over the ribs on the patient’s left side below the
left breast, also as shown.
For infants and children under 8 use child/infant
pads: Place one pad in the center of chest and back,
as shown.
Page 29

4.4.6 Follow DDU-100 AED Prompts
At this point, the DDU-100 AED will check to make sure that the pads are well connected to the
patient and that an adequate ECG signal is being received. Do not touch the patient, eliminate any
patient movement, and cease CPR at this time.
If there is a problem with the pad connection, connector connection, patient motion or other interference, the AED will guide the operator with audible and visual prompts. Visual prompts consisting of flashing LEDs with associated labeling reinforce the audio prompts and aid in high ambient
noise environments.
Pad related voice prompts:
“Plug in pads connector” – This indicates that the DDU-100 AED has determined that the
pads are not properly connected to the unit. Check that the connector is fully inserted into the
unit. If the prompts continue, try removing and reinserting the pads connector or try a new set of
pads. The “check pads” LED will flash red during this message.
“Remove pads from package in back of unit” – This indicates that the user should remove
and tear open the pad package located in the back of the unit.
“Apply pads to patient’s bare chest as shown” – This indicates that the DDU-100 AED
has determined that the pads are not placed on the patient. Place pads on the patient following
instructions on the pad package. If the prompts continue, try replacing the pads with a new set.
The “check pads” LED will flash red during this message.
“Plug in and apply pads” – This indicates that the DDU-100 AED has determined that the
pads are not plugged in and not applied to the patient. Check that the connector is fully inserted
into the unit. If the prompts continue, try removing and reinserting the pads connector or try a
new set of pads. The “check pads” LED will flash red during this message.
“Poor pad contact to patient,” “Press pads firmly” – This indicates that the pads are not
making proper contact with the patient and that the impedance is out of range for proper ECG
analysis and shock delivery. Check that the pads are properly placed and fully adhering to the
patient and that there are no air bubbles between the pads and the patient. Make sure that the
pads are not touching each other. If the pads are not sticking due to moisture, dry the patient. If
the pads are not sticking due to excessive hair, shave or clip excessive chest hair. If the prompts
continue, try replacing the pads with a new set. The “check pads” LED will flash red during this
message.
21
DAC-510E-EN-BG
Page 30

“Replace pads” – This indicates that the pads are not making proper contact with the patient
and that the impedance is out of range for proper ECG analysis and shock delivery. If another set
of pads is available, replace the pads, otherwise check that the pads are properly placed and fully
adhering to the patient. Make sure that the pads are not touching each other. If the pads are not
sticking due to moisture, dry the patient. If the pads are not sticking due to excessive hair, shave
or clip excessive chest hair. If the prompts continue, try replacing the pads with a new set. The
“check pads” LED will flash red during this message.
“Check pads” – This indicates that the pads are making improper contact with the patient or
touching each other and that the impedance is out of range for proper ECG analysis and shock delivery.
Check that the pads are not touching each other and that the patient is dry. If the prompts continue, try
replacing the pads with a new set. The “check pads” LED will flash red during this message.
“Pausing for CPR” – This indicates that the user should stop attempting to resolve problems with
the pads and assess the condition of the patient. The user will be prompted to begin CPR, if needed,
for a two-minute period.
Motion/Interference related voice prompts:
“Stop motion” – This indicates that the DDU-100 AED has detected motion in the patient. Stop all
patient motion, including CPR, in response to this message. If the patient is being transported, stop
the vehicle to stop the motion. The “do not touch patient” LED will flash red during this message.
“Stop interference” – This indicates that the DDU-100 AED has detected interference on the ECG
signal. Eliminate any radio or electrical sources of interference. Check the pads to make sure they are
adhering properly to the patient. If the environment is very dry, minimize movement around the patient
to reduce static discharges. The “do not touch patient” LED will flash red during this message.
“Pausing for CPR” – This indicates that the user should stop attempting to resolve motion and/or
interference problems and assess the condition of the patient. The user will be prompted to begin CPR,
if needed, for a two-minute period.
4.5 Heart Rhythm Analysis
Once the DDU-100 AED has determined that the pads are making a good connection to the patient,
the AED will start the ECG rhythm analysis. The unit analyzes the ECG signal and determines whether
a shockable or non-shockable rhythm is present. While analyzing, the AED will continue to monitor the
pad connections and will abort analysis if it detects any pad problems. It will also continue to monitor
for excessive motion or interference and will abort analysis if those conditions are detected.
22
DAC-510E-EN-BG
Page 31

Analysis related voice prompts:
“Analyzing heart rhythm” – This indicates that the DDU-100 AED is actively analyzing the
patient’s ECG signal. The AED will continue analyzing until it has determined whether a rhythm is
shockable or non-shockable or analyzing is interrupted for some reason. The “analyzing” LED will flash
green during this period.
“Do not touch the patient” – This indicates that the DDU-100 AED is trying to analyze the
patient’s heart rhythm and that the operator should not touch the patient. This message will be spoken
at the beginning of the analysis period and also if motion or interference has been detected. The “do
not touch patient” LED will flash red during this message.
“A nalyzing interrupted” – This indicates that the DDU-100 AED has determined that accurate
ECG analysis is not possible and has ceased analyzing. The operator is prompted to resolve the
problem – see “Follow DDU-100 AED Prompts” section. Once the problem is resolved, the unit
will enter analysis mode again. The “analyzing” LED will not be illuminated during this message.
“No shock advised” – This indicates that the DDU-100 AED has determined that a shock is
not required. The unit will not charge and the SHOCK button will not be enabled. The user will be
prompted to begin CPR, if needed, for a period of two minutes.
“Shock advised” – This indicates that the DDU-100 AED has determined that a shock is
recommended and the unit will begin charging in anticipation of a defibrillation shock. Analysis will
continue and the “analyzing” LED will continue to flash green.
4.6 Delivering the Shock
If the DDU-100 AED ECG analysis algorithm has determined that a shock is required, the unit
will automatically charge in preparation for shock delivery. While the AED charges, the unit will
continue to analyze the patient’s heart rhythm. If the unit detects that the heart rhythm has
changed to one that does not require a shock, the unit will abort the charging process and will
prompt the user to begin CPR, if needed, for a period of two minutes. Also while charging, the
AED will continue to monitor the pad connections and will abort charging if it detects any pad
problems. It will also continue to monitor for excessive motion or interference and will abort
charging if those conditions are detected. The user can abort at any time by pushing the ON/OFF
button to turn the unit off.
23
DAC-510E-EN-BG
Page 32

Shock related voice prompts:
“Charging” – This indicates that the DDU-100 AED has determined that a shock is recommended
and is charging the unit in anticipation of a defibrillation shock. Analysis will continue during this phase
and the “analyzing” LED will continue to flash green. A tone will sound to indicate charging progress.
If the unit detects a rhythm change to a non-shockable one, charging will abort and the user will be
prompted to begin CPR, if needed, for a period of two minutes.
“Stand clear” – This indicates that the DDU-100 AED is charging and that the operator and others
should stand clear of the patient. Analysis will continue during this phase and the “analyzing” LED will
continue to flash green. A tone will sound to indicate charging progress. If the unit detects a rhythm
change to a non-shockable one, charging will abort and the user will be prompted to begin CPR, if
needed, for a period of two minutes.
“Press flashing shock button” – This indicates that the DDU-100 AED has fully charged, that the
heart rhythm analysis algorithm still indicates a shock is recommended, and the unit is ready to deliver
a shock. The operator should press the SHOCK button to deliver the shock. The “Shock” button will
flash during this phase.
• Off: No Shock indicated. Button is disabled, pressing the
button will do nothing.
SHOCK
• Flashing: Shock is recommended. The device is charged
and ready to shock. Button is enabled. Press the button to
administer shock.
“Shock ‘x’ delivered” – This indicates that the DDU-100 AED has delivered the shock. The ‘x’
indicates the number of shocks that have been delivered since the unit was turned on (note: if the unit
delivers more than 15 shocks during one on period, the count will get reset to “one” on the sixteenth
shock). After each shock, the AED will enter Post-Shock CPR mode (see below).
“Shock cancelled” – This indicates that the DDU-100 AED has aborted shock mode and internally
discharged. If while waiting for the shock button to be pressed, the unit detects a rhythm change to a
non-shockable rhythm, the unit will cancel the shock. Also, if the shock button is not pressed within 30
seconds of the initial “press flashing shock button” prompt, the unit will automatically cancel the shock.
Note: The DDU-100 AED will not automatically deliver a shock – the user must press the SHOCK
button.
Note: At any time during the charging process or after the AED has been charged, the operator may
disarm the unit by pressing the ON/OFF button.
24
DAC-510E-EN-BG
Page 33

4.7 No Shock Required
If the DDU-100 AED ECG analysis algorithm has determined that a shock is not required, it will not
charge the unit and the SHOCK button will not be enabled. The operator will be prompted to begin
CPR, if needed, for a period of two minutes. The unit will not be monitoring the patient’s ECG
rhythm during this two minute CPR period.
During this two-minute period, the AED will not advise the user to “stop motion” even if motion
is present. During the two-minute period, the AED will announce time remaining in 15-second
intervals. At the end of the two-minute period, the unit will enter normal Analyzing mode.
No Shock Required Voice Prompts:
“It is safe to touch the patient” – This indicates that the DDU-100 AED analysis algorithm
has determined that no shock is required. The unit will not charge and the SHOCK button will
not be enabled. The user will be prompted to begin CPR, if needed, for a period of two minutes.
The “analyzing” LED will remain off to indicate that background rhythm monitoring has been
suspended.
“Check airway”, ”Check breathing” – This indicates that the user should check the condition
of the patient in order to determine if it is appropriate to perform CPR.
“If needed, begin CPR” – This indicates that the user should begin CPR, if needed, for two
minutes. The unit will not be monitoring the patient’s ECG rhythm during this two minute CPR
period. The “analyzing” LED will remain off to indicate that background rhythm monitoring has
been suspended.
“Continue for ‘x’ seconds” or “Continue for 1 minute ‘x’ seconds” – This indicates
that the user should continue CPR, if needed, for ‘x’ more seconds or, for 1 minute and ‘x’ more
seconds, respectively. The unit will not be monitoring the patient’s ECG rhythm during this two
minute CPR period. The “analyzing” LED will remain off to indicate that background rhythm
monitoring has been suspended.
“Continue” – This indicates that the user should continue CPR, if needed. This phrase is spoken
between the “continue for ‘x’ seconds” or “continue for 1 minute ‘x’ seconds” prompts to let the
operator know that the unit is still operating normally. The unit will not be monitoring the patient’s
ECG rhythm during this two minute CPR period. The “analyzing” LED will remain off to indicate
that background rhythm monitoring has been suspended.
“Continue for 5, 4, 3, 2, 1”, “Stop CPR” – This indicates that the user should finish
performing CPR. This phrase is spoken during the last several seconds of the two-minute CPR
period to let the operator know that the unit is still operating normally and that the two-minute
period is ending.
DAC-510E-EN-BG
25
Page 34

“Stop now”, “Do not touch the patient” – This indicates that the two-minute CPR period has
ended and the user should stop CPR. The unit will enter Analyzing mode and the “analyzing” LED will
flash.
4.8 Post-Shock CPR
If the DDU-100 AED has delivered a shock, the unit will require a mandatory two-minute CPR period.
No patient ECG rhythm monitoring will be done during this period. Once the two-minute period is
complete, the AED will continue in Analyzing mode.
Post-Shock CPR Voice Prompts:
“It is safe to touch the patient” – This indicates that it is safe for the user to touch the patient.
The unit will not be monitoring the patient’s ECG rhythm during this required two minute CPR period.
The “do not touch patient” LED will be off to indicate that it is safe to touch the patient.
“Begin CPR now” – This indicates that the user should perform CPR for two minutes. The unit
will not be monitoring the patient’s ECG rhythm during this required two-minute CPR period. The
“analyzing” LED will remain off to indicate that background rhythm monitoring has been suspended.
“Continue for ‘x’ seconds” or “Continue for 1 minute ‘x’ seconds” – This indicates that
the user should continue performing CPR for ‘x’ more seconds or, for 1 minute and ‘x’ more seconds,
respectively. The unit will not be monitoring the patient’s ECG rhythm during this required two-minute
CPR period. The “analyzing” LED will remain off to indicate that background rhythm monitoring has
been suspended.
“Continue” – This indicates that the user should continue performing CPR. This phrase is spoken
between the “continue for ‘x’ seconds” or “continue for 1 minute ‘x’ seconds” prompts to let the
user know that the unit is still operating normally. The unit will not be monitoring the patient’s ECG
rhythm during this required two-minute CPR period. The “analyzing” LED will remain off to indicate
that background rhythm monitoring has been suspended.
“Continue for 5, 4, 3, 2, 1”, “Stop CPR” – This indicates that the user should finish performing
CPR. This phrase is spoken during the last several seconds of the required two-minute CPR period to
let the user know that the unit is still operating normally and the two-minute period is ending.
“Stop now”, “Do not touch the patient” – This indicates that the mandatory two-minute
CPR period has ended and the user should stop CPR. The unit will enter Analyzing mode and the
“analyzing” LED will flash.
26
DAC-510E-EN-BG
Page 35

4.9 Post Use Procedures
After the DDU-100 AED has been used on a patient, the unit should be cleaned following
procedures in the “Cleaning” section and prepared for the next use. The following steps should be
performed:
• Remove battery pack.
• Remove DDC if installed. Replace with a new DDC.
• Reinsert battery pack. Check that the Battery Pack Insertion Self-Test passes.
• Connect a new pad package (check to make sure the package is not expired).
• Hold ON/OFF button down for at least five seconds to initiate a manually initiated Self-Test.
Unit will report status of the Self-Test and shut off.
• Check to make sure that the Active Status Indicator is flashing green.
4.10 Operational Environment
The Defibtech AED is designed to operate in a wide range of environmental conditions. To ensure
the reliability and safety of the AED in a given environment, refer to the “Environmental” section
for a detailed list of approved environmental conditions.
27
DAC-510E-EN-BG
Page 36

28
DAC-510E-EN-BG
Page 37

5 Maintaining and Troubleshooting the
DDU-100 AED
This chapter describes the maintenance and troubleshooting procedures for the DDU-100 AED.
The Self-Tests that are performed by the device are described along with the frequency and nature
of periodic maintenance for which the owner/operator is responsible. A troubleshooting guide is
provided to help diagnose user serviceable problems.
The DDU-100 AED contains no user serviceable parts except for the ASI 9V battery.
5.1 Self-Tests
Power-On Self-Tests are performed every time the unit is turned on to test the basic operation of the
unit. The unit also performs daily, weekly, monthly and quarterly self-tests automatically when a nondepleted 9V battery is present to check the integrity of the unit’s hardware and software.
Manually initiated Self-Tests may be run to test the DDU-100’s systems, including the charging and
shocking functions (the shock is internally dissipated and no voltage will be present at the pads) at
any time.
Note: Every time the manually initiated Self-Test is run, the unit does an internal shock test. This
test reduces the capacity of the battery pack by one shock.
To perform a manual Self-Test, begin with the unit powered off. Press and hold the ON/OFF button
until the unit announces that it is performing a Self-Test – this should take approximately 5 seconds.
Once you hear the announcement, release the ON/OFF button. The unit will prompt you to “press
flashing shock button.” When prompted, press and release the flashing SHOCK button. The unit will
run a series of internal tests, including charge and shock tests. The manually initiated Self-Test can
be aborted by pressing the ON/OFF button again to turn the unit off. When the Self-Test is complete,
the unit will announce its status and power off.
If the Self-Test passes: The unit will announce: “UNIT OK” and power off. The unit may then be
immediately used by pressing the ON/OFF button again.
If the Self-Test fails: The unit will announce the symptom. The user should refer to the
“Troubleshooting” section in Chapter 5 of this manual for appropriate action.
DAC-510E-EN-BG
29
Page 38

5.2 Routine Maintenance
SAMPLE
The DDU-100 AED is designed to be very low maintenance. Simple maintenance tasks are
recommended to be performed regularly by the owner/operator to ensure its dependability (see
sample maintenance table below). Different maintenance intervals may be appropriate depending
on the environment where the AED is deployed, and ultimately the maintenance program is at the
discretion of the emergency response program’s medical director.
Daily Monthly After Each Use Action
• • •
• •
•
•
•
•
Note: If the unit has been dropped, mishandled, or abused, a manually initiated self-test should
be performed.
5.2.1 Checking Active Status Indicator
The Active Status Indicator (“ASI”) is located in the upper corner of the DDU-100 AED and
indicates the operational readiness state of the unit. It will periodically flash green to indicate
a fully functional condition. If it is flashing red or not flashing at all, the AED needs attention.
Anytime the ASI is flashing red and a good 9V battery is installed, the unit will periodically emit a
“beep” to call attention to itself.
If the ASI is not flashing at all, the most likely cause is that the ASI 9V battery needs to be replaced.
Follow the directions in the “Replacing the Lithium 9V ASI Battery” section to replace the ASI
battery. Once the battery has been replaced with a fresh battery, the ASI should once again flash
green. If it does not, the battery pack may be defective. In that event, the battery pack should
be replaced. If it still does not flash after inserting a new battery pack, the DDU-100 AED is non-
operational and needs servicing.
Check that Active Status Indicator (ASI) is flashing green
Check the condition of the unit and accessories
Run manually initiated self-test
Replace pads
Check pads and battery pack expiration dates
Check the DDC, if one was installed
30
DAC-510E-EN-BG
Page 39

If the ASI is flashing red, turn the DDU-100 AED on. If the unit does not turn on or does not speak,
the AED is non-operational and requires servicing. If the unit does turn on, the voice prompts will
indicate the nature of the problem.
Maintenance Related Voice Prompts:
“Power-on self-test failed, service code ‘xxx’ ” – This indicates that the DDU-100 AED
has failed the power-on self-test and is non-operational and needs servicing. The code number will
indicate to the service personnel the type of problem that the unit is experiencing.
“Battery pack self-test failed, service code ‘xxx’ ” – This indicates that the DDU-100
AED’s battery pack is non-operational and needs servicing. The code number will indicate to the
service personnel the type of problem that the unit is experiencing.
“Service required” – This indicates that the unit has detected an error which rendered it
inoperable, and the unit requires servicing.
“Battery pack low” – This indicates that the battery pack capacity is low and should be replaced
soon. The AED will still be able to deliver at least a minimum of three defibrillation shocks the first
time this message is spoken.
“Replace battery pack” – This indicates that the battery pack is almost discharged and that the
AED may not be able to deliver defibrillation shocks. The battery pack should be replaced immediately.
“Replace 9 volt battery” – This indicates that the 9V battery in the battery pack needs to be
replaced. The unit may not provide active status indication during standby mode in this condition,
but the AED is still fully functional when turned on and may be used to treat patients. The 9V
battery should be replaced as soon as possible.
“Pads missing” – This indicates that pads were not found connected during a self test.
5.2.2 Checking the Condition of the Unit and Accessories
Inspect the unit for cracks or other signs of damage on the case, as well as dirt or contamination,
especially in the areas around the connector socket and battery pack opening.
If any cracks or other signs of damage are observed, remove the unit from service and contact an
autorized service center.
If any dirt or contamination is observed, refer to the “Cleaning” section for guidance on cleaning your unit.
DAC-510E-EN-BG
31
Page 40

5.2.3 Running a Manually Initiated Self-Test
The DDU-100 AED runs a Power-On Self-Test every time the unit is powered up to test the basic
operation of the unit. The unit also runs daily, weekly and monthly automatic Self-Tests when a
non-depleted 9V battery is present.
Note: Every time the manually initiated Self-Test is run, the unit does an internal shock test. This
test reduces the capacity of the battery pack by one shock.
To perform a manual Self-Test, begin with the unit powered off. Press and hold the ON/OFF
button until the unit announces that it is performing a Self-Test – this should take approximately 5
seconds. Once you hear the announcement, release the ON/OFF button. The unit will prompt you
to “press flashing shock button.” When prompted, press and release the flashing SHOCK button.
The unit will run a series of internal tests, including charge and shock tests. The manually initiated
Self-Test can be aborted by pressing the ON/OFF button again to turn the unit off. When the SelfTest is done, the unit will announce its status and power off.
If the Self-Test passes: The unit will announce “UNIT OK” .The unit may then be immediately used
by pressing the ON/OFF button again.
If the Self-Test fails: The user should refer to the “Troubleshooting” section in Chapter 5 of this manual.
5.2.4 Replacing Pads
The Defibtech defibrillation/monitoring pads are intended for one time use only.
The pads must be replaced after each use or if the package has been damaged.
First, check to ensure that the pad package has not expired. Pads past their expiration date
should not be used and should be discarded. Next, check to ensure that the pads package has
not been torn, opened or damaged. Dispose of the pads if the package is open or damaged.
Inspect the pads cable and replace if any nicks, cuts, or broken cables are found. Insert the
connector end of the defibrillation pad cable into the pads connector port on the corner of the
AED as shown. Press the pads connector in firmly until it is fully seated in the unit.
32
DAC-510E-EN-BG
The DDU-100 AED defibrillation/monitoring pads are supplied in a sealed
pouch with the connector and part of the cable exposed. The DDU-100 AED
is designed to be stored with the electrode cable already installed. This allows
the pads to be stored in a pre-connected state for rapid deployment during an
emergency.
Caution: DO NOT remove the defibrillation pads from the sealed package until
the pads are to be used. The packaging should be opened only immediately
prior to use, otherwise the pads may dry out and become non-functional.
Page 41

The pad package can then be stored in the pad storage slot in the back
of the DDU-100 AED. After connecting the pads connector to the
unit, push the pad package, with the pictures on the package facing up
and out, rounded end first, into the pad holder compartment on the
back of the AED. When the pad pack is fully inserted, press the pad
cable into the groove in the back of the unit to hold it in place and tuck
any excess cable behind the pad package.
Caution: The pads are intended for one time use only and must be
discarded after use or if the package has been opened.
5.2.5 Checking Pad and Battery Pack Expiration Dates
It is important that the patient pads and the battery packs not be used past their expiration dates.
The expiration date of the pad package is printed on the outside of the sealed package. The
expiration date of the battery pack is printed on the label on the pack. The battery pack should be
removed and replaced by this date; when the battery pack is used up, the unit will indicate “low
battery” or “replace battery” and will flash the Active Status Indicator red.
Once an accessory is past its expiration date, it should be replaced immediately. Follow the
instructions in the “Installing and Removing the Battery Pack” and “Connecting the Pads”
sections to replace the part with an unexpired part. Patient pads should be discarded. Battery
packs should be appropriately recycled.
5.2.6 Checking the DDC If One Was Installed
Each time the DDU-100 AED is used, an event file is created on the
DDC (if installed). If the unit was used to treat a patient, the DDC
in the unit should be removed and provided to the patient’s care
provider. A new DDC should be installed before the next use.
To remove the DDC, first remove the battery pack by pressing the
battery pack eject button on the side of the unit. The DDC card is
located in a slot directly above the battery pack opening in the unit.
To remove the DDC card, press the DDC in all the way and then
release. The DDC will be partially ejected and can be removed by pulling it the rest of the way out.
To install a new DDC, insert the DDC, label side up, in the thin slot on the top of the opening for
the battery pack. The card should click into place and be flush with the surface of the slot. If the
card does not push in all the way, it may have been inserted upside down. In that case, remove
the card, flip it over and try inserting it again.
DAC-510E-EN-BG
33
Page 42

Note: A DDC is not required for the DDU-100 AED to operate. Even if a DDC card is not
installed, basic essential information will still be recorded internally. The AED will still operate
properly even after a “replace memory card” message.
5.3 Replacing the Lithium 9V ASI Battery
The 9V ASI battery is located in the battery pack in the 9V
battery compartment (see figure). To install, remove the cover
covering the 9V battery compartment by pushing on it sideways.
The cover will slide approximately a 1/4 inch and then can be
detached from the battery pack. Insert the 9V battery into the 9V
battery compartment so that the contacts on the battery touch
the contacts in the battery pack. The orientation of the battery
contacts is shown in a picture on the inside bottom of the 9V
battery compartment. Replace the 9V battery compartment door
by reversing the process used to remove the door.
If the battery pack is stored out of the AED for an extended period of time, removal of the 9V
battery will extend the 9V battery’s life. Note that in an emergency situation, the battery pack may
be used without a 9V battery. If needed, a non-lithium based 9V battery may also be used, but
standby status indication life will be reduced.
Once the fresh 9V battery is installed, the battery pack status LED should periodically flash green
to indicate a ready state. If the indicator does not flash, either the battery pack is defective or
the 9V battery is discharged. Once the battery pack is installed into the unit, the DDU-100 AED’s
status indicator should periodically flash green.
Note: The unit will operate without a 9V battery installed, but active status indication and
automatic self-tests will not be provided. Status can still be checked by turning the unit on.
34
DAC-510E-EN-BG
Page 43

5.4 Cleaning
Periodically clean the DDU-100 AED of any dirt or contaminants on the case and connector socket.
The following are important guidelines to be adhered to when cleaning the device:
• The battery pack should be installed when cleaning the DDU-100.
• Do not immerse the DDU-100 in fluids or allow fluids to enter the unit. Use a soft cloth to
wipe the case clean.
• Do not use abrasive materials or strong solvents such as acetone or acetone based cleaning
agents. The following cleaning agents are recommended for cleaning the DDU-100 case and
the connector socket:
» Soapy water
» Ammonia based cleaners
» Hydrogen peroxide
» Isopropyl alcohol (70 percent solution)
» Chlorine bleach (30 ml / liter water)
• Ensure that the connector socket is completely dry before reinstalling the pads cable. After
cleaning the device and before returning it to service, always turn the unit on for a few
seconds, which will cause the unit to run a standard Power-On Self-Test.
5.5 Storage
The DDU-100 AED should be placed in a readily accessible location in an orientation where the
Active Status Indicator in the upper corner of the unit can be easily seen. In general, the unit should
be stored in clean, dry and moderate temperature conditions. Make sure that the environmental
conditions of the storage location are within the ranges detailed in the “Environmental” section.
35
DAC-510E-EN-BG
Page 44

5.6 Operator’s Checklist
The following checklist may be used as the basis for an Operator’s Checklist. The table should be
copied and filled out as recommend by the schedule in the “Routine Maintenance” section. As
each item is completed it should be checked off.
Defibtech DDU-100 Operator’s Checklist
Defibtech DDU-100 Serial Number: ___________________________________________________
Defibtech DDU-100 Location: __________________________________________________________
Date:
Check unit and accessories for damage, dirt and
contamination. Clean or replace as necessary.
Check that spare battery pack and pads available.
Check that battery pack and pads not past
expiration dates.
Check that the Active Status Indicator (ASI) is
flashing green.
Comments:
36
DAC-510E-EN-BG
Inspection by: (initials or signature)
Page 45

5.7 Troubleshooting
The following table lists the common causes for problems, the possible cause and the possible
corrective actions. Refer to the other sections of the User Manual for detailed explanations on how
to implement the corrective actions. If the unit continues to be non-functional, refer the unit for
servicing.
Symptom Possible Cause Corrective Action
Battery pack not inserted Insert battery pack
Unit will not turn on
Unit immediately turns off
ASI is solid red
ASI blinks red
ASI does not blink at all
Power on self-test failed,
service code ‘xxx’
Unit self-test failed,
service code ‘xxx’
Battery pack self-test failed,
service code ‘xxx’
Service required Unit needs servicing Return unit for service
“Replace battery pack”
voice prompt
“Battery pack low” voice prompt
“Replace 9 volt battery”
voice prompt
Battery pack depleted or nonfunctional
Unit is non-functional Return unit for service
Battery pack depleted Replace battery pack
Unit is non-functional Return unit for service
Unit detected an error Run manually initiated Self-Test
ASI 9V battery low Replace ASI 9V battery
Unit needs servicing
Battery pack non-functional Replace battery pack
Electrode pads are not pre-connected
to unit
ASI 9V battery depleted Replace ASI 9V battery
Battery pack not inserted Insert battery pack
Battery pack non-functional Replace battery pack
Unit is non-functional Return unit for service
Unit needs servicing
Unit needs servicing
Battery pack needs servicing
Battery pack capacity is critically low
Battery pack capacity is getting low
9V battery low or missing
Replace battery pack
Turn unit on and run manually
initiated Self-Test
Connect electrode pads to unit
Record code number and return
unit for servicing
Record code number and return
unit for servicing
Record code number and replace
with new battery pack
Unit will probably not deliver
a shock, replace battery pack
immediately
Unit will still deliver shocks,
replace battery pack as soon as
possible
Unit will still operate to treat
patients, replace 9V battery as
soon as possible
DAC-510E-EN-BG
37
Page 46

Symptom Possible Cause Corrective Action
“Plug in pads connector”
voice prompt
“Apply pads to patient’s bare
chest as shown” voice prompt
“Poor pad contact to patient”
or “Press pads firmly”
voice prompt
“Check pads” voice prompt
“Stop motion” voice prompt
“Stop interference”
voice prompt
“Analyzing interrupted”
voice prompt
“Shock cancelled” voice prompt
“Shock not delivered”
voice prompt
“Replace memory card”
voice prompt
“Pads missing” voice prompt
Unit makes periodic
“beep” sound
Connector not in properly
is oriented correctly and fully
inserted
Pad connector broken Replace pads
Unit’s connector broken Return unit for servicing
Pads not connected to patient Place pads on patient
Make sure pads connector
Pads not making good connection to
patient
Check pad connection to patient
Pads or pad cable damaged Replace pads
Dry pads Replace pads
Partial pad connection
Pads touching
Check that pads are placed
securely on patient
Separate pads and place
correctly on patient
Patient motion has been detected Stop patient motion
External interference has been
detected
Stop external interference
Motion or interference detected Stop motion or interference
Patient’s ECG rhythm changed No action necessary
Shock button not pushed within
30 seconds
Push shock button within
30 seconds
Low battery – insufficient to charge Replace battery pack
Hardware failure
Bad pad to patient connection
Run manually initiated Self-Test,
return unit for servicing
Check that pads are placed
securely on patient
Dry pads Replace pads
DDC card is full
Replace DDC card with a card
that is not full
DDC has failed Replace DDC card
Make sure pads connector
Pads not connected
is oriented correctly and fully
inserted into unit
Unit has detected a condition that
needs user attention
Turn unit on to run the power-on
Self-Test
38
DAC-510E-EN-BG
All indicator LEDs blinking,
unit does not operate
Hardware failure
Run manually initiated Self-Test,
return unit for servicing
Page 47

5.8 Repair
The DDU-100 AED contains no user serviceable parts. If the unit need servicing, return to an
authorized service center. Refer to “Contacts” section for contact information.
39
DAC-510E-EN-BG
Page 48

40
DAC-510E-EN-BG
Page 49

6 DDU-100 AED Accessories
This chapter describes the components and accessories that can be used with the Defibtech
DDU-100 AED. Information on obtaining replacement components and accessories is included
in the “Contacts” section.
6.1 Defibrillation/Monitoring Pads
The DDU-100 AED is used with Defibtech self-adhesive
defibrillation/monitoring pads for adults or with attenuated
pediatric pads for infants and children. These pads (also known
as “electrodes”) serve two functions:
• Allow the unit to read the patient’s electrocardiograph
(ECG) rhythm.
• Deliver defibrillation energy to the patient when needed.
The Defibtech self-adhesive defibrillation/monitoring pad
assembly comes in a “leads-out” sealed package that allows
the device to be stored with pads connected. When the DDU-
100 AED is used, the operator needs only to remove the pad
packaging, tear open the package and turn the device on to
administer care. The AED has a storage area in the back of the
unit that allows for storage of a single sealed pad package.
6.2 Battery Packs
The Defibtech AED uses a lithium battery pack. The battery pack
contains the main lithium battery cells, an LED status indicator, and
a 9V lithium battery. Different capacity battery packs are available.
Refer to the “Battery Packs” section for detailed information on
the available packs. The battery pack is inserted into the battery
pack opening on the side of the AED and latches into place.
The main battery is based on a lithium battery technology and
provides the AED with a long shelf and standby life. Battery pack
status indication is provided by a blinking green status LED. Status
indicator power is supplied by a user replaceable 9V lithium battery.
41
DAC-510E-EN-BG
Page 50

6.2.1 Battery Pack Active Status Indicator
The battery pack’s Active Status Indicator (“ASI”) is located on the label
face of the battery pack and is used to indicate battery pack status. A
periodically blinking green LED indicates that the battery pack status is OK
and the battery pack is ready for use. Absence of a blinking green LED
indicates a battery pack problem or a depleted or missing 9V
battery. Refer to the “Checking DDU-100 AED Status” section for
information on battery pack LED indications.
6.2.2 Active Status Indicator Battery
The Active Status Indicator (“ASI”) battery is a 9V lithium battery. It
provides power to the Active Status Indicator to prevent the main
defibrillation battery from being depleted for non-essential functions.
This provides a significantly longer standby life for the AED and battery
pack and extends the lifetime during which the DDU-100 AED can deliver
defibrillation shocks. The Active Status Indicator battery is a lithium 9 volt
battery.
6.3 Data Cards
42
DAC-510E-EN-BG
The DDU-100 AED is designed to optionally use Defibtech Data Cards
(“DDC”). The AED will operate with or without a DDC, but if a DDC is
installed, additional event storage capacity is available.
The DDU-100 AED accepts DDC cards of different capacities, each
designed to record an assortment of data for a given period of time. For
example, the DDU-100 AED can record more than ten hours of ECG only
or approximately one hour and forty minutes of audio and ECG data on
a large DDC card. Cards are available with and without audio logging
enabled.
Page 51

The DDC is inserted into a slot above the battery pack opening in the AED - refer to the “Installing
the Data Card” section. A new and initialized DDC card should be used each time the AED is
operated to maximize recording time. A new event file is created on the DDC each time the AED
is turned on and the following information is recorded (DDC cards may contain a maximum of 255
event files):
• The time the AED was turned on.
• Other data such as: ECG data, time data, audio data (audio enabled card only).
• Event milestones such as: motion detection, shock advice, shock delivery information.
When an audio enabled DDC gets low on available storage, the AED will stop recording the less
critical audio data to allow room for additional ECG data in an attempt to record at least one hour
of ECG (total recording time is limited by available space on the DDC). Data from a previous event
will NOT be erased. If the DDC fills completely, the AED will still be operable and the most critical
event documentation for the current session is still recorded internally.
Internally recorded event information can be downloaded for external review by inserting a
blank DDC card into the unit. The DDC slot is located just inside the battery pack opening. For
instructions on how to install and remove a DDC card, see Section 3.2 (“Installing the Data Card”)
of this manual. To download data from the card, refer to Section 7.2 (“Downloading the Internal
Data Log”).
6.4 Recycling Information
At the end of its useful life, recycle the defibrillator and its accessories.
6.4.1 Recycling Assistance
For recycling assistance contact your local Defibtech distributor. Recycle in accordance with local
and national regulations.
6.4.2 Preparation
Items should be clean and contaminant-free prior to being recycled. When recycling used
disposable electrodes, follow local clinical procedures.
6.4.3 Packaging
Packaging should be recycled in accordance with local and national requirements.
43
DAC-510E-EN-BG
Page 52

6.4.4 Notice to European Union Customers
The crossed-out wheeled bin symbol on this device indicates that this equipment has been
put on the market after 13 August 2005, and is included in the scope of the directive 2002/96/
EEC on Waste Electrical and Electronic Equipment (WEEE) and of the national decree(s) which
transpose provisions of such directive.
At the end of its lifetime, this device can only be disposed of in compliance with the provisions
of the above mentioned European directive (and following possible revisions) as well as with the
corresponding national regulation. Severe penalties are possible for unauthorized disposal.
Electrical and Electronic Equipment (EEE) may contain polluting components and hazardous
substances the accumulation of which could pose serious risk for the environment and human
health. It is for this reason that local Administrations provide regulations which encourage reuse
and recycling, and prohibit the disposal of WEEE as unsorted municipal waste and require the
collection of such WEEE separately (at specifically authorized treatment facilities). Manufacturer
and authorized distributors are required to supply information about a safe treatment and
disposition of the specific device.
You may also return this equipment to your distributor when purchasing a new one. As for reuse
and recycling, notwithstanding the limits imposed by the nature and the use of this device, the
manufacturer will do his best to develop recovery processes. Please contact the local distributor
for information.
44
DAC-510E-EN-BG
Page 53

7 Event Viewing
DefibView is a Windows based software application that reads data stored on a DDC and displays
it on a personal computer. DefibView serves four primary functions:
• Enables emergency care personnel to reconstruct a cardiac episode from the time the AED
was turned on and connected to the patient until the unit is turned off.
• Enables a patient’s primary care giver to review the emergency episode.
• Allows Defibtech and regulatory personnel to reconstruct a cardiac episode for review of
device performance.
• Provides maintenance personnel with additional parameter information to assist in
troubleshooting a device suspected of malfunctioning.
DefibView is a stand-alone software application. It cannot be used with the AED in operation and
exists solely to support post-event review of the data recorded on a DDC or downloaded to a DDC
from internal storage. The DDC from an event should be transported to a medical facility with the
patient, allowing medical professionals to review the data.
For details about the features and use of the application, refer to the DefibView documentation.
7.1 Defibtech Data Cards
If a DDC is installed in the unit, every time the DDU-100 is turned on the following information is
recorded on a new file on the card:
• The time the AED was turned on.
• Other data such as: ECG data, time data, audio data (audio enabled cards only).
• Event milestones such as: motion detection, shock advice, shock delivery information.
This information can be reviewed using the DefibView application.
45
DAC-510E-EN-BG
Page 54

7.2 Downloading the Internal Data Log
Regardless of whether a DDC is installed in the unit, select information is recorded internally within
the DDU-100 AED. The information recorded is limited to:
• The time the AED was turned on.
• Other data such as event milestones (motion detection, shock advice, shock delivery
information, etc).
• Eight seconds of ECG data immediately before a shock/no-shock decision, eight seconds
immediately after each shock, and all ECG data during the charging and waiting-to-shock
periods.
• Note: Audio data is not logged internally.
To download the internally logged information, perform the following procedure:
• Insert a blank DDC into the unit.
• Turn the unit on.
• Once the unit is on, turn it off in data download mode by pushing and holding the ON/OFF
button for at least five seconds.
• Allow the unit to write the contents of the internal log to the DDC by waiting for the unit to
turn off automatically.
The DDU-100 will write the contents of the internal log onto the DDC. This information can then
be reviewed using the DefibView software.
46
DAC-510E-EN-BG
Page 55

8 Technical Specifications
8.1 Defibtech DDU-100 AED
8.1.1 Physical
Category Specification
Size
Weight
8.1.2 Environmental
Category Specification
Operating / Maintenance
Standby / Storage
Altitude
Shock / Drop Abuse Tolerance
Vibration
Sealing
ESD
EMC (Emission)
EMC (Immunity)
8.5 x 11.8 x 2.7 inches (22 x 30 x 7 cm)
Approximately 4.2 lbs (1.9 kg) with DBP-1400 Battery pack
Approximately 4.4 lbs (2 kg) with DBP-2800 Batter y pack
Temperature 0 – 50°C (32 – 122°F)
One Hour Operating
Temperature Limit
(extreme cold)*
Humidity 5% – 95% (non-condensing)
Temperature 0 – 50°C (32 – 122°F)
Humidity 5% – 95% (non-condensing)
-20°C (-4°F)
-150 to 4500 meters (-500 to 15,000 feet) per
MIL-STD-810F 500.4 Procedure II
MIL-STD-810F 516.5 Procedure IV (1 meter, any edge,
corner, or surface, in standby mode)
MIL-STD-810F 514.5 Categor y 20
RTCA/DO-160D, Section 8.8.2, Cat R, Zone 2, Curve G
(Helicopter)
RTCA/DO-160D, Section 8, Cat H, Zone 2, Curves B&R
(Jet Aircraft)
IEC 60529 class IP54; Dust Protected, Splash Proof,
(Battery pack installed)
EN 61000-4-2:1998 Severity Level 4
(Open air discharges up to 8 kV or direct contact
discharges up to 6 kV)
EN 60601-1-2:2001+A1:2006, method EN 55011:1998
Group 1 Level B
(Not to exceed 30 dB μV from 30 Hz to 230 MHz and
not to exceed 37 dB μV from 230 to 1000 MHz
EN 60601-1-2:2001+A1:2006, method EN 61000-43:1998 Level 3
(Field strength: 10V/m; carrier frequency range:
26 MHz to 1 GHz; AM modulation, 80 percent index,
at 3 frequencies: 1, 5, and 20 Hz)
* From room temperature to temperature extreme, one hour duration.
47
DAC-510E-EN-BG
Page 56

8.1.3 Defibrillator
Category Specification
Waveform
Energy
Charge control
Biphasic Truncated Exponential
Adult: 150 Joules*
Infant / child: 50 Joules*
Automatic by Patient Analysis System
Typically < 6 seconds with a fresh DBP-2800 battery pack
Charge time from shock-advised
and < 9 seconds with a fresh DBP-1400 battery pack.
Charge time may increase at the end of battery life and for
temperatures below 10°C.
Charge complete indication
Shock delivery
• SHOCK button flashing
• “Press Shock button” voice prompt
Shock is delivered by a single SHOCK button
• If Patient Analysis System decides rhythm is no longer
shockable, or
Automatic
DISARM
• Within 30 seconds after Charge complete, if operator
has not pressed SHOCK button, or
• If defibrillation pads are removed from patient or
unplugged from unit.
Manual
• If operator presses the OFF/DISARM button at any time
to disarm and turns off the device.
8.1.4 Waveform Specifications
* nominal (±15%) delivered into a 50 ohm load.
The DDU-100 AED delivers a 150J Biphasic Truncated Exponential waveform to patients with
impedances ranging from 25 to 180 ohms.
The waveform is adjusted to compensate for measured patient impedance. Nominal phase times
and energy delivered are shown in the tables that follow.
48
DAC-510E-EN-BG
V
V max
V1
A
B
V1'
t
V min
Page 57

Adult
Patient Impedance
(Ohms)
100 9.0 6.0 151
125 12.0 8.0 153
150 12.0 8.0 146
175 12.0 8.0 142
Pediatric
Patient Impedance
(Ohms)
100 7. 2 4.8 53
125 7. 2 4.8 52
150 9.0 6.0 53
175 9.0 6.0 51
Phase A, Duration
(msec)
25 2.8 2.8 153
50 4.1 4.1 151
75 7. 2 4.8 152
Phase A, Duration
(msec)
25 4.1 4.1 35
50 5.8 3.8 47
75 5.8 3.8 51
Phase B, Duration
(msec)
Phase B, Duration
(msec)
Energy Delivered
(Joules)
Energy Delivered
(Joules)
8.1.5 Patient Analysis System
The Patient Analysis System ensures that the pad/patient impedance is within the proper range
and analyzes the patient’s ECG rhythm to determine whether a shock is required. On detection of
a non-shockable rhythm, the user is prompted to perform CPR. For shockable rhythms, the AED
automatically charges in preparation for shock delivery.
The patient analysis system will detect electrical “noise” or artifact in the ECG signal that may
interfere with accurate rhythm analysis. This artifact may be caused by excessive motion to the
patient or by external electrical noise. When this artifact is present, the AED will prompt the user to
“Stop motion” or “Stop Interference” until the ECG signal is free of noise and then proceed to analysis.
DAC-510E-EN-BG
49
Page 58

8.1.5.1 Shockable Rhythm Criteria
When placed on a patient meeting the indications for use criteria, the DDU-100 AED is designed to
recommend a defibrillation shock when it detects proper pad impedance and one of the following:
Peak-to-peak amplitude at least 200 µVolts.
Ventricular Fibrillation
Ventricular Tachycardia
(including ventricular flutter and
polymorphic VT)
Warning: Some very low amplitude or low frequency VF rhythms
may not be interpreted as shockable.
Cardiac rhythm rate of at least 180 bpm and peak-to-peak amplitude at
least 200 µVolts.
Warning: Some very low amplitude or low frequency VT rhythms
may not be interpreted as shockable.
The DDU-100 AED is designed to recommend no shock for all other rhythms, including Normal
Sinus Rhythms, fine Ventricular Fibrillation (<200 µVolts), and some slow Ventricular Tachycardias
and Asystole.
50
DAC-510E-EN-BG
Page 59

8.1.5.2 Patient Analysis System Performance
1
90% Lower
Specifications
2
Meets the AAMI DF39
requirement and AHA
recommendation2 of
Sensitivity > 90%
Meets the AAMI DF39
requirement and AHA
recommendation2 of
Sensitivity > 75%
Meets the AAMI DF39
requirement of Specificity
>95% and the AHA
recommendation2 of
Specificity > 99%
Meets the AAMI DF39
requirement and the AHA
recommendation2 of
Specificity > 95%
Meets the AAMI DF39
requirement and the AHA
recommendation2 of
Specificity > 95%
Rhythm Class
Shockable Rhythm –
Ventricular Fibrillation
Shockable Rhythm
– Ventricular
Tachycardia
Non-Shockable
Rhythm – Normal
Sinus Rhythm
Non-Shockable
Rhythm – Asystole
Non-Shockable
Rhythm – All other
non-shockable
rhythms
ECG Test
Sample1
Size
227 >98% >97%
100 99% >97%
213 100% 100%
113 10 0% 100 %
248 >99% >98%
Algorithm Performance
Performance
2
Confidence Limit
1. From Defibtech ECG Rhythm Databases.
2. Automatic External Defibrillators for Public Access Defibrillation: Recommendations for
Specifying and Reporting Arrhythmia Analysis Algorithm Performance, Incorporating New
Waveforms, and Enhancing Safety. American Heart Association (AHA) Task Force on
Automatic External Defibrillation, Subcommittee on AED Safety and Efficacy. Circulation,
1997;95:1677-1682.
Note: Additional information available upon request.
8.1.6 Clinical Summary
The DDU-100 AED uses a Biphasic Truncated Exponential waveform with specifications that are
substantially equivalent to the waveform specifications of the device used in the study* cited
below. The DDU-100 AED has not been the subject of a published clinical study.
8.1.6.1 Background
The objective of this study was to compare AEDs that delivered 150-J biphasic shocks with AEDs
that delivered high-energy (200- to 360-J) monophasic shocks.
*
Schneider T, Martens PR, Paschen H, et al. Multicenter, randomized, controlled trial of 150J biphasic
shocks compared with 200- to 360-J monophasic shocks in the resuscitation of out-of-hospital cardiac
arrest victims. Circulation 2000;102:1780-1787.
51
DAC-510E-EN-BG
Page 60

8.1.6.2 Methods
AEDs were prospectively randomized according to defibrillation waveform on a daily basis in four
emergency medical services systems. First responders used either the 150-J biphasic AEDs or 200- to
360-J monophasic waveform AEDs on victims where defibrillation was indicated. A sequence of up to
three defibrillation shocks was delivered: 150J-150J-150J for the biphasic units and 200J-200J-360J for
the monophasic units. Defibrillation was defined as termination of VF for > 5 seconds, without regard to
hemodynamic factors.
8.1.6.3 Results
Of 338 patients with an out-of-hospital cardiac arrest, 115 had a cardiac etiology, presented with
ventricular fibrillation, and were shocked with one of the randomized AEDs. There were no statistical
differences between the monophasic and biphasic groups in terms of age, sex, weight, primary structural
heart diseases, cause or location of arrest, bystanders who witnessed the arrest, or type of responder. A
summary of the results is presented in table below.
Biphasic Patients
Number (%)
Defibrillation Efficacy:
1 shock
< 2 shocks
< 3 shocks
Patients defibrillated 54/54 (100%) 49/58 (84%) 0.003
ROSC 41/54 (76%) 33/61 (54%) 0.01
Survival to Hospital Admission 33/54 (61%) 31/61 (51%) 0.27
Survival to Hospital Discharge 15/54 (28%) 19/61 (31%) 0.69
8.1.6.4 Conclusion
52/54 (96%)
52/54 (96%)
53/54 (98%)
Monophasic Patients
Number (%)
36/61 (59%)
39/61 (64%)
42/61 (69%)
P Value
< 0.0001
< 0.0001
< 0.0001
More patients were defibrillated with an initial biphasic shock than monophasic shock and ultimately the
biphasic waveform defibrillated at higher rates than the monophasic waveform. A higher percentage of
patients achieved Return Of Spontaneous Circulation (“ROSC”) after biphasic shocks. Rates of survival to
hospital admission and discharge did not statistically differ between the two waveforms.
52
DAC-510E-EN-BG
Page 61

8.1.7 Guidance and Manufacturer’s Declaration – Electromagnetic Emissions and Immunity
Electromagnetic conformity
The DDU-100 is intended for use in the electromagnetic environment specified below. The
customer or the user of the DDU-100 should assure that it is used in such an environment.
Electromagnetic emissions
Emissions test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/flicker
emissions
IEC 61000-3-3
Group 1
Class B
Not applicable
Not applicable
The DDU-100 uses RF energy only for its internal
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
The DDU-100 is suitable for use in all establishments,
including domestic establishments and those directly
connected to the public low-voltage power supply
network that supplies buildings used for domestic
purposes.
Electromagnetic immunity
Immunity test IEC 60601
Electrostatic discharge (ESD)
IEC 61000-4-2
Electrical fast transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
test level
±6 kV contact
±8 kV air
±2 kV for power line
supply lines
±1 kV for input/
output lines
±1 kV line(s) to
line(s)
±2 kV line(s) to earth
Compliance level Electromagnetic environment -
±6 kV contact
±8 kV air
Not applicable
Not applicable
The DDU-100 uses interference
detected and motion detected
indicators to notify the user if
conditions are not ideal. No other
ESD requirements are necessary.
guidance
53
DAC-510E-EN-BG
Page 62

Immunity test IEC 60601
Voltage dips, short
interruptions and voltage
variations on power supply
input lines
IEC 61000-4-11
Power frequency (50/60 Hz)
magnetic field
IEC 61000-4-8
Not applicable Not applicable
3 A/m 3 A/m Power frequency magnetic fields
test level
Compliance level Electromagnetic environment -
guidance
should not be greater than levels
characteristic of a typical location
in a commercial or hospital
environment.
Radiated RF
IEC 61000-4-3
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
The ISM (industrial, scientific and medical) bands between 150 kHz and 80 MHz are 6,765 MHz to 6,795 MHz;
13,553 MHz to 13,567 MHz; 26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz.
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which the DDU-100 is used exceeds the
applicable RF compliance level above, the DDU-100 should be obser ved to verify normal operation. If abnormal
performance is observed additional measures may be necessary, such as reorienting or relocating the DDU-100.
10 V/m
80 MHz to 2.5 GHz
10 V/m Portable and mobile RF
communications equipment should
be used no closer to any part of
the DDU-100, including cables,
than necessary. The recommended
separation distance calculated
from the equation applicable to
the frequency of the transmitter is
shown in the following table.
Interference may occur in the
vicinity of equipment marked with
the following symbol:
54
DAC-510E-EN-BG
Page 63

Separation Distances
The DDU-100 is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the DDU-100 can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the DDU-100 as recommended below, according to
the maximum output of the communications equipment.
Recommended separation distances between portable
and mobile RF communications equipment and the DDU-100
Separation distance according to frequency of transmitter (m)
Rated maximum
output power of
transmitter (W)
0.01 0.01 0.12 0.12 0.23
0.1 0.1 0.37 0.38 0.73
1 1 1. 17 1.20 2.30
10 10 3.69 3.79 7.27
100 10 0 11.67 12.00 23.00
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
meters (m) can be determined using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
150 kHz to 80 MHz
outside ISM bands
d = 1.16√P
150 kHz to 80 MHz
inside ISM bands
d = 1.2√P
80 MHz to
800 MHz
d = 1.2√P
800 MHz to
2.5 GHz
d = 2.3√P
Note 1: As 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2: The ISM (industrial, scientific and medical) bands between 150 kHz and 80 MHz are 6,765 MHz to
6,795 MHz; 13,553 MHz to 13,567 MHz; 26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz.
Note 3: An additional factor of 10/3 is used in calculating the recommended separation distance for transmitters
in the ISM frequency bands between 150 kHz and 80 MHz and in the frequency range 80 MHz to 2.5 GHz
to decrease the likelihood that mobile/portable communications equipment could cause interference if it is
inadvertently brought into patient areas.
Note 4: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
55
DAC-510E-EN-BG
Page 64

8.2 Battery Packs
8.2.1 High-Capacity Lithium Battery Pack
Category Specification
Model number DBP-2800
Main battery type 15VDC, 2800 mAh, Lithium/Manganese Dioxide.
Capacity 300 shocks or 16 hours of continuous operation.*
Charge time < 6 seconds*
Standby-life (installed in unit) 7 years*
Active Status Indicator (ASI) battery 9VDC, 1200 mAh, Lithium/Manganese Dioxide.
ASI battery standby-life (after installation) >1 year*
8.2.2 Standard Lithium Battery Pack
Category Specification
Model number DBP-1400
Main battery type 15VDC, 1400 mAh, Lithium/Manganese Dioxide.
Capacity 125 shocks or 8 hours of continuous operation.*
Charge Time < 9 seconds*
Standby-life (installed in unit) 5 years*
Active Status Indicator (ASI) battery 9VDC, 1200 mAh, Lithium/Manganese Dioxide.
ASI battery standby-life (after installation) >1 year*
Disposable, recyclable, non-rechargeable.
Disposable, recyclable, non-rechargeable.
*typical, new battery, at 25°C
Disposable, recyclable, non-rechargeable.
Disposable, recyclable, non-rechargeable.
*typical, new battery, at 25°C
56
DAC-510E-EN-BG
Page 65

8.3 Self-Adhesive Defibrillation/Monitoring Pads
Use only Defibtech Pads with your DDU-100 AED. Defibtech self-adhesive defibrillation/monitoring
pads have the following characteristics:
Category Specification
Model number DDP-100 DDP-200P
Type Adult Child < 8 years
Intended use Disposable Disposable
Adhesion Self-adhesive Self-adhesive
Active gel surface area 103 cm2 each (nominal) 50 cm2 each (nominal)
Cable/connector type Integrated Integrated
Cable length 122 cm (typical) 122 cm (typical)
Note: In the event of a suspected pad defect, the pads should be clearly marked “Not for Use”
and returned to Defibtech, L.L.C. for analysis. Refer to “Contacts” section for information on
defect returns.
8.4 Defibtech Data Cards (DDCs)
Use only Defibtech Data Cards in the DDU-100 AED. Defibtech Data Cards are available as follows:
Standard DDCs:
Model Number Details
DDC-6 Up to 6 hours of ECG data
DDC-12 Up to 12 hours of ECG data
Audio enabled DDCs:
Model Number Details
DDC-50AE Up to 50 minutes of Audio and 1 hour of ECG data
DDC-100AE Up to 1 hour and 40 minutes of Audio and ECG data
Note: The DDU-100 will attempt to log at least an hour of ECG data if possible. In audio enabled
DDCs, audio logging will be turned off if needed to preferentially record ECG information. If a
partially filled DDC is used, it is possible that only ECG (i.e. no Audio) will be logged. Every time
the unit is turned on, a file is created on the DDC – the DDC card can hold a maximum 255 files.
Once a card is completely filled with data or files all DDC logging will stop, but selected internal
ECG logging will continue.
DAC-510E-EN-BG
57
Page 66

8.5 DefibView
DefibView is a PC based application program that allows review of ECG data and other patient and
device performance parameters after an emergency event.
DefibView runs on various Windows platforms including Windows 98, Windows 2000, and
Windows XP. Minimum system requirements for adequate performance are as follows:
• Pentium II Processor at 300 MHz.
• 32 Mbyte System Memory.
• 100 Mbyte free space on hard disk.
Refer to the DefibView documentation for a complete description of the application.
DefibView is available for download at the Defibtech website at www.defibtech.com.
58
DAC-510E-EN-BG
Page 67

9 Glossary of Symbols
!
YYYY
YYYY
XXXX
YYYY
Symbol Meaning
High voltage present.
Caution, consult accompanying documents.
SHOCK Button – Delivers defibrillation shock to the patient when the device is ready to shock.
ON/OFF/DISARM Button –
- Turns the device ON when it is OFF.
- Turns the device OFF when it is ON.
- DISARMS the device when it is charged and then turns the device OFF.
Do not expose to high heat or open flame. Do not incinerate.
Recyclable.
Consult operating instructions.
Refer to instruction manual / booklet.
Do not damage or crush.
Follow proper disposal procedures.
Meets the requirements of the European Medical Device Directive.
Note: XXXX represents the identification number of the notified body.
Operational temperature limitation.
59
DAC-510E-EN-BG
Page 68

Symbol Meaning
YYYY
YYYY
YYYY
YYYY
Use by (yyyy-mm).
Defibrillation proof - Can withstand the effects of an externally applied defibrillation shock.
Internally powered with defibrillator-proof BF-type patient applied parts (per EN 60601-1).
Manufacturer.
Date of manufacture.
YYYY
Manufacturer and date of manufacture.
Do not reuse.
For USA users only.
DAC-510E-EN-BG
60
Federal Law (USA) restricts this device to sale by or on the order of a physician.
Catalogue number.
Keep dry.
Handle with care.
Transportation and storage requirements.
See environmental requirements.
Authorized European Representative:
EC REP
Emergo Europe
Molenstraat 15
2513 BH The Hague
The Netherlands
Page 69

Symbol Meaning
LATEX
Does not contain latex.
Lot number.
IP54
Dust protected; Protected against water jets.
Classified by TUV Rheinland of NA with respect to electric shock, fire, and mechanical hazard
only in accordance with UL 60601-1, CAN/CSA C22.2 No.601.1-M90, IEC 60601-1, and IEC
60601-2-4. Conforms to UL Standard UL 60601-1. Certified to CAN/CSA Standard C22.2 No.
601.1-M90.
Serial number.
Lithium Manganese Dioxide Battery.
Product is not sterile.
61
DAC-510E-EN-BG
Page 70

62
DAC-510E-EN-BG
Page 71

10 Contacts
Defibtech, L.L.C.
741 Boston Post Road
Guilford, CT 06437 USA
Tel.: 1-(866) 333-4241 (Toll-free within North America)
1-(203) 453-4507
Fax : 1-(203) 453-6657
Email:
sales@defibtech.com (Sales)
reporting@defibtech.com (Medical Device Reporting)
service@defibtech.com (Service and Repair)
63
DAC-510E-EN-BG
Page 72

64
DAC-510E-EN-BG
Page 73

11 Warranty Information
ORIGINAL END USER’S LIMITED WARRANTY*
COVERAGE
Defibtech, LLC provides a limited warranty that the defibrillator
and its associated accessories (e.g., batteries and pads),
whether purchased concurrently with the defibrillator as part
of a configuration or separately, shall be substantially free
from defects in material and workmanship. Defibtech’s limited
warranty shall only extend to the original end user, where
the original end user purchased the items from an authorized
Defibtech, LLC retailer. This limited warranty may not be
assigned or transferred. The terms of the Limited Warranty
in effect as of the date of original purchase shall apply to any
warranty claims.
LENGTH OF WARRANTY
The defibrillator’s limited warranty is for a period of eight (8)
years from the date of purchase. The battery’s limited warranty
is for a period of four (4) years from the date of purchase, but in
no event shall the limited warranty period extend past the date
printed on the battery. Single use accessories (e.g., the pads)
shall have a limited warranty up to use or for a period up to the
expiration date, whichever is earlier. The limited warranty for
all other accessories is for a period of one (1) year from the
date of purchase, or to the expiration date, whichever is earlier.
LIMITED WARRANTY LIMITATIONS
This limited warranty does not cover damage of any sort
resulting from, but not limited to, accidents, improper
storage, improper operation, alterations, unauthorized service,
tampering, abuse, neglect, fire, flood, war, or acts of God.
Additionally, this limited warranty does not cover damage
of any sort to the defibrillator or its associated accessories
resulting from the use of the defibrillator with unapproved
accessories or use of the accessories with unapproved medical
devices. The defibrillator and its associated accessories are
not warranted to be compatible with any other medical device.
LIMITED WARRANTY VOIDED
The limited warranty is immediately voided if: the defibrillator
or its associated accessories are serviced or repaired by any
entity, including persons, not authorized by Defibtech, LLC;
specified maintenance is not performed; the defibrillator
is used with one, or more, unauthorized accessories; the
associated accessories are used with an unauthorized
defibrillator; or the defibrillator or associated accessories
are not used in accordance with Defibtech, LLC approved
instructions.
EXCLUSIVE REMEDY
At Defibtech, LLC’s sole discretion, Defibtech shall have the
option to repair, replace, or provide a credit. In the event
of replacement, Defibtech shall have the right at its sole
discretion to replace the item with a new, or refurbished, same
or similar item. Determination of a similar item shall be at
the sole discretion of Defibtech. In the case of replacement,
the replacement at a minimum shall reflect the prorated
time remaining for the item based on the remaining limited
warranty period. In the case of a credit, the credit shall be the
prorated value of the item based on the lower of the original
item cost of the same or similar item and the remaining limited
warranty period. In no event, shall the limited warranty period
of a replacement item extend past the limited warranty period
of the item it is replacing.
WARRANTY SERVICE
In order to obtain warranty service, contact the retailer from
whom the item was purchased, or Defibtech, LLC customer
service. In the event an item must be returned, a Return
Material Authorization (RMA) number is required. Items
returned without an RMA number will not be accepted. The
item shall be shipped at the original end user’s expense to a
destination specified by the retailer or Defibtech, LLC.
OBLIGATIONS AND WARRANTY LIMITS
THE FOREGOING LIMITED WARRANTY IS IN LIEU OF
AND SPECIFICALLY EXCLUDES AND REPLACES, TO THE
DEGREE PERMITTED BY APPLICABLE STATE LAW, ALL
OTHER EXPRESS OR IMPLIED WARRANTIES, INCLUDING,
BUT NOT LIMITED TO, THE IMPLIED WARRANTIES OF
MERCHANTABILITY AND FITNESS FOR A PARTICULAR
PURPOSE.
NO PERSON (INCLUDING ANY AGENT, DEALER, OR
REPRESENTATIVE OF DEFIBTECH, LLC) IS AUTHORIZED
TO MAKE ANY REPRESENTATION OR WARRANTY
CONCERNING THE DEFIBRILLATOR OR ITS ASSOCIATED
ACCESSORIES, EXCEPT TO REFER TO THIS LIMITED
WARRANTY.
THE EXCLUSIVE REMEDY WITH RESPECT TO ANY AND
ALL LOSSES OR DAMAGES RESULTING FROM ANY
CAUSE WHATSOEVER SHALL BE AS SPECIFIED ABOVE.
DEFIBTECH, LLC SHALL IN NO EVENT BE LIABLE FOR ANY
CONSEQUENTIAL OR INCIDENTAL DAMAGES OF ANY KIND,
INCLUDING, BUT NOT LIMITED TO, EXEMPLARY DAMAGES,
SPECIAL, PUNITIVE, COMMERCIAL LOSS FROM ANY
CAUSE, BUSINESS INTERRUPTION OF ANY NATURE, LOSS
OF PROFITS OR PERSONAL INJURY, EVEN IF DEFIBTECH,
LLC HAS BEEN ADVISED OF THE POSSIBILITIES OF SUCH
DAMAGES, HOWEVER OCCASIONED, WHETHER BY
NEGLIGENCE OR OTHERWISE, UNLESS APPLICABLE STATE
LAW DOES NOT ALLOW SUCH EXCLUSION OR LIMITATION.
*Applicable to defibrillators and associated accessories
having a date of manufacture on or after January 1, 2013.
For all others, refer to warranty information in effect at the
time of manufacture.
65
DAC-510E-EN-BG
Page 74

Patents pending.
This product and its accessories are manufactured and sold under one or more of
the following United States patents: D514,951; 6,955,864; D499,183.
This product and its accessories are manufactured and sold under license to at
least one or more of the following United States patents: 5,591,213; 5,593,427;
5,601,612; 5,607,454; 5,611,815; 5,617,853; 5,620,470; 5,662,690; 5,735,879;
5,749,904; 5,749,905; 5,776,166; 5,800,460; 5,803,927; 5,836,978; 5,836,993;
5,879,374; 6,016,059; 6,047,212; 6,075,369; 6,438,415; 6,441,582.
66
DAC-510E-EN-BG
 Loading...
Loading...