
Models-1DCH01 and 1DCH02
1

325
4
TABLE OF CONTENT
INSTRUCTIONS FOR USE 7
Table of Contents 2
Introduction & Overview 5
Introduction 5
Requirements 6
Indications for Use
(Purposes of the Dechoker) 6
Contraindications
(When this device should not be used) 7
About This User’s Guide 7
Important Symbols 7
Technical Support 8
General Warnings 9
Dechoker Description & Features
Dechoker Features 11
Dechoker Components 11
Dechoker Setup 13
Dechoker Labels 17
Package Label 17
Dechoker Use 19
Dechoker Use 19
Troubleshooting 26
Troubleshooting the
Dechoker Device 26
Cleaning Specications 26
Cleaning the Dechoker 26
Device Specications 27
Dechoker Dimensions 27
Operating Conditions 27
Transportation and
Storage Conditions 28
Maintenance 28
Maintenance 28
Whom to Call for Help 28
Replaceable Parts 29
Device Disposability 29
Dechoker Limited Warranty 30
©2016 by Dechoker LLC All Rights Reserved.
Dechoker LLC
1191 Speedway Blvd
Salisbury, NC 28146
1-704-638-5445
EC Rep Ltd/M Devices Group
Health Education Centre
The Church,
Portland Street,
Southport.
PR8 1HU
United Kingdom

6
TABLE OF FIGURES
Figure 1: Dechoker storage box ........................................... 13
Figure 2: Dechoker open storage box.................................. 14
Figure 3: Dechoker removed from storage box ................. 15
Figure 4: Dechoker removed from storage bag ................. 16
Figure 5: Dechoker Package Labels .................................... 17
Figure 6: Dechoker Shipping Labels ................................... 18
7
INTRODUCTION
No part of this text shall be reproduced or transmitted in any form or by any means,
electronic or mechanical, including, but not limited to: recording, photocopying, or by
any information or retrieval system without written permission from Dechoker LLC.
The information in this manual is condential and may not be disclosed to third parties
without the prior written consent of Dechoker LLC.
The only warranty Dechoker LLC makes is the express written warranty extended on
the sale or of its products.
TABLE OF TABLES
Table 1: Import Symbols and Descriptions .......................... 8
Table 2: Warning / Caution Decriptions ............................. 10
To order additional copies of this manual
(DECH-LAB012), refer to the contact information on the front cover.
Caution: All users must read and understand the Dechoker Instructions for
Use, including indications, contraindications, operating instructions, warnings
and precautions before using the device. Failure to do so may result in
injury to the patient or caregiver or cause damage to the Dechoker Device.
8
Requirements
The Dechoker is used within the home healthcare environment, restaurants,
hospitals, and within commercial facilities. Operators of the Dechoker shall be
familiar with this manual. The Dechoker provides peace of mind that the Dechoker is
available to assist in the removal of acute obstruction of a choking victim.
Contraindications (When this device should not be used)
9
The Dechoker is contraindicated for use by patients / users with the following:
• The Dechoker should not be used without contacting
911 or emergency assistance.
Indications for Use (Purposes of the Dechoker)
The Dechoker is a medical device that creates suction to clear the acute
upper airway from obstruction in the relief of choking. The Dechoker device
can be applied to a choking victim to generate a suction to remove the acute
obstruction, and is intended for use as an aid to family members, healthcare
professionals, caregivers, EMTs, restaurant workers or any average user.
Warning: Safety and eectiveness of the Dechoker has not been
established for uses outside of those specied herein.
• The Dechoker model-1DCH01, 1DCH02 and 1DCH03
should not be used on infants.
About This User’s Guide
This manual provides general instructions for the Dechoker operations,
usability, and safety criteria. Observing the warnings and cautions
listed within this manual will provide eect use of the device.
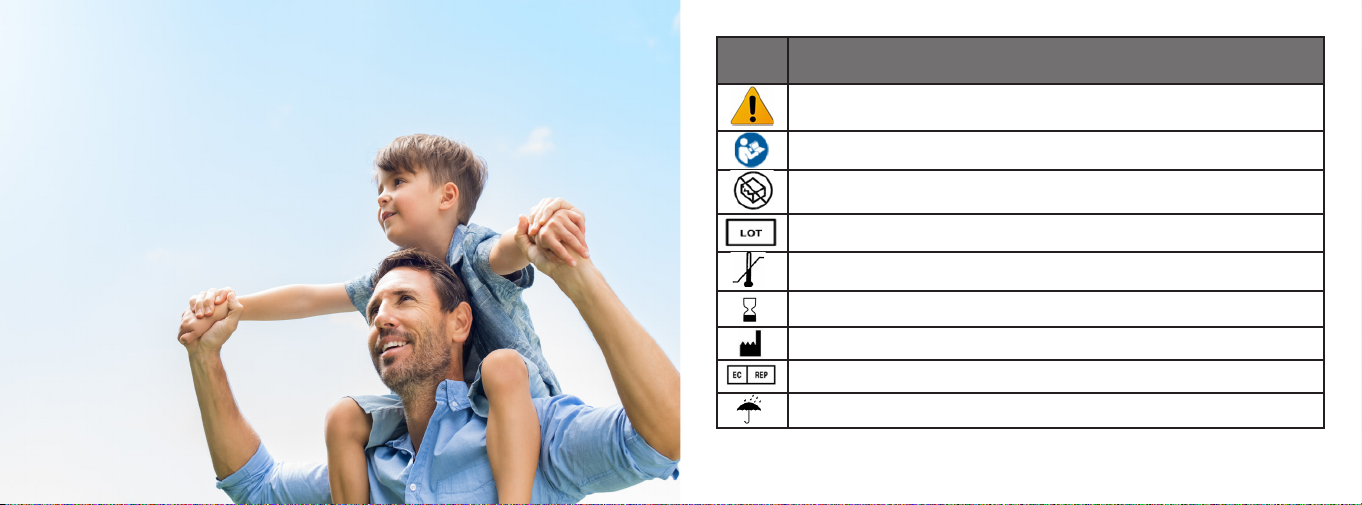
Important Symbols
The table below shows all symbols and the corresponding meaning of the symbols
used within this manual and on the Dechoker labeling. Operators must pay careful
attention to these symbols as they provide important safety precautions and warnings.
10
Technical Support
For questions regarding the Dechoker, please contact Dechoker LLC
Technical Support Team at 1-844-638-5445 in North America or
the EU Authorized
Label
Symbol
11
Meaning
Attention, Caution, Warning, Danger, Important, Note, or Refer to
Accompanying Documentation
Refer to Instruction manual / Booklet
Device has not been sterilized.
Batch code
Temperature limitation
Use by date
Manufacturer
Authorized Representative in the European Community
Keep Dry

12
Representative at +44 1704544944. Please have the Dechoker
Lot number (located on the Dechoker package label) available for
reference when contacting Dechoker LLC.
General Warnings
It is important to read all warnings before using the Dechoker.
Reference the table below for specic warnings.
Warning /
Cautions
13
Statement
All users must read and understand the Dechoker Instructions for
Use, including indications, contraindications, operating instructions,
warnings and precautions before using the device. Failure to do so
may result in injury.
Safety and eectiveness of the Dechoker has not been established for
uses outside of those specied herein.
The Dechoker is a non sterile device. Never use organic solvents (e.g.
acetone), ammonia compounds, or strong acids to clean any portion
of the Dechoker. The device can be reused on a single patient or
disposed of after use.
The Dechoker is not certied “waterproof.” Never immerse the
Dechoker in water or any other uids.
The use of accessories, other than those specied and sold by
Dechoker LLC should be used.
Do not disassemble the Dechoker. The manufacturer or an authorized
service representative only must evaluate the Dechoker for suction.
The Dechoker is a signal use device and is not repairable. Device
evaluation is used for risk mitigation and post market surveillance.

14
Warning /
Cautions
Statement
Do not interconnect the Dechoker with other equipment or accessories
that are not specied in this manual.
The Dechoker is strictly a mechanical medical device and does not
emit EMC.
Equipment is not suitable for use in the presence of ammable
anesthetic mixture with air or oxygen or nitrous oxide.
The Dechoker plastics are latex free. Skin irritation may result with
the constant handling of the device for individuals who may have
sensitivity or allergic reaction to ABS materials.
Do not open or modify the Dechoker.
If a problem occurs with the Dechoker, identify the symptom then
attempt to resolve the problem as indicated in the Instructions for Use.
If the problem cannot be resolved, under NO condition should the
device be used.
Warning /
Cautions
15
Statement
Precautions should be taken regarding the exposure of the medical
equipment to reasonably foreseeable environmental conditions
(e.g. magnetic elds, electromagnetic elds, external electrical
inuences, electrostatic discharge, pressure or variations in pressure,
acceleration, and thermal ignition sources).

16
DECHOKER DESCRIPTION & FEATURES
Dechoker Features
Dechoker is a hand held manually operated medical device comprised of seven
major components: Respiratory Mask, Suction Barrel, Pull Handle, Exhaust
valve, Enclosure, Device labeling, and device Quick Reference Guide.
This Instruction for Use document provides cautions and warning along with
device setup and use. Please read the entire manual to learn about the
features of the Dechoker prior to use.
Dechoker Components
The Dechoker is composed of the following main components as detailed in the sections
below:
1. Respiratory Mask The respiratory mask covers the mouth and nose of the individual
the device is being used on.
2. Suction Barrel The sealed suction barrel encapsulates the piston to create the
appropriate set suction level to remove the acute obstruction
3. Pull Handle The pull handle provides a simple grip to drawing back the piston
4. Exhaust valve The exhaust valve permits the pull handle to move the piston back to
its original position to allow another suction attempt in removing the acute obstruction.
5. Enclosure The enclosure is used as a protective housing separating the user from the
internal suction components of the Dechoker. Do not attempt to disassembly the device.
6. Device Labeling The device has a label on the package identifying the device
manufacturer, and reference the device lot number when making any inquiries.
7. Device Quick Reference Guide The Dechoker has an operator’s quick reference
device instructions wrapped around the barrel of the device. A hard copy quick reference
guide is also enclosed within the shipping box.
17

18
DECHOKER SETUP
Follow the simplied steps below to
prepare the Dechoker for use.
Retrieve the Dechoker storage box.
19
Open the Dechoker Bag and remove the
Dechoker Device.
Figure 1: Dechoker storage box
Open the shipping box by lifting the side
ap and pull upward.
Figure 2: Dechoker open storage box
Remove the Dechoker Package from the
box
Figure 3: Dechoker removed from storage
box
Reference Dechoker use section for usability steps on
choking individual

20
DECHOKER LABELS
Package Label
The following label is axed to the Dechoker package. This label provides
the device Lot (batch number), manufacturer contact information, and applicable symbols with explanatory text. In the event that the Dechoker needs to
be returned, please have the device lot number available for reference when
contacting Dechoker LLC or the EU authorized representative.
21
Shipping Package Label
The following label is axed to the Dechoker shipping box. This label provides the device Lot number, manufacturer, EC Authorized representative, and applicable symbols.
In the event that the Dechoker needs to be returned, please have the device lot number
available for reference when contacting Dechoker LLC.

22
DECHOKER USE
IMMEDIATELY CALL FOR EMERGENCY ASSISTANCE. OBTAIN
THE DECHOKER DEVICE FROM ITS LOCATION AND FOLLOW THE
STEPS BELOW FOR THE USE OF THE DEVICE:
23
1. Remove Dechoker from
the package and pull the
handling once or twice
2. Lay the choking
individual on their back,
tilt up the head, lifting
the chin for access to the
airway.
3. Insert the tube into the
mouth, respirator face
mask covering the mouth
and nose for no longer
than 3 second intervals.
4. Apply thumb at the bottom
of the chin, index nger on
one side of respirator, and
middle nger on the other
side of the respirator.
5. Apply light pressure
on the respirator while
pulling the plunger
upward. Repeat steps 4
and 5 if necessary.
7. Roll individual over
on side to allow debris
out of mouth to avoid
pulmonary aspiration.
6. Caution: Never
leave respirator
covering the mouth and
nose over 3 seconds at any
point in time. Countdown
1….2…..3.

24
TROUBLESHOOTING
25
DEVICE SPECIFICATIONS
Troubleshooting the Dechoker Device
The Dechoker is a non-serviceable device. It has been tested during the controlled
manufacturing process for optimum suction needed for the removal of acute obstruction
of a choking individual. If obstruction could not be removed by the Dechoker Device
use alternative medical methods for obstruction removal.
Cleaning Specications
Cleaning the Dechoker
The Dechoker is a patient individual use device. Once used on the individual to remove
the acute obstruction, the device can be cleaned to be used by the same patient. If
required dispose of the device with the regulations of the country in which it was sold.
The Dechoker is not a biohazard device.
Dechoker Dimensions
Dimensions 317.5mm x 63.5mm (12.5” x 2.5”)
Weight 16oz (1 lbs.)
Suction capability typical 34kPA
Operating Conditions
Temperature 5°C to 40°C (41°F to 104°F)
Relative Humidity 15-93% non-condensing
Atmospheric Pressure Range 700hPA to 1060hPA.
Transportation and Storage Conditions
Temperature -25°C to 70°C (-13°F to 158°F)
Relative Humidity 93% non-condensing relative humidity.
Atmospheric Pressure Range 700hPA to 1060hPA.

26
Cleaning the Dechoker cont.
Reusable Dechoker devices are required to be properly cleaned and disinfected
between each use. For normal use, Dechoker device may be cleaned many
times by disinfecting in boiling water for 3 minutes. For highly soiled devices,
the following are the cleaning instructions for the device between uses by a
single patient:
• Remove all gross soil from the device using a clean cloth moistened with an Enzol
cleaning solution made per manufacturer’s recommendations.
• Clean the device with the prepared cleaning solution and soft bristle brush. Dicult
to reach areas can be ushed with a clean syringe.
• Rinse the device under running water until no visible signs of the cleaning solution
remains. A clean syringe can be used to ush parts of the device.
• Boil the device in hot water for 3 minutes.
• Dry the device either with a clean cloth or let stand to dry overnight.
• Visually examine the device for any remaining soil and repeat the above steps if
necessary
27

28
MAINTENANCE
Whom to Call for Help
Always call 911 or the emergency call
number of the country the Dechoker
device is to be used prior to using the
device on an acute airway obstructed
individual during this emergency.
In the event that the Dechoker is
inoperable revert to other acute airway
obstruction method of relief.
Replaceable Parts
The Dechoker device does not have
CAUTION: Do not attempt to disassemble the device. The manufacturer
or an authorized representative must perform device failure evaluation.
WARNING: Do not interconnect the Dechoker Device with other equipment
or accessories that are not specied in this manual. The Dechoker is a
standalone device.
29
any replaceable parts. This device can
be reused by single patient.
DEVICE DISPOSABILITY
Dechoker device can be reused by
a single patient use device and may
also be disposed into a trash can.
The Dechoker does not contain any
biohazardous or hazardous materials.

30
DECHOKER LIMITED WARRANTY
RETURN POLICY AND AUTHORIZATION FORM
Dechoker LLC takes customer service seriously starting with returns. Dechoker
LLC oers a 30 day no questions asked return policy. We do not believe in
special rules, lots of ne print, or restocking fees. Simply complete the on line
form to begin the return process www.dechoker.com/pages/return-merchandiserequest . Once your return request is submitted, our trained team of customer
service specialist will take it from there. You will receive all the instructions on
how to complete your return via email.
31
PRIVACY
Dechoker LLC will maintain and use customer information in accordance with the
Dechoker LLC Customer Privacy Policy.
GENERAL
No Dechoker LLC reseller, agent, or employee is authorized to make any
modication, extension, or addition to this Warranty. If any term is held to be
illegal or unenforceable, the legality or enforceability of the remaining terms shall
not be aected or impaired. This Warranty is governed by and construed under
the laws of the country in which the Dechoker LLC Product purchase took place.
Dechoker LLC or its successor in title is the warrantor under this Warranty
Revision
A 5/25/16 John Popow Initial Re-
Eective
Date
Originator /
Reviser
Reason for
Release
lease
Impact of
Changes on
Reporting
Released into
doc control
and DMR

32
Contact
Dechoker LLC
North America
1-704-638-5445
EU Authorized Representative
+441704544944.
 Loading...
Loading...