Page 1

AquaLab
Water Activity Meter
Operator’s Manual
For Series 4TE, 4TEV, DUO
Version 4
Decagon Devices, Inc.
Page 2

Decagon Devices, Inc
2365 NE Hopkins Court
Pullman WA 99163
(509)332-2756
fax: (509)332-5158
www.aqualab.com
support@decagon.com
sales@decagon.com
Trademarks
AquaLab is a registered trademark of
Decagon Devices, Inc.
©2008-2009 Decagon Devices, Inc.
Page 3

Contents
1. Introduction .................................1
About this Manual ....................................1
Customer Support .....................................1
Warranty ...................................................2
Seller’s Liability ..........................................2
2. About AquaLab ...........................4
AquaLab Model and Options ....................4
AquaLab 4 Instrument Specifications ........4
AquaLab 4 DUO Specifications .................5
How AquaLab Works ................................6
AquaLab and Temperature ........................6
Chilled Mirror Dewpoint Limitations .......8
3. Water Activity eory ...................9
Moisture Content ......................................9
Water Activity............................................9
Water Potential ........................................12
Sorption Isotherms ..................................13
4. Getting Started ...........................15
Components of your AquaLab ................. 15
Choosing a Location ...............................15
Preparing AquaLab for Operation ............16
5. Menus ......................................... 18
Measurement Tab .................................... 18
i
Page 4

Configuration Tab ...................................19
Admin Settings ........................................25
Data Tab .................................................30
6. Cleaning and Maintenance ........ 32
Cleaning the Block and Sensors ...............33
Cleaning Procedure: ...............................34
Verification of Calibration .......................36
7. Verification and Calibration ........37
Water Activity Verification .......................37
Verification of Calibration .......................38
8. Sample Preparation ....................46
Preparing the Sample ...............................46
Samples Needing Special Preparation ......47
Slow Water-Emitting Samples ..................48
Volatile Samples .......................................49
Low Water Activity ..................................49
Samples not at Room Temperature ..........50
9. Taking a Reading ....................... 51
Measurement Steps ..................................51
How AquaLab Takes Readings ................ 51
10. Duo Operation (Optional) .......54
Obtaining Product Isotherm Models .......55
Loading and Organizing Product Models 55
11. Computer Interface ...................60
AquaLink RG ..........................................60
Using Windows Hyperterminal .............60
ii
Page 5

12. Troubleshooting .......................62
Diagnostic Screen ....................................72
13. Support and Repair .................. 73
Repair Costs ............................................ 74
Loaner Service .........................................74
14. Further Reading ....................... 75
Water Activity eory & Measurement ...75
Food Quality and Safety ..........................78
Water Activity and Microbiology .............80
Water Activity in Foods ...........................83
Appendix A .....................................93
Preparing Salt Solution ............................93
Appendix B .....................................95
Temperature Correction ..........................95
Appendix C .....................................96
AquaLab Calibration Standards ..............96
Declaration of Conformity ........... 101
Certificate of Traceability .............102
iii
Page 6

iv
Page 7

AquaLab
1. Introduction
1. In t r o d u c t I o n
Welcome to Decagon’s AquaLab Series 4TE, 4TEV, and DUO, the
industry standard for measuring water activity (aw). AquaLab is the
quickest, most accurate, and most reliable instrument available for
measuring water activity. Whether you are researching or working
on the production line, AquaLab will suit your needs. It is easy to
use and provides accurate and timely results.
About this Manual
Included in this manual are instructions for setting up your AquaLab,
verifying the calibration of the instrument, preparing samples, and
maintaining and caring for your instrument. Please read these instructions before operating AquaLab to ensure that the instrument
performs to its full potential.
Customer Support
If you ever need assistance with your AquaLab, or if you just have
questions or feedback, there are several ways to contact us:
NOTE: If you purchased your AquaLab through a distributor, please
contact them for assistance.
E-mail
support@decagon.com
Please include your name, contact information, instrument serial
number(s), and a description of your problem or question.
sales@decagon.com
Please include your name, address, phone number, the items you
wish to order and a purchase order number. Credit card numbers
should always be called in.
1
Page 8

AquaLab
1. Introduction
Phone
1-800-755-2751 (USA and Canada Only)
1-509-332-2756 International
Our Customer Support and Sales Representatives are available
Monday thru Friday.
Fax
1-509-332-5158
Warranty
AquaLab has a 30-day satisfaction guarantee and a three-year warranty on parts and labor. Your warranty is automatically validated
upon receipt of the instrument. We will contact you within the first
90 days of your purchase to see how the AquaLab is working for
you.
Seller’s Liability
Seller warrants new equipment of its own manufacture against defective workmanship and materials for a period of three years from
date of receipt of equipment (the results of ordinary wear and tear,
neglect, misuse, accident and excessive deterioration due to corrosion from any cause are not to be considered a defect); but Seller’s
liability for defective parts shall in no event exceed the furnishing
of replacement parts Freight On Board the factory where originally
manufactured. Material and equipment covered hereby which is
not manufactured by Seller shall be covered only by the warranty
of its manufacturer. Seller shall not be liable to Buyer for loss, dam-
2
Page 9

AquaLab
1. Introduction
age or injuries to persons (including death), or to property or things
of whatsoever kind (including, but not without limitation, loss of
anticipated profits), occasioned by or arising out of the installation,
operation, use, misuse, nonuse, repair, or replacement of said material and equipment, or out of the use of any method or process
for which the same may be employed. e use of this equipment
constitutes Buyer’s acceptance of the terms set forth in this warranty.
ere are no understandings, representations, or warranties of any
kind, express, implied, statutory or otherwise (including, but without limitation, the implied warranties of merchantability and fitness
for a particular purpose), not expressly set forth herein.
3
Page 10
AquaLab
2. About AquaLab
2. About AquaLab
AquaLab is the fastest and most accurate instrument for measuring
water activity, giving readings in five minutes or less. Its readings
are reliable, providing ±0.003 aw accuracy. e instrument is easy
to clean and checking calibration is simple.
AquaLab Model and Options
Series 4TE: User-selectable internal temperature control model,
uses thermoelectric (Peltier) components to maintain internal temperature.
Series 4TEV: Uses both a chilled-mirror dewpoint sensor and a capacitance sensor for measuring non-volatile and volatile substances,
respectively. Either sensor is easily selected using the instrument’s
menu system.
Series 4TE DUO: Uses chilled-mirror dewpoint and programmed
models obtained from isotherm data to give the user both water activity and moisture content simultaneously in five minutes or less.
AquaLab 4 Instrument Specifications
Water Activity Range: 0.050 to 1.000 a
Water Activity Accuracy: ±0.003 (4TE Dew Point Mode)
Water Activity Accuracy: ±0.015 (4TEV Capacitance Mode)
Water Activity Resolution: 0.0001
Read Time1: ≤5 min.
Sample Temperature Range: 15 to 50° C
Sample Temperature Accuracy: ±0.2° C
4
w
Page 11

AquaLab
2. About AquaLab
Sample Temperature Resolution: 0.01° C
Sample Dish Capacity: 15ml Full
Operating Environment: 5 to 50° C 20 to 80% Humidity
Case Dimensions: 26.7 x 17.8 x 12.7cm
Weight: 3.1 Kg
Case Material: Lustran 433 (ABS) with fire retardant
Display: 64 x 128 Graphical
Data Communications: RS232A Serial, 9600 to 115200 baud
Power: 110 to 220 VAC, 50/60Hz
Warranty: 3 year parts and labor
1
On samples with no significant impedance to vapor loss
AquaLab 4 DUO Specifications
Moisture Content Repeatability: 0.02%
Accuracy to Moisture Content Ref.: 0.1% to 0.5%
AquaLab and Water Activity
Water activity (aw) is a measurement of the energy status of the water
in a system. It indicates how tightly water is “bound”, structurally
or chemically, within a substance. Water activity is the relative humidity of air in equilibrium with a sample in a sealed measurement
chamber. e concept of water activity is of particular importance
in determining product quality and safety. Water activity influences
color, odor, flavor, texture and shelf-life of many products. It predicts safety and stability with respect to microbial growth, chemical
and biochemical reaction rates, and physical properties. For a more
detailed description of water activity as it pertains to products,
please refer to Chapter 3 of this manual, titled “ Water Activity
eory”.
5
Page 12

AquaLab
2. About AquaLab
How AquaLab Works
AquaLab uses the chilled-mirror dewpoint technique to measure the
water activity of a sample. In an instrument that uses the dewpoint
technique, the sample is equilibrated with the head-space of a sealed
chamber that contains a mirror and a means of detecting condensation on the mirror. At equilibrium, the relative humidity of the air
in the chamber is the same as the water activity of the sample. In
the AquaLab, the mirror temperature is precisely controlled by a
thermoelectric (Peltier) cooler. Detection of the exact point at which
condensation first appears on the mirror is observed with a photoelectric cell. A beam of light is directed onto the mirror and reflected
into a photo detector cell. e photo detector senses the change
in reflectance when condensation occurs on the mirror. A thermocouple attached to the mirror then records the temperature at which
condensation occurs. AquaLab then signals you by beeping and displays the final water activity and temperature.
In addition to the technique described above, AquaLab uses an internal fan that circulates the air within the sample chamber to reduce
equilibrium time. Since both dewpoint and sample surface temperatures are simultaneously measured, the need for complete thermal
equilibrium is eliminated, which reduces measurement times to less
than five minutes.
AquaLab and Temperature
Samples not read at room temperature during the read cycle will
equilibrate with the AquaLab’s temperature before the water activity
is displayed. Large temperature differences will cause longer reading
times, since a complete and accurate reading will not be made until
the sample and the instrument are within 2°C of each other.
6
Page 13

AquaLab
2. About AquaLab
ere are several advantages in having a temperature-controlled water activity meter. A few major reasons are:
1. Research purposes. Temperature control can be used to study
the effects of temperature on the water activity of a sample, make a
comparison of the water activity of different samples independent
of temperature, and conduct accelerated shelf-life studies or other
water activity studies where temperature control is critical. ere are
many shelf-life, packaging, and isotherm studies in which temperature control would be very beneficial.
2. To comply with government or internal regulations for specific
products. ough the water activity of most products varies by less
than ± 0.002 per °C, some regulations require measurement at a specific temperature. e most common specification is 25°C, though
20°C is sometimes indicated.
3. To minimize extreme ambient temperature fluctuations. If the
environmental and AquaLab temperatures fluctuate by as much as ±
5°C daily, water activity readings will vary by ± 0.01 aw. Temperature
control eliminates variations due to changes in ambient conditions.
Series 4TE/4TEV/4TE-DUO
e AquaLab Series 4TE models have thermoelectric components
installed to allow the instrument to maintain a set chamber temperature. e temperature is set using the configuration menu of any
of the Series 4 models.
7
Page 14

AquaLab
2. About AquaLab
Chilled Mirror Dewpoint Limitations
AquaLab’s limitation is its ability to accurately measure samples with
high concentrations (typically >1%) of certain volatiles such as ethanol or propylene glycol, which can condense on the surface of the
chilled mirror. e extent of the effect is determined by how readily
the material volatilizes, which is both concentration- and matrixdependent. erefore, even if your sample contains materials that
could volatilize, it may still be possible to make accurate readings
using the chilled mirror dewpoint sensor.
AquaLab Series 4TEV which incorporates both a chilled mirror sensor and a capacitance sensor for measuring volatile substances is
Decagon’s solution for products containing volatile materials. If you
are unsure if you need the TEV model, please call and discuss your
product with a Decagon Representative. Refer to Chapter 8’s section
titled ”Volatile Samples” or call Decagon for more details.
8
Page 15

AquaLab
3. Water Activity eory
3. Water Activity eory
Water is a major component of foods, pharmaceuticals, and cosmetics. Water influences the texture, appearance, taste and spoilage of these
products. ere are two basic types of water analysis: moisture content
and water activity.
Moisture Content
e meaning of the term moisture content is familiar to most people.
It implies a quantitative analysis to determine the total amount of
water present in a sample. Primary methods for determining moisture content are loss on drying and Karlf Fisher titration, but secondary methods such as infrared and NMR are also used. Moisture
content determination is essential in meeting product nutritional
labeling regulations, specifying recipes and monitoring processes.
However, moisture content alone is not a reliable indicator for predicting microbial responses and chemical reactions in materials.
e limitations of moisture content measurement are attributed to
differences in the intensity with which water associates with other
components.
Water Activity
Water activity is a measure of the energy status of the water in a
system, and thus is a far better indicator of perishability than water
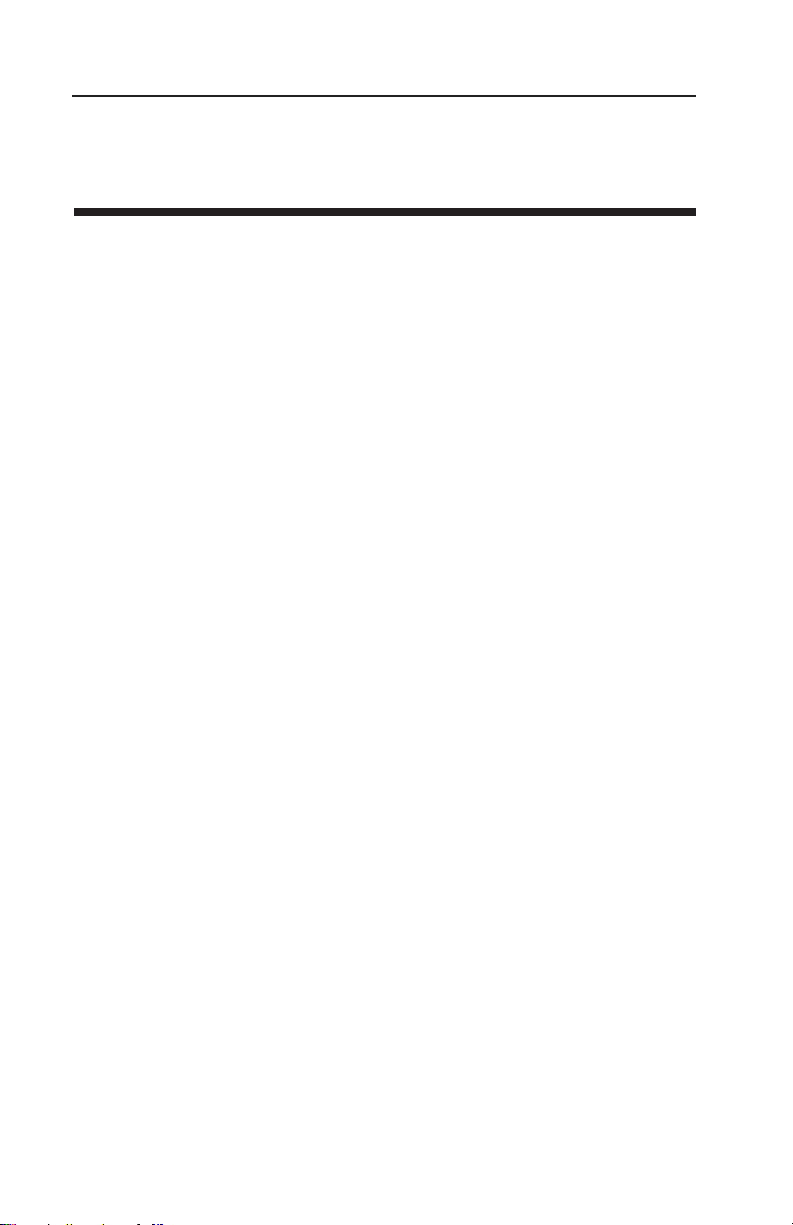
content. Figure 1 shows how the relative activity of microorganisms,
lipids and enzymes relate to water activity. While other factors, such
as nutrient availability and temperature, can affect the relationships,
water activity is the best single measure of how water affects these
processes.
9
Page 16
AquaLab
3. Water Activity eory
Fig. 1: Water Activity Diagram—adapted from Labuza
Water activity of a system is measured by equilibrating the liquid
phase water in the sample with the vapor phase water in the headspace and measuring the relative humidity of the head-space. In
the AquaLab, a sample is placed in a sample cup which is sealed
inside a sample chamber. Inside the sample chamber is a fan, a dew
point sensor, a temperature sensor, and an infrared thermometer.
e dewpoint sensor measures the dewpoint temperature of the air
in the chamber, and the infrared thermometer measures the sample
temperature. From these measurements, the relative humidity of
the head-space is computed as the ratio of dewpoint temperature
saturation vapor pressure to saturation vapor pressure at the sample
temperature. When the water activity of the sample and the relative humidity of the air are in equilibrium, the measurement of the
head-space humidity gives the water activity of the sample. e purpose of the fan is to speed equilibrium and to control the boundary
layer conductance of the dewpoint sensor.
10
Page 17

AquaLab
3. Water Activity eory
In addition to equilibrium between the liquid phase water in the
sample and the vapor phase, the internal equilibrium of the sample
is important. If a system is not at internal equilibrium, one might
measure a steady vapor pressure (over the period of measurement)
which is not the true water activity of the system. An example of
this might be a baked good or a multi-component food. Initially out
of the oven, a baked good is not at internal equilibrium; the outer
surface is at a lower water activity than the center of the baked good.
One must wait a period of time in order for the water to migrate
and the system to come to internal equilibrium. It is important to
remember the restriction of the definition of water activity to equilibrium.
Temperature Effects
Temperature plays a critical role in water activity determination.
Most critical is the measurement of the difference between sample
and dewpoint temperature. If this temperature difference were in error by 1°C, an error of up to 0.06 aw could result. In order for water
activity measurements to be accurate to 0.001, temperature difference measurements need to be accurate to 0.017°C. AquaLab’s infrared thermometer measures the difference in temperature between
the sample and the block. It is carefully calibrated to minimize temperature errors, but achieving 0.017°C accuracy is difficult when
temperature differences are large. Best accuracy is therefore obtained
when the sample is near chamber temperature.
Another effect of temperature on water activity occurs when samples are near saturation. A sample that is close to 1.0 aw and is only
slightly warmer than the sensor block will condense water within the
block. is will cause errors in the measurement, and in subsequent
measurements until the condensation disappears. A sample at 0.75 aw
needs to be approximately 4°C above the chamber temperature to
11
Page 18

AquaLab
3. Water Activity eory
cause condensation. e AquaLab warns the user if a sample is more
than 4°C above the chamber temperature, but for high water activity
samples the operator needs to be aware that condensation can occur
if a sample that is warmer than the block is put in the AquaLab.
Water Potential
Some additional information may be useful for understanding what
water activity is and why it is such a useful measure of moisture
status in products. Water activity is closely related to a thermodynamic property called the water potential, or chemical potential (µ)
of water, which is the change in Gibbs free energy (∆G) when water
concentration changes. Equilibrium occurs in a system when (µ) is
the same everywhere in the system. Equilibrium between the liquid
and the vapor phases implies that () is the same in both phases. It
is this fact that allows us to measure the water potential of the vapor
phase and use that to determine the water potential of the liquid
phase. Gradients in (µ) are driving forces for moisture movement.
us, in an isothermal system, water tends to move from regions of
high water potential (high aw) to regions of low water potential (low
aw). Water content is not a driving force for water movement, and
therefore can not be used to predict the direction of water movement, except in homogeneous materials.
Factors In Determining Water Potential
e water potential of the water in a system is influenced by factors
that effect the binding of water. ey include osmotic, matric, and
pressure effects. Typically water activity is measured at atmospheric
pressure, so only the osmotic and matric effects are important.
Osmotic Effects
Osmotic effects are well known from biology and physical chemistry. Water is diluted when a solute is added. If this diluted water is
12
Page 19

AquaLab
3. Water Activity eory
separated from pure water by a semi-permeable membrane, water
tends to move from the pure water side through the membrane to
the side with the added solute. If sufficient pressure is applied to the
solute-water mixture to just stop the flow, this pressure is a measure
of the osmotic potential of the solution. Addition of one mole of an
ideal solute to a kilogram of water produces an osmotic pressure of
22.4 atm. is lowers the water activity of the solution from 1.0 to
0.98 aw. For a given amount of solute, increasing the water content
of the systems dilutes the solute, decreasing the osmotic pressure,
and increasing the water activity. Since microbial cells are high concentrations of solute surrounded by semi-permeable membranes,
the osmotic effect on the free energy of the water is important for
determining microbial water relations and therefore their activity.
Matric Effects
e sample matrix affects water activity by physically binding water
within its structure through adhesive and cohesive forces that hold water
in pores and capillaries, and to particle surfaces. If cellulose or protein
were added to water, the energy status of the water would be reduced.
Work would need to be done to extract the water from this matrix. is
reduction in energy status of the water is not osmotic, because the cellulose or protein concentrations are far too low to produce any significant
dilution of water. e reduction in energy is the result of direct physical
binding of water to the cellulose or protein matrix by hydrogen bonding
and van der Waal forces. At higher water activity levels, capillary forces
and surface tension can also play a role.
Sorption Isotherms
Relating Water Activity to Water Content
Changes in water content affect both the osmotic and matric binding of water in a product. us a relationship exists between the
water activity and water content of a product. is relationship is
13
Page 20

AquaLab
3. Water Activity eory
called the sorption isotherm, and is unique for each product. Besides
being unique to each product, the isotherm changes depending on
whether it was obtained by drying or wetting the sample. ese factors need to be kept in mind if one tries to use water content to infer
the stability or safety of a product. Typically, large safety margins are
built into water content specifications to allow for these uncertainties.
While the sorption isotherm is often used to infer water activity from
water content, one could easily go the other direction and use the
water activity to infer the water content. is is particularly attractive because water activity is much more quickly measured than water content. is method gives particularly good precision in the
center of the isotherm. In order to infer water content from water
activity, one needs an isotherm for the particular product. Decagon
sells an Isotherm Generator called the AquaSorp IG or you can also
have Decagon run the isotherm for a fee.
For example, if one were using the AquaLab to monitor the water
content of dried potato flakes, one would measure the water activity
and water content of potato flakes dried to varying degrees using the
standard drying process for those flakes. An isotherm would be constructed using those data, and the water content would be inferred
using the measured water activity of samples and that isotherm. We
have an upgrade available to Series 4TE users that would allow you
to determine moisture content and water activity simultaneously.
is instrument is called the Series 4TE DUO.
e importance of the concept of water activity of foods, pharmaceuticals, and cosmetics cannot be over emphasized. Water activity is
a measure of the energy status of the water in a system. More importantly, the usefulness of water activity in relation to microbial growth,
chemical reactivity, and stability over water content has been shown.
14
Page 21

AquaLab
4. Getting Started
4. Getting Started
Components of your AquaLab
Your AquaLab should have been shipped with the following items:
AquaLab water activity meter•
Calibration Certificate•
Power cord•
RS-232 interface cable•
100 disposable sample cups•
Operator’s Manual•
Quick Start guide•
Cleaning Kit•
3 vials each of the following verification solutions:•
1.000 a
0.760 a
0.500 aw 8.57 molal LiCl
0.250 aw 13.41 molal LiCl
Distilled Water
w
6.0 molal NaCl
w
Choosing a Location
To ensure that your AquaLab operates correctly and consistently,
place it on a level surface. is reduces the chance that sample material will spill and contaminate the sample chamber. Also select a
location where the temperature remains fairly stable to avoid temperature changes that can affect accuracy. is location should be
well away from air conditioner and heater vents, open windows, etc.
Place the AquaLab in a location where cleanliness can be maintained
to prevent contamination of the sample chamber.
15
Page 22
AquaLab
4. Getting Started
Preparing AquaLab for Operation
After finding a good location for your AquaLab, plug the power cord
into the back of the unit. e ON/OFF switch is located on the
lower left corner of the AquaLab’s back panel. When the AquaLab
is turned on, you should see a model name/number screen and then
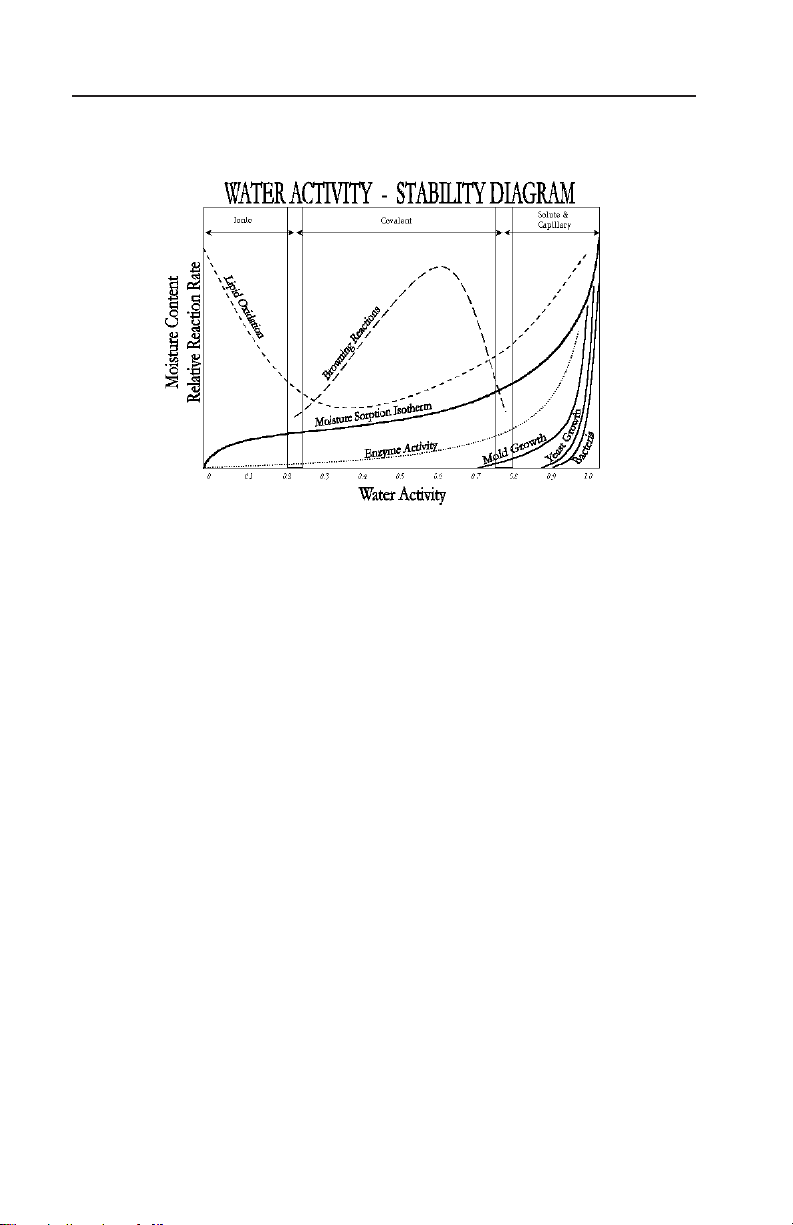
the main screen as shown below.
e main screen shows the water activity (aw) in the middle of the
screen and the sample temperature right below. On the Series 4TEV
model you will also see either DEW or CAP indicating whether you
are using the dewpoint or capacitance sensor respectively.
NOTE: In order to provide the most accurate readings, your AquaLab
should be allowed a 15 minute warm-up period.
16
Page 23
AquaLab
4. Getting Started
If users have been setup on the instrument, the following screen will
appear instead of the main screen. (See Chapter 5 for more information on administrative settings and user setup).
Select the appropriate user and login to begin.
17
Page 24
AquaLab
5. Menus
5. Menus
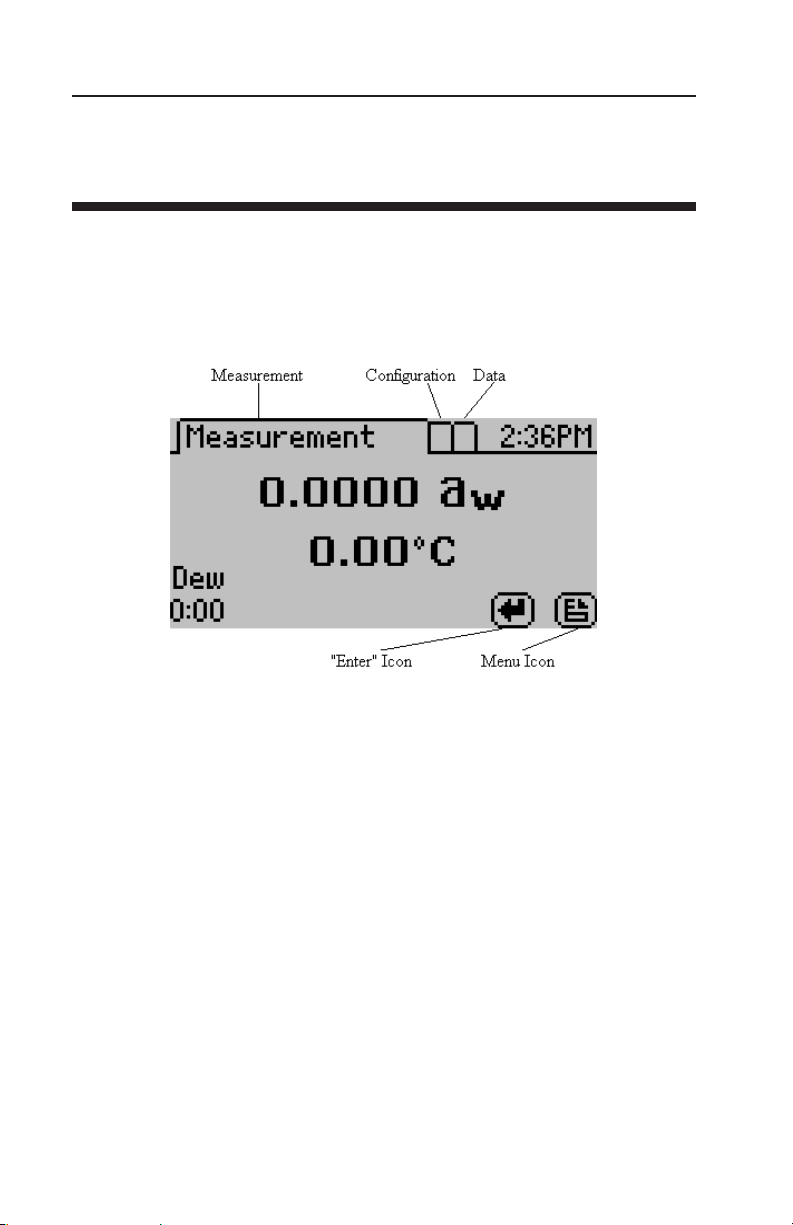
At the top of the display screen there are three tabs: Measurement,
Configuration, and Data. ese tabs indicate the three menus you
can access. To change between the tabs press the right most button
below the document icon.
e enter icon is the read or enter button. Once the latch is set to the
read position, the document icon will switch to an “X” icon, which
allows the user to stop the current reading. During a reading, pressing enter again will restart the reading.
Measurement Tab
e measurement tab, as seen above, is the main screen which displays each time you turn on your AquaLab. If this screen doesn’t
appear, refer to Chapter 12 for troubleshooting instructions. As
mentioned earlier, the water activity and sample temperature are
displayed on the screen.
18
Page 25
AquaLab
5. Menus
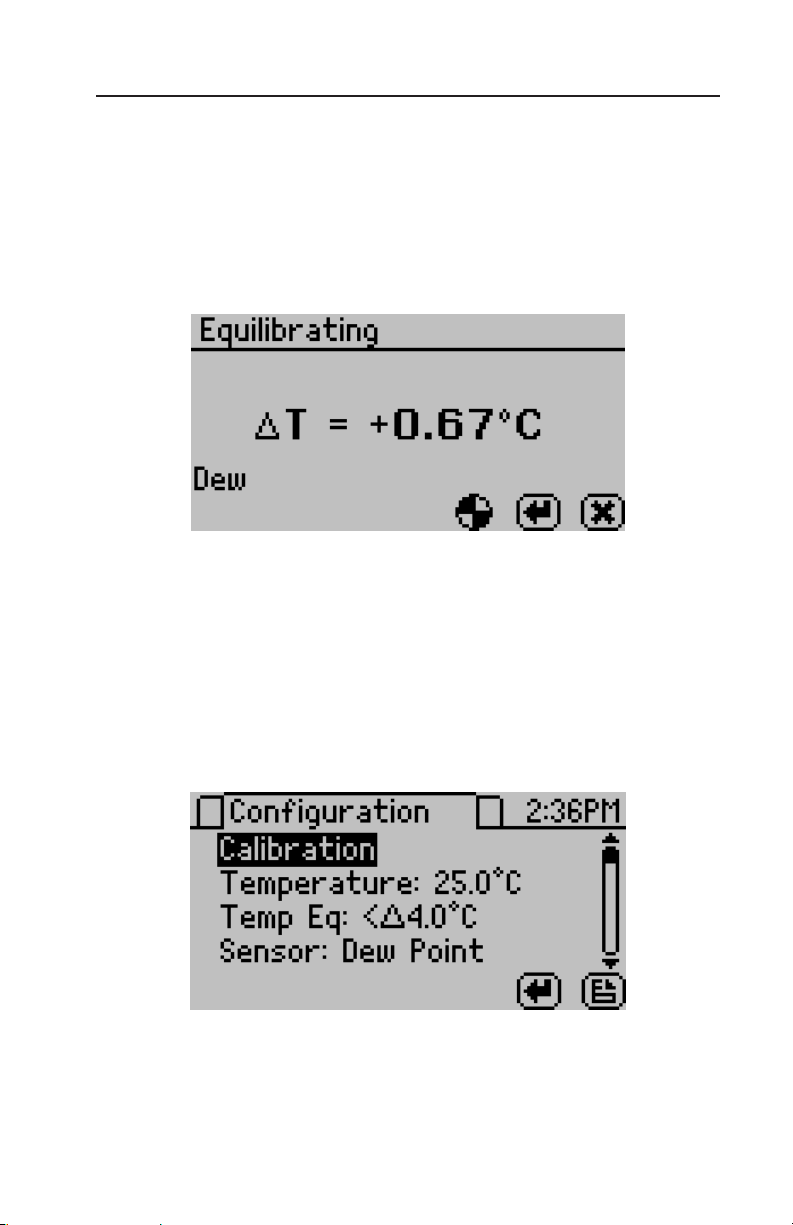
Pushing the right or left arrow keys will change the display to a
temperature equilibration screen shown below. is screen shows the
temperature difference between the sample temperature and the block
temperature.
Configuration Tab
When at the configuration screen, pressing the up and down arrow
keys moves the cursor through the various configuration options
Press the left and right arrows to page through the options. e
enter button will allow you to change the highlighted setting.
19
Page 26
AquaLab
5. Menus
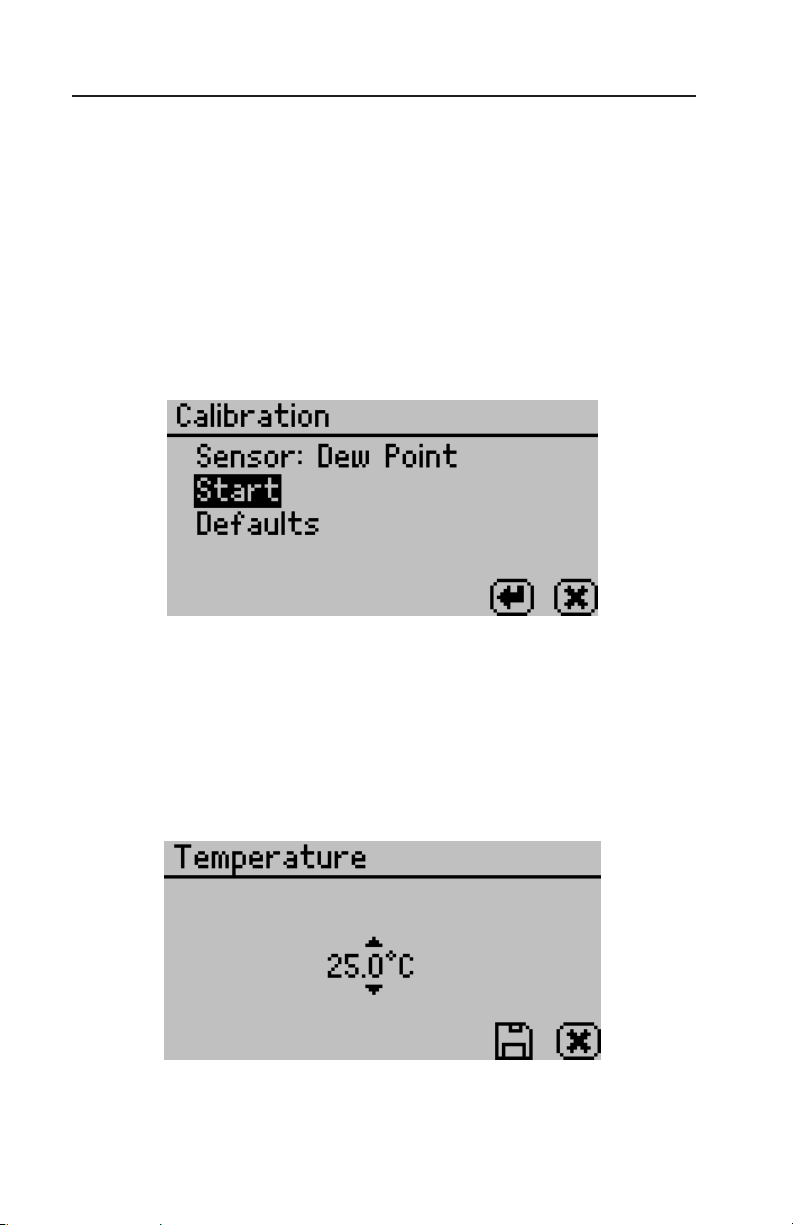
Calibration:
Pressing the Enter button when Calibration is highlighted starts the
verification process. For more details on the verification procedure
refer to Chapter 7. You may also reset the calibration to the factory defaults by highlighting the Defaults option and pressing Enter.
is will reset all options to the way they were when the instrument
arrived at your location.
Temperature:
e default temperature is 25°C. Press the enter button to change
the temperature setting. e AquaLab Series 4TE models may be set
between 15 and 50°C by 0.1°C intervals. Using the up and down
arrows, set the AquaLab to your desired temperature and press the
save button.
20
Page 27
AquaLab
5. Menus
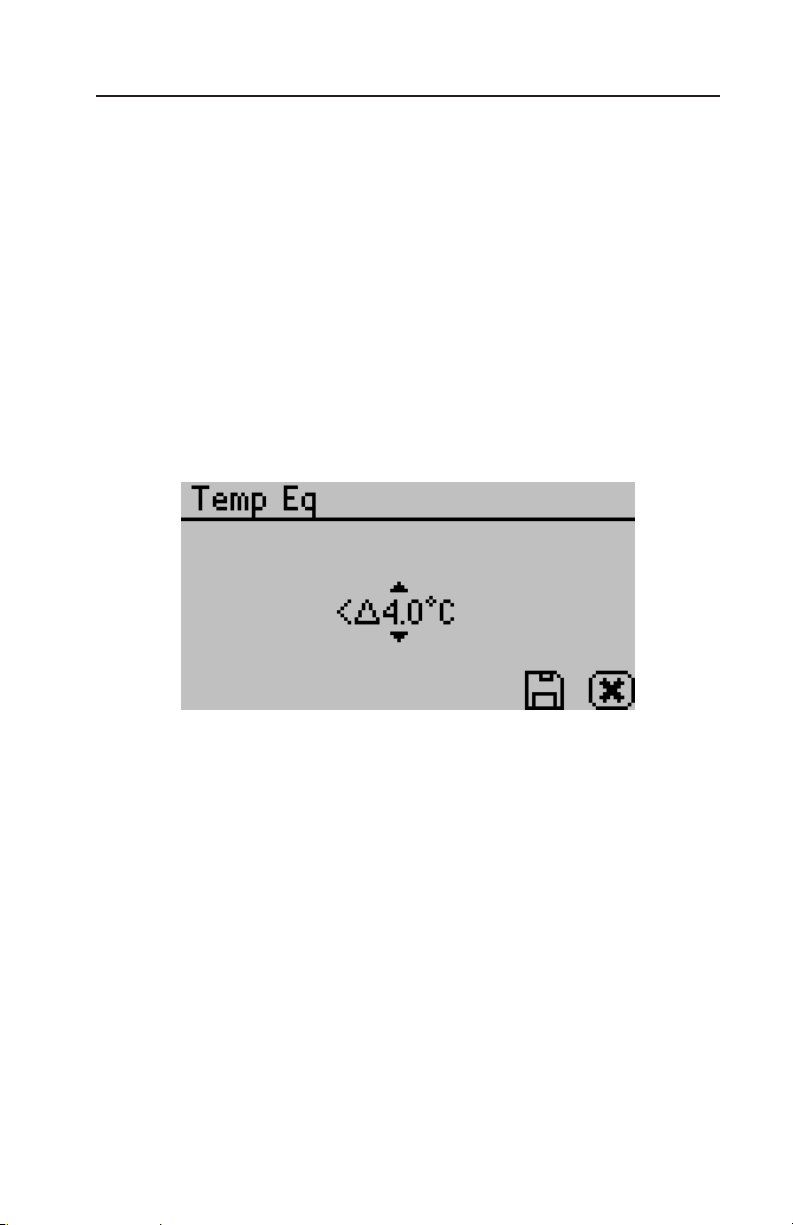
Temp Eq:
e Temperature Equilibration option allows you to set the level of
temperature equilibration desired before the water activity measurement begins. e range is 0.5 to 4.0°C. A setting of 4.0°C begins
the measurement immediately (assuming the sample is not >4.0°C
above or below the block temperature). A setting of 0.5 °C will
cause the instrument to wait until the sample temperature is within
<0.5°C of the block temperature before starting the water activity
measurement.
Sensor:
In the AquaLab Series 4TEV model only, this option indicates the
selected sensor type, either dewpoint or capacitance (e Series 4TE
models will always be Dewpoint). Pressing Enter when the Sensor
option is highlighted allows you to change between a chilled mirror
dewpoint or capacitance sensor for sampling with or without volatiles, respectively.
21
Page 28

AquaLab
5. Menus
Mode:
Users may choose between single, continuous, or custom mode by
pushing the save button.
Single Mode
Single mode reads the sample once, after which the instrument notifies you that it is finished and the water activity and temperature are
displayed on the screen.
Continuous Mode
Continuous mode reads your sample until you open the chamber
lid or stop the test using the stop button. AquaLab reads the sample,
displays the water activity and temperature, then begins another read
cycle without further input from the user. Between samples, the machine will signal you with beeps. is mode eliminates the possibility
of moisture exchange with the environment outside the chamber in
between readings. A time on the bottom left of the screen tracks the
cumulative read time. All readings taken during continuous mode
are saved on the instrument’s memory if the autosave feature is selected (see Auto Save below). If AquaLab is connected to a computer
using AquaLink RG (See Chapter 11), all readings taken during continuous mode will be downloaded to the AquaLink RG software.
Custom Mode
Custom mode allows a sample to be read multiple times until
a desired level of stability is achieved. e user determines how
many consecutive tests they want to be within a given water activity stability setting. For instance, the customer can choose to
have 4 consecutive tests be within +/- 0.001aw. e instrument
will continue to run tests until it records 4 tests that are within +/-
0.001aw and then will stop and report the value of the final test. If
autosave is turned on, all test readings will be saved to the instruments memory, but only the final reading will appear on the main
22
Page 29
AquaLab
5. Menus
measurement screen. If AquaLab is connected to a computer using
AquaLink RG (See Chapter 11), all readings taken during a custom mode test will be downloaded to the AquaLink RG software.
On the mode screen, at the top of the page, will appear the current
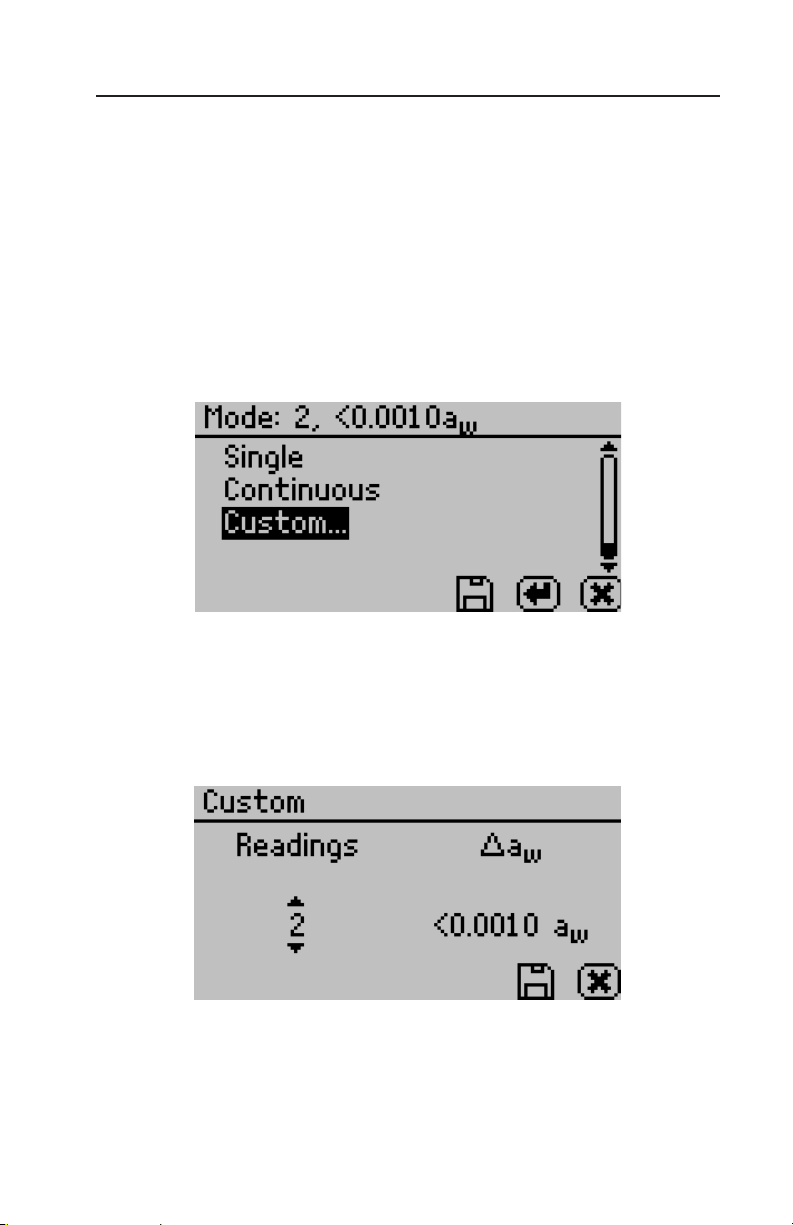
mode settings with the number of tests appearing first, followed by the
stability value (∆aw). Pressing enter with the custom mode highlighted will allow the number of tests and stability settings to be changed.
To change the number of readings, use the right/left arrow buttons to highlight the number under Readings, and then use the
up and down buttons to change to any value between 2 and 9.
To change the stability setting, use the right/left arrow buttons to
23
Page 30
AquaLab
5. Menus
highlight the number under ∆aw, and then use the up and down buttons to change to any value between 0.0005 and 0.0030. To save the
settings and finish, press the save button (to exit without updating,
press the cancel button). e mode screen will now appear with the
updated custom settings appearing at the top of the screen. Press the
save button to return to the configuration screen and begin using the
custom mode (To exit without updating, press the cancel button).
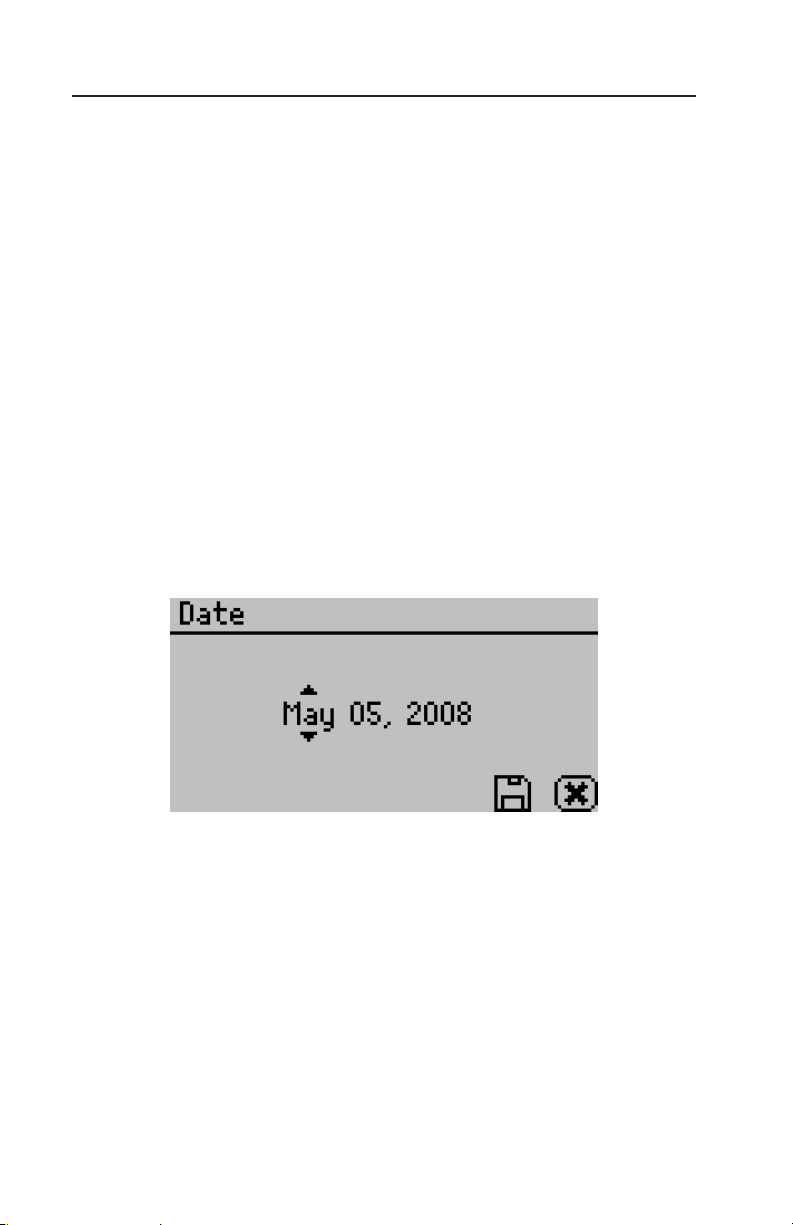
Date:
AquaLab Series 4 models now have an internal calendar and clock.
e time and date are recorded with each water activity reading.
Pressing Enter when the Date option is highlighted allows you to set
the date in the instrument. Press the left and right arrows to change
between the month, day and year. Press the up or down arrows to
change any of the individual values.
Time:
Pressing Enter when the Time option is highlighted allows you to
set the current local time. Press the up or down arrows to change
any of the individual values. Press the left or right buttons to change
between hour and minutes. e hour setting automatically changes
between AM and PM.
24
Page 31

AquaLab
5. Menus
Regional Formatting:
Allows you to configure how all Series 4 models will display information. You may choose the temperature scale (Celsius vs Fahrenheit), the date display (mm/dd/yy vs. dd/mm/yy), the hour format
(12 vs 24 hour) and the language.
Admin Settings
Allows you to create an administrator password as well as create, edit
and delete additional users.
25
Page 32

AquaLab
5. Menus
e admin option allows the administrator to grant or block access
to some or all of the configuration options in all Series 4 models. For
example: If the administrator wanted to make sure that all samples
were read at 25°C the administrator would set their temperature to
25°C and then would lock all other users out of that configuration
screen. is is accomplished by entering the Access function and
selecting the desired option to toggle it on and off.
Additionally you can lock and unlock all of them at once. For example, if you do not want John Doe changing the instruments measurement temperature, the administrator can lock that function for John.
e areas that can be locked are calibration, temperature, temperature equilibration, sensor selection, mode, date/time, region, password, auto-save, number of beeps, contrast, and delete functions.
26
Page 33

AquaLab
5. Menus
User Setup:
Users can be added, edited or deleted from this screen. An alphabet
screen will appear where a name can be entered using lower case,
upper case and accents.
NOTE: User setup is not required for instrument operation. It is in place
for users wanting to be compliant with 21 CFR Part 11 or who want to
maintain the settings they have selected.
Auto Save:
AquaLab Series 4 models have the ability to store water activity
readings within the instrument. By selecting Auto Save “On” every
water activity reading will be automatically stored in the instruments
internal memory. AquaLab Series 4 can store up to 10,000 records
before the memory is full. If you select Auto Save “off” then no
data is automatically stored, although any individual reading may be
manually stored at any time.
To manually store a water activity or append an annotation to the
active reading that has been autosaved. Press the save icon button
after the water activity measurement is completed. Pressing the icon
opens a “name” screen. You may give this reading a name by pressing the arrow buttons to highlight the letter and then pressing the
27
Page 34

AquaLab
5. Menus
“Check” icon button. Press the save icon to save this data record
with the name you have specified.
NOTE: Pressing the save icon button without giving it a name will save
the reading without a name.
Beeps:
Allows you to set the reading finished notification from 4 beeps to
continuous beeps. You may also turn the audible notification off.
Contrast:
Allows you to set the contrast of the screen to your liking. Viewing
the screen from a sitting versus a standing position may require contrast adjustment for the best visibility in that position.
Diagnostics:
For the chilled-mirror dewpoint sensor it provides you with lid, base,
sample and mirror temperature as well as an optical voltage.
28
Page 35

AquaLab
5. Menus
For the capacitance sensor (TEV Models only) it provides you lid,
base, and sample temperatures as well as relative humidity.
About:
is screen provides important information including the serial
number and code version of your instrument.
29
Page 36

AquaLab
5. Menus
Data Tab
View:
is selection will allow you to view your stored measurements. e
up/down arrows will move you through the stored data with the
most recent measurements at the top of the table. You may also press
the left and right arrows to page quickly through the data. See Chapter
11: Computer Interface for information about downloading these
readings to a computer.
When you are viewing the summary screen, you may press the enter
button on a highlighted reading to get detailed information on the
reading as shown below.
30
Page 37

AquaLab
5. Menus
e information shown is the water activity of the sample, the temperature, the time the reading took, the user who ran the test (if
setup), the date of the reading, the sensor used (4TEV only), the
time the reading was taken, and the sequence number of the stored
reading.
Delete:
Selecting this option will delete all of the information currently
stored in the instrument. If you have not backed up this information
you will be reminded of this by the following message:
NOTE: You will NOT be able to recover deleted data.
31
Page 38

AquaLab
6. Cleaning and Maintenance
6. Cleaning and Maintenance
Keeping your AquaLab clean is vital to maintaining the accuracy
of your instrument. Dust and sampling debris can contaminate the
sampling chamber and must therefore be regularly cleaned out. To
clean your instrument, carefully follow these instructions and refer
to the labeled diagram below.
32
Page 39

AquaLab
6. Cleaning and Maintenance
Purpose
e purpose for the cleaning procedure is to remove grease, dirt and
other soluble substances which can absorb/release water during verification, calibration, and/or sample testing. For a smooth and even
dew formation, it requires the mirror to be perfectly clean. If there
are any contaminants (e.g. fingerprints) on the mirror, the dew will
form unevenly and thus affect the accuracy of the reading.
Materials Needed
A thin plastic rod or other non-metal implement•
Distilled Water•
Isopropyl Alcohol (IPA) or Decagon Cleaning Solution•
Kimwipes®•
You may also purchase the AquaLab Cleaning Kit which comes
with all the above materials except the Isopropyl Alcohol and Distilled Water.
NOTE: Wash your hands with soap and water and/or use clean lab gloves
before starting the cleaning procedure. is will prevent oils from contaminating the cleaning materials, the sample chamber and/or the sensors.
Cleaning the Block and Sensors
Accessing the Sample Chamber
Turn the power off on your AquaLab. If latched, move the lever
over to the open position. Lift the chamber cover to expose the
sample chamber and sensors. e sample chamber consists of all
surfaces inside the red o-ring when the lid is closed.
NOTE for Series 4TEV: If cleaning an AquaLab Series 4TEV, follow
the cleaning procedures listed below being careful not to get cleaning
solution or alcohol on the capacitance sensor filter (see illustration on
33
Page 40

AquaLab
6. Cleaning and Maintenance
previous page) Repeated exposure of cleaning materials or contaminants
to the filter may cause inaccurate readings. If the filter appears contaminated, replace it while being careful not to disturb the sensor behind the
filter.
Cleaning Procedure:
Cleaning your AquaLab is a multi-step procedure which involves
washing, rinsing, and drying for each specific area as outlined
below:
Cleaning the Sample Chamber1.
Note: Be extremely careful not to damage the fan blades (see illustration) when cleaning the chamber.
Remove any debris that may have collected within or around a.
the sample chanber.
Wrap a NEW Kimwipe around the end of the thin plastic b.
rod (spatula) and moisten it with isopropyl alcohol or Decagon Cleaning Solution. Note: Do NOT dip a used Kimwipe
into your container of IPA or cleaning solution (the IPA or
cleaning solution will become contaminated).
WASHClean upper chamber, o-ring, and all surfaces of the c.
block within the o-ring. You may need to replace the Kimwipe if it becomes too dirty during this process.
Clean lower block with a fresh Kimwipe. Be sure to clean d.
the entire block surface.
RINSERepeat steps b-d using new Kimwipes with distilled e.
water.
DRYRepeat steps b-d using new, dry Kimwipes to help f.
remove any moisture remaining from the cleaning.
Visually inspect the sample chamber for cleanliness. Re-clean g.
if necessary. Note: Do not reuse Kimwipes.
34
Page 41

AquaLab
6. Cleaning and Maintenance
Clean the Mirror2.
Wrap a new Kimwipe around the end of the thin plastic rod a.
(spatula) and moisten it with isopropyl alcohol or Decagon
Cleaning Solution.
WASHSwipe the moistened Kimwipe across the mirror b.
once. (A single swipe is usually sufficient to remove contaminants.)
RINSERepeat steps a-b using new Kimwipes moisted with c.
distilled water instead of cleaning solution.
DRYRepeat steps a-b using new, dry Kimwipes to help d.
remove any moisture remaining from the cleaning.
Visually inspect the mirror for cleanliness. Re-clean if neces-e.
sary.
Clean the ermopile and Optical Sensor3.
Wrap a new Kimwipe around the end of the thin plastic rod a.
(spatula) and moisten it with isopropyl alcohol or Decagon
Cleaning Solution.
WASHSwipe the moistened Kimwipe across thermopile b.
and optical sensor. (A single swipe across the sensor is usually sufficient to remove contaminants.)
RINSERepeat steps a-b using new Kimwipes moistened c.
with distilled water instead of cleaning solution.
DRYRepeat steps a-b but use a new, d. dry Kimwipe to help
remove any moisture remaining from the cleaning.
Visually inspect the thermopile and optical sensor for cleanli-e.
ness. Re-clean if necessary.
Additional Drying Time4.
Visually inspect the sample chamber and sensors for contami-a.
nants, including moisture. If necessary, repeat the cleaning
process using new Kimwipes.
Let stand for about 5 minutes to ensure the sample chamber b.
is dry.
35
Page 42

AquaLab
6. Cleaning and Maintenance
Verification of Calibration
After you have cleaned the chamber and other parts of your AquaLab,
it is important to check the instrument’s performance in order to
correct for any linear offset that may have occurred during the cleaning process.
Before you check the instrument we recommend that you run a
sample of the activated charcoal pellets provided in your AquaLab
cleaning kit. is cleans the air inside the chamber, helping it come
back to a stable sampling environment.
Verify the linear offset against known verification standards according to the procedure described in the next chapter. If a linear offset
has occurred, refer to “adjust for linear offset” section in Chapter 7
for directions on how to correct for linear offset. If, after adjusting
for linear offset, your instrument is still not reading samples correctly, please contact Decagon for support.
36
Page 43

AquaLab
7. Verification and Calibration
7. Verification and Calibration
It is important to verify AquaLab’s water activity calibration against
known standards to guarantee optimal performance and accuracy.
Decagon recommends verification daily, once per shift or before
each use.
Water Activity Verification
AquaLab uses the chilled-mirror dewpoint technique to determine
water activity. Because this is a primary measurement of relative humidity, no calibration is necessary; however, it is important to verify
for linear offset periodically. e components used by the instrument to measure water activity are subject to contamination which
may affect the AquaLab’s performance. When this occurs, it changes
the accuracy of the instrument. is is what is called a “linear offset.”
erefore, frequent verification assures you that your AquaLab is
performing correctly. Linear offset is checked by using two different
verification standards.
Verification Standards
Verification standards are specially prepared unsatuarated salt solutions having a specific molality and water activity constant which are
accurately measurable. e verification standards that were sent with
your initial shipment are very accurate and readily available from
Decagon. Using verification standards to verify accuracy can greatly
reduce preparation errors. For these reasons, we recommend using
standards available through Decagon for the most accurate verification of your AquaLab’s performance.
Performance Verification Standards come in five water activity levels: 1.000, 0.984, 0.760, 0.500, and 0.250 aw. e standards are
37
Page 44

AquaLab
7. Verification and Calibration
produced under a strict quality assurance regime. Please contact
Decagon Devices to order additional standards via sales@decagon.
com or 1-800-755-2751.
Verication Standard
@ 25°C
Distilled Water 1.000 ±0.003
0.5m KCl 0.984 ±0.003
6.0m NaCl 0.760 ±0.003
8.57m LiCl 0.500 ±0.003
13.41m LiCl 0.250 ±0.003
NOTE: If you need to obtain a Material Safety Data Sheet (MSDS)
for any of these standards, a printable version is available on our website at www.decagon.com/msds.
To use a verification standard, remove the twist top and pour the
contents into an AquaLab sample cup. If for some reason you cannot obtain Decagon’s verification standards and need to make a saturated salt solution for verification, refer to Appendix A.
Water Activity
Verification of Calibration
When to Verify for Linear Offset
Linear offset should be checked against two known verification
standards daily, either once per shift or before each use. Linear offset
should never be verified solely against distilled water, since it does
not give an accurate representation of the linear offset. For batch processing, the instrument should be checked regularly against a known
standard of similar water activity. It is also a good idea to check
the offset with a standard of similar water activity when the general
38
Page 45

AquaLab
7. Verification and Calibration
water activity range of your sample is changing. Checking the water
activity of a standard solution will alert you to the possibility of unit
contamination or shifts in the linear offset from other causes.
Note: e verification process is the same for both the dewpoint and
capacitance sensors in TEV models except that the accuracy for the capacitance sensor is ± 0.015 aw .
Verification
To verify for linear offset of your AquaLab, do the following:
1. Choose a verification standard that is close to the water activity of
the sample you are measuring. Note: e AquaLab needs to warm
up for approximately 15 minutes to make accurate readings.
2. Empty a vial of solution into a sample cup and place it in the
AquaLab’s testing chamber. Make sure that your standard is as close
to the instrument temperature as possible.
Note: Make sure the rim and outside of the sample cup are clean.
3. Carefully close the lid and move the lever to the READ position.
4. Take two readings. e water activity readings should be within
± 0.003 aw of the given value for the verification standard. See Ap-
pendix B for the correct water activity value of Decagon’s standards
at temperatures other than 25°C.
5. If your AquaLab is reading within ±0.003 aw of the verification
standard, chose a second verification standard that would border the
range of water activity you plan to test. For example, if you plan
39
Page 46

AquaLab
7. Verification and Calibration
to test for water activity readings ranging between 0.713 and 0.621
you should use the 6.0M, NaCl (0.76aw)standard for your first verification and the 8.57M LiCl (0.50aw) for the second verification.
6. Prepare a sample cup of the second verification standard and
make two readings. e second water activity reading for the second
verification standard should be within ±0.003 aw.
7. If either of the verification standards is not correct, it is probably
due to contamination of the sensor chamber. For cleaning instructions, see Chapter 6. After cleaning, repeat verification from step
two.
8. If you are consistently getting readings outside the water activity of
your first verification standard by more than ±0.003 aw, a linear offset
has probably occurred. In this case, adjust the reading to match the
verification standard’s correct value as outlined in the next section.
40
Page 47
AquaLab
Correct
Not Correct
Next
Correct
Not
Correct
Measure Verification Standard
Correct
Not Correct
Repeat Process
Go to Linear
Offset Procedure
Go to Sampling
Procedure
Clean Sample
Chamber
Re-Read
Standard
Measure 2nd
Standard
Clean Sample
Chamber
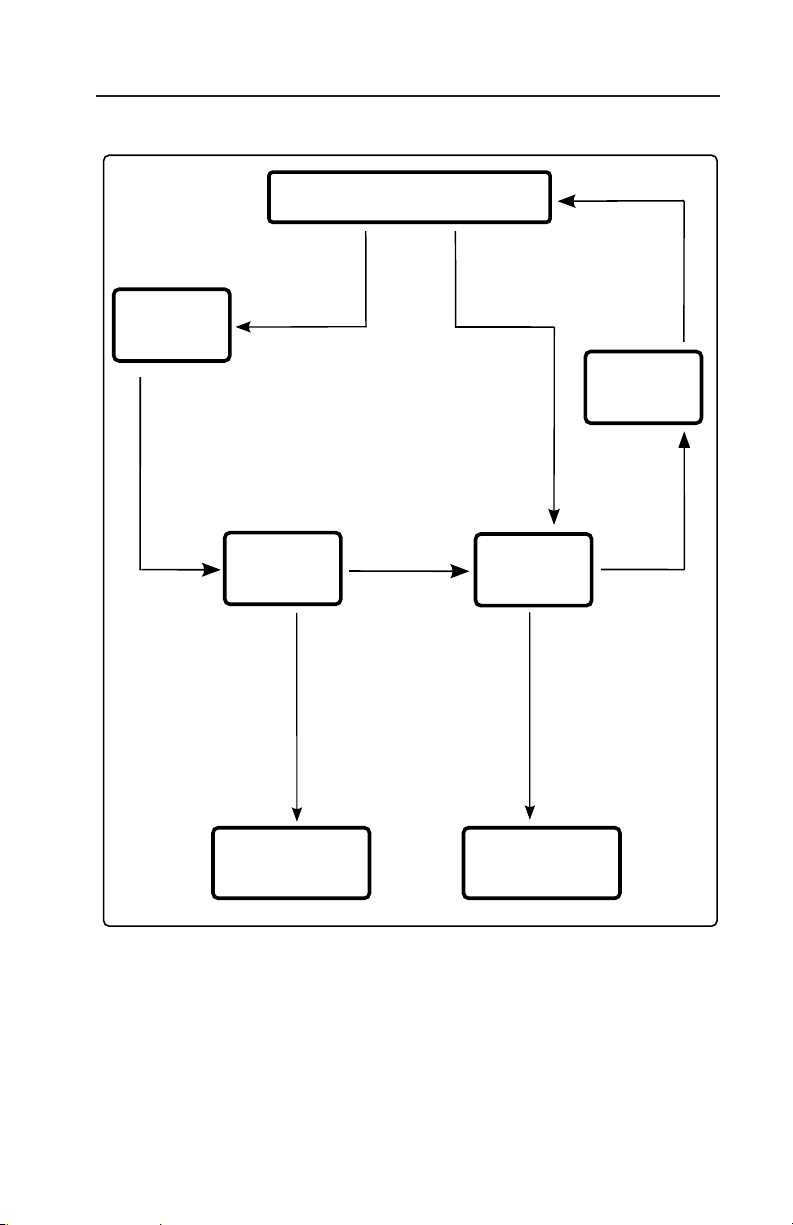
7. Verification and Calibration
is flowchart is a graphical representation of the directions given above
for checking for linear offset.
41
Page 48

AquaLab
7. Verification and Calibration
Adjust for Linear Offset
1. Once you are certain a linear offset has occurred, toggle to the
Configuration tab by pressing the Menu icon button. Calibration is
the first option highlighted in the configuration tab. Press the Enter
icon button to begin the verification process. You will be guided
through the linear offset routine through on screen commands. e
following screen will appear:
2. Press the Enter button to start the linear offset process. To
return to the main menu, press the cancel button. After pressing
the enter button, the following screen will appear:
3. Empty the whole vial of solution into a sample cup. We recommend using the 6.0 NaCl (0.76aw). Do not adjust for the offset us-
42
Page 49

AquaLab
7. Verification and Calibration
ing distilled water. Ensure the rim and outside of the cup are clean.
Place the sample cup in the AquaLab’s sample chamber.
NOTE: e same verification standard may be used to verify and
adjust the linear offset.
4. Carefully close the lid and move the lever to the READ position.
Press the Check icon button to begin testing.
NOTE: If you decide at this point not to continue with the linear offset
program, just return the lever to the OPEN position or press the cancel
button and you will be returned to the previous screen.
5. After your AquaLab has finished measuring the verification standard, it will display the following screen:
6. Press the up and down arrows to adjust the water activity reading
to its proper value for the particular verification standard you are
measuring. When the correct value is displayed, press the Save icon
button to store this new value. To cancel and return to the main
menu, press the cancel button and no changes will be made.
7. Re-measure the verification standard again in normal sampling
mode. It should read the proper value (within ±0.003 aw ) at a given
43
Page 50

AquaLab
7. Verification and Calibration
temperature for your particular standard (see Appendix B for temperatures other than 25°C ).
Measure the water activity of a second verification standard according to the verification procedure described above. If both verification readings are within ±0.003 aw then the instrument is ready to
begin testing.
If you still have incorrect verification standard readings after cleaning the chamber and adjusting for linear offset, contact Decagon
by email at support@decagon.com or by phone at 509-332-2756
(800-755-2751 in US and Canada) for further instructions. If you
purchased your Decagon instrument from one of our international
distributors, please contact them for local service and support.
How to Restore Factory Defaults
To restore original calibration settings, do the following:
1. Toggle to the Configuration tab by pressing the Menu icon button. Select Calibration and press the Enter button.
2. Scroll down to Defaults and press the Enter icon button to access
44
Page 51

AquaLab
7. Verification and Calibration
the Restore Factory Defaults routine. To cancel and return to the
main menu, press the Cancel icon button. After pushing the Enter
icon button, the following screen will appear:
NOTE: For TEV models make sure you have the correct sensor selected.
3. To restore the factory calibration values, press the Check icon
button. To cancel and return to the main menu, press the cancel
button. After pressing the Check icon button, the following screen
will appear:
4. To return to the main menu screen, press the Check icon button.
45
Page 52

AquaLab
8. Sample Preparation
8. Sample Preparation
Proper sample preparation is an important step in keeping your
AquaLab clean and achieving repeatable results. Careful preparation
and loading of samples will lengthen time between cleanings and
help you avoid downtime.
Preparing the Sample
1. Make sure the sample to be measured is homogeneous. Multi-
component samples (e.g., muffins with raisins) or samples that have
outside coatings (like deep-fried, breaded foods) can be measured,
but may take longer to equilibrate. For samples like these, AquaLab
may take more than five minutes to give an accurate reading, or may
require multiple readings of the same sample. Measuring the water
activity of these types of products is discussed in detail later in this
chapter (see Samples Needing Special Preparation).
2. Place the sample in a disposable sample cup, completely cov-
ering the bottom of the cup, if possible. AquaLab is able to accurately measure a sample that does not (or cannot) cover the bottom
of the cup. For example, raisins only need to be placed in the cup
and not flattened to cover the bottom. A larger sample surface area
increases instrument efficiency by providing more stable infrared
sample temperatures. It also speeds up the reading by shortening the
time needed to reach vapor equilibrium.
3. Do not fill the sample cup more than half full. Overfilled cups
will contaminate the sensors in the sensor chamber. Filling the
sample cup will not make the readings faster or more accurate. ere
only needs to be enough sample in the cup to allow the water in
the sample to equilibrate with the water in the vapor phase and not
46
Page 53

AquaLab
8. Sample Preparation
change the moisture content of the sample. Covering the bottom of
the sample cup provides enough sample to get an accurate reading.
4. Make sure the rim and outside of the sample cup are clean.
Wipe any excess sample material from the rim of the cup with a
clean Kimwipe. Material left on the rim or the outside of the cup
can contaminate the sensor chamber and be transferred to subsequent samples.
5. If a sample will be read at some other time, put the sample
cup’s disposable lid on the cup to restrict water transfer. For longterm storage, seal the lid by placing tape or Parafilm® completely
around the cup/lid junction. It is necessary to seal the cup if it will
not be measured immediately.
6. Be consistent in sample preparation practices.
If you crush, grind, or slice your sample, be consistent in the method
you use in order to obtain reproducible results.
Samples Needing Special Preparation
AquaLab reads most materials in five minutes or less. Some samples, however, may require longer reading times, due to their nature. ese materials need additional preparation to ensure quick,
accurate readings. To find out whether special sample preparation is
necessary, take several readings to see if readings (a
lize. If continued readings take longer than six minutes, remove the
sample and take a reading of a verification standard. is will ensure
the sample itself is causing the long read time, and that there is not a
problem with your instrument. If the verification standard also takes
longer than six minutes to sample, the chamber may be dirty. Refer
to Chapter 6 for cleaning procedures.
47
and time) stabi-
w
Page 54

AquaLab
8. Sample Preparation
Coated and Dried Samples
Samples with coatings such as sugar or fat often require multiple
readings, because it takes longer for them to equilibrate. If this is the
case for your samples, it is not a problem with your instrument; it
simply means that your particular sample takes longer than most to
equilibrate.
To reduce the time needed to take an water activity reading for coated or dried samples, you can crush or slice the sample before sampling. is increases the surface area of the sample, thus decreasing
reading times. Keep in mind, however, that modifying some samples
may alter their water activity readings.
For example, a candy may have a soft chocolate center and a hard
outer coating. e water activity reading for the center and the outer
coating are different, so one would need to evaluate which part of the
sample needed to be measured before crushing it. When the candy is
crushed, the water activity will represent the average water activity of
the entire sample; whereas leaving the candy whole will give a reading for the coating, which may act as a barrier to the center.
Slow Water-Emitting Samples
Some extremely dry, dehydrated, highly viscous water-in-oil (butter), high fat, or glassy compositions may require multiple tests due
to their slow water-emitting properties. is is because the slow
emission of water decreases the change in water activity sufficiently
that the instrument determines the test to be complete, even though
changes in water activity are still occuring. e most effective way to
test these types of samples is to run them in the AquaLab using the
continous or custom mode and wait for the water activity readings
to stablize.
48
Page 55

AquaLab
8. Sample Preparation
For faster reading, it is important to have the water activity of the
chamber at or below the water activity of these type of samples. is
causes the sample to release water to the vapor phase and equilibrate
with the chamber. If the water activity of the head-space is greater
than this type of sample, a long period of time will be required to
reach equilibrium and the water activity of the sample may be affected.
Volatile Samples
AquaLab will give accurate readings on most samples. However,
samples with certain volatiles in high enough concentrations may
give inaccurate water activity values. is is because the volatiles
condense on the mirror during the reading process, but do not
evaporate from the mirror as water does. As a result, the reading
on volatiles will not be accurate. e concentration of volatiles that
will cause interference is variable and matrix dependent. e most
effective method to determine if volatiles are a problem is to look for
incorrect standard readings after reading the sample.
Decagon’s Series 4TEV is designed for measuring volatiles such as
propylene glycol and ethanol. e Series 4TEV contains both a
chilled-mirror dewpoint and a capacitance sensor. Simply choose
the sensor you want to use from the menu in the instrument. e
only difference in operation is a lower accuracy of ±0.015 aw for
the capacitance sensor. All other operations and features will be the
same, including measurement times and adjusting for linear offset.
After measuring volatiles with the volatiles sensor it is a good idea
to clean the chamber and run charcoal before switching to the dew
point sensor.
Low Water Activity
When a sample’s water activity value is below the cooling capacity of
49
Page 56

AquaLab
8. Sample Preparation
the Series 4, your AquaLab will display an error message indicating
the lowest reading it attained on that particular sample. See Chapter 12’s
troubleshooting problem #5 for possible solutions.
If your sample is not below 0.03 aw but is still getting the error message, refer to Chapter 12 for other possible explanations.
Samples not at Room Temperature
Samples that are 4°C colder or warmer than the instrument (chamber) temperature will need to equilibrate to instrument temperature before a fast, accurate reading can be made. Rapid changes in
temperature over short periods of time will cause the water activity readings to rise or fall until the temperature stabilizes. When
the temperature stabilizes within one or two degrees of the chamber
temperature, you can proceed with normal measurements.
High-water activity samples that are warmer than the chamber temperature can cause condensation inside the measuring chamber, which
will adversely affect subsequent readings. A warning message appears
(Sample too hot) if the sample temperature is more than 4°C above
chamber temperature. If this message appears, immediately remove
the sample from the instrument, place a lid on the cup, and allow the
sample to cool to within 4°C of the instrument before measuring.
Samples that are lower than 4°C of the instrument’s temperature
will cause long read times. e sample temperature must be within
one or two degrees of the chamber temperature before fast, accurate
readings can be made.
NOTE: Powdery substances can be blown by the fan so be sure not to
overfill the sample cup and verify the cleanliness of the sample chamber
before reading a new sample.
50
Page 57

AquaLab
9. Taking a Reading
9. Taking a Reading
Measurement Steps
Once you have verified for cleanliness, calibration and prepared your
sample, you are ready to take readings. e process is simple:
Move the chamber lever to the Open position and lift the cham-•
ber lid.
Check the top lip and outside of the sample cup to make sure •
they are free from sample residue and that sample cup isn’t overfilled. (remember, an over-filled sample cup may contaminate
the chamber’s sensors).
Place your prepared sample cup in the chamber. •
Close the chamber lid and move the lever to the Read position. •
This will seal the chamber and start the reading.
In 1 to 2 minutes, the first water activity measurement will be displayed on the LCD. Length of read times may vary depending on
temperature differences between the chamber and your sample, and
other properties of your sample.
How AquaLab Takes Readings
AquaLab’s reading cycle continues until the rate of change of three
consecutive readings are less than 0.0005 aw of each other. e instrument crosses the dew threshold numerous times to ensure equilibrium and the accuracy of readings. When the instrument has finished its read cycle, the water activity is displayed, the read time is
displayed, the spinning measurement icon is replaced by the Store
51
Page 58

AquaLab
9. Taking a Reading
icon and, if enabled, you will hear a series of beeps.
Cautions!
Never leave a sample in your AquaLab after a reading has •
been taken. The sample may spill and contaminate the instrument’s chamber if the instrument is accidentally moved
or jolted.
Never try to move your instrument after a sample has been •
loaded. Movement may cause the sample material to spill
and contaminate the sample chamber.
If a sample has a temperature that is 4°C higher (or more) •
than the AquaLab’s chamber, the instrument will beep and
display a warning as shown below. Remove the sample until
it is at room temperature.
Although the instrument will measure warmer samples, the readings
may be inaccurate. Warm samples can cause condensation in the
chamber if they have a high water activity. It is best to remove the
sample from the instrument, place a lid on the cup and allow the
sample to cool before reading.
52
Page 59

AquaLab
9. Taking a Reading
The • physical temperature of the instrument should be between
15 - 50°C. Between these ambient temperatures, AquaLab will
measure samples of similar temperature quickly and accurately.
The AquaLab Series 4TE has temperature control capabilities
that enable it to read samples at temperatures different from ambient temperature, but no higher than 50°C.
If a sample has a water activity lower than about 0.03, AquaLab •
will display the < symbol (see below) notifying you that your
sample is too dry to be accurately measured by the AquaLab.
If you know that your sample’s water activity is above what the •
screen is telling you, your instrument’s sensors may have been
contaminated and will need to be cleaned (see Chapter 6) or
serviced (see Chapter 13).
53
Page 60
AquaLab
10. Duo Operation (Optional)
10. Duo Operation (Optional)
Previously, measuring moisture content and water activity required
different instruments. Now it is possible to determine both moisture
content and water activity with one machine. e Series 4TE can be
upgraded to Series 4TE DUO which can display moisture content
simultaneously with water activity. To calculate moisture content
using water activity requires an understanding of the relationship
between the two parameters. is relationship, referred to as the
moisture sorption isotherm, is complex and unique to each product
type. A product’s isotherm can be used to calculate moisture content
based on a water activity measurement. is is most easily accomplished using a model that characterizes the isotherm. For additional
information about sorption isotherms and models, please refer to
Chapter 3. e DUO generates water activity values just as a Series
4TE, but then it uses preloaded product specific isotherm models
to calculate moisture content and present it on the screen with the
water activity. For information about upgrading your Series 4TE to
a Series 4TE DUO, please contact Decagon Devices.
54
Page 61

AquaLab
10. Duo Operation (Optional)
Obtaining Product Isotherm Models
Since the isotherm relationship for each product is unique, each product’s isotherm model must be determined experimentally. is only
needs to be done once, but must be done prior to testing moisture content with the DUO. To obtain an isotherm model for a product, samples of the product need to be sent to Decagon for isotherm analysis.
Decagon will send instructions and a packing kit to assist in submitting samples for analysis. Upon completion of the isotherm, Decagon
will generate a product model file to be loaded onto the instrument.
Loading and Organizing Product Models
A Product’s model must be loaded into the Series 4TE DUO before
it can calculate moisture content. Each product must have its own
model and the model can either be loaded at the factory by Decagon
or by using the AquaLink RG software program. is software is
included with each DUO purchase or upgrade. Product model files
generated by Decagon are sent to customers via email and can then
be loaded into the instrument by connecting to the instrument using the AquaLink RG software. e software uses a model loading
tool to add and remove product models from the Series 4TE DUO,
55
Page 62

AquaLab
10. Duo Operation (Optional)
allowing the user to control and organize their product models. e
AquaLink RG software can also download data (including moisture
content) from the instrument, present the data in table form, filter
the data, and print reports.
Measuring Moisture Content
With the product models loaded into the instrument, the Series
4TE DUO can generate moisture content and water activity simultaneously.
Selecting a Product for Analysis
With the Series 4TE DUO turned on, toggle to the configura-•
tion screen by pressing the menu button.
At the configuration screen, scroll down and select moisture •
content.
56
Page 63

AquaLab
10. Duo Operation (Optional)
A list of available models will be listed by name.•
Select the model for the product to be analyzed. Selecting “None” •
will not select any model.
Taking a Reading
Readings are taken with the DUO the same as outlined in Chap-•
ter 9. First, return to the main screen.
The product chosen for analysis will be shown in the tab at the •
top of the screen. If a different product is desired to be analyzed,
it is possible to scroll through all of the available product models
on the screen by pressing the up and down buttons. This elimi-
57
Page 64

AquaLab
10. Duo Operation (Optional)
nates the need to return to the configuration screen to change
products. When the tab at the top shows “Measurement”, no
model is selected and only water activity will be displayed on the
screen.
Place a sample in the chamber and begin testing by sliding the •
lever left to the read position. For information about sample
preparation, please see Chapter 8 and for additional information
about running a test, please see Chapter 9.
When the test is complete, the screen will display the water activ-•
ity and moisture content for the product selected. If the wrong
model was selected by accident, the up and down buttons can be
used to toggle through all of the product models and the moisture content value will adjust based on the model selected.
58
Page 65

AquaLab
10. Duo Operation (Optional)
The test can be saved to the instrument’s memory by pressing •
the button under the save icon. An annotation can be added if
desired. If autosave has been selected, the data will already be
saved but without any annotation.
The results can be viewed by moving to the data screen (press the
right most button, which is below the document icon, to toggle
between tabs) as shown in Chapter 5 under the Data Tab section.
The only difference will be that moisture content data will now appear in the upper right column on the detailed information screen.
59
Page 66

AquaLab
11. Computer Interface
11. Computer Interface
Your AquaLab was shipped to you with a standard RS-232 interface
cable. Using this, you can load data to a computer using Decagon’s
AquaLink RG program or your computer’s terminal program for
further analysis and storage.
AquaLink RG
An optional software program, AquaLink Report Generator (RG),
is available for use with your AquaLab. AquaLink RG is a Windows
based program designed for data collection and customized report
generation for all Series 4 AquaLab models. AquaLink RG logs
water activity, temperature, time of measurement, and date stamps
along with other information. AquaLink RG also has sample identification and comment fields that you can use to help annotate the
data your AquaLab is gathering.
A 30 day trial cd of this program is attached to the front cover of
this manual. If you are interested in purchasing the full version of
AquaLink RG, contact Decagon or your local distributor. If you
have purchased the AquaLab 4TE DUO you will automatically
receive the full version of AquaLink RG with your manual.
Using Windows Hyperterminal
To use Hyperterminal with your AquaLab, follow these steps:
On your computer, press the Start button and select Programs •
> Accessories > Hyperterminal and click on the Hyperterminal
icon.
60
Page 67

AquaLab
11. Computer Interface
At the prompt, choose a name for this program (AquaLab is a •
good one) and choose an arbitrary icon above to represent it. In
future downloads, you will be able to click on this icon in have it
already set up for you to download. Click the OK button.
A pop-up menu labeled “Connect To” will appear. Click on the •
scroll bar on the bottom of the screen labeled “Connect Using”
and select the COM Port your RS-232 cable is connected to.
A pop-up menu labeled “• COM Properties” will appear, showing
the port settings for the COM port you selected. Make sure the
settings are the following: Bits per second, 9600; 8 databits, no
parity, 1 stop bit, and flow control set to hardware. Click OK.
Plug your RS-232 cable to the COM port you selected and con-•
nect it to your AquaLab. Begin sampling. AquaLab’s data will be
displayed on screen as it samples.
When you are finished sampling, you can print the data in the •
terminal session, or cut and paste it to a spreadsheet or text
editor. To save the data, go into the Transfers menu and select
“Capture text,” and designate where it should be saved.
61
Page 68
AquaLab
12. Troubleshooting
12. Troubleshooting
AquaLab is a high performance, low maintenance instrument, designed to have few problems if used with care. Unfortunately, sometimes even the best operators using the best instruments encounter
technical difficulties. Below is quick reference guide that will direct
you to detailed solutions of some problems that may occur. If these
remedies still don’t resolve your problem, then please contact Decagon for help (see Customer Support in Chapter 1). Here is a list of
some problems that may occur.
NOTE: If you purchased your Decagon instrument from one of our international distributors, please contact them for local service and support.
Troubleshooting Quick Guide
If this problem occurs: Refer to:
AquaLab won’t turn on ................................................ Problem #1
Readings are slow or inconsistent ................................ Problem #2
Water activity readings on solutions are ....................... Problem #3
too high/low to adjust
Screen displays “Sample too hot” ............................... Problem #4
Screen displays “aw < 0.0” ........................................... Problem #5
Dew point sensor failure ............................................. Problem #6
62
Page 69

AquaLab
12. Troubleshooting
Troubleshooting Quick Guide (Continued)
If this problem occurs: Refer to:
Verification is not correct .............................................Problem #7
Screen displays “Missing bootstrap loader” .................. Problem #8
Screen displays “Firmware is corrupted” ...................... Problem #9
DUO Model--Test was run with wrong model. ......... Problem #10
DUO Model--%MC displayed is not correct. ........... Problem #11
DUO Model--%MC is not shown on screen ............. Problem #12
DUO Model--Moisture Content is not correct .......... Problem#13
PROBLEM:1.
AquaLab won’t turn on.
SOLUTIONS:
Check to make sure your power cord is securely attached to the 1)
back of the instrument and it is plugged into the power outlet.
A power surge may have caused a 2) fuse to blow. To change the
fuses, follow these instructions:
Unplug the power cord. a.
Locate the panel where the power cord plugs in. e fuse box b.
63
Page 70

AquaLab
12. Troubleshooting
is on the right side of that panel. Press in on the release tab
and pull the fuse-holder out. Pull the broken fuse(s) out and
replace with a 1.25 Amp 250V fuse.
Caution: Do not use any other kind of fuse or you will risk damage to your instrument as well as void your warranty.
Replace the fuse-holder and push it into the fuse-well until c.
the release tab snaps in place.
Re-connect the power cord and turn your instrument on. If d.
the fuse blows again, a failed component may be causing the
problem. Contact Decagon to make arrangements for repairs.
PROBLEM:2.
Readings are slow or inconsistent.
SOLUTIONS:
e sample chamber may be dirty. Refer to Chapter 6 for direc-1)
tions on cleaning the sample chamber.
Some products absorb or desorb moisture very slowly, causing 2)
measurements to take longer than usual, and nothing can be
done to speed up the process. Refer to Chapter 8 for further
explanation.
Your sample may contain volatiles. Volatiles are known to cause 3)
unstable readings, because they condense on the surface of the
chilled mirror and alter readings. Please refer to the volatiles section in Chapter 8 for hints on reducing difficulties with measuring samples with propylene glycol. If you have further questions
regarding the measurement of volatiles contact Decagon.
64
Page 71

AquaLab
12. Troubleshooting
A fan blade in the block chamber may be broken or bent. If even 4)
salt standards take a long time to read, and the sample chamber is
clean, you may have a broken chamber fan blade. is is especially likely if you have just cleaned the chamber. If you suspect this
may have happened, contact Decagon for details on replacement.
PROBLEM:3.
Water activity readings on verification standards are too high/low
and a linear offset adjustment cannot be made any higher/lower.
SOLUTIONS:
e thermopile in your chamber, which measures sample tem-1)
perature, may have become contaminated. Refer to Chapter 6
for directions on cleaning.
e chamber mirror may be dirty. Refer to Chapter 6 for direc-2)
tions on cleaning.
PROBLEM:4.
Message on screen displays the following:
65
Page 72

AquaLab
12. Troubleshooting
SOLUTION:
Your sample’s temperature is too high for the instrument to equilibrate with it in a reasonable amount of time. e instrument and
sample need to be in temperature equilibrium before accurate
measurements can be made. erefore, very cold samples will take
a very long time to measure for the same reason. To avoid this
problem, make sure to only measure samples that are at the same
temperature as the instrument.
PROBLEM:5.
Message on screen displays the following (example):
SOLUTIONS:
e sample is too dry for the instrument to read accurately. If 1)
your sample has a water activity that is less than below the detection limits of the instrument, this message will come up. Essentially, it means that there is not enough sample moisture to
condense on the mirror and provide a reading.
e mirror may be dirty. Try cleaning the mirror and chamber 2)
and measuring the sample again.
66
Page 73

AquaLab
12. Troubleshooting
PROBLEM:6.
Message on screen displaying dew point sensor failure.
SOLUTION:
e Cooler is damaged and will need to be serviced by Decagon.
See Chapter 12 for detailed instructions.
PROBLEM7.
Verification is not correct.
SOLUTIONS:
e sample chamber and mirror need to be cleaned. See Chap-1)
ter 6 for detailed cleaning instructions. If verification is still not
correct, then linear offset has occurred.
Verify and Adjust for Linear Offset. After you have cleaned the 2)
sample chamber and mirror (Chpt. 7) you will need to use a
Verification Standard to verify and adjust for Linear Offset as
described in Chapter 7.
67
Page 74

AquaLab
12. Troubleshooting
PROBLEM:8.
Message on screen displays the following:
SOLUTION:
e instrument cannot download new firmware updates. To download new firmware to the Series 4 models, or to stop this message
from displaying, the instrument must be serviced by Decagon.
PROBLEM:9.
Message on screen displays the following:
SOLUTION:
e firmware on the instrument is corrupted and needs to be
reloaded. To download new firmware to the Series 4 models, the
instrument must be serviced by Decagon.
68
Page 75

AquaLab
12. Troubleshooting
DUO PROBLEM:10.
Test was run with wrong model.
SOLUTION:
On the measurement screen, toggle to the correct model using 1)
the up and down arrow keys. e moisture content value will be
updated to correspond with the model selected.
If the correct model is not available, the model may not be load-2)
ed on the instrument.
To determine which models are loaded on the instrument, a.
cycle to the menu tab, select Moisture Content and then the
loaded models will appear.
If the correct model is not available, load the appropriate model 3)
using Aqualink RG Software. e AquaLab DUO can only hold
a total of 20 models at any one time. You may need to remove
a model using the RG Software before you can add a new one.
Any model that is removed from the instrument will be stored
and may be reloaded again later.
DUO PROBLEM:11.
Moisture Content displayed is not correct.
SOLUTION:
Model selected may not be correct for the product being tested. 1)
Toggle through the available models to find a more appropri-a.
ate model.
If the model is correct but not giving correct moisture con-b.
tent values it may be necessary to generate a new model for
69
Page 76

AquaLab
12. Troubleshooting
the product or update an existing model. For information
about generating or updating a model, contact Decagon
Devices.
DUO PROBLEM:12.
Moisture content does not show up on the screen.
SOLUTION:
Moisture content has not been activated.
Toggle to menu tab, select moisture content, and select the ap-1)
propriate model.
If no models appear in moisture content screen, models will a.
need to be reloaded using AquaLink RG software.
If moisture content is not an active selection, the DUO fea-b.
ture may not be active. Content Decagon Devices to learn
how to activate the DUO feature.
DUO PROBLEM:13.
Message on the screen displays the following:
70
Page 77

AquaLab
12. Troubleshooting
SOLUTION:
When a moisture content reading is not shown, the water activ-1)
ity or temperature for that reading is beyond the scope of the
moisture sorption isotherm. is can happen under the following conditions:
e isotherm equation calculates a moisture content that a.
is less than 0% or greater than 100% with the given water
activity.
e control temperature is significantly different than the b.
isotherm temperature.
Make sure that the sample’s water activity and the instrument’s
controlling temperature are within the scope of the selected moisture sorption isotherm model.
71
Page 78

AquaLab
12. Troubleshooting
Diagnostic Screen
If, after cleaning your instrument and reading the other troubleshooting hints, you have reason to believe that one of the components of your AquaLab may be causing measurement error, you
may access a screen that will display values for component performance. is is done by navigating to the Configuration tab and
then by scrolling down to the diagnostics option. Press enter and
you will be given a list of components and their values.
is screen shows typical values for the dew point method. Lid,
base and sample temperatures may fluctuate but should not change
more than 0.03 degrees. Typical ranges for the lid, base and sample
temperatures is between 24.5 and 25.5 degrees.
If the mirror temperature is at lid temperature, the cooler has failed
and must be replaced. If the mirror is below the lid temperature or
appears to be random, the thermocouple wire is broken and must
be repaired.
A typical optical range is between 500 mV and 2900 mV .
For capacitance mode, not shown here, the RH percentage should
always be between 0 and 100%.
72
Page 79

AquaLab
13. Support and Repair
13. Support and Repair
NOTE: If you purchased your AquaLab from one of our international
distributors, please contact them. ey will be able to provide you with
local support and service.
When encountering problems with your AquaLab (that can’t be resolved with the help of this manual), please contact Decagon Customer Support at support@decagon.com, 800-755-2751 (US and
Canada), 509-332-2756 (International) or fax us at (509) 332-5158.
Please have the serial number and model of the instrument ready.
All AquaLabs returning to Decagon for servicing must be accompanied with a Return Material Authorization (RMA) form. Prior to
shipping the instrument, please contact a Decagon customer support representative to obtain an RMA.
Shipping Directions:
e following steps will help to ensure the safe shipping and processing of your AquaLab.
Ship your AquaLab in its original cardboard box with suspen-1)
sion packaging. If this is not possible, use a box that has at least
4 inches of space between your instrument and each wall of the
box.
Place the AquaLab in a plastic bag to avoid disfiguring marks 2)
from the packaging.
Don’t ship the power cord or serial cable.3)
If the original packaging is not available, pack the box moderate-4)
ly tight with packing material (e.g. styrofoam peanuts or bubble
wrap), ensuring the instrument is suspended in the packing material.
73
Page 80

AquaLab
13. Support and Repair
Include a copy of the RMA form in the shipment. Please verify 5)
the ship to and bill to information, contact name, and problem
description. If anything is incorrect please contact a Decagon
representative.
Tape the box in both directions for added support.6)
Include the RMA number in the attention line on the shipping 7)
label.
Ship to:
Decagon Devices Inc.
ATTN: RMA (insert your RMA #)
2365 NE Hopkins Court
Pullman, WA 99163
Repair Costs
Manufacturer’s defects and instruments within the three-year warranty
will be repaired at no charge. Non-warranty repair charges for parts,
labor and shipping will be billed to you. An extra fee may be charged for
rush work. Decagon will provide an estimated repair cost, if requested.
Loaner Service
Decagon has loaner instruments to keep you measuring water
activity while your instrument is being serviced. If your AquaLab is still under calibration warranty or you have a service plan
with your instrument, there is no charge for the loaner service.
74
Page 81

AquaLab
14. Further Reading
14. Further Reading
Water Activity eory & Measurement
Bousquet-Ricard, M, G. Qualyle, T. Pharm, and J.C. Cheftel.
(1980). Comparative study of three methods of determining water
activity in intermediate moisture foods. Lebensmittel-Wissenschaftund-Technologie.13:169-173.
Chirife, J., G. Favetto, C. Ferro-Fontan, and S. Resnik. (1983). e
water activity of standard saturated salt solutions in the range of
intermediate moisture foods. Lebensmittel-Wissenschaft und-Technologie. 16:36-38.
Duckworth, R. (1975). Water Relations of Foods. Academic Press, New York.
Gomez-Diaz, R. (1992). Water activity in foods: Determination
methods. Alimentaria. 29:77-82.
Gómez, R. and Fernandez-Salguero J. (1992). Water activity and chemical composition of some food emulsions. Food Chemistry. 45:91-93.
Greenspan, L. (1977). Humidity fixed points of binary saturated
aqueous solutions. Journal of Research of the National Bureau of
Standards - A.Physics and Chemistry. 81A:89-96.
Karmas, E. (1981). Measurement of moisture content. Cereal Foods
World. 26(7):332-334.
Kitic, D., D.C. Pereira-Jardim, G. Favetto, S.L. Resnik, and J.
Chirife. (1986). eoretical prediction of the water activity of standard saturated salt solutions at various temperatures. Journal of Food
Science. 51:1037-1042.
75
Page 82

AquaLab
14. Further Reading
Labuza, T.P. and R. Contreras-Medellin. (1981). Prediction of moisture
protection requirements for foods. Cereal Foods World. 26(7):335-343.
Labuza, T.P., K. Acott, S.R. Tatini, and R.Y. Lee. (1976). Water
activity determination: A collaborative study of different methods.
Journal of Food Science. 41:910-917.
Prior, B.A. (1979). Measurement of water activity in foods: A review. Journal of Food Protection. 42(8):668-674.
Reid, D.S. (1976). Water activity concepts in intermediate moisture
foods. In: Intermediate Moisture Foods.
Davies, R., G.G. Birch, and K.J. Parker (ed.) Applied Science Publishers, London. pp. 54-65.
Richard, J. and T.P. Labuza. (1990). Rapid determination of the water activity of some reference solutions, culture media and cheese
using a new dew point apparatus. Sciences des Aliments. 10:57-64.
Roa, V. and M.S. Tapia de Daza. (1991). Evaluation of water activity measurements with a dew point electronic humidity meter.
Lebensmittel-Wissenschaft und-Technologie. 24(3):208-213.
Roos, K.D. (1975). Estimation of water activity in intermediate
moisture foods. Food Technology. 29:26-30.
Scott, V.N. and D.T. Bernard. (1983). Influence of temperature on
the measurement of water activity of food and salt systems. Journal
of Food Science. 48:552-554.
Snavely, M.J., J.C. Price, and H.W. Jun. (1990). A comparison of
three equilibrium relative humidity measuring devices. Drug Devel-
76
Page 83

AquaLab
14. Further Reading
opment and Industrial Pharmacy. 16(8):1399-1409.
Stamp, J.A., S. Linscott, C. Lomauro, and T.P. Labuza. (1984).
Measurement of water activity of salt solutions and foods by several
electronic methods as compared to direct vapor pressure measurement. Journal of Food Science. 49:1139-1142.
Stoloff, L. (1978). Calibration of water activity measuring instruments and devices: Collaborative study. Journal of Association of
Official Analytical Chemists. 61:1166-1178.
Troller, J.A. (1983). Methods to measure water activity. Journal of
Food Protection. 46:129-134.
Troller, J.A. and J.H.B. Christian. (1978). Water Activity and Food.
Academic Press, New York.
Troller, J.A. and V.N. Scott. (1992). Measurement of water activity
(aw) and acidity. In: Compendium of Methods for the Microbiological Examination of Foods. Vanderzant, C. and D.F. Splittstoesser
(ed.) American Public Health Association, Washington, D.C. pp.
135-151.
Van den Berg, C. (1985). Water activity. In: Concentration and Drying of Foods. MacCarthy, D. (ed.) Elsevier, London. pp. 11-35.
Van den Berg, C. (1991). Food-water relations: Progress and integration, comments and thoughts. In: Water Relationships in Foods.
Levine, H. and L. Slade (ed.) Plenum Press, New York.
Van den Berg, C. and Bruin. (1981). Water activity and its estimation in food systems: eoretical aspects. In: Water Activity: Influ-
77
Page 84

AquaLab
14. Further Reading
ences on Food Quality. Rockland, L.B. and G.F. Stewart (ed.) Academic Press, New York. pp. 1-61.
Vega, M.H. and G.V. Barbosa-Canovas. (1994). Prediction of water
activity in food systems: A review on theoretical models. Revista Espanola De Ciencia Y Tecnologia De Alimentos. 34:368-388.
Vega, M.H., B. Romanach, and G.V. Barbosa-Canovas. (1994). Prediction of water activity in food systems: A computer program for
predicting water activity in multicomponent foods. Revista Espanola De Ciencia Y Tecnologia De Alimentos. 34:427-440.
Vos, P.T. and T.P. Labuza. (1974). Technique for measurements of
water activity in the high aw range. Journal of Agricultural and Food
Chemistry. 22:326-327.
Voysey, P. (1993). An evaluation of the AquaLab CX-2 system for
measuring water activity. Digest, Microbiology Section. 124 Food
Quality and Safety
Food Quality and Safety
Brandt, L. (1996). Bound for success. Controlling water activity
gives technologists the edge in developing safe, shelf-stable foods.
Food Formulating. September:41-48.
Chirife, J. and B.M. Del-Pilar. (1994). Water activity, glass transition and microbial stability in concentrated/semimoist food systems.
Journal of Food Science. 59:921-927.
Chirife, J. and M.P. Buera. (1995). A critical review of some nonequilibrium situations and glass transitions on water activity values
of foods in the microbiological growth range. Journal of Food Engineering. 25:531-552.
78
Page 85

AquaLab
14. Further Reading
Chirife, J. and M.P. Buera. (1996). Water activity, water glass dynamics, and the control of microbiological growth in foods. Critical
Reviews in Food Science and Nutrition. 36(5):465-513.
Franks, F. (1982). Water activity as a measure of biological viability
and quality control. Cereal Foods World. 27(9):403-407.
Franks, F. (1991). Water activity: a credible measure of food safety
and quality? Trends in Food Science and Technology. March:68-72.
Hardman, T.M. (1988). Water and Food Quality. Elseiver Press,
London.
Kress-Rogers, E. (1993). Food quality measurement. Food Industry
News. September:23-26.
Levine, H. and L. Slade. (1991). Water Relationships in Foods. Plenum Press, New York.
Mannheim, C.H., J.X. Liu, and S.G. Gilbert. (1994). Control of
water in foods during storage. Journal of
Food Engineering. 22:509-532.
McMeekin, T.A. and T. Ross. (1996). Shelf life prediction: Status and future possibilities. International Journal of Food Microbiology. 33:65-83.
Nelson, K.A. and T.P. Labuza. (1994). Water activity and food polymer science: Implications of state on arrhenius and WLF models in
predicting shelf life. Journal of Food Engineering. 22:271-289.
Rockland, L.B. and G.F. Stewart. (1981). Water Activity: Influences
on Food Quality. Academic Press, New York.
79
Page 86

AquaLab
14. Further Reading
Rockland, L.B. and S.K. Nishi. (1980). Influence of water activity
on food product quality and stability. Food Technology. 34:42-59.
Seow, C.C., T.T. Teng, and C.H. Quah. (1988). Food Preservation
by Moisture Control. Elsevier, New York.
Taoukis, P., W. Breene, and T.P. Labuza. (1988). Intermediate moisture foods. Advances in Cereal Science and Technology. 9:91-128.
Water Activity and Microbiology
Water Activity and Microbiology
Beuchat, L.R. (1981). Microbial stability as affected by water activity. Cereal Foods World. 26(7):345-349.
Chen, H.C. (1995). Seafood microorganisms and seafood safety.
Journal of Food and Drug Analysis. 3:133-144.
Farber, J.M., F. Coates, and E. Daley. (1992). Minimum water activity requirements for the growth of Listeria monocytogenes. Letters
In Applied Microbiology. 15:103-105.
Garcia de Fernando, G.D., O. Diaz, M. Fernandez, and J.A. Ordonez. (1992). Changes in water activity of selected solid culture
media throughout incubation. Food Microbiology. 9:77-82.
Gibson, A.M., J. Baranyi, J.I. Pitt, M.J. Eyles, and T.A. Roberts.
(1994). Predicting fungal growth: e effect of water activity on
Aspergillus flavus and related species. International Journal of Food
Microbiology. 23:419-431.
Goaleni, N., J.E. Smith, J. Lacey, and G. Gettinby. (1997). Effects
80
Page 87

AquaLab
14. Further Reading
of temperature, water activity, and incubation time on production
of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface agar culture. Applied and Environmental Microbiology. 63:1048-1053.
Hocking, A.D. and B.F. Miscamble. (1995). Water relations of some
Zygomycetes isolated from food. Mycological Research. 99:1113-1118.
Hocking, A.D., B.F. Miscamble, and J.I. Pitt. (1994). Water relations of Alternaria alternata, Cladosporium cladosporioides, Cladosporium sphaerospermum, Curvularia lunata and Curvularia
pallescens. Mycological Research. 98:91-94.
Houtsma, P.C., A. Heuvelink, J. Dufrenne, and S. Notermans.
(1994). Effect of sodium lactate on toxin production, spore germination and heat resistance of proteolytic Clostridium botulinum
strains. Journal of Food Protection. 57:327-330.
Kuntz, L.A. (1992). Keeping microorganisms in control. Food Product Design. August:44-51.
Li, K.Y. and J.A. Torres. (1993). Water activity relationships for selected mesophiles and psychrotrophs at refrigeration temperature.
Journal of Food Protection. 56:612-615.
Marauska, M., A. Vigants, A. Klincare, D. Upite, E. Kaminska, and
M. Bekers. (1996). Influence of water activity and medium osmolality on the growth and acid production of Lactobacillus casei var.
alactosus. Proceedings of the Latvian Academy of Sciences Section B
Natural Exact and Applied Sciences. 50:144-146.
Miller, A.J. (1992). Combined water activity and solute effects on
81
Page 88

AquaLab
14. Further Reading
growth and survival of Listeria monocytogenes Scott A. Journal of
Food Protection. 55:414-418.
Nakajo, M. and Y. Moriyama. (1993). Effect of pH and water activity on heat resistance of spores of Bacillus coagulans. Journal of the
Japanese Society for Food Science and Technology. 40:268-271.
Nolan, D.A., D.C. Chamblin, and J.A. Troller. (1992). Minimal
water activity levels for growth and survival of Listeria monocytogenes and Listeria innocua. International Journal of Food Microbiology. 16:323-335.
Petersson, S. and J. Schnuerer. (1995). Biocontrol of mold growth
in high-moisture wheat stored under airtight conditions by Pichia
anomala, Pichia guilliermondii, and Saccharomyces cerevisiae. Applied and Environmental Microbiology. 61:1027-1032.
Pitt, J.I. and B.F. Miscamble. (1995). Water relations of Aspergillus flavus and closely related species. Journal of Food Protection. 58:86-90.
Quintavalla, S. and G. Parolari. (1993). Effects of temperature, aw
and pH on the growth of Bacillus cells and spore: A response surface
methodology study. International Journal of Food Microbiology.
19:207-216.
Saad, R.R. (1992). Effect of water activity on growth and lipids of
xerophilic fungi, Aspergillus repens and Aspergillus amstelodami.
Zentralblatt Fuer Mikrobiologie. 147:61-64.
Santos, J., T.M. Lopez-Diaz, M.L. Garcia-Lopez, M.C. GarciaFernandez, and A. Otero. (1994). Minimum water activity for the
growth of Aeromonas hydrophila as affected by strain, temperature
82
Page 89

AquaLab
14. Further Reading
and humectant. Letters In Applied Microbiology. 19:76-78.
Tapia de Daza, M.S., Y. Villegas, and A. Martinez. (1991). Minimal
water activity for growth of Listeria monocytogenes as affected by
solute and temperature. International Journal of Food Microbiology. 14:333-338.
Tokuoka, K. and T. Ishitani. (1991). Minimum water activities for
the growth of yeasts isolated from high-sugar foods. Journal of General and Applied Microbiology. 37:111-119.
Ucar, F. and I. Guneri. (1996). e effect of water activity (aw), pH
and temperature on the growth of osmophilic yeasts. Turkish Journal of Biology. 20:37-46.
Wijtzes, T., P.J. Mcclure, M.H. Zwietering, and T.A. Roberts. (1993).
Modelling bacterial growth of Listeria monocytogenes as a function
of water activity, pH and temperature. International Journal of Food
Microbiology. 18:139-149.
Zwietering, M.H., T. Wijtzes, J.C. De-Wit, and R.K. Van’T. (1992).
A decision support system for prediction of the microbial spoilage in
foods. Journal of Food Protection. 55:973-979.
Water Activity in Foods
Meat and Seafood
Chen, N. and L.A. Shelef. (1992). Relationship between water activity, salts of lactic acid, and growth of Listeria monocytogenes in a
meat model system. Journal of Food Protection. 55:574-578.
Clavero, M.R.S. and L.R. Beuchat. (1996). Survival of Escherichia
coli O157:H7 in broth and processed salami as influenced by pH,
83
Page 90

AquaLab
14. Further Reading
water activity, and temperature and suitability of media for its recovery. Applied and Environmental Microbiology. 62:2735-2740.
Duffy, L.L., P.B. Vanderlinde, and F.H. Grau. (1994). Growth of
Listeria monocytogenes on vacuum-packed cooked meats: Effects of
pH, a w, nitrite and ascorbate. International Journal of Food Microbiology. 23:377-390.
Fernandez-Salguero J., R. Gómez, and M.A. Carmona. (1994). Water activity of Spanish intermediate-moisture meat products. Meat
Science. 38:341-346.
Gómez, R. and Fernandez-Salguero J. (1993). Note: Water activity
of Spanish intermediate moisture fish products. Revista Espanola
De Ciencia Y Tecnologia De Alimentos. 33:651-656.
Hand, L. (1994). Controlling water activity and pH in snack sticks.
Meat Marketing and Technology. May:55-56.
Lee, M.B. and S. Styliadis. (1996). A survey of pH and water activity levels in processed salamis and sausages in Metro Toronto. Journal of Food Protection. 59:1007-1010.
Luecke, F.K. (1994). Fermented meat products. Food Research International. 27:299-307.
Minegishi, Y., Y. Tsukamasa, K. Miake, T. Shimasaki, C. Imai, M.
Sugiyama, and H. Shinano. (1995). Water activity and microflora in
commercial vacuum-packed smoked salmons. Journal of the Food
Hygienic Society of Japan. 36:442-446.
Rocha-Garza, A.E. and J.F. Zayas. (1996). Quality of broiled beef
84
Page 91

AquaLab
14. Further Reading
patties supplemented with wheat germ protein flour. Journal of Food
Science. 61:418-421.
Shimasaki, T., K. Miake, Y. Tsukamasa, M.A. Sugiyama, Y. Minegishi, and H. Shinano. (1994). Effect of Water Activity and Storage
Temperature on the Quality and Microflora of Smoked Salmon.
Nippon Suisan Gakkaishi. 60:569-576.
Untermann, F. and C. Muller. (1992). Influence of aw value and
storage temperature on the multiplication and enterotoxin formation of staphylococci in dry-cured raw hams. International Journal
of Food Microbiology. 16:109-115.
Williams, S.K., G.E. Rodrick, and R.L. West. (1995). Sodium lactate affects shelf life and consumer acceptance of fresh (Ictalurus
nebulosus, marmoratus) fillets under simulated retail conditions.
Journal of Food Science. 60:636-639.
Dairy Products
Fresno, J.M., M.E. Tornadijo, J. Carballo, P.J. Gonzalez, and A.
Bernardo. (1996). Characterization and biochemical changes during
the ripening of a Spanish craft goat’s milk cheese (Armada variety).
Food Chemistry. 55:225-230.
Hong, Y.H. (1991). Physical and chemical properties of the process
cheese on the domestic market. Korean Journal of Animal Science.
33:387-391.
Kombila, M.E. and C. Lacroix. (1991). e effect of combinations
of salt, lactose and glycerol on the water activity (AW) of cheese
spreads. Canadian Institute of Food Science and Technology Journal. 24:233-238.
85
Page 92

AquaLab
14. Further Reading
Pisecky, J. (1992). Water activity of milk powders. Milchwissenschaft.
47:3-7.
Tornadijo, E., J.M. Fresno, J. Carballo, and S.R. Martin. (1993).
Study of Enterobacteriaceae throughout the manufacturing and ripening of hard goats’ cheese. Journal of Applied Bacteriology. 75:240-
246.
Valik, L. and F. Gorner. (1995). Effect of water activity adjusted
with different solutes on growth and Lactic acid production by Lactobacillus helveticus. Folia Microbiologica. 40:472-474.
Vivier, D., M. Rivemale, J.P. Reverbel, R. Ratomahenina, and P.
Galzy. (1994). Study of the growth of yeasts from feta cheese. International Journal of Food Microbiology. 22:207-215.
Vivier, D., R. Ratomahenina, and P. Galzy. (1994). Characteristics
of micrococci from the surface of Roquefort cheese. Journal of Applied Bacteriology. 76:546-552.
Fruits and Vegetables
Ayub, M., R. Khan, S. Wahab, A. Zeb, and J. Muhammad. (1995).
Effect of crystalline sweeteners on the water activity and shelf stability of osmotically dehydrated guava. Sarhad Journal of Agriculture.
11:755-761.
Beveridge, T. and S.E. Weintraub. (1995). Effect of blanching pretreatment on color and texture of apple slices at various water activities. Food Research International. 28:83-86.
Hubinger, M., F.C. Menegalli, R.J. Aguerre, and C. Suarez. (1992).
Water vapor adsorption isotherms of guava, mango and pineapple.
Journal of Food Science. 57:1405-1407.
86
Page 93

AquaLab
14. Further Reading
Jimenez, M., M. Manez, and E. Hernandez. (1996). Influence of
water activity and temperature on the production of zearalenone in
corn by three Fusarium species. International Journal of Food Microbiology. 29:417-421.
Kiranoudis, C.T., Z.B. Maroulis, E. Tsami, and K.D. Marinos.
(1993). Equilibrium moisture content and heat of desorption of
some vegetables. Journal of Food Engineering. 20:55-74.
Makower, B. and G.L. Dehority. (1943). Equilibrium moisture content of dehydrated vegetables. Industrial and Engineering Chemistry. 35(2):193-197.
Maltini, E., D. Torreggiani, B.R. Brovetto, and G. Bertolo. (1993).
Functional properties of reduced moisture fruits as ingredients in
food systems. Food Research International. 26:413-419.
Marin, S., V. Sanchis, and N. Magan. (1995). Water activity, temperature, and pH effects on growth of Fusarium moniliforme and
Fusarium proliferatum isolates from maize. Canadian Journal of Microbiology. 41:1063-1070.
Monsalve-Gonzalez, A., G.V. Barbosa-Canovas, and R.P. Cavalieri.
(1993). Mass transfer and textural changes during processing of
apples by combined methods. Journal of Food Science. 58:1118-
1124.
Tapia de Daza, M.S., C.E. Aguilar, V. Roa, and R.V. Diaz de Tablante. (1995). Combined stress effects on growth of Zygosaccharomyces rouxii from an intermediate moisture papaya product. Journal of
Food Science. 60:356-359.
87
Page 94

AquaLab
14. Further Reading
Zeb, A., R. Khan, A. Khan, M. Saeed, and S.A. Manan. (1994).
Influence of crystalline sucrose and chemical preservatives on the
water activity and shelf stability of intermediate banana chips. Sarhad Journal of Agriculture. 10:721-726.
Zhang, X.W., X. Liu, D.X. Gu, W. Zhou, R.L. Wang, and P. Liu.
(1996). Desorption isotherms of some vegetables. Journal of the Science of Food and Agriculture. 70:303-306.
Baked Goods and Cereals
Aramouni, F.M., K.K. Kone, J.A. Craig, and D.-Y.C. Fung. (1994).
Growth of Clostridium sporogenes PA 3679 in home-style canned
quick breads. Journal of Food Protection. 57:882-886.
Cahagnier, B., L. Lesage, and M.D. Richard. (1993). Mould growth
and conidiation in cereal grains as affected by water activity and
temperature. Letters In Applied Microbiology. 17:7-13.
Clawson, A.R. and A.J. Taylor. (1993). Chemical changes during
cooking of wheat. Food Chemistry. 47:337-343.
Gómez, R., Fernandez-Salguero J., M.A. Carmona, and D. Sanchez.
(1993). Water activity in foods with intermediate moisture levels: Bakery and confectionery products: Miscellany. Alimentaria. 30:55-57.
Harris, M. and M. Peleg. (1996). Patterns of textural changes in
brittle cellular cereal foods caused by moisture sorption. Cereal
Chemistry. 73:225-231.
Michniewicz, J., C.G. Biliaderis, and W. Bushuk. (1992). Effect of
added pentosans on some properties of wheat bread. Food Chemistry.
43:251-257.
88
Page 95

AquaLab
14. Further Reading
Ramanathan, S. and S. Cenkowski. (1995). Sorption isotherms of
flour and flow behaviour of dough as influenced by flour compaction. Canadian Agricultural Engineering. 37:119-124.
Roessler, P.F. and M.C. Ballenger. (1996). Contamination of an unpreserved semisoft baked cookie with a xerophilic Aspergillus species. Journal of Food Protection. 59:1055-1060.
Seiler, D.A.L. (1979). e mould-free shelf life of bakery products.
FMBRA Bulletin. April(2):71-74.
Sumner, S.S., J.A. Albrecht, and D.L. Peters. (1993). Occurrence of
enterotoxigenic strains of Staphylococcus aureus and enterotoxin production in bakery products. Journal of Food Protection. 56:722-724.
Tesch, R., M.D. Normand, and M. Peleg. (1996). Comparison of
the acoustic and mechanical signatures of two cellular crunchy cereal foods at various water activity levels. Journal of the Science of
Food and Agriculture. 70:347-354.
Weegels, P.L., J.A. Verhoek, A.M.G. de Groot, and R.J. Hamer. (1994).
Effects of gluten of heating at different moisture contents: I. Changes
in functional properties. Journal of Cereal Science. 19:31-38.
Beverages/Soups/Sauces/Preserves
Carson, K.J., J.L. Collins, and M.P. Penfield. (1994). Unrefined, dried
apple pomace as a potential food ingredient. Journal of Food Science.
59:1213-1215.
Durrani, M.J., R. Khan, M. Saeed, and A. Khan. (1992). Development of concentrated beverages from Anna apples with or without
added preservatives by controlling activity of water for shelf stability.
89
Page 96

AquaLab
14. Further Reading
Sarhad Journal of Agriculture. 8:23-28.
Ferragut, V., J.A. Salazar, and A. Chiralt. (1993). Stability in the
conservation of emulsified sauces low in oil content. Alimentaria.
30:67-69.
Ibarz, A., J. Pagan, and R. Miguelsanz. (1992). Rheology of clarified
fruit juices: II. Blackcurrant juices. Journal of Food Engineering.
15:63-74.
Kusumegi, K., T. Takahashi, and M. Miyagi. (1996). Effects of addition of sodium citrate on the pasteurizing conditions in “Tuyu”,
Japanese noodle soup. Journal of the Japanese Society for Food Science and Technology. 43:740-747.
Sa, M.M. and A.M. Sereno. (1993). Effect of temperature on sorption isotherms and heats of sorption of quince jam. International
Journal of Food Science and Technology. 28:241-248.
Pharmaceuticals/Cosmetics
Ahlneck, C. and G. Zografi. (1990). e molecular basis of moisture effects on the physical and chemical stability of drugs in the
solid state. International Journal of Pharmaceutics. 62:87-95.
Cochet, N. and A.L. Demain. (1996). Effect of water activity on
production of beta-lactam antibiotics by Streptomyces clavuligerus
in submerged culture. Journal of Applied Bacteriology. 80:333-
337.
Costantino, H.R., R. Langer, and A.M. Klibanov. (1994). Solidphase aggregation of proteins under pharmaceutically relevant conditions. Journal of Pharmaceutical Sciences. 83:1662-1669.
90
Page 97

AquaLab
14. Further Reading
Enigl, D.C. and K.M. Sorrels. (1997). Water Activity and Self-Preserving Formulas. In: Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practice. Kabara, J.J. and D.S. Orth
(ed.) Marcel Dekker, pp. 45-73.
Hageman, M.J. (1988). e role of moisture in protein stability.
Drug Development and Industrial Pharmacy. 14(14):2047-2070.
Heidemann, D.R. and P.J. Jarosz. (1991). Performulation studies
involving moisture uptake in solid dosage forms. Pharmaceutical
Research. 8(3):292-297.
Friedel, R.R. and A.M. Cundell. (1998). e application of water activity
measurement to the microbiological attributes testing of nonsterile overthe-counter drug products. Pharmacopeial Forum. 24(2):6087-6090.
Kontny, M.J. (1988). Distribution of water in solid pharmaceutical systems. Drug Development and Industrial Pharmacy. 14(14):1991-2027.
Zografi, G. (1988). States of water associated with solids. Drug Development and Industrial Pharmacy. 14(14):1905-1926.
Zografi, G. and M.J. Kontny. (1986). e interactions of water with
cellulose- and starch-derived pharmaceutical excipients. Pharmaceutical Research. 3(4):187-193.
Miscellaneous
Bell, L.N. and T.P. Labuza. (1992). Compositional influence on the pH
of reduced-moisture solutions. Journal of Food Science. 57:732-734.
Bell, L.N. and T.P. Labuza. (1994). Influence of the low-moisture
state on pH and its implication for reaction kinetics. Journal of Food
Engineering. 22:291-312.
91
Page 98

AquaLab
14. Further Reading
Bell, L.N. (1995). Kinetics of non-enzymatic browning in amorphous solid systems: Distinguishing the effects of water activity and
the glass transition. Food Research International. 28:591-597.
Brake, N.C. and O.R. Fennema. (1993). Edible coatings to inhibit
lipid migration in a confectionery product. Journal of Food Science.
58:1422-1425.
Dole, M. and L. Faller. (1950). Water sorption by synthetic high
polymers. Journal of the American Chemical Society. 12:414-419.
Fernandez-Salguero J., R. Gómez, and M.A. Carmona. (1993). Water activity in selected high-moisture foods. Journal of Food Composition and Analysis. 6:364-369.
Lomauro, C.J., A.S. Bakshi, and T.P. Labuza. (1985). Evaluation of
food moisture sorption isotherm equations. Part I: Fruit, vegetable
and meat products. Lebensmittel-Wissenschaft und-Technologie.
18:111-117.
Lomauro, C.J., A.S. Bakshi, and T.P. Labuza. (1985). Evaluation of
food moisture sorption isotherm equations. Part II: Milk, coffee, tea,
nuts, oilseeds, spices and starchy foods. Lebensmittel-Wissenschaft
und-Technologie. 18:118-124.
Yasuda, H., H.G. Olf, B. Crist, C.E. Lamaze, and A. Peterlin.
(1972). Movement of water in homogeneous water-swollen polymers. In: Water Structure at the Water Polymer Interface. Jellinek,
H.H.G. (ed.) Plenum Press, New York/London.
92
Page 99

AquaLab
Appendix A
Appendix A
Preparing Salt Solution
If you choose to mix a saturated salt solution for use as a verification
standard, we recommend that you use the approved AOAC method.
is method is as follows:
1. Select a reagent-grade salt and place it in a test container to a
depth of about 4cm for more soluble salts (lower aw), to a depth of
about 1.5 cm for less soluble salts (high aw), and to an intermediate
depth for intermediate salts.
2. Add distilled water in increments of about 2mL, stirring constantly.
3. Add water until the salt can absorb no more water, evidenced
by the presence of free liquid. Keep the amount of free liquid to
the minimum needed to keep the solution saturated with water. If
you plan on using this solution over a long term period, seal the
solution well to prevent losses from evaporation. Below is a table of
saturated salt solutions and their respective water activities at various temperatures. Please note that these values are based on averaged
published data, and the standard errors shown reflect Greenspan’s
standard error for each salt solution, not the AquaLab’s accuracy in
measuring the salt. AquaLab measures all samples with an accuracy
of ±0.003 aw.
93
Page 100

AquaLab
Appendix A
Table 2: Water Activity of Selected Salt Solutions
Saturated
Solution
Lithium
Chloride
Magnesium
Chloride
Potassium
Chloride
Magnesium
Nitrate
Sodium
Chloride
Potassium
Chloride
Potassium
Sulfate
Adapted from Greenspan (1977). Rounded to nearest thousandth.
aw at 20° C aw at 25° C
0.113 ± 0.003 0.113 ± 0.003
0.331 ± 0.002 0.328 ± 0.002
0.432 ± 0.003 0.432 ± 0.004
0.544 ± 0.002 0.529 ± 0.002
0.755 ± 0.001 0.753 ± 0.001
0.851 ± 0.003 0.843 ± 0.003
0.976 ± 0.005 0.973 ± 0.005
4. Saturated salt solutions are very temperature-sensitive and their
values are not as accurate as the verification standards offered by
Decagon.
94
 Loading...
Loading...