Page 1

Danfoss District Heating
Page 1 of 9
Guideline of Water Quality for copper brazed Plate Heat Exchangers
0 Summary
Danfoss District Heating has prepared this guideline for the water quality of tap water and
district heating water used in plate heat exchangers of stainless steel (1.4404, X2CrNiMo17-12-2
acc. to EN 10088-2:2005 ~ AISI 316L) brazed with pure copper.
The water flowing in these brazed plate heat exchangers (PHEX) varies a lot from application to
application and corrosion can become a problem in some situations. This guideline is based on a
comprehensive literature survey and on our experiences from many years using copper brazed
stainless steel PHEX.
It is important to point out that this water specification is not a guarantee against corrosion, but
must be considered as a tool to avoid the most critical water applications. A summary of the
parameters and their recommended limits are listed in table 2 for water on the secondary side
(tap water, drinking water) and table 3 for primary water (heat supply, district heating water).
These limits are only valid for PHEX made of stainless steel 1.4404 brazed with pure copper.
1 Introduction
Danfoss District Heating has prepared this guideline for the water quality of tap water and
district heating water used in heat exchangers of stainless steel (1.4404, X2CrNiMo17-12-2 acc.
to EN 10088-2:2005 ~ AISI 316L) brazed with pure copper. Normally, tap water (drinking water)
flows in the secondary side and a heating media (e.g. district heating water) flows in the primary
side of the heat exchanger.
Surfaces in contact with water can be subject to two problems, scale formation and corrosion.
Gases and salts being dissolved in the water play the major role; besides that, component design
(e.g. design, materials used, fabrication processes) and operating conditions (e.g. temperature,
flow conditions, times of stagnation) influence the risk for scaling and/or corrosion.
Furthermore it must be kept in mind that the reaction rate of chemical reactions, e.g. the
corrosion rate, increases with increasing temperature. According to van’t Hoff’s rule, the increase
is in the order of factor 2 to 3, for every 10 °C of temperature increase.
Knowing the chemical water composition and the operating conditions of a heating system, the
risk for scaling and corrosion can be evaluated. Based on that, recommendations in order to
avoid scaling and/or corrosion problems in components can be given. This is the intention of
this water specification.
Page 2

Danfoss District Heating
Page 2 of 9
1.1 Scale formation
Raw water used for the production of drinking water (tap water) contains more or less high
amounts of dissolved gases and salts depending of the geological properties of the extraction
area. These differences cause a different composition also in the finally produced drinking water.
For the formation of scale, especially carbonate hardness (= content of hydrogen carbonate) and
total hardness, i.e. sum of calcium- and magnesium ions, are determinant; besides that, other
ions like e.g. sulphate can have an influence.
From the compounds mentioned above, lime scale (boiler scale, calcium carbonate, CaCO3) can
be formed under increasing temperatures and/or loss of carbon dioxide, e.g. by degassing).
Further temperature increase might lead to deposition of different salts, e.g. gypsum (CaSO4).
Other compounds being able to cause blocking of components are iron containing deposits like
„rust“, i.e. iron oxides and –hydroxides, or magnetite. These can be built in the PHEX itself, but
can also be flushed in from other parts of the entire system, being formed due to corrosion
processes elsewhere in the system.
1.2 Corrosion
Corrosion can be caused by different mechanisms resulting in many types of corrosion. Some of
these can take place in a PHEX during service. Most of the corrosion mechanisms are caused
chemically, whereas the chemical composition of the water influences the different materials
differently.
Besides of the factors mentioned above (material, operating conditions…), oxygen content plays
a major role in corrosion of metals. Furthermore, pH-value (acid concentration), acid capacity
(buffering capacity), salt content, are important parameters for corrosion to occur. Insofar,
knowledge of these is crucial for the evaluation of possible corrosion risks.
A detailed explanation of the different types of corrosion would go beyond the scope of this
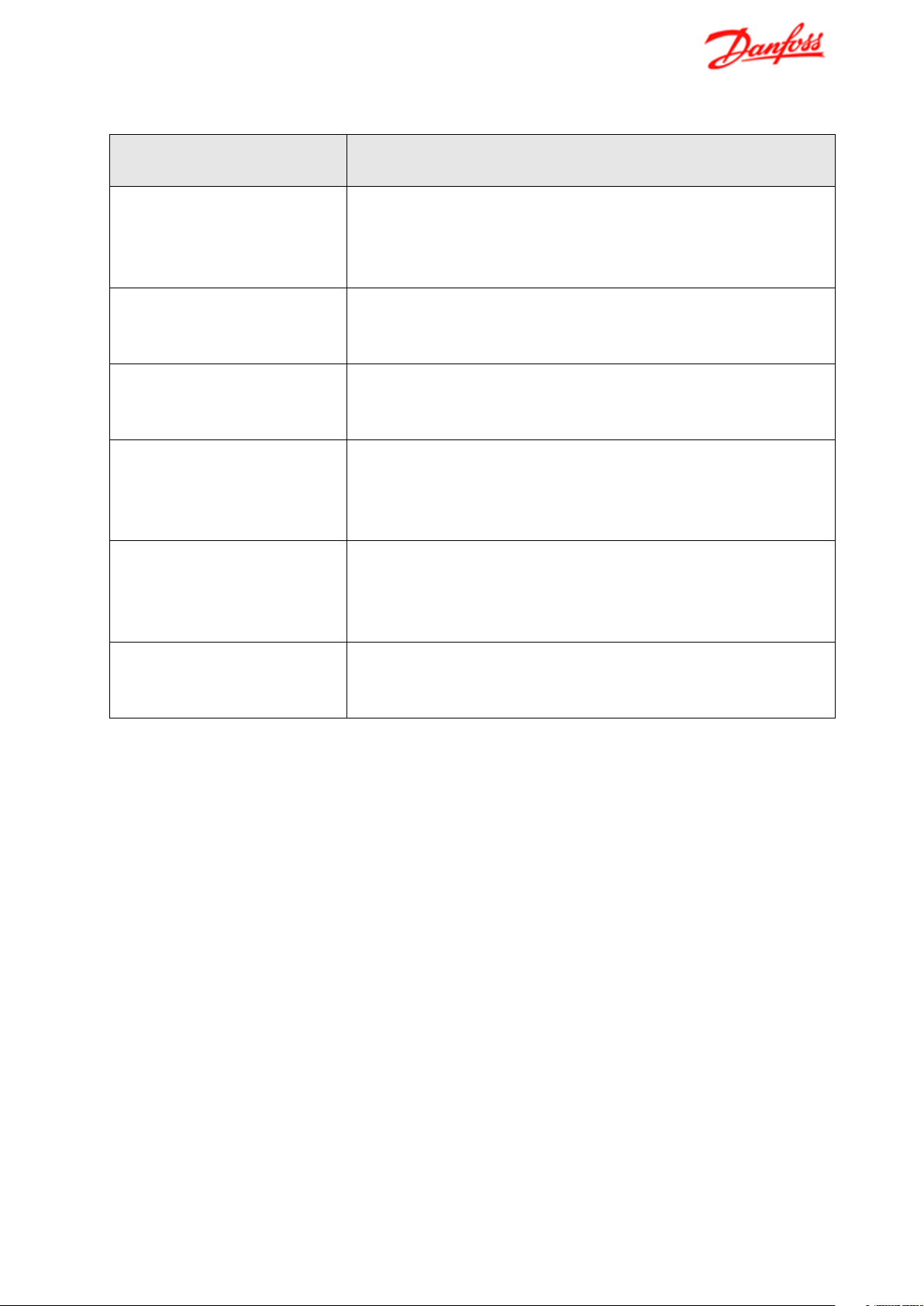
report; however, an overview of the most typical corrosion types is given in the following table 1.
Page 3
Danfoss District Heating
Page 3 of 9
Table 1 Typical corrosion types in copper brazed stainless steel plate heat exchangers
Corrosion type
Description
General corrosion
If general corrosion takes place in a PHEX it is typical copper
possibly leaks in the heat exchanger.
Crevice corrosion
Normally the heat exchanger is free of crevices, but crevices
deposits as well as imperfect brazing joints.
Galvanic corrosion
Metallic contact between copper and stainless steel in water of
high electrical conductivity can initiate a corrosive attack of the
more electronegative metal, in this case copper.
Stress corrosion cracking
Stress corrosion cracking (SCC) can occur in stainless steel if
SCC; it will often take place at temperatures above 60 °C.
Intergranular corrosion
Stainless steel can experience intergranular corrosion due to
Liquid metal embrittlement
If the brazing process takes place at too high brazing
decrease the strength of the stainless steel plates.
that will corrode and not stainless steel. If the copper brazing
corrodes it will result in loss of mechanical strength and
can be formed under deposits from scaling and other kinds of
tensile stresses and a high amount of chloride are present. An
increase in temperature will furthermore increase the risk of
[12]
[14]
formation of chromium carbide in the grain boundaries during
improper heat treatment. Areas with decreased chromium
content will become sensible towards corrosion.
temperatures, copper can diffuse into stainless steel and
Page 4

Danfoss District Heating
Page 4 of 9
2 Water specifications
2.1 Secondary side – Tap water
Parameters of normal tap water determining the overall corrosion stability of a PHEX are:
Temperature, pH, carbonate hardness (alkalinity), total hardness as well as chloride, sulphate and
nitrate concentration; conductivity is often used as sum parameter for the total ion (salt) content.
Since copper in general has lower corrosion stability than stainless steel 1.4404 in tap water, these
water specifications are mainly determined by copper corrosion. In general, corrosion of stainless
steel only occurs in tap waters containing high chloride concentrations at high temperature.
A description of the most important water parameters and their specifications are stated in the
following.
• Temperature: In general, an increase in temperature will increase the corrosion rate of most
metals. For copper in heated water, the likelihood of pitting is higher at temperatures above 60°C.
Also the risk of stress corrosion cracking of stainless steel will increase at temperatures above
60°C, and pitting and crevice corrosion in stainless steel is also temperature dependent (see the
section about chloride).
[1, 2, 14]
• pH: General corrosion of copper mainly depends on pH and the risk of corrosion is lowest if pH
is kept above 7.5 and below 9.0.
[1, 10, 12]
However, one must expect a pH around 7 in normal tap
water, but it is recommendable to avoid water with a pH below 7. Water of district heating
systems will often be alkaline up to pH 10.
• Alkalinity: If the content of hydrogen carbonate (HCO
[4, 5, 6, 8]
-
) in the water is very low, i.e. below 60
3
mg/l, corrosion products of copper may dissolve and be released into the system. It is also
-
recommendable not to exceed a HCO
concentration of 300 mg/l.
3
• Conductivity: A high conductivity in the tap water means that the water has a high concentration
[1, 10, 12]
of ionic substances. In general, an increase in conductivity of tap water will increase the corrosion
rate of most metals. A maximum conductivity of 500 µS/cm is in general a desirable value.
[9, 12]
2+
, Mg2+] / [HCO
• Hardness: Copper is susceptible to corrosion in soft water; the [Ca
(calculated in molar amounts) must therefore be greater than 0.5.
• Chloride: Presence of chloride in the drinking water will increase the risk of localized corrosion
of stainless steel. The limit value will depend on temperature according to tables 2 and 3.
• Sulphate: High concentrations of sulphate will increase the risk of pitting in copper. A maximum
[13]
-
] ratio
3
[14, 15]
sulphate concentration of 100 mg/l is recommendable, but corrosion can also take place at lower
concentrations if the [HCO
-
] / [SO
3
2-
] (calculated in molar amounts) ratio is below 1.
4
[1, 10]
• Nitrate: Nitrate ions have an influence similar to that of sulphate, and a maximum nitrate
concentration of 100 mg/l is recommendable.
• Chlorine: In many tap water installations, addition of chlorine is added for bacteriological
reasons. Chlorine is highly oxidizing and lowers the corrosion resistance of stainless steel.
Investigations of the stainless steel supplier Outukumpu Oyj have proven that the concentration
of free active chlorine should be kept below 0.5 mg/l in order to avoid corrosion of stainless steel
1.4404.
[10, 13]
[15]
Page 5

Danfoss District Heating
Page 5 of 9
The following table shows summarized the specifications being recommended for copper brazed
Parameter
Remarks
Value
Appearance
clear
Smell
no smell
Content of impurities
free of sediments/particles
Oil and grease
< 1 mg/L
pH
between 7 and 10
El. conductivity
2500 μS/cm
Carbonate hardness *)
1 mmol/L < Ks
< 5 mmol/L **)
Total hardness ***)
[Ca2+, Mg2+]/[HCO
] > 0.5
Chloride
at T ≤ 20 °C
1000 mg/l
at T ≤ 50 °C
400 mg/L
at T ≤ 80 °C
200 mg/L
at T > 100 °C
100 mg/L
Sulphate
[SO
] < 100 mg/L and
[HCO
]/[ SO
] > 1.5
Nitrate
< 100 mg/L
Nitrite
not allowed
Ammonium
< 2.0 mg/L
Free chlorine
< 0.5 mg/L
Total iron
< 0.2 mg/L
Manganese
< 0.05 mg/L
plate heat exchangers of stainless steel for the secondary side, i.e. drinking water side.
Table 2 Recommended water quality limits for water on secondary side of PHEX
4.3
-
3
2-
4
-
3
2-
4
*) = hydrogen carbonate content, temporary hardness, (carbonate) alkalinity
**) Ks
= acid capacity
4.3
***) = sum of calcium and magnesium ions
Page 6

Danfoss District Heating
Page 6 of 9
2.2 Primary side – District heating water
Water specifications for district heating water are given in several national guidelines being
evaluated for this specification
[4, 5, 6, 7, 8]
. All these guidelines deal with aspects of corrosion and
scale prevention in district heating systems.
The limits stated in the following table 3 are a reasonable compromise to avoid corrosion and
scaling on the primary side of the plate heat exchanger; they are widely identical with those for
tap water used on the secondary side.
The most important parameters influencing corrosion resistance of stainless steel in district
heating water are chloride, temperature and oxygen content. The acceptable chloride level will
depend on the maximum temperature which the PHEX is exposed to.
The most important parameters to limit the corrosion risk of copper is providing a virtually
oxygen free (below 0.1 mg/L) and alkaline environment (below pH 10) and keeping contents of
ammonia and sulphide below minimum limits (see table 3).
In district heating water, often softened or desalinated water conditioned to a pH around 9-9.5 is
used; oxygen content is either removed or chemically bonded. Special concerns should be made
about some of the chemicals which are used for pH-conditioning and/or as oxygen binders.
The use of ammonia for pH conditioning should be avoided due to the risk of copper (and brass)
corrosion. Instead, use sodium hydroxide (NaOH) or tri sodium phosphate (Na3PO4) to increase
the pH of the water.
Sodium sulphite (Na2SO3) has been widely used as an oxygen binder, but should be avoided in
systems containing copper and stainless steel. Due to the oxygen binding process, sulphite is
transformed to sulphate. Sulphate can be used by some bacteria which reduce sulphate to
sulphide, thus creating a corrosive environment towards copper and stainless steel. Instead,
organic oxygen binders such as tannins should be used.
Generally, increased concentrations of sulphide in the water could indicate bacterial
contamination in the district heating water system. Therefore it is recommended to keep a
minimum of sulphide in the water.
Other oxygen binders are sometimes added to the water. Some examples are C-vitamin and
Methyl-ethyl-ketoxim (MEKO). Biocides can also be added to the water in order to control the
formation of bacteria in the system. Tensides are sometime added to the water in order to reduce
friction in the system.
Page 7
Danfoss District Heating
Page 7 of 9
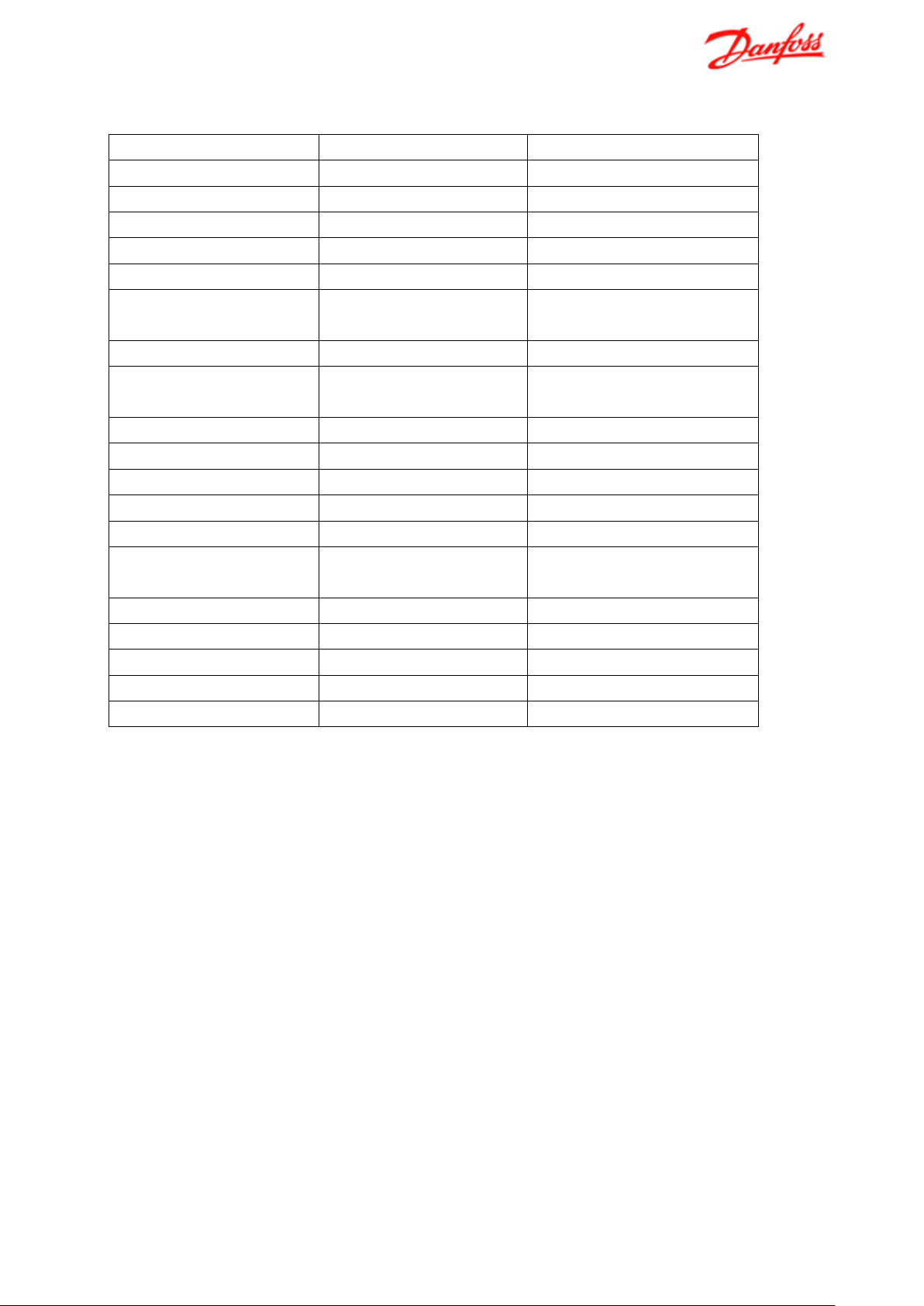
Table 3 Recommended water quality limits for district heating water on primary side
Parameter
Remarks
Value
Appearance
clear
Smell
no smell
Content of impurities
free of sediments/particles
Oil and grease
< 1 mg/l
pH at 25°C
7 to 10
Residual water hardness
[Ca2+, Mg2+]/[HCO
] > 0.5 ,
< 0.5 mmol/L (2.8 °dH)
Conductivity at 20°C
2500 µS/cm
Oxygen
<0.1 mg/L
(as low as possible)
Chloride
at T ≤ 20 °C
1000 mg/l
at T ≤ 50 °C
400 mg/L
at T ≤ 80 °C
200 mg/L
at T > 100 °C
100 mg/L
Sulphate
[SO
2-
] < 100 mg/L and
[HCO
]/[ SO
] > 1.5
Sulphite
e.g. use of oxygen binder
< 10 mg/L
Sulphide
< 0.02 mg/L
Nitrate
< 100 mg/l
Ammonium
< 2.0 mg/L
Total Org. Carbon TOC
< 30 mg/L
-
3
4
-
3
2-
4
2.3 Hardness, scaling and warranty
The ability to transfer heat in plate heat exchangers will be reduced by precipitation of contents
of the water (scaling) and deposition of impurities. Scaling is usually caused by the presence of
calcium and magnesium salts.
Total Hardness, is primarily the sum of calcium (Ca++) and magnesium (Mg++) ions in the water.
It is commonly expressed in milligram per litre (mg/L) or parts per million (ppm) of calcium
carbonate (CaCO3) or degrees hardness (°dH). A German °dH is equivalent to 17.8 ppm CaCO3.
Since 2004, water hardness is classified in the European Community acc. the EC-Regulation No
648/2004 on detergents
[16]
as shown in the following table.
Page 8

Danfoss District Heating
Page 8 of 9
Table 4 Classification of water hardness acc. to EC-Regulation No. 648/2004 on detergents
Range of hardness
Calcium carbonate
[mmol/L] 1)
Calcium carbonate
[mg/L] 2)
°dH 2)
soft Less than 1.5 Less than 150 Less than 8,4 °dH
medium 1.5 to 2.5 150 to 250 8,4 to 14 °dH
hard More than 2.5 More than 250 More than 14 °dH
1
) Acc. to the Système international d’unités from 1971, sum of earth alkali is given in mmol/L.
2
) Statement of values in mg/L and “degree of German Hardness °dH” is only informative.
Heating of water with high hardness causes precipitation of lime scale (CaCO3). This will appear
as a layer on the plate surface. Heating above 55°C may cause extensive precipitation of lime scale.
This will reduce the ability to transfer heat in plate heat exchangers.
It is therefore important to select Danfoss heat exchangers in sizes which secure that flow speed
is as high as possible. This will help to reduce scaling.
Content of impurities can also be deposited as a layer on the plate surface.
Impurities and lime scaling can be removed by flushing the heat exchanger with different types
of chemicals, depending on the composition of the deposit. Danfoss recommends using suppliers
with a proven technology and experience in cleaning heat exchangers.
Flushing can remove the depositions and increase the ability to transfer heat, but it can also cause
reduction of the life of the heat exchanger.
Danfoss District Heating cannot take over the warranty responsibility for heat exchangers:
• With reduced capacity caused by lime precipitation and scaling
• Leaking externally or internally after flushing to remove precipitation and scaling.
• Leaking externally or internally caused by water induced corrosion if the recommendations
for the water quality in this guideline are not fulfilled.
Page 9

Danfoss District Heating
Page 9 of 9
3 References
[1] EN 12502-2:2004. Protection of metallic materials against corrosion – Guidance on the
assessment of corrosion likelihood in water distribution and storage systems – Part 2:
Influencing factors for copper and copper alloys
[2] EN 12502-4:2004. Protection of metallic materials against corrosion – Guidance on the
assessment of corrosion likelihood in water distribution and storage systems – Part 4:
Influencing factors for stainless steels
[3] EN 14868:08-2005 Protection of metallic materials against corrosion – Guidance on the
assessment of corrosion likelihood in closed water circulation systems.
[4] VDI 2035-2:08-2009 Prevention of damage in water heating installations, Part 2: Water-
side corrosion.
[5] AGFW-Arbeitsblatt FW 510:06-2011 Requirements for circulation water in industrial and
district heating systems and recommendations for their operation.
[6] ÖNORM H 5195-1:12-2010 Heat medium for technical building equipment, Part 1:
Prevention of damage by corrosion and scale formation in closed warm-water-heating
systems.
[7] SWKI BT 102-01:04-2012, Richtlinie “Wasserbeschaffenheit für Gebäudetechnik-Anlagen“
Ed.: Schweizerischer Verein von Gebäudetechnik-lngenieuren, www.swki.ch
[8] DFF-guideline “Vandbehandling og korrosionsforebyggelse i fjernvarmesystemer”. DFF
Danske Fjernvarmeværkers Forening, 1999.
[9] Mattsson, E., 1988. Counteraction of pitting in copper water pipes by bicarbonate dosing.
Werkstoffe und Korrosion 39,499-503
[10] Mattsson, E., 1990. Tappvattensystem av kopparmaterial. Korrosionsinstitutet, ISBN 91-
7332-558-9.
[11] Anonymus, 2004. Fachthema Gelötete Plattenwärmeüberträger. Euroheat & Power 33, 3,
96-104
[12] Nilsson, K., Klint, D., Johansson, M., 2007. Corrosion aspects of compact heat exchangers
consisting of stainless steel plates brazed with copper filler metal in water applications”,
14th Nordic Corrosion Congress, , Copenhagen, Denmark.
[13] Pajonk, G., undated. “Korrosionsschäden an gelöteten Plattenwärmetauschern”,
Materialprüfungsamt Nordrhein-Westfalen, Dortmund. http://www.vau-thermotech.de/
mediapool/40/409506/data/Korrosionsschaeden_an_geloeteten_Plattenwaermetauschern.pdf
[14] Outukumpu Corrosion Handbook for Stainless Steels”, Tenth edition, 2009
[15] Mameng, S., Pettersson, R., 2011. “Localised corrosion of stainless steels depending on
chlorine dosage in chlorinated water”. Outukumpu acom 03-2011.
[16] Regulation (EC) No 648/2004 of the European parliament and of the council of 31 March
2004 on detergents
 Loading...
Loading...