Cytomedix Angel Operator's Manual

CONCENT RATED PLATELET RICH
PL ASMA (cPRP) SYSTEM
Angel® Concentrated Platelet Rich
Plasma (cPRP) System - Operator’s Manual
Software Version 1.21
966030107 Rev. E

Table of Contents
Before You Get Started
Introduction................................................................... i
Indications for Use ............................................................. i
Contraindications for Use ........................................................ i
Warnings ....................................................................ii
Precautions...................................................................iv
Symbols and Certication........................................................vi
Service Information............................................................vii
Return of Used Product ........................................................vii
Chapter 1: Overview
Product Description .......................................................... 1-1
Description of the Angel® Concentrated Platelet Rich Plasma (cPRP) System ............. 1-1
How the Angel® Concentrated Platelet Rich Plasma (cPRP) System Works .......... 1-1
Angel® Concentrated Platelet Rich Plasma (cPRP) System Components ............ 1-2
Shipping and Storage......................................................... 1-3
Installation ................................................................. 1-3
Special tools, equipment and environmental requirements ....................... 1-3
Visual Inspection........................................................ 1-4
Unpacking/Assembly .................................................... 1-4
Operational Checks ..................................................... 1-4
Setting the Date and Time ................................................ 1-5
Chapter 2: Installing the Disposables
Angel® Concentrated Platelet Rich Plasma (cPRP) System ............................2-1
Description .............................................................2-1
Warnings and Precautions .................................................2-3
Setup and Blood/Bone Marrow Preparation.........................................2-3
Blood Collection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Chapter 3: Processing
Before You Begin ............................................................3-1
Loading the Angel® cPRP System ..........................................3-1
Collecting the Blood or Mixture of Blood and Bone Marrow .......................3-1
Running the Separation Process ................................................3-1
Saving Case Data............................................................3-5
Entering Optional Case Data Fields .........................................3-6
Modifying Optional Data Field Values........................................3-7
Selecting Past Cases ....................................................3-8
Angel® cPRP System Operator’s Manual

Saving a Tally Table to a USB Storage Device .................................3-9
Saving a Case Log .....................................................3-10
Touch Screen User Interface ..................................................3-11
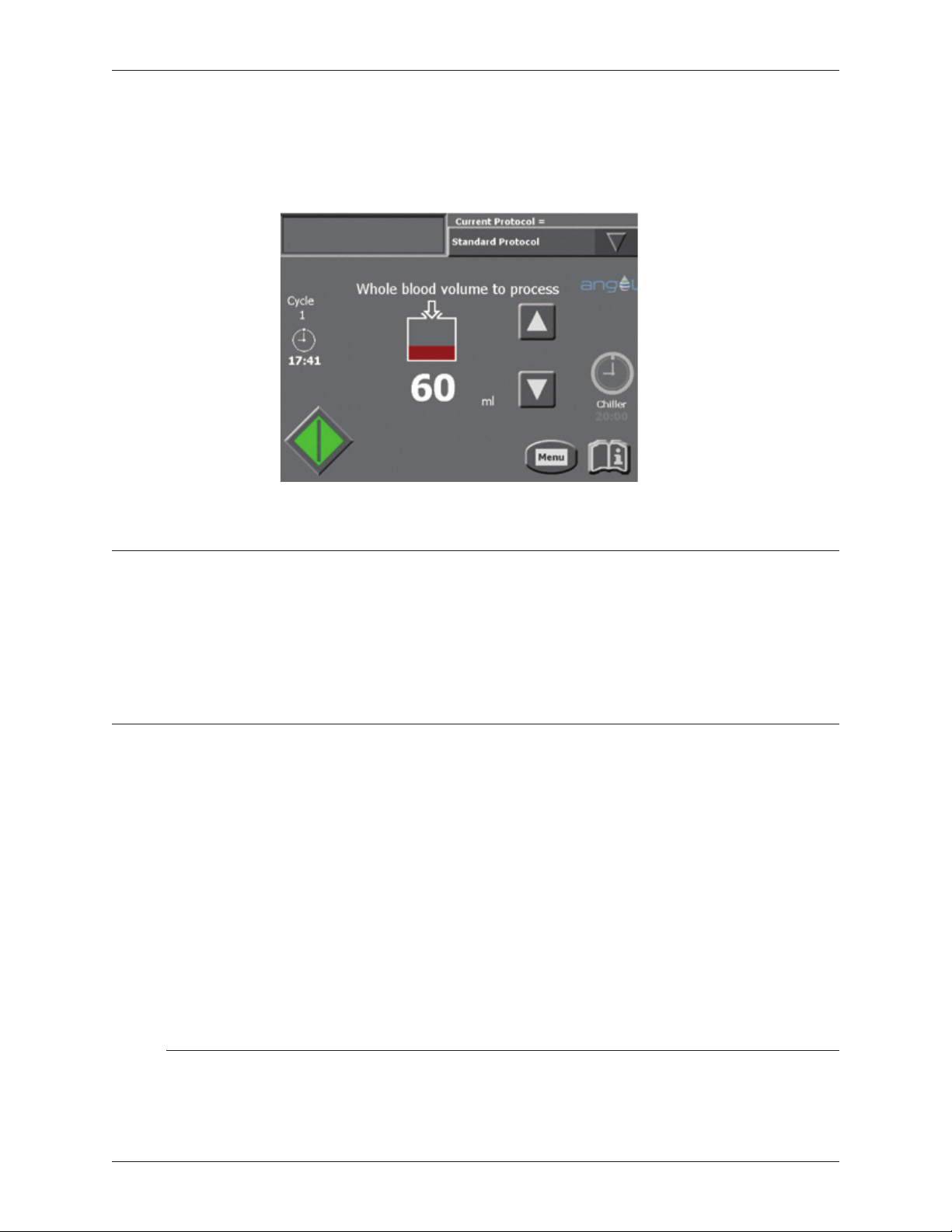
Start Screen ..........................................................3-11
Run Screen...........................................................3-13
End of Cycle Screen ....................................................3-15
End of Case Screen ....................................................3-15
Menu Screen .........................................................3-17
Info Screen ...........................................................3-20
Past Cases Screen .....................................................3-21
Output Screen.........................................................3-22
Stop Button................................................................3-24
Power Loss................................................................3-25
Chapter 4: Programmability Option
Creating Custom Protocols.....................................................4-1
Entering Values and Text .................................................4-2
Creating a New Protocol..................................................4-3
Editing the Parameters of a Protocol ........................................4-4
Restoring the Parameters of a Protocol ......................................4-4
Renaming a Protocol ....................................................4-4
Changing the Wakeup Protocol ............................................4-5
Deleting a Protocol ......................................................4-5
The Protocols Tab............................................................4-5
1. Protocol Buttons ......................................................4-6
2. Protocol Parameters and Buttons.........................................4-7
3. Protocol Pull-Down Button ..............................................4-8
4. Close Button .........................................................4-8
5-6. Up and Down Arrow Buttons ...........................................4-8
7. Info Button ..........................................................4-8
Chapter 5: Troubleshooting
Alarms and Notications.......................................................5-1
Troubleshooting the Save Process...............................................5-7
Troubleshooting the Software Update Process .....................................5-7
Chapter 6: Routine Care
Precaution Statement......................................................... 6-1
New Software ...............................................................6-1
New Software Screen .................................................... 6-4
Visual Inspection ............................................................ 6-5
Routine Care ............................................................... 6-5
External Surfaces .......................................................6-5
Angel® cPRP System Operator’s Manual
Non-Routine Care............................................................ 6-6
Cleaning the Centrifuge Well .............................................. 6-6
Cleaning the Pump Rotor .................................................6-8
Preventive Maintenance Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-9
Data Integrity Accessories ..................................................... 6-9
List of Operator Replaceable Parts .............................................. 6-9
Fuse Replacement ...........................................................6-9
Chapter 7: Technical Data
Specications ................................................................7-1
Performance Characteristics ...............................................7-1
Physical Characteristics ...................................................7-1
Environmental Limitations..................................................7-2
Classication according to EN 60601-1/IEC601-1 ...............................7-6
Chapter 8: Warranty
Limited warranty and contractual conditions for Cytomedix, Inc. medical devices............8-1
Warranty Expiration ......................................................8-1
Contents and necessary requirements of the warranty ...........................8-1
Cases in which the warranty shall not be effective ...............................8-2
Warranty for replaced parts ................................................8-2
Availability of spare parts ..................................................8-2
Technical documentation ..................................................8-2
Maintenance contracts ....................................................8-2
Limits to the warranty with regard to product use by the buyer and/or user ............8-3
Technical safety standards applicable for the purpose of the warranty ...............8-3
Absolute prohibition to amend the conditions of this warranty ......................8-4
Applicable law and jurisdiction ..............................................8-4
Identication of manufacturer ...............................................8-4
Angel® cPRP System Operator’s Manual
List of Figures
Chapter 1: Overview
Figure 1-1 Front-view of the Angel® Concentrated Platelet Rich Plasma (cPRP) System ............1-2
®
Figure 1-2 Rear-view of the Angel
Figure 1-3 Start Screen ..............................................................1-3
Figure 1-4 Load Screen ..............................................................1-4
Figure 1-5 Date and Time Settings......................................................1-5
Chapter 2: Installing the Disposables
Figure 2-1 Angel® cPRP Processing Set .................................................2-2
Figure 2-2 Rear-view of the Angel® Concentrated Platelet Rich Plasma (cPRP) System ............2-4
Figure 2-3 Mounting the Separation Chamber .............................................2-5
Figure 2-4 Centrifuge Stator Arm Aligned with Variable Volume Separation Chamber ..............2-6
Figure 2-5 Valve Assembly............................................................2-7
Concentrated Platelet Rich Plasma (cPRP) System ............1-2
Chapter 3: Processing
Figure 3-1 Load Screen ..............................................................3-1
Figure 3-2 Start Screen ..............................................................3-2
Figure 3-3 Run Screen ...............................................................3-3
Figure 3-4 End of Cycle Screen ........................................................3-3
Figure 3-5 End of Case Screen ........................................................3-4
Figure 3-6 “Tally” tab of the Menu Screen ................................................3-5
Figure 3-7 Keyboard Screen (Text Entry).................................................3-6
Figure 3-8 Past Cases Screen .........................................................3-8
Figure 3-9 Output Screen.............................................................3-9
Figure 3-10 Start Screen ............................................................3-13
Figure 3-11 Run Screen .............................................................3-15
Figure 3-12 End of Cycle Screen ......................................................3-18
Figure 3-13 End of Case Screen ......................................................3-19
Figure 3-14 “Language” tab of the Menu Screen ..........................................3-20
Figure 3-15 “Tally” tab of the Menu Screen ..............................................3-21
Figure 3-16 “Settings” tab of the Menu Screen ...........................................3-22
Figure 3-17 Info Screen .............................................................3-23
Figure 3-18 Past Cases Screen .......................................................3-24
Figure 3-19 Output Screen...........................................................3-25
Figure 3-20 Empty Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-27
Figure 3-21 Correct Valve Assembly Handle Position ......................................3-28
Angel® cPRP System Operator’s Manual

Chapter 4: Programmability Option
Figure 4-1 The “Protocols” tab .........................................................4-2
Figure 4-2 The Keyboard Screen (Protocol Name) .........................................4-2
Figure 4-3 “Protocols” tab of the Menu Screen ............................................4-6
Chapter 6: Routine Care
Figure 6-1 Unlock Code Screen ........................................................6-2
Figure 6-2 Software Validated Screen ...................................................6-3
Figure 6-3 Install Screen .............................................................6-3
Figure 6-4 New Software Screen .......................................................6-4
Figure 6-5 Removing the Centrifuge Adaptor Plate .........................................6-6
Figure 6-6 Removing the Spring Plate and Springs .........................................6-7
Figure 6-7 Cleaning On and Around the Adaptor Base ......................................6-7
Figure 6-8 Removing the Pump Rotor ...................................................6-8
Figure 6-9 Reinstalling the Pump into the Rotor Housing ....................................6-9
Figure 6-10 Fuse Replacement: Pry Open Cover .........................................6-10
Figure 6-11 Fuse Replacement: Slide Out Holders ........................................6-10
Angel® cPRP System Operator’s Manual

Before You Get Started
Introduction
The Angel® Concentrated Platelet Rich Plasma (cPRP) System (Angel) is
designed to separate autologous blood or a mixture of blood and bone marrow.
The primary blood components that the Angel® Concentrated Platelet Rich
Plasma (cPRP) System separates and collects are red blood cells (RBC), platelet
poor plasma (PPP) and platelet rich plasma (PRP).
®
The Angel
volume separation chamber that is capable of processing between 40 ml - 180 ml
of anticoagulated whole blood or mixture of blood and bone marrow in a single
cycle. A total of 180 ml can be processed over three (3) cycles per disposable.
This manual is intended for users of the Angel® Concentrated Platelet Rich
Plasma (cPRP) System. The procedures recommended in this Operator’s
Manual have been developed and tested to provide safe, reliable and efcient
operation of the Angel
It is important that the operator thoroughly understand the information in this
Operator’s Manual before attempting to use the Angel® Concentrated Platelet
Rich Plasma (cPRP) System.
Concentrated Platelet Rich Plasma (cPRP) System utilizes a variable
®
Concentrated Platelet Rich Plasma (cPRP) System.
Before You Get Started
Indications for Use
The Angel® Concentrated Platelet Rich Plasma (cPRP) System is intended to
be used in the clinical labaratory or interoperatively at the point-of-care for the
safe and rapid preparation of platelet poor plasma and concentrate (platelet rich
plasma) from a small sample of whole blood or a small mixture of blood and bone
marrow. The platelet rich plasma from the
Plasma (cPRP) System
prior to the application to an orthopedic site.
Disclaimer
Platelet Rich Plasma prepared from a mixture of whole blood and bone marrow
may contain higher levels of plasma free hemoglobin than Platelet Rich Plasma
prepared from whole blood.
Contraindications for Use
The Angel® Concentrated Platelet Rich Plasma (cPRP) System may be
contraindicated in cases where there is active systemic infections or
systemic heparinization.
Angel® Concentrated Platelet Rich
can also be mixed with autograft and/or allograft bone
Angel® cPRP System Operator’s Manual i

Before You Get Started
Warnings
1. The use of operating or maintenance procedures other than those published by
Cytomedix, Inc., or the use of accessory devices not recommended by Cytomedix,
Inc. may result in poor equipment performance.
Cytomedix, Inc. will not be responsible for patient safety or equipment performance
if the procedures to operate and maintain the Angel® Concentrated Platelet Rich
Plasma (cPRP) System are other than those specied by Cytomedix, Inc. Persons
performing the procedures must be properly trained and qualied.
Any equipment modications must be performed by qualied persons and be
approved by Cytomedix, Inc. in writing.
All electrical installations must comply with all applicable local electrical codes and
Cytomedix, Inc. specications.
This equipment meets the following standards in the United States and the user
does not need to provide additional efforts regarding electromagnetic emissions
or immunity:
• EN 55011, Class A standards (for electromagnetic emissions)
• EN 61000 (for electromagnetic emissions)
• IEC 60601-1-2 (2007) (for electromagnetic immunity)
• Canadian Warning: This equipment is intended for use by healthcare
professionals only. The Angel® Concentrated Platelet Rich Plasma (cPRP)
System may cause radio interference or may disrupt the operation of nearby
equipment. It may be necessary to take mitigation measures, such as reorienting or relocating the Angel® Concentrated Platelet Rich Plasma (cPRP)
System or shielding the location.
2. The operator should never touch the data port on the Angel® cPRP System, while at
the same time making contact with the patient, as potential for electrical shock may
result.
3. Place the Angel® Concentrated Platelet Rich Plasma (cPRP) System on a at, stable
surface. Never try to move the Angel® Concentrated Platelet Rich Plasma (cPRP)
System while the device is in operation. Failure to comply may result in damage to
the Angel® Concentrated Platelet Rich Plasma (cPRP) System and injury may result.
4. Do not use the Angel® Concentrated Platelet Rich Plasma (cPRP) System in the
presence of ammable agents because an explosion and/or re may result.
5. A trained operator should be present at all times to operate and monitor the Angel®
Concentrated Platelet Rich Plasma (cPRP) System during processing.
6. Do not contact any moving parts of the centrifuge or pump while the Angel®
Concentrated Platelet Rich Plasma (cPRP) System is in operation. Injury may result.
7. To prevent risk of electrical shock, do not use alternate power plugs or adapters that
disconnect the safety ground.
8. Only Angel® cPRP Processing Sets are approved for patient use with the Angel®
Concentrated Platelet Rich Plasma (cPRP) System.
9. Do not use the Angel® cPRP Processing Set if the sterile packaging barrier has
been broken.
Angel® cPRP System Operator’s Manualii

Before You Get Started
10. Carefully examine the Angel® cPRP Processing Set for damage prior to use. Should any
evidence of damage to the Processing Set be evident, do not use the Processing Set.
11. Carefully observe the Angel® cPRP Processing Set for leaks during use. Leakage
may result in loss of sterility of the device or loss of blood product.
12. Use of this product for pediatric patients is at the discretion of a physician. Blood
withdrawal from a pediatric patient should be performed in the presence and at the
direction of a physician to prevent signicant reduction of the circulating blood volume.
13. When collecting and processing autologous blood products it is recommended
that the following precautions be followed to insure that the autologous product is
not contaminated:
• Use sterile technique when setting up the Angel® cPRP Processing Set
• Thoroughly clean and disinfect the donation site
• Use sterile technique whenever handling autologous blood products
14. The whole blood or the mixture of blood and bone marrow must be anticoagulated
before it can be processed for separation. Inadequate anticoagulation may result
in clotting, interfering with the processing of the blood products. Blood containing
clots will not pass through the syringe-activated valve located on the Whole Blood
compartment of the three-compartment reservoir bag.
15. Failure to properly load the centrifuge plate prior to processing may lead to
exposure to blood and blood-borne pathogens.
16. If centrifugation is discontinued before the completion of a processing cycle, the
variable volume separation chamber is pressurized and presents the risk for
exposure to blood and blood-borne pathogens if the variable volume separation
chamber is not properly removed. Please refer to “Stop Button” on page 3-27 for
emptying a variable volume separation chamber containing blood.
17. If a power loss occurs, and there is blood or a mixture of blood and bone marrow
in the variable volume separation chamber, follow the instructions under “Power
Loss” on page 3-28.
18. Failure to properly secure the luer lock syringe to the valve assembly may result in
a leakage of uids.
19. Do not directly connect the patient to the three-compartment reservoir bag. Direct
connection to the patient could lead to vascular damage, shock, or air embolism.
20. Disposal of used equipment and/or used Angel® cPRP Processing Sets should be
in accordance with federal, state, and local regulations. These materials should be
considered biohazardous. Universal precautions for blood-borne pathogens should
be practiced when disposing of these items.
21. Do not place objects in pump during pump rotation. Damage to machine and
disposable may result.
22. Do not use the separated blood products if the Angel® Concentrated Platelet Rich
Plasma (cPRP) System fails to operate as intended.
23. The platelet rich plasma is not intended for transfusion.
Angel® cPRP System Operator’s Manual iii

Before You Get Started
Warnings (Cont.)
Precautions
24. The Angel® Concentrated Platelet Rich Plasma (cPRP) System is not intended to
be used directly by the patient. As such a mains power switch is not available to the
user. In case of an emergency, power from the unit can be removed by unplugging
the unit from the electrical socket.
25. Only devices or cables meeting IEC 60950 should be connected to the USB and/
or Ethernet port. Failure to do so may result in operator shock. All cables used in
conjunction with the device should be no longer than 1m (3ft) in length.
1. Carefully read this Operator’s Manual for complete instructions.
2. This product is intended for use by trained medical personnel only.
3. When cleaning the platelet sensor, do not use bleach, abrasive materials or
solvents as they may cause damage to the sensor. Use a mild detergent.
4. Do not place external light sources within 1 meter (3 feet) of the unit when
operating the Angel® Concentrated Platelet Rich Plasma (cPRP) System.
External light sources may interfere with the operation of the platelet sensor
and may result in reduced processing efciency.
5. Do not immerse the pump rotor in cleaning solution. Do not autoclave.
Component damage may result.
6. Prior to rst use follow the installation instructions included in this manual.
7. Any device connected to the data port must comply with the applicable IEC
standard for that device.
8. To prevent risk of electrical shock, shut OFF power and unplug the system
from the electrical outlet before performing cleaning procedures or replacing
the fuses.
9. Report immediately to the Cytomedix, Inc. Customer Service Response
Center any of the following conditions. Do not use the Angel® Concentrated
Platelet Rich Plasma (cPRP) System until corrective action has been taken:
• Damaged or worn power cord, plug or receptacle
• Switches that are loose or do not operate with a positive action
• A system that has been subject to signicant physical damage
• A system that has given anyone an electrical shock while in use
• A system that appears to be overheating
10. It is the responsibility of the health care institution to adequately prepare and
identify the product for return shipment. Do not return products that have
been exposed to blood-borne infectious diseases.
11. Due to the possibility of operator exposure to blood-borne pathogens (such
as HIV, hepatitis viruses, bacteria, etc.), Universal Precautions for bloodborne pathogens should be practiced.
Angel® cPRP System Operator’s Manualiv

Before You Get Started
12. The Angel® cPRP Processing Set is intended for single patient use. Each set
can be used on the same patient for up to three processing cycles. Do not
resterilize any part of this Processing Set.
13. Failure to properly load the Angel® cPRP Processing Set per the enclosed
instructions may affect the performance of the system.
14. Luer lock syringes should be used with the Angel® cPRP Processing Set.
15. Pressing the Stop button during separation may reduce processing efciency.
16. The physician ordering the collection of PRP shall use discretion when any of
the following conditions exist:
• sepsis
• preoperative hematocrit less than 30%
• preoperative platelet count less than 195,000 per ul
• hemodynamically unstable
• prolonged clotting times
• recent use of anti-platelet drugs
• inability to maintain stable oncotic pressure
17. Attach the power cord only to a power receptacle which is properly grounded.
18. Replace the mains fuses only with fuses of the same type and rating.
19. There are no user-serviceable parts inside this device. To avoid the risk of
electrical shock, do not remove the cover. Refer all servicing to qualied
service personnel.
20. Take care not to misplace the screws and springs when disassembling the
centrifuge adaptor plate. If springs or screws are misplaced, you may order
replacements by contacting Cytomedix, Inc.
21. Federal law (USA) restricts this device to sale by or on the order of a physician.
22. The user of the Angel® Concentrated Platelet Rich Plasma (cPRP) System
is responsible for the monitoring of the system performance when using
custom protocols.
23. The Angel® Concentrated Platelet Rich Plasma (cPRP) System has been
tested and veried to meet applicable Electromagnetic Compatibly (EMC)
and Electrical Safety standards when used in conjunction with the specied
Data Integrity Accessories. (Refer to Section 6.)
24. The Angel® Concentrated Platelet Rich Plasma (cPRP) System has the
ability to save data using both the USB and Ethernet Network connections.
When using either of these connections, it is recommended that the
applicable Data Integrity Accessory be used. (Refer to Section 6.)
Angel® cPRP System Operator’s Manual v
Before You Get Started
Symbols and Certications
If applicable, the following symbols may appear on the device labeling.
MODEL
Catalog number.
Model number.
Attention. Consult
accompanying documents.
(IEC 60601-1, 2nd Edition)
Caution. (IEC 60601-1, 3rd. Edition)
Follow instructions for use.
Date of manufacture.
This symbol indicates that the
device requires an alternating
supply current.
2X F 5A 250V
This symbol species the type
of fuse(s).
To reduce the risk of electrical
shock, do not remove cover.
There are no user-serviceable
parts inside. Refer servicing to
qualied service personnel.
This side up
Fragile
Keep dry
SN
This symbol identies the point
of connection of a potential
equalization conductor. The
location of this connection point
is at the rear of the machine,
below the access door.
This symbol indicates the power
OFF position on the main power
switch.
This symbol indicates the power
ON position on the main power
switch.
Manufacturer.
Caution: Federal law (USA)
restricts this device to sale by or
on the order of a physician.
SN Serial number.
-20°C
10°C
10%
10%
37.7°C
Ship and store between these
temperatures: -20 and 37.7
degrees C (-4 and 100 degrees F).
30°C
Install and operate between 10
and 30 degrees C (50 and 86
degrees F).
90%
Ship and store between 10%
and 90% relative humidity.
70%
Install and operate between
10% and 70% relative humidity.
Angel® cPRP System Operator’s Manualvi

Service Information
The company accepts responsibility for the safety, reliability and performance of this
equipment only if operational procedures, calibrations and repairs are performed by
appropriately qualied persons; if all equipment modications are authorized in writing
by the company and performed by appropriately qualied persons; if the electrical
installation of the relevant room complies with all applicable local electrical codes;
and if the equipment is used in accordance with the published instructions for use.
Should you require technical assistance, contact your Customer Service Representative.
Cytomedix, Inc.
Customer Service Response Center
209 Perry Parkway, Suite 7
Gaithersburg, MD 20877 USA
Telephone: +1 877-865-9927
Telephone: +1 301-917-6860
Fax: +1 240-499-2690
CustomerCare@Cytomedix.com
www.Cytomedix.com
Before You Get Started
Return of Used Product
If for any reason this product must be returned to Cytomedix, Inc., a returned goods
authorization (RGA) number is required from Cytomedix, Inc. prior to shipping.
If the product has been in contact with blood or body uids, it must be thoroughly
cleaned and disinfected before packing. It should be shipped in either the original carton,
or an equivalent carton, to prevent damage during shipment; and it should be properly
labeled with an RGA number and an indication of the biohazardous nature of the
contents of the shipment.
Instructions for cleaning and materials, including appropriate shipping containers, proper
labeling and an RGA number may be obtained from the Cytomedix, Inc. Customer
Service Response Center (877-865-9927) or CustomerCare@Cytomedix.com.
It is the responsibility of the health care institution to adequately
prepare and identify the product for return shipment. Do not return
products that have been exposed to blood-borne infectious diseases.
The shipping address for returned goods is:
Cytomedix, Inc.
Customer Service Response Center
209 Perry Parkway, Suite 7
Gaithersburg, MD 20877 USA
PRECAUTION
Telephone: +1 877-865-9927
Telephone: +1 301-917-6860
Fax: +1 240-499-2690
CustomerCare@Cytomedix.com
www.Cytomedix.com
Angel® cPRP System Operator’s Manual vii

Overview
Chapter 1: Overview
Product Description
The Angel® Concentrated Platelet Rich Plasma (cPRP) System (Angel® cPRP
System) consists of a blood processing device and disposable products used for
separation of whole blood or mixture of blood and bone marrow into red cells,
platelet poor plasma, and platelet rich plasma. The processing disposable, the
Angel® Concentrated Platelet Rich Plasma (cPRP) System Processing Set,
is designed for single-patient use. The variable volume separation chamber
allows the clinician to process from 40 ml to 180 ml of autologous whole blood
or mixture of blood and bone marrow in a single cycle. A total of 180 ml can be
processed over three (3) cycles per disposable.
Description of the Angel® Concentrated Platelet Rich Plasma (cPRP) System
How the Angel® Concentrated Platelet Rich Plasma (cPRP) System Works
The Angel® Concentrated Platelet Rich Plasma (cPRP) System processes a
determined volume of anticoagulated whole blood or mixture of blood and bone
marrow from a patient and separates the blood/bone marrow into its primary
components: red blood cells (RBC), platelet poor plasma (PPP), and platelet rich
plasma (PRP).
The basic steps are:
Blood Collection: The whole blood or mixture of blood and bone marrow is
drawn from a patient and mixed with a citrate anticoagulant. The collected whole
blood/bone marrow is mixed in a 7:1 ratio (7 parts whole blood to 1 part citrate
anticoagulant (ACD-A)).
Processing: The Angel® (cPRP) Processing Set utilizes a variable volume
separation chamber (40 ml – 180 ml) which allows the clinician to determine the
amount of preoperative blood/bone marrow volume to be processed. The Angel®
cPRP Processing Set allows for up to three processing cycles per disposable.
Once the anticoagulated whole blood or mixture of blood and bone marrow has
been dispensed into the whole blood compartment of the three-compartment
reservoir bag, the clinician selects the desired volume of autologous whole
blood/bone marrow they elect to process and presses the “Start” button on the
touch screen display. The Angel® Concentrated Platelet Rich Plasma (cPRP)
System will ll the variable volume disposable with the pre-determined volume of
anticoagulated whole blood or mixture of blood and bone marrow, separate the
whole blood/bone marrow through centrifugation, and collect the primary blood
components (RBC, PPP, and PRP) in their respective collection compartments.
Administration: Reinfusion of blood components is under the control
and supervision of the physician in charge. Follow your institution’s blood
administration protocol for appropriate handling and labeling of blood components.
Angel® cPRP System Operator’s Manual1-1
Overview
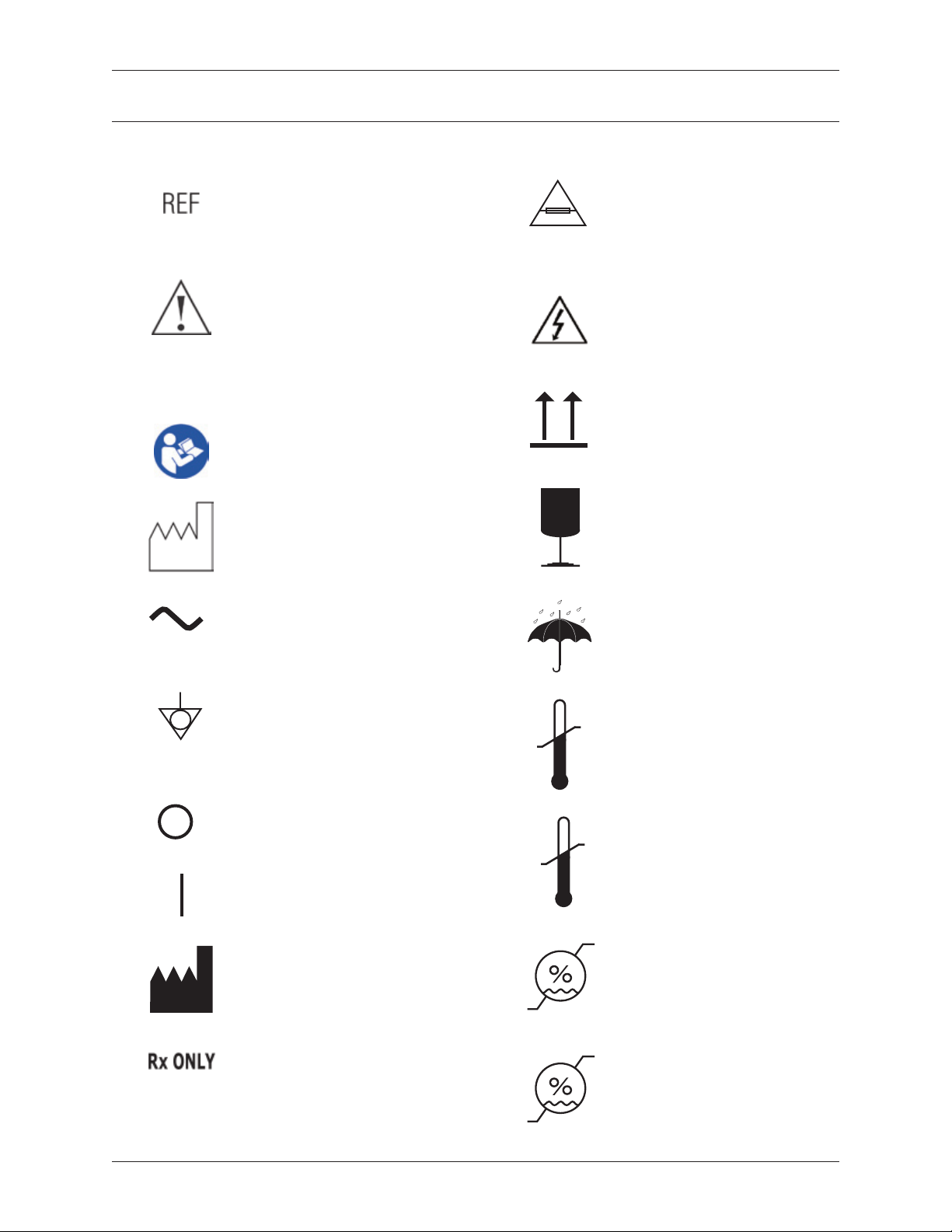
Angel® Concentrated Platelet Rich Plasma (cPRP) System Components
1. Centrifuge Well
2. Lid Latch Release Handle
3. Lid
4. Pump Rotor
Figure 1-1 Front-view of the Angel® Concentrated Platelet Rich Plasma (cPRP) System
5. Valve Assembly Driver
6. Stop Button
7. Touch Screen Display
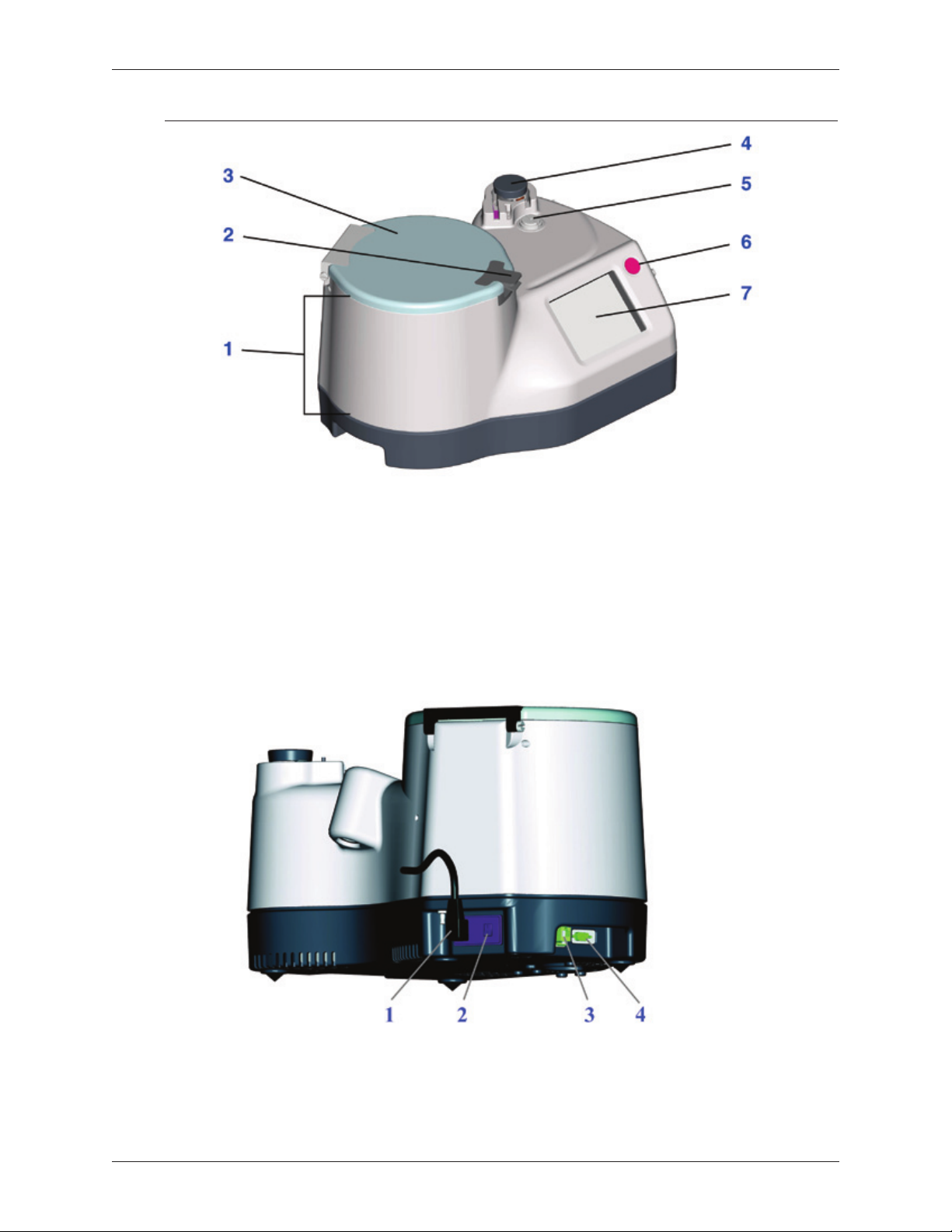
1. Power Cord
2. Power Switch
Figure 1-2 Rear-view of Angel® Concentrated Platelet Rich Plasma (cPRP) System
Angel® cPRP System Operator’s Manual 1-2
3. Ethernet
4. USB Port
Overview
Touch Screen Display User Interface
The color touch screen provides both the controls and the necessary information
for operating the Angel® Concentrated Platelet Rich Plasma (cPRP) System.
Below is a typical screen.
Figure 1-3 Start Screen
Shipping and Storage
1. Ship and store carton in an upright position.
2. The contents are fragile. Do not drop or jar carton.
3. Keep dry. Ship and store between 10% and 90% relative humidity.
4. Store between -20 and 37.7 degrees Celsius (-4 to 100 degrees Fahrenheit).
Installation
This section contains installation instructions for the Angel® Concentrated Platelet
Rich Plasma (cPRP) System. The Angel® Concentrated Platelet Rich Plasma
(cPRP) System has been designed to be essentially a “plug and play” device,
and, as such, requires very little preparation to get started.
However, before proceeding, please note the following:
1. Read the installation procedure in its entirety.
2. Become familiar with any precautionary indications noted in this procedure.
Note:
If at any time you encounter problems with the installation of the Angel®
Concentrated Platelet Rich Plasma (cPRP) System, contact Cytomedix, Inc. Customer
Service Response Center at 877-865-9927 or CustomerCare@Cytomedix.com.
Special tools, equipment and environmental requirements
There are no special tools required to install and set up this device.
PRECAUTION
Prior to rst use follow the installation instructions included in this manual.
Angel® cPRP System Operator’s Manual1-3
Visual Inspection
1. Inspect the exterior of the shipping container and verify that no visual
damage is present.
A. If damage is present, notify Cytomedix, Inc. Customer Service Response
Center at 877-865-9927 to report the damage.
B. If no damage is present, proceed with the installation.
Unpacking/Assembly
1. Open the shipping carton and remove the Angel® Concentrated Platelet
Rich Plasma (cPRP) System and all ancillary components from the shipping
carton. Install the device in an operating environment between 10 and 30
degrees C (50-86 degrees F) and 10%-75% relative humidity.
2. Place the Angel® Concentrated Platelet Rich Plasma (cPRP) System on a
solid, at work surface.
3. Install the power cord supplied with the equipment into the Angel®
Concentrated Platelet Rich Plasma (cPRP) System. Plug the power into an
available power outlet
Overview
Operational Checks
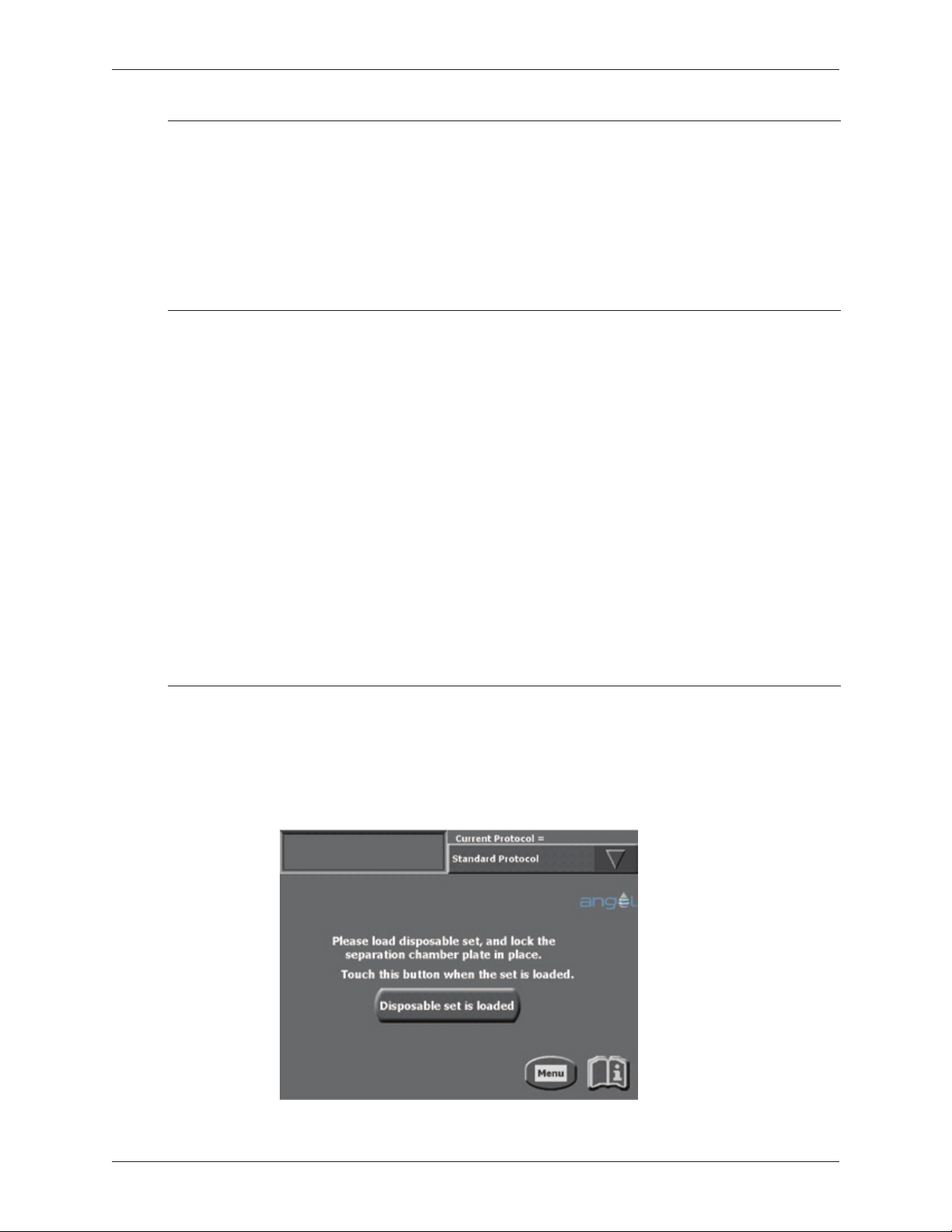
Power-up the Angel® Concentrated Platelet Rich Plasma (cPRP) System by moving
the power switch located at the back of the machine to the on position. On powerup, the Angel® Concentrated Platelet Rich Plasma (cPRP) System will perform an
automatic self-test. At this time, the valve assembly will also calibrate and reposition
itself. Successful completion of that self-test will be evident by visual conrmation of
the following start-up screen:
PRECAUTION
There are no user-serviceable parts inside this device. To avoid
the risk of electrical shock, do not remove the cover. Refer all
servicing to qualied service personnel.
Figure 1-4 Load Screen
No other checks or tests are required as part of this installation procedure.
Angel® cPRP System Operator’s Manual 1-4

Overview
Setting the Date and Time
The date and time used internally by the Angel® Concentrated Platelet Rich Plasma (cPRP)
System may be set from within the “Settings” tab of the Menu Screen (see Figure 1-5).
Figure 1-5 Date and Time Settings
To access the date and time settings, do the following:
1. Touch the Menu button from any screen.
2. From the Menu Screen, touch the “Settings” tab.
3. From within the “Settings” tab, touch the “Adjust date, time, or time zone” button.
The date and time settings will appear within the “Settings” tab. The settings consist
of seven elds which may be adjusted: “Year”, “Month”, “Day”, “Hour”, “Min” (for
minute), “Sec” (for second), and “Time zone:”
To adjust a eld:
1. Touch the eld you wish to adjust. For example, if you wish to change the
month, click on the rectangular eld labelled “Month”.
The eld will become highlighted, and the Up Arrow button and Down Arrow
button will appear at the right side of the screen.
2. Touch the Up Arrow button and Down Arrow button to increase or decrease,
respectively, the value of the highlighted eld.
When the “Time zone:” eld is the highlighted eld, touching the Up Arrow button
and Down Arrow button will scroll through a list of time zones. Select your region
from this list.
When a time zone which observes Daylight Saving Time is selected, the option
is given to automatically adjust the clock. This featured may be toggled on or off
by touching the check-box located below the “Time zone:” eld.
Angel® cPRP System Operator’s Manual1-5

Setting the Date and Time (Cont.)
3. Once you are satised with the value of the eld, you may lock in its new value
in two ways: either touch the highlighted eld a second time, or touch another
eld which needs modifying.
When the highlighted eld is touched to lock in its value, it will become not-
highlighted, and the Up Arrow button and Down Arrow button will disappear.
4. Once the value of all elds have been set correctly and locked in, touch the
Close button to exit the Menu Screen.
Overview
Angel® cPRP System Operator’s Manual 1-6

Installing the Disposables
Chapter 2: Installing the Disposables
Angel® cPRP Processing Set
Description
The Angel® cPRP Processing Set consists of a pre-connected variable volume
separation chamber, a tubing set with a platelet sensor/valve assembly, and
a three-compartment reservoir bag for the collection of blood products (whole
blood, red blood cells, and platelet poor plasma). The Angel® cPRP Processing
Set also contains a 20 ml luer lock syringe for the collection of platelet rich
plasma (PRP), two 60 ml specimen cups for use in a sterile eld, a whole
blood bag spike adapter, male-female luer plugs, and labels for collected blood
components.
Major Components
(see Figure 2-1)
Variable Volume Separation Chamber: The Angel® cPRP Processing Set
utilizes a variable volume separation chamber that can process from 40 ml
– 180 ml of anticoagulated autologous whole blood or mixture of blood and
bone marrow in a single cycle. The Angel® cPRP Processing Set is capable of
processing up to three (3) cycles per disposable.
The top half of the variable volume separation chamber (the hard plastic
component) is the separation chamber plate. The separation chamber plate is
used to seat the variable volume separation chamber in the centrifuge.
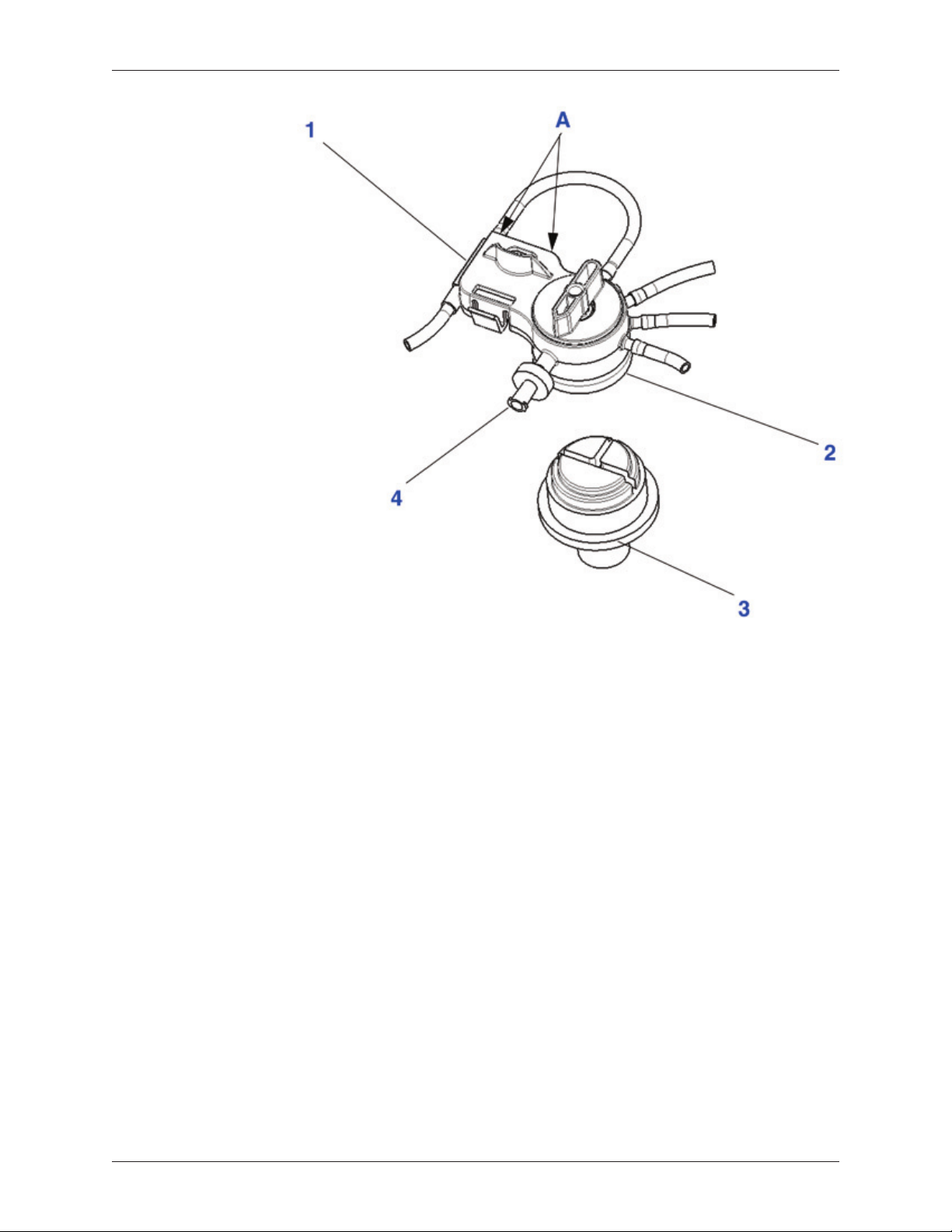
Platelet Cuvette/Valve Assembly: The platelet cuvette/valve assembly contains
three major components: (1) the platelet cuvette, (2) the pump loop tubing and
(3) the rotating valve. The platelet cuvette is seated in the platelet sensor and is
used to optimize the collection of the separated blood components. The pump
loop tubing is inserted around the pump, which moves volume from the whole
blood compartment of the three-compartment reservoir bag to the variable volume
separation chamber and from the variable volume separation chamber to the
various collection compartments for the separated blood products (PRP, PPP and
RBC). The rotating valve rotates throughout the course of the processing cycle
to direct the whole blood, the mixture of blood and bone marrow, or its separated
components through the appropriate pathway.
The platelet cuvette/valve assembly has been designed so that the operator can
easily install the platelet cuvette/valve assembly while insuring that the platelet
cuvette is properly seated in the platelet sensor and that the rotating valve is
properly seated on the valve assembly driver.
Angel® cPRP System Operator’s Manual2-1
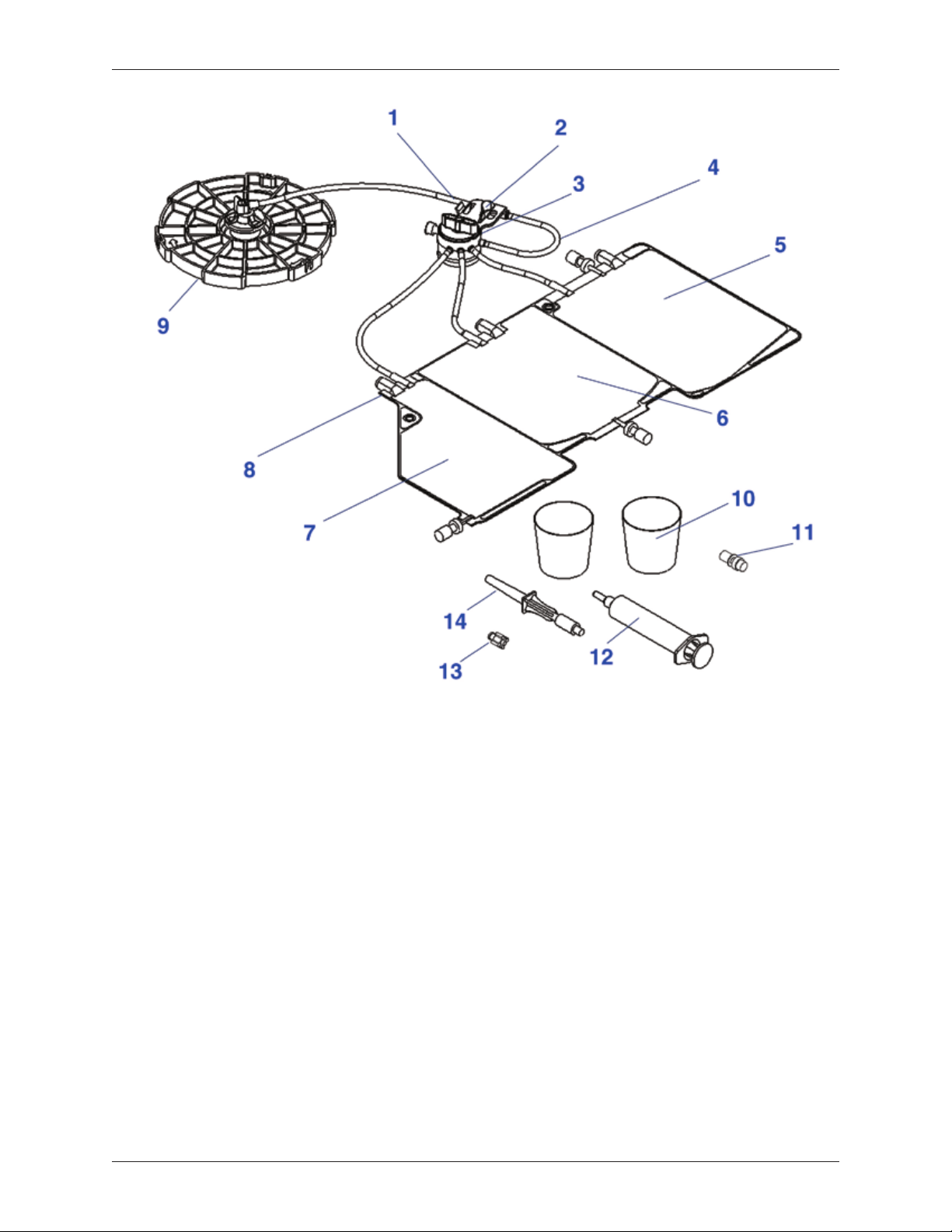
Installing the Disposables
1. Platelet Cuvette / Valve
Assembly Clip
2. Platelet Cuvette
3. Rotating Valve
4. Pump Loop Tubing
5. Whole Blood Compartment
6. RBC Compartment
PRP Valve Port: A luer lock syringe is attached to the PRP valve port to collect
PRP. At the end of a processing cycle, the PRP valve port can also be used to
collect PPP. A Syringe-activated Valve is included as an accessory and can be
attached to maintain a closed port when the PRP syringe is removed.
Three-Compartment Reservoir Bag: The three-compartment reservoir bag is
used to collect anticoagulated whole blood, mixture of blood and bone marrow
and separated blood/bone marrow components. The whole blood compartment
is used as a reservoir for collected anticoagulated whole blood from a patient.
The clinician may use syringes or whole blood bags to collect the anticoagulated
whole blood or mixture of blood and bone marrow from a patient. The RBC
Compartment is used to collect the concentrated red cells at the end of the
7. PPP Compartment
8. Three-Compartment
Reservoir Bag
9. Variable Volume
Separation Chamber
10. 60 ml Specimen Cups
11. Syringe-activated PRP Valve
Figure 2-1
Angel® cPRP Processing Set
12. 20 ml Luer Lock Syringe
13. Male-Female Luer Plug
(quantity, 7 each)
14. Whole Blood Spike Adapter
Angel® cPRP System Operator’s Manual 2-2

Installing the Disposables
processing cycle. The PPP Compartment is used to collect platelet poor plasma;
the PPP is the rst blood component collected after separation has been completed.
Syringe-activated valves are used to access the PPP and whole blood
compartments of the three-compartment reservoir bag.
Other accessory items in the Angel® cPRP Processing Set are:
20 ml Luer Lock Syringe: The 20 ml luer lock syringe is used for the
collection of platelet rich plasma. However, the syringe-activated PRP valve will
accommodate most luer tting syringes.
60 ml Specimen Cups (2 ea.): Two 60 ml specimen cups for use in a sterile eld.
Male/Female Luer Plugs: The male / female luer plugs are used during and at
the end of procedure to seal open luer lock connections.
Whole Blood Bag Spike Adapter: The whole blood spike adapter is used to
transfer the blood or mixture of blood and bone marrow from a whole blood bag
to the whole blood compartment of the three-compartment reservoir bag.
Labels: Appropriate labels to label collected whole blood/bone marrow and
separated components.
Contents of this set have been sterilized by ethylene oxide gas and have
nonpyrogenic uid pathways.
Warnings and Precautions
Please see “Warnings” on page ii in the chapter “Before You Get Started”; see
“Precautions” on page iv in the same chapter.
Setup and Blood/Bone Marrow Preparation
Turning on the Angel® cPRP System
1. Turn on the Angel® cPRP System be pressing the power switch on the back
of the machine. The message “Self test in progress. Please stand by.” will
be displayed on the Angel® cPRP System’s touch screen display, and the
machine will orientate the valve assembly driver to the loading position.
Angel® cPRP System Operator’s Manual2-3

Installing the Disposables
Figure 2-2 Rear-view of Angel® Concentrated Platelet Rich Plasma (cPRP) System
1. Power Switch
Initial Setup
With the Angel® cPRP System turned on, do the following:
Note: An instructional animation which illustrates the installation of the
disposable set (described in the steps below) is available from the Info Screen
immediately after powering on the Angel® cPRP System. (Refer to “Info Screen”
on page 3-23)
1. Open the centrifuge lid cover and lift the centrifuge stator arm to lock the
centrifuge adapter within the centrifuge well.
2. Remove the Angel® cPRP Processing Set from the tray.
3. Lay the Angel® cPRP Processing Set on the top of the machine. Insert the
variable volume separation chamber into the centrifuge adapter by aligning
the notches in the separation chamber plate with the aligning feature on the
centrifuge adapter. Once aligned, press the separation chamber plate down
near the location of the position indicator and turn clockwise until the position
indicator snaps into place (see Figure 2-3).
Rotate the centrifuge to a position so that the interlock mechanism shown
in Figure 2-3, item 2 does not interfere with the stator arm. If the interlock
mechanism interferes with the stator arm the separation chamber plate will
not load properly.
Note: Loading the variable volume separation chamber should always be
the rst step in the setup process. Loading the variable volume separation
chamber and pressing down on the separation chamber plate will remove
excess air volume from the chamber. If excess air is not removed, the
separation chamber plate will not load properly.
Angel® cPRP System Operator’s Manual 2-4
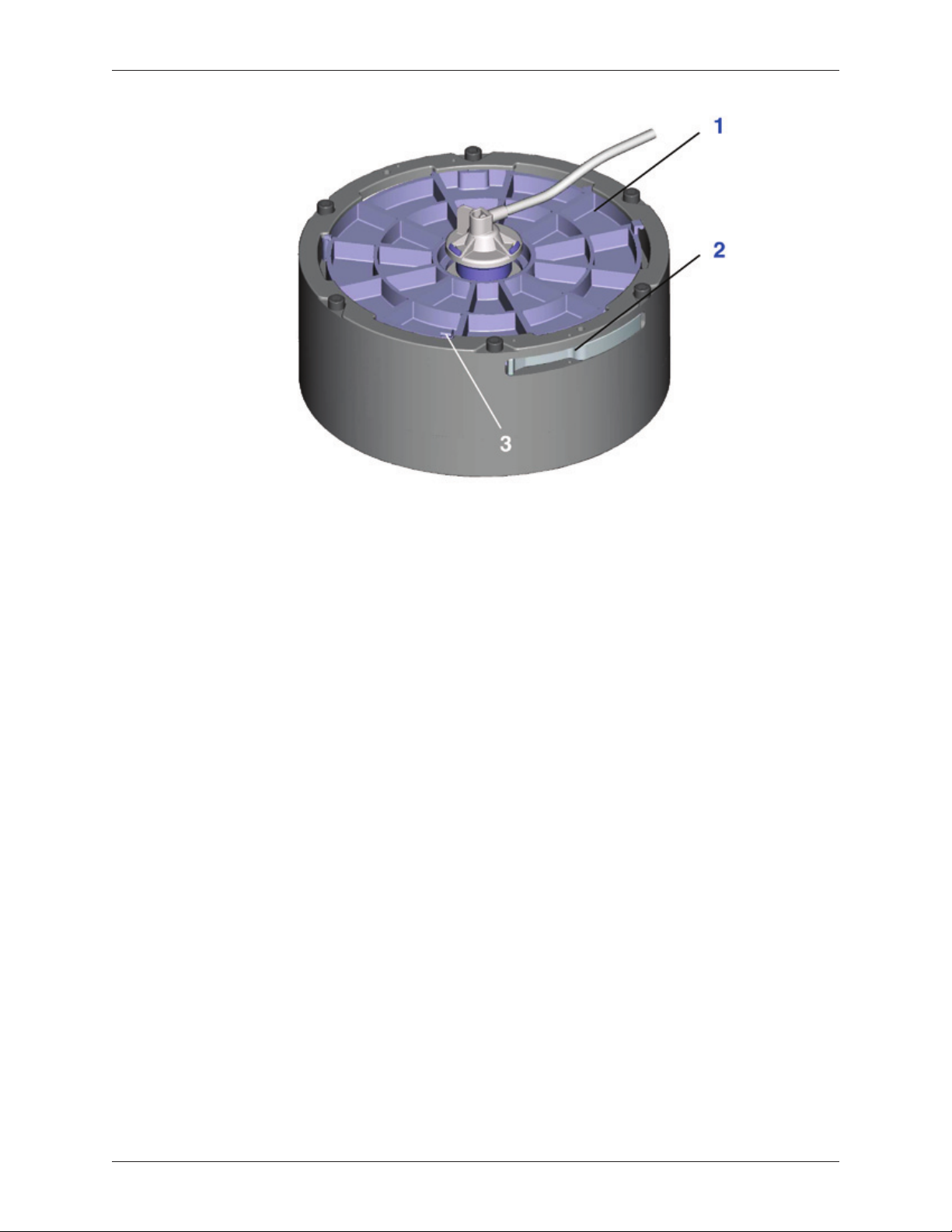
Installing the Disposables
1. Separation Chamber Plate
2. Separation Chamber Plate Interlock Mechanism
3. Position Indicators
Figure 2-3 Mounting the Separation Chamber
4. Place the tube leading from the variable volume separation chamber through
the slot on the rim of the centrifuge well.
5. Lower the centrifuge stator arm and align it with the mating feature on the top of
the rotating seal of the variable volume separation chamber (see Figure 2-4).
Angel® cPRP System Operator’s Manual2-5

Installing the Disposables
1. Stator arm lowered and aligned
Figure 2-4 Centrifuge Stator Arm Aligned with
Variable Volume Separation Chamber
6. Close the centrifuge lid. After closing the centrifuge lid, make sure that the
tubing remains in the slot on the rim of the centrifuge and is not occluded by
the centrifuge lid.
7. Place the pump loop tubing over the pump rotor. The pump loop will
automatically load when the processing cycle is initiated. Seat the platelet
cuvette/valve assembly by aligning the platelet cuvette and the valve
assembly with the platelet sensor body and the valve assembly driver. Press
down rmly on the back side of the platelet cuvette/valve assembly, at position
A near the pump loop, until the assembly is snapped in place (see Figure 2-5).
Note: It is essential that the platelet cuvette/valve assembly seats fully on the
machine to obtain proper sensing of blood components.
Angel® cPRP System Operator’s Manual 2-6
Installing the Disposables
1. Platelet Cuvette
2. Valve Assembly
3. Valve Assembly Driver
4. Syringe-activated PRP Valve
Figure 2-5 Valve Assembly
8. Hang the three-compartment reservoir bag on the two support pins located
on the side of the Angel® Concentrated Platelet Rich Plasma (cPRP) System.
9. Remove breather cap from the PRP valve port located on the valve
assembly. If desired, attach the Syringe-activated Valve to the PRP valve
port. Attach the 20 ml luer lock syringe (or alternate syringe, if desired) to the
PRP valve port.
Note: The luer on the PRP valve port will accommodate most
luer-lock syringes.
10. After set-up, inspect the circuit to make sure there are no kinks or occlusions.
Angel® cPRP System Operator’s Manual2-7
Installing the Disposables
Blood Collection
The Angel® Concentrated Platelet Rich Plasma (cPRP) System utilizes a variable
volume separation chamber that is capable of processing between 40 ml and 180
ml of anticoagulated whole blood or mixture of blood and bone marrow in a single
cycle. A total of 180 ml can be processed over three (3) cycles per disposable.
The Angel® Concentrated Platelet Rich Plasma (cPRP) System can
accommodate anticoagulated whole blood that has been collected preoperatively
from a patient in either syringes or blood collection bags. In either situation, the
patient’s whole blood should be collected in a citrate anticoagulant (ACD-A) in
a 7:1 ratio (seven parts whole blood to one part citrate anticoagulant (ACD-A)).
The following table denes the appropriate mixture of whole blood and citrate
anticoagulant (ACD-A):
Whole Blood vs. Citrate Anticoagulant Mixture
(7:1 ratio; seven parts blood to one part citrate anticoagulant)
Total Volume of
Anticoagulated
Whole Blood (ml)
i
40
50 6 44
60 8 52
70 9 61
80 10 70
90 12 78
100 13 87
110 14 96
120 15 105
130 16 114
140 18 122
150 19 131
160 20 140
170 21 149
Volume of ACD-A (ml)
5 35
Volume of Whole
Blood Drawn (ml)
180 23 157
i
40 ml anticoagulated whole blood or mixture of blood and bone marrow
volumes require a patient hematocrit of 30% or greater. The recommended
minimum patient hematocrit for anticoagulated whole blood volumes of 50 ml or
greater is 28%.
During and after collection, gently mix the whole blood/bone marrow with the
citrate anticoagulant (ACD-A) for a thorough distribution of the anticoagulant.
Failure to properly mix the collected blood/bone marrow with anticoagulant may
cause blood clot formation. Blood clot formation may interfere with the loading of
Angel® cPRP System Operator’s Manual 2-8
 Loading...
Loading...