Critikon Dinamap Pro1000 Service manual

PRO 1000
SERVICE MANUAL
DINAMAP
PRO 1000 SERVICE MANUAL

DINAMAP PRO 1000 Monitor
Service Manual

List of Effective Pages
Part No./Rev. Page No. Date of Latest Revision
2008072 All Original Oct. 2001)
U.S. Patent 5,170,795 U.S. Patent 4,349,034
U.S. Patent 5,052,397 U.S. Patent 4,360,029
U.S. Patent 4,754,761 U.S. Patent 4,501,280
U.S. Patent 4,638,810 U.S. Patent 4,546,775
U.S. Patent 4,543,962 U.S. Patent 5,518,000
U.S. Patent 5,704,362 Patents Pending
CAUTION: Federal (U.S.A.) law restricts this device to sale
by or on the order of a health care practitioner
The content of this document, including all figures and
drawings, is proprietary information of General Electric
Medical Systems Information Technologies, provided
solely for purposes of operation, maintenance or repair of
PRO 1000 Monitors. Dissemination for other purposes or
copying thereof without the prior written consent of General
Electric Medical Systems Information Technologies,
Tampa, Florida, is prohibited. Illustrations may show
design models; production units may incorporate changes.
CRITIKON 2001 TAMPA, FL 33614
Printed in the U.S.A. All rights reserved.
United States
Critikon, L.L.C.
4502 Woodland Corporate Boulevard
Tampa, FL 33614
EU Representative
GE Medical Systems
Information Technologies GmbH
Munzinger Strasse,
Freiburg, Germany
ii

TABLE OF CONTENTS
SECTION 1 INTRODUCTION
1.1. Scope of Manual........................................................................................1-1
1.2. Manual Changes .......................................................................................1-2
1.3 Service Policy ............................................................................................ 1-2
1.3.1 Extended Warranties ................................................................................. 1-2
1.3.2 Assistance ................................................................................................. 1-2
1.3.3 Service ......................................................................................................1-3
1.3.4 Service Loaners.........................................................................................1-4
1.3.5 Repair Parts ..............................................................................................1-4
1.3.6 Replacement Accessories ......................................................................... 1-5
1.4 Product Description ...................................................................................1-5
1.4.1 General Description...................................................................................1-5
1.4.2 Storage Batteries.......................................................................................1-6
Table 1-1 Specifications.......................................................................................... 1-7
SECTION 2. PRODUCT DESCRIPTION
2.1. Introduction ................................................................................................ 2-3
2.2. Product Configurations ..............................................................................2-3
2.3. Controls, Indicators, and Connectors.........................................................2-3
2.3.1. PRO Monitor Rear Panel Connections ......................................................2-4
2.3.2. Front Panel Controls and Indicators...........................................................2-5
2.4. Host Port Connector (rear panel)...............................................................2-7
2.4.1. Pin Assignments ........................................................................................2-7
2.5. Compatible Parts .......................................................................................2-8
2.6. Specifications.............................................................................................2-9
2.6.1. Power Requirements .................................................................................2-9
2.6.2. Environmental ............................................................................................2-9
2.6.3. Mechanical.............................................................................................. 2-10
2.6.4. NIBP ....................................................................................................... 2-10
2.6.5. Temperature ........................................................................................... 2-10
2.6.6. SpO2....................................................................................................... 2-11
2.6.7. ECG ........................................................................................................ 2-12
SECTION 3. PRINCIPLES OF OPERATION
3.1. Introduction ............................................................................................... 3-3
3.2. Overall Principle Of Operation ..................................................................3-3
3.2.1. Nellcor SPO2.............................................................................................3-3
3.2.2. Cuff Blood Pressure (BP) and Pulse .........................................................3-3
3.2.3. Alaris Oral and Rectal Thermometry .........................................................3-4
3.2.4. ECG with Heart Rate and Respiration ....................................................... 3-4
iii

3.2.5. Host Communication Ports........................................................................3-4
3.3. Functional Description ..............................................................................3-5
3.3.1. PSU PWA..................................................................................................3-5
3.3.2. Mains Converter Module ...........................................................................3-6
3.3.3. Main Board ................................................................................................ 3-6
3.3.4. Keyboard PWA ..........................................................................................3-7
3.3.5. ECG PWA .................................................................................................3-8
3.3.6. Pneumatic Control .....................................................................................3-8
3.3.7. LCD Assembly...........................................................................................3-9
3.3.8. Printer (Optional) .................................................................................... 3-10
List of Figures
3-1 General System Diagram .............................................................................. 3-12
SECTION 4. GENERAL MAINTENANCE
4.1. Introduction................................................................................................ 4-3
4.2. Configuring the PRO 1000 Monitor for the First Time................................4-3
4.2.1 Unpacking and Preparation for Installation................................................4-3
4.2.2 Set the Date and the Clock........................................................................ 4-5
4.2.3 Parameter Level Functional Testing ..........................................................4-6
4.3. Periodic Maintenance ................................................................................ 4-7
4.3.1. As Required............................................................................................... 4-7
4.3.1.1 Integrity of Cuffs and Hoses ..................................................................4-7
4.3.1.2 External DC Supply and Battery ............................................................ 4-7
4.3.1.3 Cleaning of Accessories ........................................................................4-7
4.3.1.4 Long Term Storage................................................................................ 4-8
4.3.2 Annual Procedures .................................................................................... 4-8
4.4. Care of Storage Batteries ..........................................................................4-9
4.4.1. Procedures for First Use............................................................................ 4-9
4.4.2 Battery Charging........................................................................................4-9
4.5 Safety Resistance Testing ...................................................................... 4-12
4.6. Alarm Code Interpretation ...................................................................... 4-14
4.6.1. System Failures...................................................................................... 4-14
4.6.2. Hardware Errors ..................................................................................... 4-15
4.6.3. Parameter Failures ................................................................................. 4-15
4.6.3.1 ECG/RESP/TEMP Errors ................................................................... 4-15
4.6.3.2 NIBP Messages.................................................................................. 4-15
4.6.3.3 Temperature Messages...................................................................... 4-16
4.6.3.4 SpO2 Messages ................................................................................. 4-16
4.7. Service Mode Operation......................................................................... 4-16
4.7.1 SpO2 Tests ............................................................................................ 4-19
4.7.2 NIBP Tests ............................................................................................. 4-20
4.7.2.1 Leak Test............................................................................................ 4-21
4.7.2.2 NIBP Calibration Check ...................................................................... 4-23
iv
4.7.2.3 Pressure Recalibration ....................................................................... 4-24
4.7.2.4 Overpressure Test.............................................................................. 4-25
4.7.3 EKG Tests .............................................................................................. 4-27
4.7.4 Temp Tests ............................................................................................ 4-28
4.7.5 Recorder Tests ....................................................................................... 4-30
4.7.6 Battery Tests .......................................................................................... 4-31
4.7.7 Test Failsafe Logic ................................................................................. 4-32
4.7.8 Keypad LED Test ................................................................................... 4-33
4.7.9 Keypad Key Test .................................................................................... 4-33
4.7.10 Sound Test............................................................................................. 4-33
4.7.11 Turn off the System................................................................................ 4-33
4.8 Service Mode Exit................................................................................... 4-33
Chapter 4 Appendices
Test Record ......................................................................................... Appendix A
Monitor Configuration Log ................................................................... Appendix B
SECTION 5 ASSEMBLY DRAWINGS & ELECTRICAL SCHEMATICS
Assembly Drawings (Monitor Assembly & Disassembly)
Front Case 1 ......................................................................................................5-1/2
Front Case 2 ......................................................................................................5-3/4
Rear Case 1.......................................................................................................5-5/6
Rear Case 2.......................................................................................................5-7/8
Electrical Schematics
ECG Board – 315589........................................................................ 5-9 through 5-18
Main Board – 315592...................................................................... 5-19 through 5-42
Power Supply Board – 315593 ....................................................... 5-43 through 5-52
Keyboard ........................................................................................................ 5-53/54
Probe Warmer ................................................................................................ 5-55/56
v

SECTION 1 INTRODUCTION
Contents
1.1. Scope of Manual .............................................................................................. 1-1
1.2. Manual Changes..............................................................................................1-2
1.3 Service Policy ...................................................................................................1-2
1.3.1 Extended Warranties...................................................................................1-2
1.3.2 Assistance...................................................................................................1-2
1.3.3 Service ........................................................................................................1-3
1.3.4 Service Loaners ..........................................................................................1-4
1.3.5 Repair Parts ................................................................................................1-4
1.3.6 Replacement Accessories...........................................................................1-5
1.4 Product Description........................................................................................... 1-5
1.4.1 General Description.....................................................................................1-5
1.4.2 Storage Batteries.........................................................................................1-6
Table 1-1 Specifications.......................................................................................... 1-7

This page intentionally left blank.

SECTION 1. INTRODUCTION
1.1
SCOPE OF MANUAL
This Service Manual provides service and parts repair information about the
DINAMAP PRO 1000
technicians who are familiar with electromechanical devices and digital and
analog circuit techniques.
CAUTION
Only qualified service-technicians should perform repairs to this
equipment.
Voltages dangerous to life exist in this unit. Take care when servicing
power supply and display assembly.
For information about operating the Monitor in a clinical environment, refer to the
separate Operation Manual.
This Service Manual consists of the following four sections:
Section 1 describes this volume and tells you how to use it. Information is
also provided about the physical and functional characteristics of the Monitor,
and how to get assistance in the event the unit fails to function properly.
Section 2 provides a general overview of the PRO 1000 including user
controls, external connections, and product/ parameter specifications.
Section 3 presents principles of operation for the Monitor, including an overall
system description and principles of operation at the component level.
Section 4 provides information about periodic and corrective maintenance of
the Monitor. Procedures include module performance tests and calibration
procedures. Information is provided to facilitate isolating faults to the
subassembly level.
Section 5 provides component information about the Monitor, including
disassembly and reassembly procedures, parts lists, and assembly drawings,
and electrical schematics.
Monitor. This manual is intended for use by trained service
WARNING
To reduce the risk of electric shock, do not remove
cover or back of any component. Refer servicing to
qualified service personnel.
1-1

1.2 MANUAL CHANGES
If, in the normal use of this manual, you notice errors, omissions, incorrect data,
or if you can suggest comments that may help improve this manual, please
complete the Publications Change Request form in the back of this manual.
Submit the form to:
General Electric Medical Systems Information Technologies
Technical Publications
4502 Woodland Corporate Boulevard
Tampa, Florida 33614
Changes to the Service Manual, either in response to user input or to reflect
continuing product improvements, are accomplished through reissue.
Changes occurring between reissues are addressed through Change
Information Sheets and replacement pages. If a Change Information Sheet does
not accompany your manual, the manual is correct as printed.
1.3
SERVICE POLICY
The warranty for this product is enclosed with the product in the shipper carton.
All repairs on products under warranty must be performed or approved by
Product Service personnel. Unauthorized repairs will void the warranty. Only
qualified electronics service personnel should repair products not covered by
warranty.
1.3.1
Extended Warranties
Extended warranties may be purchased on most products. Contact your Sales
Representative for details and pricing.
1.3.2
Assistance
If the product fails to function properly, or if assistance, service or spare parts
are required, contact Customer Support. Before contacting Customer Support,
it is helpful to attempt to duplicate the problem and to check all accessories to
ensure that they are not the cause of the problem. If you are unable to resolve
the problem after checking these items, contact General Electric Medical
Systems Information Technologies. Prior to calling, please be prepared to
provide:
1-2

1.3.3
Service
• product name and model number
• a complete description of the problem
If the repair parts or service are necessary, you will also be asked to provide
• the product serial number
• the facility's complete name and address
• a purchase order number if the product is to need of repair or when you
order spare parts
• the facility's account number, if possible
• the 6-digit part number for spare or replacement parts
If your product requires warranty, extended warranty or non-warranty repair
service, call Customer Support and a representative will assist you. Estimates
for non-warranty repairs are provided at no charge; however, the product must
be sent to the General Electric Medical Systems Service Center in order to
provide you with an estimate.
To facilitate prompt service in cases where the product has external chassis or
case damage, please advise the Customer Support representative when you
call.
The Customer Support representative will record all necessary information and
will provide you with a Return Merchandise Authorization Number (RMA). Prior
to returning any product for repair, you must have a RMA number. Contact
technical support at 1-877-274-8456
Monday through Friday, 8:00 a.m. to 7:00 p.m. EST, excluding holidays.
Packing Instructions
Follow these recommended packing instructions.
• Remove all hoses, cables, sensors, and power cords from the monitor
before packing.
• Pack only the accessories you are requested to return; place them in a
separate bag and insert the bag and the product inside the shipping carton.
• Use the original shipping carton and packing materials, if available.
If the original shipping carton is not available
• Place the product in a plastic bag and tie or tape the bag to prevent
loose particles or materials from entering openings such as hose ports.
1-3

• Use a sturdy corrugated container to ship the product; tape securely to
seal the container for shipping.
• Pack with 4 to 6 in. of padding on all sides of the product.
Insurance
Insurance is at the customer's discretion. The shipper must initiate claims for
damage to the product.
1.3.4
Service Loaners
A loaner unit is provided at no charge during the service life of the product
when we perform the repair service. Within 48 hours of your request, a loaner
will be shipped to your facility.
• General Electric Medical Systems will pay shipping charges for a loaner
sent to the customer for product repairs under the warranty.
• Shipping charges for a loaner sent to the customer for product repairs not
under warranty will be billed to the customer.
• The customer will pay shipping charges to return a loaner.
All loaners provided to customers must be returned within the specified time
stated on the loaner agreement or a rental fee will be incurred.
1.3.5
Repair Parts
Repair parts can be ordered from General Electric Medical Systems:
Via phone 1-877-274-8456, or
Via FAX 1-813-887-2430
Exchange replacement assemblies such as Circuit Board Assemblies also are
available; ask the Customer Support representative for details.
Please allow one working day for confirmation of your order. All orders must
include the following information.
• Facility's complete name, address, and phone number
• FAX number
• Your purchase order number
• Your account number
1.3.6
Replacement Accessories
1-4

Replacements such as hoses, sensors, etc. must be purchased from General
Electric Medical Systems at 1-877-274-8456. Please have the 4-digit or 6-digit
Reorder/Product Code of the item you wish to order, your purchase order and
account number available.
1.4
PRODUCT DESCRIPTION
The Monitor and storage batteries are described below. Refer to Table 1-1 for
specifications.
1.4.1
General Description
The DINAMAP PRO 1000 is designed for patient monitoring in acute care
settings such as critical care, emergency room, radiology, labor and delivery,
and operating room. It allows the clinician to view, record, and recall clinical
data derived from each parameter. This data includes heart rate, respiration
rate, oxygen saturation, noninvasive blood pressure, and temperature. Alarm
limit conditions are also detected.
The recorder provides numeric and waveform printouts of monitored data. Up
to 2 waveforms can be traced simultaneously. Each monitor can monitor one
patient at the bedside.
Patient sensor connections are made at the side of the unit, and network and
device connectors are at the rear.
Indicators for external DC operation (from AC mains), battery operation, and
battery charging are at the front of the unit.
At the time of publication, the available functioning parameters included the
following:
• NIBP
• Nellcor
™
Pulse oximetry (SpO2)
• 3-lead ECG, with respirations
• 2-channel thermal recorder
™
• Alaris
Oral and Rectal thermometry
The PRO 1000 Monitor series uses a TFT active-matrix-color liquid display.
The 10.4” diagonal display area contains 640 x 480 pixels and can display
262,144 colors simultaneously.
1-5

The LCD has the following specific characteristics. These are neither defects
nor malfunctions:
• The ambient temperature may affect the display condition of the LCD.
• The LCD uses replaceable cold cathode tubes for backlighting. Optical
characteristics, like luminance or uniformity will change during time.
• Uneven brightness and/or small spots may be noticed depending on
different display patterns.
Other DINAMAP PRO 1000 features include:
• The ability to uses industry standard accessories
• Remote alarm capability
• An intuitive graphical user interface, with a simple Select Knob that moves
the user through menus in a logical, and easy to understood format
• Five single-function keys for quick access to Alarm Silence, Record,
Freeze, NIBP Start/Stop, and STAT NIBP
1.4.2
Storage Batteries
The PRO 1000 Monitor operates from AC mains power, an external DC power
supply, or from the internal Nickel Metal Hydride storage battery. When
external DC power becomes available, the system rapidly switches from
battery power to external power.
1-6

Table 1-1. Specifications
Mechanical
Monitor 14.8 in (H) x 8.7 in (D) x 13.8 in (W)
37.0 cm (H) x 21.8 cm (D) x 34.4 cm (W)
Weight Less than 12 lb (9.5 kg)
Environmental*
Operating Temperature +41º F to +104º F (+5° C to +40° C)
Storage Temperature -40º F to +158º F (-20º C to +60º C)
Operating Humidity 5% to 95%, noncondensing
Storage Humidity 5% to 95%, noncondensing
Operating Atmospheric Pressure 700 hPa to 1060 hPa
Storage Atmospheric Pressure 500 hPa to 1060 hPa
Electrical
Power Supply
The PRO 1000 Monitor can be operated from AC power, external DC power, or the
rechargeable internal battery.
AC Input Voltage 120 - 240
AC Input Frequency 50 - 60 Hz
AC Input Power 60 - 120 Volt Amperes
AC Power Cable Detachable, 16-gauge, 10 ft (3 meters) long
DC Input Voltage 18-24 V (supplied from a source conforming to IEC 601-1)
DC Input Power 60 Watts (supplied from a source conforming to IEC 601-1)
Internal Battery 12 Volts, nickel-metal-hydride (NiMH)
Battery Life 120 minutes (± 10 minutes) using fully charged internal
battery, under specified load **
Charge time,
internal charger
The PRO Monitor typically charges the battery to within
90% capacity within 3 hours.
Fuse (Battery) 10A 250V slow-blow
* The Monitor may not meet Performance Specifications (ANSI/AAMI SP10) if it
is stored or used out of environmental specification ranges.
** Monitor shall be capable of operating on battery power for 2 hours minimum
(NIBP @ 5 min., ECG/Resp. SpO
2
, temp, dual channel recording once every
20 minutes.
1-7

SECTION 2. PRODUCT DESCRIPTION
CONTENTS
2.1. Introduction ................................................................................................ 2-3
2.2. Product Configurations .............................................................................. 2-3
2.3. Controls, Indicators, and Connectors.........................................................2-3
2.3.1. PRO Monitor Rear Panel Connections ...................................................... 2-4
2.3.2. Front Panel Controls and Indicators........................................................... 2-5
2.4. Host Port Connector (rear panel)............................................................... 2-7
2.4.1. Pin Assignments ........................................................................................ 2-7
2.5. Compatible Parts .......................................................................................2-8
2.6. Specifications.............................................................................................2-9
2.6.1. Power Requirements ................................................................................. 2-9
2.6.2. Environmental ............................................................................................ 2-9
2.6.3. Mechanical...............................................................................................2-10
2.6.4. NIBP ........................................................................................................2-10
2.6.5. Temperature ............................................................................................ 2-10
2.6.6. SpO
2.6.7. ECG .........................................................................................................2-12
........................................................................................................ 2-11
2
2-1

This page intentionally left blank.
2-2

2.1. INTRODUCTION
2.2. PRODUCT
CONFIGURATIONS
SECTION 2. PRODUCT DESCRIPTION
DINAMAP PRO Monitors provide non-invasive
determination of systolic blood pressure, diastolic
blood pressure, mean arterial pressure, pulse rate, 3lead ECG, temperature, and oxygen saturation.
These portable AC and DC operated monitors are
primarily intended for use in hospital acute care
settings such as outpatient surgery, accident and
emergency, labor and delivery, GI/endoscopy, and
medical/surgical units.
Each PRO Monitor is supplied with an accessory
pack. The contents of the pack vary according to
model. Unpack the items carefully, and check them
against the checklists enclosed within the accessory
boxes. If an accessory is missing or if an item is in a
nonworking condition, contact Critikon immediately.
2.3. CONTROLS,
INDICATORS, AND
CONNECTORS
It is recommended that all the packaging be retained,
in case the PRO Monitor must be returned for service
in the future.
Descriptions of the items shown are listed on the
pages that follow. For symbol definitions, refer to
paragraph: 2.3.2 of this section.
2-3
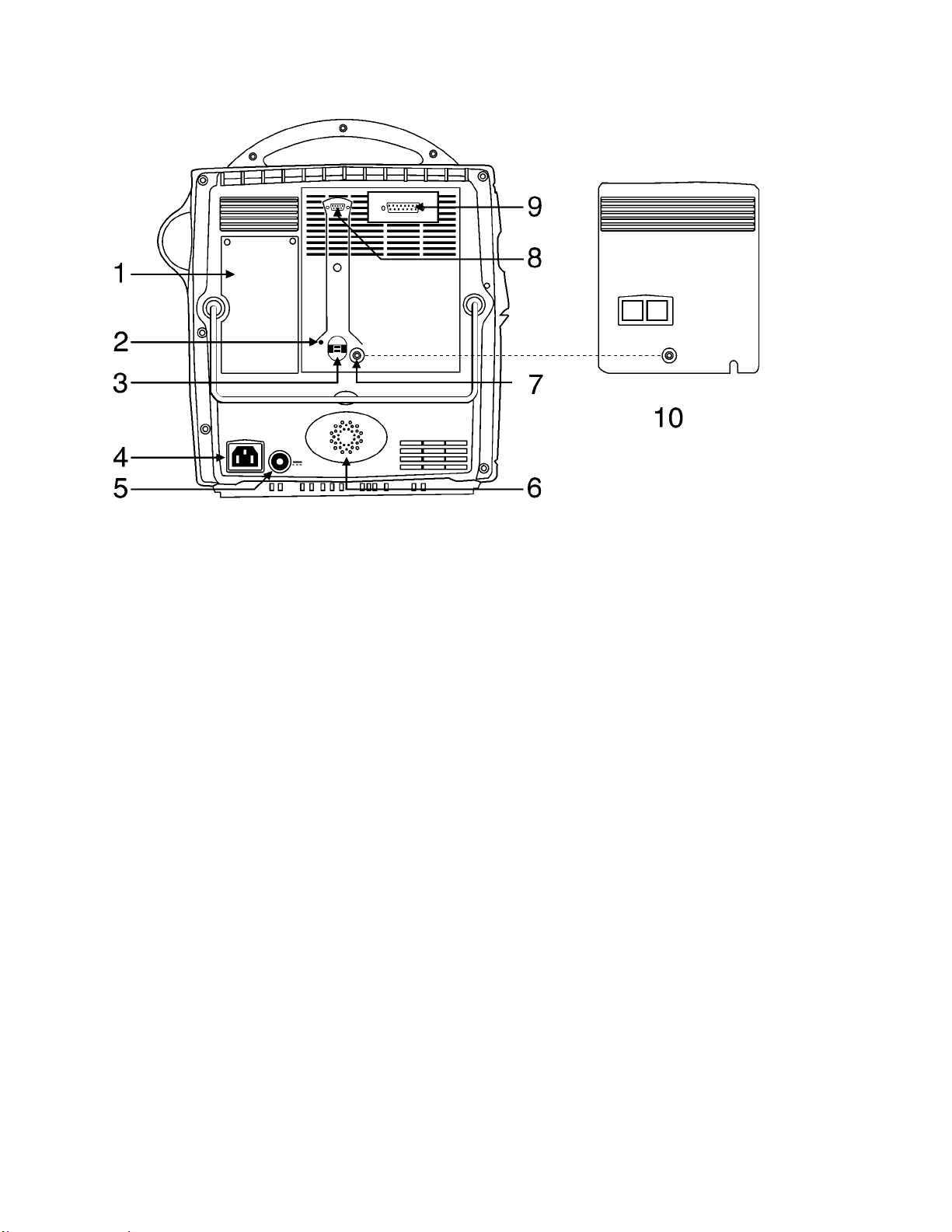
2.3.1. PRO Monitor Rear Panel Connections
1. Serial Number/ Manufacturer labeling
2. Earth Ground (safety test compatible)
3. Battery fuse (10A 250V)
4. Mains input (Used to connect to AC power supply)
5. External DC Input: 18-24 VDC only.
6. Main speaker opening.
7. Socket to secure removable rear cover (see 10)
8. DB9 connection used for Host Communication.
9. DB15 used for Host Communication/ remote alarm.
10. Removable rear protective cover.
2-4
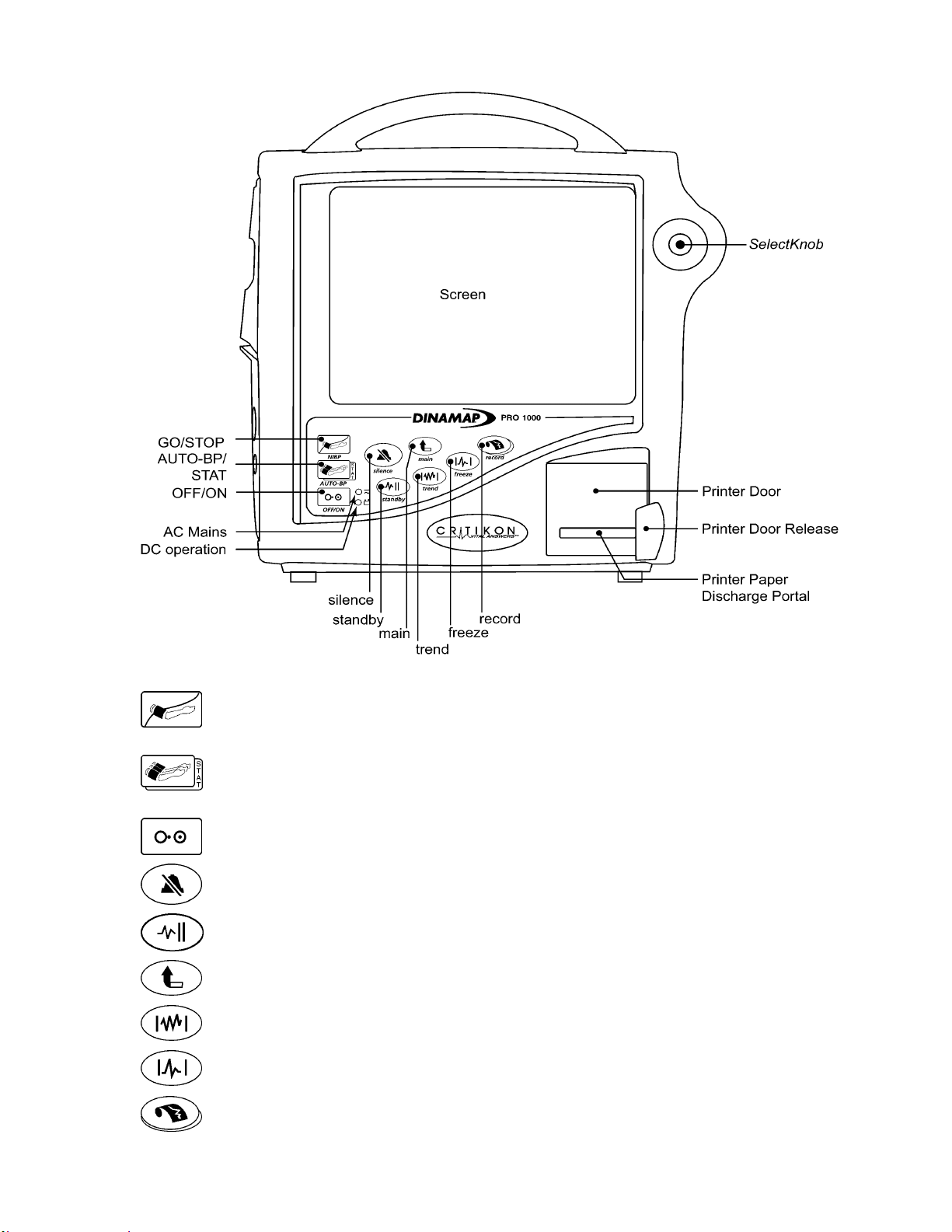
2.3.2. Front Panel Controls and Indicators
GO/STOP – Starts and stops any determination of noninvasive blood pressure.
AUTO-BP/STAT – Dual-function hardkey. Starts and stops auto BP determinations
by a single-press and gives you access to change the NIBP cycle time. Starts and
stops stat determinations with a double-press (5 minutes of continuous NIBP
cycles.)
OFF/ON – Turns Monitor off and on.
Silence – Temporarily silences alarms; acknowledges alarming crisis conditions.
Standby – Enters and exits standby mode.
Main – Closes the menu system and takes you back to the main screen.
Trend – Enters and exits trends (view patient trends data.) This hardkey can be
configured through config mode two ways: to view mini trends or to view full trends.
Freeze – Captures up to 16.8 seconds of waveforms on the screen (seconds vary
depending on the chosen sweep speed.)
Record – Prints with a single-press for a snapshot (timed recording) and a doublepress for a continuous recording of the chosen waveforms.
2-5
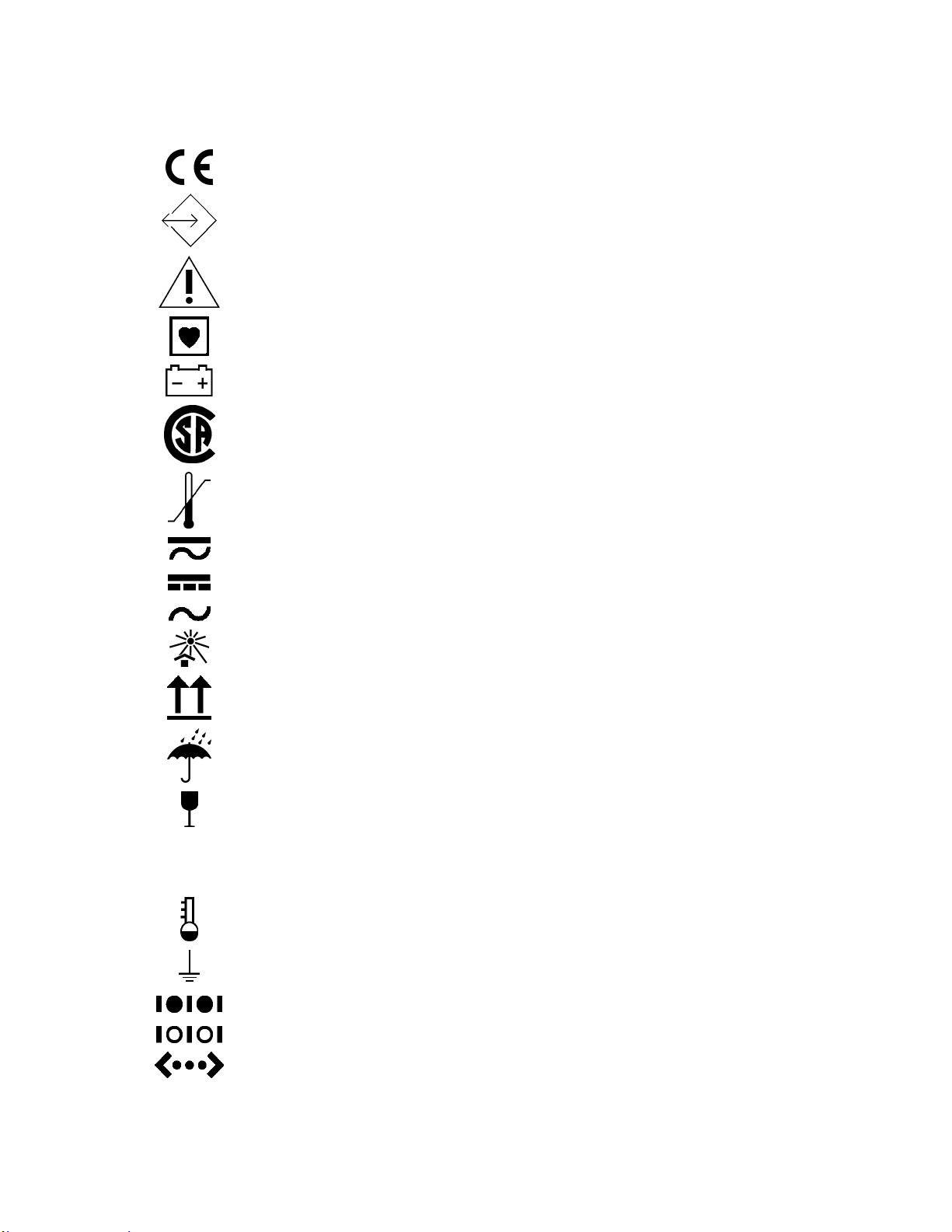
Optional Components
Note: Interconnected equipment must be installed by a qualified service person.
Symbols
CE Mark
External Communications Port Connector
Attention, consult accompanying documents
Type CF applied part
Battery in use
Canadian Standards Association
Storage temperature
External AC or DC power indicator
External DC power input
External AC power input
SN
REF
Keep away from heat
This way up
Keep dry
Fragile, handle with care
Serial number
Catalog number
Predictive temperature
Functional earth terminal (ground lug)
Serial Port 1
Serial Port 2
Ethernet Connector
2-6
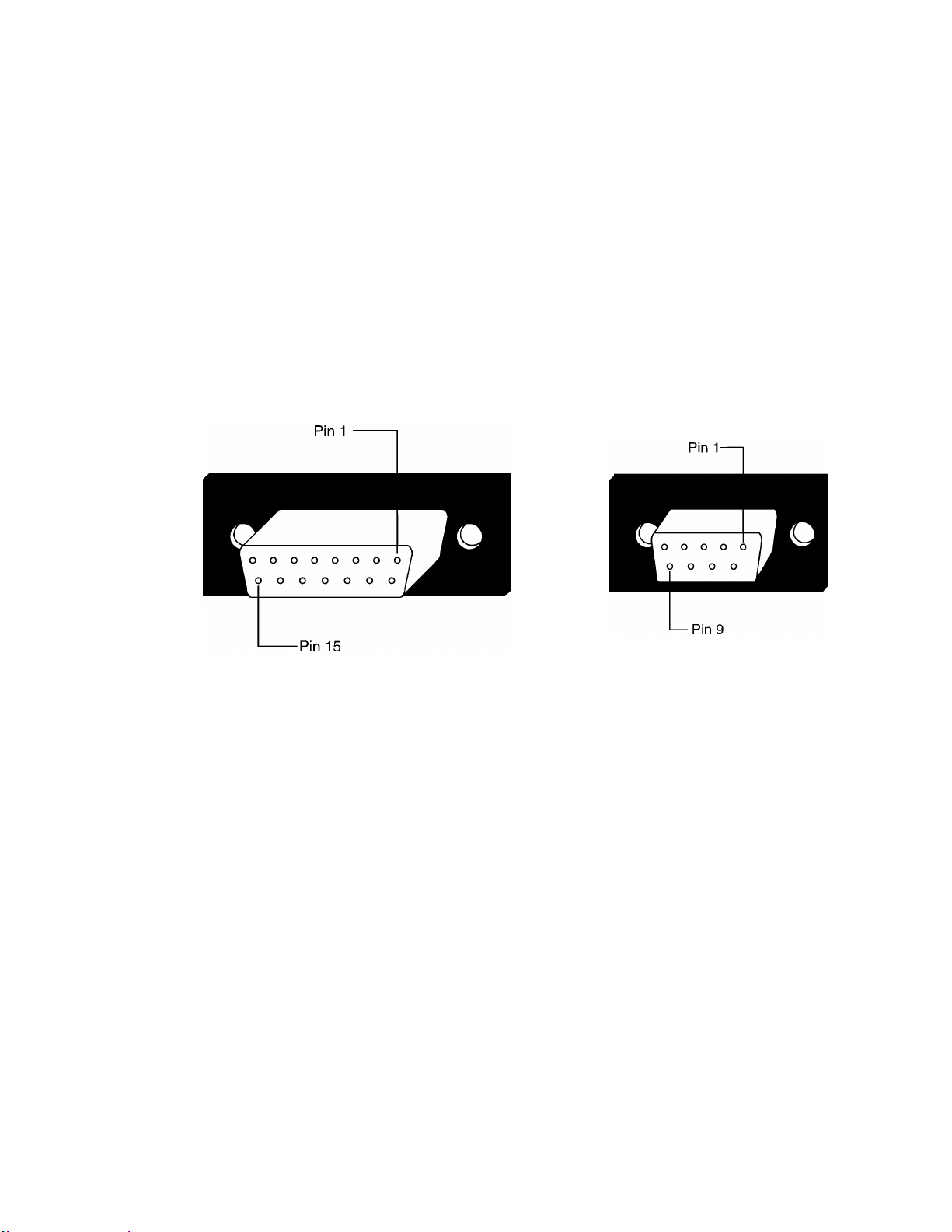
2.4. HOST PORT CONNECTORS
(BENEATH REAR PANEL)
All host port signals are NON-ISOLATED and should be connected to equipment
conforming to IEC 601-1-1 ONLY. Where isolation of data communication is
required, the Critikon isolated level converter should be used. If external alarm
control is required, Critikon part number 487208 (Isolated Remote Alarm Cable
Assembly) should ALWAYS be used. Please refer to the Information Sheet included
with the isolated remote alarm cable for operational details.
Note: When using remote alarm, the PRO Monitor should be considered the
primary alarm source. The secondary alarm is used for secondary purposes
only.
2.4.1. DB15/ DB9 Connector Pin Assignments
Pin Function Pin Function
Ground
1
TX2_Inverted TTL Data
2
RX2_Inverted TTL Data
3
AUX5V (600mA max.)
4
AUX12V (250mA max.)
5
Serial Level Control (High=TTL Low=-RS-232)
6
Ground
7
Remote Alarm (open collector, 75mA Max Sink)
8
No Connection
9
No Connection
10
TX2_RS232
11
Port Enable Control <low=port 2> (when in use, DB9 4 & 5 disabled)
12
RX2_RS232
13
No connection
14
No connection
15
Ground
1
TX1 Inverted TTL Data
2
RX1
3
TX2
4
RX2
5
+5V (600mA Max)
6
+12V (400mA Max)
7
No Connection
8
No Connection
9
2-7

2.5. COMPATIBLE PARTS
The following parts are available from Customer Service.
Description of Compatible Part Code
SOFT-CUF, Cuff, Infant
SOFT-CUF, Cuff, Child
SOFT-CUF, Cuff, Small Adult
SOFT-CUF, Cuff, Adult
SOFT-CUF, Cuff, Large Adult
SOFT-CUF, Cuff, Thigh
SOFT-CUF, Cuff, Neonatal type 1
SOFT-CUF, Cuff, Neonatal type 2
SOFT-CUF, Cuff, Neonatal type 3
SOFT-CUF, Cuff, Neonatal type 4
SOFT-CUF, Cuff, Neonatal type 5
DURA-CUF Cuff, Infant
DURA-CUF Cuff, Child
DURA-CUF Cuff, Small Adult
DURA-CUF Cuff, Adult
DURA-CUF Cuff, Large Adult
DURA-CUF Cuff, Thigh
DURA-CUF Cuff, Assortment cuff pack
DURA-CUF Cuff, Child pack
CLASSIC-CUF , Cuff, Infant
CLASSIC-CUF, Cuff, Child
CLASSIC-CUF, Cuff, Small Adult
CLASSIC-CUF, Cuff, Adult
CLASSIC-CUF, Cuff, Large Adult
CLASSIC-CUF, Cuff, Thigh
CLASSIC-CUF, Cuff, Neonatal type 1
CLASSIC-CUF, Cuff, Neonatal type 2
CLASSIC-CUF, Cuff, Neonatal type 3
CLASSIC-CUF, Cuff, Neonatal type 4
CLASSIC-CUF, Cuff, Neonatal type 5
12 Foot (approx. 3.7 meters) Long Adult / Pediatric Hose 107365
24 Foot (approx. 7.3 meters) Long Adult / Pediatric Hose 107366
12 Foot (approx. 3.7 meters) Long Neonatal Hose 107368
12 Foot (approx. 3.7 meters) Long A/P Hose Quick Disconn. 107368
IVAC** Oral Temperature Probe 088012
IVAC** Rectal Temperature Probe 088013
IVAC** Temperature Probe Covers 088015
DINAMAP PRO Monitor Operation Manual
DINAMAP PRO Monitor Service Manual
Accessory Pole/Basket/Base 3215
Printer Paper (Box of 10) 089100
Power Cable 316579
NELLCOR*** SpO2 Extension Cable SCP10*
NELLCOR Finger Sensor DS100A
NIBP Calibration Kit 320246
2500
2501
2502
2503
2504
2505
2521
2422
2523
2524
2525
2783
2781
2779
2774
2791
2796
2699
2697
2618
2613
2608
2603
2643
2648
2638
2633
2628
2623
2619
776995*
777358*
* PRO Monitor unique parts
** IVAC is a trademark of ALARIS Medical Systems
*** NELLCOR is a trademark of Mallinckrodt, Inc.
2-8
2.6.
SPECIFICATIONS
0 0 8 6
IPX1
MAINS
AC INPUT VOLTAGE
ALTERNATE SOURCES
DC INPUT VOLTAGE
EXTERNAL DC FUSE
BATTERY
This product conforms with the essential requirements of the
Medical Device Directive. Accessories without the CE Mark are not
guaranteed to meet the Essential requirements of the Medical
Device Directive.
The PRO Monitor is protected against vertically falling drops of
water and conforms to the IEC 529 standard at level of IPX1. No
harmful effects will come of vertically falling drops of water making
contact with the monitor.
2.6.1. Power Requirements
Protection against electrical shock - Class 1
115 / 230 VAC, 50 / 60 Hz (nominal),
90 ~ 253 VAC, 47 ~ 63 Hz (range)
Protection against electrical shock – Class 1
24 VDC (nominal), 12-30 VDC from supplied power converter
Internal, auto-resetting.
12 volt, 2.3 amp-hours. Protected by auto-resetting fuse.
Minimum operation time: 2 hours (5 minute auto cycle with adult
cuff at 25°C (77°F) with power save mode enabled) from full
charge. Time for full recharge: 1 hr 50 min from full discharge when
the Monitor is switched off and 8 hrs when Monitor is switched on.
OPERATING TEMPERATURE
OPERATING ATMOSPHERIC
PRESSURE RANGE
STORAGE TEMPERATURE
STORAGE / TRANSPORTATION
ATMOSPHERIC PRESSURE
HUMIDITY RANGE
RADIO FREQUENCY
INGRESS OF LIQUIDS
2.6.2. Environmental
+ 5° C to + 40° C (+ 41° F to + 104° F)
700 to 1060 hectoPascal
– 20° C to + 50° C (– 4° F to + 122° F)
500 to 1060 hectoPascal
0 % to 95 % non-condensing
Complies with IEC Publication 601-1-2 (April 1993) Medical
Electrical Equipment, Electromagnetic Compatibility
Requirements and Tests, and CISPR 11 (Group 1, Class A)
for radiated and conducted emissions.
The Monitor is protected against vertically falling drops of
water and conforms with the IEC 529 standard at level of
IPX1. No harmful effects will come of vertically falling drops
of water making contact with the Monitor.
2-9

DIMENSIONS
WEIGHT including battery
MOUNTINGS
PORTABILITY
CLASSIFICATION
INFORMATION
CUFF PRESSURE RANGE
DEFAULT TARGET: CUFF
INFLATION
TARGET CUFF INFLATION
ADJUSTMENT RANGE
2.6.3. Mechanical
Height 9.8 in. (25.0 cm)
Width 9.8 in. (24.8 cm)
Depth 6.9 in. (17.5 cm)
7.8 lb (3.5 kg)
Self-supporting on rubber feet or pole mountable
Carried by recessed handle or pole mounted
Mode of Operation: Continuous Degree of Protection against
harmful ingress of water: Drip-proof IPX1
2.6.4. NIBP
Adult 0 mmHg to 290 mmHg
Neonate 0 mmHg to 145 mmHg
Adult 150 ± 15 mmHg
Neonate 110 ± 15 mmHg
Adult
Neonate
100 to 250 mmHg
5 mmHg increments
100 to 140 mmHg
5 mmHg increments
BLOOD PRESSURE
DETERMINATION TIME
PULSE RATE RANGE
OVERPRESSURE CUT-OFF
BLOOD PRESSURE
MEASUREMENT RANGES
Adult 30 - 290 20 – 260 10 - 220
Neonate 30 - 140 20 – 125 10 - 110
BLOOD PRESSURE ACCURACY
PULSE RATE ACCURACY
SCALES
RANGE
Max
Min
MONITOR MODE ACCURACY
PREDICTIVE MODE ACCURACY
DETERMINATION TIME
Adult
Neonate
Adult
Neonate
120 seconds maximum
85 seconds maximum
30 – 200 BPM ±3%
30 – 220 BPM ±3%
Adult 300 – 330 mmHg
Neonate 150 – 165 mmHg
Systolic
mmHg
MAP
mmHg
Meets AAMI/ANSI standard SP-10
AAMI/ANSI standard: ± 5 mmHg mean error
Intra-arterial method: ± 8 mmHg standard deviation
± 3.5 percent
2.6.5. Temperature
Celsius Fahrenheit
42.2 °Celsius
31.6°Celsius
o
C
± 0.1
o
C
± 0.6
Less than 60 seconds
108.0° Fahrenheit
88.9° Fahrenheit
o
F (when tested in a calibrated liquid
± 0.2
bath; meets ASTM E1112, Table 1, in
range specified)
o
F
± 1.0
Diastolic
mmHg
2-10

2.6.6. SpO2
SpO2 RANGE AND
ACCURACY
PULSE RATE RANGE AND
ACCURACY
SATURATION PITCH
INDICATOR
WAVEFORMS
SENSOR CONNECT /
DISCONNECT FROM
PATIENT
SENSOR CONNECT /
DISCONNECT FROM
MONITOR
PULSE DETECTION
LOSS OF PULSE
NELLCOR SENSORS
ADULT ACCURACY (70% - 100%) ACCURACY
OXICLIQ-P pediatric sensor 2.5 digits
OXICLIQ-I infant sensor 2.5 digits
OXICLIQ-N neonatal/adult sensor 2.5 digits
OXICLIQ-A adult sensor 2.5 digits
OXIBAND pediatric/infant sensor 3.0 digits
OXIBAND adult/neonatal sensor 3.0 digits
DURA-Y ear clip 3.5 digits
REFLECTANCE sensor 3.5 digits
DURASENSOR adult 3.5 digits
PEDI-CHECK pediatric spot-check clip 3.5 digits
OXISENSOR II D-20 pediatric sensor 2.0 digits
OXISENSOR R-15 adult nasal sensor 3.5 digits
OXISENSOR II D-25 adult sensor 2.0 digits
OXISENSOR II N-25 neonatal/adult sensor 2.0 digits
OXISENSOR II I-20 infant sensor 2.0 digits
OXISENSOR II D-25L adult sensor, long cable 2.0 digits
Neonatal Accuracy
NOTE: Refer to NELLCOR
sensor specifications
adult/neonate: 70% to 100% ± 3.5 digits
adult/neonate: 0% to 69% ± (unspecified)
30 BPM - 250 BPM ± 3 BPM
Pitch changes with saturation
Volume selectable from 0 (off) to 9
Pulse plethysmograph waveform on LCD gain compensated
The monitor detects the attachment or disconnection of a sensor
from the patient within 15 seconds
The monitor detects the attachment or disconnection of a sensor
from the Monitor within 5 seconds
The monitor detects a pulse or enters a no signal state within 15
seconds of being attached to the patient
The monitor detects loss of pulse from patient and enters a no
signal state within 10 seconds
When sensors are used on neonatal subjects as recommended,
the specified accuracy range is increased by ± 1 digit to account
for the theoretical effect on oximeter measurements of fetal
hemoglobin in neonatal blood, e.g., N-25 accuracy on neonates
is ± 3, rather than ± 2.
2-11
2.6.7 ECG
Leads Available 3-lead configuration:
I, II, III, MCL1
QRS amplitude 0.2 to 5.0 mV
QRS duration range 15 to 200ms (does not reject 10 ms, 1mV QRS)
Heart rate accuracy 10 to 300 (adult) / 10 to 350 (neonate) beats/min ±3 beats/min or
3% of reading, whichever is greater
Heart rate resolution 1 beat/min
Bandwidth:
Display/Recorder
Standardizing voltage 1 mV marker
Common mode rejection 1 mV RTI or 10 mm peak-to-peak maximum displayed noise
Input impedance: > 2.5 MΩ @ 10 Hz
60 Hz tolerance Up to 10 mV (with artifact detector off)
Pacemaker detection/rejection
Pacer amplitude
Pacer width
With under or overshoot of
Pacer amplitude
Pacer width
Tall T wave rejection: 100%
0.5 to 40 Hz
0.05 to 40 Hz
0.05 to 100 Hz
allowed with 20 Vrms, 50-60 Hz input
Up to 300µV (at 1 mV QRS and artifact detector on)
± 2 mV to ±700 mV
0.1 ms to 2 ms
2 mV, 70 ms duration
± 2 mV to ± 700 mV
0.1 ms to 2 ms
Lead off sensing current: <0.1 µA DC signal leads
< 1 µA DC driven lead
Time to alarm: High heart rate < 10 s per AAMI EC13 – 1992
Low heart rate < 10 s per AAMI EC13 – 1992
Cardiac standstill < 10 s per AAMI EC13 – 1992
Tachycardia waveforms < 10 s per AAMI EC13 - 1992
2-12

SECTION 3. PRINCIPLES OF OPERATION
CONTENTS
3.1. Introduction............................................................................................... 3-3
3.2. Overall Principles Of Operation................................................................ 3-3
3.2.1. Nellcor SPO2............................................................................................3-3
3.2.2. Cuff Blood Pressure (BP) and Pulse......................................................... 3-3
3.2.3. Alaris Oral and Rectal Thermometry......................................................... 3-4
3.2.4. ECG with Heart Rate and Respriation....................................................... 3-4
3.2.5. Host Communication Ports....................................................................... 3-4
3.3. Functional Description.............................................................................. 3-5
3.3.1. PSU PWA ................................................................................................. 3-5
3.3.2. Mains Converter Module...........................................................................3-6
3.3.3. Main Board................................................................................................ 3-6
3.3.4. Keyboard PWA ......................................................................................... 3-7
3.3.5. ECG PWA................................................................................................. 3-8
3.3.6. Pneumatic Control .................................................................................... 3-8
3.3.7. LCD Assembly..........................................................................................3-9
3.3.8. Printer (Optional)..................................................................................... 3-10
LIST OF FIGURES
3-1 General System Diagram.......................................................................... 3-11/12
3-1

This page intentionally left blank.
3-2
 Loading...
Loading...