Coviden Kangaroo User Manual

Kangaroo
TM
Feeding Tube with IRIS Technology,
Console and Accessories
User Manual

Table of Contents
Section I: System Overview
Safety and Cautions ................................................................. 2
System Components ................................................................. 3
Section II: Assembly and Initial Administrator Use
Assembling System Parts ......................................................... 9
Initial Use ............................................................................... 10
Section III: Setting Up and Using the Kangaroo
Feeding Tube with IRIS Technology for Placement
Set Up and Use ...................................................................... 13
Reconnecting ......................................................................... 17
Section IV: Cleaning, Charging, and Storage
General Console, Interface Cable and Power Cord
Cleaning Directions ............................................................... 19
Battery and Storage .............................................................. 20
Section V: User Interface Features
On Screen Keyboard .............................................................. 22
Logging In and Automatic Log-Out ...................................... 23
Main Menu ............................................................................. 24
Procedure ............................................................................... 25
Options for Viewing Patient Folders and Files ..................... 26
Exporting Images to USB Flash Drive .................................... 27
Opening Images from USB Flash Drive ................................. 28
Rename Images ...................................................................... 29
Delete Images ......................................................................... 29
Viewing Captured Images ..................................................... 30
Annotating Images ................................................................ 31
Operator and Reviewer Settings ........................................... 32
Administrator Settings ........................................................... 33
Changing Date, Time, Formatting, Language,
and Device Settings ................................................................ 34
Adding and Editing Users ...................................................... 35
Creating and Editing Encryption Password .......................... 36
Icon Glossary ........................................................................... 37
Section VI: Additional Kangaroo Feeding Tube with
IRIS TechnologyInformation
Order Information ................................................................. 39
Troubleshooting ..................................................................... 40
Definition of Symbols ............................................................ 41
Warranty ................................................................................. 42
Specifications .......................................................................... 43
MRI Safety Information ......................................................... 43
Maintenance and Safety ........................................................ 44
Electronic Specification Tables .........................................45-49

Section I: System Overview

Kangaroo™ Console | 2
Safety and Cautions
Safety notice
This section summarizes information basic to the safe operation of the equipment
described in this manual. All safety precautions and operating instructions should be
read and understood before installation, operation, maintenance, or repair of this device.
Consult with Covidien-trained personnel before attempting to operate this equipment.
Always follow product labeling and manufacturer’s recommendations. If in doubt as to
how to proceed in any situation, contact your Covidien representative.
Note: For Enteral Fluids Only
Description
The Kangaroo™ feeding tube with IRIS technology is a single use device with a camera
embedded in the distal end to aid in placement. The tube is made of radiopaque
polyurethane material and features a Hydromer™* coated tip.
For use with ENFit™* Connection System.
Indications
The Kangaroo feeding tube with IRIS technology utilizes a video stream to aid a trained
user during placement into the stomach or small bowel for the administration of nutrition,
fluids, and medications by the naso-enteric route for patients aged 18 years and older
who have an intact gastrointestinal tract, but are physically unable to manage nutritional
intake through normal mastication and deglutition.
A trained user, as defined per facility protocol or determined by clinical privileging, should
read the Kangaroo Feeding Tube with IRIS Technology User Manual, Instructions for Use
and review the training program provided. Facility protocol for insertion of any feeding
tube should also be followed. Placement of the tip of the device into the small bowel
should only be attempted by clinicians with expertise in small bowel placement.
Prior to commencing administration, confirm correct tube placement per
institutionalprotocol.
Actions
• Acts as a conduit for food, fluids, and medications to a patient’s stomach or smallbowel.
• Avoid administration of light sensitive medications while the tube is connected to the
interface cable.
Contraindications
• Use caution with patients who have anomalies or diseases of the nose, throat,
oresophagus.
• The use of this product is contraindicated in patients with known sensitivities or
allergies to its components.
Warnings
• Coughing or any other symptom of respiratory distress would likely indicate that the
device had been misplaced in the trachea. If this is suspected, remove the tube and
stylet and reinsert.
• At any point during the procedure if continuous resistance is felt the device
should be withdrawn and then reinserted. The operator should discontinue all
attempts at placement after repetitive unsuccessful attempts (such as 5 or more) at
deviceplacement.
• The presence of an endotracheal device tends to guide the feeding tube into the trachea.
Should the feeding tube and stylet (if stylet is used) enter the tracheobrochial tree
during tube placement, damage to the lung or esophagus could occur. If any resistance
is felt during placement, remove the tube and stylet and reinsert. Coughing or any
other symptom of respiratory distress would likely
indicate that the device had been misplaced in the
trachea. Misplacement of tubes into the lungs resulting
in pneumothorax has been reported in neurologically
impaired patients and those with endotracheal tubes in
place. The operator should discontinue all attempts at
placement after repetitive unsuccessful attempts (such
as 5 or more) at device placement.
• This device should only be inserted by a trained user.
• The feeding tube is a disposable device intended for
single use. Do notreuse.
• Maintaining the patient in a High-Fowlers or SemiFowlers position may reduce regurgitation or aspiration.
If using this position, do not lean patientforward.
• The stylet must be removed prior to a patientMRI.
• No modification of this equipment is allowed.
• Do not use this device near flammableanesthetics or in
oxygen rich environments.
• The Kangaroo feeding tube with IRIS technology is
intended for enteral feeding, fluids and medication
administration, but has the potential to misconnect with
small bore connectors of other healthcare applications.
This nasogastric feeding tube should not be used with
connectors from other healthcare applications. The
feeding tube is specifically for the purpose of enteral
fluids. The insufflation device is for use with the viewing
system for connection with the feeding tube during
placement, and is to be disconnected from the feeding
tube after placement. Please avoid connection of these
devices to devices of other applications and ensure
that the tubing is appropriately connected in order to
provide enteral nutrition to the patient.
• Additional components or equipment connected to
medical electrical equipment must comply with the
respective IEC or ISO standards. All configurations
shall comply with the requirement for medical
electrical systems (see clause 16 of IEC 60601-1 Ed
3.1). If the operator connects additional components
or equipment to the medical electrical equipment, the
operator configures a medical system, and it is the
operator’s responsibility that the system complies with
the requirements for medical electrical systems. If in
doubt, consult your local representative or the technical
servicedepartment.
• When light is emitted from tube tip, do not point directly
at eye.
• To minimize heat exposure of tissue, the Kangaroo
feeding tube with IRIS technology should be
disconnected from the interface cable after the
placement is complete.
Precautions
• This device is not intended for diagnosis. Consult the
appropriate service for diagnostic evaluation if there is
concern regarding an image observed duringplacement.
Section I: System Overview
• The user should be aware of patients who have
photosensitivity due to administered medications or
other conditions since the device exposes internal
tissues to light.
• Do not autoclave.
• Feeding tubes should be flushed frequently to prevent
clogging. Suggested flushing schedule:
a) before and after each feeding
b) before and after administering medication
c) once every four hours during continuous feeding or
between intermittentfeedings
d) each time the feeding set is disconnected
e) each time the feeding container is filled/changed
f) each time the pump is stopped
• Use only water to flush. Do not use solutions containing
meat tenderizer to flush or open a clogged feeding tube.
• Tube replacement may be considered at four (4) week
intervals to ensure optimum tube patency.
• Use a Kangaroo™ enteral feeding pump for accuracy
and control of nutritional formula delivery. Infusion
pumps that deliver in excess of 40 psi should not be
used as excessive pressure is capable of causing tubes
and pump sets to balloon and/or rupture. Consult pump
manufacturer’s specifications andrecommendations.
• Administration of medications should be guided by
hospital policy. Many liquid preparations contain
Sorbitol which tends to interact with enteral formulas
and clog the feeding tube. Thoroughly crush tablets,
excluding enteric tablets which should never be crushed;
however, always consult with your pharmacist regarding
which tablets should be crushed for feeding tube
administration.
• The device generates light, the user should be aware of
patients who have taken light sensitive medications or
who may have photosensitivity.
• Images from the camera going to the console may
be lost or temporarily suspended during placement
if performed near sources of electromagnetic energy
such as RFID, diathermy equipment or hand-held
metaldetectors.
Adverse events
Pneumothorax, intestinal perforation, and aspiration
pneumonia have been reported during the use of this type
of device.
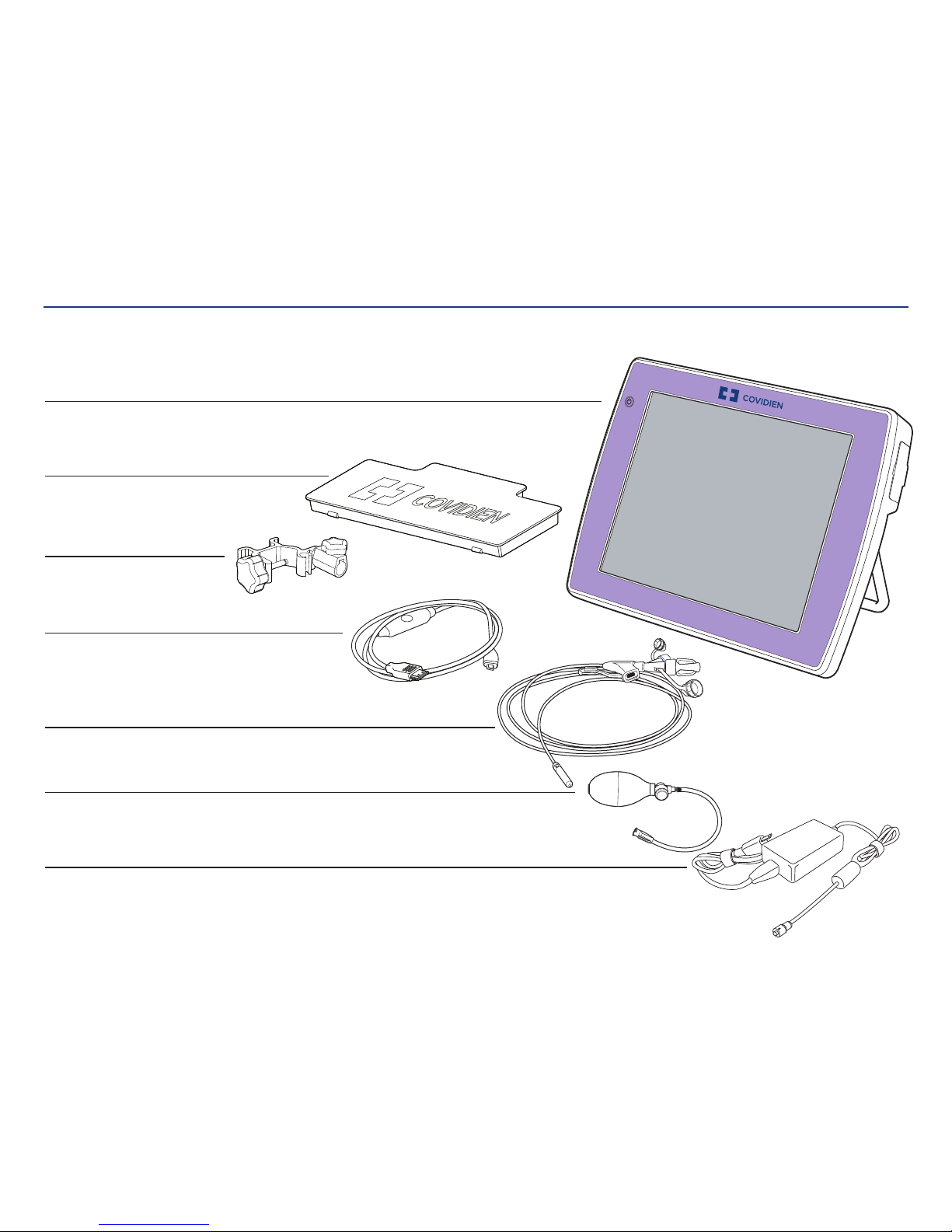
Kangaroo™ Console | 3
The Kangaroo feeding tube with IRIS technology is supplied in a semi-ready-to-use state.
Console with touch screen interface
Mounting clamp
Interface cable
Kangaroo feeding tube with IRIS technology and stylet
Detachable power adapter
Rechargeable battery
Insufflation device (packaged separately)
Optional accessories (see section regarding optional accessories):
Battery charging station, mounting cart, and carrying case.
Section I: System Overview
System Components

Kangaroo™ Console | 4
Kangaroo Feeding Tube with IRIS Technology and Stylet (single use devices):
The Kangaroo feeding tube with IRIS technology and stylet is a single use device with a camera embedded in the distal end to aid in placement. The tube is made of radiopaque polyurethane
material and features a Hydromer coated tip.
Insufflation device (single use device):
The insufflation device is to aid in feeding tube placement.
The insufflation device is connected to the feeding tube through the side port.
Section I: System Overview
Port for interface cable
Feeding port
Stylet
Side port
Camera
Feeding eyelet
cm marks
Insufflation bulb
Pressure relief valve
Insufflation connector
Insufflation connector
Side port

Kangaroo™ Console | 5
Interface cable:
The interface cable is a re-usable cable that connects the console to the tube.
Mounting clamp:
The mounting clamp is used to attach the console to a pole, if desired.
For detailed instructions, refer to the assembly section.
Power adapter:
The power adapter is supplied with four AC power cord options. Plug the power
adapter into the console to charge the battery.
It is important to choose the appropriate AC power cord for your region.
The interface cable has a push-button used to capture images of theprocedure.
Connects to feeding tube
Connects to console
Image capture button
Section I: System Overview
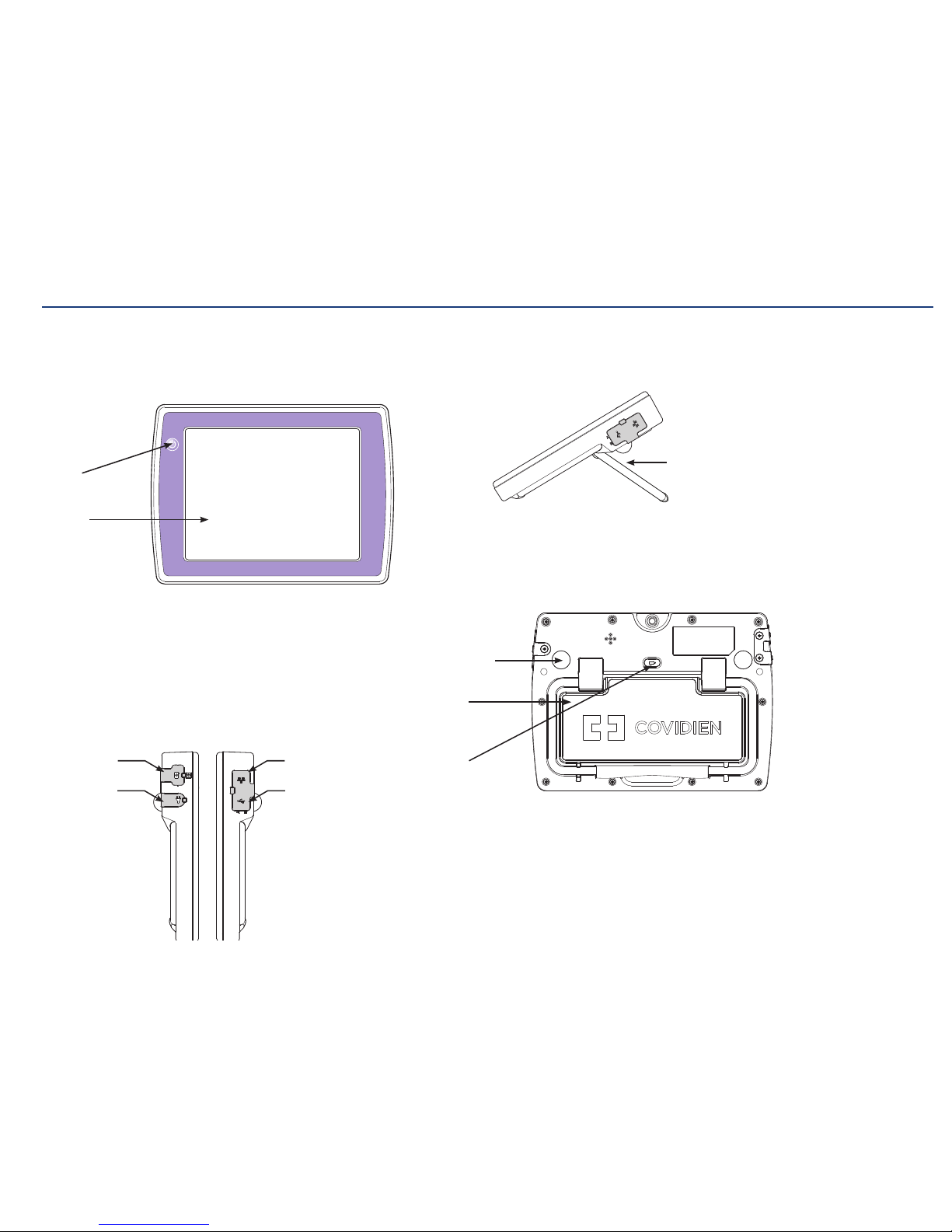
Kangaroo™ Console | 6
Console and battery:
The console has a full-color touch screen that displays a real time image as the
feeding tube travels through the patient during the placement procedure. Images can
be captured, stored, and annotated on the console.
Power
On/Off
Rechargeable
battery
Rubber
console feet
Touch
screen
Kickstand
for use on
flat surface
Battery
release
button
Ethernet port
(non-functional)
Interface
cable port
USB ports
Power cord
connector
Section I: System Overview
Mounting clamp:
The mounting clamp can be attached to the back of the console and is easily
removable. For information on attaching, using, and removing the mounting clamp,
see section regarding assembly.
Image capture:
The console has security features to help protect the information from being accessed
by other people. The device requires a login name and password to use the system.
Stored images can be exported as .bmp or .jpg files onto a USB flash drive and added
to a patient’s file. Images are password-protected for security and privacy purposes.
The USB ports are also used for software updates to the system.
Kickstand:
The console can be used on a tabletop or mounted on a pole or cart. A built-in
kickstand props the console up at an ideal viewing angle when on a flatsurface.
Rechargeable battery:
A replaceable, rechargeable Li-Ion battery is included. The console recharges when
the power adapter is plugged in to an AC outlet, or by using the battery charger
(optional accessory).
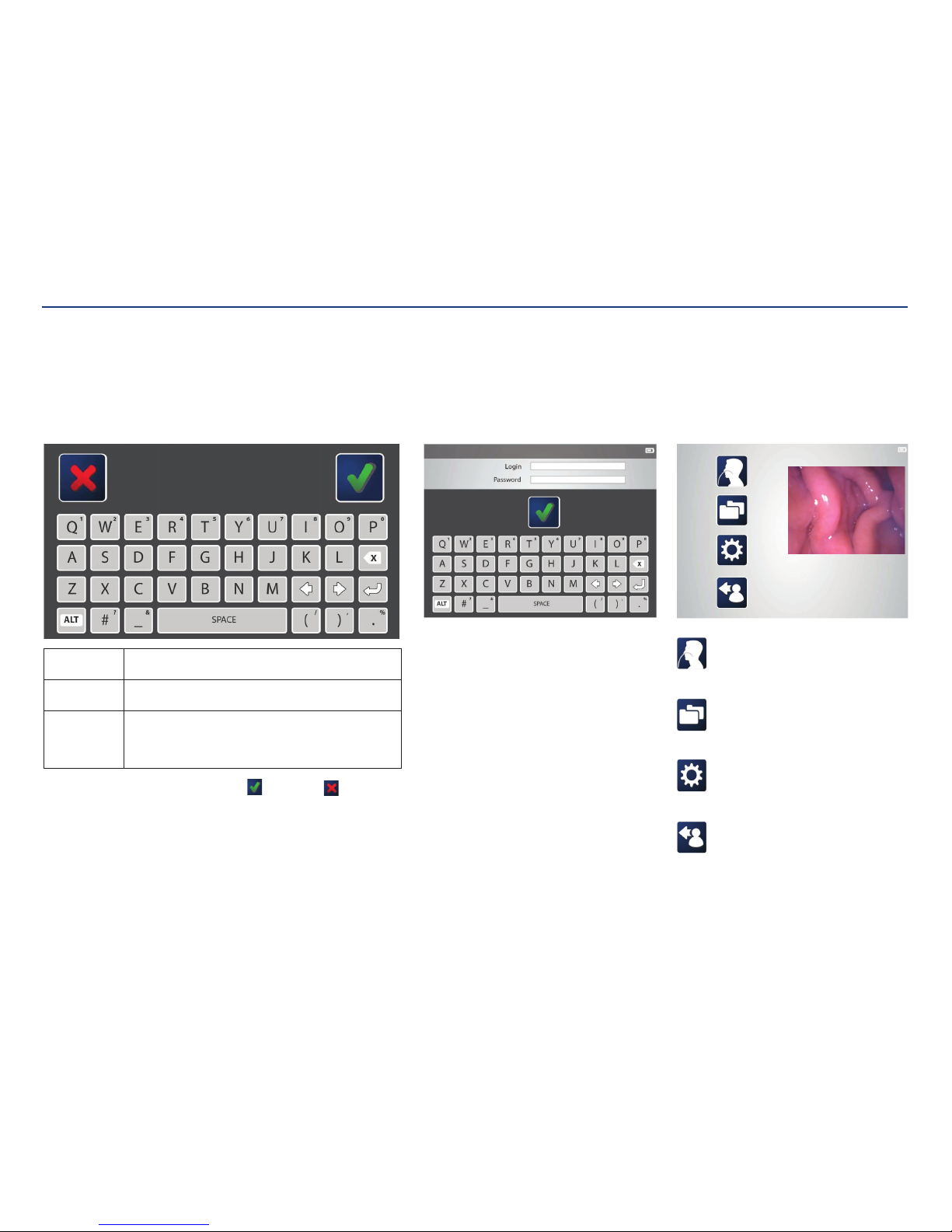
Kangaroo™ Console | 7
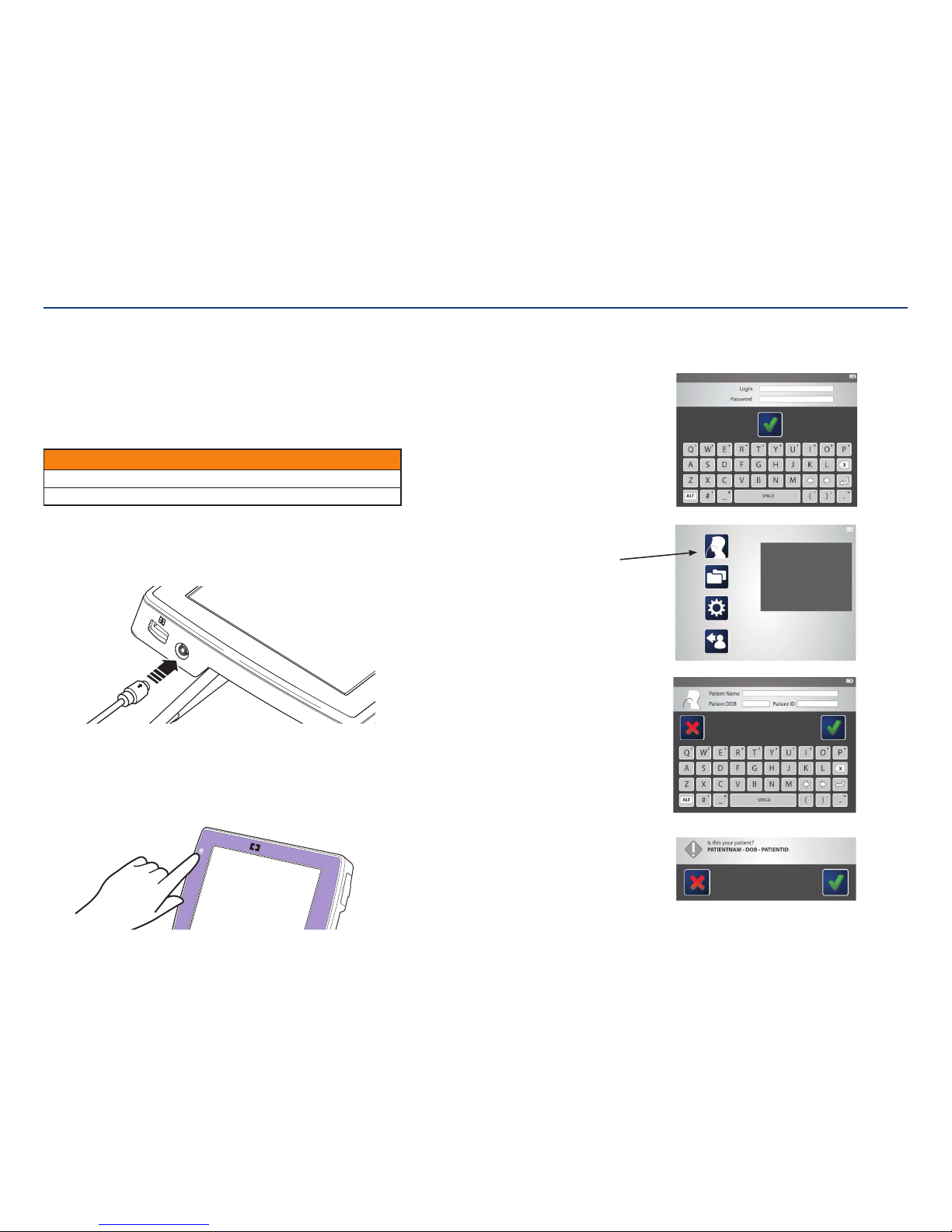
Console user interface:
The console has a color-display touch screen with step-by-step prompts to guide you
through setup andoperation.
On screen keyboard:
An on screen keyboard will appear when typing is necessary. Use the keyboard to
enter text, such as a login name, patient information, or to annotate images.
To enter text: Tap a text field (such as a login name or password) and then use
the on screen keyboard to type.
To edit text: Tap the text field and use the arrow keys to navigate to desired
text location.
To enter
numbers or
secondary
symbols:
Press the ALT key. Tap the key(s) that contains the desired
number or symbol (displayed in the upper, right corner). When
finished, press the ALT key again.
When finished typing, press the check mark icon
to save or the to cancel and
return to the previousscreen.
Procedure:
Use the procedure feature to launch
the functional flow for entering patient
information and procedure activities.
Folders menu:
The folders menu launches the data browser
of patient folders. Each folder contains images
captured during theprocedure.
Settings menu:
The settings menu provides links to modify
language, date/time, file formats, passwords,
device options, and usersettings.
Log out:
Clicking the log out icon ends thesession.
Section I: System Overview
Main menu:
The main menu provides links to log out, settings, file
management, and procedure initiation. Clicking any of
these icons brings you to thosescreens.
System login:
After the initial set up, users will be prompted for a
login name and password to access the system. First
time users should receive their login information from
an administrator. After logging in, the screen will show
the main menu.

Section II: Assembly and Initial Administrator Use

Kangaroo™ Console | 9
Section II: Assembly and Initial Administrator Use
Assembling System Parts
Attach battery:
The battery provided is both removable and rechargeable. To place it into the console, align it with the space on the
back, then lightly press it in.
Attach mounting clamp (optional):
The console can be attached to a vertical pole using the mounting clamp, included with the systemcomponents.
Attach the clamp to the console by aligning the hole on the pole clamp with the mounting hole on the back of the
console. Use a 3/8” - 16 bolt to fasten together.
Attach the mounting clamp to a pole by fitting it in between. Then, tighten the knob so that the clamp and console
are securely attached.
Connecting power adapter:
The removable power adapter connects to the console to charge the battery.
To connect, arrow on power adapter should be facing up (same side asscreen).
To disconnect, grasp cord by the locking sleeve and pull away from console.
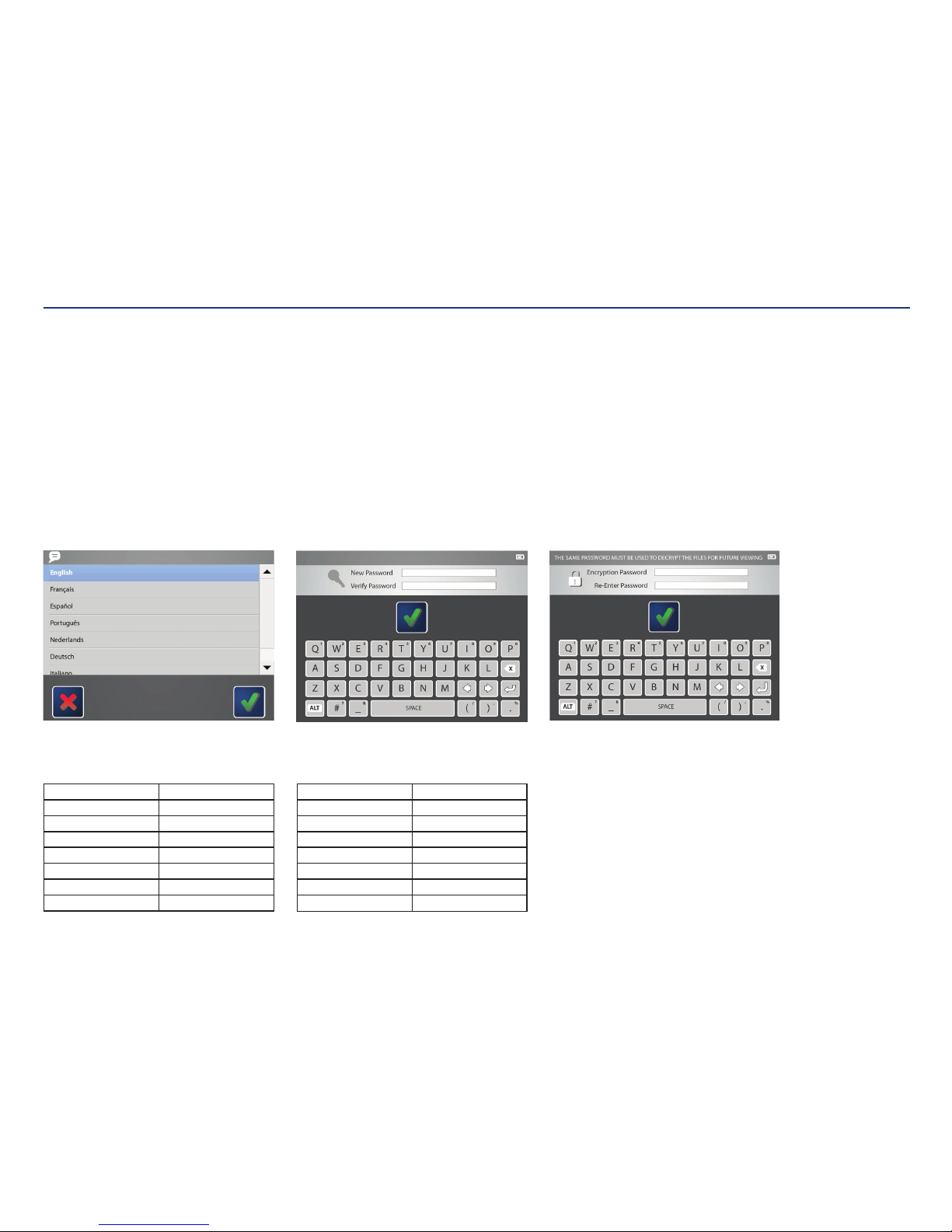
Kangaroo™ Console | 10
Choose language:
Choose the language for the console interface.
Initial Administrator Use
When the console is powered on for the first time, or after being reset to factory settings, the administrator account needs to set up the console settings.
This administrator login name is “ADMINISTRATOR” when English is selected.
If another language is selected, see table below.
Administrator password:
Create a password that will be used only for the system
administrator account. Use the on screen keyboard to
enter the password into the text fields. The password
must be between 8 and 12 characters. Password can
include uppercase letters, numbers, and symbols.
This administrator login name is “ADMINISTRATOR”.
Other administrator accounts can be created in
addition to this.
Encryption password:
The password created will be needed to open any files
exported from this console. The same password will be
used for any files exported.
Tap on the first text field to enter the desired password.
Then, re-enter the password in the text field below.
When satisfied, touch the check mark to save
thechanges.
Note: The password is case-sensitive and will be in all
upper-case.
Section II: Assembly and Initial Administrator Use
Language Administrator Login
English ADMINISTRATOR
Français ADMINISTRATEUR
Español ADM INISTRADOR
Português ADMINISTRADOR
Nederlands BEHEERDER
Deutsch ADMINISTRATOR
Italiano AMMINISTRATORE
Language Administrator Login
Dansk ADMINISTRATOR
Suomi PÄÄ KÄYT TÄ JÄ
Norsk ADMINISTRATOR
Polski ADMINISTRATOR
Русский АДМИНИСТРАТОР
Svenska ADMINISTRATÖR
日本語 カンリシャ

Kangaroo™ Console | 11
Initial Administrator Use
When the console is powered on for the first time, or after being reset to factory
settings, the administrator account needs to set up the console settings. A settings
menu will appear once the language preference, administrator password and
encryption password have been set.
Settings menu:
Tap on the icons at the bottom of the screen to make changes to the date/time,
language, file format, device, and users.
It is recommended that the current time and date is set prior to use. New user logins
and passwords should be added at this point.
Section II: Assembly and Initial Administrator Use
Back:
Return to previous screen.
Time and date:
Set or change the time and date.
Language:
Change the preferred language.
Format:
Choose the preferred image type (.jpg or .bmp) for export.
Edit the way the time and date are written on the patient files and images.
Device:
Erase patient data, update software, or reset console back to
factorysettings.
Password:
Change the login password.
Passwords must be reset after 90 days.
User groups:
Create logins and passwords for operators and reviewers.
Change login names and/or passwords for other users.
Encryption password:
Create password to decrypt and open exported files.

Section III: Setting Up and Using the Kangaroo Feeding Tube with IRIS Technology for Placement
Kangaroo™ Console | 13
Set Up and Use
1. Read all warnings and precautions prior to tube insertion.
2. Explain procedure to the conscious patient. Prepare supplies (ENFit™*
syringe, feeding tube water, wipes, and stethoscope).
Position console in a direct line of sight. Console can be pole mounted, propped on
bedside table, or handheld.
CAUTION
Do not place console on patient’s bed during procedure.
Check battery life of console. If low, connect console to AC power outlet.
3. Plug in the power cable (if necessary).
If desired, connect the power cord to the console. To connect, arrow on power
adapter should be facing up (same side as screen).
4. Power the console on.
Press the power button. The system should start up shortly after and display a
loginscreen.
Note: After a period of inactivity (no user input to the console), the console will
automatically log the user out. This log-out will not occur during aprocedure.
COVIDIEN
5. Enter login, password, and patient information.
The system will request a login name and password. After
typing, tap the check mark to proceed to the mainmenu.
Note: First time users need to have an administrator set
up a login name and password prior to use.
The main menu will appear.
Tap on the procedure icon to begin the
placementprocedure.
Follow the screen commands to enter the patient
information. When complete, tap the check mark
toproceed.
The system will ask for confirmation that the
information is entered correctly. If yes, tap the check
mark. If not, tap the red “x” to go back and re-enter
theinformation.
Section III: Setting Up and Using the Kangaroo Feeding Tube with IRIS Technology for Placement
Kangaroo™ Console | 14
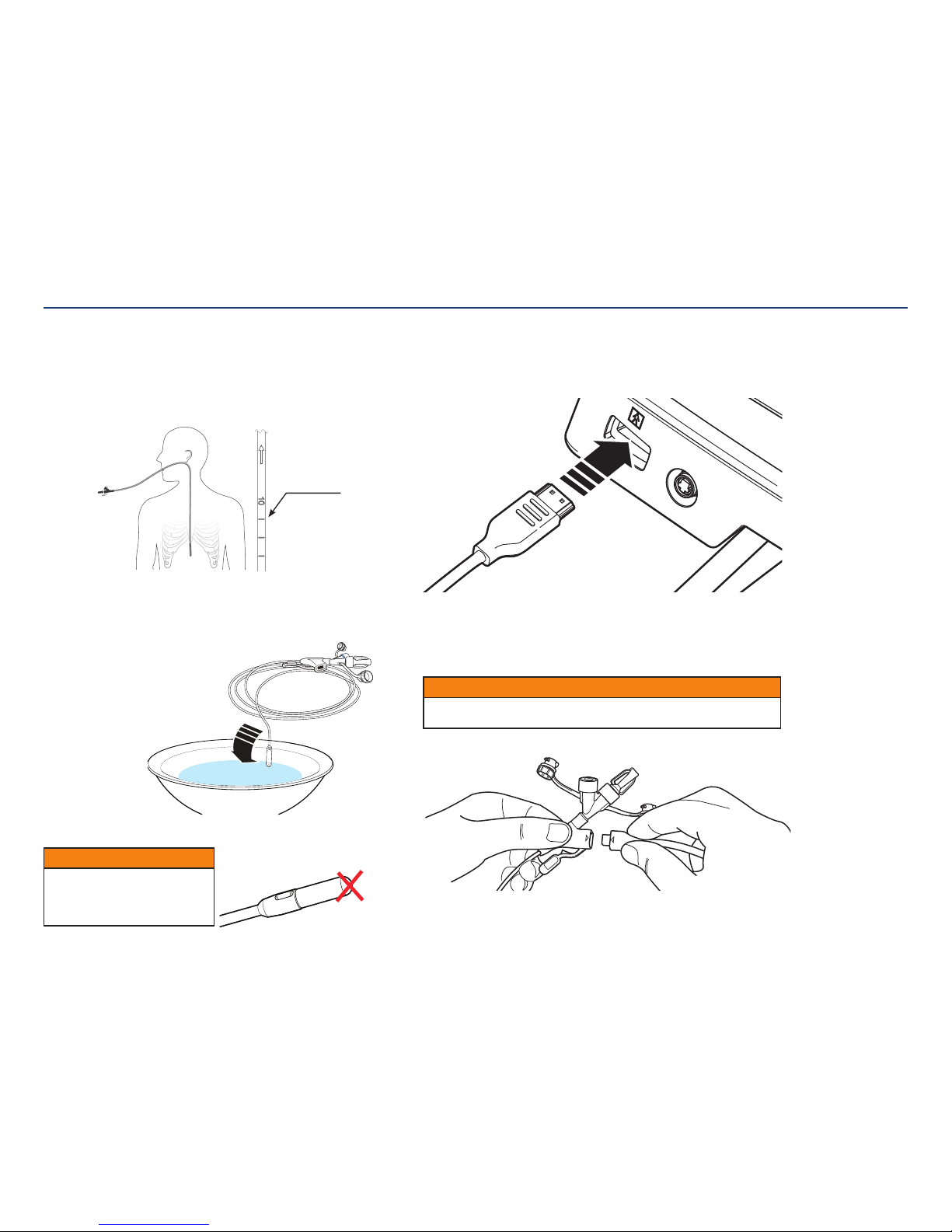
8. Connect interface cable to console.
After entering patient information, the console will request a feeding tube to
beattached.
Start by connecting the larger end of the interface cable to the console.
9. Connect interface cable to feeding tube.
A screen on the console will request that a feeding tube be attached. Connect the
smaller end of the interface cable to the feeding tube. Once the feeding tube and
interface cable are connected to the console, a live feed from the camera will display
on the screen.
CAUTION
Allow enough slack so that there is no stress on the tube or console. Strain on the
tube or console could cause droppage, breakage, or discomfort to thepatient.
CAUTION
If applying lubricant, do not put
lubricant on or near the camera-side
of the feeding tube. The camera vision
may become blocked orblurred.
6. Position patient and estimate feeding tube length.
Position patient in accordance with facility protocol for feeding tubeplacement.
To estimate insertion depth, use the tube to measure the distance from the tip of the
patient’s nose to the earlobe and from the earlobe to the xiphoid process for gastric
placement. Add approximately 10 (ten) inches (25 cm) for intestinal placement.
Spontaneous transpyloric passage of the tip often occurs within 24 to 48 hours.
7. Activate Hydromer coating.
Use water to activate the Hydromer coating on the Kangaroo feeding tube with IRIS
technology. Submerge tip for about 5 seconds to activate the Hydromer coating.
Avoid applying additional
lubricant to tip
Section III: Setting Up and Using the Kangaroo Feeding Tube with IRIS Technology for Placement
cm marks
 Loading...
Loading...