Coulter HmX with Autoloader Reference guide
COULTER® HmX Hematology Analyzer
COULTER
®
HmX Hematology Analyzer with
Autoloader
B
ECKMAN
OULTER
C
AVOID EXPOSURE, LASER RADIATION
EMITTED FROM THIS APERTURE
Reference
B
ECKMAN
C
OULTER
HmX
COULTER
®
HmX
HmX
COULTER
®
HmX
PN 4237523A (July 1999)
COULTER CORPORATION
A Beckman Coulter Company
Miami, Florida 33196-2500 USA
LEGAL NOTICES
READ ALL PRODUCT MANUALS AND CONSULT WITH BECKMAN COULTER-TRAINED PERSONNEL
BEFORE ATTEMPTING TO OPERATE INSTRUMENT.
HAZARDS AND OPERATIONAL PRECAUTIONS AND LIMITATIONS
WARNINGS, CAUTIONS, and IMPORTANTS alert you as follows:
WARNING - Might cause injury.
CAUTION - Might cause damage to the instrument.
IMPORTANT - Might cause misleading results.
CAUTION System integrity might be compromised and operational failures might occur if:
r This equipment is used in a manner other than specified. Operate the instrument as instructed in the Product
Manuals.
r You introduce software that is not authorized by Beckman Coulter into your computer. Only operate your system’s
computer with software authorized by Beckman Coulter.
r You install software that is not an original copyrighted version. Only use software that is an original copyrighted
version to prevent virus contamination.
Beckman Coulter, Inc. urges its customers to comply with all national health and safety standards such as the use of barrier
protection. This may include, but it is not limited to, protective eyewear, gloves, and suitable laboratory attire when
operating or maintaining this or any other automated laboratory analyzer.
WARNING Risk of operator injury if all covers are not secured in place prior to instrument operation or you attempt to
replace a part without carefully reading the replacement instructions. Do not attempt to replace any component until you
carefully read the instructions for replacing the component.
IMPORTANT If you purchased this product from anyone other than Beckman Coulter or an authorized Beckman Coulter
distributor, and, if it is not presently under a Beckman Coulter service maintenance agreement, Beckman Coulter cannot
guarantee that the product is fitted with the most current mandatory engineering revisions or that you will receive the most
current information bulletins concerning the product. If you purchased this product from a third party and would like
further information concerning this topic, call your Beckman Coulter Representative.

Initial Issue, A 7/99
Software version 1.0.
REVISION STATUS
This document applies to the latest software listed and higher versions. When a subsequent software version changes the
information in this document, a new issue will be released.
PN 4237523A
iii

REVISION STATUS
iv
PN 4237523A

LEGAL NOTICES
REVISION STATUS, iii
CONTENTS, v
INTRODUCTION, xv
HOW TO USE YOUR COULTER® HmX HEMATOLOGY ANALYZER AND HmX
HEMATOLOGY ANALYZER WITH AUTOLOADER DOCUMENTATION SET, xv
ABOUT THIS MANUAL, xv
CONVENTIONS, xvi
LIST OF ICONS, xvii
COMPUTER PROGRAM STATEMENT, xvii
1 USE AND FUNCTION , 1-1
1.1 INTENDED USE, 1-1
General, 1-1
Parameters, 1-2
CLIA Complexity Categories, 1-3
CONTENTS
1.2 QUALITY CONTROL (QC), 1-3
1.3 METHOD HISTORY, 1-4
Development, 1-4
Hemoglobinometry, 1-4
Differential Measurement, 1-5
Volume Analysis, 1-5
Conductivity Analysis, 1-5
Light Scatter Analysis, 1-5
Reticulocyte (Retic) Analysis, 1-5
% Analysis, 1-5
1.4 SYSTEM COMPONENTS, 1-6
Main Unit, 1-6
Sample Handler, 1-6
Diluter, 1-6
Analyzer/Systems Control Module, 1-6
Electronic Power Supply, 1-6
Pneumatic Power Supply, 1-6
Data Management System (DMS), 1-7
1.5 OPTIONS, 1-7
Printers, 1-7
PN 4237523A
v

CONTENTS
1.6 REAGENTS, 1-7
Diluent, 1-7
CBC Lytic Reagent, 1-8
HmX PAK, 1-8
PAK LYSE, 1-8
PAK PRESERVE, 1-8
ReticPrep™ Reagent Kit, 1-8
Reagent A, 1-8
Reagent B, 1-8
Cleaning Agent, 1-8
1.7 CONTROLS AND CALIBRATOR, 1-9
Controls, 1-9
Calibrator, 1-9
1.8 MATERIAL SAFETY DATA SHEETS (MSDS), 1-9
2 INSTALLATION, 2-1
2.1 GENERAL, 2-1
2.2 SPECIAL REQUIREMENTS, 2-1
Space and Accessibility, 2-1
Electrical Input, 2-1
Ambient Temperature and Humidity, 2-2
Ventilation, 2-2
Air Conditioning, 2-2
Drainage, 2-2
Date Format, 2-3
2.3 INTERUNIT CONNECTIONS, 2-3
Reagent and Waste Connections, 2-3
Power and Signal Cables, 2-5
3 OPERATION PRINCIPLES, 3-1
3.1 GENERAL PRINCIPLES, 3-1
CBC Analysis, 3-1
Differential Analysis, 3-1
Effect of Reagents, 3-2
Retic Analysis, 3-2
3.2 SPECIMEN TRANSPORT - HmX HEMATOLOGY ANALYZER WITH
AUTOLOADER, 3-3
vi
3.3 SPECIMEN TRANSPORT - HmX HEMATOLOGY ANALYZER, 3-4
3.4 SAMPLE FLOW, 3-5
Normal Sample Flow, 3-5
Retic Sample Flow, 3-9
PN 4237523A

3.5 COUNTING AND SIZING, 3-10
Red and White Blood Cell Counting, 3-10
Routine Counting, 3-10
Extended Counting, 3-10
Coincidence Correction, 3-10
Triplicate Counting/Voting, 3-10
Sweep Flow, 3-11
Pulse Editing, 3-11
RBC Count and Size Distribution, 3-11
Plt Count and Size Distribution, 3-11
Plt Fitting Process, 3-12
Scatterplot Development, 3-12
Differential-Related, 3-12
Retic-Related, 3-13
Retic Parameters, 3-14
Derived and Computed Parameters, 3-14
3.6 Hgb CONCENTRATION MEASUREMENT, 3-14
CONTENTS
3.7 PARAMETERS AND THEIR DERIVATION, 3-14
White Blood Cell (WBC) Count, 3-15
Red Blood Cell (RBC), 3-15
Hemoglobin (Hgb) Concentration, 3-15
Mean Corpuscular Volume (MCV), 3-15
Hematocrit (Hct), 3-15
Mean Corpuscular Hemoglobin (MCH), 3-15
Mean Corpuscular Hemoglobin Concentration (MCHC), 3-16
Red Distribution Width (RDW), 3-16
Platelet (Plt) Count, 3-16
Mean Platelet Volume (MPV), 3-16
Differential Counts, 3-16
Percentages, 3-16
Absolute Numbers, 3-17
Reticulocyte (Retic) Parameters, 3-17
Reticulocyte Percent (RET%), 3-17
Reticulocyte Absolute Number (RET#), 3-17
PN 4237523A
vii

CONTENTS
4 SPECIFICATIONS/CHARACTERISTICS, 4-1
4.1 INSTRUMENT SPECIFICATIONS, 4-1
Dimensions, 4-1
Power, 4-1
Installation Category, 4-1
Input - Dedicated Line, 4-1
Consumption, 4-1
Pneumatic Supplies, 4-1
Pressure, 4-1
Vacuum, 4-2
Temperature (Ambient Operating Range for Patient Samples), 4-2
Humidity, 4-2
Recommended Reagents, 4-2
Reagent Usage, 4-2
Recommended Commercial Controls, 4-2
Recommended Calibrator, 4-2
Recommended Anticoagulant, 4-3
Minimum Sample Volume Required, 4-3
Tube Sizes for Closed-Vial Mode, 4-3
Recommended Bar-Code Labels, 4-3
DMS Storage, 4-3
Patient Results, 4-3
Controls, 4-3
Conditions of Measurement, 4-3
Hemoglobin Measurement, 4-3
Aperture Size, 4-4
Electronic Stability, 4-4
Throughput, 4-4
viii
4.2 SOFTWARE SPECIFICATONS, 4-4
Date Format, 4-4
4.3 CBC AND DIFFERENTIAL PERFORMANCE SPECIFICATIONS, 4-4
Precision, 4-4
Accuracy, 4-5
CBC, 4-5
WBC Differential, 4-5
Linearity, 4-5
Mode-to-Mode Matching, 4-6
Background Counts, 4-6
Carryover, 4-7
4.4 RETICULOCYTE PERFORMANCE SPECIFICATIONS, 4-7
Precision, 4-7
Accuracy, 4-7
Reportable Range, 4-8
Operating Range, 4-8
Flagging, 4-8
Limitations, 4-8
PN 4237523A

CONTENTS
4.5 PERFORMANCE CHARACTERISTICS - HmX HEMATOLOGY ANALYZER , 4-8
Precision of the CBC Parameters, 4-8
Imprecision Analysis by Paired Sample of the Differential Parameters, 4-10
Accuracy of CBC Parameters, 4-10
Accuracy of Differential Parameters, 4-11
Clinical Sensitivity, 4-11
4.6 PERFORMANCE CHARACTERISTICS - HmX HEMATOLOGY ANALYZER WITH
AUTOLOADER, 4-13
Precision of the CBC Parameters, 4-13
Precision of the Differential Parameters - HmX Hematology Analyzer with
Autoloader, 4-13
Accuracy of CBC Parameters - HmX Hematology Analyzer with Autoloader, 4-14
4.7 RETICULOCYTE PERFORMANCE CHARACTERISTICS, 4-15
Imprecision Analysis by Replication, 4-15
Accuracy, 4-15
Reference Interval, 4-16
4.8 INTERFERING SUBSTANCES, 4-16
WBC, 4-16
RBC, 4-16
Hgb, 4-16
MCV, 4-16
RDW, 4-16
Plt, 4-17
MPV, 4-17
Hct, 4-17
MCH, 4-17
MCHC, 4-17
Diff Parameters, 4-17
Reticulocytes, 4-17
5 LASER SAFETY, 5-1
5.1 LASER SAFETY PRECAUTIONS, 5-1
5.2 GENERAL LASER SAFETY WARNINGS, 5-1
5.3 WARNING LABELS, 5-1
6 REPORTING OPTIONS, 6-1
PN 4237523A
6.1 TICKET FORMAT ON A GRAPHIC PRINTER, 6-1
6.2 GRAPHIC FORMAT, 6-2
ix

CONTENTS
7 BAR-CODE SPECIFICATIONS, 7-1
7.1 BAR-CODE LABELS, 7-1
Optical Characteristics at 670 nm ±10%, 7-2
Label Specifications, 7-3
Code-Related Specifications, 7-4
Printing Methods, 7-4
7.2 BAR-CODE READER, 7-4
Description, 7-4
Settings and Defaults, 7-4
7.3 BAR-CODE DECODER, 7-5
Normal Operation, 7-5
HmX Hematology Analyzer, 7-5
HmX Hematology Analyzer with Autoloader, 7-5
A SAMPLE TUBE SIZES, A-1
A.1 BECKMAN COULTER, A-1
A.2 BECTON DICKINSON, A-1
Worldwide, A-1
U.S.A., A-1
Europe, A-2
U.K. and Australia, A-2
Japan, A-2
A.3 GREINER, A-2
Vacuette Brand, A-2
A.4 JOHNS, A-3
A.5 LABCO, A-3
Exetainer Brand, A-3
A.6 LABO EXPRESS SERVICE (L.E.S.), A-3
A.7 LDM, A-3
A.8 L.I.P., A-3
A.9 SARSTEDT, A-3
A.10 SHERWOOD MEDICAL, A-4
x
A.11 TERUMO (Worldwide), A-4
PN 4237523A

B DIFF COMPARISON, B-1
B.1 INTRODUCTION, B-1
B.2 PROCEDURE, B-1
B.3 LOG SHEETS, B-5
LIMITED WARRANTY
REFERENCES, REFERENCES-1
GLOSSARY, GLOSSARY-1
INDEX, 9
TRADEMARKS
CONTENTS
PN 4237523A
xi

CONTENTS
ILLUSTRATIONS
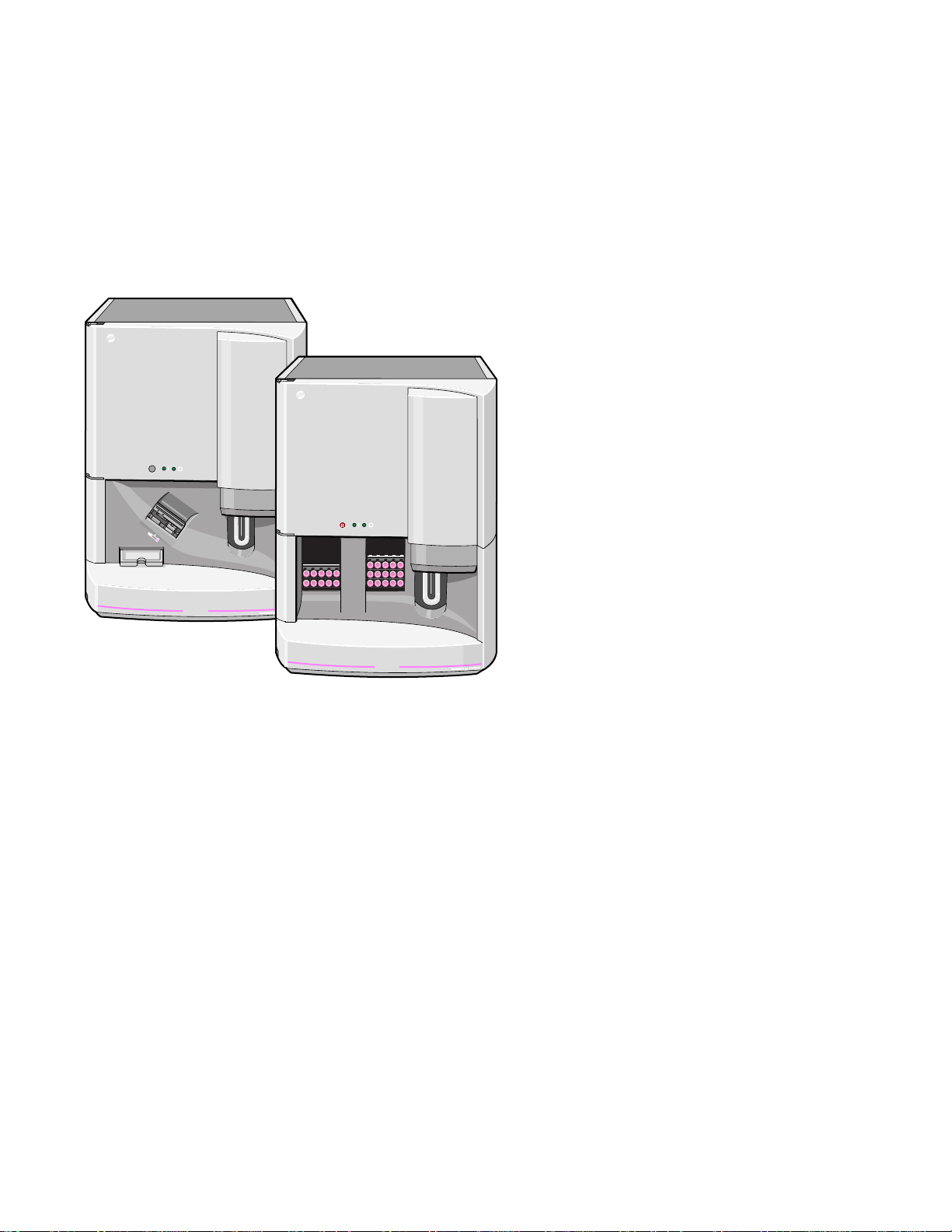
1.1 The HmX Hematology Analyzer, 1-1
1.2 The HmX Hematology Analyzer with Autoloader, 1-2
1.3 System Version Display, 1-7
2.1 Reagent and Waste Container Tubing Connections, 2-4
2.2 Interunit Connections, 2-5
3.1 Coulter Method of Counting and Sizing, 3-1
3.2 Flow Cell, 3-2
3.3 Loading a Cassette, 3-3
3.4 Reading a Bar-Code Label, 3-4
3.5 BSV, 3-5
3.6 Baths Draining, 3-6
3.7 Sample Flow, 3-7
3.8 Erythrolyse II (PAK LYSE) and StabiLyse (PAK PRESERVE) Reagent Pumps, 3-8
3.9 Aperture Baths and Vacuum Isolators, 3-8
3.10 Sample Movement to the Flow Cell, 3-9
3.11 Sweep Flow, 3-11
3.12 Scatterplot, DF 1 View, 3-12
3.13 Scatterplot, DF 2 View, 3-13
3.14 Scatterplot, DF 3 View, 3-13
3.15 Scatterplot, DF 5 View, 3-14
3.16 Scatterplot, DF 6 View, 3-14
5.1 Laser Safety Label, 5-2
5.2 Safety Labels on the TTM, 5-3
5.3 Laser Safety Labels for Bar-Code Reader on the HmX Hematology Analyzer, 5-4
5.4 Laser Safety Labels for Bar-Code Reader on the HmX Hematology Analyzer with
Autoloader, 5-4
6.1 Ticket Format, 6-1
6.2 Graphic (Wide/Large Page) Format, 6-2
6.3 Graphic (Narrow/Small Page) Format, 6-3
7.1 Bar-Code Label Specifications, 7-3
xii
PN 4237523A

CONTENTS
TABLES
1.1 CLIA Complexity Table, 1-3
4.1 Precision Specifications, 4-4
4.2 Precision, WBC Differential Parameters, 4-5
4.3 CBC Accuracy Limits, 4-5
4.4 Linearity Limits, 4-6
4.5 Mode-to-Mode Matching, 4-6
4.6 Background Counts for CBC Analysis, 4-6
4.7 Reportable Range, 4-8
4.8 Imprecision Analysis by Replication WHOLE BLOOD - HmX Hematology Analyzer, 4-9
4.9 Imprecision Analysis by Paired Sample - HmX Hematology Analyzer, 4-9
4.10 Imprecision Analysis (Diff Parameters) by Paired Sample - HmX Hematology Analyzer, 4-10
4.11 Imprecision Analysis (Diff Parameters) by Replication - HmX Hematology Analyzer, 4-10
4.12 Reference Range (Diff Parameters) for 160 Subjects - HmX Hematology Analyzer, 4-10
4.13 Accuracy Analysis by Compared Specimens - HmX Hematology Analyzer, 4-11
4.14 Accuracy Analysis (Diff Parameters) by Compared Specimens - HmX Hematology
Analyzer, 4-11
4.15 Flagging Criteria - H2O Method, 4-12
4.16 Morphologic Abnormalities - HmX Hematology Analyzer, 4-12
4.17 Clinical Sensitivity for Morphologic Abnormals - HmX Hematology Analyzer, 4-12
4.18 Imprecision Analysis by Replication: WHOLE BLOOD - HmX Hematology Analyzer with
Autoloader, 4-13
4.19 Imprecision Analysis by Paired Sample - HmX Hematology Analyzer with Autoloader, 4-13
4.20 Imprecision Analysis (Diff Parameters) by Paired Sample - HmX Hematology Analyzer with
Autoloader, 4-14
4.21 Accuracy Analysis by Compared Specimens - HmX Hematology Analyzer with
Autoloader, 4-14
4.22 Imprecision Analysis by Replication, 4-15
4.23 Imprecision Analysis by Paired Samples, 4-15
4.24 Accuracy Analysis by Compared Specimens, 4-15
4.25 Reference Intervals, 4-16
7.1 Code 128 Characters, 7-1
7.2 Code-Related Specifications, 7-4
7.3 Bar-Code Reader Settings and Defaults , 7-4
xiii
PN 4237523A

CONTENTS
xiv
PN 4237523A

INTRODUCTION
This introductory section contains the following topics:
r How to use your COULTER HmX Hematology Analyzer and HmX Hematology Analyzer
with Autoloader Documentation set
r About this Manual
r Conventions
r List of Icons.
HOW TO USE YOUR COULTER® HmX HEMATOLOGY ANALYZER AND HmX
HEMATOLOGY ANALYZER WITH AUTOLOADER DOCUMENTATION SET
Use the
methods it uses, its specifications, and information on installation, safety, and software options.
Use the Special Procedures and Troubleshooting Manual to run a calibration, perform
reproducibility and carryover checks, and to clean, replace, or adjust a component of the
instrument. The troubleshooting tables appear at the back of the manual.
Reference
manual for in-depth information about what the instrument does, the
Use the
Overview chapter to become familiar with the different parts of your system. Then go through
the detailed step-by-step procedures of start up, running controls and samples, reviewing data,
and shutdown.
Use the Host Specifications Manual to locate information about transmission to a host
computer.
Use the Master Index to locate a subject in your documentation set.
See the Documentation page on the back cover of this manual for the contents of each manual.
It can help you to determine quickly which manual contains the information you need.
Operator's Guide
ABOUT THIS MANUAL
Your COULTER HmX Hematology Analyzer and HmX Hematology Analyzer with Autoloader
Reference manual provides in-depth information about what the instrument does, the
methods it uses, its specifications, and information on installation, safety, and software
options.
This manual covers the total HmX system. Use this manual for reference if you have a HmX
Hematology Analyzer or a HmX Hematology Analyzer with Autoloader.
This information is organized as follows:
for the day-to-day running of your instrument. Read the System
PN 4237523A
s Chapter 1, Use and Function
Contains the intended use of the instrument, a brief history of the methods used by the
instrument, the reagents, calibrator, and controls used, and a short description of the
major components and options.
s Chapter 2, Installation
Contains the instrument requirements, the diagrams of the reagent tubing connections,
and the interunit cable connections.
xv

INTRODUCTION
CONVENTIONS
s
Chapter 3, Operation Principles
Contains the descriptions of the Coulter Method, the normal sample flow through the
instrument, how counting and sizing are accomplished, and how the parameters are
derived.
s Chapter 4, Specifications/Characteristics
Details the instrument and performance specifications, the performance characteristics,
and the interfering substances.
s Chapter 5, Laser Safety
Describes laser safety precautions and the location of the laser-related labels.
s Chapter 6, Reporting Options
Shows examples of printouts you can select from your graphic printer.
s Chapter 7, Bar-Code Specification
Describes the specifications for bar-code labels to be used with the system.
s Appendices
The appendices provide reference material on the following topics:
r Tu b e S i z e s
s References
s Glossary
s Index
CONVENTIONS
This manual uses the following conventions:
r ITALICS indicate screen messages such as RESET THE SYSTEM or Press any key.
r
r The software path to access the needed function or screen appears in a series separated
r Diff Comparison.
Lists the references, by number, as used throughout this manual.
Contains the definitions for words and terms used in the set of manuals.
Contains terms and where you can easily locate information about them in this manual.
Bold indicates
t a menu item such as
Run Samples
t or a function such as F3 Run.
by double arrow heads. For example, the path to the
Special Functions
Set Uptt System Set Uptt Reagents.
tt
REAGENTS set up screen is:
To select a menu item, highlight it then press Û or press the alphabetic key on the
keyboard that corresponds to the letter displayed in black within the name of the menu
item.
xvi
r ë indicates a key (such as Û).
r ë ë indicates to press and release the first key listed, then press and release the next
key listed.
r ë+ë indicates to press and hold the first key listed, then press the next key.
PN 4237523A

LIST OF ICONS
INTRODUCTION
LIST OF ICONS
Read this section if you
have a HmX Hematology
Analyzer with
Autoloader.
COMPUTER PROGRAM STATEMENT
About the HmX Hematology Analyzer Computer Program
HmX Hematology Analyzer Computer Program, Version 1.0
©
Copyright
All Rights Reserved.
This Computer program is protected by international copyright laws, and unauthorized
copying, use, distribution, transfer or sale is a violation of those laws that may result in civil
or criminal penalties. This computer program may also be subject to additional restrictions
contained in a license granted by Beckman Coulter, Inc. to the authorized user of this
computer program or to the authorized owner or other authorized user of the system onto
which this computer program is installed. Any violation of the license provisions may result
in additional civil penalties, including an injunction and damages. Please refer to the
computer program or system agreement or to the computer program or system
documentation for the terms and conditions of that license.
1999 Beckman Coulter, Inc.
Read this section if you
have a HmX
Hematology Analyzer.
PN 4237523A
xvii

INTRODUCTION
COMPUTER PROGRAM STATEMENT
xviii
PN 4237523A
1.1 INTENDED USE
General
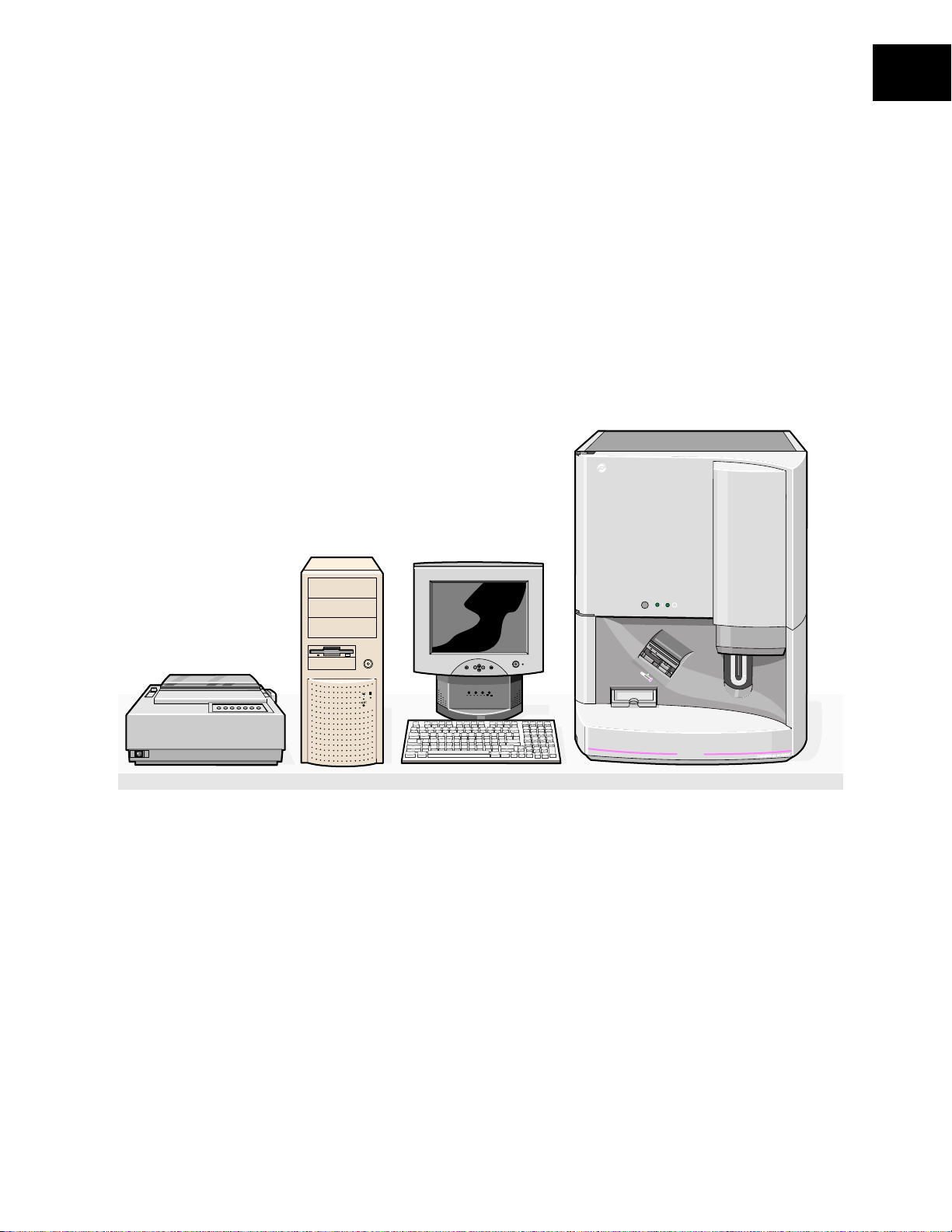
The COULTER HmX Hematology Analyzer, Figure 1.1, and the HmX Hematology Analyzer
with Autoloader, Figure 1.2, are quantitative, automated hematology analyzers and leukocyte
differential cell counters For In Vitro Diagnostic Use in clinical laboratories.
The purpose of the HmX Hematology Analyzer is to separate the normal patient, with all
normal system-generated parameters, from the patient who needs additional studies. These
studies include further measurements of cell size and cell distribution, biochemical
investigation or any other test that helps diagnose the abnormality.
Figure 1.1 The HmX Hematology Analyzer
USE AND FUNCTION
1
1
Main Unit
B
ECKMAN
C
OULTER
Graphic Printer
ON
OFF
Computer
LCD Display
EXIT MENU
+
-
ON/OFF
MICTREEBLEBASS
VOLUME
ON OFF
MAX MIN MAX MIN MAX MIN
AVOID EXPOSURE, LASER RADIATION
EMITTED FROM THIS APERTURE
HmX
COULTER
®
HmX
PN 4237523A
1-1
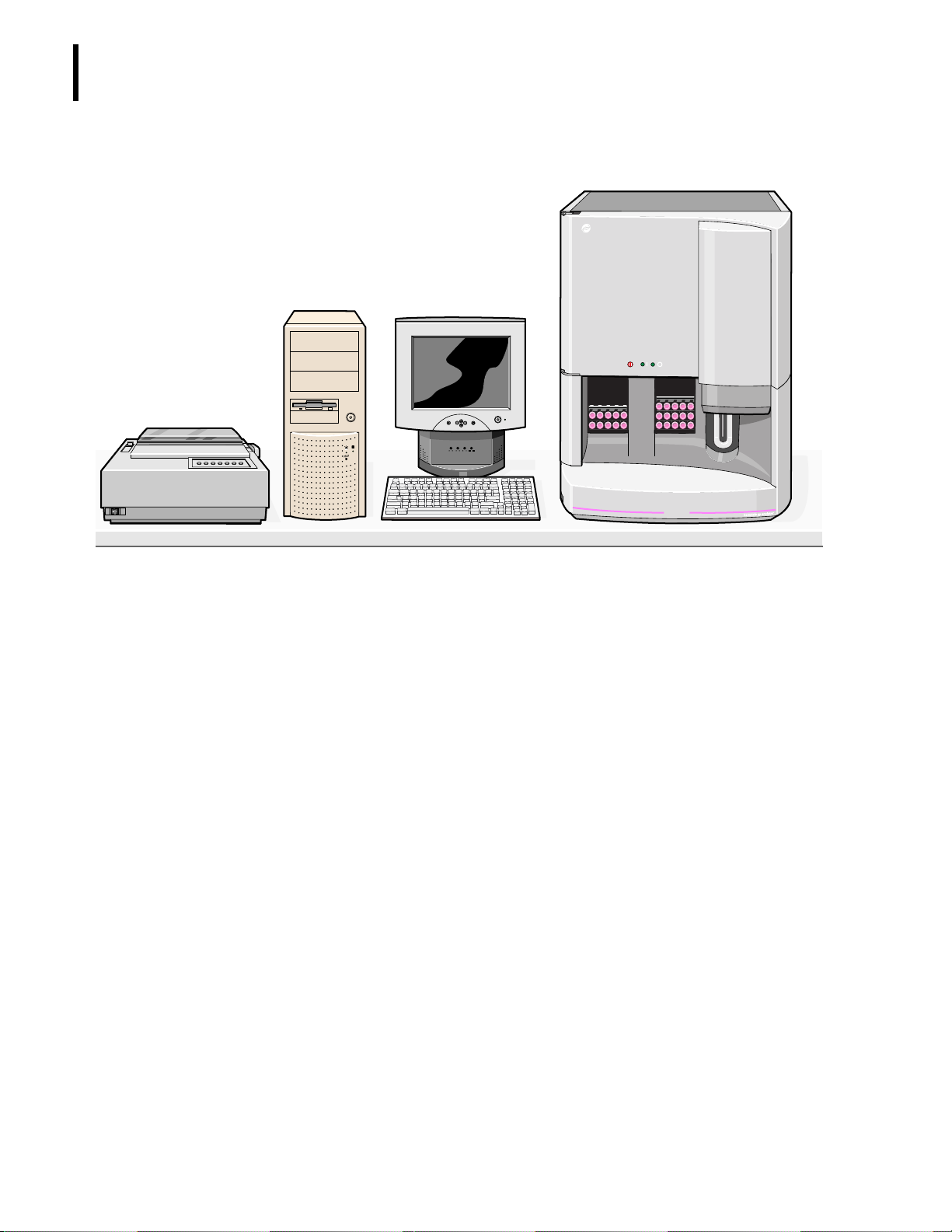
USE AND FUNCTION
INTENDED USE
Figure 1.2 The HmX Hematology Analyzer with Autoloader
Main Unit
B
ECKMAN
C
OULTER
Graphic Printer
ON
OFF
Parameters
Computer
LCD Display
EXIT MENU
+
-
ON/OFF
MICTREEBLEBASS
VOLUME
ON OFF
MAX MIN MAX MIN MAX MIN
HmX
COULTER
The systems measure these hematologic parameters of whole-blood specimens:
WBC White Blood Cell (leukocyte) Count
NE% Neutrophil percent
NE# Neutrophil number
LY% Lymphocyte percent
LY# Lymphocyte number
MO% Monocyte percent
MO# Monocyte number
EO% Eosinophil percent
EO# Eosinophil number
BA% Basophil percent
BA# Basophil number
RBC Red Blood Cell (erythrocyte) count
Hgb Hemoglobin concentration
Hct Hematocrit
MCV Mean Corpuscular Volume
MCH Mean Corpuscular Hemoglobin
MCHC Mean Corpuscular Hemoglobin Concentration
RDW Red Cell Distribution Width
Plt Platelet count
MPV Mean Platelet Volume
*PDW Platelet Distribution Width
*Pct Plateletcrit
®
HmX
1-2
PN 4237523A
USE AND FUNCTION
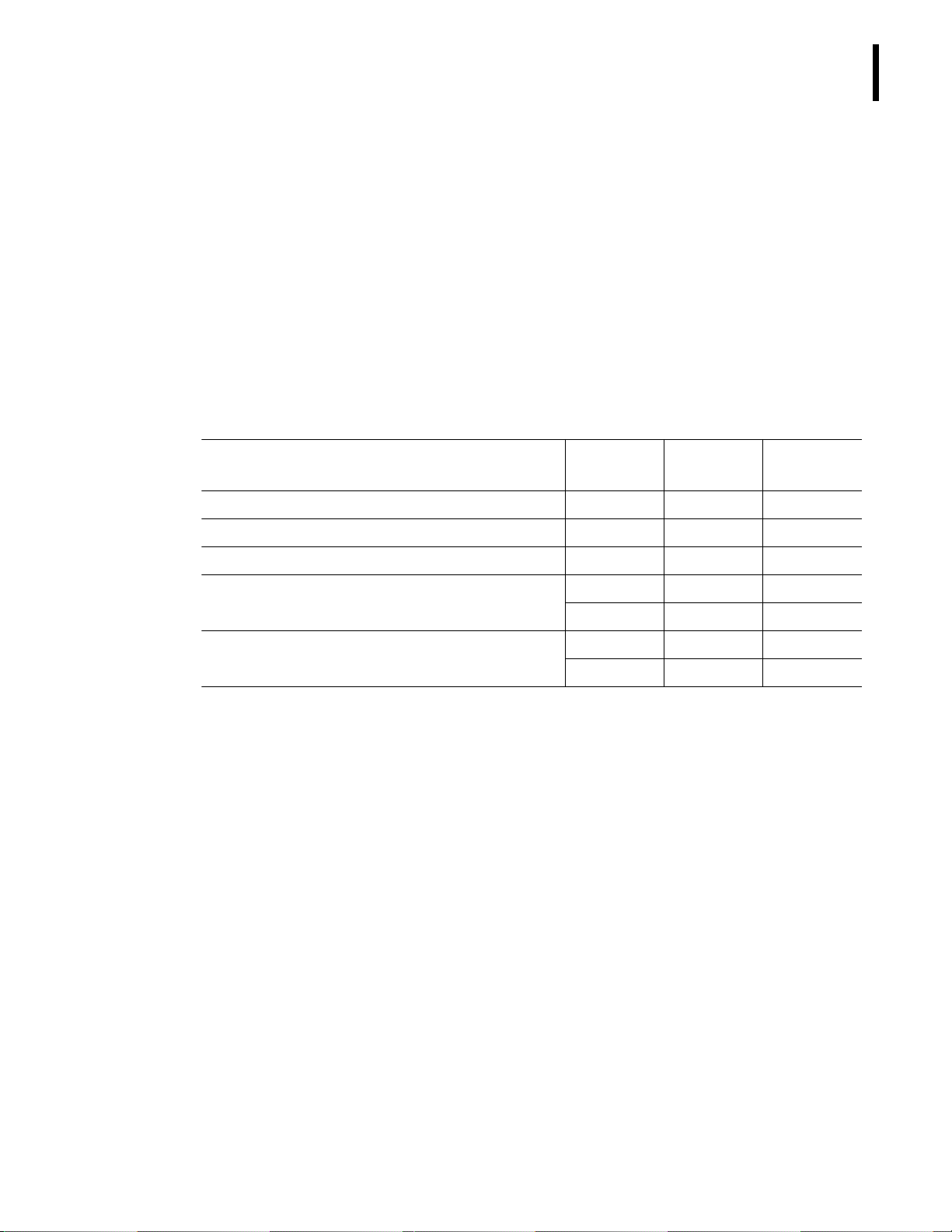
Table 1.1 CLIA Complexity Table
Analyte Category
Analyte
Identifier
CDC Test
System Code
Hematocrit (Hct)
Moderate 2514 10254
Hemoglobin (Hgb)
Moderate 2515 10254
Platelet count (Plt)
Moderate 4908 10254
Red blood cell count, erythrocyte count (RBC)
Moderate 5502 10254
Reticulocyte
Moderate 5506 10078
White blood cell count, leukocyte count (WBC)
Moderate 7002 10254
White blood cell differential, (WBC Diff)
Moderate 7001 10254
QUALITY CONTROL (QC)
RET% Reticulocyte percent
RET# Reticulocyte number
*In the USA, the PDW and Pct parameters are Not for Diagnostic Use. The value for PDW is
used as an internal check on the reported platelet parameters Plt and MPV.
Unless otherwise stated, all parameter results are shown in the US unit format throughout the
manuals.
1, 2, 3
CLIA Complexity Categories
See Table 1.1 for the CLIA complexity categories of the HmX Hematology Analyzer and HmX
Hematology Analyzer with Autoloader.
1
1.2 QUALITY CONTROL (QC)
Your laboratory can use these QC techniques with the HmX Hematology Analyzer:
r Daily instrument checks
r Commercial controls
r% Analysis
r Patient sample review
r Interlaboratory comparison (IQAP)
Quality Assurance (QA) can include a combination of these methods to provide complete
PN 4237523A
QC. Beckman Coulter manufactures commercial controls for monitoring performance of CBC
and differential parameters as well as monitoring flow cell alignment, gains, and VCS for
flow-cell volume, conductivity, and light scatter.
You can perform manual differentials as a measure of good QC practice or as recommended
by your laboratory, state, or federal protocol.
1-3

USE AND FUNCTION
METHOD HISTORY
1.3 METHOD HISTORY
Development
W.H. Coulter (1956) describes the Coulter Principle:
A suspension of blood cells is passed thru a small orifice simultaneously with an electric
current. The individual blood cells passing thru the orifice introduce an impedance
change in the orifice determined by the size of the cell. The system counts the individual
cells and provides cell size distribution. The number of cells counted per sample is
approximately 100 times greater than the usual microscope count to reduce the
statistical error by a factor of approximately 10 times.
This substantial improvement in precision over previous methods helped to establish the
erythrocyte count as a sensitive index of erythropoietic dyscrasia, particularly when
considered together with Hct and Hgb measurements.
The COULTER COUNTER® Model S analyzer was the first instrument that automated
simultaneous multiparameter measurements on blood. Brittin et al., Gottmann, and Hamilton
and Davidson, reviewed the performance and clinical value of the Model S.
4
5
6, 7, 8
Refinements of the COULTER COUNTER analyzer to provide accurate size (volume)
distribution data led to a reawakening of interest in pathological erythrocyte size distribution,
first aroused by Price-Jones in 1922.
9, 10
Among the advantages offered by the Coulter method of counting and sizing was the ability
to derive an accurate Hct measurement by summing the electronic volume of erythrocytes.
England et al. speculated that electronic Hct measurements did not have the trapped plasma
error of centrifugal Hct measurements.
11
Bull et al. described the use of a COULTER COUNTER analyzer for counting thrombocytes.12
This method, useful as it was, depended on preparing thrombocyte-rich plasma to avoid
counting erythrocytes as thrombocytes. Mundschenk et al. and Schulz and Thom discussed
the possibility of counting thrombocytes in the presence of erythrocytes and classifying them
13, 14
by size.
Electronic refinements in the Model S-PLUS™ enhanced the accuracy of the
hydrodynamic method. Von Behrens and Paulus also cited the feasibility of counting
thrombocytes by the Coulter method.
15, 16
Hemoglobinometry
The lytic reagent used for the complete blood count (CBC) parameters prepares the blood so
the system can count leukocytes and sense the amount of hemoglobin. The lytic reagent
rapidly and simultaneously destroys the erythrocytes and converts a substantial proportion of
the hemoglobin to a stable pigment while it leaves leukocyte nuclei intact. The absorbance of
the pigment is directly proportional to the hemoglobin concentration of the sample.
1-4
The accuracy of this method equals that of the hemiglobincyanide method, the reference
method of choice for hemoglobinometry recommended by the International Committee for
Standardization in Hematology (ICSH).
17
PN 4237523A

USE AND FUNCTION
METHOD HISTORY
Differential Measurement
The COULTER VCS established WBC differential technology using three measurements:
individual cell volume, high-frequency conductivity, and laser-light scatter.
The combination of low-frequency current, high-frequency current, and light-scattering
technology provides abundant cell-by-cell information that is translated by the instrument
into conventional stained film leukocyte categories. Correlation between the frequency of the
different cell types using stained film microscopy and this system is greater than 0.9 for
lymphocytes and granulocytes, and 0.7 for mononuclear cells.
Volume Analysis
Electronic leukocyte volume analysis, using low-frequency current, has been used since
18
1967.
count.
Conductivity Analysis
Cell walls act as conductors to high-frequency current. As the current passes through the cell
walls and through each cell interior, it detects differences in the insulating properties of cell
components. The current characterizes the nuclear and granular constituents and the
chemical composition of the cell interior.
It has been evaluated as a possible adjunct to the differential white cell
19, 20, 21, 22
23, 24, 25
1
Light Scatter Analysis
Beckman Coulter's experience in flow cytometry dates back decades to Fulwyler's pioneering
use of light scatter for cell analysis.
particle size and refractivity to the angle of light scattered from a laser beam.
26
Loken et al. and Jovin et al. discuss the relationship of
27
Reticulocyte (Retic) Analysis
Reticulocytes are immature, nonnucleated erythrocytes retaining a small network of
basophilic organelles, comprised of RNA and protoporphyrin. The enumeration of
reticulocytes provides a simple, effective means to determine red cell production and
regeneration.
28, 29, 30, 31
The most common means of measuring reticulocytes is to use supravital dyes, such as New
Methylene Blue or Brilliant Cresyl Blue. These dyes precipitate and aggregate the basophilic
substances within the reticulocyte, resulting in a granular, staining pattern easily seen with
light microscopy.
32
%Analysis
Dennis B. Dorsey, MD, proposed in 1963 that the relatively constant blood cell indices could
be used to follow the performance of hematology instrumentation.
improved the technique and named it % Analysis.
%
Analysis uses a "weighted moving average" of patient sample results because Koepke said
34
that QC materials "ideally should be similar in structure and in reactivity to the patient
constituent being measured, [and] therefore freshly drawn patient blood samples seem to be
35
the most appropriate [QC material]."
Bull explains, "The analyser [sic] is considered to be
‘in control’ when the MCV, MCH, and MCHC determined on a batch of 20 patients by use of
the % algorithm are within 3% of the expected mean indices of the population."
33
Brian Bull, MD,
36
PN 4237523A
1-5

USE AND FUNCTION
SYSTEM COMPONENTS
1.4 SYSTEM COMPONENTS
Main Unit
The Main Unit includes:
r A sample handler
r A Diluter for:
t The complete blood count (CBC)
t Leukocyte differential analysis (DIFF) or Reticulocyte analysis
r An Analyzer/Systems Control Module
r An Electronic Power Supply, and
r A Pneumatic Power Supply.
Sample Handler
All HmX Hematology Analyzer instruments have:
An automated, cassette-based transport for Primary mode -
r
HmX Hematology Analyzer with Autoloader.
OR
r An automated, closed-vial Primary mode of a rotary
cap-piercer - HmX Hematology Analyzer.
r An open-vial Secondary mode that uses a self-cleaning
manual aspirator tip.
r A bubble/blood detector.
r A bar-code reader.
Diluter
The Diluter is the primary mechanical operating unit of the system. It aspirates, pipets,
dilutes, mixes, lyses, and senses.
Analyzer/Systems Control Module
This module controls the timing and sequencing of the operating cycles. As it receives pulses
and raw data from both the CBC and VCS (diff) diluters, it counts, measures, and computes
parameters. It then sends this information to the DMS.
Electronic Power Supply
This unit supplies the necessary power for all instrument functions.
1-6
Pneumatic Power Supply
This unit supplies all air pressures and vacuums needed to operate the system.
PN 4237523A
USE AND FUNCTION
Data Management System (DMS)
The DMS controls instrument operation, displays, stores, and recalls sample data, and allows
the operator to perform quality control and calibration procedures. It stores patient and
quality-control data on the hard drive and allows bidirectional communication with a host
computer.
The System Version screen, Figure 1.3, displays the version number of the enabled software
and the features. If a feature is not enabled, the system displays *****.
Figure 1.3 System Version Display
Sample Analysis Controls Diluter Functions Special Functions
Diagnostics
Set UP
HmX SYSTEM VERSION 1.XXX
DMS SOFTWARE
INSTRUMENT SOFTWARE
SAMPLE HANDLER SOFTWARE
DIGIBOARD SOFTWARE
DILUTER TABLE
376 FIRMWARE
196 FIRMWARE
ANALYSIS ALGORITHM
RETICULOCYTE SOFTWARE
BI-DIREC HOST SOFTWARE
DATA PACKAGE SOFTWARE
002HL
03X93
03C60
03a00
01s4l
01B01
03A17
7.00
2.0ST
1.1
1.1
Control set up
Sample analysis set up
System set up
OPTIONS
1
1.5 OPTIONS
Printers
Up to two graphics printers can be added to your system to produce hard copy reports of
sample data.
1.6 REAGENTS
Beckman Coulter recommends these reagents or their equivalents. All stated performance
characteristics in this manual refer to the HmX Hematology Analyzer and HmX Hematology
Analyzer with Autoloader using these reagents.
Diluent
ISOTON® III (or ISOTON 4, Japan only) diluent is an isotonic electrolyte that:
r Dilutes the whole-blood samples.
r Stabilizes cell membranes for accurate counting and sizing.
r Conducts aperture current.
r Carries and focuses the sample stream in the flow cell to enable the WBC differential
measurements.
r Rinses the system between samples.
PN 4237523A
1-7

USE AND FUNCTION
REAGENTS
CBC Lytic Reagent
LYSE S® III diff (or LYSE S 4, Japan only) lytic reagent is a lytic reagent used for the CBC
mode. It:
r Rapidly lyses erythrocytes (RBCs), freeing hemoglobin (Hgb), and reducing the size of
r Causes a substantial conversion of the Hgb to a stable pigment, the absorbance of which
HmX PAK
The HmX Pak contains the PAK LYSE (Erythrolyse™ II erythrocyte lytic reagent) and the
PAK PRESERVE (StabiLyse™ leukocyte preservative) used for the differential measurement.
PAK LYSE
The PAK LYSE (also called the diff lytic reagent), while maintaining leukocytes (WBCs) in
near-native state:
cellular debris to a level that does not interfere with the leukocyte (WBC) count.
is directly proportional to the Hgb concentration over the clinical range.
Note: If you use LYSE S III diff lytic reagent you must use ISOTON III diluent. If you use
LYSE S 4 lytic reagent you must use ISOTON 4 diluent.
r Dilutes the blood samples.
r Rapidly lyses erythrocytes (RBCs).
r Reduces cellular debris to an insignificant level.
PAK PRESERVE
The PAK PRESERVE preserves the leukocytes (WBCs) in near-native state. It allows the
leukocytes to be differentiated into their subpopulations through the volume, conductivity,
and light-scatter measurements.
ReticPrep™ Reagent Kit
The COULTER ReticPrep reagent kit (see package insert) includes two reagents: Reagent A
and Reagent B. Use these reagents when preparing samples for reticulocyte analysis. Follow
the preparation instructions supplied with the kit.
Reagent A
Reagent A is a specially formulated, New Methylene Blue dye that stains the reticulum.
Reagent B
Reagent B is a clearing reagent that removes hemoglobin from the cell without removing the
precipitated stain-RNA complex, keeping the cell and its membranes intact. Reagent B needs
to be used with the repipetter dispenser available from Beckman Coulter, Inc.
1-8
Cleaning Agent
COULTER CLENZ® cleaning agent cleans and rinses the internal surfaces of the Diluter
components. Daily use prevents protein buildup and eliminates routine aperture bleaching.
PN 4237523A

1.7 CONTROLS AND CALIBRATOR
Controls
COULTER 5C® cell control monitors the CBC and differential parameters.
LATRON™ primer prepares the tubing and instrument components for the LATRON control.
LATRON control monitors the performance of the volume, conductivity, and light scatter
measurements.
Retic-C™ cell control monitors the Reticulocyte (Retic) parameters.
®
LIN-C
Calibrator
The S-CAL® calibrator kit calibrates Primary mode CBC parameters and is an acceptable
alternative to the whole-blood reference method of calibration.
S-CAL calibrator meets the requirements recommended by the International Committee for
the Standardization of Hematology (ICSH).
linearity control verifies the reportable range of the instrument’s CBC parameters.
USE AND FUNCTION
CONTROLS AND CALIBRATOR
1
The diff/retics measurement device is calibrated for optimum performance at the factory.
1.8 MATERIAL SAFETY DATA SHEETS (MSDS)
To obtain an MSDS for reagents used on the HmX Hematology Analyzer:
1. In the USA, either call Coulter Customer Operations (800-526-7694) or write to:
Coulter Corporation
Attn: MSDS Requests
P. O. Box 169015
Miami, FL 33116-9015
2. Outside the USA, call your Coulter Representative.
PN 4237523A
1-9

USE AND FUNCTION
MATERIAL SAFETY DATA SHEETS (MSDS)
1-10
PN 4237523A

2.1 GENERAL
INSTALLATION
2
2
CAUTION
Keep the system in its packaging until your Beckman Coulter Representative uncrates it for installation and
set up.
Your instrument is tested before it is shipped from the factory. International symbols and
special handling instructions on the cartons tell the carrier how to handle this electronic
system.
Carefully inspect all cartons when they arrive. If you see any sign of mishandling or damage,
file a claim with the carrier immediately. If the system is insured separately, file a claim with
the insurance company.
Possible system damage could occur if you improperly uncrate the system, install it, or set it up.
2.2 SPECIAL REQUIREMENTS
The system is intended for installation and operation in a conventional clinical laboratory
setting. Because the components are interrelated, you must determine the system location and
layout before your local Beckman Coulter Representative arrives to install the system.
Consider the following special requirements.
Space and Accessibility
In addition to the space required for the individual components and their interconnection,
consider:
r Comfortable working height.
r Access to the rear of the system for maintenance and service. Allow:
t 30 cm (12 in.) behind
t 30 cm (12 in.) on the sides.
Electrical Input
This system requires:
r An independent protected circuit.
r A ground path capable of carrying the full current of the circuit (confirmed third-wire,
earth ground).
r A female outlet:
t For the 110/120 V, 60 Hz model, it needs to furnish 120 ±10 Vac, 60 Hz, 15 A,
single-phase input power.
t For the 220/240 V model, it needs to furnish 220/240 ±10 Vac, 50/60 Hz, 8 A,
single-phase input power.
PN 4237523A
2-1

INSTALLATION
SPECIAL REQUIREMENTS
CAUTION
r Introduction of electrical interference can occur and cause instrument performance problems
r Overheating, melting, and burning of the extension cord can occur.
Plug the primary power cable directly into the electrical outlet. Position the system close enough to an
electrical outlet so you do not need to use an extension cord.
Either of these two hazards can occur if you use an extension cord:
(frequent lock ups and resets), or
Do not use an extension cord.
CAUTION
If you plan to use a power strip other than the one recommended by Beckman Coulter, call your Beckman
Coulter Representative to be sure that your power strip is compatible with your instrument.
Possible damage can occur if you use a power strip that is not compatible with your instrument.
Ambient Temperature and Humidity
Operate the system in a room with a temperature between 16° and 32°C (60° and 90°F) and
humidity no higher than 95% without condensation.
Ventilation
Arrange for the ventilation fan on the rear panel to be at least 12 cm (5 in.) from any walls or
obstructions that could interfere with the flow of air.
Air Conditioning
Compensate for system-generated heat in air-conditioned environments with an additional
5,000 Btus.
Drainage
CAUTION
than the recommended length. Contact your Beckman Coulter Representative if you need to increase the
length of the waste line supplied with the system
The maximum waste line length is 3.7 m (12 ft).
WARNING
tubing if not handled with care. Avoid skin contact. Clean up spills immediately. Dispose of the contents of
the waste container in accordance with local environmental regulations and with acceptable laboratory
procedures.
The waste line supplied with the instrument can be connected to either:
r A drain less than 76 cm (30 in.) above the floor.
r A waste container with a recommended minimum capacity of 20 L (5 gal.).
If you use an open drain, mechanically secure the waste tube into the drain so the tube cannot
accidentally come out of the drain. This prevents spillage.
Incomplete drainage and overflow into the vacuum system can occur if the waste line is longer
Biohazardous contamination can occur from contact with the waste container and its associated
2-2
PN 4237523A
 Loading...
Loading...