Coulter 5diff User manual
BECKMAN COULTER
™
AC•T™ 5diff Hematology Analyzer
Operator’s Guide
PN 4237615B (July 2000)
COULTER CORPORATION
A Beckman Coulter Company
Miami, Florida 33196-2500 USA
READ ALL PRODUCT MANUALS AND CONSULT WITH BECKMAN COULTER-TRAINED PERSONNEL
BEFORE ATTEMPTING TO OPERATE INSTRUMENT.
HAZARDS AND OPERATIONAL PRECAUTIONS AND LIMITATIONS
WARNINGS, CAUTIONS, and IMPORTANTS alert you as follows:
WARNING - Might cause injury.
CAUTION - Might cause damage to the instrument.
IMPORTANT - Might cause misleading results.
CAUTION System integrity might be compromised and operational failures might occur if:
r This equipment is used in a manner other than specified. Operate the instrument as instructed in the Product
Manuals.
r You introduce software that is not authorized by Beckman Coulter into your computer. Only operate your system’s
computer with software authorized by Beckman Coulter.
r You install software that is not an original copyrighted version. Only use software that is an original copyrighted
version to prevent virus contamination.
Beckman Coulter, Inc. urges its customers to comply with all national health and safety standards such as the use of barrier
protection. This may include, but it is not limited to, protective eyewear, gloves, and suitable laboratory attire when
operating or maintaining this or any other automated laboratory analyzer.
WARNING Risk of operator injury if all covers are not secured in place prior to instrument operation or you attempt to
replace a part without carefully reading the replacement instructions. Do not attempt to replace any component until you
carefully read the instructions for replacing the component.
IMPORTANT If you purchased this product from anyone other than Beckman Coulter or an authorized Beckman Coulter
distributor, and, if it is not presently under a Beckman Coulter service maintenance agreement, Beckman Coulter cannot
guarantee that the product is fitted with the most current mandatory engineering revisions or that you will receive the most
current information bulletins concerning the product. If you purchased this product from a third party and would like
further information concerning this topic, call your Beckman Coulter Representative.

Initial Issue, 03/00
Software version 0.11
Issue B, 07/00
Software version 1.0
REVISION STATUS
This document applies to the latest software listed and higher versions. When a subsequent software version changes the
information in this document, a new issue will be released.
PN 4237615B
iii

REVISION STATUS
iv
PN 4237615B

REVISION STATUS
INTRODUCTION, xvii
C
HOW TO USE YOUR A
•T 5diff HEMATOLOGY ANALYZER MANUALS, xvii
ABOUT THIS MANUAL, xvii
CONVENTIONS, xix
GRAPHICS, xix
SYMBOLS, xx
Safety Symbols, xx
Tab Symbols, xx
MENU TREE, xxi
1 USE AND FUNCTION, 1-1
1.1 INTENDED USE, 1-1
General, 1-1
Purpose, 1-1
Instrument Description, 1-1
Control Panel, 1-3
Back Panel, 1-4
Warning and Caution Labels, 1-4
Modes, 1-5
Parameters, 1-5
CBC Mode, 1-5
CBC/DIFF Mode, 1-6
Features, 1-6
Reports, 1-6
CONTENTS
1.2 CONTROLS AND CALIBRATORS, 1-7
Cell Controls, 1-7
Calibrator, 1-7
1.3 REAGENTS, 1-7
C
A
•T 5diff Diluent, 1-8
C
A
•T 5diff Fix, 1-8
C
A
•T 5diff WBC Lyse, 1-8
C
A
•T 5diff Hgb Lyse, 1-8
C
A
•T 5diff Rinse, 1-8
Waste Handling Procedures, 1-9
Neutralizing the Waste and Treating for Biohazards, 1-9
Handling Expired Reagents, 1-10
1.4 PRINTER, 1-10
1.5 RANGES, 1-10
PN 4237615B
v

CONTENTS
1.6 WORKING WITH THE SOFTWARE, 1-11
Moving the Cursor, 1-11
Selecting Menu Items, 1-11
Erasing Saved Text, 1-12
Selecting/De-selecting Software Fields, 1-12
1.7 PRESENTING SAMPLES TUBES (OR VIALS) AND STARTING ANALYSIS, 1-13
1.8 ORDERING MATERIAL SAFETY DATA SHEETS (MSDS), 1-13
2 OPERATION PRINCIPLES, 2-1
2.1 OVERVIEW, 2-1
2.2 MEASUREMENT PRINCIPLES, 2-1
Coulter Principle, 2-1
Aperture Sensor System, 2-1
Applying the Coulter Principle, 2-2
2.3 A
C
V TECHNOLOGY, 2-3
Dual Focused Flow (DFF), 2-3
Flowcell, 2-3
Focused Flow Impedance, 2-3
Absorbance Cytochemistry, 2-4
Signal Processing, 2-4
Thresholds, 2-4
2.4 WBC/BASO METHODOLOGY, 2-5
2.5 SAMPLE ANALYSIS OVERVIEW, 2-5
Aspiration, 2-5
Dilution, 2-6
CBC Mode, 2-7
CBC/DIFF Mode, 2-7
Delivery, 2-7
2.6 SAMPLE ANALYSIS, 2-8
RBC and Platelet Analysis, 2-8
Parameter Results Obtained from the RBC/Plt Dilution, 2-9
Hgb Measurement, 2-9
WBC Count and Differential, 2-10
Parameter Results Obtained from the WBC/BASO Dilution, 2-11
Differential, 2-11
Parameter Results Obtained from the DIFF Dilution, 2-12
Dilution Summary, 2-13
vi
PN 4237615B

2.7 PARAMETER DEVELOPMENT, 2-13
RBC Parameters, 2-13
RBC Count, 2-13
RBC Histogram, 2-14
Parameter Results Obtained Using the RBC Histogram, 2-14
MCH and MCHC Calculations, 2-15
Plt Parameters, 2-15
Interference on the Lower End of the Platelet Distribution Curve, 2-15
Microcytic Interferences on the Upper End of the Platelet Distribution Curve, 2-16
Parameter Results Obtained Using the Plt Histogram, 2-16
Hgb Determination, 2-17
WBC Count, BASO Count, and DiffPlot Development, 2-17
WBC Count, 2-17
BASO Count, 2-17
DiffPlot Development, 2-18
3 SPECIFICATIONS/CHARACTERISTICS, 3-1
3.1 INSTRUMENT SPECIFICATIONS, 3-1
Dimensions and Weight, 3-1
Power, 3-1
Supply, 3-1
Consumption, 3-1
Installation Category, 3-1
Grounding Requirements, 3-1
Temperature, Ambient Operating, 3-1
Altitude Range, 3-2
Recommended Location, 3-2
Electromagnetic Environment Check, 3-2
Recommended Reagents, 3-2
Recommended Controls, 3-2
Recommended Calibrator, 3-2
Recommended Anticoagulant, 3-2
Sample Volume Aspirated, 3-2
Dilution Ratios, 3-2
Throughput, 3-3
Sample Stability, 3-3
Sample Identification, 3-3
Output, 3-3
Measurements and Computation, 3-4
Counting Aperture Diameters, 3-4
Reagent Consumption, 3-4
Environmental Protection, 3-4
CONTENTS
3.2 PERFORMANCE SPECIFICATIONS, 3-5
Reproducibility, 3-5
Linearity, 3-5
Accuracy, 3-5
Carryover, 3-6
Reportable Range, 3-6
PN 4237615B
vii

CONTENTS
3.3 PERFORMANCE CHARACTERISTICS, 3-7
Reproducibility, 3-7
Accuracy, 3-8
Carryover, 3-8
3.4 LIMITATIONS, 3-8
Maintenance, 3-8
Blood Specimens, 3-8
3.5 INTERFERING SUBSTANCES, 3-9
4 PRECAUTIONS/HAZARDS, 4-1
4.1 DEFINITIONS, 4-1
Warnings, 4-1
Cautions, 4-1
Importants, 4-1
Attention, 4-1
4.2 SAFETY PRECAUTIONS, 4-1
Electronic, 4-1
Biological, 4-1
Moving Parts, 4-1
4.3 OPERATIONAL HAZARDS, 4-2
5 RUNNING SAMPLES, 5-1
5.1 BEFORE ANALYSIS, 5-1
Waste Container Level Check, 5-1
Printer Check, 5-1
Startup, 5-2
Startup During Power Up, 5-2
Startup After Power Up, 5-3
Specimen Collection and Mixing, 5-5
Running Cell Controls to Verify Calibration, 5-5
5.2 ANALYSIS, 5-8
Running Whole-Blood Samples, 5-8
5.3 AFTER ANALYSIS, 5-10
Results, 5-10
Printing Results for Last Sample Analyzed, 5-10
Auto-Clean, 5-11
5.4 SHUTDOWN, 5-11
5.5 ENTERING THE SAMPLE IDENTIFICATION (ID), 5-11
Auto-Numbering, 5-11
Manual Sample ID, 5-12
Scanning the Sample ID with the Barcode Reader, 5-13
viii
PN 4237615B

6 REVIEWING RESULTS, 6-1
6.1 GENERAL, 6-1
6.2 FLAGS AND INTERPRETIVE MESSAGES, 6-1
Flags, 6-1
Definition, 6-1
Types of Flags, 6-1
Types of Flag Printout Formats, 6-1
Interpretive Messages, 6-2
Definition, 6-2
6.3 FLAGS GENERATED BY THE INSTRUMENT, 6-2
Results Exceeding Instrument Capacity, 6-2
Hemoglobin Errors, 6-2
Hgb Blank Error, 6-2
Hgb Read Error, 6-2
Voteout Flag, 6-3
WBC Count Flag, 6-3
DiffPlot Flags, 6-3
CBC Flags, 6-8
Suspect or Detailed Flag Format, 6-11
Suspect Flag Format, 6-11
Detailed Flag Format, 6-12
Patient Ranges and Action Ranges, 6-12
CONTENTS
6.4 INTERPRETIVE MESSAGES, 6-12
WBC Interpretive Messages, 6-13
RBC Interpretive Messages, 6-13
Plt Interpretive Messages, 6-14
Combination WBC/RBC/Plt Interpretive Messages, 6-14
7CALIBRATION,7-1
7.1 GENERAL, 7-1
Recommended Calibration Conditions, 7-1
When to Calibrate, 7-1
When to Verify Calibration, 7-1
7.2 PRE-CALIBRATION CHECKS, 7-1
7.3 AUTO-CALIBRATION, 7-3
Calibration Setup, 7-3
Running Calibration, 7-5
Interpreting Calibration Results, 7-9
Forced Calibration, 7-9
7.4 MANUAL CALIBRATION FACTOR ADJUSTMENT, 7-11
7.5 PRINTING CALIBRATION FACTORS, 7-14
PN 4237615B
ix

CONTENTS
8 DIAGNOSTICS, 8-1
8.1 GENERAL MAINTENANCE, 8-1
8.2 MAINTENANCE SCHEDULE, 8-1
8.3 CLEANING PROCEDURES, 8-2
Cleaning the Outside of the Instrument, 8-2
Cleaning the Inside of the Instrument, 8-2
Extended Cleaning Procedure, 8-2
Auto-Clean, 8-4
Shutdown, 8-5
System Cleaning, 8-5
8.4 SYSTEM RESET CYCLE, 8-9
8.5 COMPONENT LOCATIONS, 8-10
8.6 SYSTEM TROUBLESHOOTING PROCEDURES, 8-12
Diluter System, 8-12
Backflush, 8-12
Bath and Flowcell Rinse, 8-13
Draining the Baths and/or the Diluent Reservoir, 8-14
Hardware System, 8-15
Hardware Reset, 8-15
Checking the Valves, 8-15
Checking the Motors, 8-15
8.7 REPLACEMENT PROCEDURES, 8-15
Replacing Reagents, 8-15
Viewing Reagent Levels, 8-16
Replacing the Diluent Reagent, 8-17
Replacing Fix, WBC Lyse, Hgb Lyse, and Rinse Reagents, 8-21
Priming the Reagents, 8-26
Replacing the Waste Container, 8-27
Replacing the Flowcell Lamp, 8-29
8.8 SYSTEM ERRORS, 8-34
What Error Messages Mean, 8-34
8.9 TROUBLESHOOTING GUIDES, 8-36
A INSTRUMENT SETUP, A-1
A.1 INSTALLATION, A-1
A.2 DEFAULT CONFIGURATION, A-1
A.3 CHANGES TO INSTRUMENT SETUP, A-2
A.4 LANGUAGE AND USA FIELD SELECTION, A-2
A.5 PASSWORD SETUP, A-3
x
PN 4237615B

A.6 DATE/TIME SETUP, A-4
Date Setup, A-4
Selecting the Date Format, A-4
Selecting the Time Format, A-5
Setting a New Date and Time, A-5
A.7 REPORTING UNIT SELECTION, A-7
A.8 LABORATORY LIMITS SETUP, A-8
Patient Ranges, A-8
Changing CBC Patient Ranges, A-9
Changing DIFF Patient Ranges, A-11
Action Ranges, A-13
Changing CBC Action Ranges, A-13
Changing DIFF Action Ranges, A-15
A.9 SETTING FLAG SENSITIVITY AND THRESHOLDS, A-17
A.10 PRINTER CONFIGURATION, A-18
Configuring the Instrument’s Printer Settings, A-18
Printing Options, A-20
CONTENTS
A.11 ENTERING/EDITING THE INSTITUTIONAL HEADER, A-21
A.12 PRINTING A SYSTEM SETUP REPORT, A-22
A.13 CALIBRATION SETUP, A-23
Changing CV% Limits, A-23
Defining the Operator, A-25
A.14 SELECTING THE SAMPLE IDENTIFICATION (ID) MODE, A-27
A.15 DISPLAYING DIFF # OR DIFF %, A-28
A.16 ENABLING ATL, IMM, PCT, AND PDW, A-28
A.17 RESETTING THE MANUAL SAMPLE ID NUMBER AND INSTRUMENT SEQUENCE
NUMBER TO “1”,A-29
A.18 SELECTING BARCODE WITH CHECKSUM, A-30
A.19 AUTO-CLEAN FREQUENCY SETTING, A-31
A.20 CHANGING THE DAILY WORKLOAD, A-32
A.21 REAGENT VOLUMES SETUP, A-33
A.22 VIEWING THE CYCLE COUNT, A-34
PN 4237615B
xi

CONTENTS
B LOG SHEETS, B-1
ACTION LOG, B-2
MAINTENANCE LOG, B-3
REAGENT LOG, B-4
CMANUAL CALIBRATION,C-1
C.1 ANALYSIS PROCEDURE, C-1
C.2 CALCULATIONS PROCEDURE, C-2
C.3 CALCULATING NEW CALIBRATION FACTORS, C-3
Calibration Worksheet, C-4
D TROUBLESHOOTING FLOWCHART, D-1
D.1 TROUBLESHOOTING FLOWCHART, D-1
E TRAINING CHECKLIST, E-1
E.1 INSTALLATION, E-1
E.2 GENERAL, E-1
E.3 SAMPLE HANDLING, E-1
E.4 INSTRUMENT COMPONENTS, E-1
E.5 SOFTWARE MENU, E-1
E.6 REAGENTS, E-1
E.7 INSTRUMENT SETUP/CUSTOMIZATION, E-2
E.8 CALIBRATION, E-2
E.9 CONTROLS, E-2
E.10 SYSTEM OPERATION OVERVIEW, E-2
E.11 DAILY PROCEDURES, E-2
E.12 SPECIAL PROCEDURES, E-3
E.13 MAINTAINING AND SERVICING THE INSTRUMENT, E-3
E.14 PAPERWORK, E-3
F BARCODE SPECIFICATIONS, F-1
F.1 OVERVIEW, F-1
Definition, F-1
xii
PN 4237615B

F.2 BARCODE LABELS, F-1
Symbologies, F-1
F.3 BARCODE SPECIFICATIONS, F-1
F.4 BARCODE LABEL TEST PAGES, F-3
F.5 BARCODE SCANNER CONFIGURATION, F-4
F.6 CODE 39 AND CODABAR BARCODE SCANNER OPTIONS, F-5
F.7 I 2-OF-5 PROGRAMMING OPTIONS AND TEST LABELS, F-7
REFERENCES, REFERENCES-1
LIST OF REFERENCES, REFERENCES-1
GLOSSARY, GLOSSARY-1
DEFINITIONS, GLOSSARY-1
CONTENTS
ABBREVIATIONS, ABBREVIATIONS-1
LIST OF ABBREVIATIONS, ABBREVIATIONS-1
INDEX, INDEX-1
TRADEMARKS
ILLUSTRATIONS
1.1 AC•T 5diff Analyzer, 1-1
1.2 Outside View of the Instrument, 1-2
1.3 Control Panel Buttons, 1-3
1.4 Back Panel, 1-4
1.5 Warning and Caution Labels on the Instrument, 1-4
2.1 Coulter Principle, 2-2
2.2 Dual Focused Flow Process, 2-3
2.3 Signal Processing, 2-4
2.4 BASO Thresholds, 2-5
2.5 Sample Partitions Inside the Probe - CBC/DIFF Mode, 2-6
2.6 Sample Partitions Inside the Probe - CBC Mode, 2-6
2.7 Bath Assembly, 2-6
2.8 Sample Delivery Using Tangential Flow, 2-7
2.9 Bath Assembly, 2-8
2.10 Bath Assembly, 2-10
2.11 Flowcell Operation, 2-11
2.12 DiffPlot Regions, 2-12
2.13 Typical RBC Histogram, 2-14
2.14 Typical Plt Histogram, 2-15
2.15 Area of the Plt Histogram Used to Determine the PDW Parameter Result, 2-16
2.16 Areas Used to Determine WBC and BASO Parameter Results, 2-17
2.17 DiffPlot Regions, 2-18
PN 4237615B
xiii

CONTENTS
3.1 Instrument Dimensions and Weight, 3-1
3.2 Sample Report, 3-3
5.1 Sample Report, 5-10
6.1 WBC/BASO Histogram Flags: CBC Mode, 6-8
6.2 WBC/BASO Histogram Flags: CBC/DIFF Mode, 6-8
6.3 MICRO and MACRO Regions on RBC Histogram, 6-9
6.4 Plt Flags, 6-10
6.5 Mobile Threshold Positioned in the Standard Regions (Between 18fL and 25fL), 6-10
6.6 Mobile Threshold Cannot Be Positioned in the Standard Region, 6-10
6.7 Mobile Threshold Cannot Be Positioned, 6-10
7.1 Out of Range Calibration Factors, 7-9
8.1 View of the Pneumatics Area (Right Side), 8-10
8.2 Bath Assembly, 8-11
8.3 View Behind Motherboard (Left Side), 8-11
8.4 Motherboard, 8-12
8.5 Reagent Bottle Location, 8-16
8.6 Waste Sensor Alarm Unit Location, 8-27
A.1 Sample Results Report: Areas Defined, A-19
D.1 Troubleshooting Flowchart, D-1
TABLES
1.1 CBC Parameters, 1-5
1.2 CBC/DIFF Parameters, 1-6
2.1 A
2.2 Technical Characteristics for Obtaining RBC and Platelet Counts, 2-8
2.3 Technical Characteristics for the Measurement of the Hemoglobin, 2-9
2.4 Characteristics Required to Obtain WBC/BASO Results, 2-10
2.5 Technical Characteristics for Acquisition of the DiffPlot, 2-12
2.6 Summary of Dilutions, 2-13
2.7 DiffPlot Regions Defined, 2-19
2.8 Immature White Blood Cells, 2-20
3.1 Reagent Consumption by Cycle in mL, 3-4
3.2 Reproducibility Specifications, 3-5
3.3 Linearity Specifications, 3-5
3.4 Accuracy Specifications, 3-5
3.5 Carryover Specifications, 3-6
3.6 Reportable Range, 3-6
3.7 Reproducibility Characteristics From a Normal Sample with a Low Normal WBC Count, 3-7
3.8 Reproducibility Characteristics From a Normal Sample with a High Normal WBC Count, 3-7
3.9 Accuracy Characteristics, 3-8
3.10 Carryover Characteristics, 3-8
3.11 Interfering Substances, 3-9
6.1 Definition of DIFF Flags, 6-4
6.2 CBC Histogram Flags, 6-8
6.3 Patient Range and Action Range Flags, 6-12
6.4 WBC Interpretive Messages from Action Ranges, 6-13
6.5 WBC Interpretive Messages from DiffPlot, 6-13
6.6 RBC Interpretive Messages from Action Ranges, 6-13
6.7 RBC Interpretive Messages from Flag Sensitivity, 6-14
C
•T 5diff Analyzer: Measurement Technologies, 2-1
xiv
PN 4237615B

6.8 Plt Interpretive Messages from Action Ranges, 6-14
6.9 Plt Interpretive Messages from the Plt Histogram, 6-14
6.10 Interpretive Messages from a Combination of WBC/RBC/Plt Action Ranges, 6-14
6.11 NRBCs and PLATELET AGGREGATES Interpretive Messages, 6-15
7.1 Calibration Factors Range, 7-13
8.1 Maintenance Schedule, 8-1
8.2 Error Messages, 8-34
8.3 Troubleshooting Guide, 8-36
A.1 Instrument Default Settings, A-1
A.2 Reporting Unit Format, A-7
A.3 CBC Default Patient Ranges, A-9
A.4 DIFF Default Patient Ranges, A-11
A.5 CBC Default Action Ranges, A-13
A.6 DIFF Default Action Ranges, A-15
A.7 Default CV Limits, A-23
A.8 Daily Workload Runs by Mode, A-32
A.9 Default Reagent Volumes, A-33
F.1 Default Barcode Settings, F-2
F.2 Test Labels With the Check Digit (Checksum), F-3
F.3 Test Labels Without the Check Digit, F-3
F.4 Barcode Scanner Configuration Sheet, F-4
F.5 Code 39 Barcode Scanner Options, F-5
F.6 Codabar Barcode Scanner Options, F-6
F.7 Interleaved 2-of-5 Options With Fixed Length Characters Test Labels, F-7
CONTENTS
PN 4237615B
xv

CONTENTS
xvi
PN 4237615B

This introductory section contains the following topics:
r HOW TO USE YOUR AC•T 5diff HEMATOLOGY ANALYZER MANUALS,
r ABOUT THIS MANUAL,
r CONVENTIONS,
r GRAPHICS
r SYMBOLS, and
r MENU TREE.
HOW TO USE YOUR AC•T 5diff HEMATOLOGY ANALYZER MANUALS
Use this Operator’s Guide to find information about:
r Getting started,
r Running your instrument,
r Reviewing results,
r Performing special procedures, such as cleaning, replacing, or adjusting an instrument
component,
r Troubleshooting problems,
INTRODUCTION
r Determining what the instrument does,
r Understanding how to safely operate the instrument,
r Powering up the instrument,
r Customizing the setup, and
r Running controls and samples.
Use the Host Transmission Specification manual (PN 4277065) to find out information about
interfacing your A
ABOUT THIS MANUAL
The information in this manual is organized as follows:
r Chapter 1, USE AND FUNCTION
Contains the intended use of the instrument, a brief history of the methods used by the
instrument, the reagents, calibrators, and controls used, a brief description of the major
components, and how to work with the software.
r Chapter 2, OPERATION PRINCIPLES
Contains the descriptions for cell counting and voting and how the parameters are
derived.
r Chapter 3, SPECIFICATIONS/CHARACTERISTICS
Details instrument specifications, characteristics, and interfering substances.
C
•T 5diff analyzer to your laboratory’s host computer.
PN 4237615B
r Chapter 4, PRECAUTIONS/HAZARDS
Provides information about key safety issues and contains information on biological
hazards and hazards pertaining to moving parts.
r Chapter 5, RUNNING SAMPLES
Provides information on how to run patient blood samples.
xvii

INTRODUCTION
ABOUT THIS MANUAL
r Chapter 6, REVIEWING RESULTS
Provides information on reviewing flagged sample results.
r Chapter 7, CALIBRATION
Provides procedures for calibrating the instrument, including manually adjusting the
calibration factors.
r Chapter 8, DIAGNOSTICS
Provides information about special procedures and troubleshooting procedures for the
instrument. Includes topics such as a maintenance schedule, cleaning and replacement
procedures, and what error messages mean.
r Appendix A, INSTRUMENT SETUP
Provides procedures on customizing the instrument’s settings, such as date/time,
reporting units, laboratory limits, and others.
r Appendix B, LOG SHEETS
Contains log sheets for your laboratory’s use.
r Appendix C, MANUAL CALIBRATION
Provides a procedure for manually calibrating the instrument.
r Appendix D, TROUBLESHOOTING FLOWCHART
Provides supplemental troubleshooting information.
r Appendix E, TRAINING CHECKLIST
Summarizes what must be done after the instrument is installed.
r Appendix F, BARCODE SPECIFICATIONS
Defines the specifications that barcode labels must meet for use with the instrument.
r REFERENCES
Lists references used in this manual.
r GLOSSARY
Defines terminology used in this manual.
r ABBREVIATIONS
Defines abbreviations used in this manual.
r INDEX
Provides page numbers for indexed information.
xviii
PN 4237615B

CONVENTIONS
This manual uses the following conventions:
r Main Menu refers to the initial menu
r When instructed to make a menu selection, the text appears in bold with two symbols
INTRODUCTION
CONVENTIONS
displayed on the instrument after
16:0512/07/99MAIN MENU
Startup.
1 - RUN SAMPLES
2 - CALIBRATION
3 - REAGENTS
4 - DIAGNOSTICS
5 - SETUP
to distinguish the menu path. For example, if instructed to choose Calibration, then
Autocalibration, the text will appear as
CALIBRATION
AUTOCALIBRATION.
tt
GRAPHICS
r
Bold font
indicates a menu option, such as
SETUP
.
r Italics font indicates screen text displayed on the instrument, such as
Calibration Passed.
Bold, italics font
r
be instructed to do the Startup procedure, which would appear as “Do
r Instrument refers to the A
indicates a heading name within this document. For example, you may
Startup
”.
C
•T 5diff hematology analyzer.
r A Note contains information that is important to remember or helpful when
performing a procedure.
r Motherboard refers to the main card (board) in the instrument.
r RBC bath is sometimes referred to as RBC/Plt bath.
C
r A
r A
r A
r A
r A
•T 5diff Rinse reagent is sometimes referred to as Rinse.
C
•T 5diff Fix reagent is sometimes referred to as Fix.
C
•T 5diff Hgb Lyse reagent is sometimes referred to as Hgb Lyse.
C
•T 5diff WBC Lyse reagent is sometimes referred to as WBC Lyse.
C
•T 5diff Diluent reagent is sometimes referred to as Diluent.
All graphics, including screens and printouts, are for illustration purposes only and must not
be used for any other purpose.
PN 4237615B
xix

INTRODUCTION
SYMBOLS
SYMBOLS
Safety Symbols
Safety symbols alert you to potentially dangerous conditions. These symbols, together with
text, apply to specific procedures and appear as needed throughout this manual.
Symbol Warning Condition
Biohazard
!
!
!
(specimens, reagents, controls, and
calibrators, and so forth) and areas
these materials come into contact
with as being potentially infectious.
Probe hazard.
may contain biohazardous materials,
such as controls and calibrators.
Electrical shock hazard
electrical shock when instrument is
plugged in to the power source.
.
Consider all materials
The probe is sharp and
.
Possibility of
Action
Wear standard laboratory attire and
follow safe laboratory procedures
when handling any material in the
laboratory.
Avoid any unnecessary contact with
the probe and probe area.
Before continuing, unplug the
C
A
•T 5diff analyzer from the
electrical outlet.
Tab Symbols
Tabs divide this document into four sections: reference, operation, special procedures and
troubleshooting, and appendices. Each tab reflects a unique symbol.
Symbol Definition
Identifies the reference section.
xx
Identifies the operating instructions section.
Identifies the special procedures and troubleshooting section.
Identifies the appendices section.
PN 4237615B
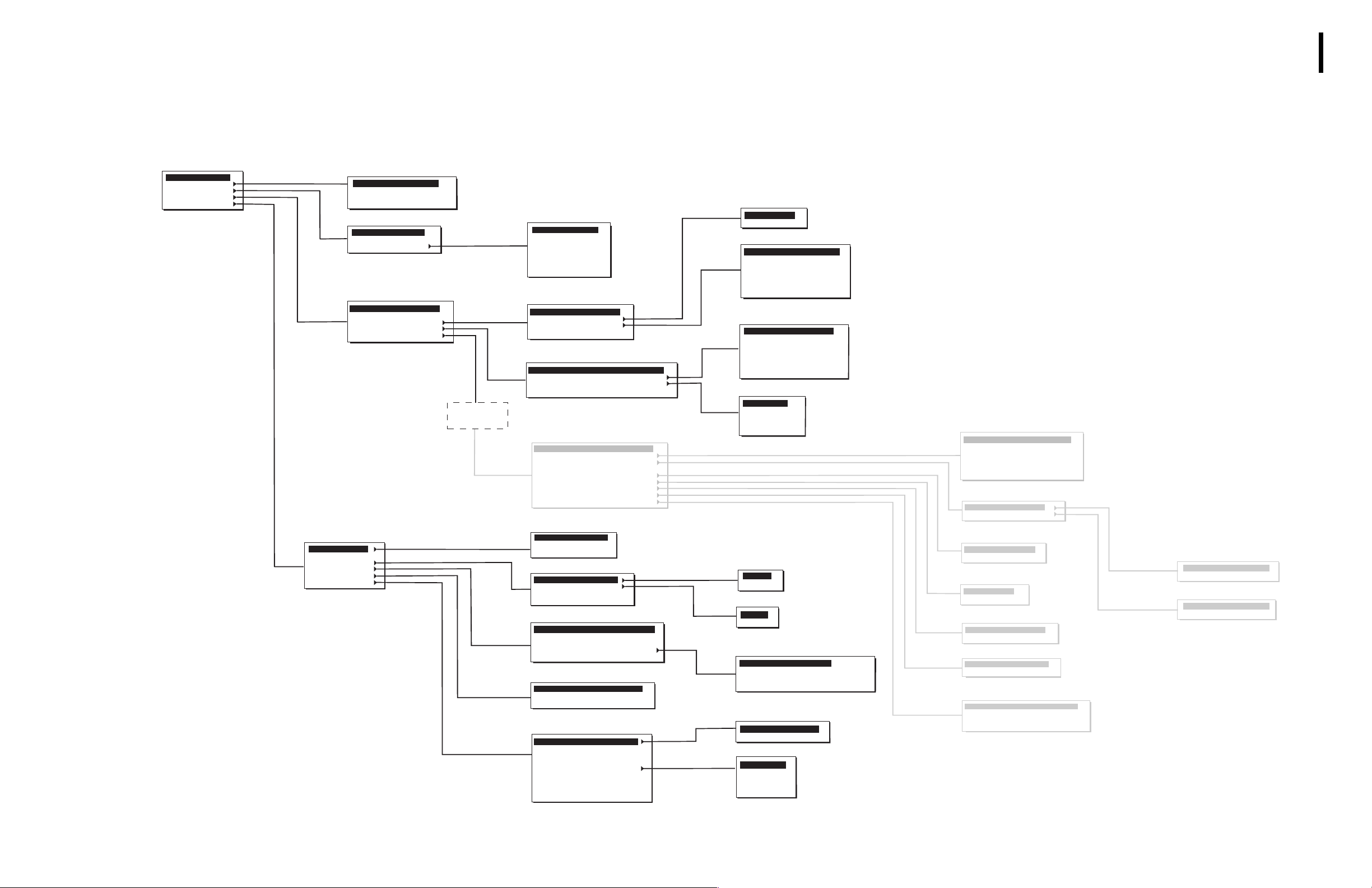
MENU TREE
The functions of the instrument are programmed into its software.
MENU TREE
MAIN MENU
1 - RUN SAMPLES
2 - CALIBRATION
3 - REAGENTS
4 - DIAGNOSTICS
5 - SETUP
SETUP
1 - DATE / TIME
2 - UNITS
3 - LAB. LIMITS
4 - HOST SETUP
5 - PRINTER
6 - OTHERS
* Consult a Beckman Coulter
representative before selecting this
option.
Service password required
CALIBRATION
1 - AUTOCALIBRATION
2 - CAL FACTORS
3 - PRINT CAL FACTORS
4 - REPRODUCIBILITY
REAGENTS
1 - LEVEL-CHANGE
2 - DAILY WORKLOAD
3 - PRIME
DIAGNOSTICS
1-SYSTEM RESET CYCLE
2-MINI PRIME
3-DILUTER SYSTEMS
4-HARDWARE SYSTEMS
5-SERVICE
SERVICE
PASSWORD
REQUIRED
PRIME
1- DILUENT
2 - FIX
3 - WBC LYSE
4 - HGB LYSE
5 - RINSE
6 - ALL REAGENTS
7 - UNPRIME ALL
DILUTER SYSTEMS
1-BACKFLUSH
2-RINSE
3-DRAIN BATHS
4-EXTENDED CLEANING
HARDWARE SYSTEMS
1-HARDWARE RESET
2-MOTORS *
3-VALVES *
4-TRAVERSE SERVICE POSITION
SERVICE
1 - DILUTION
2 - MEASUREMENT
3 - HEATING SYSTEMS
4 - MIXING
5 - SENSOR CHECK
6 - VACUUM CHECK
7 - BURN-IN
8 - FLOWCELL WBC CALIBRATION
9 - OTHERS
SET DATE / TIME
1 - TIME FORMAT
2 - DATE FORMAT
3 - SET DATE & TIME
LAB. LIMITS
1 - PATIENT RANGES
2 - ACTION RANGES
3 - FLAGS SENSITIVITY
4 - THRESHOLDS
HOST SETUP
1 - HOST SETUP CONFIGURATION
2 - SENDING CONFIGURATION
3 - SENDING OPTIONS
4 - VARIABLE FORMAT SETUP
5 - SEND LATEST RESULT
PRINTER
1 - PRINTER CONFIGURATION
2 - INSTITUTIONNAL HEADER
3 - PRINT LATEST RESULT
OTHERS
1 - CALIBRATION
2 - IDENTIFICATION MODE
3 - AUTOCLEAN FREQUENCY
4 - CHANGE PASSWORD
5 - LANGUAGE
6 - REAGENT VOLUMES
7 - CYCLE COUNTS
8 - PRINT SYSTEM SETUP
9 - SEND SETUP
RINSE
1 - BATHS
2 - FLOWCELL
DRAIN BATHS
1 - RINSE
2 - FIRST DILUTION
3 - DIFF
4 - WBC / BASO
5 - RBC / PLT
6 - ALL BATHS
7 - DILUENT RESERVOIR
MOTORS
1 - SAMPLING PROBE
2 - TRAVERSE
3 - SAMPLING SYRINGE
4 - DRAINING SYRINGE
5 - COUNTING SYRINGE
6 - FLOWCELL SYRINGES
7 - DILUTION SYRINGES
VALVES
1 - 1 TO 11
2 - 12 TO 16
3 - 17 AND 18
4 - 20 TO 26
5 - 27 TO 31
PATIENT RANGES
1 - CBC
2 - DIFF
ACTION RANGES
1 - CBC
2 - DIFF
VARIABLE FORMAT SETUP
1 - NUMERICAL RESULTS
2 - FLAGS AND MESSAGES
3 - HISTOGRAMS AND THRESHOLDS
4 - PATIENT FILE
CALIBRATION
1 - CV% L I M I TS
2 - DEFINE OPERATOR
LANGUAGE
1 - ENGLISH
2 - FRENCH
3 - GERMAN
4 - SPANISH
5 - ITALIAN
MEASURMENT
1 - HGB BLANK ADJUSTMENT
2 - APERTURE CURRENT
3 - RBC / PLT GAIN
4 - WBC / BASO GAIN
5 - DIFF ADJUSTMENT
6 - PULSE ADJUSTMENT
HEATING SYSTEMS
1 - HEATING COIL
2 - BATH ENCLOSURE
SENSOR CHECK
1 - DRAINING
2 - DIFF TRANSFER
VACUUM CHECK
1 - COUNTING
2 - DRAINING
BURN - IN
1 - BURN - IN CYCLES
2 - ANALYSIS CYCLES
FLOWCELL WBC CALIBRATION
1 - AUTOCALIBRATION
2 - CAL FACTORS
OTHERS
1 - USER MODE
2 - CYCLE COUNTS
3 - PARK SYRINGES
4 - RESET TO DEFAULT VALUES
HEATING COIL
1 - ADJUSTMENT
2 - REFERENCE
BATH ENCLOSURE
1 - ADJUSTMENT
2 - REFERENCE
7615022B
PN 4237615B
xxi

MENU TREE
xxii
xxii
PN 4237615B

1.1 INTENDED USE
General
USE AND FUNCTION
1
1
C
The Beckman Coulter A
hematology analyzer (Figure 1.1) is a
26-parameter, fully automated hematology
analyzer, including a five-part leukocyte
differential counter.
Of the 26 reported parameters:
r 20 parameters are
Diagnostic Use
MCV, MCH, MCHC, RDW, Plt, MPV,
NE%, NE#, LY%, LY#, MO%, MO#,
EO%, EO#, BA%, and BA#.
r 6 parameters are qualitative and are
For Research Use Only. Not for use in
diagnostic procedures
IMM%, IMM#, ATL%, and ATL#.
•T 5diff
For In Vitro
: WBC, RBC, Hgb, Hct,
.: Pct, PDW,
Figure 1.1 AC•T 5diff Analyzer
Purpose
The purpose of the AC•T 5diff hematology analyzer is to identify normal patient results with
all normal system-generated parameters and to flag or identify patient results that require
additional studies.
PN 4237615B
Instrument Description
r Figure 1.2 shows the outside of the instrument.
r Figure 1.3 shows the control panel.
r Figure 1.4 shows the back panel.
r Figure 1.5 shows the warning and caution labels on the instrument.
WARNING
operate the instrument. Ensure that all covers and doors are closed and secured before operating the
instrument.
Risk of operator injury when covers and doors are not closed and secured in place before you
1-1

USE AND FUNCTION
g
1
INTENDED USE
Figure 1.2 Outside View of the Instrument
)
b
c
d
e
LCD (liquid crystal display) screen
b
Control panel: allows you to
c
interface with the instrument. See
Control Panel
Door to reagents: allows you to
d
access the reagent bottles on
board.
Top cover
e
Door to pneumatics: allows you to
f
access the hydraulic parts for
maintenance procedures.
: The system will not operate
Note
when this door is open.
Aspirate switch: allows you to
g
start an analysis cycle.
for details.
Aspirate (sample) probe: aspirates
h
sample or control material from
tubes or vials.
Green LED (light-emitting diode):
i
f
j
i
indicates the instrument is ready.
Red LED: indicates the instrument
j
is busy.
ON/OFF switch
1)
h
1-2
PN 4237615B

USE AND FUNCTION
Control Panel
Use the control panel buttons (Figure 1.3) to setup and operate the instrument.
INTENDED USE
1
Figure 1.3 Control Panel Buttons
bcd efg
2
ESC
RANGE
1
4
7
0
3
5
6
8
CBC
DIFF
9
DEL
hi
2
1
4
7
0
3
5
6
8
9
j
2
1
4
7
0
1)
3
5
6
8
9
1!
7615006A
b
procedure, followed by a background count.
performs a prime and rinsing
Startup
c
Shutdown
typically done at the end of the day. The
instrument remains in stand-by mode with
the Rinse.
d
ESC
Escape
executing it and goes to the previous screen.
CBC
e
DIFF
Mode
CBC/DIFF modes.
performs a cleaning,
exits a function without
allows you to select CBC and
f
allows you to print the last sample
Print
result, calibration results, laboratory limits,
and so forth.
g
DEL
deletes the entered information.
Delete
h
executes a function or enters
Enter
data.
i
RANGE
used.
Cursor keys
j
and allow you to scroll through the alphabet
when entering information.
selects the flagging range to be
Range
move the cursor on the screen
PN 4237615B
Numeric keypad
1)
allows you to enter
numbers for dates, values, limits, sample
IDs, and to select menu items.
. Allows you to enter the decimal
Period
1!
number separator and to select/de-select
software options.
1-3

USE AND FUNCTION
INTENDED USE
Back Panel
Figure 1.4 shows the instrument’s back panel.
Figure 1.4 Back Panel
MOD
NO.
ASSY
S/N
xxxxxx
xxxxxx
NO.
50/60100-240
VOLTS
HZ AMPS
MANUFACTURED BY COULTER CORPORATION
A BECKMAN COULTER COMPANY
B
ECKMAN
11800 SW 147 AVENUE, MIAMI, FLORIDA 33196-2500 U.S.A.
OULTER
C
PATTENTS ISSUED AND/OR PENDING
AUTOMATED DIFFERENTIAL CELL COUNTER
FOR IN VITRO DIAGNOSTIC USE
CAUTION:
TO REDUCE THE RISK OF ELECTRICAL SHOCK DO NOT REMOVE THE COVER
OR BACK.
REFER SERVICING TO QUALIFIED SERVICE PERSONNEL.
ELECTRIC SHOCK HAZARD. DISCONNECT UNIT FROM POWER SOURCE
PRIOR TO SERVICING.
FOR CONTINUED PROTECTION AGAINSTR FIRE HAZARD, REPLACE ONLY
WITH SAME TYPE AND RATING OF FUSE.
FOR SAFETY REASONS, EQUIPMENT REQUI4RES CONNECTION TO
PROTECTIVE EARTH GROUND.
Serial number label
b
Barcode reader connector
c
b
Printer connector
d
WATTS
c
d
Host RS 232 C Output connector
e
Power supply cord connector
f
Waste output connector
g
e
Diluent input connector
h
h
g
Warning and Caution Labels
Pay close attention to the labels on the instrument (Figure 1.5).
Figure 1.5 Warning and Caution Labels on the Instrument
MOD
NO.
ASSY
xxxxxx
NO.
VOLTS
B
ECKMAN
C
OULTER
AUTOMATED DIFFERENTIAL CELL COUNTER
FOR IN VITR O D IAG N O ST IC US E
CAUTION:
TO REDUCE THE RISK OF ELECTRICAL SHOCK DO NOT REMOVE THE COVER
OR BACK.
REFER SERVICING TO QUALIFIED SERVICE PERSONNEL.
ELECTRIC SHOCK HAZARD. DISCONNECT UNIT FROM POWER SOURCE
PRIOR TO SERVICING.
FOR CONTINUED PROTECTION AGAINSTR FIRE HAZARD, REPLACE ONLY
WITH SA M E TYPE A ND RA T IN G OF FUS E.
FOR SAFETY REASONS, EQUIPMENT REQUI4RES CONNECTION TO
PROTECTIVE EARTH GROUND.
f
MOD
c
T 5diff
A
•
S/N
xxxxxx
50/60100-240
WATTS
HZ AMPS
MANUFACTURED BY COULTER CORPORATION
A BECKMAN COULTER COMPANY
11800 SW 147 AVENUE, MIAMI, FLORIDA 33196-2500 U.S.A.
PATTENTS ISSUED AND/OR PENDING
NO.
ASSY
xxxxxx
NO.
VO LTS
CAUTIO N:
T O R E D U C E TH E R IS K O F E LE C T R IC A L S H O C K D O N O T R E M O V E T H E C O V E R
OR BACK.
REFER SERVICING TO QUALIFIED SERVICE PERSONNEL.
ELECTRIC SHOCK HAZARD. DISCONNECT UNIT FROM POW ER SOURCE
PRIO R TO SERVICING .
FOR CONTINUED PROTECTION AG AINSTR FIRE HAZARD, REPLACE O NLY
W IT H S A M E T Y P E A N D R A T IN G O F F U S E .
FOR SA FETY REASO NS, EQUIPMEN T REQU IRES CONNECTIO N TO
PROTECTIVE EARTH G ROUND.
50-60100-240
B
ECKMAN
C
OULTER
AUTOMATED DIFFERENTIA L CELL CO UNTER
F O R IN V IT R O D IA G N O S T IC U S E
S/N
xxxxxx
0.9-2.0 200
HZ AMPS
MANUFACTURED FOR BECKMAN COULTER INC.
11800 SW 147 AVENUE, MIAMI, FLORIDA 33196-2500 U.S.A.
PATENTS ISSUED AND/OR PENDING
MADE IN FRANCE
WATTS
1-4
T H IS A R E A M A Y C O N T A IN
B IO H A Z A R D O U S M A T E R IA L
REFER TO PRODUICT REFERENCE
MANUAL FOR PROPER HANDLING
ALL CO VER S/PANELS M UST BE
SECUR ED IN PLACE PRIO R TO
INSTRUM ENT OPER ATIO N.
REFER TO PRODUCT REFERENCE
MANUAL FOR PROPER INSTALLATIO N.
2429555
CAUTIO N
PN 4237615B

USE AND FUNCTION
Modes
The instrument has two modes of analysis: CBC and CBC/DIFF. For information on the
parameters of each mode, see
Parameters
.
Parameters
CBC Mode
Table 1.1 lists the 12 parameters analyzed in the CBC mode.
Table 1.1 CBC Parameters
Parameter Definition
WBC White Blood Cell or leukocyte count
RBC Red Blood Cell or erythrocyte count
Hgb Hemoglobin concentration
Hct Hematocrit (relative volume of erythrocytes within the whole-blood
sample)
INTENDED USE
1
MCV Mean Corpuscular (erythrocyte) Volume
MCH Mean Corpuscular (erythrocyte) Hemoglobin
MCHC Mean Corpuscular (erythrocyte) Hemoglobin Concentration
RDW Red Cell (erythrocyte) Distribution Width
Plt Platelet or thrombocyte count
MPV Mean Platelet Volume
†
PDW
†
Pct
†
Pct and PDW are derived parameters and are For Research Use Only. Not for use in diagnostic procedures.
Platelet Distribution Width
Plateletcrit
PN 4237615B
1-5

USE AND FUNCTION
INTENDED USE
CBC/DIFF Mode
Table 1.2 lists the 26 parameters analyzed in the CBC/DIFF mode:
Table 1.2 CBC/DIFF Parameters
Parameter Definition
WBC White Blood Cell or leukocyte count
NE%: Neutrophil percentage
NE#: Neutrophil number,
LY%: Lymphocyte percentage,
LY#: Lymphocyte number,
MO%: Monocyte percentage,
MO#: Monocyte number
EO%: Eosinophil percentage,
EO#: Eosinophil number,
BA%: Basophil percentage,
BA#: Basophil number
†
IMM%
IMM#
ATL%
ATL#
: Immature cell percentage
†
: Immature cell number
†
: Atypical lymphocyte percentage
†
: Atypical lymphocyte number
RBC Red Blood Cell or erythrocyte count
Hgb Hemoglobin concentration
Hct Hematocrit (relative volume of erythrocytes within the whole-blood sample)
MCV Mean Corpuscular (erythrocyte) Volume
MCH Mean Corpuscular (erythrocyte) Hemoglobin
MCHC Mean Corpuscular (erythrocyte) Hemoglobin Concentration
RDW Red Cell (erythrocyte) Distribution Width
Plt Platelet or thrombocyte count
MPV Mean Platelet (thrombocyte) Volume
PDW
Pct
†
†
Platelet Distribution Width
Plateletcrit
†
Derived parameters are For Research Use Only. Not for use in diagnostic procedures.
Features
Features of the instrument include automated calibration, one-button aspiration with probe
wipe, 12- or 26-parameter analysis with histograms and DiffPlots, and alphanumeric or
barcode patient sample identification.
Reports
Patient sample reports are printed based on your instrument setup.
1-6
PN 4237615B

1.2 CONTROLS AND CALIBRATORS
Cell Controls
AC•T 5diff Control is available in three levels (low, normal, and high) to provide a stable
reference control for use with this instrument.
Calibrator
AC•T 5diff Cal Calibrator is a recommended alternative to the whole-blood reference method
of calibration and is traceable to reference methods and materials. Use A
Calibrator to ensure accurate instrument measurements for WBC, RBC, Plt, Hct, and Hgb.
1.3 REAGENTS
Beckman Coulter recommends these reagents:
C
r A
r A
r A
r A
r A
•T 5diff Diluent,
C
•T 5diff Fix,
C
•T 5diff WBC Lyse,
C
•T 5diff Hgb Lyse, and
C
•T 5diff Rinse.
USE AND FUNCTION
CONTROLS AND CALIBRATORS
C
•T 5diff Cal
1
These reagents are:
r Registered by the AFSSAPS (Agence Francaise de sécurité sanitaire des produits de
santé) and are For In Vitro Diagnostic Use.
r Manufactured by Coulter Corporation, Inc., Miami, Florida USA, and distributed by
Beckman Coulter France, SA 33 rue des Vanesses BP 50359 Villepinte 95942 Roissy CDG
Cedex.
All stated performance characteristics in this manual are based on the use of the A
C
•T 5diff
analyzer with the above-referenced reagents. Refer to the reagent’s bottle/container label for
detailed information, such as stability, before using the reagent.
ATTENTION:
The open container stability on the reagent labeling applies only to the reagent
when connected to the instrument with approved reagent pickups and caps.
For information on handling reagent waste, see
Waste Container
.
Waste Handling Procedures
Replacing the
and
PN 4237615B
1-7

USE AND FUNCTION
REAGENTS
AC•T 5diff Diluent
WARNING
water. Sodium azide preservative may form explosive compounds in metal drain lines. [See National
Institute for Occupational Safety and Health Bulletin: Explosive Azide Hazards (8/16/76).] When disposing
of reagents down the drain, flush with large volumes of water.
Risk of explosion if sodium azide is not properly flushed down the drain with large volumes of
Used for counting and differentiating blood cells, AC•T 5diff Diluent is clear and odorless.
Composed of stabilized saline solution containing an organic buffer and less than 0.1%
sodium azide, A
Dilutes whole-blood samples,
r
Stabilizes cell membranes for accurate counting and sizing,
r
Conducts aperture current, and
r
Rinses instrument components between analyses.
r
C
•T 5diff Diluent:
Handle as indicated in this manual. To be used at ambient temperature from 18°C to 25ºC up
to the expiration date indicated on the packaging.
AC•T 5diff Fix
Used to lyse erythrocytes, fix leukocytes, and differentially stain granules of monocytes,
neutrophils, and eosinophils, A
alcohol. A
C
•T 5diff Fix is composed of an alcohol solution containing propylene-glycol, a
formic dye, buffers, alkaline salts, wetting agents, and an aldehyde preservative.
C
•T 5diff Fix is a deep blue aqueous solution that smells like
Handle as indicated in this manual. To be used at ambient temperature from 18°C to 25ºC up
to the expiration date indicated on the packaging.
AC•T 5diff WBC Lyse
Used to lyse red blood cells for the leukocyte count and to differentiate poly-nuclear
basophils, A
C
•T 5diff WBC Lyse is a colorless, aqueous solution. It is composed of an acidic
solution containing a lytic agent.
Handle as indicated in this manual. To be used at ambient temperature from 18°C to 25ºC up
to the expiration date indicated on the packaging.
AC•T 5diff Hgb Lyse
Used to lyse blood cells and to determine hemoglobin concentration, AC•T 5diff Hgb Lyse is a
clear, aqueous solution and is composed of potassium cyanide at 0.035, a quarternary
ammonium salt, and a saline phosphate buffer containing less than 0.1% sodium azide.
Handle as indicated in this manual. To be used at ambient temperature from 18°C to 25ºC up
to the expiration date indicated on the packaging.
AC•T 5diff Rinse
Used as a rinsing agent, AC•T 5diff Rinse is a transparent liquid composed of an enzymatic
solution with proteolytic action.
1-8
Handle as indicated in this manual. To be used at ambient temperature from 18°C to 25ºC up
to the expiration date indicated on the packaging.
PN 4237615B
