COOK Medical Zilver Instructions For Use Manual

0123
EN
Zilver® Vascular Stent
5
Instructions for Use
FR
Endoprothèse vasculaire Zilver®
17
Mode d’emploi
IFU0043-9
*IFU0043-9*

a. Handle
d
e
hh
c
a
b
g
f
i
j
j
b. Hub
c. Safety Lock
d. Delivery System: Outer Sheath
e. Tip of Delivery System Inner Catheter
f. Side-arm Flushing Port
g. Metal Cannula
h. Radiopaque Markers on the Delivery System
(635 has no proximal radiopaque marker)
i. Inner Support Stylet
j. Gold Radiopaque Markers
a. Poignée
b. Embase
c. Verrou de sécurité
d. Système de largage : gaine externe
e. Extrémité du cathéter interne du système de largage
f. Orifice de rinçage du raccord latéral
g. Canule métallique
h. Marqueurs radio-opaques sur le système de largage
(635 ne porte pas de marqueur radio-opaque proximal)
i. Stylet interne de support
j. Marqueurs radio-opaques en or
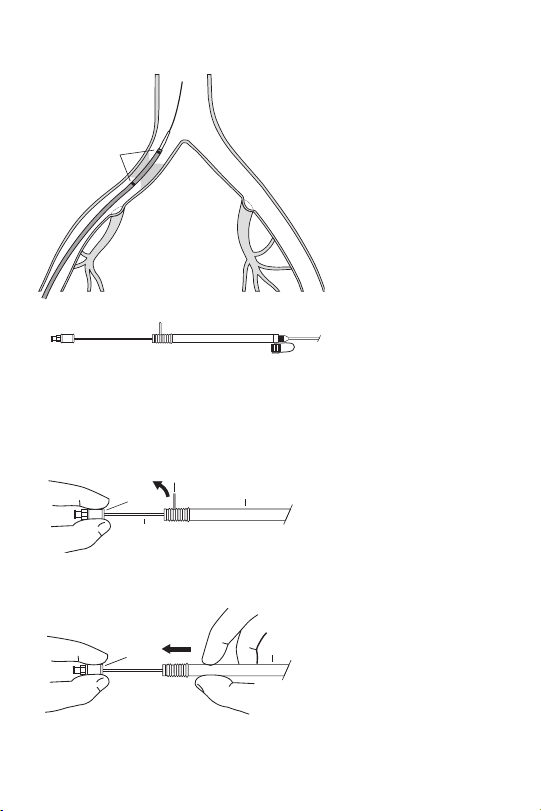
h
Fig. 1
Fig. 2
Fig. 3
c
b
a
g
b
a
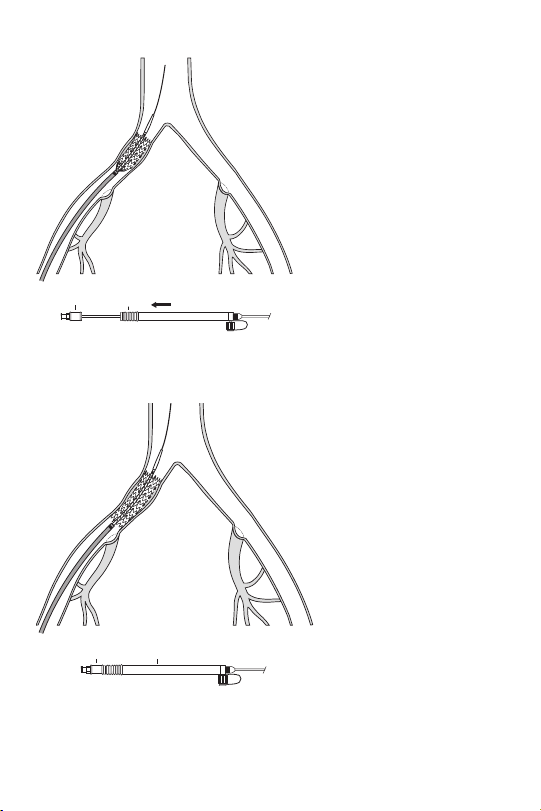
Fig.4
Fig. 5
b
a
b
a
ENGLISH
ZILVER® VASCULAR STENT
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician
(or properly licensed practitioner).
Do not re-sterilize.
DEVICE DESCRIPTION
The Zilver® Vascular Stent is a self-expanding stent made of nitinol. It is a flexible, slotted
tube that is designed to provide support while maintaining flexibility in the vessel upon
deployment. Post-deployment, the stent is designed to impart an outward radial force upon
the inner lumen of the vessel, establishing patency in the stented region.
The Zilver Vascular Stent is available in the following sizes:
5 French (1.67 mm)
Stent
Outer
Diameter
(mm)
The Zilver stent comes preloaded in 7.0, 6.0 and 5.0 French (2.3, 2.0 and 1.67 mm) delivery
catheters. Hand-loading of the stent is not possible. Stent deployment is controlled by
retraction of the handle while holding the metal cannula stationary.
INDICATIONS FOR USE
The Zilver Vascular Stent is intended for use as an adjunct to percutaneous transluminal
angioplasty (PTA) in the treatment of symptomatic vascular disease of the iliac arteries up to
100 mm in length with a reference vessel diameter of 5 to 9 mm. Patients should be suitable
candidates for PTA and/or stent treatment.
CONTRAINDICATIONS
There are no contraindications known at this time based on the clinical data.
WARNINGS
Persons allergic to nitinol (nickel titanium) may suffer an allergic reaction to this implant.
PRECAUTIONS
• This product should only be used by physicians trained and experienced in diagnostic
Delivery System
20 30 40 60 80 20 30 40 60 80 20 30 40 60 80
6 × × × × × × × × × × × × × × ×
7 × × × × × × × × × × × × × × ×
8 × × × × × × × × × × × × × × ×
9 × × × × × × × × × × × × × × ×
10 × × × × × × × × × × × × × × ×
and interventional vascular techniques. Standard techniques for interventional vascular
procedures should be employed.
6 French (2.0 mm)
Delivery System
Stent Length (mm)
5
7 French (2.3 mm)
Delivery System

• Manipulation of the Zilver Vascular Stent requires fluoroscopic control.
• Do not use power injection systems with the delivery system.
• Before insertion of the dilation catheter, appropriate antiplatelet and anticoagulant
therapy should be administered.
• Use in patients with a history of contrast sensitivity is not recommended unless the
patient can be adequately premedicated.
• Bench testing suggests that an increased potential for strut fracture may be associated with
overlapping of Zilver Vascular Stents in the peripheral vasculature, while animal studies
involving implantation of overlapped Zilver Vascular Stents in iliac ar teries did not result in
any detected strut fractures. Clinical data characterizing the incidence of fractures in
implanted Zilver Vascular Stents are not available.
• The long-term outcome following repeat dilatation of endothelialized stents is
unknown at present.
• Safety and effectiveness have not been demonstrated in:
• Patients with a history of bleeding diathesis or coagulopathy
• Patients with a history of iliac aneurysm
• Patients with a known pregnancy
• Lesions located within or beyond a bypass graft
• Pediatric patients
Stent Handling
• Do not attempt to remove the stent from the delivery system before use.
• Do not expose any part of the delivery system to organic solvents (e.g., alcohol).
• This device is designed for single use only. Attempts to reprocess, re-sterilize, and/or
reuse may lead to device failure and/or transmission of disease.
• Carefully inspect the sterile package and stent system prior to use to verify that neither
has been damaged during shipment.
• Use the stent system prior to the expiration date specified on the package.
Stent Placement
• Ensure that the red safety lock is not removed until ready for final stent release.
• Deploy the stent over an extra stiff or ultra stiff wire guide.
• Do not push the hub toward the handle during deployment.
• Do not rotate any part of the system during deployment.
• Avoid stent placement that may obstruct access to a vital side branch.
• If placement of multiple stents is required in a patient, to cover the length of the lesion,
the distal area of narrowing should be stented first, followed by the proximal locations
(i.e., a second stent should be placed proximally to the previously placed stent). Stents
placed in tandem must overlap to allow for complete coverage of the lesion.
• When more than one stent is required, resulting in stent-to-stent contact, stent
materials should be of similar composition to avoid the possibility of dissimilar metal
corrosion.
• Once stent deployment has begun, the stent must be fully deployed.
• Repositioning of the Zilver Vascular Stent is not possible since the delivery system’s
outer sheath cannot be re-advanced over the stent once deployment begins.
• Overstretching of the artery may result in rupture and life-threatening bleeding. Do not
overstretch the stent.
Stent/System Removal
Do not advance sheath after stent has been deployed. Delivery system can be removed
without the need to recapture tip.
6

Post Implant
• Appropriate antiplatelet/anticoagulant therapy should be administered post procedure.
• Use caution when re-crossing a stent to avoid stent damage or migration.
MR CONDITIONAL
Non-clinical testing has demonstrated that the Zilver Vascular Stent is MR Conditional. It can
be scanned safely under the following conditions:
• Static magnetic field of 3 Tesla or less
• Spatial gradient field of 720 Gauss/cm or less
• Whole body averaged specific absorption rate (SAR) of 1.5 W/kg (for a single stent
at 1.5 Tesla) and 3 W/kg (for a single stent at 3 Tesla and a pair of overlapping stents
at 1.5 and 3 Tesla) for 20 minutes (for a single stent at 1.5 Tesla) and 15 minutes of
scanning (for a single stent at 3 Tesla and a pair of overlapping stents at 1.5 and 3 Tesla),
respectively.
In non-clinical testing, the Zilver Vascular Stent produced maximum temperature rises of
0.1, 3.8, 0.8, and 0.1 degrees C (for a single stent at 1.5 Tesla, a pair of overlapping stents at
1.5 Tesla, a single stent at 3 Tesla, and a pair of overlapping stents at 3 Tesla, respectively) at
whole body averaged specific absorption rates (SAR) of 1.5 W/kg (for a single stent at 1.5 Tesla)
and 3 W/kg (for a single stent at 3 Tesla and a pair of overlapping stents at 1.5 and 3 Tesla)
for 20 minutes (for a single stent at 1.5 Tesla) and 15 minutes (for a single stent at 3 Tesla and
a pair of overlapping stents at 1.5 and 3 Tesla) of MR scanning in a 1.5 Tesla/64 MHz General
Electric MR scanner, a 1.5 Tesla Magnetom Siemens Medical Solutions MR Scanner (to evaluate
a pair of overlapping stents), and a 3 Tesla Excite General Electric MR scanner.
MR image quality may be compromised if the area of interest is in the exact same area or
relatively close to the position of the Zilver Vascular Stent. Therefore, it may be necessary to
optimize MR imaging parameters for the presence of this metallic implant.
Heating in the MRI environment for stents with fractured struts is not known.
POTENTIAL ADVERSE EVENTS
Potential adverse events that may occur include, but are not limited to, the following:
• Abrupt stent closure
• Allergic reaction to nitinol
• Amputation
• Angina/coronary ischemia
• Arrhythmia
• Arterial aneurysm
• Arterial rupture
• Arteriovenous fistula
• Atheroembolization (Blue Toe Syndrome)
• Death
• Embolism
• Fever
• Hematoma/hemorrhage
• Hypersensitivity reactions
• Hypotension/hypertension
• Infection/abscess formation at access site
• Intimal injury/dissection
7

• Ischemia requiring intervention (bypass or amputation of toe, foot
or leg)
• Myocardial infarction (MI)
• Pseudoaneurysm formation
• Pulmonary embolism
• Renal failure
• Restenosis of the stented artery
• Septicemia/bacteremia
• Stent malapposition
• Stent migration
• Stent strut fracture
• Stroke
• Spasm
• Tissue necrosis
• Worsened claudication/rest pain
SUMMARY OF CLINICAL INVESTIGATIONS
A pilot study of the safety of the Zilver Vascular Stent enrolled 20 patients at four investigative
sites and provided justification for initiation of a pivotal study to assess the safety and
effectiveness of the Zilver Vascular Stent.
A total of 151 patients at 24 U.S. investigative sites were enrolled in a pivotal study to evaluate
the safety and effectiveness of the Zilver Vascular Stent for use as an adjunct to percutaneous
transluminal angioplasty (PTA) in the treatment of symptomatic vascular disease of the iliac
arteries. The following is a summary of the pivotal study.
Study Endpoints
This prospective, non-randomized study of the Zilver Vascular Stent for the treatment of
stenotic or occlusive lesions of the external or common iliac arteries was intended to establish
the rate of major adverse events (MAE) at 9-month clinical follow-up as the primary study
endpoint compared to an Objective Performance Criterion (OPC) derived from literature
of recent studies in similar patient populations. The MAE rate of the OPC was set to be not
greater than 16%, with a 9% delta. Secondary endpoints included acute procedure success,
30-day clinical success, 9-month patency rate based on ultrasound examination, anklebrachial index, and 9-month functional status as measured by the walking impairment
questionnaire.
Patient Population
Patients eligible to enroll in this study had up to two documented stenotic (≤10 cm long) or
occluded (≤5 cm long) atherosclerotic lesions of the external iliac or common iliac artery on
opposite sides. Lesions could be either de novo or restenotic. Patients with previously stented
lesions were excluded. Characteristics of the patients enrolled in this study, including age,
gender, medical history as well as angiographic characteristics of the treated lesions (preprocedure), are included in Tables 2 and 3.
8

Table 2: Characteristics of Patients Implanted with the Zilver Vascular Stent
Baseline Characteristics
Age (Mean years +/- SD) 67 ± 8.9
Male Gender 93 61.6%
Smoking Status
Diabetes 46 30.5%
Hypercholesterolemia 109 72.2%
Hypertension 117 77.5%
Carotid Disease 49 32.5%
Renal Disease 23 15.2%
Pulmonary Disease 50 33.1%
Use of Antiplatelets 116 76.8%
CHF Class 3 or 4 7 4.6%
Previous MI 47 31.1%
Table 3: Angiographic Characteristics of the Lesions Prior to Treatment with the Zilver
Vascular Stent
Angiographic Characteristics
Lesion Length (mm) 168 32.9 ± 18.8
RVD (mm) 171 7.4 ± 1.5
In-Stent MLD (mm) 171 2.7 ± 1.4
% Diameter In-Stent Stenosis 171 64.5 ± 15.2
Methods
All patients underwent PTA (predilatation) of the target lesion prior to deployment of the
stent. Up to two lesions per patient on opposite sides were stented with no more than
two stents per lesion. Patients had an angiogram prior to and immediately following stent
placement. Duplex ultrasound to assess patency of the stented artery and common femoral
artery was performed no more than three days following the procedure. The protocol
recommended each hospital follow its standard protocol with respect to pre- and postprocedure medication; based on previous published studies, clopidogrel was suggested
before and post-procedure for 6 months. Patients underwent clinical follow-up at 1 and
9 months post-procedure. Clinical follow-up at 1 month included measurement of ABI on
the treated side as well as completion of a walking impairment questionnaire. Follow-up
Past 79 52.3%
Current 65 43.0%
Lesions
(N=177) Mean ± S.D.
Patients
(N=151)
9
at 9 months included measurement of ABI on the treated side as well as completion of the
walking impairment questionnaire, and an ultrasound to evaluate patency. In addition,
patients were contacted by telephone at 6 months post-procedure. All data were recorded
on case report forms at the investigative sites. Independent core laboratories were to analyze
angiographic and ultrasonic imaging.
Results
The primary study endpoint is the major adverse event (MAE) rate occurring within nine
months post-procedure. Major adverse events include death, MI (non-Q-wave and Q-wave),
target lesion revascularization, and limb loss on the same side as the treated lesion. Success
of the study required that the MAE rate be less than or equal to a predetermined objective
performance criterion (OPC) of 16%. All MAEs were also adjudicated with respect to their
relationship to the study device by an independent Clinical Events Committee.
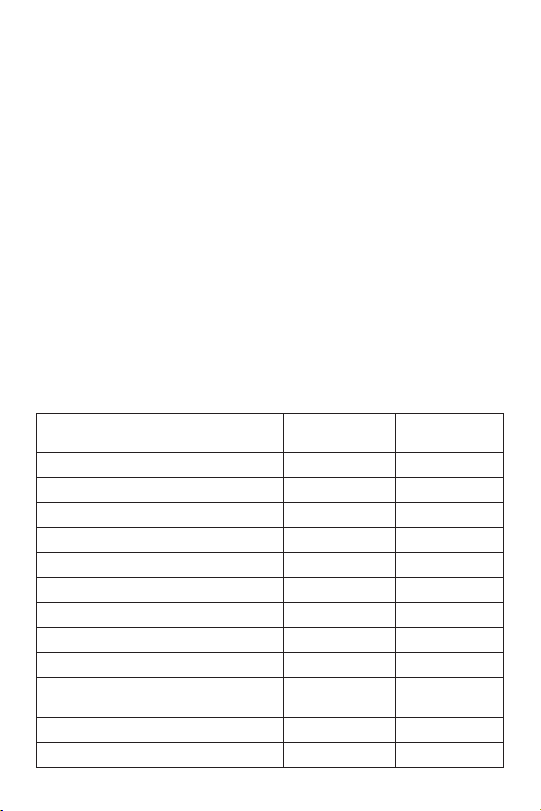
Table 4 presents the adverse events and complications reported in the pivotal study. Events
that occurred while the patients were hospitalized and cumulative events through 9 months
post-implant are presented. There were a total of 8 deaths, 3 myocardial infarctions (MI), 1
target lesion revascularization, and 1 limb loss. Two patients experienced 2 events each as
discussed below. All patients have completed their 9-month follow-up or reached a study
endpoint. Five (5) of the 151 patients (3.3%) have been confirmed as withdrawn or lost to
follow-up. Therefore, there were 146 evaluable patients available for assessment of MAE within
the entire 9-month follow-up period. This number (146) exceeds the sample size of 130 patients
determined a priori to be necessary to provide at least 80% power for this measure.
Table 4: Adverse Events/Complications Observed in Patients Implanted with the Zilver®
Vascular Stent
Adverse Event/Complication In-Hospital
(1)
Death
MI (Non-Q-Wave and Q-Wave)
(1)
2.6% (4/151) 5.3% (8/151)
0.7% (1/151) 2.0% (3/151)
Cumulative thru
9 Months
Target Lesion Revascularization 0.0% (0/151) 0.7% (1/151)
(1)
Limb Loss
0.0% (0/151) 0.7% (1/151)
Arterial Aneurysm/Rupture 0.0% (0/151) 0.0% (0/151)
Blood Loss Requiring Transfusion 3.3% (5/151) 4.6% (7/151)
Blue Toe Syndrome 0.0% (0/151) 0.7% (1/151)
Drug/Allergic Reactions Requiring Antibiotics 0.7% (1/151) 0.7% (1/151)
Embolism 0.0% (0/151) 0.0% (0/151)
Hematoma at Access Site Requiring
Intervention
1.3% (2/151) 1.3% (2/151)
Hemorrhagic Stroke with Deficit 0.0% (0/151) 0.0% (0/151)
Iliac Artery Spasm 0.0% (0/151) 0.0% (0/151)
10
 Loading...
Loading...