COOK Medical Hemospray Series Manual

MEDICAL
EN
Hemospray® Endoscopic Hemostat
3
Produit hémostatique
FR
6
endoscopiqueHemospray®
Dispositivo hemostático
ES
10
endoscópicoHemospray®
1.
2.
3.
4.
5.
2
1-2 sec
6.
7.
ENGLISH
INTENDED USE
This device is used for hemostasis of nonvariceal gastrointestinal bleeding.
NOTES
Do not use this device for any purpose other than stated intended use.
Store in a dry location, away from temperature extremes.
Use of this device is restricted to a trained healthcare professional.
DEVICE DESCRIPTION
Hemospray is an inert, bentonite powder developed for endoscopic
hemostasis. The powder is delivered by use of a carbon dioxide powered
delivery system and through a catheter inserted through the working
channel of an endoscope which provides access to the site of the bleed.
Each device contains approximately 20g of powder.
CONTRAINDICATIONS
Those specic to primary endoscopic procedure to be performed in
gaining access to desired target site. Also contraindicated in patients who
have gastrointestinal stulas, are suspected of having a gastrointestinal
perforation, or are at high risk of gastrointestinal perforation during
endoscopic treatment.
WARNING
Keep catheter tip at least 1 cm away from bleeding site to minimize risk of
embolization.
Hemospray has not been approved for use in pediatric populations, no
safety or eectiveness data exists and would be considered o-label usage,
to do so is at the professional risk of the surgeon/healthcare professional.
Patients with gastrointestinal bleeding that are on antithrombotic
medication may be at an increased risk of rebleeding. Follow the
3

relevant clinical guidelines for management of antithrombotic agents for
endoscopic procedures.
This device is designed for single use only. Attempts to reprocess,
resterilize, and/or reuse may lead to device failure and/or transmission of
disease.
If package is opened or damaged when received, do not use. Visually
inspect with particular attention to kinks, bends and breaks. If an
abnormality is detected that would prohibit proper working conditions, do
not use. Please notify Cook for return authorization.
PRECAUTIONS
For best results, Hemospray must be delivered to the source of bleeding.
Ensure gastrointestinal lumen is not distended because Hemospray
adds volume in excess of insuation volumes during procedure. Closely
monitor bowel distension and balance insuation and Hemospray
volumes as necessary.
Use of more than (3) Hemospray devices per patient may result in
impaction in colon and is not recommended.
Product contains 16 g CO cartridge. Contents under pressure. Do not
puncture or heat above 120° F/ 49° C. Do not inhale or discharge towards
face/body. Keep CO dispenser and cartridge out of reach of children.
Hemospray is inert and non-toxic.
As a granular material, unintentional exposure to the powder may cause
potential irritation to the skin, eyes and lungs.
In the event of unintended exposure to the powder refer to the following
First Aid measures:
Skin: Wash with soap and water until clean.
Eyes: Flush with water until irritation ceases.
Inhalation: Move to area free from powder. If symptoms of irritation
persist, contact physician. Inhalation may aggravate existing respiratory
illness.
Refer to package label for minimum channel size required for this device.
All endoscopic hemostatic therapies, including Hemospray, have an
associated risk of rebleeding,particularly in situations where the cause
of bleeding is an unresolved underlying disease. After hemostasis has
been achieved, monitor patients for rebleeding per the relevant Clinical
Guidelines.
Although not seen in clinical practice, there remains a theoretical risk of
aspiration of Hemospray powder resulting in respiratory complications. It is
prudent to restrict the use of Hemospray to 5cm below the UES.
PROCEDURAL PRECAUTIONS
Like other modalities, Hemospray may not be eective for all types of
bleeds. Gastrointestinal bleeding may exacerbate existing comorbidities,
increasing the potential for adverse events including patient mortality.
4

POTENTIAL COMPLICATIONS
Those associated with gastrointestinal endoscopy include, but are not
limited to: perforation, hemorrhage, aspiration, fever, infection, allergic
reaction to medication, hypotension, respiratory depression or arrest,
cardiac arrhythmia or arrest.
Use of Hemospray in the presence of bowel obstruction and/or an
anastomosis may pose a risk of injury due to over-distention.
Hemospray may occlude ducts and orices which communicate with the
main bowel lumen. Use caution when using Hemospray in the vicinity of
these orices.
Others include, but are not limited to: powder impaction in colon or
embolization.
When spraying in retroexed position, Hemospray powder may adhere to
the outside of the endoscope. This may result in diculty repositioning/
removing the endoscope, particularly if passing through a strictured area.
MRI SAFETY INFORMATION
While Adverse Eects related to MR safety have not been observed in
clinical use, Hemospray has not been evaluated for safety and compatibility
in the MR environment. It has not been tested for heating, migration, or
image artifact in the MR environment. The safety of Hemospray in the MR
environment is unknown. Scanning a patient who has this device may
result in patient injury.
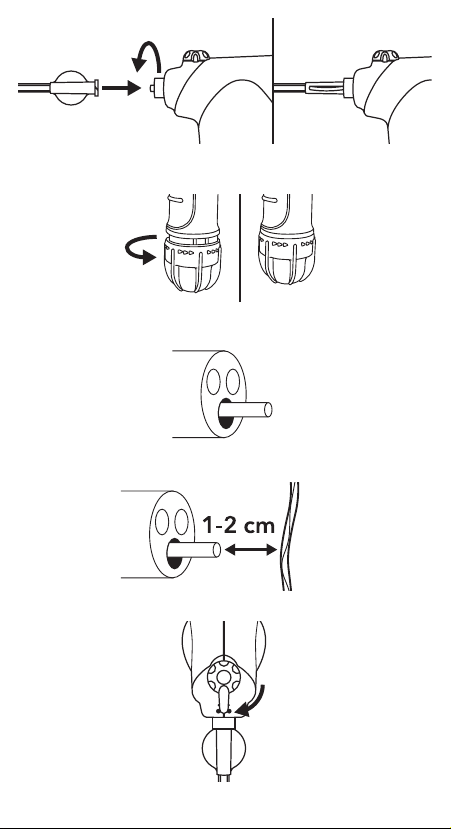
SYSTEM PREPARATION
1. Remove device from package and attach catheter to handle, ensuring
connection is secure (See Fig 1).
2. Activate CO cartridge by turning red activation knob until it stops.
Note: Do not over rotate knob as this could damage the device (See
Fig. 2). Note: Do not test device prior to insertion into endoscope
accessory channel as this may increase risk of catheter occlusion.
INSTRUCTIONS FOR USE
1. Before inserting catheter into accessory channel, identify bleeding site,
remove as much blood as possible, then ush accessory channel with
air. Caution: Ensure gastrointestinal lumen is not distended because
Hemospray adds volume during procedure.
2. Slowly advance catheter through accessory channel in short
increments until catheter tip is visualized endoscopically (See Fig. 3).
Precaution: To avoid catheter occlusion, do not place catheter directly
in contact with blood and/or mucosa, including any pooled blood and
do not aspirate blood while catheter is in accessory channel.
3. To ensure proper visibility, catheter tip should be 1-2 cm away from
bleeding site at all times (See Fig. 4).
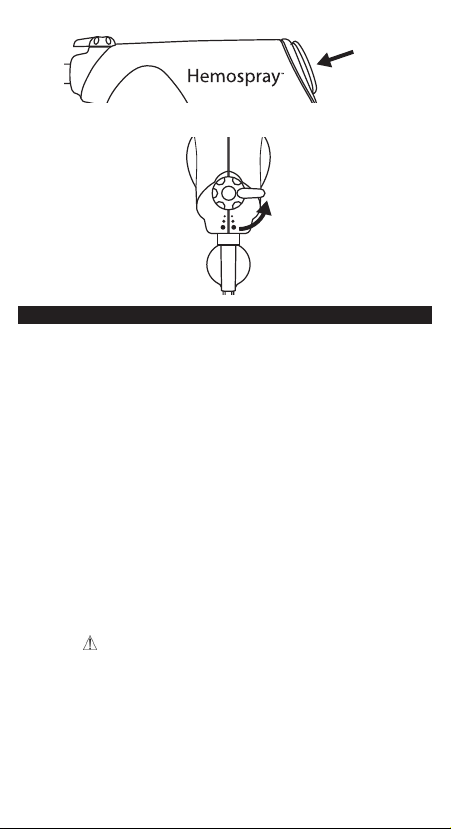
4. To allow powder deployment, turn red valve to open position (See Fig.
5). Note: Device is now active and ready for use. Do not press trigger
button until powder deployment is desired.
5. To deploy powder, hold handle upright and depress red trigger
button for 1-2 seconds and release (See Fig. 6). Continue applying
5
 Loading...
Loading...