COOK Medical Fusion OASIS Quick Reference Manual
®
OASIS
®
ONE
ACTION STENT INTRODUCTION SYS TEM
www.cookmedical.com
MEDICAL
QUICK REFERENCE GUIDE
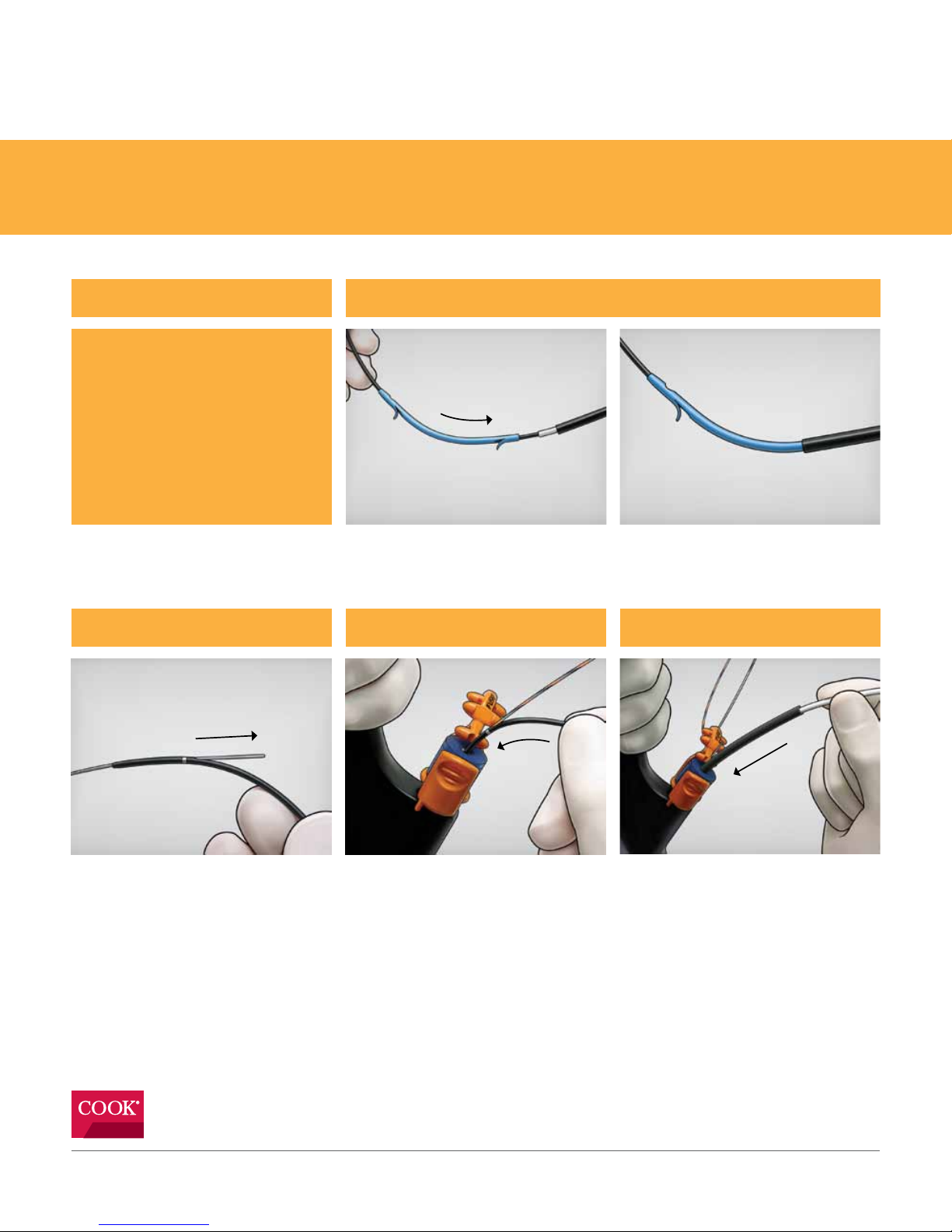
Step 1Tips
Place the desired stent and positioning sleeve onto the device, making sure the distal flap of the
stent is covered.
1) Fusion Oasis is compatible with
Cotton-Leung®, Cotton-Huibregste® and
ST-2 Soehendra Tannenbaum®.
2) The tapered tip end of the stent or
sideholes (if any) must be positioned in
the common bile duct while the other
end remains in the duodenum.
Step 2 Step 3
Backload the device onto pre-positioned wire
guide, ensuring the wire guide exits the IDE
port.
Unlock the wire guide from the Wire Guide
Locking Device and advance the device into
the endoscope accessory channel. Once the
IDE port is inside of the accessory channel,
relock the wire guide.
Step 4
Advance the device in short increments until
the stent is inside of the accessory channel,
then slide the positioning sleeve over the
pushing catheter, keeping it clear of the
accessory channel. Continue advancing
the device into the appropriate duct.
Customer Service
EMEA: EDI – www.cookmedical.com/edi.do
Distributors: +353 61239240, ssc.distributors@cookmedical.com
Austria: +43 179567121, oe.orders@cookmedical.com
Belgium: +32 27001633, be.orders@cookmedical.com
Denmark: +45 38487607, da.orders@cookmedical.com
Finland: +358 972519996, fi.orders@cookmedical.com
France: +33 171230269, fr.orders@cookmedical.com
Germany: +49 6950072804, de.orders@cookmedical.com
Hungary: +36 17779199, hu.orders@cookmedical.com
Ireland: +353 61239252, ie.orders@cookmedical.com
Italy: +39 0269682853, it.orders@cookmedical.com
Netherlands: +31 202013367, nl.orders@cookmedical.com
Norway: +47 23162968, no.orders@cookmedical.com
Spain: +34 912702691, es.orders@cookmedical.com
Sweden: +46 858769468, se.orders@cookmedical.com
Switzerland – French: +41 448009609, fr.orders@cookmedical.com
Switzerland – Italian: +41 448009609, it.orders@cookmedical.com
Switzerland – German: +41 448009609, de.orders@cookmedical.com
United Kingdom:
+44 2073654183, uk.orders@cookmedical.com
www.cookmedical.com
Americas:
EDI – www.cookmedical.com/edi.do
Phone: +1 812.339.2235, 800.457.4500, Fax: 800.554.8335
E-mail: orders@cookmedical.com
Australia:
Phone: +61 738411188, 1800777222, Fax: +61 738411288, 1800077283
E-mail: cau.custserv@cookmedical.com
MEDICAL
© COOK 2014 ESC-WM -19546- EN-201403
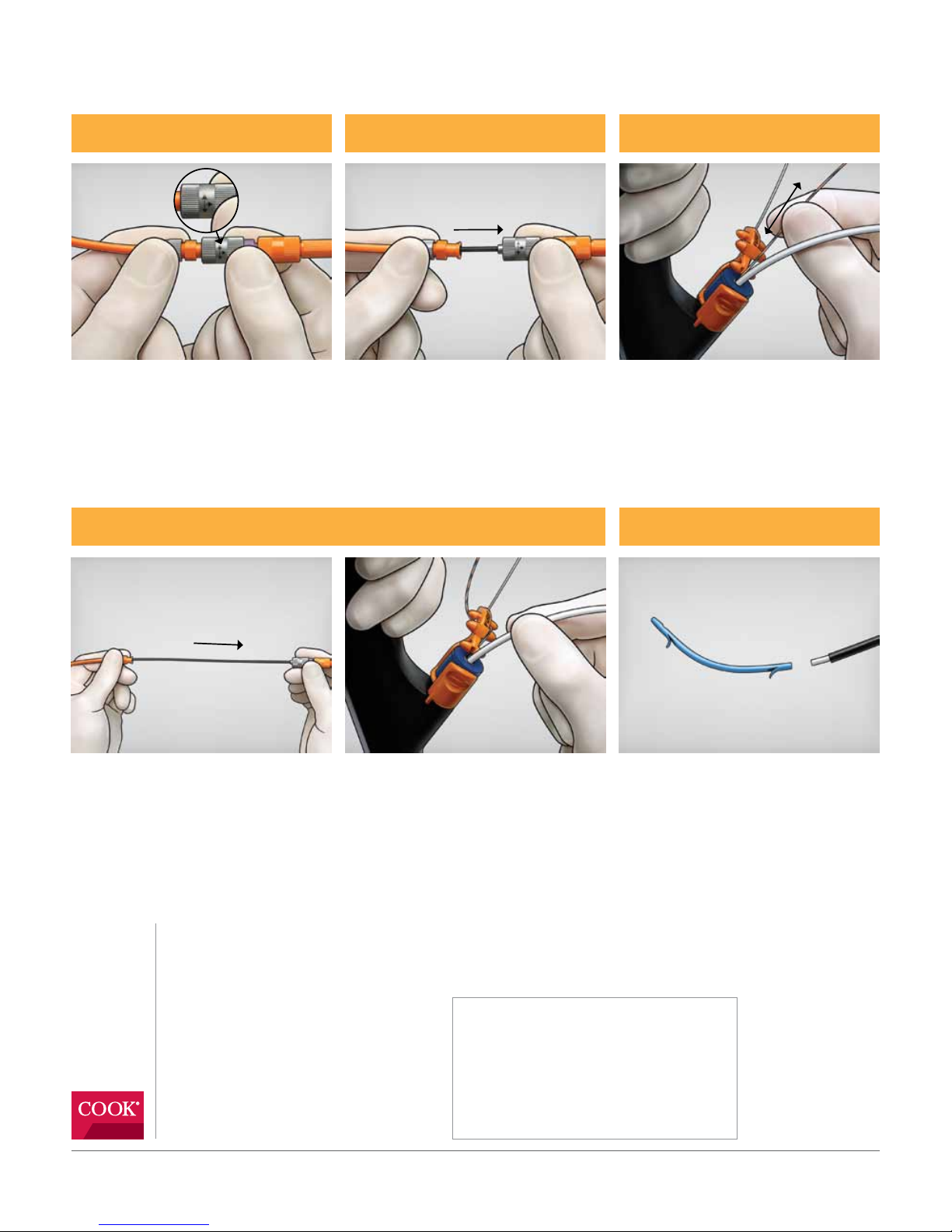
Step 9
The introducer, separated from the stent.
Step 8
To deploy the stent, retract the guiding catheter into the endoscope while maintaining the position
of the pushing catheter. Confirm the position of the stent once the guiding catheter is removed,
then remove the pushing catheter from the accessory channel.
Refer to current instructions for detailed system use.
Step 5 Step 6 Step 7
Fluoroscopically monitor the radiopaque band
on the distal end of the guiding catheter. When
a sufficient length of the guiding catheter is
above the stricture, disconnect the Luer lock
fitting (the gray connection with black arrows)
on the device.
Maintain position of the guiding catheter and
advance the stent into the desired position
using the pushing catheter. Fluoroscopically
and endoscopically confirm desired
stent placement.
Unlock the wire guide from the Wire Guide
Locking Device. Using fluoroscopy, retract the
wire guide until it exits the guiding catheter
at the IDE port. For multiple stent placement,
advance the disengaged wire guide to maintain
ductal access and relock the wire guide.
 Loading...
Loading...