CONTEC MEDICAL SYSTEMS CO.,LTD
Pulse Oximeter Probe user manual
Product name:
Product model: ESA0008,ESA0015,ESC0029,ESA0004,ESA0014,ESA0005,ESA0016,ESA0061
Scope of application:
This product is used to match CONTEC Patient Monitor 、Pulse Oximeter and Electronic sphygmomanometer ,collect and transmit the
signal from patient with continuance and no trauma. It is inapplicable to monitor the weak perfusion moving state and monitor for
SpO
2
long, so check the measuring position or change for another position per 4 hours.
Taboo disease:
Don't fix the product on the position with tissue injury. It is inapplicable for the patient or users allergic to PVC、TPU、TPE、ABS plastic.
Product performance:
1) The range of SpO2 measurement: 70%~100%;
Accuracy: 70~100%:±2%; Below 70%: unspecified.
2) The range of pulse measurement: 30~250bpm;
Accuracy: ±2bpm or ±2%(select larger).
3) Optical Sensor:
Red light (wavelength is 650~670nm, 6.65mW)
Infrared (wavelength is 880~910nm, 6.75mW)
Main configuration: Consisting of plug, cable and probe.
Power supply requirement:
and Electronic sphygmomanometer which are applicable to the requirements of IEC60601-1.
Directions for use:
This product is type BF applied part;
Note:
Pulse Oximeter Probe
The special power is supplied by from the equipments of CONTEC Patient Monitor 、Pulse Oximeter
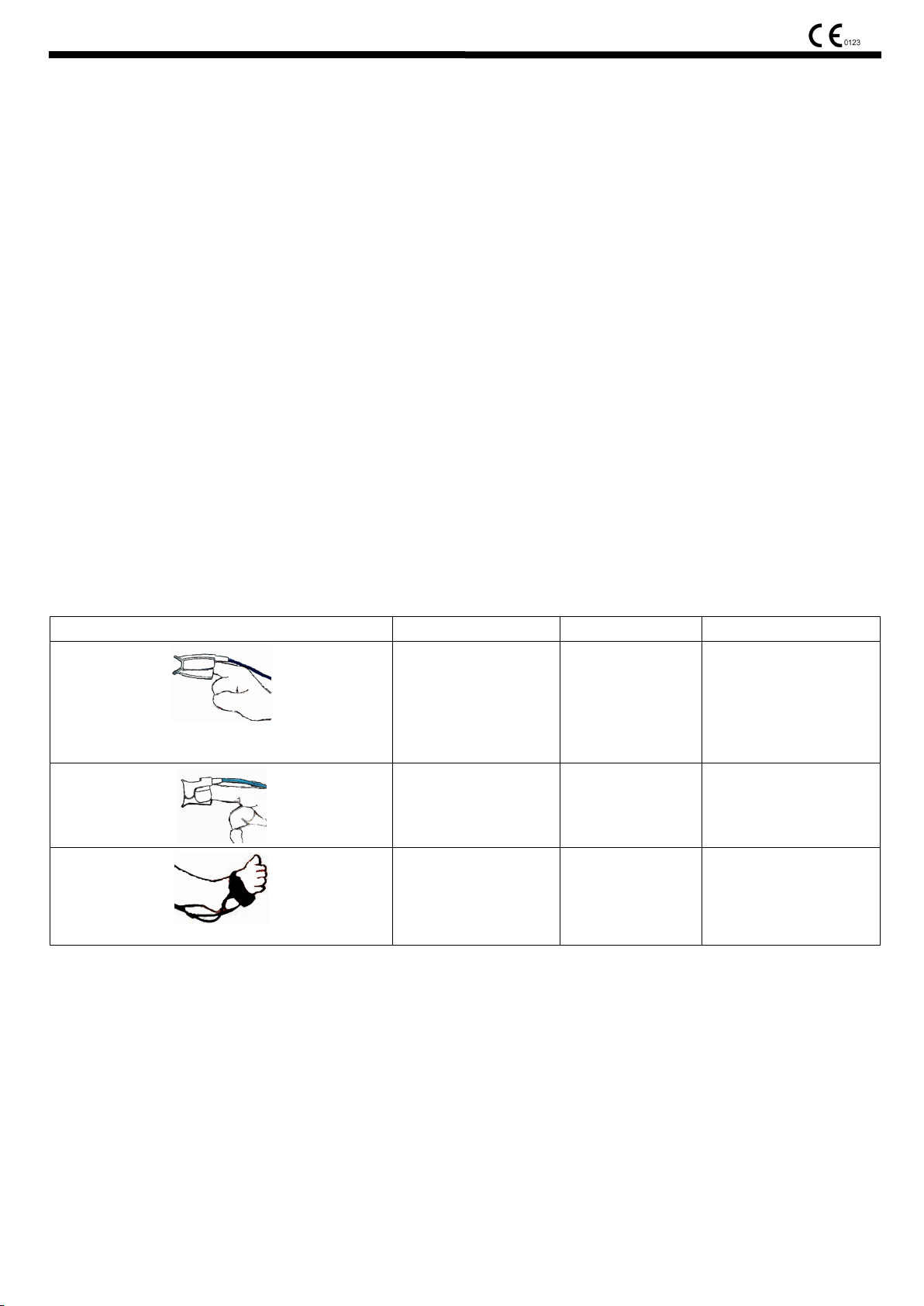
Sketch map Model explanation Applied crowds placement
Reusable adult
finger-clip SpO2 probe
(ESA0008,ESA0004,
ESA0005,ESA0014,
ESA0061)
Reusable wrap SpO2
Reusable child finger-clip
SpO2 probe
(ESA0015,ESA0016)
probe(type Y probe)
(ESC0029)
Weight>40Kg adult
Weight10~40kg
child
Weight3-10kg
neonate
Recommendatory
placement:forefinger
Recommendatory
placement:forefinger
Recommendatory
placement:sole of foot
Figure 1
1) As Figure 1, the pulse oximeter probe of different types is applied to different crowds.
2) Select proper probe and put recommendatory placement according to Figure 1.
3) Arrange the cable along the back of hand when place the pulse oximeter probe.
4) Connect Pulse oximeter probe with Pulse oximeter ,Patient Monitor or Electronic sphygmomanometer and check if the operating
procedure accords with the procedure introduced in user manual.
5) Pulse Oximeter probe ESC0029 needs the help of the FST0001 Pulse Oximeter probe extension cable to be connected in to the jack
of the Pulse Oximeter CMS60D,CMS70A or Patient Monitor CMS8000(old model),Pulse Oximeter probe ESC0029 needs the help of the
FST0004 Pulse Oximeter probe extension cable to be connected in to the jack of the Electronic Sphygmomanometer
CONTEC08A,Pulse Oximeter probe ESC0029 needs the help of the FST0002 Pulse Oximeter probe extension cable to be connected in
to the jack of the Patient Monitor PM50. Pulse Oximeter probe ESC0029 needs the help of the FST0014 Pulse Oximeter probe
extension cable to be connected in to the jack of the Patient Monitor CMS8000(new model)
1

CONTEC MEDICAL SYSTEMS CO.,LTD
Notice items:
1) pulse oximeter probe placement, the position without ductus arteriosus, BP cuff and vein input pipe is top-priority.
2) If the pulse oximeter probe can't monitor the state of pulsation, it shows that the position of probe is improper, or the position is too
thick, too thin or having too deep pigment to reach a proper translucidus effect. If above things has happened, place the probe again or
select probe of other type.
3) This pulse oximeter probe should be applied to the special medical equipment. Operator is responsible to check the compatibility
Incompatible fittings or device will influence the measuring result.
4) The disposal of scrap instrument and its accessories and packing (including battery, plastic bags, foams and paper boxes) should
follow the local laws and regulations.
Maintenance/cleaning/disinfection:
1) Check if the product is undamaged and clean before using.
2) This product is not allow to use disinfection liquid for disinfection, this probe belong to one-off products.
Note: Don't immerse the product in the liquid, and don't expose it under the strong ultra-violet radiation
Service life: Suggest this product use only once, don't use again.
Environment requirements:
Transport and storage
1) Temperature: -10℃~+40℃
2) Humidity: less than 80%
3) Pressure:86kPa~106kPa
Operating
1) Temperature: 10℃~+40℃
2) Humidity: 30% ~ 75%
3) Pressure: 700hPa~1060Pa
Statement:
1) pulse oximeter probe needs special precautions regarding EMC and needs to be installed and put into service according to the
EMC information provided in User Manual and test report.
2) Portable and mobile RF communications equipment can affect pulse oximeter probe.
Warning:
1) The use of cables other than those specified, with the exception of cables sold by CONTEC as replacement parts for internal
components, may result in increased emissions or decreased immunity of pulse oximeter probe.
2) pulse oximeter probe should not be used adjacent to or stacked with other equipment and that if adjacent or stacked use is necessary,
the pulse oximeter probe should be observed to verify normal operation in the configuration in which it will be used.
3) Improper usage can result in inaccurate measurement.
4) Using it under too strong light will cause inaccurate measurement, in case of that, please set a opaque stuff around the probe to cut
light off.
5) You should move the probe to other position per 4 hours at least. Because the state of local skin can influence the ability of skin to
enduring probe, it is necessary to replace the position of probe according to the state of patient. Please do that when skin integrity
changes.
6) The dyestuff in blood vessel cab cause the inaccurate measurement.
7) The performance of pulse oximeter probe is influenced by movement easily, so it is not suitable for active patient to use it.
8) Don't fix the probe with belt or bundle it tightly, because the vein pulsation can cause inaccurate SpO
9) Same as other medical equipment, the cable should be set properly to avoid enlacing or asphyxiate patient.
10) Don't use it in the process of MRI scan, because the conductor current may burn the skin of patient, moreover, the probe will
influence MRI image and MRI set will also influence the accuracy of SpO
11) Don't change the product at will, otherwise the capability or accuracy of product will be influenced.
12) The probe is not intended for use during patient transport outside the healthcare facility.
13) DO NOT use the probe while the patient is being scanned by MRI or CT.
2
measurement.
2
measurement.
2

CONTEC MEDICAL SYSTEMS CO.,LTD
Explanation about graphs and symbols used on the product:
Follow
instructions
for use
TYPE BF
APPLIED
PAR T
Lot number
SpO
Manufacturer
2
Date of
manufacture
Product
code
The pulse
oximeter
saturation
(%)
Authorized representative
in the community European
BPM
Keep away
from sunlight
WEEE
disposal
Pulse rate
(bpm)
Medical Device complies
IPX1
with Directive 93/42/EEC
Keep in a
cool, dry
Covering
Protection
rate
3
place

CONTEC MEDICAL SYSTEMS CO.,LTD
Guidance and manufacturer’s declaration – electromagnetic emission
The
is intended for use in the electromagnetic environment specified below. The
Emission test
Compliance
Electromagnetic environment – guidance
not likely to cause any interference in nearby electronic
table for use in all
establishments, including domestic establishments and
voltage power
Guidance and manufacture’s declaration – electromagnetic immunity
Electromagnetic
environment - guidance
synthetic material, the relative
(50/60Hz) magnetic
Power frequency magnetic fields
a typical location in a typical
Guidance and manufacturer’s declaration – electromagnetic emissions-for
pulse oximeter probe
pulse oximeter probe
customer of the user of the pulse oximeter probe should assure that it is used in such and environment.
RF emissions
CISPR 11
RF emission
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/ flicker
emissions
IEC 61000-3-3
Group 1
Class B
Not Applicable
Not Applicable
The pulse oximeter probe uses RF energy only for its internal
function. Therefore, its RF emissions are very low and are
equipment.
The pulse oximeter probe is sui
those directly connected to the public low-
supply network that supplies buildings used for domestic
purposes.
Guidance and manufacture’s declaration – electromagnetic immunity –
for pulse oximeter probe
The pulse oximeter probe is intended for use in the electromagnetic environment specified below. The customer or the user
of pulse oximeter probe should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level
Electrostatic
discharge (ESD)
IEC 61000-4-2
Power frequency
field
IEC61000-4-8
±6 kV contact
±8 kV air
3A/m 3A/m
±6 kV contact
±8 kV air
Floors should be wood, concrete or
ceramic tile. If floor are covered with
humidity should be at least 30%.
Should be at levels characteristic of
commercial or hospital environment.
4

CONTEC MEDICAL SYSTEMS CO.,LTD
Guidance and manufacturer’s declaration – electromagnetic immunity
The pulse oximeter probe is intended for use in the electromagnetic environment specified below. The customer or the
user of pulse oximeter probe should assure that it is used in such an environment.
level
Portable and mobile RF communications equipment
, including cables, than the
recommended separation distance calculated from
equation applicable to the frequency of the
Pd 2.1=
Pd 2.1=
Pd 3.2=
Field strengths from fixed RF transmitters, as
Interference may occur in the vicinity of equipment
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
reflection from structures, objects and people.
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
ent due to fixed RF transmitters, an electromagnetic site survey should be
d be observed to verify normal operation. If abnormal
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Guidance and manufacturer’s declaration – electromagnetic immunity –for
pulse oximeter probe that are not LIFE-SUPPORTING
Immunity test IEC 60601 test level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Compliance
3 V
3 V/m
Electromagnetic environment - guidance
should be used no closer to any part of the pulse
oximeter probe
the
transmitter.
Recommended separation distance
80 MHz to 800 MHz
800 MHz to 2.5 GHz
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in metres (m).
determined by an electromagnetic site survey,a should
be less than the compliance level in each frequency
b
range.
marked with the following symbol:
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
a
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To
assess the electromagnetic environm
considered. If the measured field strength in the location in which the pulse oximeter probe is used exceeds the applicable
RF compliance level above, the pulse oximeter probe shoul
performance is observed, additional measures may be necessary, such as reorienting or relocating the pulse oximeter
probe.
b
5

CONTEC MEDICAL SYSTEMS CO.,LTD
pulse oximeter probe
The pulse oximeter probe is intended for use in an electromagnetic environment in which radiated RF disturbances are
can help prevent electromagnetic interference by
oximeter probe as recommended below, according to the maximum output power of the communications equipment.
Pd
2
.1
=
Pd 2.1=
Pd 3.2=
0.12
0.12
0.23
0.37
0.37
0.74
1.17
1.17
2.33
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m)
reflection from structures, objects and people.
Address
Tel :
Fax: +86
E
Recommended separation distances between portable and mobile
RF communications equipment and pulse oximeter probe–
for pulse oximeter probe that are not LIFE-SUPPORTING
Recommended separation distances between
portable and mobile RF communications equipment and the
controlled. The customer or the user of the pulse oximeter probe
maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the pulse
Rated maximum output
power of transmitter
(W)
0.01
0.1
1
10
100
can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power
rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
150 kHz to 80 MHz
Separation distance according to frequency of transmitter
(m)
3.69
11.67
80 MHz to 800 MHz
3.69
11.67
800 MHz to 2.5 GHz
7.38
23.33
No.112 Qinhuang West Street, Economic&Technical Development Zone,
:
066004,Qinhuangdao,Hebei Province,PEOPLE'S REPUBLIC OF CHINA
+86-335-8015430
-335-8015430
CONTEC MEDICAL SYSTEMS CO., LT D
Shanghai International Holding Corp. GmbH (Europe)
-mail: cms@contecmed.com.cn
Address: Eiffestrasse 80, 20537, Hamburg, Germany
Tel : +49-40-2513175
Fax: +49-40-255726
E-mail: shholding@hotmail.com
File No.: CMS2.782.G002(CE)ESS/1.6
File Ver.: 1.6
6
Release Date: 2020.06
 Loading...
Loading...